Utilization of Ground Eggshell as a Biofiller of Plasticized PVC-Based Materials Fabricated Using Melt Blending
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Eggshell Filler (ES) Preparation
2.3. Materials Processing
2.4. Testing Methods of ES Filler and pPVC Composites
2.4.1. ES Analysis
2.4.2. Methodology for Testing pPVC and Composites
3. Results
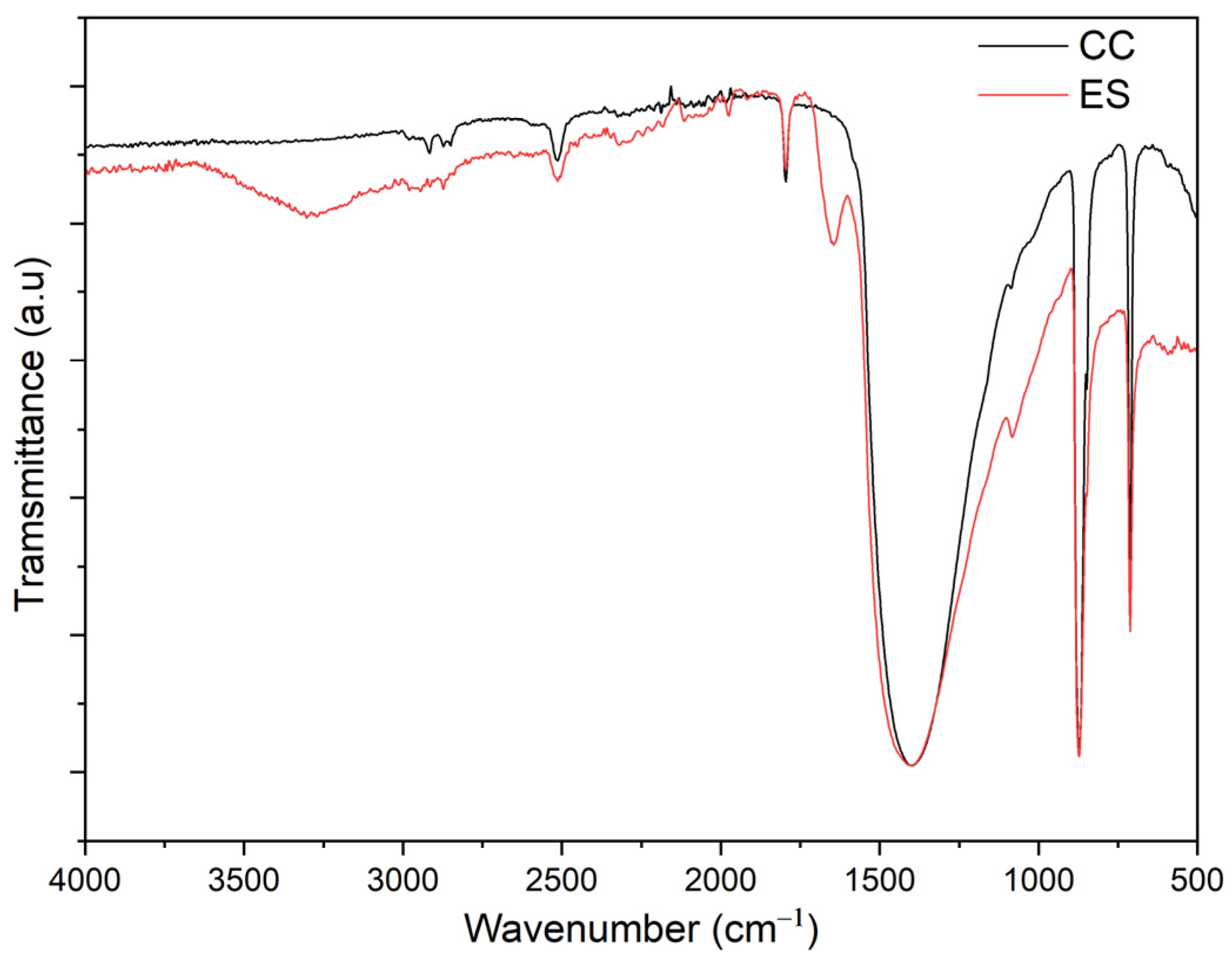
3.1. FTIR Analysis of ES
3.2. Particle Size Analysis of ES
3.3. TGA Analysis of ES
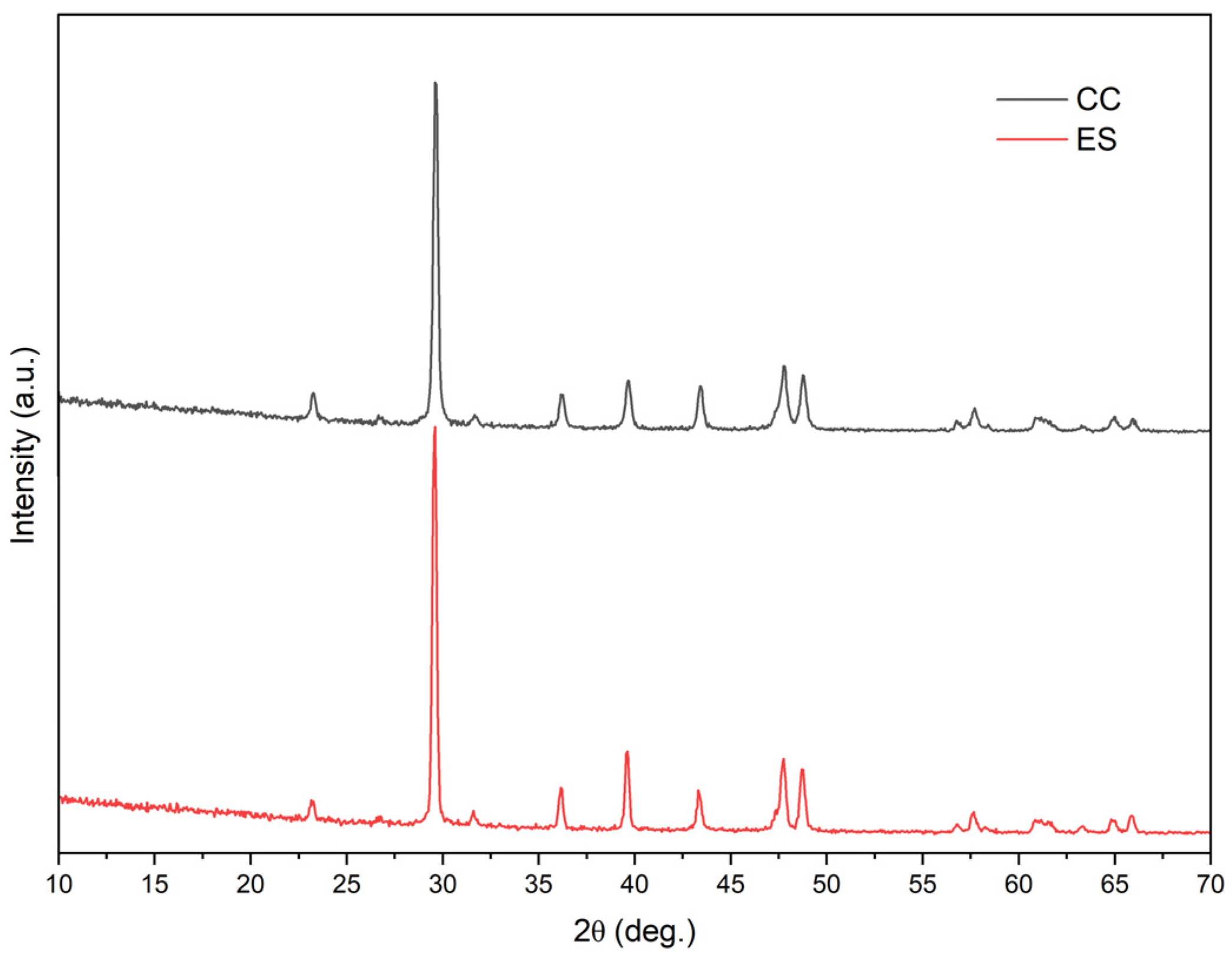
3.4. XRD Results
3.5. Plastographometric Analysis of pPVC and Composites
3.6. Chromatic Properties of Fillers and Polymeric Materials
- 0 < ΔE < 1—does not notice the difference,
- 1 < ΔE < 2—only an experienced observer will notice the difference,
- 2 < ΔE < 3.5—the inexperienced observer also notices the difference,
- 3.5 < ΔE < 5—notices a clear colour difference,
- 5 < ΔE—the observer gets the impression of two different colours.
