Local Relaxation Phenomena in Epoxy Resins in the Temperature Range from −150 °C to +150 °C
Abstract
1. Introduction

Relaxation Properties
- –
- –
- a low-temperature β-transition, the nature of which is still debatable for various polymers; however, according to most authors, the β-transition is caused by a smaller scale and cooperative movement (compared with the α–transition) of relatively small sections of the polymer chain [26,76,77,78]—for example, links or segments [74,79];
- –
- the low-temperature γ-transition is associated with the smallest-scale processes among the listed transitions and, according to many authors, is caused by the movement of the smallest sections of the polymer chain (side group [76], aliphatic groups –CH2– in the main chain [80], etc.). It is observed at fairly low temperatures (below −100 °C) and rarely falls within the temperature range of studies. In addition, not all methods make it possible to visualize the γ-relaxation process (for example, by high-frequency experiments [81]). In dielectric spectra, γ-transitions are often not resolved and merge into a single blurred maxim [76].
- –
- hydroxyester (glyceryl) group
 [84,88,89,90], as the only common fragment of epoxy–amine meshes based on epoxy oligomers of various nature.
[84,88,89,90], as the only common fragment of epoxy–amine meshes based on epoxy oligomers of various nature.- –
- fragment of diphenylolpropane (bisphenol A)
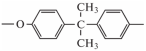 [88,91,92], in particular, the movement of diester bonds in it [88], non-rotational bending movements of fragments containing benzene ring [88], the movement of aromatic rings themselves [85], etc. In the first case, it can be a crankshaft motion [84,88], the movement of a hydroxyester group bound by hydrogen bonds [88], etc. According to [83], the activation energy of the crank shaft motion is 46–63 kJ/mol, and for its implementation it is necessary to have a free volume where the “crank shaft” can rotate. According to [93], for epoxy–amine systems, relaxation of this fragment should be observed at about −93 °C; however, as can be seen from [75], these may be higher temperatures (up to −50 °C [92,94,95] and even higher).
