Alkali Metal Ion Insertion in Polypyrrole Polyoxometalates for Multifunctional Actuator–Sensor–Energy Storage Devices
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Formation of PPyDBS with PTA
2.3. Linear Actuation Measurements
2.4. Characterizations
3. Results and Discussions
3.1. Polymerization and Characterization of PPyDBS-PT4 and PPyDBS-PT8
3.2. Linear Actuation Studies of PPyDBS-PT Samples
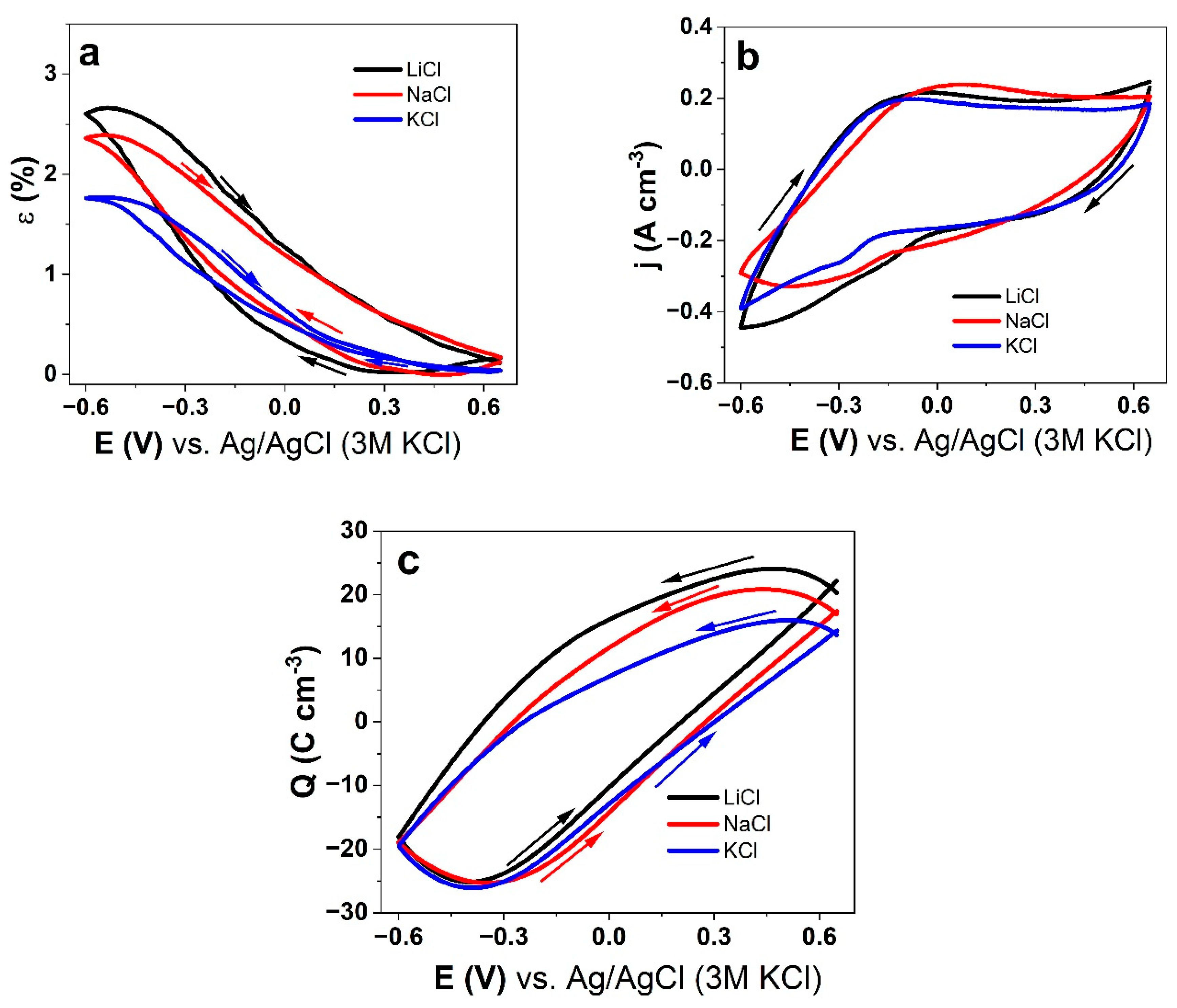
3.3. Cyclic Voltammetry
3.4. Energy Storage of PPyDBS-PT4 and PPyDBS-PT8
3.5. Sensor Calibration
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Bay, L.; Jacobsen, T.; Skaarup, S.; West, K. Mechanism of Actuation in Conducting Polymers: Osmotic Expansion. J. Phys. Chem. B 2001, 105, 8492–8497. [Google Scholar] [CrossRef]
- Jager, E.W.H.; Inganas, O.; Lundstrom, I. Microrobots for Micrometer-Size Objects in Aqueous Media: Potential Tools for Single-Cell Manipulation. Science 2000, 288, 2335–2338. [Google Scholar] [CrossRef] [PubMed]
- Smela, E. Conjugated Polymer Actuators for Biomedical Applications. Adv. Mater. 2003, 15, 481–494. [Google Scholar] [CrossRef]
- Martinez, J.G.; Richter, K.; Persson, N.K.; Jager, E.W.H. Investigation of Electrically Conducting Yarns for Use in Textile Actuators. Smart Mater. Struct. 2018, 27, 074004. [Google Scholar] [CrossRef]
- Ortega-santos, A.B.; Mart, J.G.; Jager, E.W.H. Synchronous Cation-Driven and Anion-Driven Polypyrrole-Based Yarns toward In-Air Linear Actuators. Chem. Mater. 2024, 36, 9391–9405. [Google Scholar] [CrossRef] [PubMed]
- Bay, L.; West, K.; Sommer-Larsen, P.; Skaarup, S.; Benslimane, M. A Conducting Polymer Artificial Muscle with 12% Linear Strain. Adv. Mater. 2003, 15, 310–313. [Google Scholar] [CrossRef]
- Khuyen, N.Q.; Kiefer, R.; Zondaka, Z.; Anbarjafari, G.; Peikolainen, A.; Otero, T.F.; Tamm, T. Multifunctionality of Polypyrrole Polyethyleneoxide Composites: Concurrent Sensing, Actuation and. Polymers 2020, 12, 2060. [Google Scholar] [CrossRef] [PubMed]
- Zondaka, Z.; Harjo, M.; Khan, A.; Khanh, T.T.; Tamm, T.; Kiefer, R. Optimal Phosphotungstinate Concentration for Polypyrrole Linear Actuation and Energy Storage. Multifunct. Mater. 2018, 1, 14003. [Google Scholar] [CrossRef]
- Dubal, D.P.; Ballesteros, B.; Mohite, A.A.; Gómez-Romero, P. Functionalization of Polypyrrole Nanopipes with Redox-Active Polyoxometalates for High Energy Density Supercapacitors. ChemSusChem 2017, 10, 731–737. [Google Scholar] [CrossRef]
