Marine Microbial Exopolysaccharides (EPSs): Untapped Bio-Reserves
Abstract
1. Introduction
Literature Search Strategy and Selection Criteria
- (i)
- Studies that described the isolation, biochemical characterization, regulatory pathways, or functional applications of EPS from marine microorganisms;
- (ii)
- Microorganisms were to be marine bacteria, cyanobacteria, microalgae, and marine fungi only;
- (iii)
- Full-length peer-reviewed publications;
- (iv)
- Articles in English.
- (i)
- Studies about the EPS from terrestrial microbes;
- (ii)
- Patents, conference abstracts, or papers without experimental details;
- (iii)
- Studies on polysaccharides that are purely synthetic or chemically synthesized.
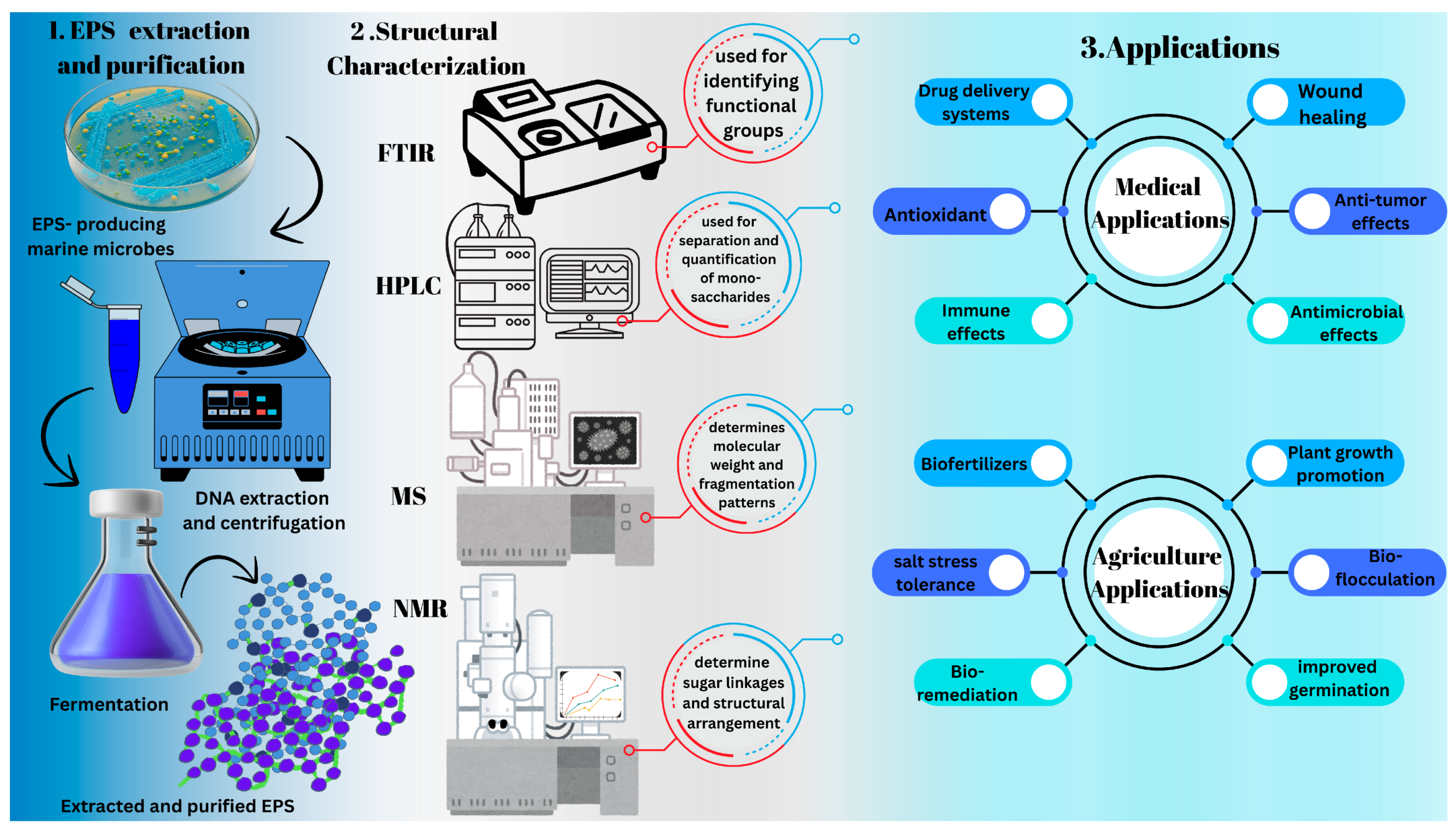
2. Biochemical Properties of Marine Exopolysaccharides
3. Marine Microbiome
4. Molecular Mechanisms of EPS Regulation in Marine Microorganisms
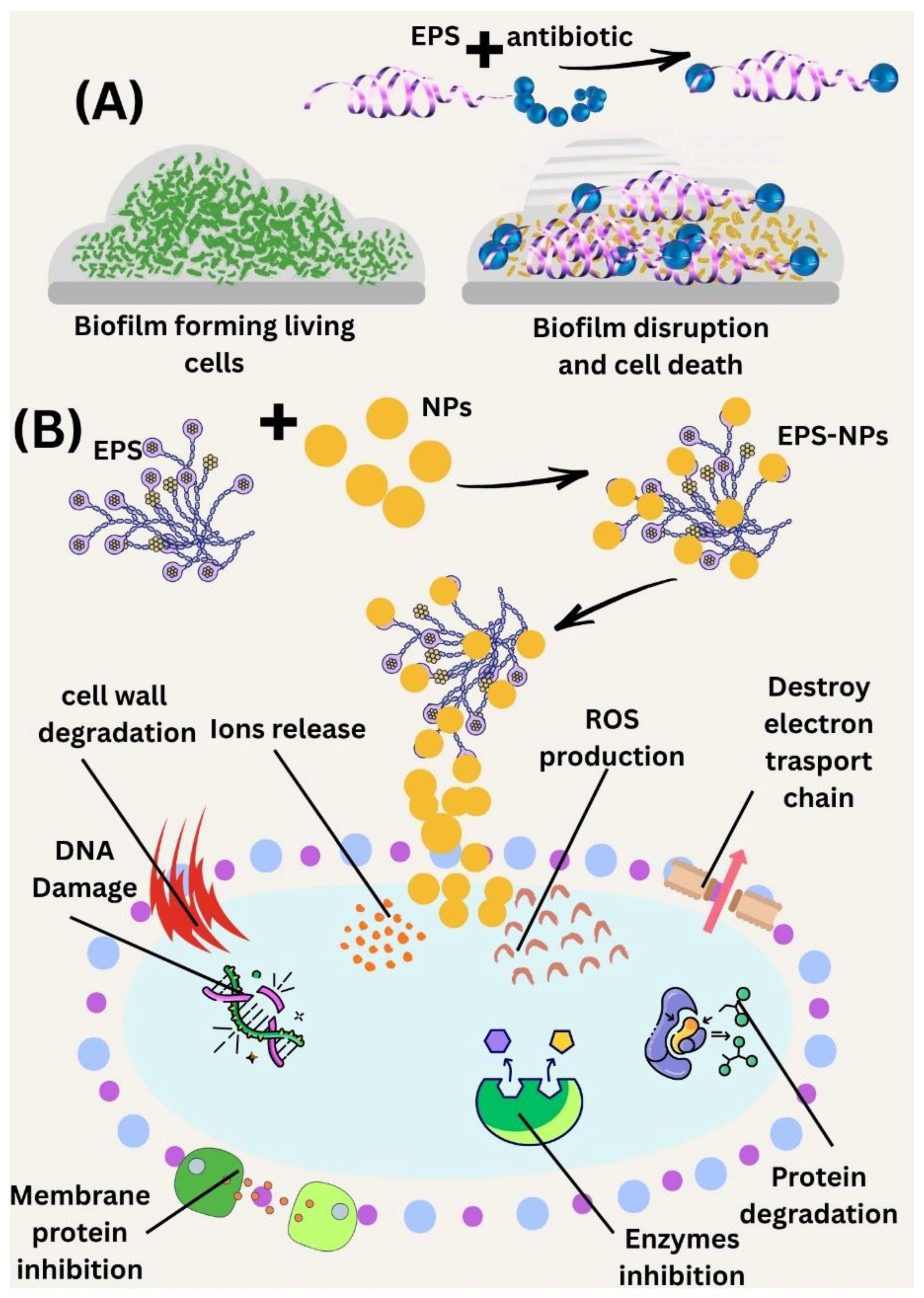
5. Mechanisms of Antibacterial and Antibiofilm Activity of Marine EPS
6. Marine EPS: Synergy in Antimicrobial and Antibiofilm Activities
7. Novel Insights: Exploring Under-Researched Aspects
8. AI-Driven Insights into Marine Exopolysaccharides
9. Potential Application of Marine EPS in Biomedicine
9.1. Wound Healing Potential as Coatings
9.2. Marine EPS as Drug Delivery Systems
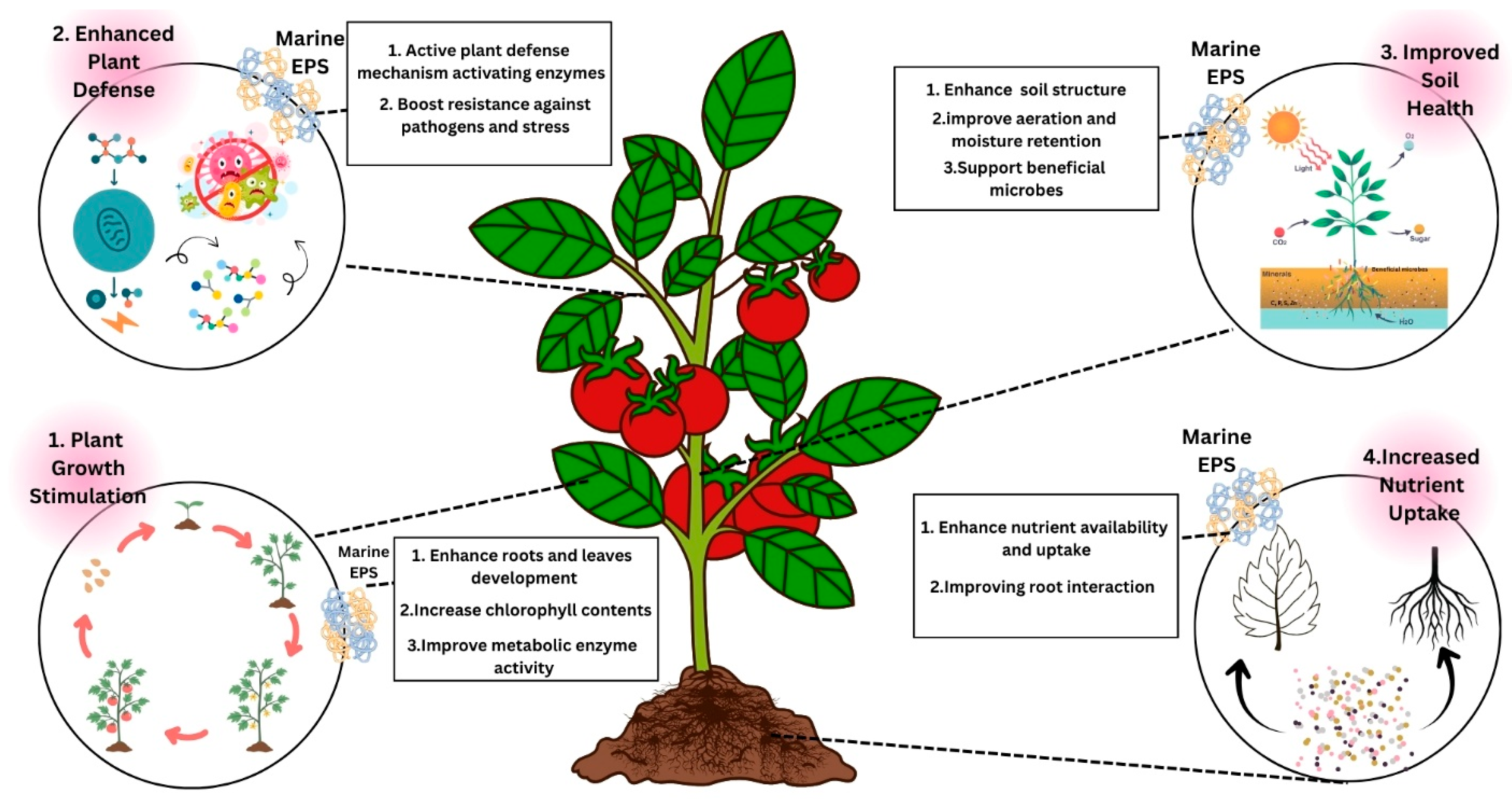
9.3. Marine EPS Potential in Agriculture
10. Impact of Climate Change on Marine EPS-Producing Microbiota
11. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| EPSs | Exopolysaccharides |
| AMR | Antimicrobial resistance |
| NPs | Nanoparticles |
| SEC | Size Exclusion Chromatography |
| MIC | Minimum inhibitory concentration |
References
- Casillo, A.; Lanzetta, R.; Parrilli, M.; Corsaro, M.M. Exopolysaccharides from Marine and Marine Extremophilic Bacteria: Structures, Properties, Ecological Roles and Applications. Mar. Drugs 2018, 16, 69. [Google Scholar] [CrossRef]
- Baria, D.M.; Patel, N.Y.; Yagnik, S.M.; Panchal, R.R.; Rajput, K.N.; Raval, V.H. Exopolysaccharides from marine microbes with prowess for environment cleanup. Environ. Sci. Pollut. Res. 2022, 29, 76611–76625. [Google Scholar] [CrossRef]
- Laroche, C. Exopolysaccharides from Microalgae and Cyanobacteria: Diversity of Strains, Production Strategies, and Applications. Mar. Drugs 2022, 20, 336. [Google Scholar] [CrossRef]
- Al-Nabulsi, A.A.; Jaradat, Z.W.; Al Qudsi, F.R.; Elsalem, L.; Osaili, T.M.; Olaimat, A.N.; Esposito, G.; Liu, S.-Q.; Ayyash, M.M. Characterization and bioactive properties of exopolysaccharides produced by Streptococcus thermophilus and Lactobacillus bulgaricus isolated from labaneh. LWT 2022, 167, 113817. [Google Scholar] [CrossRef]
- Tan, X.; Ma, B.; Wang, X.; Cui, F.; Li, X.; Li, J. Characterization of Exopolysaccharides from Lactiplantibacillus plantarum PC715 and Their Antibiofilm Activity Against Hafnia alvei. Microorganisms 2024, 12, 2229. [Google Scholar] [CrossRef] [PubMed]