[88,91,92], in particular, the movement of diester bonds in it [88], non-rotational bending movements of fragments containing benzene ring [88], the movement of aromatic rings themselves [85], etc. In the first case, it can be a crankshaft motion [84,88], the movement of a hydroxyester group bound by hydrogen bonds [88], etc. According to [83], the activation energy of the crank shaft motion is 46–63 kJ/mol, and for its implementation it is necessary to have a free volume where the “crank shaft” can rotate. According to [93], for epoxy–amine systems, relaxation of this fragment should be observed at about −93 °C; however, as can be seen from [75], these may be higher temperatures (up to −50 °C [92,94,95] and even higher).2. Samples and Methods
2.1. Samples
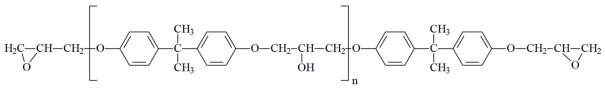 ,
,- where n can range from 0 to 15 and beyond (up to ~200). As n increases, the viscosity of the oligomer also increases. The brand designations consist of the following: E—epoxy; D—diane; and numerical digits denoting the upper limit of the normative epoxy group content.
Sample Preparation
- No significant dissipative losses within −150 °C to +150 °C that could obscure oligomer peaks;
- Minimal moment of inertia Is to avoid influence on the oscillation process;
- No chemical interaction with the applied oligomer layer;
- It is necessary to take into account the adhesive contact interactions between the surface of the substrate (matrix) and the composite oligomer.
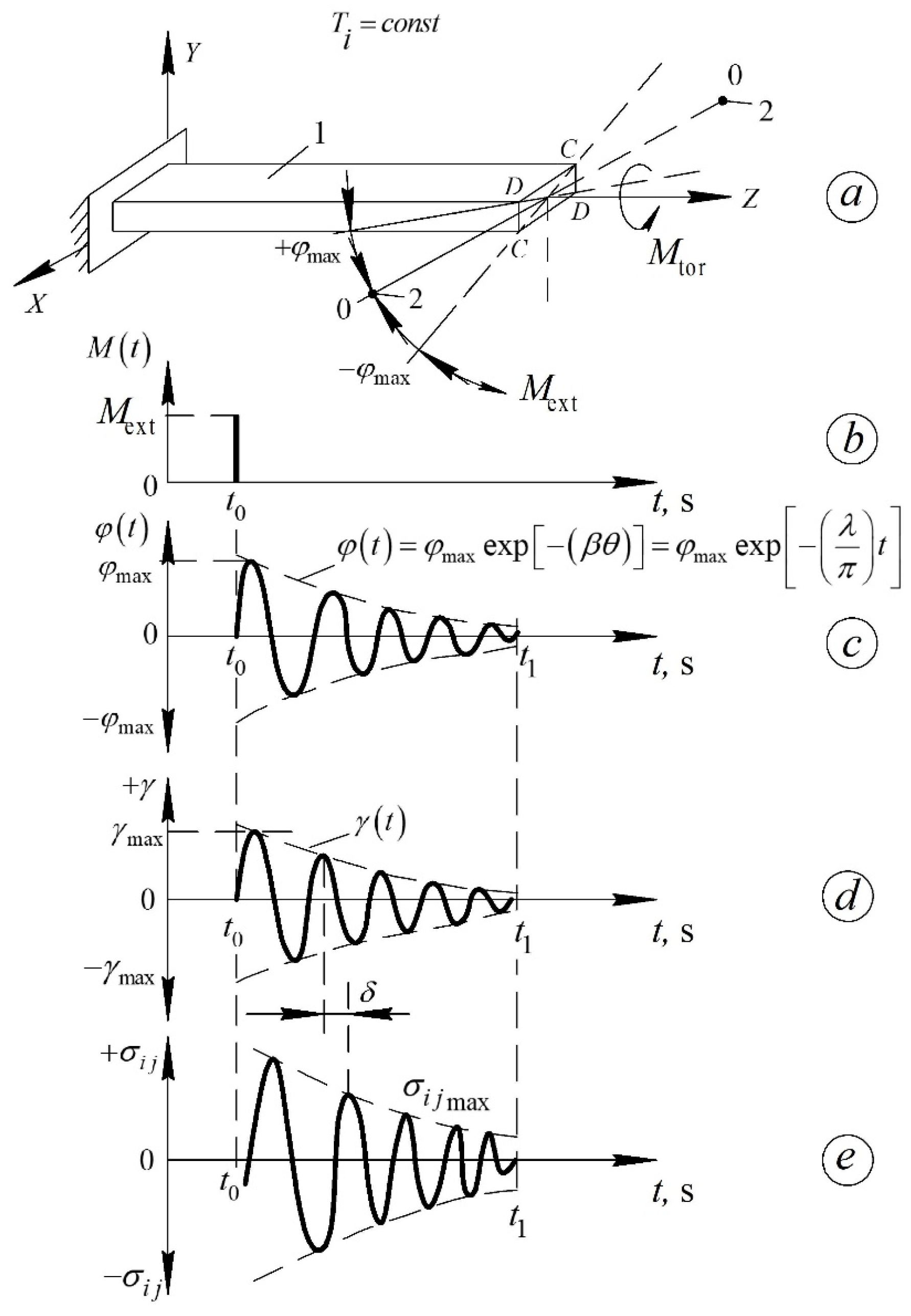
2.2. Methods
- the internal-friction spectrum —which reflects energy dissipation due to molecular motion, and
- the oscillation frequency —which reflects the elastic stiffness and inertia of the system.
3. Results and Discussion
3.1. Materials
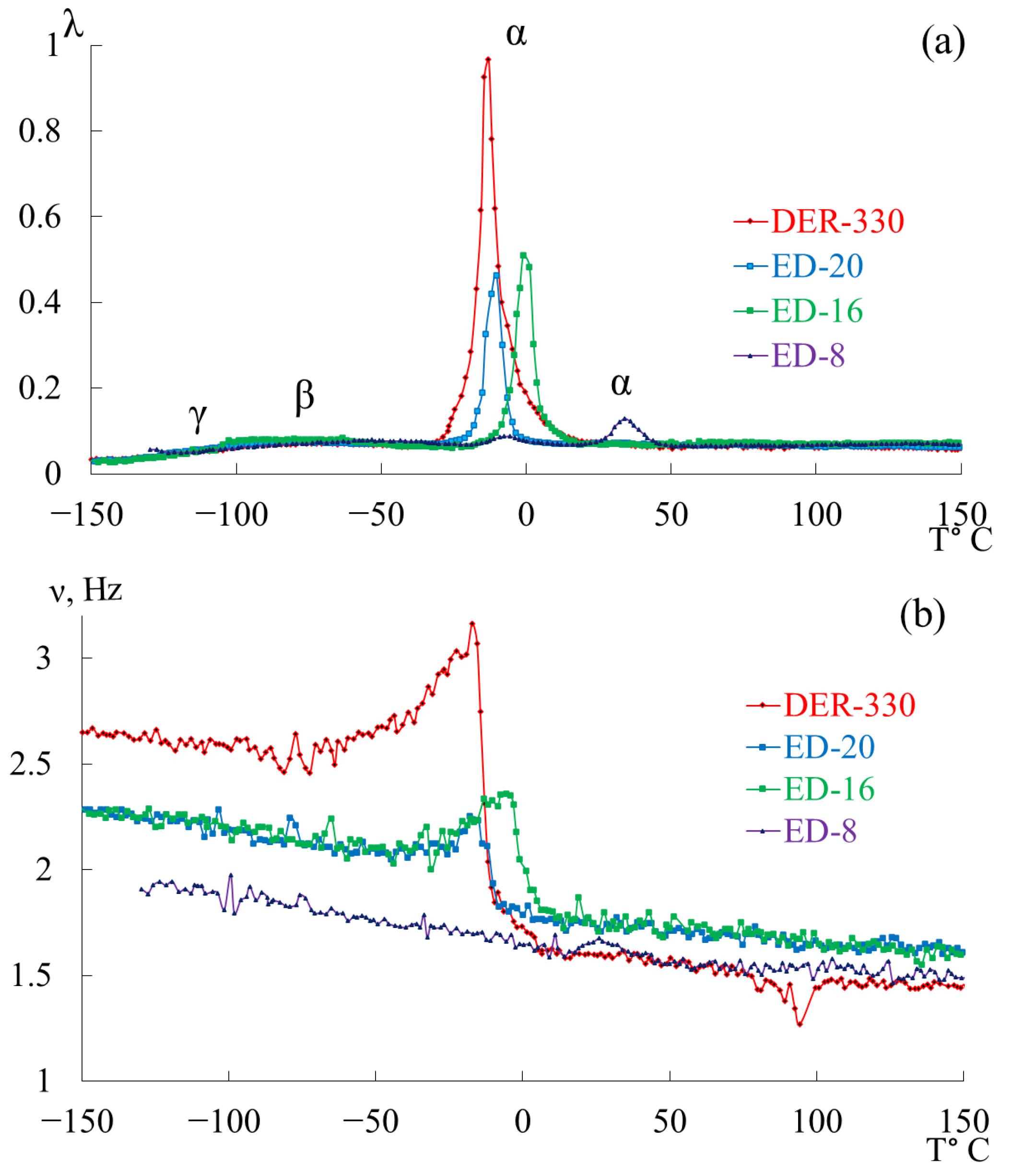
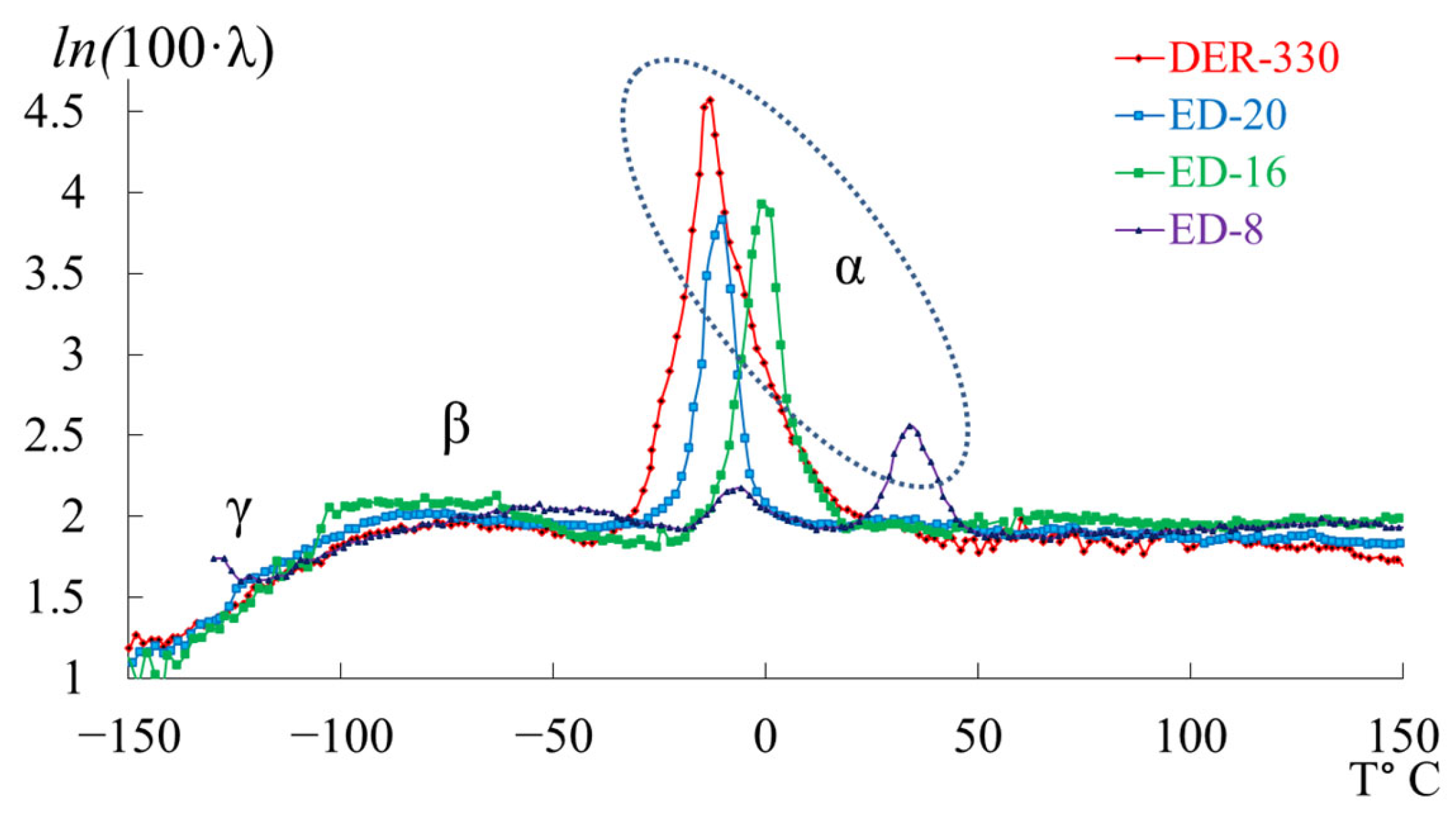
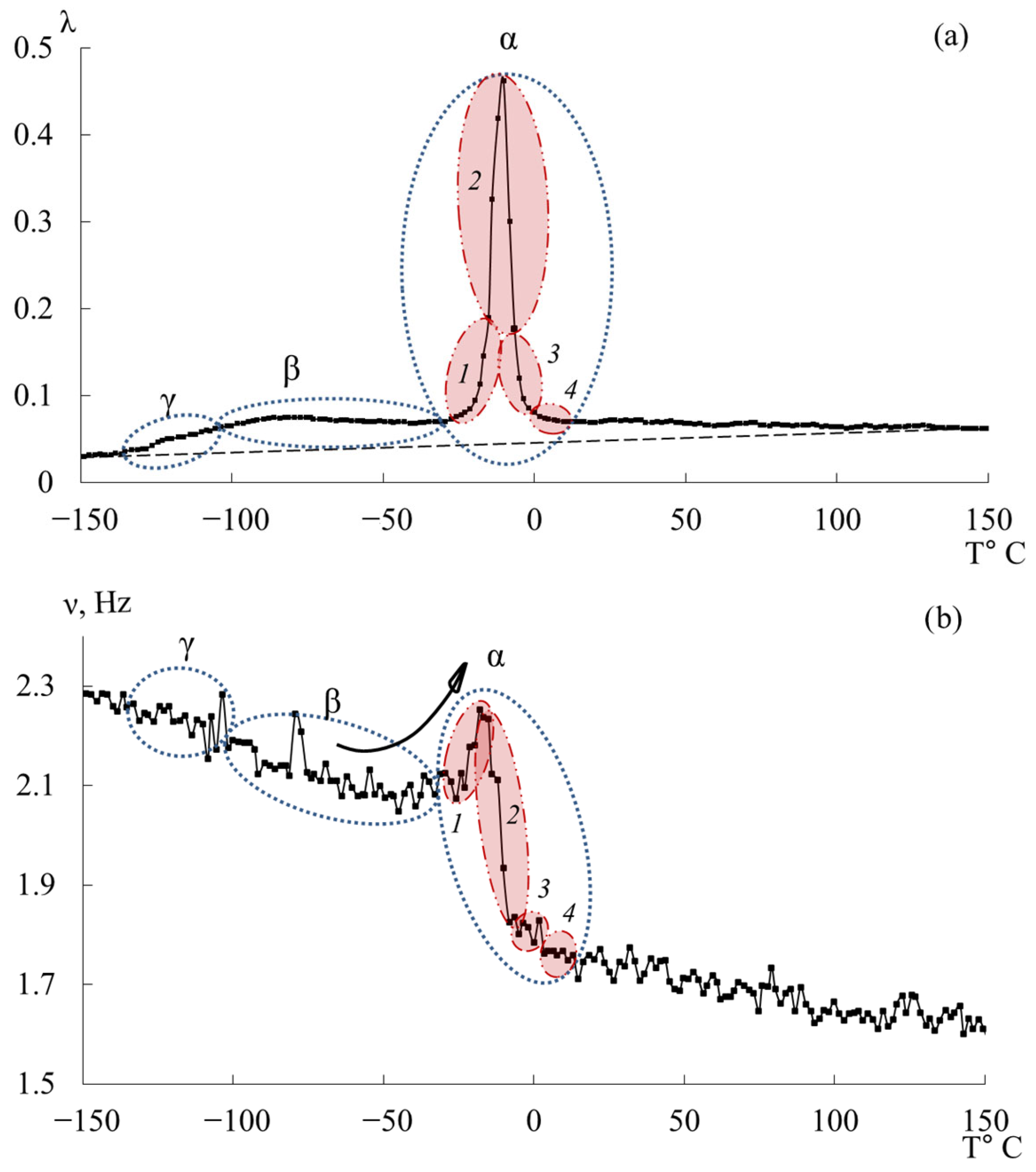
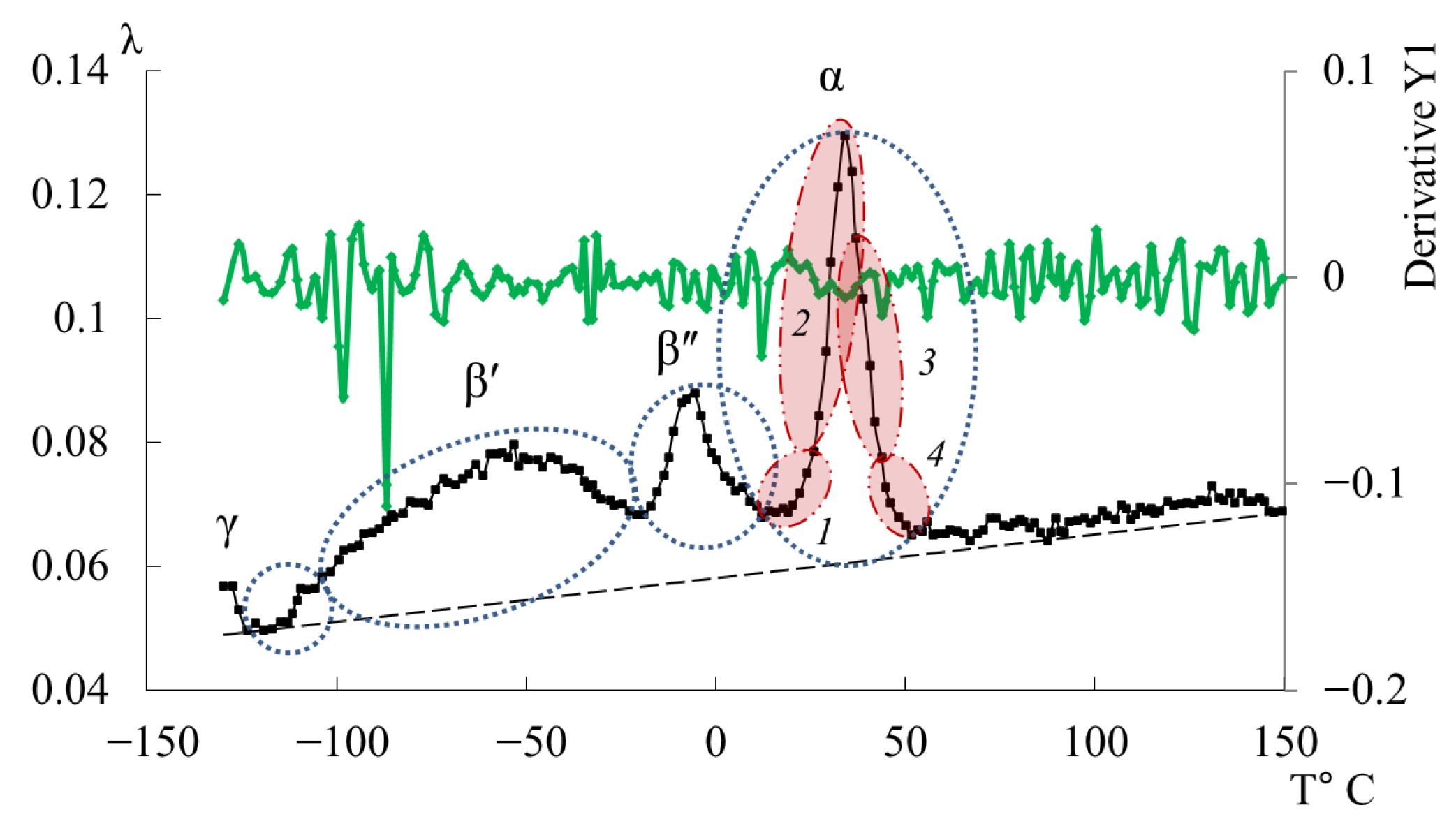
- in the range from −120 °C to −40 °C, a low-intensity dissipative process (, type relaxation) is observed;
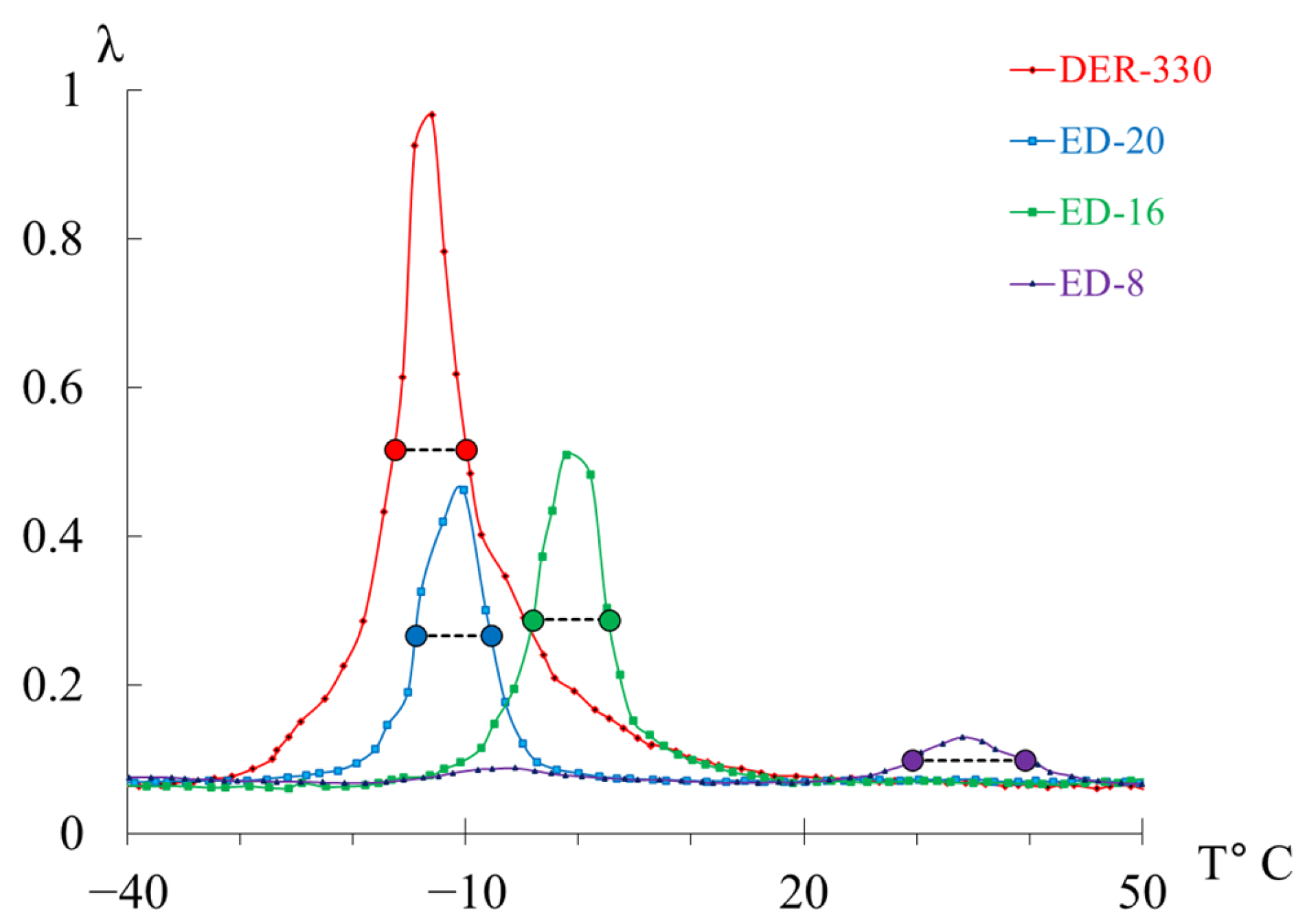
- in the range from −30 °C to +20 °C, a high-intensity process (, type relaxation) is detected.