- Suppes, G.M.; Cameron, C.G.; Freund, M.S. A Polypyrrole/Phosphomolybdic Acid∣Poly(3,4-ethylenedioxythiophene)/Phosphotungstic Acid Asymmetric Supercapacitor. J. Electrochem. Soc. 2010, 157, A1030. [Google Scholar] [CrossRef]
- Cuentas-Gallegos, A.K.; Martínez-Rosales, R.; Baibarac, M.; Gómez-Romero, P.; Rincón, M.E. Electrochemical Supercapacitors Based on Novel Hybrid Materials Made of Carbon Nanotubes and Polyoxometalates. Electrochem. Commun. 2007, 9, 2088–2092. [Google Scholar] [CrossRef]
- Wang, D.; Liu, L.; Jiang, J.; Chen, L.; Zhao, J. Polyoxometalate-Based Composite Materials in Electrochemistry: State-of-the-Art Progress and Future Outlook. Nanoscale 2020, 12, 5705–5718. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Zhou, Y.; Zhang, J.; Foroughi, J.; Peng, S.; Baughman, R.H.; Wang, Z.L.; Wang, C.H. Advanced Energy Harvesters and Energy Storage for Powering Wearable and Implantable Medical Devices. Adv. Mater. 2024, 36, 2404492. [Google Scholar] [CrossRef] [PubMed]
- Khanh, T.T.; Kesküla, A.; Zondaka, Z.; Harjo, M.; Kivilo, A.; Khorram, M.S.; Tamm, T.; Kiefer, R. Role of Polymerization Temperature on the Performance of Polypyrrole/dodecylbenzenesulphonate Linear Actuators. Synth. Met. 2019, 247, 53–58. [Google Scholar] [CrossRef]
- Otero, T.F.; Cortés, M.T. A Sensing Muscle. Sensors Actuators, B Chem. 2003, 96, 152–156. [Google Scholar] [CrossRef]
- Martinez, J.G.; Otero, T.F. Three Electrochemical Tools (Motor-Sensor-Battery) with Energy Recovery Work Simultaneously in a Trilayer Artificial Muscle. Electrochim. Acta 2019, 294, 126–133. [Google Scholar] [CrossRef]
- Martinez, J.G.; Otero, T.F.; Jager, E.W.H. Effect of the Electrolyte Concentration and Substrate on Conducting Polymer Actuators. Langmuir 2014, 30, 3894–3904. [Google Scholar] [CrossRef] [PubMed]
- Beheshtian, J.; Baei, M.T.; Bagheri, Z.; Peyghan, A.A. Carbon Nitride Nanotube as a Sensor for Alkali and Alkaline Earth Cations. Appl. Surf. Sci. 2013, 264, 699–706. [Google Scholar] [CrossRef]
- Merhebi, S.; Mohammad, M.; Mayyas, M.; Abbasi, R.; Zhang, C.; Cai, S.; Centurion, F.; Xie, W.; Cao, Z.; Tang, J.; et al. Post-Transition Metal/polymer Composites for the Separation and Sensing of Alkali Metal Ions. J. Mater. Chem. A 2021, 9, 19854–19864. [Google Scholar] [CrossRef]
- Le, Q.B.; Zondaka, Z.; Nguyen, N.T.; Kiefer, R. Ion-Selectivity of Polypyrrole Carbide-Derived Carbon Films in Aqueous Electrolytes. J. Appl. Polym. Sci. 2023, 140, e53522. [Google Scholar] [CrossRef]