- Sayahi, N.; Othmani, B.; Mnif, W.; Algarni, Z.; Khadhraoui, M.; Rebah, F.B. Microbial Extracellular Polymeric Substances as Corrosion Inhibitors: A Review. Surfaces 2025, 8, 49. [Google Scholar] [CrossRef]
- Salimi, F.; Farrokh, P. Recent advances in the biological activities of microbial exopolysaccharides. World J. Microbiol. Biotechnol. 2023, 39, 213. [Google Scholar] [CrossRef]
- Dave, S.R.; Upadhyay, K.H.; Vaishnav, A.M.; Tipre, D.R. Exopolysaccharides from marine bacteria: Production, recovery and applications. Environ. Sustain. 2020, 3, 139–154. [Google Scholar] [CrossRef]
- Champion, M.; Portier, E.; Vallée-Réhel, K.; Linossier, I.; Balnois, E.; Vignaud, G.; Moppert, X.; Hellio, C.; Faÿ, F. Anti-biofilm activity of a hyaluronan-like exopolysaccharide from the marine Vibrio MO245 against pathogenic bacteria. Mar. Drugs 2022, 20, 728. [Google Scholar] [CrossRef]
- Cerezo, I.M.; Pérez-Gómez, O.; Rohra-Benítez, S.; Domínguez-Maqueda, M.; García-Márquez, J.; Arijo, S. Postbiotics of Marine Origin and Their Therapeutic Application. Mar. Drugs 2025, 23, 335. [Google Scholar] [CrossRef]
- Amer, M.S.; Barakat, K.M.; Ibrahim, H.A.; Matsuo, K.; Ibrahim, M.I. An overview on marine bacterial exopolysaccharides and their industrial applications. J. Carbohydr. Chem. 2025, 44, 95–132. [Google Scholar] [CrossRef]
- Moo, C.-L.; Yang, S.-K.; Yusoff, K.; Ajat, M.; Thomas, W.; Abushelaibi, A.; Lim, S.-H.-E.; Lai, K.-S. Mechanisms of antimicrobial resistance (AMR) and alternative approaches to overcome AMR. Curr. Drug Discov. Technol. 2020, 17, 430–447. [Google Scholar] [CrossRef] [PubMed]
- Bhat, R.; Altinok, I. Antimicrobial resistance (AMR) and alternative strategies for combating AMR in aquaculture. Turk. J. Fish. Aquat. Sci. 2023, 23, 11. [Google Scholar] [CrossRef]
- Zhang, F.; Cheng, W. The Mechanism of Bacterial Resistance and Potential Bacteriostatic Strategies. Antibiotics 2022, 11, 1215. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, R.; Duan, J.; Shi, X.; Zhang, Y.; Guan, F.; Sand, W.; Hou, B. Extracellular Polymeric Substances and Biocorrosion/Biofouling: Recent Advances and Future Perspectives. Int. J. Mol. Sci. 2022, 23, 5566. [Google Scholar] [CrossRef]
- Yildiz, H.; Karatas, N. Microbial exopolysaccharides: Resources and bioactive properties. Process Biochem. 2018, 72, 41–46. [Google Scholar] [CrossRef]
- Wang, W.; Ju, Y.; Liu, N.; Shi, S.; Hao, L. Structural characteristics of microbial exopolysaccharides in association with their biological activities: A review. Chem. Biol. Technol. Agric. 2023, 10, 137. [Google Scholar] [CrossRef]
- Tho, N.; Son, L.; Tho, N.; Cuong, B.; Toan, H.; Khanh, H.; Thanh, N. Enhancing the production and monosaccharide composition of exopolysaccharides of Lactobacillus plantarum VAL6 by applying thermal stress and increased carbon dioxide concentration. Microbiology 2021, 90, 527–537. [Google Scholar] [CrossRef]
- Halfawy, N.M.E.; Zaghloul, E.H. Functional and genomic evaluation of novel exopolysaccharide produced by marine Pediococcus pentosaceus E3 with antidiabetic, anticancer, and anti-inflammatory potentials. BMC Microbiol. 2025, 25, 628. [Google Scholar] [CrossRef]