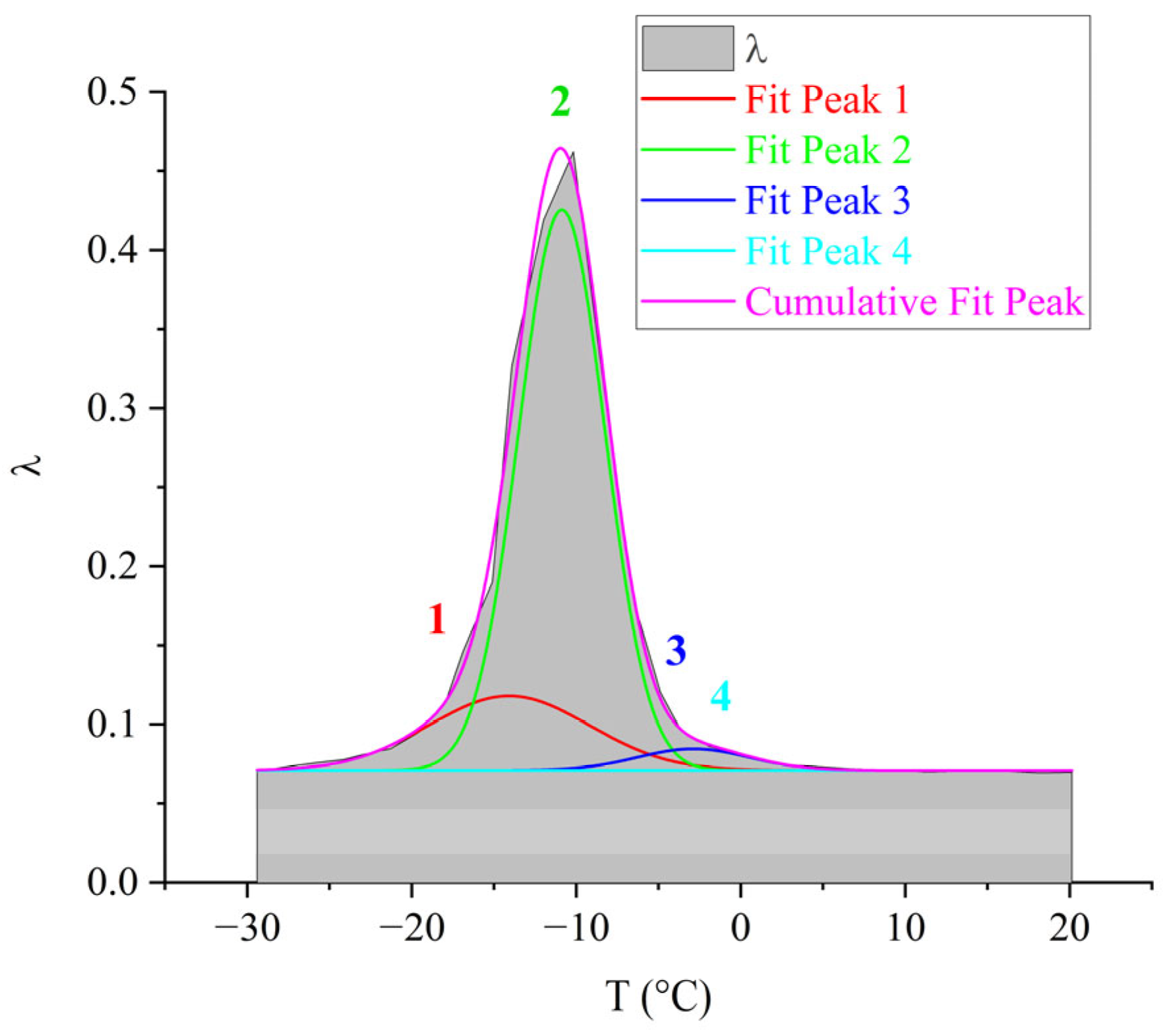
3.2. DER-330
3.3. ED-20
3.4. ED-16
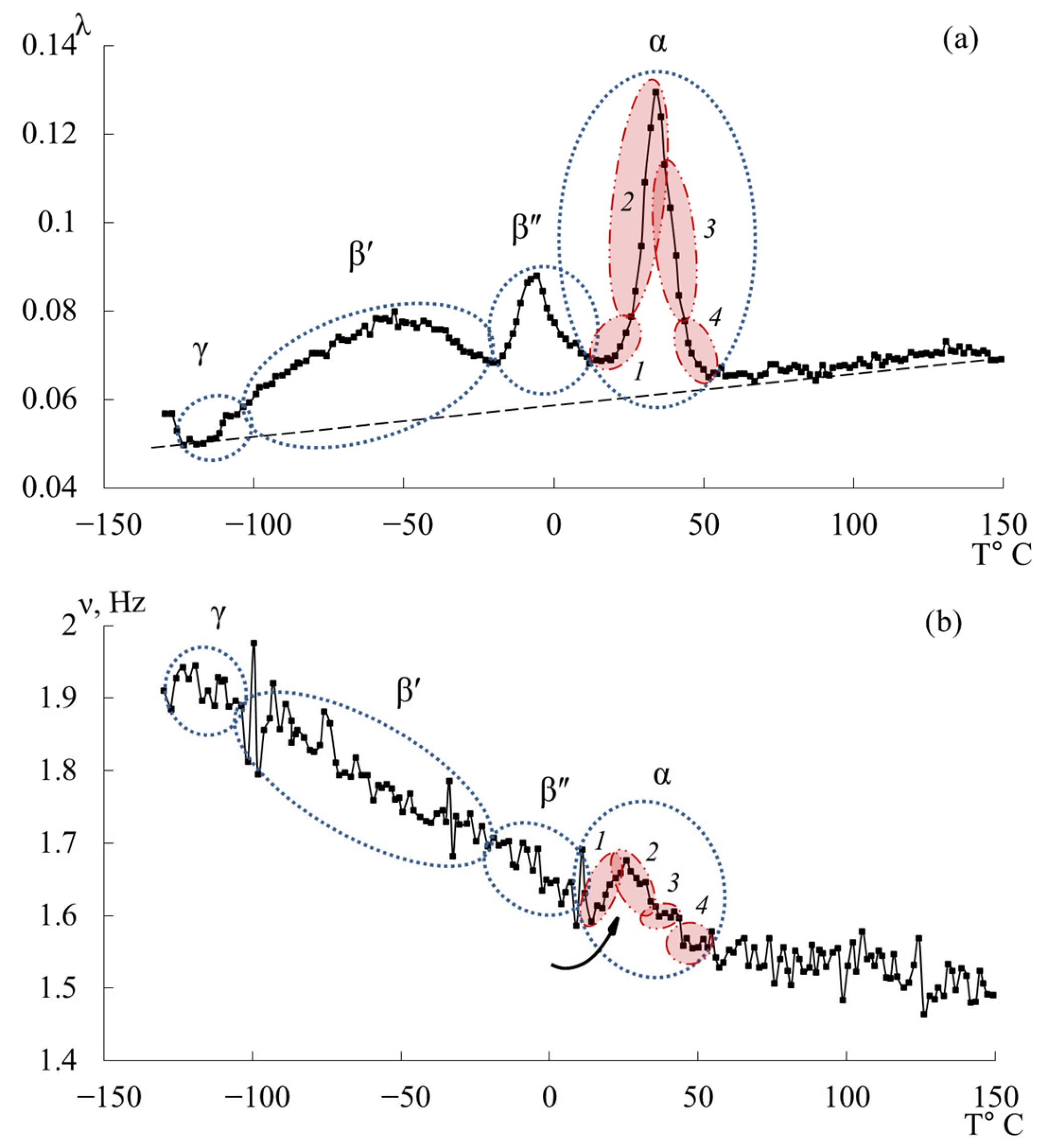
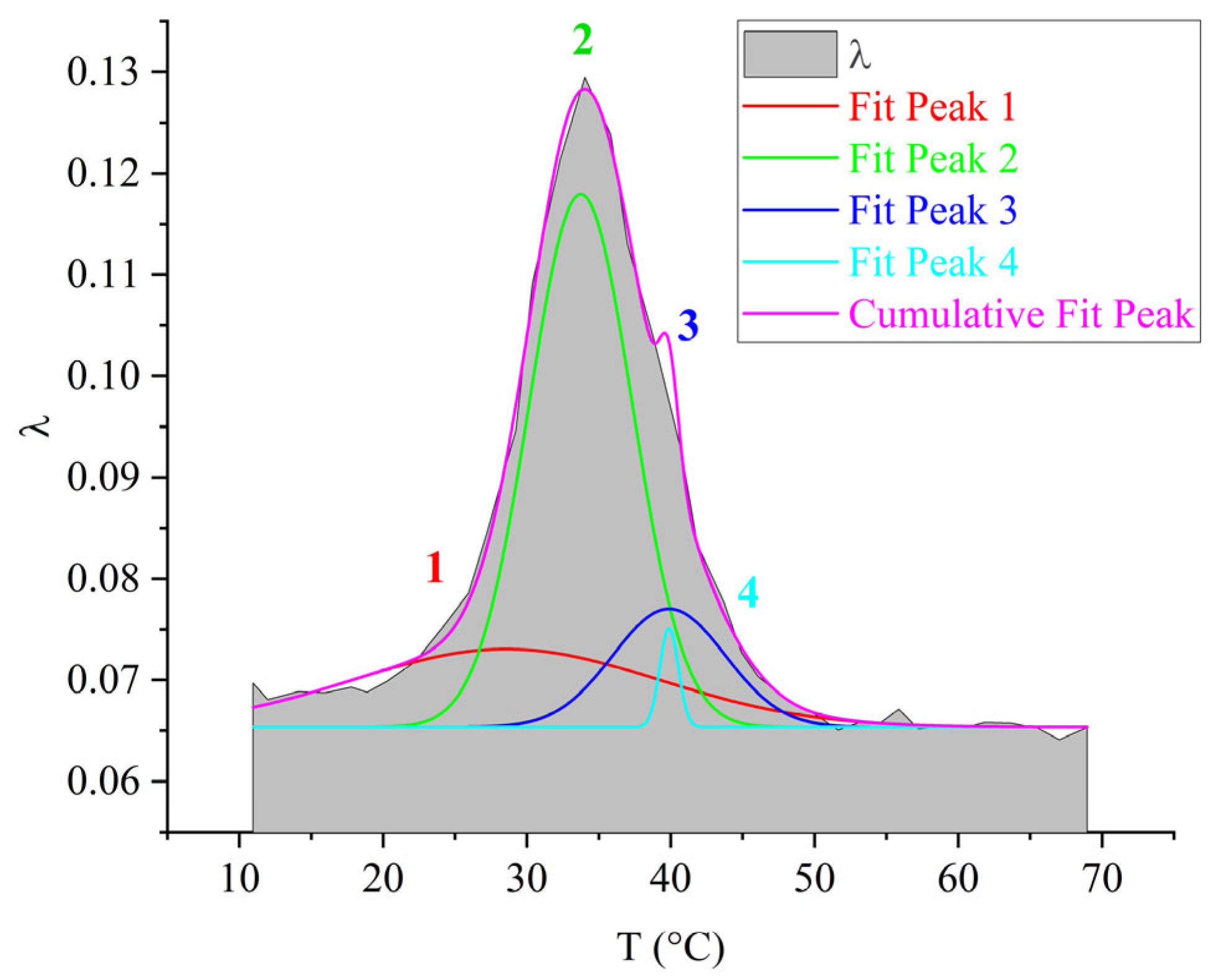
3.5. ED-8
3.6. Calculation of Physicochemical and Physicomechanical Characteristics of Dissipative Processes
4. Conclusions
- A deep literature analysis was conducted on studies of the relaxation behavior of epoxy oligomers. An experimental investigation was performed, followed by theoretical analysis of the obtained results for uncured epoxy oligomers, taking into account their aggregation state over a wide temperature range (from −150 °C to +150 °C) in dynamic mode.
- From the obtained experimental results, it was established that three processes of dissipative losses are detected on the internal friction spectra: γ-, β-, α-, and the l-l region. The structural rationale for the manifestation of each of these local dissipative processes is considered as follows: the γ-process–oscillatory-rotational movements of side groups; the β-process–oscillatory-rotational movements of atoms and atomic groups around the axis of the main polymer chain; the α-process is associated with the defrosting of the mobility of macromolecular segments and corresponds to mechanical and structural glass transition processes. These processes, in turn, represent a set of dissipative processes superimposed on each other, which manifests itself in the splitting of these loss peaks into components.
