Eco-Friendly Biosorbents from Biopolymers and Food Waste for Efficient Dye Removal from Wastewater
Abstract
1. Introduction
2. Materials and Methods
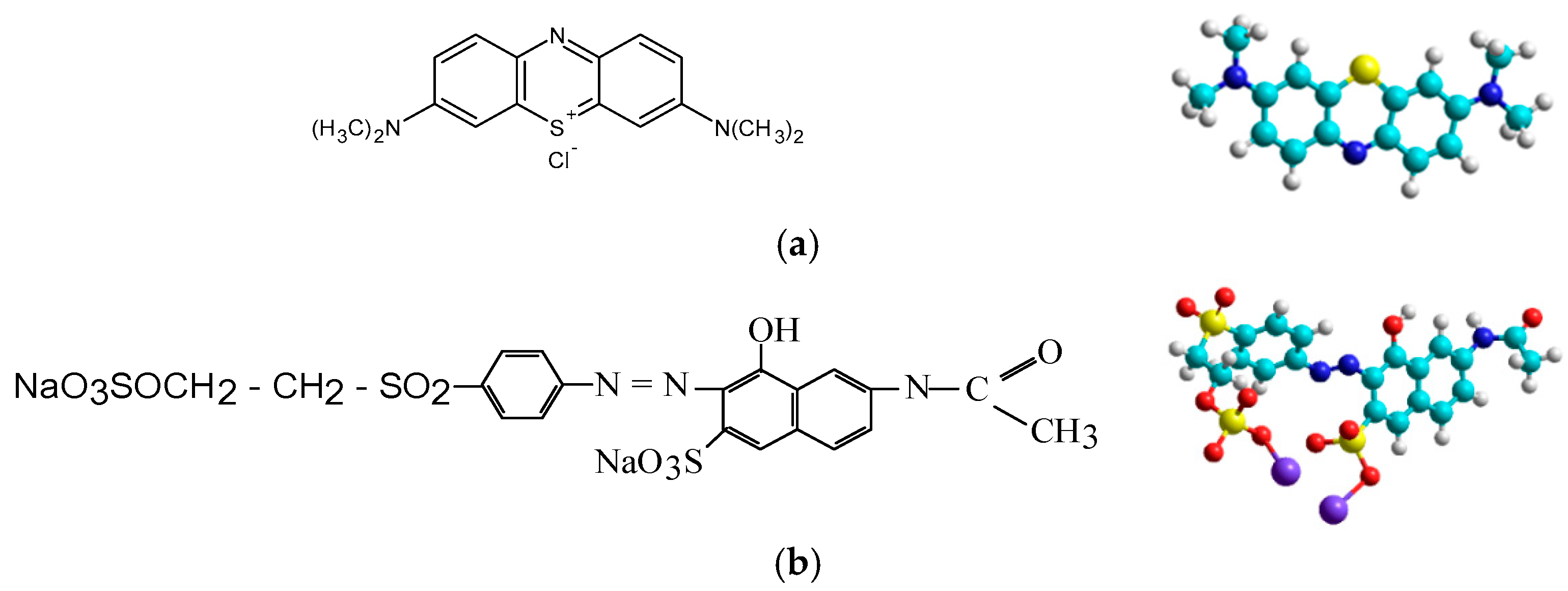
2.1. Materials
2.2. Methods
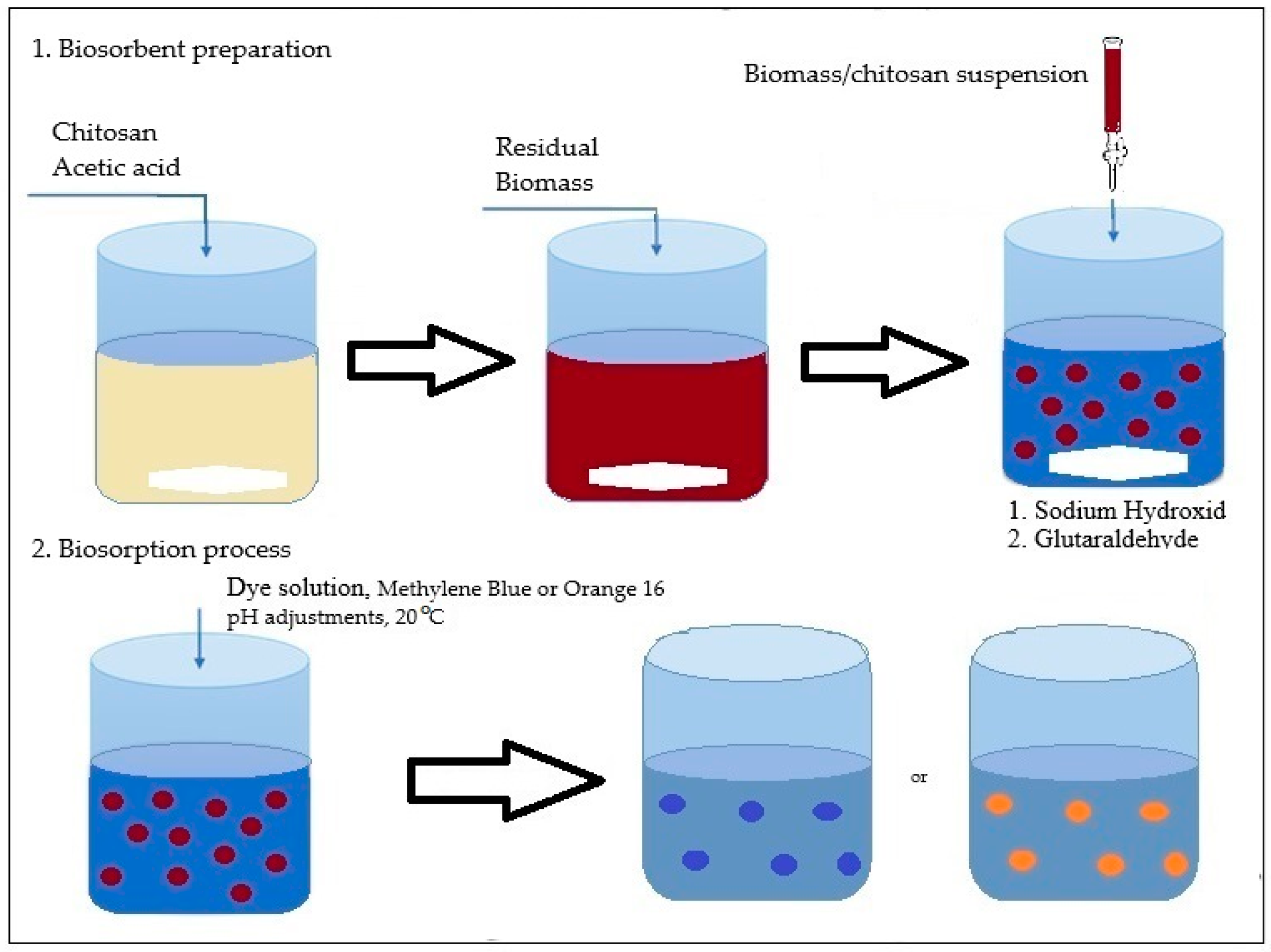
2.2.1. Batch Biosorption Methodology
2.2.2. Characterization of Biosorbents
2.2.3. The Biosorption Equilibrium Data Analysis
3. Results
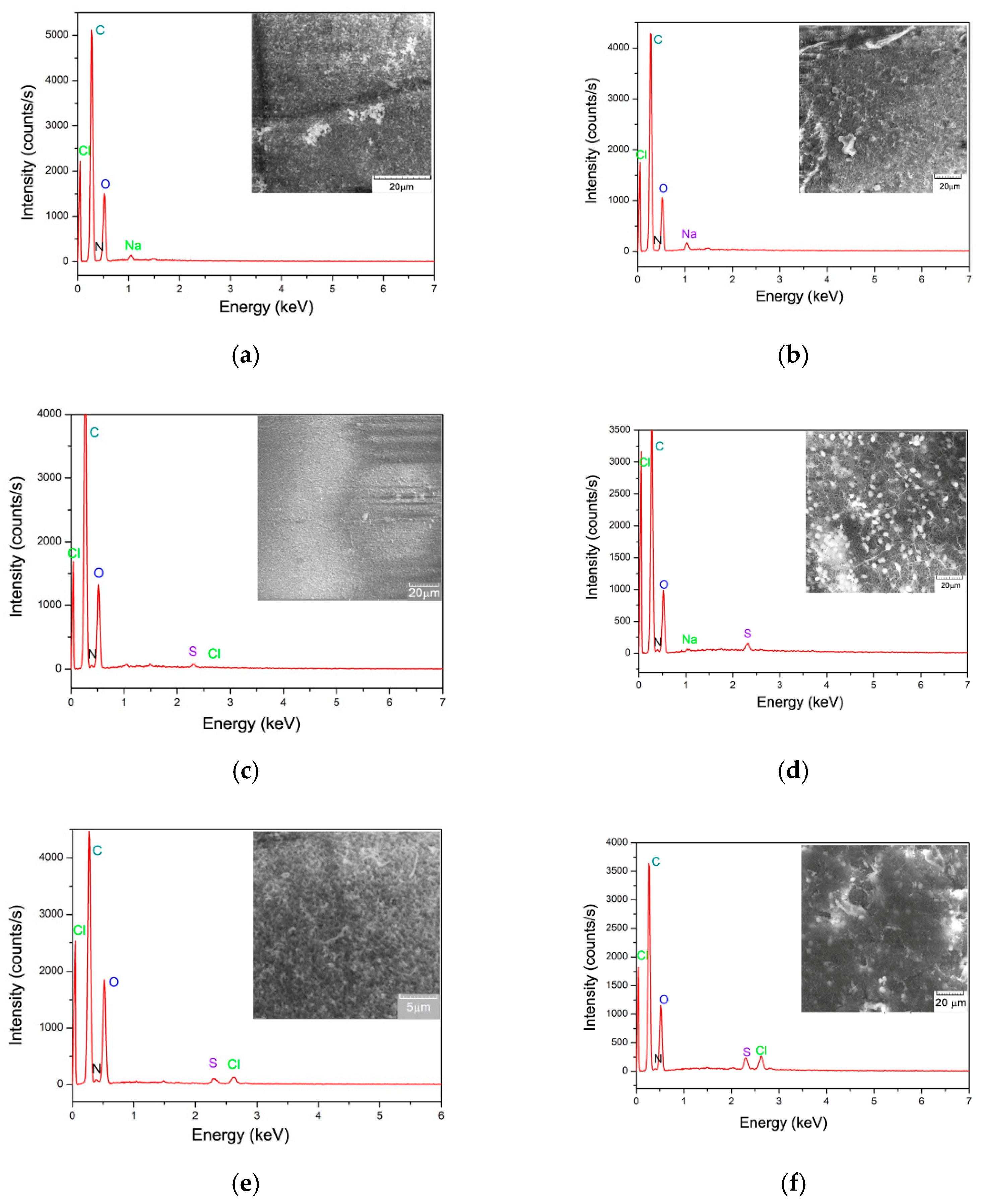
3.1. The Biosorbent Physical-Chemical Characterization Before and After Dyes Biosorbtion
3.2. Evaluation of the Biosorbent Potential of the Studied Materials
3.2.1. Impact of Key Physico-Chemical Operating Parameters on the Biosorption of Dyes onto Analyzed Biosorbents