| Sample | Colour Parameters | |||||
|---|---|---|---|---|---|---|
| L* | a* | b* | ΔE | ΔE (ES:CC) | Colour | |
| ES | 89.07 | 0.74 | 6.23 | - | ||
| CC | 95.13 | 0.14 | 4.12 | - | ||
| pPVC | 74.64 | 0.84 | 33.51 | 0 | ||
| pPVC/10ES | 72.48 | 2.77 | 25.93 | 8.1 | 4.5 | |
| pPVC/20ES | 73.02 | 2.99 | 23.13 | 10.7 | 5.4 | |
| pPVC/30ES | 71.53 | 3.00 | 21.67 | 12.4 | 7.4 | |
| pPVC/40ES | 71.57 | 3.04 | 20.79 | 13.3 | 6.0 | |
| pPVC/10CC | 76.57 | 1.15 | 26.60 | 7.2 | 4.5 | |
| pPVC/20CC | 76.64 | 0.81 | 19.82 | 13.8 | 5.4 | |
| pPVC/30CC | 77.01 | 0.55 | 17.28 | 16.4 | 7.4 | |
| pPVC/40CC | 75.45 | 0.45 | 16.97 | 16.6 | 6.0 | |
3.7. Density and Porosity
3.8. Mechanical Properties
3.9. Analysis of Thermal Properties
4. Summary
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CC | Commercial Calcium Carbonate |
| CIE L*a*b* | Commission Internationale De l’Eclairage (CIE) By Measuring The L*a*b* Colour |
| Cl• | Chlorine Radical |
| DTG | Derivative Thermogravimetry |
| EOS | Epoxidised Soybean Oil |
| ES | Filler From Eggshell Waste |
| Et | Modulus Of Elasticity |
| FTIR | Fourier-Transform Infrared Spectroscopy |
| GCC | Ground Calcium Carbonate |
| HCL | Hydrogen Chloride |
| M | Torque |
| PCC | Precipitated Calcium Carbonate |
| phr | Part Per Hundred |
| pPVC | Plasticized PVC |
| PVC | Poly(Vinyl Chloride) |
| T | Temperature |
| TGA | Thermogravimetric Analysis |
| tts | Time of Thermal Stability |
| XRD | X-ray Diffraction |
| ΔE | Total Colour Difference Parameter |
| ΔT5 | Differences In T5 Values Between The Unfilled Matrix And The Composites |
| εB | Elongation At Brake |
| σM | Tensile Strength |
| CC | Commercial Calcium Carbonate |
References
- Vandeginste, V. Food Waste Eggshell Valorization through Development of New Composites: A Review. Sustain. Mater. Technol. 2021, 29, e00317. [Google Scholar] [CrossRef]
- Quina, M.J.; Soares, M.A.R.; Quinta-Ferreira, R. Applications of Industrial Eggshell as a Valuable Anthropogenic Resource. Resour. Conserv. Recycl. 2017, 123, 176–186. [Google Scholar] [CrossRef]
- Chen, W.; Wu, Y.; Xie, Z.; Li, Y.; Tang, W.; Yu, J. Calcium Hydroxide Recycled from Waste Eggshell Resources for the Effective Recovery of Fluoride from Wastewater. RSC Adv. 2022, 12, 28264–28278. [Google Scholar] [CrossRef]
- Ummartyotin, S.; Manuspiya, H. A Critical Review of Eggshell Waste: An Effective Source of Hydroxyapatite as Photocatalyst. J. Met. Mater. Miner. 2018, 28, 124–135. [Google Scholar] [CrossRef]
- Mignardi, S.; Archilletti, L.; Medeghini, L.; De Vito, C. Valorization of Eggshell Biowaste for Sustainable Environmental Remediation. Sci. Rep. 2020, 10, 2436. [Google Scholar] [CrossRef] [PubMed]
- Adams, S.M.; Atikpo, E.; Aigbodion, V.S.; Romanus, N.; Odo, L.I. CaCO3 Derived from Eggshell Waste for Improving the Hardness Values and Wear Behavior of Composite Coating on Mild Steel via Co-Deposition. Int. J. Adv. Manuf. Technol. 2022, 119, 5483–5496. [Google Scholar] [CrossRef]
- Chi, Y.; Lin, M.; Zuo, D.; Wang, H.; Chi, Y. Eggshell Waste Separation Process Assisted with Pressure-Vacuum: Process Conditions and Optimization. J. Food Sci. 2023, 88, 356–366. [Google Scholar] [CrossRef]
- Ngayakamo, B.; Onwualu, A.P. Recent Advances in Green Processing Technologies for Valorisation of Eggshell Waste for Sustainable Construction Materials. Heliyon 2022, 8, e09649. [Google Scholar] [CrossRef]
- Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 30 November 2024).
- Kowalska, E.; Kucharska-Gaca, J.; Kuźniacka, J.; Lewko, L.; Gornowicz, E.; Biesek, J.; Adamski, M. Egg Quality Depending on the Diet with Different Sources of Protein and Age of the Hens. Sci. Rep. 2021, 11, 2638. [Google Scholar] [CrossRef]
- Ogundipe, O.L.; Adediran, A.A.; Ikubanni, P.P.; Adekanye, T.A.; Ajisegiri, E.S.A.; Ikumapayi, O.M.; Afolalu, S.A. Influence of Eggshell Addition on the Properties of Natural Fibre-Reinforced Epoxy Composite—A Mini-Review. In Proceedings of the 2024 International Conference on Science, Engineering and Business for Driving Sustainable Development Goals (SEB4SDG), Omu-Aran, Nigeria, 2–4 April 2024; pp. 1–7. [Google Scholar] [CrossRef]
- Strelec, I.; Ostojčić, M.; Brekalo, M.; Hajra, S.; Kim, H.J.; Stanojev, J.; Maravić, N.; Budžaki, S. Transformation of Eggshell Waste to Egg White Protein Solution, Calcium Chloride Dihydrate, and Eggshell Membrane Powder. Green Process. Synth. 2023, 12, 20228151. [Google Scholar] [CrossRef]
- Ahmed, T.A.E.; Wu, L.; Younes, M.; Hincke, M. Biotechnological Applications of Eggshell: Recent Advances. Front. Bioeng. Biotechnol. 2021, 9, 675364. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, M.M. Sustainable and Environmentally Friendly Microwave Synthesis of Nano-Hydroxyapatite from Decarbonized Eggshells. Materials 2024, 17, 1832. [Google Scholar] [CrossRef]
- Bezerra, E.B.; Wellen, R.M.R.; Barreto Luna, C.B.; da Silva Barbosa Ferreira, E.; do Nascimento, E.P.; Araújo, E.M. Toward the Production of Biopolyethylene-Based Ecocomposites with Improved Performance: The Potential of Eggshell Particles as an Ecological Additive. J. Appl. Polym. Sci. 2024, 141, e55439. [Google Scholar] [CrossRef]
- Szymandera-Buszka, K.; Kobus-Cisowska, J.; Szymanowska-Powałowska, D.; Rezler, R.; Szczepaniak, O.; Marciniak, G.; Piechocka, J.; Jarzębski, M. Synergic Effect of Selected Ingredients and Calcium Chloride on the Technological, Molecular and Microbial Usefulness of Eggshells and Their Impact on Sensory Properties in a Food Model System. Int. J. Mol. Sci. 2021, 22, 2029. [Google Scholar] [CrossRef]
- Sowińska-Baranowska, A.; Maciejewska, M. Potential Utilization of Ground Eggshells as a Biofiller for Natural Rubber Biocomposites. Materials 2023, 16, 2988. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.D.C.; Brites, P.; Martins, C.; Nunes, C.; Coimbra, M.A.; Ferreira, P.; Gonçalves, I. Starch Consolidation of Calcium Carbonate as a Tool to Develop Lightweight Fillers for LDPE-Based Plastics. Int. J. Biol. Macromol. 2023, 226, 1021–1030. [Google Scholar] [CrossRef] [PubMed]