- Mathematical processing of the temperature dependence of the frequency of the free damped oscillatory process made it possible to assume mechanisms of internal friction (relaxation and phase) and to calculate the defect of the shear modulus for the main α-relaxation process. It was established that with increasing molecular mass, the ability of the uncured oligomer to elastically resist external shear influences increases in the temperature range of the manifestation of the α-relaxation process.
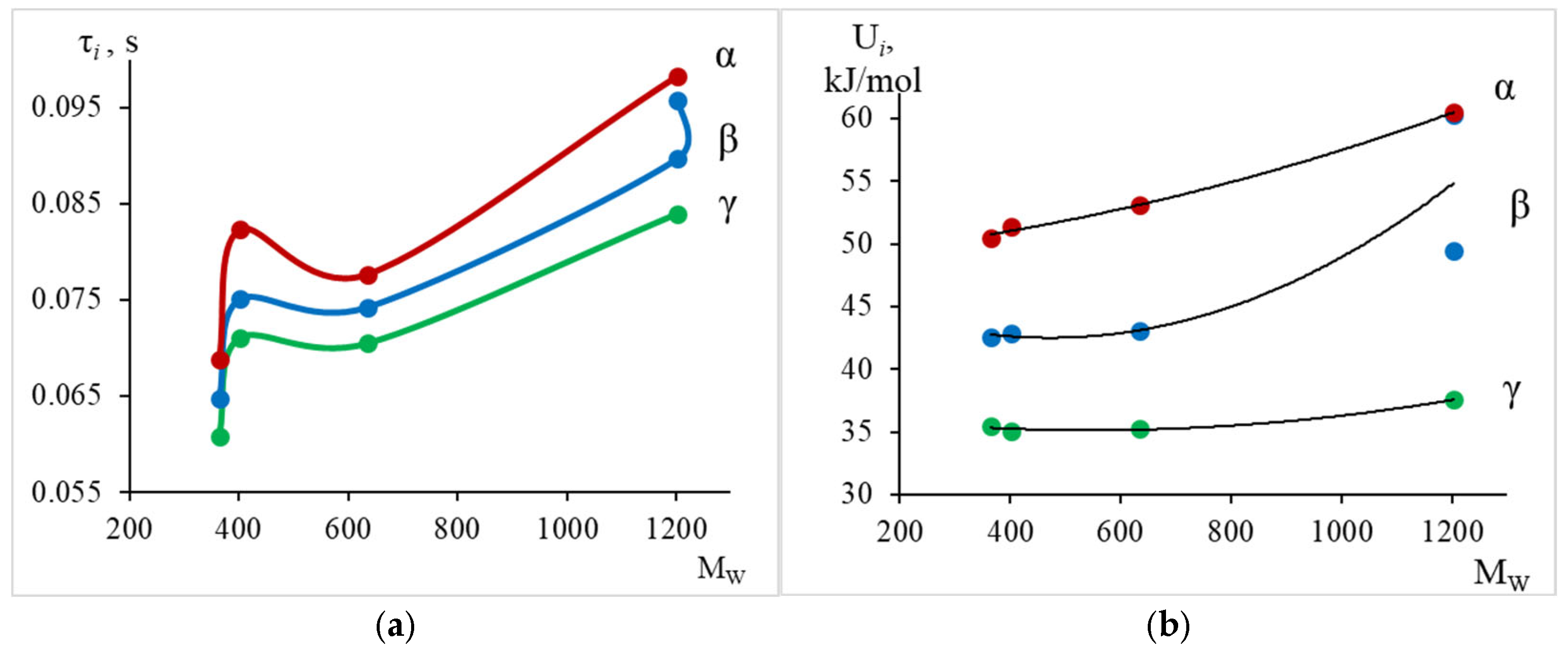
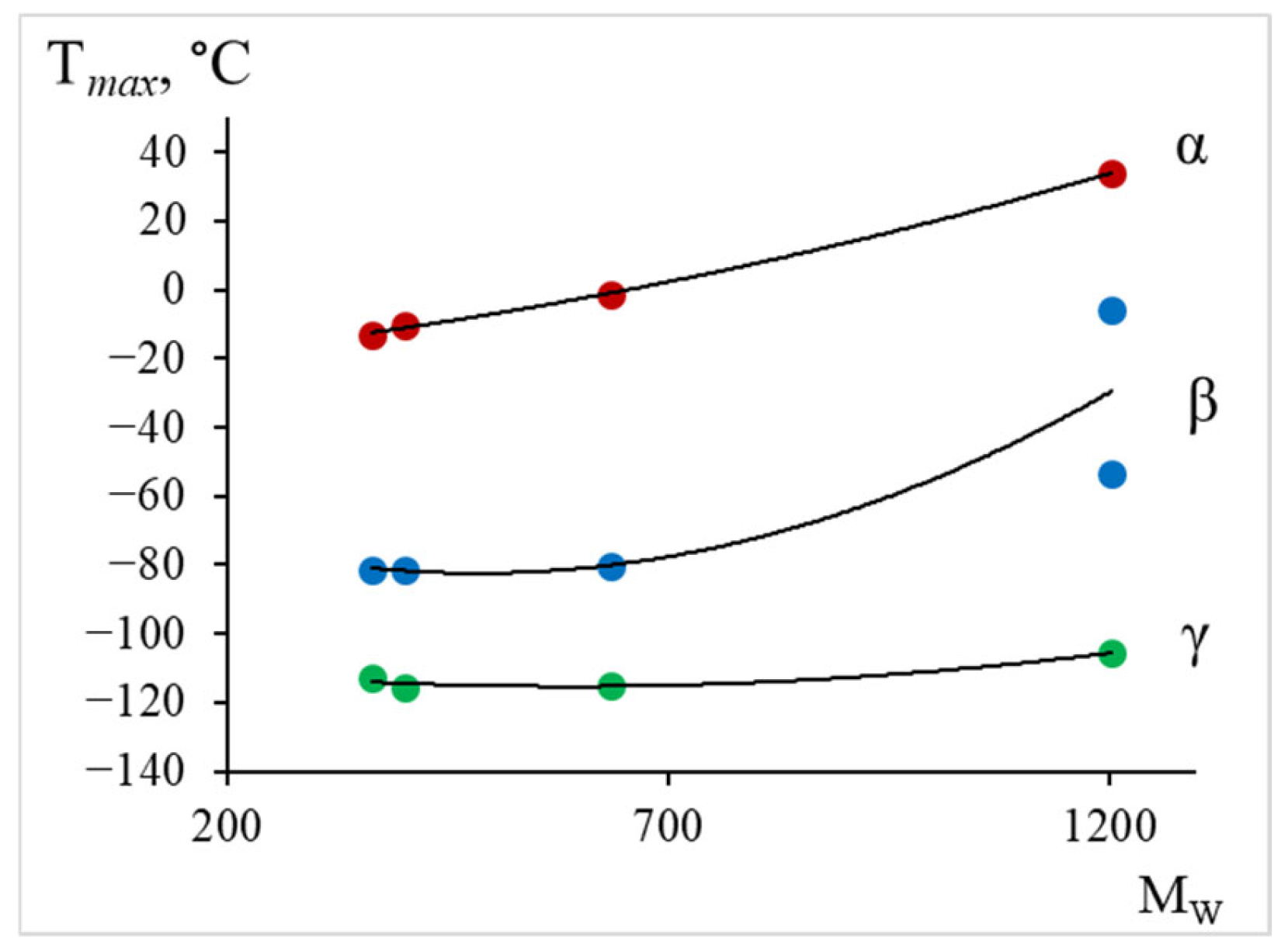
- The physicochemical characteristics (relaxation time and activation energy) of the local dissipative processes were calculated. The obtained activation energy values are as follows: process–~35–40 kJ/mol; process–~40–60 kJ/mol; process–~50–60 kJ/mol. The greatest changes in the values of temperature and activation energy (increase in values) with increasing molecular mass of the oligomer, decreasing proportion of epoxy groups, are observed in the region of the relaxation process manifestation: temperature increases from −13 °C to +34 °C, activation energy increases from 50 kJ/mol to 60 kJ/mol.
- It was established that the higher values of the activation energy of the γ-relaxation process (compared to the literature data) are associated not only with the temperature position of this process, but also with the frequency value and, to a greater extent, with the value of the pre-exponential coefficient in the Arrhenius equation.
- An assumption is given that, from the point of view of structural structure, higher values of the activation energy of the γ-relaxation process may be associated with the complication of the oligomer system’s structure and a change in the number of intermolecular interactions due to the presence of mono- and non-functional molecules.
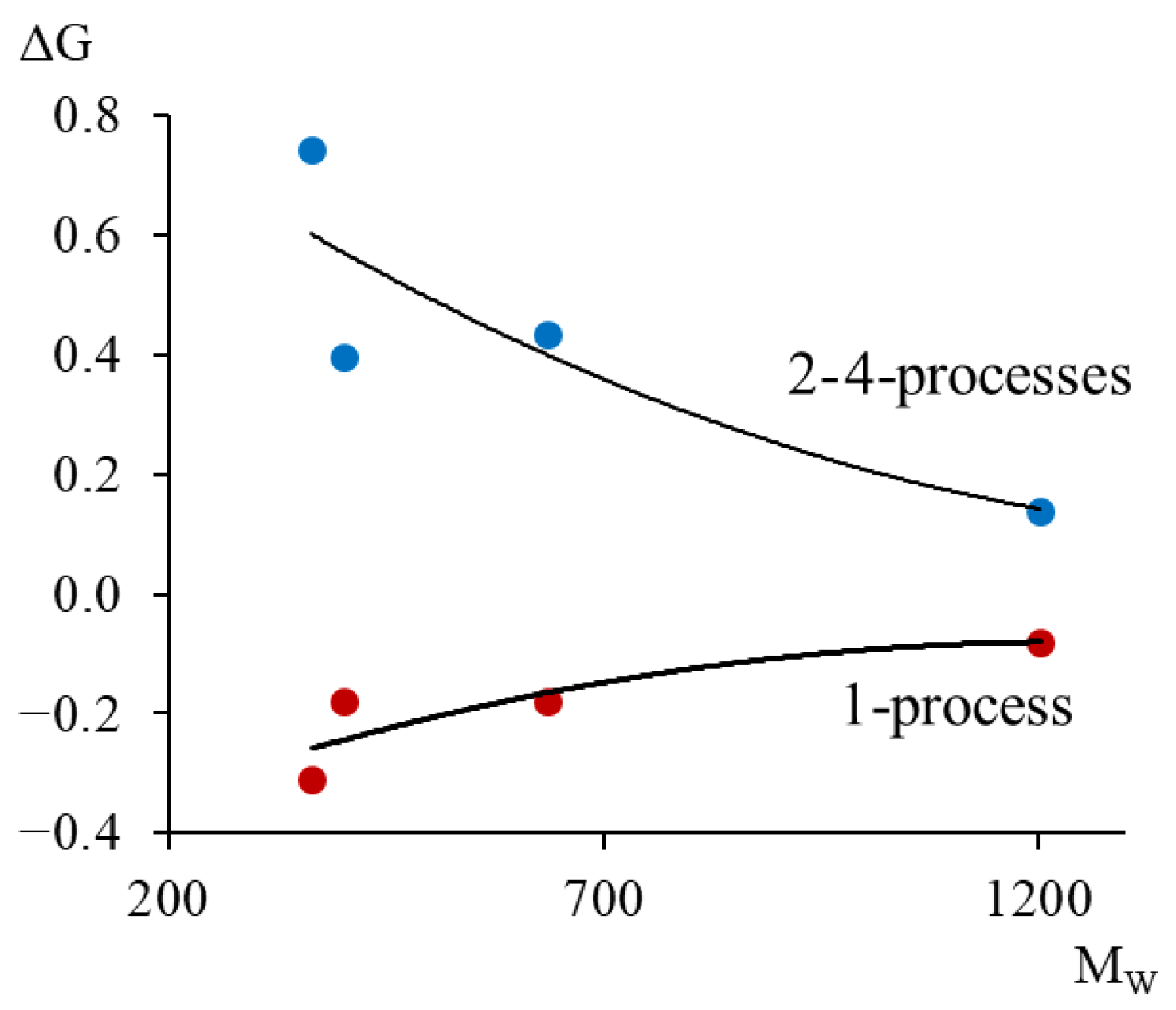
- The possibility of describing the relaxation microinhomogeneity of the α-relaxation process depending on the molecular mass of the oligomer is considered.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, H.; Neville, K. Handbook of Epoxy Resins; McGraw-Hill: New York, NY, USA, 1981; ISBN 0070369976. [Google Scholar]
- Irzhak, V.I. Epoxy Polymers and Epoxy Matrix Composites; Russian Academy of Sciences: Moscow, Russia, 2022. (In Russian) [Google Scholar]
- Irzhak, V.I. Epoxy Polymers and Nanocomposites; Editorial and Publishing Department of ICP RAS: Chernogolovka, Russia, 2021. (In Russian) [Google Scholar]
- Zhuravleva, I.I.; Akopyan, V.A. High Molecular Compounds Part VI. Synthetic Polymers: Textbook; Samara University Press: Samara, Russia, 2014. (In Russian) [Google Scholar]
- Ma, H.; Hu, N.; Wu, C.; Zhu, Y.; Cao, Y.; Chen, Q.Q. Synthesis and Research of Epoxy Resin Toughening Agent. Springerplus 2016, 5, 1049. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Akhtarul Islam, M. Application of Epoxy Resins in Building Materials: Progress and Prospects. Polym. Bull. 2022, 79, 1949–1975. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, X.; Chen, H.; Li, J.; Wang, X.; Zhu, J. Application of Epoxy Resin in Cultural Relics Protection. Chin. Chem. Lett. 2024, 35, 109194. [Google Scholar] [CrossRef]
- Peerzada, M.; Abbasi, S.; Lau, K.T.; Hameed, N. Additive Manufacturing of Epoxy Resins: Materials, Methods, and Latest Trends. Ind. Eng. Chem. Res. 2020, 59, 6375–6390. [Google Scholar] [CrossRef]
- Mohan, P. A Critical Review: The Modification, Properties, and Applications of Epoxy Resins. Polym. Plast. Technol. Eng. 2013, 52, 107–125. [Google Scholar] [CrossRef]
- Gorbatkina, Y.A. Adhesive Strength in Polymer–Fiber Systems; Khimiya: Moscow, Russia, 1987. (In Russian) [Google Scholar]
- Water-Based Epoxy Technologies for Metal Coating Applications. PCI Mag. 2021. Available online: https://www.pcimag.com/articles/108596-water-based-epoxy-technologies-for-metal-coating-applications (accessed on 11 October 2025).