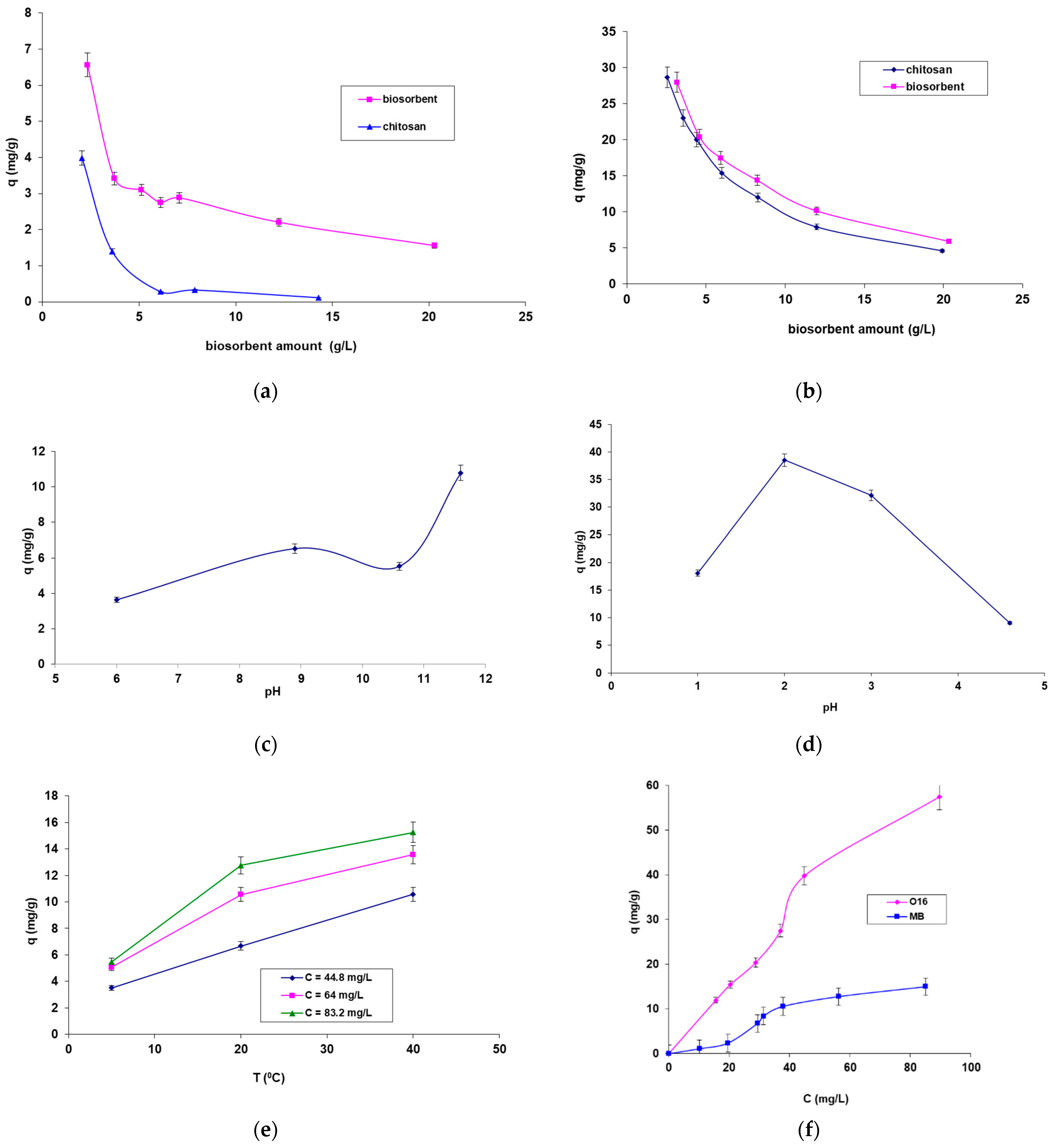
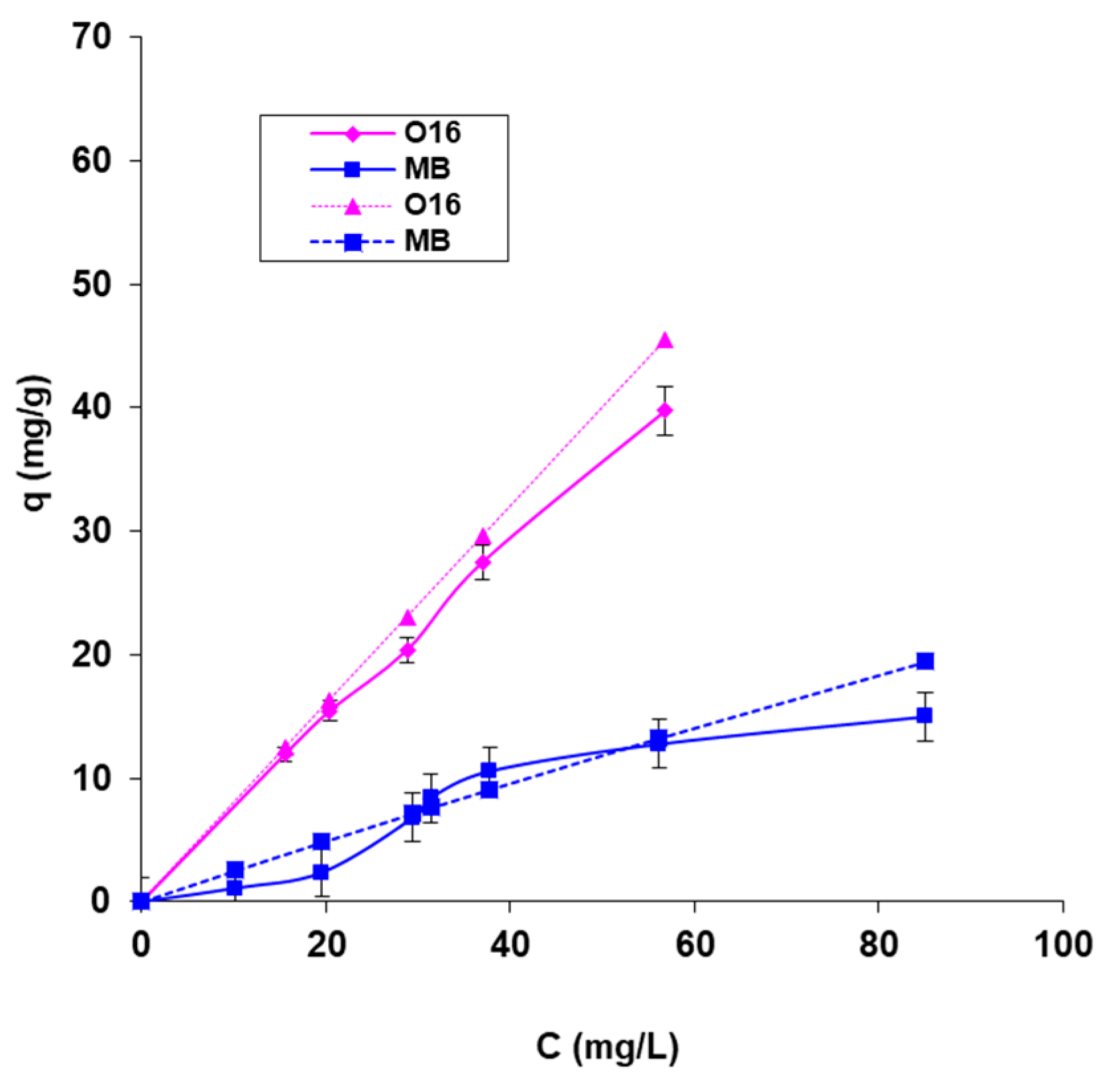
- Figure 5a,b illustrates the influence of biosorbent amount (for both CB and SpCB) on the biosorption of the two selected dyes. The results indicate that both types of biosorbents are capable of adsorbing both dyes, but the biosorption capacity is significantly higher for SpCB. This can be attributed to the cumulative effect of the adsorption capacity of the chitosan matrix and the immobilized residual biomass. Thus, the immobilization of residual biomass allowed bringing it into a form that can be easily manipulated during the technological process but also led to an increased biosorption capacity. Due to these results, the influence