- Ben Aribia, W.; Trigui, A.; Alshammari, N.K.; Abdelmoleh, M. Development of Phase Change Eco-Composite Materials from Eggshell Waste. Green Chem. Lett. Rev. 2024, 17, 380060. [Google Scholar] [CrossRef]
- Kuciel, S.; Rusin-Żurek, K.; Kurańska, M. The Influence of Filler Particle Size on the Strength Properties and Mechanical Energy Dissipation Capacity of Biopoly(Ethylene Terephthalate) BioPET/Eggshell Biocomposites. Recycling 2024, 9, 88. [Google Scholar] [CrossRef]
- Dev, B.; Rahman, M.A.; Khan, A.N.; Siddique, A.B.; Alam, M.R.; Rahman, M.Z. Eggshell Bio-Filler Integration in Jute/Banana Fiber-Reinforced Epoxy Hybrid Composites: Fabrication and Characterization. J. Appl. Polym. Sci. 2024, 141, e56155. [Google Scholar] [CrossRef]
- Wibowo, C.H.; Ubaidillah, U.; Ariawan, D.; Surojo, E.; Nugroho, K.C.; Sunardi, S. A Scientometric Analysis of Eggshell-Based Composite Literature with Research Mapping Knowledge. Discov. Appl. Sci. 2024, 6, 401. [Google Scholar] [CrossRef]
- Trigui, A.; Aribia, W.B.; Akrouti, A.; Znaidia, S.; Alshammari, N.K.; Abdelmouleh, M. Latent Heat Storage Bio-Composites from Egg-Shell/PE/PEG as Feasible Eco-Friendly Building Materials. Polym. Compos. 2024, 45, 11416–11433. [Google Scholar] [CrossRef]
- Thomas, S.P. Interaction of Silica with Polystyrene: Mechanical Properties, Polymer/Filler Adhesion and Failure Behavior. Chim. Techno Acta 2024, 11, 202411105. [Google Scholar] [CrossRef]
- Cree, D.; Soleimani, M. Bio-Based White Eggshell as a Value-Added Filler in Poly(Lactic Acid) Composites. J. Compos. Sci. 2023, 7, 278. [Google Scholar] [CrossRef]
- Klapiszewski, Ł.; Tomaszewska, J.; Skórczewska, K.; Jesionowski, T. Preparation and Characterization of Eco-Friendly Mg(OH)2/Lignin Hybrid Material and Its Use as a Functional Filler for Poly(Vinyl Chloride). Polymers 2017, 9, 258. [Google Scholar] [CrossRef] [PubMed]
- Schiller, M. PVC Additives Performance, Chemistry, Developments, and Sustainability; Hanser Publications: Cincinnati, OH, USA, 2013; ISBN 978-1-56990-543-2. [Google Scholar]
- Erdogan, N.; Eken, H.A. Precipitated Calcium Carbonate Production, Synthesis and Properties. Physicochem. Probl. Miner. Process. 2017, 53, 57–68. [Google Scholar] [CrossRef]
- Jembere, A.L.; Genet, M.B.; Sintayehu, B. Evaluation of Precipitated CaCO3 Produced from Locally Available Limestone as a Reinforcement Filler for PVC Pipe. Sci. Rep. 2024, 14, 11234. [Google Scholar] [CrossRef]
- Grossman, R.F. Handbook of Vinyl Formulating, 2nd Edition; Wiley: Hoboken, NJ, USA, 2008; ISBN 978-0-470-25354-0. [Google Scholar]
- Owuamanam, S.; Cree, D. Progress of Bio-Calcium Carbonate Waste Eggshell and Seashell Fillers in Polymer Composites: A Review. J. Compos. Sci. 2020, 4, 70. [Google Scholar] [CrossRef]
- Boronat, T.; Fombuena, V.; Garcia-Sanoguera, D.; Sanchez-Nacher, L.; Balart, R. Development of a Biocomposite Based on Green Polyethylene Biopolymer and Eggshell. Mater. Des. 2015, 68, 177–185. [Google Scholar] [CrossRef]
- Arzate-Vázquez, I.; Méndez-Méndez, J.V.; Flores-Johnson, E.A.; Nicolás-Bermúdez, J.; Chanona-Pérez, J.J.; Santiago-Cortés, E. Study of the Porosity of Calcified Chicken Eggshell Using Atomic Force Microscopy and Image Processing. Micron 2019, 118, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Wang, S.; Nie, F.; Feng, L.; Zhu, G.; Jiang, L. Elaborate Architecture of the Hierarchical Hen’s Eggshell. Nano Res. 2011, 4, 171–179. [Google Scholar] [CrossRef]
- Baláž, M. Ball Milling of Eggshell Waste as a Green and Sustainable Approach: A Review. Adv. Colloid Interface Sci. 2018, 256, 256–275. [Google Scholar] [CrossRef]
- Kiryakova, D.; Kolchakova, G. Effect of Vinyltrimethoxysilane Surface Treatment and Immersion in Water on the Tensile Behaviors of Eggshells Polyvinyl Chloride Films Prepared By Solution Casting. Chem. Chem. Technol. 2024, 18, 30–36. [Google Scholar] [CrossRef]
- Skórczewska, K.; Lewandowski, K.; Szewczykowski, P.; Wilczewski, S.; Szulc, J.; Stopa, P.; Nowakowska, P. Waste Eggshells as a Natural Filler for the Poly(Vinyl Chloride) Composites. Polymers 2022, 14, 4372. [Google Scholar] [CrossRef]
- Khaleghi, M. Experimental and Computational Study of Thermal Behavior of PVC Composites Based on Modified Eggshell Biofiller for UPVC Product. J. Polym. Res. 2022, 29, 2. [Google Scholar] [CrossRef]
- Sharmeeni, M.; Munusamy, Y.; Ismail, H. The Effect of Blending Sequence on the Structure and Properties of Poly(Vinyl Chloride)/Chicken Eggshell Powder Composites. J. Vinyl Addit. Technol. 2017, 23, 298–304. [Google Scholar] [CrossRef]
- Murugan, S.; Munusamy, Y.; Ismail, H. Effects of Chicken Eggshell Filler Size on the Processing, Mechanical and Thermal Properties of PVC Matrix Composite. Plast. Rubber Compos. 2017, 46, 42–51. [Google Scholar] [CrossRef]
- Kiryakova, D.; Kolchakova, G. Preparation of Silane-Treated Eggshells Polyvinyl Chloride Films by Co-Precipitation: Effect of Vinyltrimethoxysilane Surface Treatment on the Tensile Properties. Key Eng. Mater. 2024, 981, 125–134. [Google Scholar] [CrossRef]
- Marceneiro, S.; Lobo, I.; Dias, I.; de Pinho, E.; Dias, A.M.A.; de Sousa, H.C. Eco-Friendlier and Sustainable Natural-Based Additives for Poly(Vinyl Chloride)-Based Composites. J. Ind. Eng. Chem. 2022, 110, 248–261. [Google Scholar] [CrossRef]
- Jia, P.; Zhang, M.; Hu, L.; Zhou, Y. Green Plasticizers Derived from Soybean Oil for Poly(Vinyl Chloride) as a Renewable Resource Material. Korean J. Chem. Eng. 2016, 33, 1080–1087. [Google Scholar] [CrossRef]
- Available online: http://Familyhome.by/Produktsiya/40-Plastiki/Produkty-Proizvodimye-v-Chekhii/89-Omyacarb-2t-va.Html (accessed on 10 December 2024).