- Epoxy Components for the Production of Construction and Finishing Materials. Available online: https://baltimix.ru/confer_archive/reports/doclad06/soldatov.php (accessed on 11 October 2025). (In Russian).
- Rehbinder, P.A. Selected Works. Surface Phenomena in Disperse Systems: Physicochemical Mechanics; Nauka: Moscow, Russia, 1979. (In Russian) [Google Scholar]
- Tamrakar, S.; Ganesh, R.; Sockalingam, S.; Haque, B.Z.; Gillespie, J.W. Experimental Investigation of Strain Rate and Temperature Dependent Response of an Epoxy Resin Undergoing Large Deformation. J. Dyn. Behav. Mater. 2018, 4, 114–128. [Google Scholar] [CrossRef]
- Bartenev, G.M.; Barteneva, A.G. Relaxation Properties of Polymers; Khimiya: Moscow, Russia, 1992; ISBN 5-7245-0371-9. (In Russian) [Google Scholar]
- Blanter, M.S.; Golovin, I.S.; Neuhäuser, H.; Sinning, H.-R. Introduction to Internal Friction: Terms and Definitions. In Internal Friction in Metallic Materials; Springer: Berlin/Heidelberg, Germany, 2007; Volume 90, pp. 1–10. ISBN 978-3-540-68757-3. [Google Scholar]
- Bartenev, G.M. Structure and Relaxation Properties of Elastomers; Khimiya: Moscow, Russia, 1979. (In Russian) [Google Scholar]
- Irzhak, V.I. Topological Structure and Relaxation Properties of Polymers. Russ. Chem. Rev. 2005, 74, 937–956. [Google Scholar] [CrossRef]
- Lomovskoy, V.A.; Shatokhina, S.A.; Chalykh, A.E.; Matveev, V.V. Spectra of Internal Friction in Polyethylene. Polymers 2022, 14, 675. [Google Scholar] [CrossRef]
- Khanna, Y.P.; Turi, E.A.; Taylor, T.J.; Vickroy, V.V.; Abbott, R.F. Dynamic Mechanical Relaxations in Polyethylene. Macromolecules 1985, 18, 1302–1309. [Google Scholar] [CrossRef]
- Bershtein, V.A.; Yegorov, V.M.; Marikhin, V.A.; Myasnikova, L.P. Specific Features of Molecular Motion in Lamellar Polyethylene between 100 and 400 K. Polym. Sci. USSR 1985, 27, 864–874. [Google Scholar] [CrossRef]
- Salakhov, I.I.; Chalykh, A.E.; Shaidullin, N.M.; Shapagin, A.V.; Budylin, N.Y.; Khasbiullin, R.R.; Nifant’ev, I.E.; Gerasimov, V.K. Phase Equilibria and Interdiffusion in Bimodal High-Density Polyethylene (HDPE) and Linear Low-Density Polyethylene (LLDPE) Based Compositions. Polymers 2021, 13, 811. [Google Scholar] [CrossRef]
- Salakhov, I.I.; Shaidullin, N.M.; Chalykh, A.E.; Matsko, M.A.; Shapagin, A.V.; Batyrshin, A.Z.; Shandryuk, G.A.; Nifant’ev, I.E. Low-Temperature Mechanical Properties of High-Density and Low-Density Polyethylene and Their Blends. Polymers 2021, 13, 1821. [Google Scholar] [CrossRef] [PubMed]
- Shut, N.I.; Bartenev, G.M.; Kaspersky, A.V. Relaxation Transitions in Polyethylene According to Structural and Mechanical Relaxation Data. Acta Polym. 1989, 40, 529–532. [Google Scholar] [CrossRef]
- Bartenev, G.M.; Aliguliyev, R.M.; Khiteyeva, D.M. Relaxation Transitions in Polyethylene. Polym. Sci. USSR 1981, 23, 2183–2193. [Google Scholar] [CrossRef]
- Lomovskoy, V.A.; Shatokhina, S.A.; Alekhina, R.A.; Lomovskaya, N.Y. Temperature Areas of Local Inelasticity in Polyoxymethylene. Polymers 2024, 16, 3582. [Google Scholar] [CrossRef]
- Zamboni, V.; Zerbi, G. Vibrational Spectrum of a New Crystalline Modification of Polyoxymethylene. J. Polym. Sci. Part C Polym. Symp. 1964, 7, 153–161. [Google Scholar] [CrossRef]
- Lüftl, S.; Visakh, P.M.; Chandran, S. (Eds.) Polyoxymethylene Handbook. Structure, Properties, Applications and Their Nanocomposites; Wiley: Hoboken, NJ, USA, 2014; ISBN 9781118385111. [Google Scholar]
- Kurosu, H.; Komoto, T.; Ando, I. 13C NMR Chemical Shift and Crystal Structure of Polyoxymethylene in the Solid State. J. Mol. Struct. 1988, 176, 279–284. [Google Scholar] [CrossRef]
- Raimo, M. An Overview on the Processing of Polymers Growth Rate Data and on the Methods to Verify the Accuracy of the Input Parameters in Crystallization Regime Analysis. Prog. Cryst. Growth Charact. Mater. 2011, 57, 65–92. [Google Scholar] [CrossRef]
- Raimo, M. Analysis of Layer-by-Layer Phase Transformation of a Polyoxymethylene Copolymer Film. Acta Mater. 2008, 56, 4217–4225. [Google Scholar] [CrossRef]
- Read, B.E.; Williams, G. The Dielectric and Dynamic Mechanical Properties of Polyoxymethylene (Delrin). Polymer 1961, 2, 239–255. [Google Scholar] [CrossRef]
- McCrum, N.G. Internal Friction in Polyoxymethylene. J. Polym. Sci. 1961, 54, 561–568. [Google Scholar] [CrossRef]
- Kazen, M.E.; Geil, P.H. Electron Microscopy Studies of Relaxation Behavior of Polyoxymethylene. J. Macromol. Sci. Part B 1977, 13, 381–404. [Google Scholar] [CrossRef]
- Yamada, N.; Orito, Z.; Minami, S. Relaxation Phenomena of Polyoxymethylene Obtained from Solid-State Polymerization. J. Polym. Sci. Part A Gen. Pap. 1965, 3, 4173–4179. [Google Scholar] [CrossRef]
- Bershtein, V.A.; Egorova, L.M.; Egorov, V.M.; Peschanskaya, N.N.; Yakushev, P.N.; Keating, M.Y.; Flexman, E.A.; Kassal, R.J.; Schodt, K.P. Segmental Dynamics in Poly(Oxymethylene) as Studied via Combined Differential Scanning Calorimetry/Creep Rate Spectroscopy Approach. Thermochim. Acta 2002, 391, 227–243. [Google Scholar] [CrossRef]
- Abaturova, N.A.; Lomovskaya, N.Y.; Shatokhina, S.A.; Lomovskoy, V.A. Influence of the Degree of Hydration on the Relaxation Microheterogeneity of Segmental Mobility in PVA. Key Eng. Mater. 2021, 899, 619–627. [Google Scholar] [CrossRef]
- Nekrasova, N.V.; Khlebnikova, O.A.; Lomovskoy, V.A.; Kadyko, M.I.; Vysotskii, V.V.; Galushko, T.B.; Kazberov, R.Y. The Study of the Behavior of Aqueous Poly(Vinyl Alcohol) Solutions Exposed to UV Radiation. Colloid J. 2023, 85, 66–71. [Google Scholar] [CrossRef]
- Lomovskoy, V.A.; Abaturova, N.A.; Lomovskaya, N.Y.; Khlebnikova, O.A. Internal-Friction Spectra of Poly(Vinyl Alcohol) with Various Molecular Masses. Polym. Sci. A 2015, 57, 123–130. [Google Scholar] [CrossRef]
- Lomovskoy, V.A.; Chugunov, Y.V.; Shatokhina, S.A. Methodology for the Study of Internal Friction in the Mode of Free Damped Oscillatory Process. Part 1. Sci. Instrum. 2023, 33, 60–71. [Google Scholar]
- Lomovskoy, V.A.; Chugunov, Y.V.; Shatokhina, S.A. Methodology for the Study of Internal Friction in the Mode of Free Damped Oscillatory Process. Part 2. Theoretical Analysis of Experimental Results. Sci. Instrum. 2024, 34, 3–18. [Google Scholar]
- Lomovskoy, V.A. Structural Formation Problems in Dispersed Systems. In Scientific Publication “Modern Problems of Physical Chemistry”; Publishing House “Granitsa”: Moscow, Russia, 2005; pp. 193–209. (In Russian) [Google Scholar]
- Lomovskoy, V.A. Internal Friction Spectra and Dissipative Mobility of Elements of Aggregate and Modifying Subsystems. Mater. Sci. 2007, 2, 3–10. (In Russian) [Google Scholar]
- Bartenev, G.M.; Aliguliyev, R.M. Relaxational Spectrometry of Low Density Polyethylene. Polym. Sci. USSR 1982, 24, 2106–2114. [Google Scholar] [CrossRef]
- Bugayev, N.M.; Gorshkov, A.A.; Lomovskoy, V.A.; Fomkina, Z.I. Nature and Possible Mechanisms of the Origin of the Dissipative Losses Background on the Internal Friction Spectra of Amorphous Polymeric Materials. In Abstracts of the XXVI International Conference Mathematical and Computer Modeling in the Mechanics of Deformable Media and Structures; Publishing House “Farmindeks”: Saint Petersburg, Russia, 2015; pp. 346–348. (In Russian) [Google Scholar]
- Bassett, D.C. Principles of Polymer Morphology; Cambridge University Press: New York, NY, USA, 1981. [Google Scholar]
- Lebedev, I.I. Molecular Mobility in Near-Surface Polymer Nanostructures. Ph.D. Thesis, Ioffe Physical-Technical Institute of RAS, Saint Petersburg, Russia, 2011; 191p. (In Russian). [Google Scholar]
- Askadsky, A.A. Deformation of Polymers; Khimiya: Moscow, Russia, 1973. (In Russian) [Google Scholar]
- Askadsky, A.A.; Khokhlov, A.R. Introduction to the Physicochemistry of Polymers; Nauchny Mir: Moscow, Russia, 2009. (In Russian) [Google Scholar]
- Kerber, M.L.; Bukanov, A.M.; Volfson, S.I.; Gorbunova, I.Y.; Kandyrin, L.B.; Sirota, A.G.; Sheryshev, M.A. Physical and Chemical Processes During Polymer Processing: Scientific Foundations and Technologies; Nauchnye Osnovy I Tekhnologii: Saint Petersburg, Russia, 2013. (In Russian) [Google Scholar]
- Shevchenko, A.A. Physical Chemistry and Mechanics of Composite Materials: Textbook for Universities; TsOB “Professiya”: Saint Petersburg, Russia, 2010. (In Russian) [Google Scholar]
- Askadskii, A.A. Lectures on the Physicochemistry of Polymers; Nova Science Publishers, Inc.: New York, NY, USA, 2003. [Google Scholar]
- Tager, A. Physical Chemistry of Polymers, 2nd ed.; Gordon and Breach Science Publishers: New York, NY, USA, 1978. [Google Scholar]
- Zinoviev, P.A.; Ermakov, Y.N. Energy Dissipation in Composite Materials; Routledge: New York, NY, USA, 2018; ISBN 9780203757529. [Google Scholar]
- Dwyer, D.B.; Isbill, S.; Brubaker, Z.E.; Keum, J.K.; Bras, W.; Niedziela, J.L. Thermally Induced Structural Transitions in Epoxy Thermoset Polymer Networks and Their Spectroscopic Responses. ACS Appl. Polym. Mater. 2023, 5, 5961–5971. [Google Scholar] [CrossRef]
- Lv, G.; Shen, C.; Shan, N.; Jensen, E.; Li, X.; Evans, C.M.; Cahill, D.G. Odd–Even Effect on the Thermal Conductivity of Liquid Crystalline Epoxy Resins. Proc. Natl. Acad. Sci. USA 2022, 119, e2211151119. [Google Scholar] [CrossRef] [PubMed]
- Lv, G.; Jensen, E.; Shen, C.; Yang, K.; Evans, C.M.; Cahill, D.G. Effect of Amine Hardener Molecular Structure on the Thermal Conductivity of Epoxy Resins. ACS Appl. Polym. Mater. 2021, 3, 259–267. [Google Scholar] [CrossRef]
- Morgan, R.J.; O’Neal, J.E. Effect of Epoxy Monomer Crystallization and Cure Conditions on Physical Structure, Fracture Topography, and Mechanical Response of Polyamide-Cured Bisphenol-A-Diglycidyl Ether Epoxies. J. Macromol. Sci. Part B 1978, 15, 139–169. [Google Scholar] [CrossRef]
- Lomovskoy, V.A.; Shatokhina, S.A.; Simonov-Emelyanov, I.D. Phenomenological Description of the “Structure–Property” Relation for Epoxy Oligomer Hardeners on the Basis of Internal Friction Spectra. Colloid J. 2024, 86, 418–430. [Google Scholar] [CrossRef]
- Chernin’s, I.Z.; Smehov, F.M.; Zherdev, Y.V. Epoxy Polymers and Compositions; Khimiya: Moscow, Russia, 1982. (In Russian) [Google Scholar]
- Kataev, V.M.; Popov, V.A.; Sazhin, B.I. Handbook on Plastics; In 2 Volumes; Khimiya: Moscow, Russia, 1975; Volume 2. (In Russian) [Google Scholar]
- Lidaoik, M. Epoxydove Priskioice; SNTL: Praha, Czechoslovakia, 1983. [Google Scholar]
- Brojer, Z.; Hertz, Z.; Penczek, R. Ziwice Epoksydowe; WNT: Warszawa, Poland, 1981. [Google Scholar]
- Postnikov, V.S. Internal Friction in Metals; Metallurgiya: Moscow, Russia, 1969. (In Russian) [Google Scholar]