of the main factors on biosorption for SpCB was analyzed. When comparing biosorption efficiencies for the two dyes, a higher capacity was observed for the reactive anionic dye O16, likely due to its structure and functional groups, which are more compatible with the surface of the biosorbent. Figure 5a,b show a decrease in the amount of dyes retained per unit mass of biosorbents from 6.559 mg/g to 1.560 mg/g in the case of MB dye onto SpCB (Figure 5a) and, respectively, from 27.956 mg/g to 5.896 mg/g (Figure 4b) in the case of O16 dye onto the same biosorbent as the amount of biosorbent increases from 2.32 g/L to 20.28 g/L.
- The influence of pH (Figure 5c,d) demonstrates that the biosorption of the two dyes is most efficient at distinctly different pH values, depending on the structure of the dye molecules. Specifically, the biosorption of the cationic MB dye on the SpCB-based biosorbent occurs at a strongly basic pH (11.6), while the biosorption of the anionic O16 dye takes place at a strongly acidic pH (2).
- Figure 5e shows that increasing temperature has a positive effect on the biosorption process of the dyes, with MB biosorption on SpCB serving as an example. This variation suggests an endothermic biosorption process. In addition, Figure 5f demonstrates that increasing the initial concentration of the dye causes an increase in the biosorption capacity up to the saturation point of the biosorbent.