- Lewandowski, K.; Skórczewska, K.; Piszczek, K.; Manikowski, M.; Mirowski, J. Modification of Rigid Poly(Vinyl Chloride) for Application in Three-Layer Feed Pipes. Polimery/Polymers 2020, 65, 304–310. [Google Scholar] [CrossRef]
- Lewandowski, K.; Skórczewska, K.; Piszczek, K.; Urbaniak, W. Recycled Glass Fibres from Wind Turbines as a Filler for Poly(Vinyl Chloride). Adv. Polym. Technol. 2019, 2019, 8960503. [Google Scholar] [CrossRef]
- Bociaga, E.; Trzaskalska, M. Influence of Polymer Processing Parameters and Coloring Agents on Gloss and Color of Acrylonitrile-Butadiene-Styrene Terpolymer Moldings. Polimery/Polymers 2016, 61, 544–550. [Google Scholar] [CrossRef]
- Hejna, A.; Barczewski, M.; Skórczewska, K.; Szulc, J.; Chmielnicki, B.; Korol, J.; Formela, K. Sustainable Upcycling of Brewers’ Spent Grain by Thermo-Mechanical Treatment in Twin-Screw Extruder. J. Clean. Prod. 2021, 285, 124839. [Google Scholar] [CrossRef]
- Barczewski, M.; Hejna, A.; Sałasińska, K.; Aniśko, J.; Piasecki, A.; Skórczewska, K.; Andrzejewski, J. Thermomechanical and Fire Properties of Polyethylene-Composite-Filled Ammonium Polyphosphate and Inorganic Fillers: An Evaluation of Their Modification Efficiency. Polymers 2022, 14, 2501. [Google Scholar] [CrossRef]
- PN-EN ISO 527; Plastics—Determination of Tensile Properties. ISO: Geneva, Switzerland, 2019.
- Rodríguez-Navarro, A.B.; Domínguez-Gasca, N.; Muñoz, A.; Ortega-Huertas, M. Change in the Chicken Eggshell Cuticle with Hen Age and Egg Freshness. Poult. Sci. 2013, 92, 3026–3035. [Google Scholar] [CrossRef] [PubMed]
- Torit, J.; Phihusut, D. Phosphorus Removal from Wastewater Using Eggshell Ash. Environ. Sci. Pollut. Res. 2019, 26, 34101–34109. [Google Scholar] [CrossRef] [PubMed]
- Tizo, M.S.; Blanco, L.A.V.; Cagas, A.C.Q.; Dela Cruz, B.R.B.; Encoy, J.C.; Gunting, J.V.; Arazo, R.O.; Mabayo, V.I.F. Efficiency of Calcium Carbonate from Eggshells as an Adsorbent for Cadmium Removal in Aqueous Solution. Sustain. Environ. Res. 2018, 28, 326–332. [Google Scholar] [CrossRef]
- Mishra, S.; Shimpi, N.G.; Mali, A.D. Investigation of Photo-Oxidative Effect on Morphology and Degradation of Mechanical and Physical Properties of Nano CaCO 3 Silicone Rubber Composites. Polym. Adv. Technol. 2012, 23, 236–246. [Google Scholar] [CrossRef]
- Omya Poland. Available online: https://www.omya.com/pl-pl (accessed on 7 December 2024).
- Tsai, W.T.; Yang, J.M.; Hsu, H.C.; Lin, C.M.; Lin, K.Y.; Chiu, C.H. Development and Characterization of Mesoporosity in Eggshell Ground by Planetary Ball Milling. Microporous Mesoporous Mater. 2008, 111, 379–386. [Google Scholar] [CrossRef]
- Ahmad, W.; Sethupathi, S.; Munusamy, Y.; Kanthasamy, R. Valorization of Raw and Calcined Chicken Eggshell for Sulfur Dioxide and Hydrogen Sulfide Removal at Low Temperature. Catalysts 2021, 11, 295. [Google Scholar] [CrossRef]
- Jadhav, S.D.; Patil, R.C.; Jagdale, A.A.; Patil, S.S. Revisit to Henry Reaction by Non Conventional Heterogeneous and Efficient Catalyst for Nitroalcohol Synthesis. Res. Chem. Intermed. 2022, 48, 593–606. [Google Scholar] [CrossRef]