- Skaarup, S.; Jafeen, M.J.M.; Careem, M.A. Determination of Membrane Hydration Numbers of Alkali Metal Ions by Insertion in a Conducting Polymer. Solid State Ion. 2010, 181, 1245–1250. [Google Scholar] [CrossRef]
- Zondaka, Z.; Kesküla, A.; Tamm, T.; Kiefer, R. Polypyrrole Linear Actuation Tuned by Phosphotungstic Acid. Sens. Actuators B Chem. 2017, 247, 742–748. [Google Scholar] [CrossRef]
- Valero, L.; Otero, T.F.; Martinez, J.G.; Martínez, J.G. Exchanged Cations and Water during Reactions in Polypyrrole Macroions from Artificial Muscles. ChemPhysChem 2014, 15, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Otero, T.F.; Boyano, I. Comparative Study of Conducting Polymers by the ESCR Model. J. Phys. Chem. B 2003, 107, 6730–6738. [Google Scholar] [CrossRef]
- Harjo, M.; Tamm, T.; Anbarjafari, G.; Kiefer, R. Hardware and Software Development for Isotonic Strain and Isometric Stress Measurements of Linear Ionic Actuators. Polymers 2019, 11, 1054. [Google Scholar] [CrossRef]
- Kaempgen, M.; Chan, C.K.; Ma, J.; Cui, Y.; Gruner, G. Printable Thin Film Supercapacitors Using Single-Walled Carbon Nanotubes. Nano Lett. 2009, 9, 1872–1876. [Google Scholar] [CrossRef]
- Tran, C.B.; Otero, T.F.; Travas-Sejdic, J.; Bao Le, Q.; Kiefer, R. A Comparison of Poly (3,4-Ethylenedioxythiophene) Polymerized Potentiostatically and Galvanostatically. Synth. Met. 2023, 299, 117466. [Google Scholar] [CrossRef]
- Kim, Y.; Shanmugam, S. Polyoxometalate-Reduced Graphene Oxide Hybrid Catalyst: Synthesis, Structure, and Electrochemical Properties. ACS Appl. Mater. Interfaces 2013, 5, 12197–12204. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, S.; Ritchie, C.; Streb, C. Polyoxometalate—Conductive Polymer Composites for Energy Conversion, Energy Storage and Nanostructured Sensors. Dalt. Trans. 2015, 44, 7092–7104. [Google Scholar] [CrossRef]
- Tanaka, Y.; Hasegawa, T.; Shimamura, T.; Ukeda, H.; Ueda, T. Potentiometric Evaluation of Anti-oxidant Capacity Using Polyoxometalate-Immobilized Electrodes. J. Electroanal. Chem. 2018, 828, 102–107. [Google Scholar] [CrossRef]
- Anandan Vannathan, A.; Chandewar, P.R.; Shee, D.; Sankar Mal, S. Asymmetric Polyoxometalate-Polypyrrole Composite Electrode Material for Electrochemical Energy Storage Supercapacitors. J. Electroanal. Chem. 2022, 904, 115856. [Google Scholar] [CrossRef]
- Genovese, M.; Lian, K. Polyoxometalate Modified Inorganic-Organic Nanocomposite Materials for Energy Storage Applications: A Review. Curr. Opin. Solid State Mater. Sci. 2015, 19, 126–137. [Google Scholar] [CrossRef]