3.2.2. Evaluation of Some Characteristic Quantitative Parameters of the Biosorbtion of the Selected Dyes onto the Studied Biosorbent
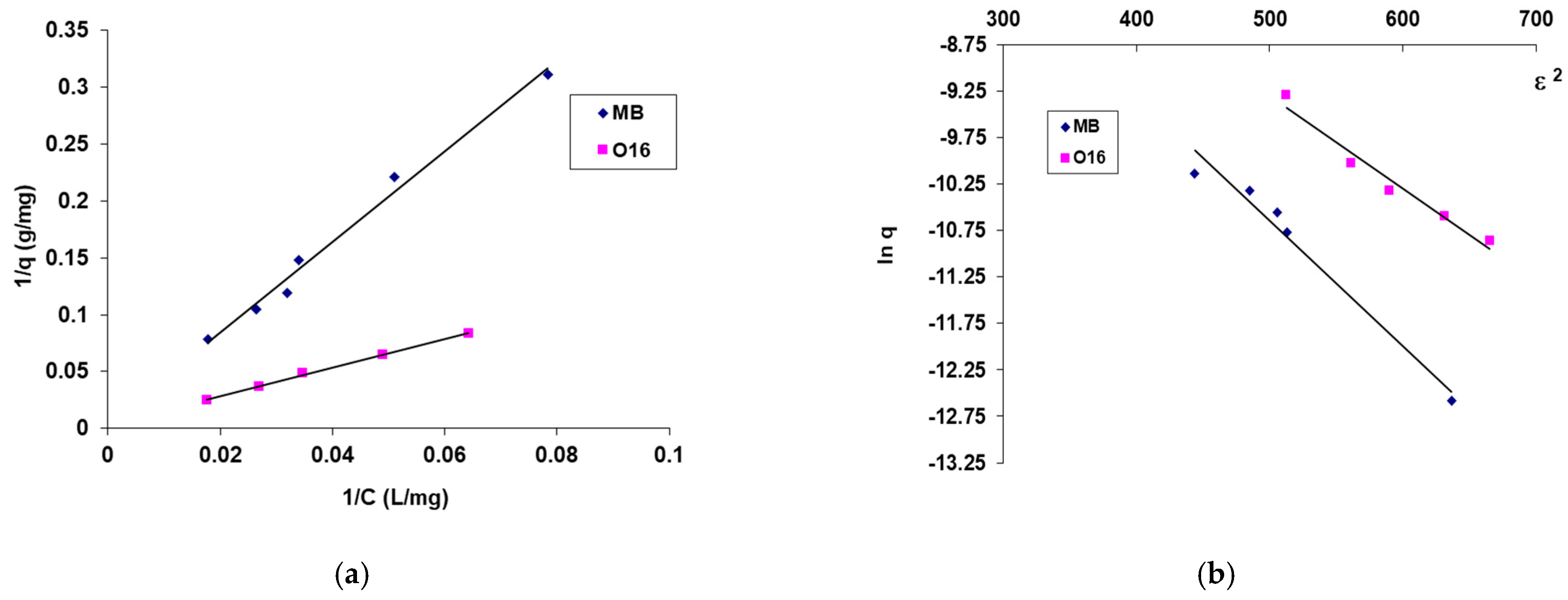
- It is observed that for SpCB, the maximum biosorption capacity for the reactive dye O16 (285.71 mg/g) is significantly higher than for the cationic dye MB (212.77 mg/g). These findings support previous results that the higher biosorption capacity for O16 is due to a stronger affinity between the functional groups of the anionic dye molecule (SO32−) and the functional groups on the surface of the biosorbent, derived from both the chitosan matrix and the immobilized microbial biomass.
- The mean free biosorption energy, E, calculated by the DR equation, can be useful to estimate the nature of the biosorption process (physical or chemical) [41]. In this case, the energy value, E, is 6.086 kJ/mol in the case of MB dye biosorption and 7.071 kJ/mol in the case of O16 dye biosorption, suggesting for the studied dyes biosorption, a physical mechanism as a result of the electrostatic interaction bonds (the sorption energy is less than 8 kJ/mol) [40,41].
- If we take into account the superior value of the maximum absorption capacity presented in Table 2 in the case of the MB dye obtained in the case of SpCB (212 mg/g) and the one from the previous study [39] performed on biosorbents based on the same biomass of Saccharomyces pastorianus immobilized in a sodium alginate matrix by a simple entrapment technique (40.8 mg/g) and by microencapsulation with the Buchi equipment (200 mg/g), current results prove the fact that the biosorbent obtained by immobilization of this biomass in a chitosan matrix (even in the simple version of immobilization) is much more efficient. This fact can be attributed to the biosorption capacity of the matrix itself.
- Similar results have been reported in the literature, such as in the biosorption of Orange II and Indigo Carmine dyes on biosorbents made by immobilizing Saccharomyces pastorianus biomass in the sodium alginate and chitosan matrices. The dye retention for the alginate matrix was 27.8% and 58.2%, respectively, while for the chitosan matrix, it was 40.8% and 77.9%, respectively [42]. Additionally, studies by Kim S. demonstrate that using chitosan as an immobilization matrix for industrial fermentation waste biomass of Escherichia coli in biosorbents for retaining the reactive dye Reactive Yellow 2 from an aqueous medium resulted in a retention capacity of 679 ± 23 mg/g [38]. Compared to studies realized using chitosan or modified chitosan [35,36,37] and the results presented in Table 3, the obtained results showed superior values for adsorption capacity.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, H.; Xue, K.; Wang, B.; Ren, W.; Sun, D.; Shao, C.; Sun, R. Advances in lignin-based biosorbents for sustainable wastewater treatment. Bioresour. Technol. 2024, 395, 130347. [Google Scholar] [CrossRef] [PubMed]
- Zavala-Flores, E.; Flores-López, L.Z.; Alonso-Nuñez, G.; Espinoza-Gómez, H. Removal and adsorption kinetics of methylene blue dye by pristine cotton husk bracts (Gossypium hirsutum L.) from agroindustrial waste. Ind. Crops Prod. 2024, 209, 117947. [Google Scholar] [CrossRef]
- An, D.; Sun, Y.; Yang, Y.-L.; Shi, X.-L.; Chen, H.-J.; Zhang, L.; Suo, G.; Hou, X.; Ye, X.; Lu, S.; et al. A strategy-purifying wastewater with waste materials: Zn2+ modified waste red mud as recoverable adsorbents with an enhanced removal capacity of congo red. J. Colloid Interface Sci. 2023, 645, 694–704. [Google Scholar] [CrossRef]
- Samuel, M.S.; John, A.; Ravikumar, J.M.; Raizada, P.; Wan Azelee, N.I.; Selvarajan, E.; Selvasembian, R. Recent progress on the remediation of dyes in wastewater using cellulose-based adsorbents. Ind. Crops Prod. 2023, 206, 117590. [Google Scholar] [CrossRef]
- Li, B.; Yang, H.; Li, C.; He, X.; Zhang, Y. Preparation of novel (MgCoNiCuZn)O high-entropy ceramic membrane and its dye separation. J. Eur. Ceram. Soc. 2023, 43, 3437–3446. [Google Scholar] [CrossRef]