- Toibah, A.; Misran, F.; Shaaban, A.; Mustafa, Z. Effect of PH Condition during Hydrothermal Synthesis on the Properties of Hydroxyapatite from Eggshell Waste. J. Mech. Eng. Sci. 2019, 13, 4958–4969. [Google Scholar] [CrossRef]
- Spelta, J.S.d.O.; Galdino, A.G. de S. Bioceramic Composite: Hen’s Eggshell Characterization And Main Applications. Rev. Ifes Ciência 2018, 4, 9–20. [Google Scholar] [CrossRef]
- Putra, R.S.; Liyanita, A.; Arifah, N.; Puspitasari, E.; Sawaludin; Hizam, M.N. Enhanced Electro-Catalytic Process on the Synthesis of FAME Using CaO from Eggshell. Energy Procedia 2017, 105, 289–296. [Google Scholar] [CrossRef]
- Suresh, D.S.; Ba Shbil, A.; Sharanappa, S.; Vijaykumar, S.P.; Ganesha, H.; Veeresh, S.; Nagaraju, Y.S.; Devendrappa, H. Enhancement of Crystallinity with Porosity Material through Solvent and Thermal Treated Eggshell Waste for High-Performance Supercapacitor Applications. J. Mater. Sci. Mater. Electron. 2024, 35, 330. [Google Scholar] [CrossRef]
- Intharapat, P.; Kongnoo, A.; Kateungngan, K. The Potential of Chicken Eggshell Waste as a Bio-Filler Filled Epoxidized Natural Rubber (ENR) Composite and Its Properties. J. Polym. Environ. 2013, 21, 245–258. [Google Scholar] [CrossRef]
- Fathi, M.B.; Taghizadeh RahmatAbadi, Z. Eggshell Microstructure, Shell Quality Indices, Mineralogy, and UV–Vis Absorbance of Domestic Eggs of Iran. J. Photochem. Photobiol. 2024, 21, 100235. [Google Scholar] [CrossRef]
- Baláž, M.; Boldyreva, E.V.; Rybin, D.; Pavlović, S.; Rodríguez-Padrón, D.; Mudrinić, T.; Luque, R. State-of-the-Art of Eggshell Waste in Materials Science: Recent Advances in Catalysis, Pharmaceutical Applications, and Mechanochemistry. Front. Bioeng. Biotechnol. 2021, 8, 612567. [Google Scholar] [CrossRef] [PubMed]
- Cain, C.J.; Heyn, A.N.J. X-Ray Diffraction Studies of the Crystalline Structure of the Avian Egg Shell. Biophys. J. 1964, 4, 23–39. [Google Scholar] [CrossRef]
- Lewandowski, K.; Altmajer, P.; Borkowska, Z.; Skórczewska, K. Mechanical and Processing Properties of Plasticised PVC/Wood Composites. Polymers 2024, 16, 2204. [Google Scholar] [CrossRef] [PubMed]
- Piszczek, K.; Tomaszewska, J. Processing of Precipitated Nongranular PVC in the Brabender Measuring Mixer. J. Vinyl Addit. Technol. 2012, 18, 147–152. [Google Scholar] [CrossRef]
- Klapiszewski, Ł.; Pawlak, F.; Tomaszewska, J.; Jesionowski, T. Preparation and Characterization of Novel Pvc/Silica-Lignin Composites. Polymers 2015, 7, 1767–1788. [Google Scholar] [CrossRef]
- Diani, J.; Liu, Y.; Gall, K. Finite Strain 3D Thermoviscoelastic Constitutive Model for Shape Memory Polymers. Polym. Eng. Sci. 2006, 46, 486–492. [Google Scholar] [CrossRef]
- Barczewski, M.; Lewandowski, K.; Rybarczyk, D.; Kloziński, A. Rheological and Single Screw Extrusion Processability Studies of Isotactic Polypropylene Composites Filled with Basalt Powder. Polym. Test. 2020, 91, 106768. [Google Scholar] [CrossRef]