- Krishtal, M.A.; Golovin, S.A. Internal Friction and Structure of Metals; Metallurgiya: Moscow, Russia, 1976. (In Russian) [Google Scholar]
- Meshkov, S.I. Viscous-Elastic Properties of Metals; Metallurgiya: Moscow, Russia, 1974. (In Russian) [Google Scholar]
- Tavadze, F.N.; Postnikov, V.S.; Gordienko, L.K. Internal Friction in Metallic Materials. Mechanisms of Internal Friction. In Collection of Articles; Nauka: Moscow, Russia, 1970; p. 208. (In Russian) [Google Scholar]
- Baranov, V.V. (Ed.) Internal Friction in Metals, Semiconductors, Dielectrics and Ferromagnets; Naukova Dumka: Kiev, Ukraine, 1978; p. 240. (In Russian) [Google Scholar]
- Gridnev, S.A. Mechanisms of Internal Friction in Ferroelectrics and Ferroelastics; Voronezh State University Publishing: Voronezh, Russia, 1983. (In Russian) [Google Scholar]
- Postnikov, V.S. (Ed.) Mechanisms of Relaxation Phenomena in Solids; Metallurgiya: Moscow, Russia, 1974; p. 364. [Google Scholar]
- Shut’lin, Y.F. Physical Chemistry of Polymers; Voronezh Regional Printing House: Voronezh, Russia, 2012; ISBN 978-5-4420-0044-3. (In Russian) [Google Scholar]
- Panovko, Y.G. Internal Friction in Elastic Systems Vibrations; Fizmatgiz: Moscow, Russia, 1960. (In Russian) [Google Scholar]
- Granato, A.V.; Lücke, K. Dislocation Theory of Absorption. In Ultrasonic Methods for Studying Dislocations; IL: Moscow, Russia, 1963; pp. 27–57. (In Russian) [Google Scholar]
- Diogo, H.P.; Ramos, J.J.M. Slow Molecular Mobility in the Amorphous Thermoplastic Polysulfone: A TSDC Investigation. J. Therm. Anal. Calorim. 2013, 111, 773–779. [Google Scholar] [CrossRef]
- Zhavoronok, E.S.; Senchikhin, I.N.; Roldughin, V.I. Physical Aging and Relaxation Processes in Epoxy Systems. Polym. Sci.—Ser. A 2017, 59, 159–192. [Google Scholar] [CrossRef]
- Bartenev, G.M.; Shut, N.I.; Dushchenko, V.P.; Sichkar, T.G. Relaxation Transitions in Epoxide Polymers. Polym. Sci. USSR 1986, 28, 699–707. [Google Scholar] [CrossRef]
- Kochergin, Y.S.; Zolotareva, V.V. Comparative Study of Composite Materials Based on Diglycylic Derivatives of Diphenylmethane and Diphenylpropane. Bull. BSTU Named After V.G. Shukhov 2018, 3, 91–98. [Google Scholar] [CrossRef]
- Lomovskoy, V.A.; Shatokhina, S.A. Relaxation Phenomena in Low-Density and High-Density Polyethylene. Polymers 2024, 16, 3510. [Google Scholar] [CrossRef]
- Bershtein, V.A.; Egorov, V.M. Differential Scanning Calorimetry of Polymers: Physics, Chemistry, Analysis, Technology. In Ellis Horwood Series in Polymer Science and Technology; Ellis Horwood Ltd.: Chichester, UK, 1994; p. 253. [Google Scholar]
- Urbaczewski-Espuche, E.; Galy, J.; Gerard, J.-F.; Pascault, J.-P.; Sautereau, H. Influence of Chain Flexibility and Crosslink Density on Mechanical Properties of Epoxy/Amine Networks. Polym. Eng. Sci. 1991, 31, 1572–1580. [Google Scholar] [CrossRef]
- Kramarenko, V.Y. Relaxations in Heterocyclic Polymer Networks. Polym. J. 2007, 29, 106–112. [Google Scholar]
- Slutsker, A.I.; Polikarpov, Y.I.; Vasilyeva, K.V. On the Determination of the Energy of Activation of Relaxation Transitions in Polymers by Differential Scanning Calorimetry. Tech. Phys. 2002, 47, 880–885. [Google Scholar] [CrossRef]
- Kong, E.S.-W. Physical Aging in Epoxy Matrices and Composites. Adv. Polym. Sci. 1986, 71, 125–171. [Google Scholar] [CrossRef]
- Wan, J.; Bu, Z.Y.; Xu, C.J.; Li, B.G.; Fan, H. Learning about Novel Amine-Adduct Curing Agents for Epoxy Resins: Butyl-Glycidylether-Modified Poly(Propyleneimine) Dendrimers. Thermochim. Acta 2011, 519, 72–82. [Google Scholar] [CrossRef]
- Hölck, O.; Bauer, J.; Wittler, O.; Michel, B.; Wunderle, B. Comparative Characterization of Chip to Epoxy Interfaces by Molecular Modeling and Contact Angle Determination. Microelectron. Reliab. 2012, 52, 1285–1290. [Google Scholar] [CrossRef]
- Fragiadakis, D.; Roland, C.M. Characteristics of the Johari-Goldstein Process in Rigid Asymmetric Molecules. Phys. Rev. E 2013, 88, 042307. [Google Scholar] [CrossRef]
- Adrjanowicz, K.; Kaminski, K.; Wlodarczyk, P.; Grzybowska, K.; Tarnacka, M.; Zakowiecki, D.; Garbacz, G.; Paluch, M.; Jurga, S. Molecular Dynamics of the Supercooled Pharmaceutical Agent Posaconazole Studied via Differential Scanning Calorimetry and Dielectric and Mechanical Spectroscopies. Mol. Pharm. 2013, 10, 3934–3945. [Google Scholar] [CrossRef]
- Pangrle, S.; Wu, C.S.; Geil, P.H. Low Temperature Relaxation of DGEBA Epoxy Resins: A Thermally Stimulated Discharge Current (TSDC) Study. Polym. Compos. 1989, 10, 173–183. [Google Scholar] [CrossRef]
- Wan, J.; Li, C.; Bu, Z.Y.; Fan, H.; Li, B.G. Acrylonitrile-Capped Poly(Propyleneimine) Dendrimer Curing Agent for Epoxy Resins: Model-Free Isoconversional Curing Kinetics, Thermal Decomposition and Mechanical Properties. Mater. Chem. Phys. 2013, 138, 303–312. [Google Scholar] [CrossRef]
- Maxwell, I.D.; Pethrick, R.A. Dielectric Studies of Water in Epoxy Resins. J. Appl. Polym. Sci. 1983, 28, 2363–2379. [Google Scholar] [CrossRef]
- Dean, K.M.; Cook, W.D.; Lin, M.Y. Small Angle Neutron Scattering and Dynamic Mechanical Thermal Analysis of Dimethacrylate/Epoxy IPNs. Eur. Polym. J. 2006, 42, 2872–2887. [Google Scholar] [CrossRef]
- Williams, J.G. The Effect of Boiling Water on Dynamic Mechanical Properties of Composites. J. Mater. Sci. 1982, 17, 1427–1433. [Google Scholar] [CrossRef]
- Bunton, L.G.; Daly, J.H.; Maxwell, I.D.; Pethrick, R.A. Investigation of Cure in Epoxy Resins: Ultrasonic and Thermally Stimulated Current Measurements. J. Appl. Polym. Sci. 1982, 27, 4283–4294. [Google Scholar] [CrossRef]
- Williams, J.G. The Beta Relaxation in Epoxy Resin-based Networks. J. Appl. Polym. Sci. 1979, 23, 3433–3444. [Google Scholar] [CrossRef]
- Chatterjee, A.; Gillespie, J.W. Room Temperature-Curable VARTM Epoxy Resins: Promising Alternative to Vinyl Ester Resins. J. Appl. Polym. Sci. 2010, 115, 665–673. [Google Scholar] [CrossRef]
- Yurechko, N.A.; Lipskaya, V.A.; Nesolenaya, L.G.; Yevtushenko, G.T.; Shologon, I.M.; Irzhak, V.I.; Rozenberg, B.A. Effect of the Crosslinking Density of Epoxide Polymers on Their Relaxation and Strain Characteristics. Polym. Sci. USSR 1980, 22, 1–9. [Google Scholar] [CrossRef]
- Wan, J.; Li, C.; Bu, Z.Y.; Xu, C.J.; Li, B.G.; Fan, H. A Comparative Study of Epoxy Resin Cured with a Linear Diamine and a Branched Polyamine. Chem. Eng. J. 2012, 188, 160–172. [Google Scholar] [CrossRef]
- Wan, J.; Li, C.; Bu, Z.Y.; Fan, H.; Li, B.G. Evaluating a Four-Directional Benzene-Centered Aliphatic Polyamine Curing Agent for Epoxy Resins: Isothermal Curing Behavior and Dynamic Mechanical Property. J. Therm. Anal. Calorim. 2013, 114, 365–375. [Google Scholar] [CrossRef]
- Djourelov, N.; Suzuki, T.; Shantarovich, V.P.; Dobreva, T.; Ito, Y. Transitions and Relaxations in Gamma-Irradiated Polypropylene Studied by Positron Annihilation Lifetime Spectroscopy. Radiat. Phys. Chem. 2005, 72, 13–18. [Google Scholar] [CrossRef]
- Takahama, T.; Geil, P.H. The β Relaxation Behavior of Bisphenol-Type Resins. J. Polym. Sci. Polym. Phys. Ed. 1982, 20, 1979–1986. [Google Scholar] [CrossRef]
- Chang, T.D.; Carr, S.H.; Brittain, J.O. Studies of Epoxy Resin Systems: Part A: A Study of the Origins of the Secondary Relaxations of Epoxy Resins by Thermally Stimulated Depolarization. Polym. Eng. Sci. 1982, 22, 1205–1212. [Google Scholar] [CrossRef]
- Pogany, G.A. The β-Relaxation in Epoxy Resins; the Temperature and Time-Dependence of Cure. J. Mater. Sci. 1969, 4, 405–409. [Google Scholar] [CrossRef]
- Ballesteros, D.; Walters, C. Detailed Characterization of Mechanical Properties and Molecular Mobility within Dry Seed Glasses: Relevance to the Physiology of Dry Biological Systems. Plant J. 2011, 68, 607–619. [Google Scholar] [CrossRef] [PubMed]
- Puértolas, J.A.; Martínez-Morlanes, M.J.; Mariscal, M.D.; Medel, F.J. Thermal and Dynamic Mechanical Properties of Vitamin E Infused and Blended Ultra-High Molecular Weight Polyethylenes. J. Appl. Polym. Sci 2011, 120, 2282–2291. [Google Scholar] [CrossRef]
- Noshay, A.; Robeson, L.M. Sulfonated Polysulfone. J. Appl. Polym. Sci. 1976, 20, 1885–1903. [Google Scholar] [CrossRef]
- Mulliken, A.D.; Boyce, M.C. Mechanics of the Rate-Dependent Elastic-Plastic Deformation of Glassy Polymers from Low to High Strain Rates. Int. J. Solids Struct. 2006, 43, 1331–1356. [Google Scholar] [CrossRef]
- Jacobs, J.D.; Arlen, M.J.; Wang, D.H.; Ounaies, Z.; Berry, R.; Tan, L.S.; Garrett, P.H.; Vaia, R.A. Dielectric Characteristics of Polyimide CP2. Polymer 2010, 51, 3139–3146. [Google Scholar] [CrossRef]
- Bartenev, G.M.; Shut, N.I.; Baglyuk, S.V.; Rupyshev, V.G. Relaxational Transitions in Polystyrene and Their Classification. Polym. Sci. USSR 1988, 30, 2448–2456. [Google Scholar] [CrossRef]
- Slutsker, A.I.; Polikarpov, Y.I.; Vasilyeva, K.V. Determination of the Activation Energy for Complicated Relaxation Processes. Phys. Solid State 2002, 44, 1604–1610. [Google Scholar] [CrossRef]
- Shinyashiki, N.; Shinohara, M.; Iwata, Y.; Goto, T.; Oyama, M.; Suzuki, S.; Yamamoto, W.; Yagihara, S.; Inoue, T.; Oyaizu, S.; et al. The Glass Transition and Dielectric Secondary Relaxation of Fructose–Water Mixtures. J. Phys. Chem. B 2008, 112, 15470–15477. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.N.; Zhang, C.Z.; Yue, Y.Z.; Bian, X.F. A New Threshold of Uncovering the Nature of Glass Transition: The Slow β Relaxation in Glassy States. Chin. Sci. Bull. 2010, 55, 457–472. [Google Scholar] [CrossRef]
- Irzhak, V.I. Topological Structure and Relaxation Properties of Branched Polymers. Russ. Chem. Rev. 2006, 75, 919–934. [Google Scholar] [CrossRef]
- Bershtein, V.A.; Egorov, V.M. Differential Scanning Calorimetry in the Physicochemistry of Polymers; Khimiya: Leningrad, Russia, 1990. (In Russian) [Google Scholar]
- Kiselev, M.R.; Bardyshev, I.I. The Structural Organization of Liquid Low-Molecular-Weight Epoxy Oligomers. Russ. J. Phys. Chem. A 2007, 81, 210–215. [Google Scholar] [CrossRef]
- May, C.A. (Ed.) Epoxy Resins: Chemistry and Technology, 2nd ed.; Marcel Dekker: New York, NY, USA, 1988; ISBN 9780824776909. [Google Scholar]
- Jyotish, J.; Mahapatra, B.K.; Moharana, S.; Mahaling, R.N. Chemistry and Types of Epoxy Resins. In Materials Horizons: From Nature to Nanomaterials; Springer: Cham, Switzerland, 2025; pp. 9–35. [Google Scholar] [CrossRef]
- Paluvai, N.R.; Mohanty, S.; Nayak, S.K. Synthesis and Modifications of Epoxy Resins and Their Composites: A Review. Polym. Plast. Technol. Eng. 2014, 53, 1723–1758. [Google Scholar] [CrossRef]
- GOST 10587-84; Uncured Epoxy Dianol Resins. Technical Conditions. State Committee for Standards of the USSR: Moscow, Russia, 1984.