- Osman, A.I.; El-Monaem, E.M.A.; Elgarahy, A.M.; Aniagor, C.O.; Hosny, M.; Farghali, M.; Rashad, E.; Ejimofor, M.I.; López-Maldonado, E.A.; Ihara, I.; et al. Methods to prepare biosorbents and magnetic sorbents for water treatment: A review. Environ. Chem. Lett. 2023, 21, 2337–2398. [Google Scholar] [CrossRef]
- Shankar, S.; Joshi, S.; Srivastava, R.K. A review on heavy metal biosorption utilizing modified chitosan. Environ. Monit. Assess. 2023, 195, 1350. [Google Scholar] [CrossRef]
- Wang, J.; Zhuang, S. Chitosan-based materials: Preparation, modification and application. J. Clean. Prod. 2022, 355, 131825. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, F.; Xu, K.; Che, Y.; Qi, M.; Song, C. Modified magnetic chitosan materials for heavy metal adsorption: A review. RSC Adv. 2023, 13, 6713–6736. [Google Scholar] [CrossRef] [PubMed]
- Ayach, J.; Duma, L.; Badran, A.; Hijazi, A.; Martinez, A.; Bechelany, M.; Baydoun, E.; Hamad, H. Enhancing Wastewater Depollution: Sustainable Biosorption Using Chemically Modified Chitosan Derivatives for Efficient Removal of Heavy Metals and Dyes. Materials 2024, 17, 2724. [Google Scholar] [CrossRef]
- Harugade, A.; Sherje, A.P.; Pethe, A. Chitosan: A review on properties, biological activities and recent progress in biomedical applications. React. Funct. Polym. 2023, 191, 105634. [Google Scholar] [CrossRef]
- Elgarahy, A.M.; Elwakeel, K.Z.; Mohammad, S.H.; Elshoubaky, G.A. A critical review of biosorption of dyes, heavy metals and metalloids from wastewater as an efficient and green process. Clean. Eng. Technol. 2021, 4, 100209. [Google Scholar] [CrossRef]
- Roșca, M.; Silva, B.; Tavares, T.; Gavrilescu, M. Biosorption of Hexavalent Chromium by Bacillus megaterium and Rhodotorula sp. Inactivated Biomass. Processes 2023, 11, 179. [Google Scholar] [CrossRef]
- Nascimento, J.M.; Otaviano, J.J.S.; Sousa, H.S.; Oliveira, J.D. Biological method of heavy metal management: Biosorption and bioaccumulation. Heavy Met. Environ. Manag. Strat. Glob. Pollut. 2023, 1456, 315–360. [Google Scholar]
- Kandeel, E.M.; Mohamed, M.K.; Hasanien, Y.A.; Abdel-Galil, E.A. Removal of 4-(2-pyridylazo) resorcinol and Arsenazo-III from liquid waste solutions using Penicillium oxalicum: Gamma irradiation studies. Desalin. Water Treat. 2024, 320, 100872. [Google Scholar] [CrossRef]
- Tripathi, M.; Singh, P.; Singh, R.; Bala, S.; Pathak, N.; Singh, S.; Chauhan, R.S.; Singh, P.K. Microbial biosorbent for remediation of dyes and heavy metals pollution: A green strategy for sustainable environment. Front. Microbiol. 2023, 14, 1168954. [Google Scholar] [CrossRef] [PubMed]
- Ghaedi, M.; Hajati, S.; Barazesh, B.; Karimi, F.; Ghezelbash, G. Saccharomyces cerevisiae for the biosorption of basic dyes from binary component systems and the high order derivative spectrophotometric method for simultaneous analysis of Brilliant green and Methylene blue. J. Ind. Eng. Chem. 2013, 19, 227–233. [Google Scholar] [CrossRef]
- Ghodsi, S.; Kamranifar, M.; Fatehizadeh, A.; Taheri, E.; Bina, B.; Hublikar, L.V.; Ganachari, S.V.; Nadagouda, M.; Aminabhavi, T.M. New insights on the decolorization of waste flows by Saccharomyces cerevisiae strain—A systematic review. Environ. Res. 2024, 249, 118398. [Google Scholar] [CrossRef]
- Al-Tohamy, R.; Ali, S.S.; Li, F.; Okasha, K.M.; Mahmoud, Y.A.G.; Elsamahy, T.; Jiao, H.; Fu, Y.; Sun, J. A critical review on the treatment of dye-containing wastewater: Ecotoxicological and health concerns of textile dyes and possible remediation approaches for environmental safety. Ecotoxicol. Environ. Saf. 2022, 231, 113160. [Google Scholar] [CrossRef]
- Abidli, A.; Huang, Y.; Ben Rejeb, Z.; Zaoui, A.; Park, C.B. Sustainable and efficient technologies for removal and recovery of toxic and valuable metals from wastewater: Recent progress, challenges, and future perspectives. Chemosphere 2021, 292, 133102. [Google Scholar] [CrossRef] [PubMed]
- Fathollahi, A.; Khasteganan, N.; Coupe, S.J.; Newman, A.P. A meta-analysis of metal biosorption by suspended bacteria from three phyla. Chemosphere 2021, 268, 129290. [Google Scholar] [CrossRef]