- Kloziński, A.; Szczepańska, M.; Jakubowska, P.; Samujło, B.; Barczewski, M.; Lota, G. The Influence of Calcium Carbonate and Its Modifications on the Extrusion Process and Selected Properties of Polypropylene Cast Films. Polimery/Polymers 2022, 67, 509–521. [Google Scholar] [CrossRef]
- Sun, S.; Li, C.; Zhang, L.; Du, H.L.; Burnell-Gray, J.S. Interfacial Structures and Mechanical Properties of PVC Composites Reinforced by CaCO3 with Different Particle Sizes and Surface Treatments. Polym. Int. 2006, 55, 158–164. [Google Scholar] [CrossRef]
- Kloziński, A.; Lewandowski, K.; Mirowski, J.; Barczewski, M.; Jakubowska, P. Rheological Properties of Polypropylene Composites with Calcium Carbonate under High Shear Rates. Polimery/Polymers 2023, 68, 403–412. [Google Scholar] [CrossRef]
- Castro, M.A.; Pereira, F.J.; Aller, A.J.; Littlejohn, D. Raman Spectrometry as a Screening Tool for Solvent-Extracted Azo Dyes from Polyester-Based Textile Fibres. Polym. Test. 2020, 91, 106765. [Google Scholar] [CrossRef]
- Atiganyanun, S.; Kumnorkaew, P. Effects of Pigment Volume Concentration on Radiative Cooling Properties of Acrylic-Based Paints with Calcium Carbonate and Hollow Silicon Dioxide Microparticles. Int. J. Sustain. Energy 2023, 42, 612–626. [Google Scholar] [CrossRef]
- Elgharbawy, A. Poly Vinyl Chloride Additives and Applications—A Review. J. Risk Anal. Cris. Response 2022, 12, 143–151. [Google Scholar] [CrossRef]
- Rijavec, T.; Strlič, M.; Cigić, I.K. Plastics in Heritage Collections: Poly(Vinyl Chloride) Degradation and Characterization. Acta Chim. Slov. 2020, 67, 993–1013. [Google Scholar] [CrossRef]
- Wypych, A. Plasticizers Databook; Elsevier Science: Burlington, NJ, USA, 2013; ISBN 978-1-895198-58-4. [Google Scholar]
- Zeng, L.; Xu, G.; Jiang, C.; Li, J.; Zheng, J. Research Note: L*a*b* Color Space for Prediction of Eggshell Pigment Content in Differently Colored Eggs. Poult. Sci. 2022, 101, 101942. [Google Scholar] [CrossRef] [PubMed]
- Samiullah, S.; Roberts, J.R.; Chousalkar, K. Eggshell Color in Brown-Egg Laying Hens—A Review. Poult. Sci. 2015, 94, 2566–2575. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, X.; Li, C. Mechanical Properties of PVC/Nano-CaCO3 Composites. J. Mater. Sci. 2005, 40, 2097–2098. [Google Scholar] [CrossRef]
- Sangsawang, M.; Kaewdook, D. Influence of Physical Characteristics and Mechanical Properties on Polyvinylchloride Flexible Sheet with Calcium Carbonate from Shells. In Proceedings of the 2018 5th International Conference on Business and Industrial Research (ICBIR), Bangkok, Thailand, 17–18 May 2018; pp. 230–235. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, M.; Zhang, H.; Liu, H.; Xu, S. Surface Modification of Calcium Carbonate by Thiol-Ene Click Reaction and Its Effect on the Performance of PVC Composite. J. Appl. Polym. Sci. 2024, 141, e54798. [Google Scholar] [CrossRef]
- Karmalm, P.; Hjertberg, T.; Jansson, A.; Dahl, R. Thermal Stability of Poly(Vinyl Chloride) with Epoxidised Soybean Oil as Primary Plasticizer. Polym. Degrad. Stab. 2009, 94, 2275–2281. [Google Scholar] [CrossRef]