- Liu, S.; Diao, B.; Shen, X.; Wei, X.; Wang, Y.; Jiang, S.; Wang, A.; Bozorovich, H.I.; Blanchard, C.; Zhou, Z. Glucomannan polysaccharides derived from Pediococcus pentosaceus: From molecular characteristics to inhibition on colonic cancer cells proliferation. Carbohydr. Polym. 2025, 368, 124113. [Google Scholar] [CrossRef]
- Jiang, G.; He, J.; Gan, L.; Li, X.; Xu, Z.; Yang, L.; Li, R.; Tian, Y. Exopolysaccharide produced by Pediococcus pentosaceus E8: Structure, bio-activities, and its potential application. Front. Microbiol. 2022, 13, 923522. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Li, X.; Tian, R.; Tang, R.; Zhang, J. Characterization and Biological Activity of a Novel Exopolysaccharide Produced by Pediococcus pentosaceus SSC–12 from Silage. Microorganisms 2022, 10, 18. [Google Scholar] [CrossRef] [PubMed]
- Marimuthu, S.; Rajendran, K. Structural and functional characterization of exopolysaccharide produced by a novel isolate Bacillus sp. EPS003. Appl. Biochem. Biotechnol. 2023, 195, 4583–4601. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.I.A.; Ibrahim, H.A.H.; Haga, T.; Ishida, A.; Nehira, T.; Matsuo, K.; Gad, A.M. Potential Bioactivities, Chemical Composition, and Conformation Studies of Exopolysaccharide-Derived Aspergillus sp. Strain GAD7. J. Fungi 2024, 10, 659. [Google Scholar] [CrossRef]
- El Awady, M.E.; Eldin, M.A.N.; Ibrahim, H.M.; Al Bahnasy, M.E.; Aziz, S.H.A. In Vitro evaluation of antioxidant, anticancer, and antiviral activities of exopolysaccharide from Streptomyces hirsutus NRC2018. J. Appl. Pharm. Sci. 2019, 9, 010–018. [Google Scholar] [CrossRef]
- Spanò, A.; Zammuto, V.; Macrì, A.; Agostino, E.; Nicolò, M.S.; Scala, A.; Trombetta, D.; Smeriglio, A.; Ingegneri, M.; Caccamo, M.T.; et al. Arsenic Adsorption and Toxicity Reduction of An Exopolysaccharide Produced by Bacillus licheniformis B3-15 of Shallow Hydrothermal Vent Origin. J. Mar. Sci. Eng. 2023, 11, 325. [Google Scholar] [CrossRef]
- Wei, M.; Geng, L.; Wang, Q.; Yue, Y.; Wang, J.; Wu, N.; Wang, X.; Sun, C.; Zhang, Q. Purification, characterization and immunostimulatory activity of a novel exopolysaccharide from Bacillus sp. H5. Int. J. Biol. Macromol. 2021, 189, 649–656. [Google Scholar] [CrossRef]
- Gonzalez-Garcia, Y.; Heredia, A.; Meza-Contreras, J.C.; Escalante, F.M.; Camacho-Ruiz, R.M.; Cordova, J. Biosynthesis of extracellular polymeric substances by the marine bacterium Saccharophagus degradans under different nutritional conditions. Int. J. Polym. Sci. 2015, 2015, 526819. [Google Scholar] [CrossRef]
- Ramamoorthy, S.; Gnanakan, A.; Lakshmana, S.S.; Meivelu, M.; Jeganathan, A. Structural characterization and anticancer activity of extracellular polysaccharides from ascidian symbiotic bacterium Bacillus thuringiensis. Carbohydr. Polym. 2018, 190, 113–120. [Google Scholar] [CrossRef]