- Gade, V.K.; Shirale, D.J.; Gaikwad, P.D.; Kakde, P.; Savale, P.A.; Kharat, H.J. Synthesis and Characterization of Ppy-PVS, Ppy-pTS, and Ppy- DBS Composite Films. Int. J. Polym. Mater. Polym. Biomater. 2007, 56, 107–114. [Google Scholar] [CrossRef]
- Cherevan, A.S.; Nandan, S.P.; Roger, I.; Liu, R.; Streb, C.; Eder, D. Polyoxometalates on Functional Substrates: Concepts, Synergies, and Future Perspectives. Adv. Sci. 2020, 7, 1903511. [Google Scholar] [CrossRef]
- Lim, H.K.; Lee, S.O.; Song, K.J.; Kim, S.G.; Kim, K.H. Synthesis and Properties of Soluble Polypyrrole Doped with Dodecylbenzenesulfonate and Combined with Polymeric Additive Poly (ethylene Glycol). J. Appl. Polym. Sci. 2005, 97, 1170–1175. [Google Scholar] [CrossRef]
- Haspulat Taymaz, B.; Kamiş, H.; Yoldaş, Ö. Photocatalytic Degradation of Malachite Green Dye Using Zero Valent Iron Doped Polypyrrole. Environ. Eng. Res. 2022, 27, 200638. [Google Scholar] [CrossRef]
- Maruthamuthu, S.; Chandrasekaran, J.; Manoharan, D.; Magesh, R. Conductivity and Dielectric Analysis of Nanocolloidal Polypyrrole Particles Functionalized with Higher Weight Percentage of Poly(styrene Sulfonate) Using the Dispersion Polymerization Method. J. Polym. Eng. 2017, 37, 481–492. [Google Scholar] [CrossRef]
- Otero, T.F.; Cheng, S.A.; Alonso, D.; Huerta, F. Hybrid Materials polypyrrole/PW12O403-. 2. Physical, Spectroscopic and Electrochemical Characterization. J. Phys. Chem. B 2000, 104, 10528–10533. [Google Scholar] [CrossRef]
- Najafi-Ashtiani, H.; Bahari, A.; Gholipour, S.; Hoseinzadeh, S. Structural, Optical and Electrical Properties of WO3–Ag Nanocomposites for the Electro-Optical Devices. Appl. Phys. A Mater. Sci. Process. 2018, 124, 24. [Google Scholar] [CrossRef]
- Zuend, A.; Marcolli, C.; Luo, B.P.; Peter, T. A Thermodynamic Model of Mixed Organic-Inorganic Aerosols to Predict Activity Coefficients. Atmos. Chem. Phys. 2008, 8, 4559–4593. [Google Scholar] [CrossRef]
- Varma, S.; Rempe, S.B. Coordination Numbers of Alkali Metal Ions in Aqueous Solutions. Biophys. Chem. 2006, 124, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Skaarup, S.; Bay, L.; Vidanapathirana, K.; Thybo, S.; Tofte, P.; West, K. Simultaneous Anion and Cation Mobility in Polypyrrole. Solid State Ion. 2003, 159, 143–147. [Google Scholar] [CrossRef]
- Zondaka, Z.; Valner, R.; Tamm, T.; Aabloo, A.; Kiefer, R. Carbide-Derived Carbon in Polypyrrole Changing the Elastic Modulus with a Huge Impact on Actuation. RSC Adv. 2016, 6, 26380–26385. [Google Scholar] [CrossRef]
- Sung, H.; So, H.; Paik, W.K. Polypyrrole Doped with Heteropolytungstate Anions. Electrochim. Acta 1994, 39, 645–650. [Google Scholar] [CrossRef]