- Liu, P.; Zhao, M.; Guo, J. Thermal Stabilities of Poly(Vinyl Chloride)/Calcium Carbonate (PVC/CaCO3) Composites. J. Macromol. Sci. Part B Phys. 2006, 45, 1135–1140. [Google Scholar] [CrossRef]
- Barkoula, N.M.; Alcock, B.; Cabrera, N.O.; Peijs, T. Flame-Retardancy Properties of Intumescent Ammonium Poly(Phosphate) and Mineral Filler Magnesium Hydroxide in Combination with Graphene. Polym. Polym. Compos. 2008, 16, 101–113. [Google Scholar]
- Chen, C.H.O.; Teng, C.C.; Su, S.F.; Wu, W.C.; Yang, C.H. Effects of Microscale Calcium Carbonate and Nanoscale Calcium Carbonate on the Fusion, Thermal, and Mechanical Characterizations of Rigid Poly(Vinyl Chloride)/Calcium Carbonate Composites. J. Polym. Sci. Part B Polym. Phys. 2006, 44, 451–460. [Google Scholar] [CrossRef]
- Gerstner, P.; Paltakari, J.; Gane, P.A.C. Measurement and Modelling of Heat Transfer in Paper Coating Structures. J. Mater. Sci. 2009, 44, 483–491. [Google Scholar] [CrossRef]
- Abbasnezhad, B.; Hamdami, N.; Monteau, J.Y.; Vatankhah, H. Numerical Modeling of Heat Transfer and Pasteurizing Value during Thermal Processing of Intact Egg. Food Sci. Nutr. 2016, 4, 42–49. [Google Scholar] [CrossRef]
- Ambli, K.G.; Angadi, B.M.; Vanarotti, M.B. Thermal Behaviour of Tamarind Seed Kernel Based Bio-Composites Intended for Thermal Insulation. Indian J. Sci. Technol. 2022, 15, 927–937. [Google Scholar] [CrossRef]
- Song, L.; Huo, H.; Zhang, W.; Xia, H.; Niu, Y. The Facile Strategy of Improving the Long-Term Stability of Highly Transparent Polyvinyl Chloride by Introducing Unsaturated Zn Oleate and Uracil Derivatives. Materials 2022, 15, 2672. [Google Scholar] [CrossRef] [PubMed]
- Beneš, M.; Plaček, V.; Matuschek, G.; Kettrup, A.A.; Györyová, K.; Emmerich, W.D.; Balek, V. Lifetime Simulation and Thermal Characterization of PVC Cable Insulation Materials. J. Therm. Anal. Calorim. 2005, 82, 761–768. [Google Scholar] [CrossRef]
- Merlo, A.; Lavagna, L.; Suarez-Riera, D.; Pavese, M. Mechanical Properties of Mortar Containing Waste Plastic (PVC) as Aggregate Partial Replacement. Case Stud. Constr. Mater. 2020, 13, e00467. [Google Scholar] [CrossRef]
- Schlickmann, K.P.; Leite Howarth, J.L.; Kasper Silva, D.A.; Testa Pezzin, A.P. Effect of the Incorporation of Micro and Nanoparticles of Calcium Carbonate in Poly (Vinyl Chloride) Matrix for Industrial Application. Mater. Res. 2019, 22, e20180870. [Google Scholar] [CrossRef]
- Folarin, O.M.; Sadiku, E.R. Thermal Stabilizers for Poly(Vinyl Chloride): A Review. Int. J. Phys. Sci. 2011, 6, 4323–4330. [Google Scholar] [CrossRef]

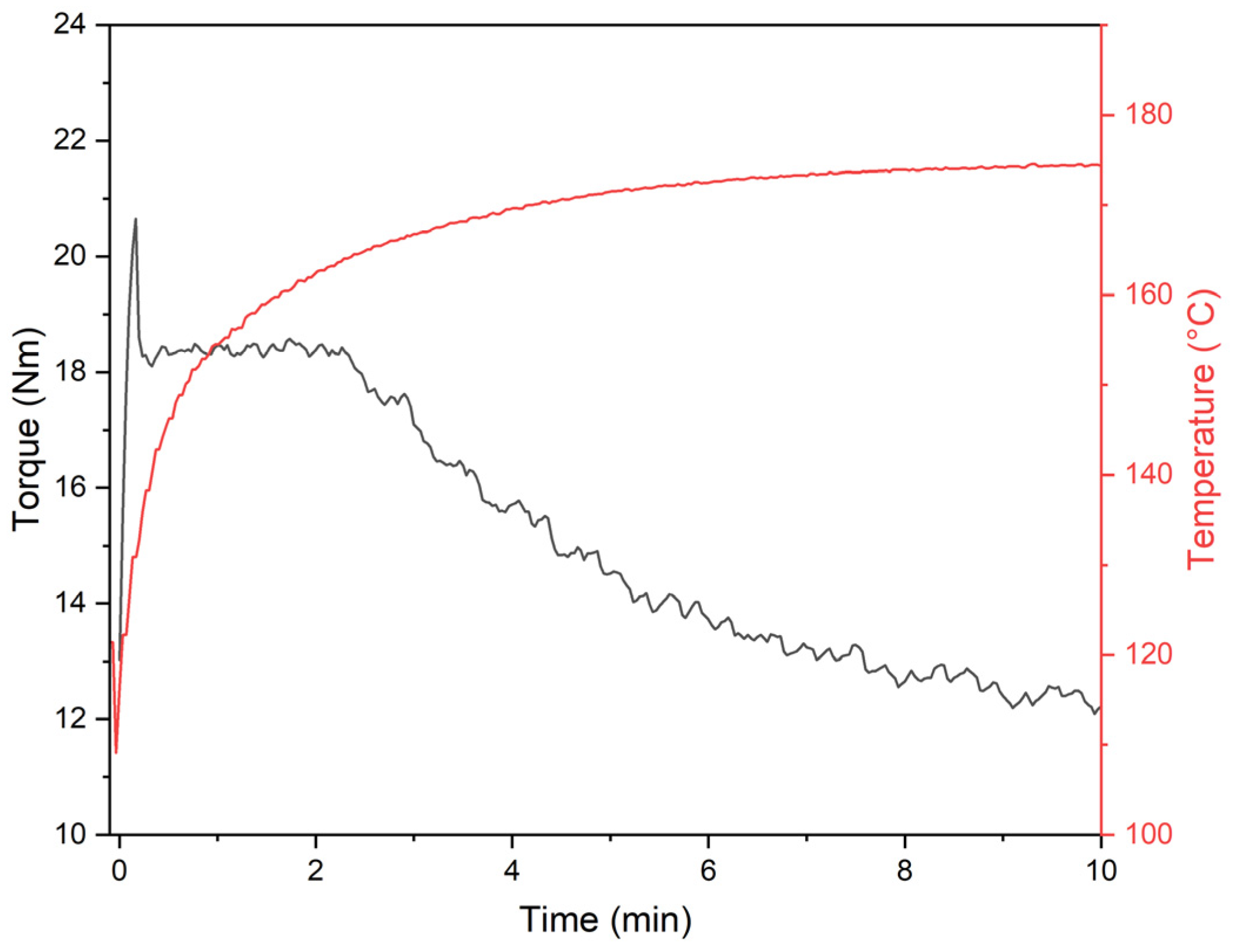

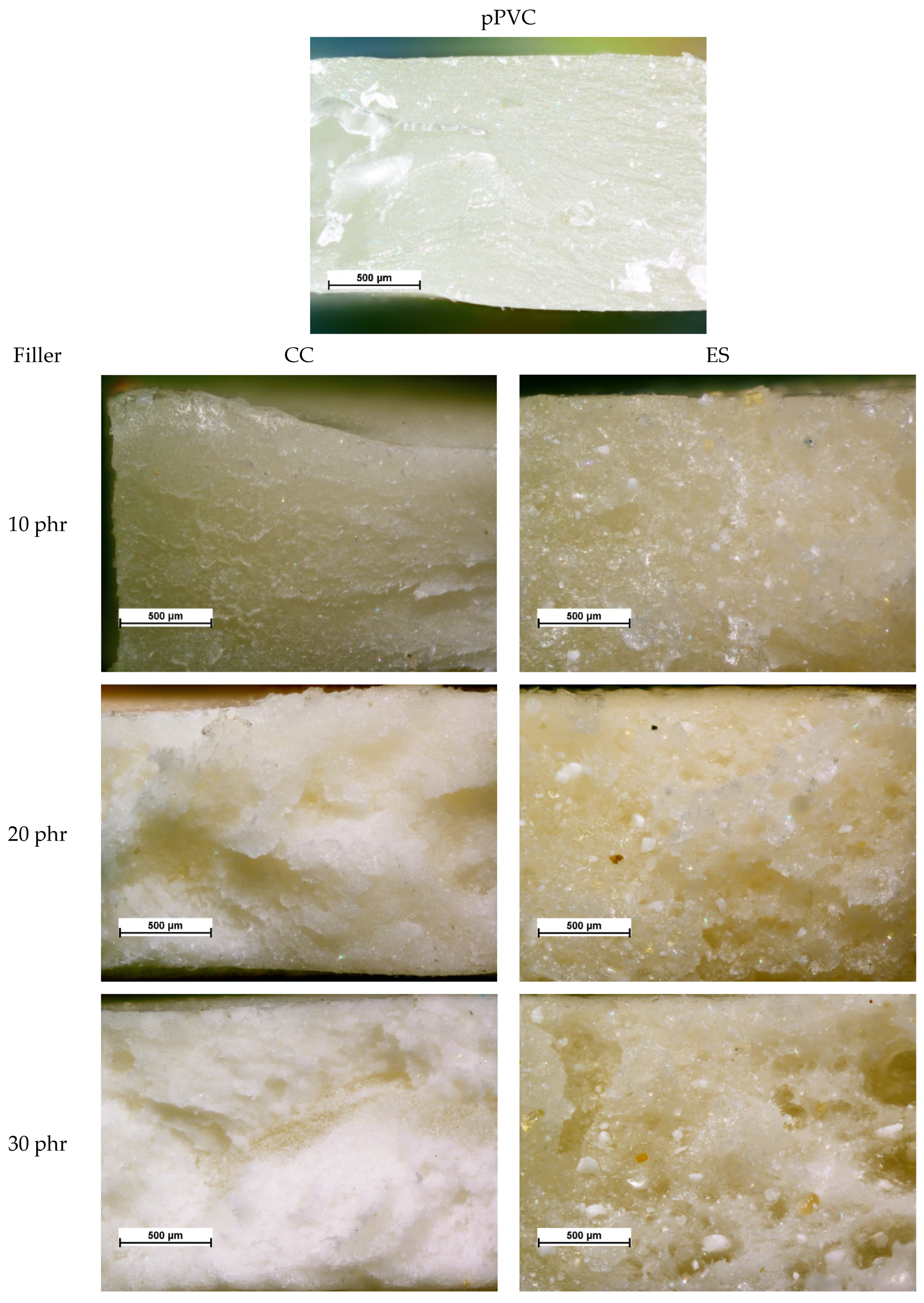
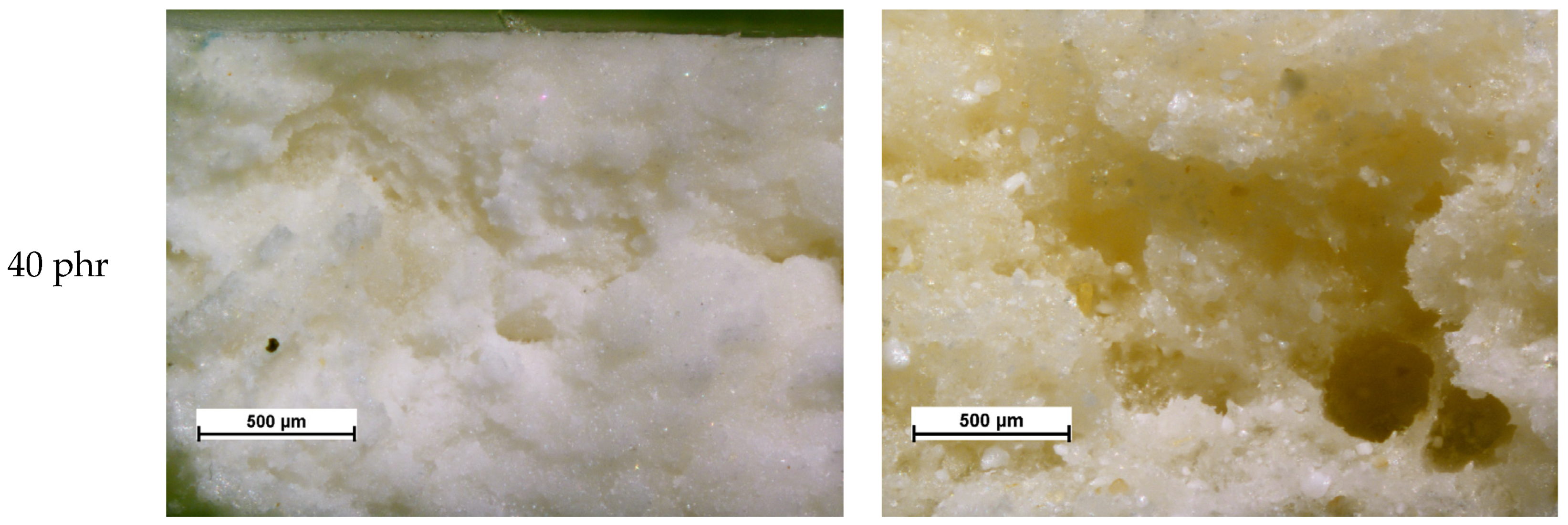
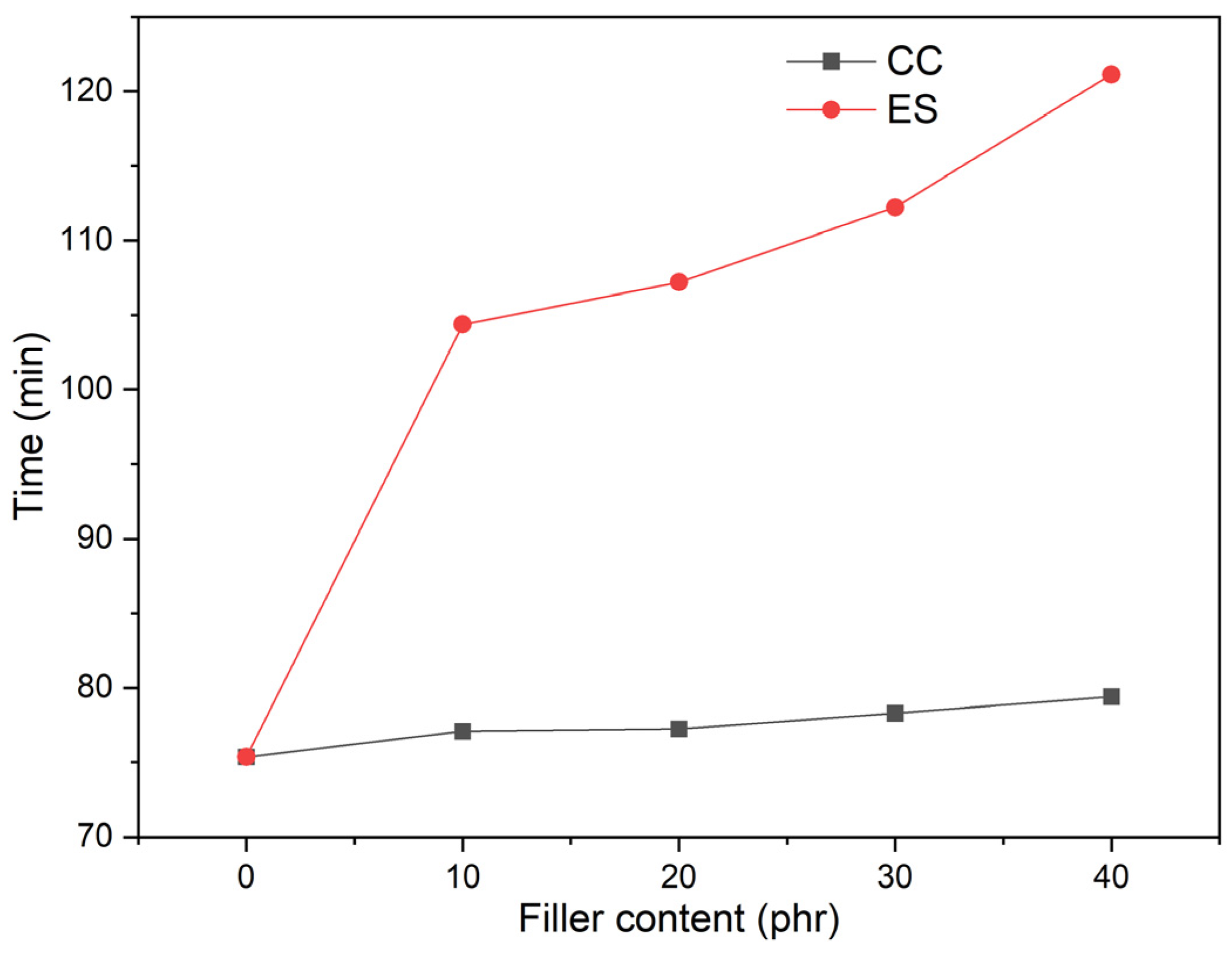
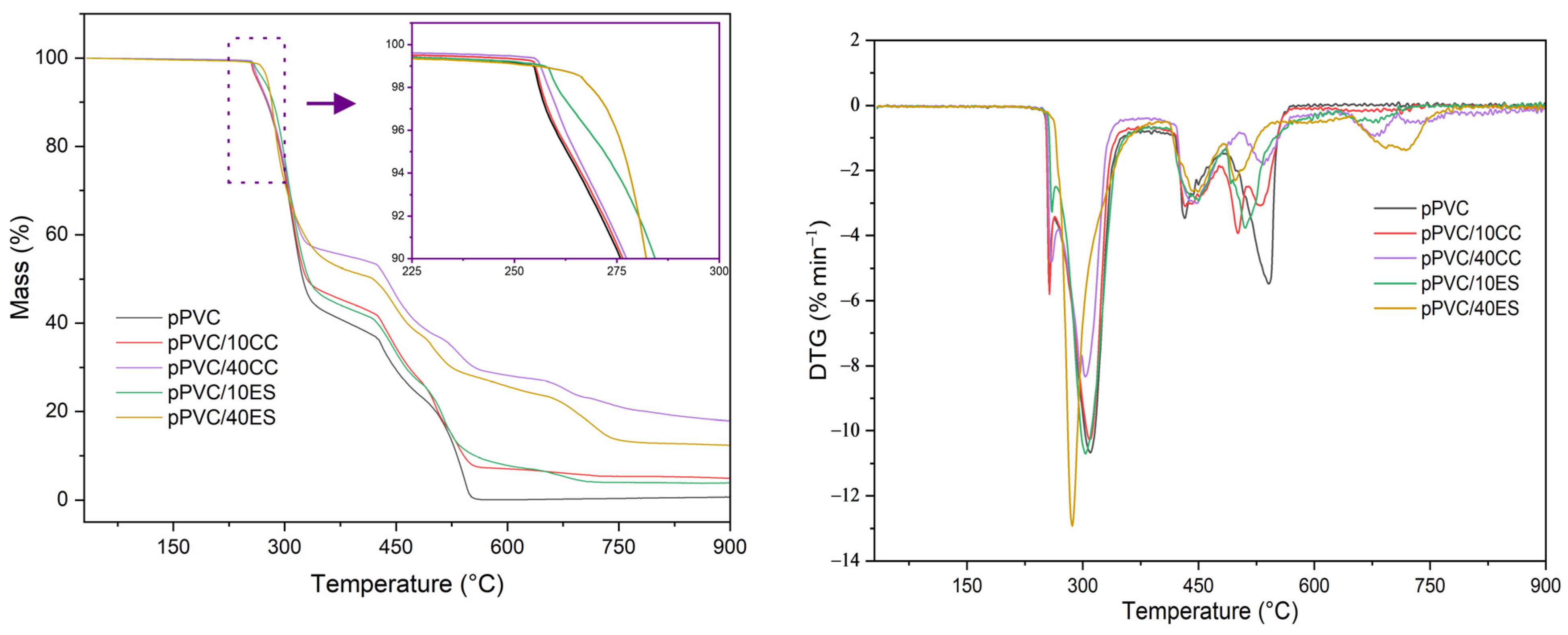
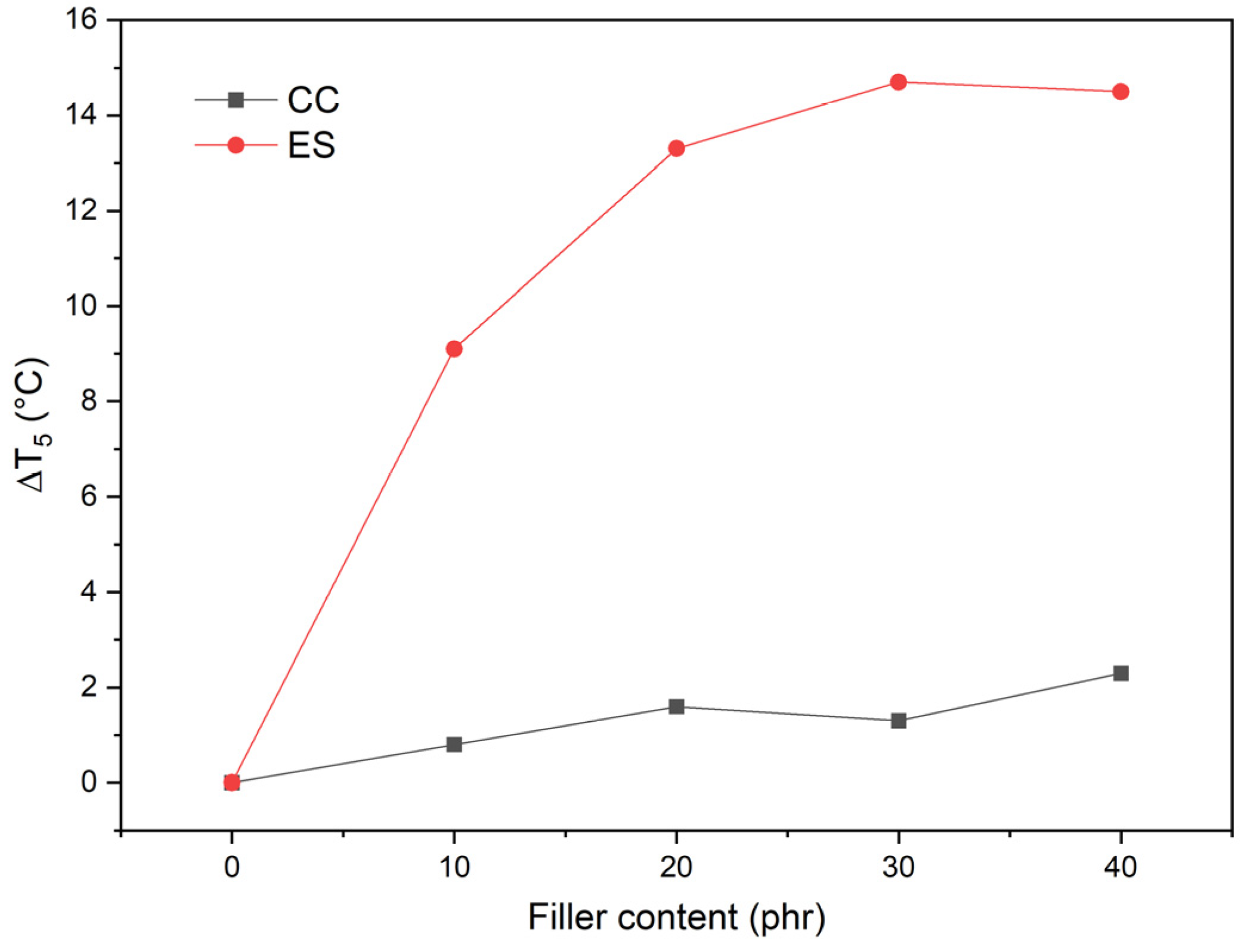
| Material | Kneading Time | |||||
|---|---|---|---|---|---|---|
| 2 min | 5 min | 10 min | ||||
| M (Nm) | T (°C) | M (Nm) | T (°C) | M (Nm) | T (°C) | |
| pPVC | 18.4 (0.9) | 162.0 (0.3) | 14.5 (0.5) | 171.4 (0.2) | 12.2 (0.3) | 174.3 (0.2) |