- Shyam, K.P.; Rajkumar, P.; Ramya, V.; Sivabalan, S.; Kings, A.J.; Miriam, L.M. Exopolysaccharide production by optimized medium using novel marine Enterobacter cloacae MBB8 isolate and its antioxidant potential. Carbohydr. Polym. Technol. Appl. 2021, 2, 100070. [Google Scholar] [CrossRef]
- Selim, M.S.; Amer, S.K.; Mohamed, S.S.; Mounier, M.M.; Rifaat, H.M. Production and characterisation of exopolysaccharide from Streptomyces carpaticus isolated from marine sediments in Egypt and its effect on breast and colon cell lines. J. Genet. Eng. Biotechnol. 2018, 16, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Roychowdhury, R.; Srivastava, N.; Kumari, S.; Pinnaka, A.K.; Choudhury, A.R. Isolation of an exopolysaccharide from a novel marine bacterium Neorhizobium urealyticum sp. nov. and its utilization in nanoemulsion formation for encapsulation and stabilization of astaxanthin. LWT 2021, 151, 112105. [Google Scholar] [CrossRef]
- Caccamo, M.T.; Gugliandolo, C.; Zammuto, V.; Magazù, S. Thermal properties of an exopolysaccharide produced by a marine thermotolerant Bacillus licheniformis by ATR-FTIR spectroscopy. Int. J. Biol. Macromol. 2020, 145, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Paidi, M.K.; Dhinakarasamy, I.; Sivakumar, M.; Clements, C.; Thirumurugan, N.K.; Sivakumar, L. Adaptive mechanism of the marine bacterium Pseudomonas sihuiensis-BFB-6S towards pCO2 variation: Insights into synthesis of extracellular polymeric substances and physiochemical modulation. Int. J. Biol. Macromol. 2024, 261, 129860. [Google Scholar] [CrossRef]
- Sran, K.S.; Sundharam, S.S.; Krishnamurthi, S.; Choudhury, A.R. Production, characterization and bio-emulsifying activity of a novel thermostable exopolysaccharide produced by a marine strain of Rhodobacter johrii CDR-SL 7Cii. Int. J. Biol. Macromol. 2019, 127, 240–249. [Google Scholar] [CrossRef]
- Feng, J.; Mazzei, M.; Di Gregorio, S.; Niccolini, L.; Vitiello, V.; Ye, Y.; Guo, B.; Yan, X.; Buttino, I. Marine copepods as a microbiome hotspot: Revealing their interactions and biotechnological applications. Water 2023, 15, 4203. [Google Scholar] [CrossRef]
- Reineke, W.; Schlömann, M. Microorganisms at different sites: Living conditions and adaptation strategies. In Environmental Microbiology; Springer: Berlin/Heidelberg, Germany, 2023; pp. 349–396. [Google Scholar]
- Barzkar, N.; Sukhikh, S.; Babich, O. Study of marine microorganism metabolites: New resources for bioactive natural products. Front. Microbiol. 2024, 14, 1285902. [Google Scholar] [CrossRef]
- Maldonado-Ruiz, K.; Pedroza-Islas, R.; Pedraza-Segura, L. Blue Biotechnology: Marine Bacteria Bioproducts. Microorganisms 2024, 12, 697. [Google Scholar] [CrossRef]
- García, A.; Fernández-Sandoval, M.T.; Morales-Guzmán, D.; Martínez-Morales, F.; Trejo-Hernández, M.R. Advances in exopolysaccharide production from marine bacteria. J. Chem. Technol. Biotechnol. 2022, 97, 2694–2705. [Google Scholar] [CrossRef]