- Simonov-Emelianov, I.D.; Zaytseva, Z.A.; Nesolenaya, T.A.; Veselova, S.P.; Khek, S.L. Rheokinetics Curing of Epoxy Oligomers Produced by Industry with Amine Hardener. Fine Chem. Technol. 2010, 5, 102–107. [Google Scholar]
- Kochnova, Z.A.; Zhavoronok, E.S.; Chalykh, A.E. Epoxy Resins and Hardeners: Industrial Products; LLC “Paint-Media”: Moscow, Russia, 2006. (In Russian) [Google Scholar]
- Khozin, V.G. Reinforcement of Epoxy Polymers; PIK “Dom pechati”: Kazan, Russia, 2004. [Google Scholar]
- Surikov, P.V.; Trofimov, A.I.; Kohan, E.I.; Sheulova, L.K.; Simonov-Emelianov, I.D. Influence of MM and MMD on Rheological Properties of Epoxy Resins. Fine Chem. Technol. 2009, 4, 87–90. [Google Scholar]
- Irzhak, V.I.; Mezhikovsky, S.M. Chemical Physics of Oligomer Curing: Monograph, 2nd ed.; Chalykh, A.E., Ed.; Yurait Publishing House: Moscow, Russia, 2019. (In Russian) [Google Scholar]
- Surikov, P.V.; Trofimov, A.I.; Kohan, E.I.; Simonov-Emelianov, I.D.; Sheulova, L.K.; Kandyrin, L.B. The Molecular Characteristics Influence on Rheological Properties of Epoxides and Their Blends. Plast. Massy 2009, 9, 3–7. [Google Scholar]
- Zaytsev, Y.S.; Kocherin, Y.S.; Pakter, M.K.; Kucher, R.V. Epoxy Oligomers and Adhesive Compositions; Grekov, A.P., Ed.; Naukova Dumka: Kiev, Ukraine, 1990; ISBN 5120014313. (In Russian) [Google Scholar]
- Babaevsky, P.G. Curing Oligomer-Oligomer and Oligomer-Polymer Compositions. Plast. Massy 1984, 4, 20–23. (In Russian) [Google Scholar]
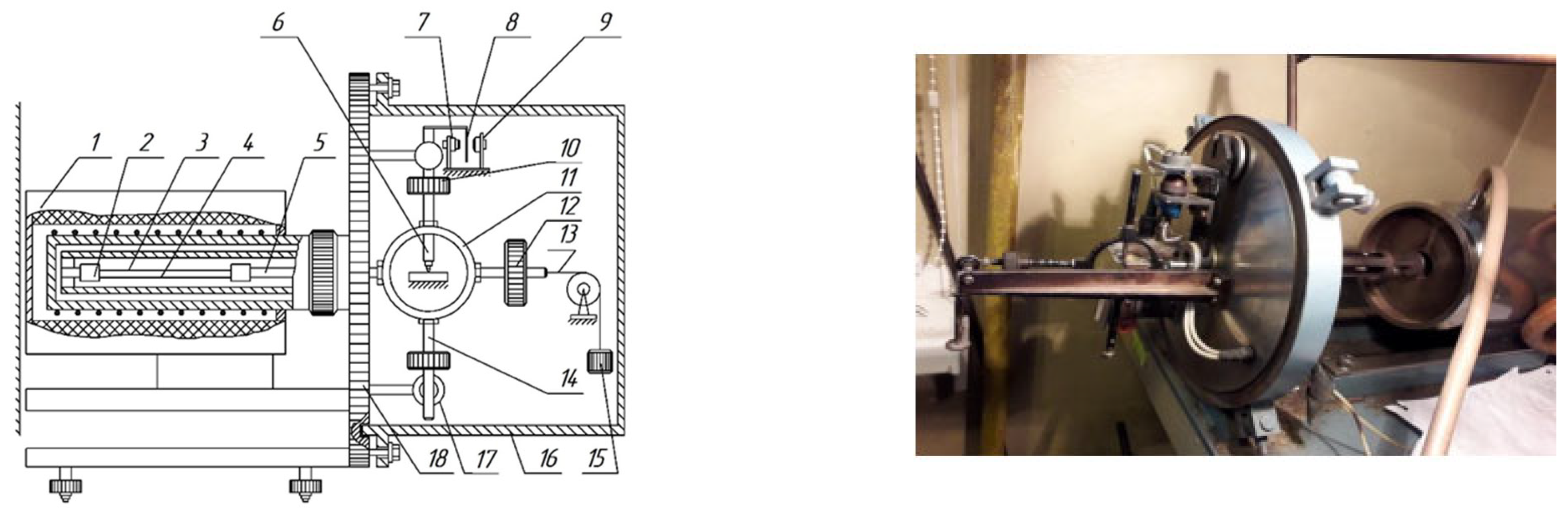
- Lomovskoy, V.A. The Device for Researching of Local Dissipative Processes in Solid Materials of Various Chemical Origin, Composition and Structure. Sci. Instrum. 2019, 29, 33–46. [Google Scholar] [CrossRef]
- Terent’eva, E.P.; Udoenko, N.K.; Pavlova, E.A. Chemistry of Wood, Cellulose and Synthetic Polymers: Textbook. Part 1; SPbSTURP: Saint Petersburg, Russia, 2014. [Google Scholar]
- Aslamazova, T.R.; Lomovskoy, V.A.; Shorshina, A.S.; Zolotarevskii, V.I.; Kotenev, V.A.; Lomovskaya, N.Y. Temperature–Frequency Domains of Inelasticity in the Rosin–Copper and Rosin–Cellulose Composites. Russ. J. Phys. Chem. A 2022, 96, 222–229. [Google Scholar] [CrossRef]
- Aslamazova, T.R.; Kotenev, V.A.; Lomovskaya, N.Y.; Lomovskoy, V.A.; Tsivadze, A.Y. Dissipative Processes in an Acrylic Polymer on Metal Substrates. Russ. J. Phys. Chem. A 2022, 96, 1062–1069. [Google Scholar] [CrossRef]
- Askadsky, A.A.; Bartenev, G.M.; Belyakov, A.L.; Pastukhov, A.V. Various Forms of Molecular Mobility and Relaxation Transitions in Epoxydian Polymer. Polym. Sci. USSR 1988, 30, 868–873. (In Russian) [Google Scholar]
- Lomovskoy, V.A.; Chugunov, Y.V.; Shatokhina, S.A. Methodology for the Study of Internal Friction in the Mode of Free Damped Oscillatory Process. Part 3. Internal Friction Mechanisms. Sci. Instrum. 2024, 34, 17–29. [Google Scholar]
- Lomovskoy, V.A.; Mazurina, S.A.; Simonov-Emel’yanov, I.D.; Kiselev, M.R.; Konstantinov, N.Y. Relaxation Spectroscopy of Polyethylenes of Different Molecular Weight. Inorg. Mater. Appl. Res. 2019, 10, 174–183. [Google Scholar] [CrossRef]
- Lomovskoy, V.A.; Nekrasova, N.V.; Abaturova, N.A.; Lomovskaya, N.Y. Physical and Chemical Characteristics of Local Dissipative Losses in Polymethylmethacrylate. Rev. J. Chem. 2017, 7, 64–78. [Google Scholar] [CrossRef]
- Khozin, V.G. Oligomeric Prehistory of the Structure Formation of Epoxy Polymers. Butlerov Commun. 2006, 8, 36–49. [Google Scholar]
- ASTM D1763-00(2013); Standard Specification for Epoxy Resins. ASTM International: West Conshohocken, PA, USA, 2013.