- Baughman, R.H. Conducting Polymer Artificial Muscles. Synth. Met. 1996, 78, 339–353. [Google Scholar] [CrossRef]
- Fleischmann, S.; Mitchell, J.B.; Wang, R.; Zhan, C.; Jiang, D.E.; Presser, V.; Augustyn, V. Pseudocapacitance: From Fundamental Understanding to High Power Energy Storage Materials. Chem. Rev. 2020, 120, 6738–6782. [Google Scholar] [CrossRef] [PubMed]
- Bonastre, J.; Molina, J.; Galván, J.C.; Cases, F. Characterization of Polypyrrole/phosphotungstate Membranes by Electrochemical Impedance Spectroscopy. Synth. Met. 2014, 187, 37–45. [Google Scholar] [CrossRef]
- Martinez, J.G.; Otero, T.F. Structural Electrochemistry. Chronopotentiometric Responses from Rising Compacted Polypyrrole Electrodes: Experiments and Model. RSC Adv. 2014, 4, 29139–29145. [Google Scholar] [CrossRef]
- Chen, Y.; Han, M.; Tang, Y.; Bao, J.; Li, S.; Lan, Y.; Dai, Z. Polypyrrole-Polyoxometalate/reduced Graphene Oxide Ternary Nanohybrids for Flexible, All-Solid-State Supercapacitors. Chem. Commun. 2015, 51, 12377–12380. [Google Scholar] [CrossRef] [PubMed]
- Anees, M.; Puniyanikkottil, P.; Rajendra Chandewar, D.S.; Mal, S.S. Synergistic Enhancement of Supercapacitor Performance: Vanadium-Substituted Phosphotungstic and Molybdic Acid Combined with Polypyrrole Using Pyridinium and Ammonium Ionic Containing Organic Cation Linkers with Improved Conductivity. Energy Technol. 2024, 12, 2400708. [Google Scholar] [CrossRef]
- Cheng, D.; Li, K.; Zang, H.; Chen, J. Recent Advances on Polyoxometalate-Based Ion-Conducting Electrolytes for Energy-Related Devices. Energy Environ. Mater. 2023, 6, e12341. [Google Scholar] [CrossRef]
- Otero, T.F.; Martinez, J.G. Physical and Chemical Awareness from Sensing Polymeric Artificial Muscles. Experiments and Modeling. Prog. Polym. Sci. 2015, 44, 62–78. [Google Scholar] [CrossRef]
- Jafeen, M.J.M.; Careem, M.A.; Skaarup, S. Speed and Strain of Polypyrrole Actuators: Dependence on Cation Hydration Number. Ionics 2010, 16, 1–6. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, W.; Zeng, X.; Deng, T.; Wang, J. Membranes for Separation of Alkali/alkaline Earth Metal Ions: A Review. Sep. Purif. Technol. 2022, 278, 119640. [Google Scholar] [CrossRef]
- Novák, P.; Müller, K.; Santhanam, K.S.V.; Haas, O. Electrochemically Active Polymers for Rechargeable Batteries. Chem. Rev. 1997, 97, 207–281. [Google Scholar] [CrossRef]