- Rizvi, A.; Ahmed, B.; Zaidi, A.; Khan, M.S. Biosorption of heavy metals by dry biomass of metal tolerant bacterial biosorbents: An efficient metal clean-up strategy. Environ. Monit. Assess. 2020, 192, 12. [Google Scholar] [CrossRef] [PubMed]
- Bibbins-Martínez, M.; Juárez-Hernández, J.; López-Domínguez, J.Y.; Nava-Galicia, S.B.; Martínez-Tozcano, L.J.; Juárez-Atonaľ, R.; Cortés-Espinosa, D.; Díaz-Godinez, G. Potential application of fungal biosorption and/or bioaccumulation for the bioremediation of wastewater contamination: A review. J. Environ. Biol. 2023, 44, 135–145. [Google Scholar] [CrossRef]
- Al-Tohamy, R.; Kenawy, E.R.; Sun, J.; Ali, S.S. Performance of a newly isolated salt-tolerant yeast strain Sterigmatomyces halophilus SSA-1575 for azo dye decolorization and detoxification. Front. Microbiol. 2020, 11, 1163. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.A.; Mehmood, S.; Iqbal, A.; Hamayun, M. Industrial polluted soil borne fungi decolorize the recalcitrant azo dyes Synozol red HF–6BN and Synozol black B. Ecotoxicol. Environ. Saf. 2020, 206, 111381. [Google Scholar] [CrossRef]
- Younes, M.K.; Algburi, S.; Al Omari, R.H.; Abdulhameed, A.S. Application of chitosan/acid-treated biomass composite for dye wastewater treatment: Adsorption modeling using Box-Behnken design. Desalin Water Treat. 2024, 320, 100795. [Google Scholar] [CrossRef]
- Zaharia, C.; Suteu, D. Organic Pollutants Ten Years After the Stockholm Convention—Environmental and Analytical Update; Puzyn, T., Mostrag-Szlichtyng, A., Eds.; InTech: London, UK, 2012; Chapter 3. [Google Scholar] [CrossRef]
- Adelaja, O.A.; Babaniyi, B.R.; Aransiola, S.A.; Rodríguez-Díaz, J.M.; Maddela, N.R.; Prasad, R. Biosorption of dyes in wastewater using chitosan/polyethylene nanoparticle as adsorbent. Discov. Catal. 2024, 1, 3. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Zekker, I.; Zhang, B.; Hendi, A.H.; Ahmad, A.; Ahmad, S.; Zada, N.; Ahmad, H.; Shah, L.A.; et al. Review on methylene blue: Its properties, uses, toxicity and photodegradation. Water 2022, 14, 242. [Google Scholar] [CrossRef]
- Bouras, H.D.; Isik, Z.; Arikan, E.B.; Yeddou, A.R.; Bouras, N.; Chergui, A.; Favier, L.; Amrane, A.; Dizge, N. Biosorption characteristics of methylene blue dye by two fungal biomasses. Int. J. Environ. Stud. 2021, 78, 365–381. [Google Scholar] [CrossRef]
- Bharti, V.; Vikrant, K.; Goswami, M.; Tiwari, H.; Sonwani, R.K.; Lee, J.; Tsang, D.C.W.; Kim, K.-H.; Saeed, M.; Kumar, S.; et al. Biodegradation of methylene blue dye in a batch and continuous mode using biochar as packing media. Environ. Res. 2019, 171, 356–364. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.K.S.; Gupta, R.K.; Karuppanan, K.K.; Padhi, D.K.; Ranganathan, S.; Paramanantham, P.; Lee, J.-K. Trametes versicolor Laccase-Based Magnetic Inorganic-Protein Hybrid Nanobiocatalyst for Efficient Decolorization of Dyes in the Presence of Inhibitors. Materials 2024, 17, 1790. [Google Scholar] [CrossRef]
- Mazzeo, L.; Marzi, D.; Bavasso, I.; Bracciale, M.P.; Piemonte, V.; Di Palma, L. Characterization of waste roots from the as hyperaccumulator Pteris vittata as low-cost adsorbent for methylene blue removal. ChERD 2022, 186, 13–21. [Google Scholar] [CrossRef]
- Castro, F.D.; Bassin, J.P.; Alves, T.L.M.; Sant’Anna, G.L., Jr.; Dezotti, M. Reactive Orange 16 dye degradation in anaerobic and aerobic MBBR coupled with ozonation: Addressing pathways and performance. Int. J. Environ. Sci. Technol. 2021, 18, 1991–2010. [Google Scholar] [CrossRef]
- Chatterjee, S.; Chatterjee, T.; Lim, S.-R.; Woo, S.H. Adsorption of a cationic dye, methylene blue, on to chitosan hydrogel beads generated by anionic surfactant gelation. Environ. Technol. 2011, 32, 1503–1514. [Google Scholar] [CrossRef]
- Tasdelen, B.; Cifci, D.I.; Meric, S. Preparation and characterization of chitosan/AMPS/kaolinite composite hydrogels for adsorption of methylene blue. Polym. Bull. 2022, 79, 9643–9662. [Google Scholar] [CrossRef]
- Wu, R.; Abdulhameed, A.S.; Jawad, A.H.; Musa, S.A.; De Luna, Y.; ALOthman, Z.A.; Algburi, S. An eco-friendly chitosan-genipin/SiO2 composite for reactive orange 16 dye removal: Insights into adsorption statistical modeling and mechanism. Int. J. Biol. Macromol. 2024, 270, 132329. [Google Scholar] [CrossRef] [PubMed]