- Kasterina, T.N.; Kalinina, L.S. Chemical Methods for the Investigation of Synthetic Resins and Plastics; State Scientific-Technical Publishing House of Chemical Literature: Moscow, Russia, 1963. [Google Scholar]
- Prokopova, L.A.; Golovina, E.Y. About the Technological Properties Stability of Type I Epoxy Resins after the Warranty Period Expiration. Proc. VIAM 2019, 1, 13–20. [Google Scholar] [CrossRef]









| Characteristic | DER-330 | ED-20 | ED-16 | ED-8 | |||
|---|---|---|---|---|---|---|---|
| Grade | – | Extra Class | Class I | Extra Class | Class I | Extra Class | Class I |
| Appearance | Viscous transparent liquid | Viscous transparent liquid | Highly viscous transparent liquid | Solid transparent oligomer | |||
| Color (Co–Fe scale, max) | – | 3 | 8 | 3(4) | 8 | 2 | 6 |
| Density at 25 °C, g/cm3 | 1.13 | 1.166 | 1.15 | 1.160 | 1.160 | – | – |
| Dynamic viscosity at 25 °C, Pa·s | 7–10 | 13–20 | 12–25 | 5–18 (50 °C) | 5–20 (50 °C) | – | – |
| Dynamic viscosity at 50 °C, Pa·s | – | 5 | 18 | 5 | 20 | – | – |
| Weight-average molecular weight | 364 | 403 | 635 | 1203 | |||
| Number of fractions | 2 | 3 | 8 | 13 | |||
| Epoxy equivalent, g/mol | 176–185 | 195–216 | 195–216 | 239–269 | 239–269 | 430–506 | 430–537 |
| Mass fraction of epoxy groups, % | 23.2–24.4 | 20–22.5 | 20–22.5 | 16–18 | 16–18 | 8.5–10 | 8–10 |
| Chlorine ion content, %, ≤ | 0.005 | 0.001 | 0.005 | 0.002 | 0.004 | 0.001 | 0.003 |
| Washable chlorine, %, ≤ | 0.5 | 0.3 | 0.8 | 0.3 | 0.5 | 0.2 | 0.3 |
| Hydroxyl group content, %, ≤ | – | 17 | – | 25 | – | – | – |
| Volatile content, %, ≤ | 0.7 | 0.2 | 0.8 | 0.2 | 0.4 | 0.2 | 0.3 |
| Gelation onset at 30 °C, min | 57 | 24 | 15 [119] | – | |||
| Activation energy E, kJ/mol | 91 | 102 | 144 | 135 [120] | |||
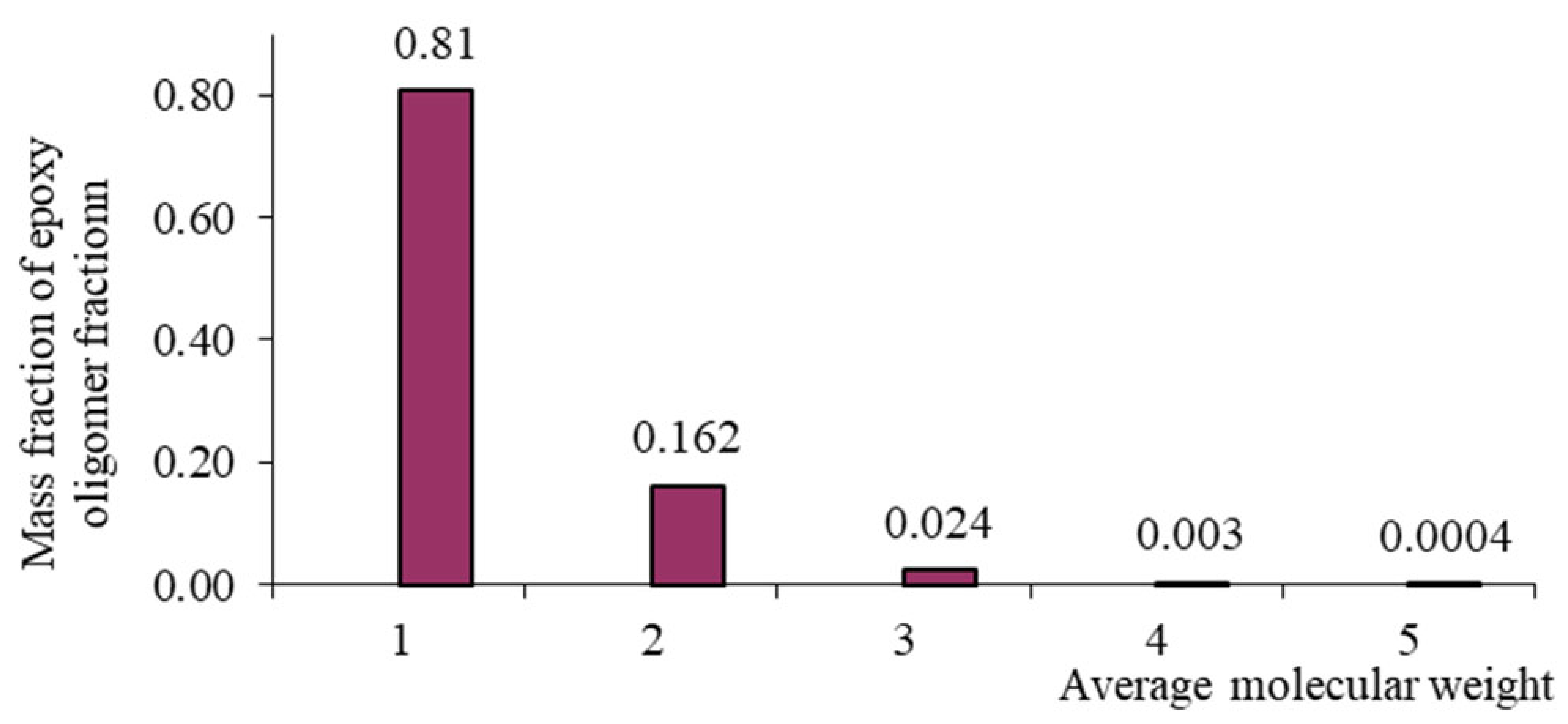
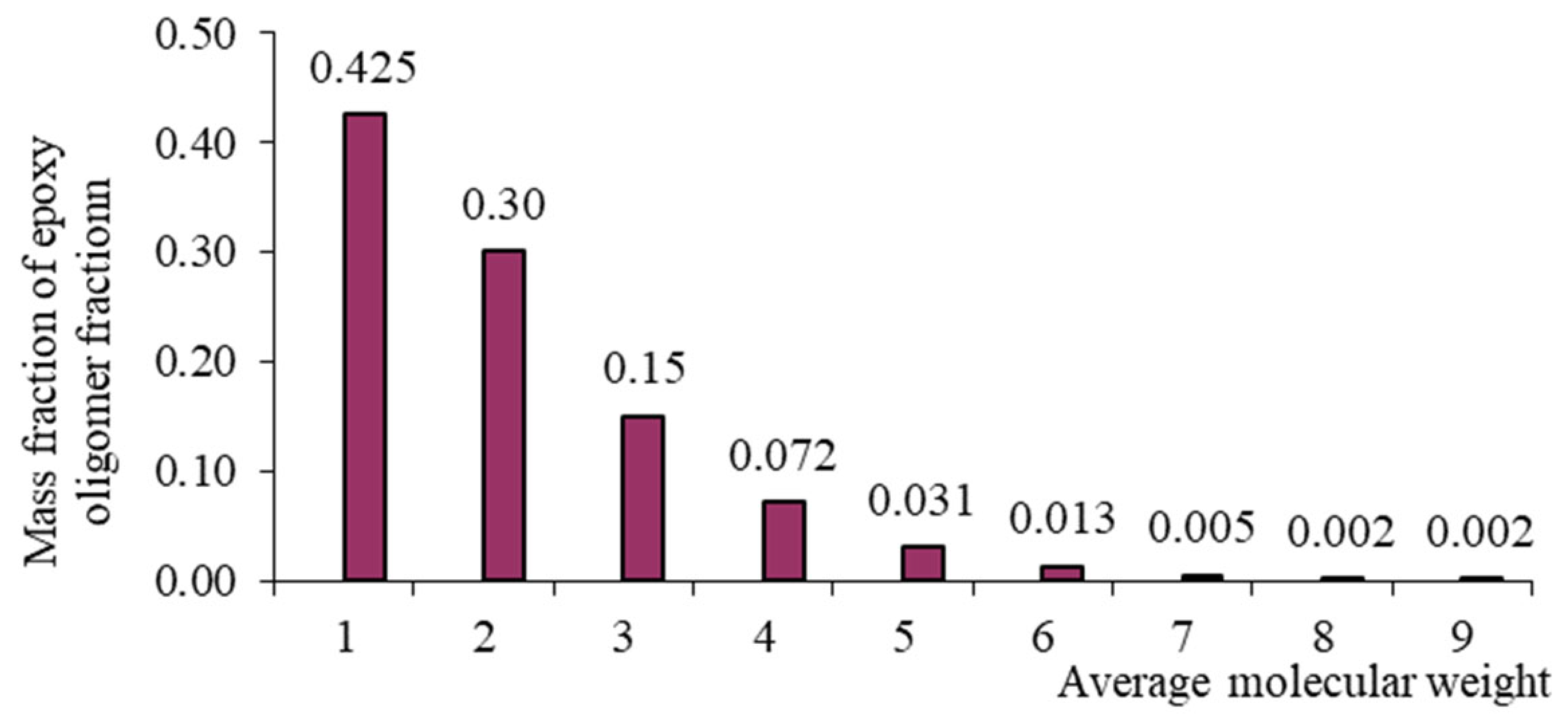
| n | Mass Fraction of Epoxy Oligomer Fraction | ||||
|---|---|---|---|---|---|
| DER-330 | ED-20 | ED-16 | ED-8 | ||
| 0 | 340 | 0.92 | 0.81 | 0.425 | 0.14 |
| 1 | 624 | 0.076 | 0.162 | 0.30 | 0.176 |
| 2 | 908 | 0.004 | 0.0243 | 0.15 | 0.165 |
| 3 | 1192 | - | 0.0032 | 0.072 | 0.14 |
| 4 | 1476 | - | 0.0004 | 0.031 | 0.11 |
| 5 | 1760 | - | - | 0.013 | 0.08 |
| 6 | 2044 | - | - | 0.005 | 0.06 |
| 7 | 2328 | - | - | 0.002 | 0.04 |
| ≥8 | - | - | - | 0.002 | 0.089 |
| Average value | 364 | 403 | 635 | 1203 | |
| Grade | Tmax, (K) | Tmax, (°C) | U, kJ/mol | |||
|---|---|---|---|---|---|---|
| process | ||||||
| DER-330 | 160 | −113 | 0.052 | 2.62 | 35.5 | 0.061 |
| ED-20 | 157 | −116 | 0.053 | 2.24 | 35.0 | 0.071 |
| ED-16 | 158 | −115 | 0.056 | 2.26 | 35.2 | 0.070 |
| ED-8 | 167 | −106 | 0.056 | 1.90 | 37.6 | 0.084 |
| process | ||||||
| DER-330 | 192 | −81 | 0.069 | 2.46 | 42.6 | 0.065 |
| ED-20 | 192 | −81 | 0.075 | 2.12 | 42.8 | 0.075 |
| ED-16 | 193 | −80 | 0.083 | 2.14 | 43.1 | 0.074 |
| ED-8 | 220 | −53 | 0.080 | 1.78 | 49.4 | 0.090 |
| 267 | −6 | 0.088 | 1.66 | 60.3 | 0.096 | |
| process | ||||||
| DER-330 | 260 | −13 | 0.966 | 2.31 | 50.5 | 0.069 |
| ED-20 | 263 | −10 | 0.462 | 1.94 | 51.4 | 0.082 |
| ED-16 | 272 | −1 | 0.510 | 2.05 | 53.1 | 0.078 |
| ED-8 | 307 | 34 | 0.129 | 1.62 | 60.5 | 0.098 |
| Grade | Frequency Range, Hz | ||
|---|---|---|---|
| 1-process | |||
| DER-330 | 2.763 | 3.165 | −0.312 |
| ED-20 | 2.074 | 2.253 | −0.180 |
| ED-16 | 2.172 | 2.358 | −0.178 |
| ED-8 | 1.613 | 1.676 | −0.079 |
| 2–4-process | |||
| DER-330 | 3.165 | 1.602 | 0.744 |
| ED-20 | 2.253 | 1.749 | 0.397 |
| ED-16 | 2.358 | 1.774 | 0.434 |
| ED-8 | 1.676 | 1.556 | 0.138 |
| Grade | T1, °C | T2, °C | Δ T, °C | τ1, Hz | τ2, Hz | Δτ, Hz |
|---|---|---|---|---|---|---|
| DER-330 | −16 | −10 | 6 | 0.093 | 0.053 | 0.040 |
| ED-20 | −14 | −8 | 7 | 0.121 | 0.066 | 0.055 |
| ED-16 | −4 | 3 | 7 | 0.100 | 0.056 | 0.044 |
| ED-8 | 30 | 40 | 10 | 0.139 | 0.064 | 0.075 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lomovskoy, V.A.; Trofimov, D.A.; Shatokhina, S.A.; Lomovskaya, N.Y.; Simonov-Emelyanov, I.D. Local Relaxation Phenomena in Epoxy Resins in the Temperature Range from −150 °C to +150 °C. Polymers 2025, 17, 3318. https://doi.org/10.3390/polym17243318
Lomovskoy VA, Trofimov DA, Shatokhina SA, Lomovskaya NY, Simonov-Emelyanov ID. Local Relaxation Phenomena in Epoxy Resins in the Temperature Range from −150 °C to +150 °C. Polymers. 2025; 17(24):3318. https://doi.org/10.3390/polym17243318
Chicago/Turabian StyleLomovskoy, Viktor A., Dmitry A. Trofimov, Svetlana A. Shatokhina, Nadezhda Yu. Lomovskaya, and Igor D. Simonov-Emelyanov. 2025. "Local Relaxation Phenomena in Epoxy Resins in the Temperature Range from −150 °C to +150 °C" Polymers 17, no. 24: 3318. https://doi.org/10.3390/polym17243318
APA StyleLomovskoy, V. A., Trofimov, D. A., Shatokhina, S. A., Lomovskaya, N. Y., & Simonov-Emelyanov, I. D. (2025). Local Relaxation Phenomena in Epoxy Resins in the Temperature Range from −150 °C to +150 °C. Polymers, 17(24), 3318. https://doi.org/10.3390/polym17243318