- Cheng, Y.; Hao, Z.; Hao, C.; Deng, Y.; Li, X.; Li, K.; Zhao, Y. A Review of Modification of Carbon Electrode Material in Capacitive Deionization. RSC Adv. 2019, 9, 24401–24419. [Google Scholar] [CrossRef]
- Wang, J.; Guo, X.; Apostol, P.; Liu, X.; Robeyns, K.; Gence, L.; Morari, C.; Gohy, J.F.; Vlad, A. High Performance Li-, Na-, and K-Ion Storage in Electrically Conducting Coordination Polymers. Energy Environ. Sci. 2022, 15, 3923–3932. [Google Scholar] [CrossRef]





| Electrolytes Cation | r (Å) [40] | N [40] | [41] | NPPy [21] |
|---|---|---|---|---|
| Li+ | 0.76 | 0.58 | 4 | 5.3–5.5 |
| Na+ | 1.02 | 0.22 | 5 | 4.3–4.5 |
| K+ | 1.38 | 0 | 6 | 2.0–2.2 |
| PPyDBS-PT4 Applied Aqueous Electrolytes | ε (%) | Q (C cm−3) | σe (S cm−1) | Y (MPa) | |
|---|---|---|---|---|---|
| Before | After | ||||
| LiCl | 6.6 ± 0.4 | 149.2 ± 12.2 | 13.5 ± 0.9 | 4.8 ± 0.3 | 1.4 ± 0.1 |
| NaCl | 4.7 ± 0.3 | 137.6 ± 11.1 | 10.3 ± 0.6 | 4.4 ± 0.4 | 1.1 ± 0.1 |
| KCl | 2.4 ± 0.2 | 114.5 ± 9.4 | 7.4 ± 0.4 | 5.0 ± 0.4 | 4.2 ± 0.3 |
| PPyDBS-PT8 Applied Aqueous Electrolytes | ε (%) | Q (C cm−3) | σe (S cm−1) | Y (MPa) | |
|---|---|---|---|---|---|
| Before | After | ||||
| LiCl | 2.6 ± 0.2 | 41.2 ± 2.8 | 7.2 ± 0.5 | 18.5 ± 1.3 | 11.3 ± 0.8 |
| NaCl | 2.3 ± 0.1 | 36.4 ± 2.2 | 6.2 ± 0.4 | 17.2 ± 1.2 | 11.5 ± 0.7 |
| KCl | 1.8 ± 0.1 | 32.6 ± 2.1 | 3.8 ± 0.3 | 20.4 ± 1.4 | 18.5 ± 1.4 |
| Electrolytes | Ue (J g−1) | Eox (V) | ε (%) |
|---|---|---|---|
| * PPyDBS-PT4 | |||
| LiCl | 0.60 ± 0.050 | ||
| NaCl | 0.41 ± 0.037 | ||
| KCl | 0.16 ± 0.015 | ||
| ** PPyDBS-PT8 | |||
| LiCl | 0.85 ± 0.079 | ||
| NaCl | 0.52 ± 0.048 | ||
| KCl | 0.44 ± 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kiefer, R.; Nguyen, N.T.; Le, Q.B. Alkali Metal Ion Insertion in Polypyrrole Polyoxometalates for Multifunctional Actuator–Sensor–Energy Storage Devices. Polymers 2025, 17, 262. https://doi.org/10.3390/polym17030262
Kiefer R, Nguyen NT, Le QB. Alkali Metal Ion Insertion in Polypyrrole Polyoxometalates for Multifunctional Actuator–Sensor–Energy Storage Devices. Polymers. 2025; 17(3):262. https://doi.org/10.3390/polym17030262
Chicago/Turabian StyleKiefer, Rudolf, Ngoc Tuan Nguyen, and Quoc Bao Le. 2025. "Alkali Metal Ion Insertion in Polypyrrole Polyoxometalates for Multifunctional Actuator–Sensor–Energy Storage Devices" Polymers 17, no. 3: 262. https://doi.org/10.3390/polym17030262
APA StyleKiefer, R., Nguyen, N. T., & Le, Q. B. (2025). Alkali Metal Ion Insertion in Polypyrrole Polyoxometalates for Multifunctional Actuator–Sensor–Energy Storage Devices. Polymers, 17(3), 262. https://doi.org/10.3390/polym17030262