| pPVC/10ES | 20.1 (1.0) | 150.1 (1.8) | 18.5 (1.2) | 161.1 (0.9) | 16.5 (0.8) | 164.5 (0.4) |
| pPVC/20ES | 17.6 (1.3) | 154.3 (1.0) | 16.1 (0.6) | 163.3 (0.8) | 14.4 (0.2) | 166.8 (0.3) |
| pPVC/30ES | 18.4 (0.7) | 155.1 (1.8) | 16.8 (0.5) | 163.9 (0.3) | 14.6 (0.2) | 167.5 (0.4) |
| pPVC/40ES | 19.3 (1.0) | 155.5 (1.1) | 17.1 (0.5) | 164.0 (0.3) | 14.7 (0.3) | 167.4 (0.2) |
| pPVC/10CC | 16.6 (0.8) | 163.0 (0.6) | 13.8 (0.3) | 171.8 (0.3) | 11.1 (0.1) | 174.7 (0.1) |
| pPVC/20CC | 16.1 (1.1) | 160.2 (0.4) | 13.1 (0.3) | 170.0 (0.2) | 11.5 (0.2) | 173.4 (0.2) |
| pPVC/30CC | 16.6 (0.6) | 161.5 (0.3) | 13.0 (0.4) | 170.6 (0.3) | 11.6 (0.1) | 174.1 (0.1) |
| pPVC/40CC | 17.5 (0.5) | 161.4 (0.2) | 13.5 (0.3) | 171.1 (0.4) | 12.1 (0.2) | 174.5 (0.1) |
| Sample | Density (g/cm3) | Volume Fraction of Filler | Porosity (%) |
|---|---|---|---|
| CC | 2.668 | - | - |
| ES | 2.266 | - | - |
| pPVC | 1.284 | - | - |
| pPVC/10ES | 1.299 | 0.060 | 3.29 |
| pPVC/20ES | 1.327 | 0.128 | 5.83 |
| pPVC/30ES | 1.387 | 0.204 | 6.59 |
| pPVC/40ES | 1.428 | 0.292 | 9.11 |
| pPVC/10CC | 1.327 | 0.051 | 0.61 |
| pPVC/20CC | 1.372 | 0.110 | 1.48 |
| pPVC/30CC | 1.424 | 0.178 | 2.38 |
| pPVC/40CC | 1.487 | 0.257 | 3.23 |
| Sample | The Temperature of Weight Loss | Temperature of the Maximal Degradation Rate (TDTG) (°C) | Residual Mass at 900 °C (%) | ||
|---|---|---|---|---|---|
| T1 (°C) | T5 (°C) | T50 (°C) | |||
| pPVC | 254.7 | 262.6 | 323.4 | 309.8 | 0.2 |
| pPVC/10ES | 257.4 | 271.7 | 332.7 | 303.5 | 3.8 |
| pPVC/20ES | 259.3 | 275.9 | 342.1 | 292.2 | 8.7 |
| pPVC/30ES | 262.5 | 277.3 | 371.5 | 289.3 | 9.4 |
| pPVC/40ES | 255.1 | 277.1 | 418.9 | 286.2 | 12.4 |
| pPVC/10CC | 255.3 | 263.4 | 329.8 | 309.2 | 5.0 |
| pPVC/20CC | 255.9 | 264.2 | 355.0 | 307.1 | 9.3 |
| pPVC/30CC | 255.9 | 263.9 | 423.1 | 304.3 | 13.6 |
| pPVC/40CC | 256.2 | 264.9 | 437.5 | 303.4 | 17.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skórczewska, K.; Lewandowski, K.; Wilczewski, S.; Szulc, J.; Rakowska, P. Utilization of Ground Eggshell as a Biofiller of Plasticized PVC-Based Materials Fabricated Using Melt Blending. Polymers 2025, 17, 434. https://doi.org/10.3390/polym17040434
Skórczewska K, Lewandowski K, Wilczewski S, Szulc J, Rakowska P. Utilization of Ground Eggshell as a Biofiller of Plasticized PVC-Based Materials Fabricated Using Melt Blending. Polymers. 2025; 17(4):434. https://doi.org/10.3390/polym17040434
Chicago/Turabian StyleSkórczewska, Katarzyna, Krzysztof Lewandowski, Sławomir Wilczewski, Joanna Szulc, and Paulina Rakowska. 2025. "Utilization of Ground Eggshell as a Biofiller of Plasticized PVC-Based Materials Fabricated Using Melt Blending" Polymers 17, no. 4: 434. https://doi.org/10.3390/polym17040434
APA StyleSkórczewska, K., Lewandowski, K., Wilczewski, S., Szulc, J., & Rakowska, P. (2025). Utilization of Ground Eggshell as a Biofiller of Plasticized PVC-Based Materials Fabricated Using Melt Blending. Polymers, 17(4), 434. https://doi.org/10.3390/polym17040434