- Kim, S. Blending of waste biomass for cost-effective chitosan-based biosorbents for removal of reactive dye from aqueous solution. Environ. Eng. Res. 2022, 27, 210457. [Google Scholar] [CrossRef]
- Blaga, A.C.; Tanasa, A.M.; Cimpoesu, R.; Tataru-Farmus, R.-E.; Suteu, D. Biosorbents based on biopolymers from natural sources and food waste to retain the Methylene Blue dye from the aqueous medium. Polymers 2022, 14, 2728. [Google Scholar] [CrossRef] [PubMed]
- Crini, G.; Badot, P.M. Application of Chitosan, a Natural Aminopolysaccharide, for Dye Removal from Aqueous Solutions by Adsorption Processes Using Batch Studies: A Review of Recent Literature. Prog. Polym. Sci. 2008, 33, 399–447. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Insights into the modeling of adsorption isotherm systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Rusu, L.; Grigoras, C.-G.; Suceveanu, E.M.; Simion, A.-I.; Dediu Botezatu, A.V.; Istrate, B.; Doroftei, I. Eco-friendly biosorbents based on microbial biomass and natural polymers: Synthesis, characterization and application for the removal of drugs and dyes from aqueous solutions. Materials 2021, 14, 4810. [Google Scholar] [CrossRef] [PubMed]
- Bilal, M.; Ihsanullah, I.; Shah, M.U.H.; Reddy, A.V.B.; Aminabhavi, T.M. Recent advances in the removal of dyes from wastewater using low-cost adsorbents. J. Environ. Manag. 2022, 321, 115981. [Google Scholar] [CrossRef] [PubMed]
- Marrakchi, F.; Ahmed, M.J.; Khanday, W.A.; Asif, M.; Hameed, B.H. Mesoporous carbonaceous material from fish scales as low-cost adsorbent for reactive orange 16 adsorption. J. Taiwan Inst. Chem. Eng. 2017, 71, 47–54. [Google Scholar] [CrossRef]
- Won, S.W.; Yun, H.J.; Yun, Y.-S. Effect of pH on the binding mechanisms in biosorption of Reactive Orange 16 by Corynebacterium glutamicum. J. Colloid Interface Sci. 2009, 331, 83–89. [Google Scholar] [CrossRef] [PubMed]

| Parameters | Studied Limits of Variation | |
|---|---|---|
| MB | O-16 | |
| pH | 6–11 | 1–7 |
| Biosorbent dose (g/L) | 0.2–2.028 | 0.256–1.992 |
| Initial dye concentration in solution (mg/L) | 12.8–83.2 | 21–232 |
| Temperature (°C) | 5; 20; 40 | |
| Isotherm | Dyes | |
|---|---|---|
| MB | O16 | |
| Langmuir | ||
| q0 (mg/g) | 212.77 | 285.71 |
| q0 (mmol/g) | 20.26 | 13.41 |
| KL (L/g) | 0.0012 | 0.0028 |
| R2 | 0.988 | 0.997 |
| Dubinin–Radushkevich (DR) | ||
| q0 (mg/g) | 6478.9 | 8280.8 |
| β (mol2/kJ2) | 0.0135 | 0.01 |
| E (kJ/mol) | 6.09 | 7.07 |
| R2 | 0.969 | 0.962 |
| No. | Dye | Biosorbent | qmax (mg/g) |
|---|---|---|---|
| 1 | Methylene blue | Millimeter-sized chitosan/carboxymethyl cellulose hollow capsule | 64.6 |
| 2 | Sargassum dentifolium | 66.6 | |
| 3 | Pseudomonas aeruginosa USM-AR2/SiO2 | 75.7 | |
| 4 | Chlorella pyrenoidosa | 212 | |
| 5 | Aspergillus carbonarius | 21.88 | |
| 6 | Orange 16 | Psyllium seed powder | 100 |
| 7 | Labeo rohita | 114.2 | |
| 8 | Pine shell-char | 314 | |
| 9 | Corynebacterium glutamicum | 156.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blaga, A.C.; Cimpoesu, R.; Tataru-Farmus, R.-E.; Suteu, D. Eco-Friendly Biosorbents from Biopolymers and Food Waste for Efficient Dye Removal from Wastewater. Polymers 2025, 17, 291. https://doi.org/10.3390/polym17030291
Blaga AC, Cimpoesu R, Tataru-Farmus R-E, Suteu D. Eco-Friendly Biosorbents from Biopolymers and Food Waste for Efficient Dye Removal from Wastewater. Polymers. 2025; 17(3):291. https://doi.org/10.3390/polym17030291
Chicago/Turabian StyleBlaga, Alexandra Cristina, Ramona Cimpoesu, Ramona-Elena Tataru-Farmus, and Daniela Suteu. 2025. "Eco-Friendly Biosorbents from Biopolymers and Food Waste for Efficient Dye Removal from Wastewater" Polymers 17, no. 3: 291. https://doi.org/10.3390/polym17030291
APA StyleBlaga, A. C., Cimpoesu, R., Tataru-Farmus, R.-E., & Suteu, D. (2025). Eco-Friendly Biosorbents from Biopolymers and Food Waste for Efficient Dye Removal from Wastewater. Polymers, 17(3), 291. https://doi.org/10.3390/polym17030291