- Upadhyaya, C.; Patel, H.; Patel, I.; Upadhyaya, T. Extremophilic Exopolysaccharides: Bioprocess and Novel Applications in 21st Century. Fermentation 2025, 11, 16. [Google Scholar] [CrossRef]
- Ghareeb, A.; Fouda, A.; Kishk, R.M.; El Kazzaz, W.M. Unlocking the therapeutic potential of bioactive exopolysaccharide produced by marine actinobacterium Streptomyces vinaceusdrappus AMG31: A novel approach to drug development. Int. J. Biol. Macromol. 2024, 276, 133861. [Google Scholar] [CrossRef] [PubMed]
- Manivasagan, P.; Sivasankar, P.; Venkatesan, J.; Senthilkumar, K.; Sivakumar, K.; Kim, S.-K. Production and characterization of an extracellular polysaccharide from Streptomyces violaceus MM72. Int. J. Biol. Macromol. 2013, 59, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Wahab, B.A.; F. Abd El-Kareem, H.; Alzamami, A.; A. Fahmy, C.; H. Elesawy, B.; Mostafa Mahmoud, M.; Ghareeb, A.; El Askary, A.; H. Abo Nahas, H.; G. M. Attallah, N.; et al. Novel Exopolysaccharide from Marine Bacillus subtilis with Broad Potential Biological Activities: Insights into Antioxidant, Anti-Inflammatory, Cytotoxicity, and Anti-Alzheimer Activity. Metabolites 2022, 12, 715. [Google Scholar] [CrossRef] [PubMed]
- Selim, S.; Almuhayawi, M.S.; Alharbi, M.T.; Nagshabandi, M.K.; Alanazi, A.; Warrad, M.; Hagagy, N.; Ghareeb, A.; Ali, A.S. In Vitro Assessment of Antistaphylococci, Antitumor, Immunological and Structural Characterization of Acidic Bioactive Exopolysaccharides from Marine Bacillus cereus Isolated from Saudi Arabia. Metabolites 2022, 12, 132. [Google Scholar] [CrossRef]
- Alharbi, M.A.; Alrehaili, A.A.; Albureikan, M.O.I.; Gharib, A.F.; Daghistani, H.; Bakhuraysah, M.M.; Aloraini, G.S.; Bazuhair, M.A.; Alhuthali, H.M.; Ghareeb, A. In Vitro studies on the pharmacological potential, anti-tumor, antimicrobial, and acetylcholinesterase inhibitory activity of marine-derived Bacillus velezensis AG6 exopolysaccharide. RSC Adv. 2023, 13, 26406–26417. [Google Scholar] [CrossRef]
- Aloraini, G.S.; Albureikan, M.O.I.; Shahlol, A.M.; Shamrani, T.; Daghistani, H.; El-Nablaway, M.; Tharwat, N.A.; Elazzazy, A.M.; Basyony, A.F.; Ghareeb, A. Biomedical and therapeutic potential of marine-derived Pseudomonas sp. strain AHG22 exopolysaccharide: A novel bioactive microbial metabolite. Rev. Adv. Mater. Sci. 2024, 63, 20240016. [Google Scholar] [CrossRef]
- Alshawwa, S.Z.; Alshallash, K.S.; Ghareeb, A.; Elazzazy, A.M.; Sharaf, M.; Alharthi, A.; Abdelgawad, F.E.; El-Hossary, D.; Jaremko, M.; Emwas, A.-H.; et al. Assessment of Pharmacological Potential of Novel Exopolysaccharide Isolated from Marine Kocuria sp. Strain AG5: Broad-Spectrum Biological Investigations. Life 2022, 12, 1387. [Google Scholar] [CrossRef]
- Hassan, S.W.; Ibrahim, H.A. Production, characterization and valuable applications of exopolysaccharides from marine Bacillus subtilis SH1. Pol. J. Microbiol. 2017, 66, 449. [Google Scholar] [CrossRef]
- El-Newary, S.A.; Ibrahim, A.Y.; Asker, M.S.; Mahmoud, M.G.; El Awady, M.E. Production, characterization and biological activities of acidic exopolysaccharide from marine Bacillus amyloliquefaciens 3MS 2017. Asian Pac. J. Trop. Med. 2017, 10, 652–662. [Google Scholar] [CrossRef]
- Sathishkumar, R.; Kannan, R.; Jinendiran, S.; Sivakumar, N.; Selvakumar, G.; Shyamkumar, R. Production and characterization of exopolysaccharide from the sponge-associated Bacillus subtilis MKU SERB2 and its in-vitro biological properties. Int. J. Biol. Macromol. 2021, 166, 1471–1479. [Google Scholar] [CrossRef]
- Bouzaiene, T.; Mohamedhen Vall, M.; Ziadi, M.; Ben Rejeb, I.; Yangui, I.; Aydi, A.; Ouzari, I.; Moktar, H. Exopolysaccharides from Lactiplantibacillus plantarum C7 Exhibited Antibacterial, Antioxidant, Anti-Enzymatic, and Prebiotic Activities. Fermentation 2024, 10, 339. [Google Scholar] [CrossRef]
- Ravindran, A.; Manivannan, A.C.; Bharathi, G.S.J.; Balasubramanian, V.; Velmurugan, P.; Sivasubramanian, K.; Muruganandham, M.; Arumugam, N.; Almansour, A.I.; Kumar, R.S. Production and characterization of exopolysaccharide (EPS) from marine Bacillus halotolerans and its antibacterial activity against clinical pathogens. Biologia 2024, 79, 605–619. [Google Scholar] [CrossRef]
- Brian-Jaisson, F.; Molmeret, M.; Fahs, A.; Guentas-Dombrowsky, L.; Culioli, G.; Blache, Y.; Cérantola, S.; Ortalo-Magné, A. Characterization and anti-biofilm activity of extracellular polymeric substances produced by the marine biofilm-forming bacterium Pseudoalteromonas ulvae strain TC14. Biofouling 2016, 32, 547–560. [Google Scholar] [CrossRef] [PubMed]
- Gargouch, N.; Elleuch, F.; Karkouch, I.; Tabbene, O.; Pichon, C.; Gardarin, C.; Rihouey, C.; Picton, L.; Abdelkafi, S.; Fendri, I.; et al. Potential of Exopolysaccharide from Porphyridium marinum to Contend with Bacterial Proliferation, Biofilm Formation, and Breast Cancer. Mar. Drugs 2021, 19, 66. [Google Scholar] [CrossRef]
- Almutairi, M.H.; Helal, M.M. Biological and microbiological activities of isolated Enterobacter sp. ACD2 exopolysaccharides from Tabuk region of Saudi Arabia. J. King Saud Univ.-Sci. 2021, 33, 101328. [Google Scholar] [CrossRef]
- Soundararajan, D.; Natarajan, L.; Trilokesh, C.; Harish, B.; Ameen, F.; Islam, M.A.; Uppuluri, K.B.; Anbazhagan, V. Isolation of exopolysaccharide, galactan from marine Vibrio sp. BPM 19 to template the synthesis of antimicrobial platinum nanocomposite. Process Biochem. 2022, 122, 267–274. [Google Scholar] [CrossRef]
- Amer, M.S.; Zaghloul, E.H.; Ibrahim, M.I. Characterization of exopolysaccharide produced from marine-derived Aspergillus terreus SEI with prominent biological activities. Egypt. J. Aquat. Res. 2020, 46, 363–369. [Google Scholar] [CrossRef]
- Jenkinson, I.R. Plankton Genes and Extracellular Organic Substances in the Ocean. J. Mar. Sci. Eng. 2023, 11, 783. [Google Scholar] [CrossRef]
- Sengupta, D.; Datta, S.; Biswas, D. Towards a better production of bacterial exopolysaccharides by controlling genetic as well as physico-chemical parameters. Appl. Microbiol. Biotechnol. 2018, 102, 1587–1598. [Google Scholar] [CrossRef]
- Nagaraj, A.; Ghate, S.D.; Arun, A.; Rao, S.S.; Kumar, S.A.; Kandiyil, M.K.; Saptami, K.; Rekha, P. Genome analysis of a halophilic bacterium Halomonas malpeensis YU-PRIM-29T reveals its exopolysaccharide and pigment producing capabilities. Sci. Rep. 2021, 11, 1749. [Google Scholar]
- Wei, Y.; Zhou, F.; Feng, Z.; Qi, M.; Xiang, R.; Yi, H.; Yang, Q.; Yang, X. High-throughput and genome-guided optimization of exopolysaccharide production in marine bacteria for sustainable biotechnology. Appl. Environ. Microbiol. 2025, 91, e00837-25. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Zhang, J. Bacterial exopolysaccharides: Chemical structures, gene clusters and genetic engineering. Int. J. Biol. Macromol. 2021, 173, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Behzadnia, A.; Moosavi-Nasab, M.; Oliyaei, N. Anti-biofilm activity of marine algae-derived bioactive compounds. Front. Microbiol. 2024, 15, 1270174. [Google Scholar] [CrossRef] [PubMed]
- Zayed, A.; Mansour, M.K.; Sedeek, M.S.; Habib, M.H.; Ulber, R.; Farag, M.A. Rediscovering bacterial exopolysaccharides of terrestrial and marine origins: Novel insights on their distribution, biosynthesis, biotechnological production, and future perspectives. Crit. Rev. Biotechnol. 2022, 42, 597–617. [Google Scholar] [CrossRef]
- Nehal, F.; Sahnoun, M.; Smaoui, S.; Jaouadi, B.; Bejar, S.; Mohammed, S. Characterization, high production and antimicrobial activity of exopolysaccharides from Lactococcus lactis F-mou. Microb. Pathog. 2019, 132, 10–19. [Google Scholar] [CrossRef]
- Kavita, K.; Singh, V.K.; Mishra, A.; Jha, B. Characterisation and anti-biofilm activity of extracellular polymeric substances from Oceanobacillus iheyensis. Carbohydr. Polym. 2014, 101, 29–35. [Google Scholar] [CrossRef]
- Mohamed, S.S.; Amer, S.K.; Selim, M.S.; Rifaat, H.M. Characterization and applications of exopolysaccharide produced by marine Bacillus altitudinis MSH2014 from Ras Mohamed, Sinai, Egypt. Egypt. J. Basic Appl. Sci. 2018, 5, 204–209. [Google Scholar] [CrossRef]
- Zammuto, V.; Spanò, A.; Agostino, E.; Macrì, A.; De Pasquale, C.; Ferlazzo, G.; Rizzo, M.G.; Nicolò, M.S.; Guglielmino, S.; Gugliandolo, C. Anti-Bacterial Adhesion on Abiotic and Biotic Surfaces of the Exopolysaccharide from the Marine Bacillus licheniformis B3-15. Mar. Drugs 2023, 21, 313. [Google Scholar] [CrossRef]
- Rizzo, M.G.; Zammuto, V.; Spanò, A.; Gugliandolo, C.; Calabrese, G.; Guglielmino, S. Anti-inflammatory effects in LPS-induced macrophages and antibiofilm activity of the mannose-rich exopolysaccharide produced by Bacillus licheniformis B3-15. Heliyon 2024, 10, e38367. [Google Scholar] [CrossRef]
- Sayem, S.A.; Manzo, E.; Ciavatta, L.; Tramice, A.; Cordone, A.; Zanfardino, A.; De Felice, M.; Varcamonti, M. Anti-biofilm activity of an exopolysaccharide from a sponge-associated strain of Bacillus licheniformis. Microb. Cell Factories 2011, 10, 74. [Google Scholar] [CrossRef]
- Wan, C.; Ju, X.; Xu, D.; Ou, J.; Zhu, M.; Lu, G.; Li, K.; Jiang, W.; Li, C.; Hu, X. Escherichia coli exopolysaccharides disrupt Pseudomonas aeruginosa biofilm and increase its antibiotic susceptibility. Acta Biomater. 2024, 185, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Zammuto, V.; Agostino, E.; Macrì, A.; Spanò, A.; Grillo, E.; Nicolò, M.S.; Gugliandolo, C. Synergistic Antibiofilm Effects of Exopolymers Produced by the Marine, Thermotolerant Bacillus licheniformis B3-15 and Their Potential Medical Applications. J. Mar. Sci. Eng. 2023, 11, 1660. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, Y.; Pan, B. Exploring the structural, functional, and biocompatibility aspects of marine bacterial extracellular polysaccharides for biopharmaceutical applications. Theor. Nat. Sci. 2024, 37, 277–282. [Google Scholar] [CrossRef]
- Garza-Cervantes, J.A.; Escárcega-González, C.E.; Barriga Castro, E.D.; Mendiola-Garza, G.; Marichal-Cancino, B.A.; López-Vázquez, M.A.; Morones-Ramirez, J.R. Antimicrobial and antibiofilm activity of biopolymer-Ni, Zn nanoparticle biocomposites synthesized using R. mucilaginosa UANL-001L exopolysaccharide as a capping agent. Int. J. Nanomed. 2019, 14, 2557–2571. [Google Scholar] [CrossRef]
- Garza-Cervantes, J.A.; Mendiola-Garza, G.; León-Buitimea, A.; Morones-Ramírez, J.R. Synergistic antibacterial effects of exopolysaccharides/nickel-nanoparticles composites against multidrug-resistant bacteria. Sci. Rep. 2023, 13, 21519. [Google Scholar] [CrossRef]
- Zhang, M.; Wu, Y.; Qu, C.; Huang, Q.; Cai, P. Microbial extracellular polymeric substances (EPS) in soil: From interfacial behaviour to ecological multifunctionality. Geo-Bio Interfaces 2024, 1, e4. [Google Scholar] [CrossRef]
- Palomares-Navarro, J.J.; Bernal-Mercado, A.T.; González-Aguilar, G.A.; Ortega-Ramirez, L.A.; Martínez-Téllez, M.A.; Ayala-Zavala, J.F. Antibiofilm Action of Plant Terpenes in Salmonella Strains: Potential Inhibitors of the Synthesis of Extracellular Polymeric Substances. Pathogens 2023, 12, 35. [Google Scholar] [CrossRef]
- Pham, T.-T.; Nguyen, T.-D.; Nguyen, T.-T.; Pham, M.-N.; Nguyen, P.-T.; Nguyen, T.-U.T.; Huynh, T.-T.N.; Nguyen, H.-T. Rhizosphere bacterial exopolysaccharides: Composition, biosynthesis, and their potential applications. Arch. Microbiol. 2024, 206, 388. [Google Scholar] [CrossRef]
- Benhadda, F.; Zykwinska, A.; Colliec-Jouault, S.; Sinquin, C.; Thollas, B.; Courtois, A.; Fuzzati, N.; Toribio, A.; Delbarre-Ladrat, C. Marine versus Non-Marine Bacterial Exopolysaccharides and Their Skincare Applications. Mar. Drugs 2023, 21, 582. [Google Scholar] [CrossRef]
- Ibrahim, H.A.; Abou Elhassayeb, H.E.; El-Sayed, W.M. Potential functions and applications of diverse microbial exopolysaccharides in marine environments. J. Genet. Eng. Biotechnol. 2022, 20, 151. [Google Scholar] [CrossRef]
- Jeewon, R.; Aullybux, A.A.; Puchooa, D.; Nazurally, N.; Alrefaei, A.F.; Zhang, Y. Marine Microbial Polysaccharides: An Untapped Resource for Biotechnological Applications. Mar. Drugs 2023, 21, 420. [Google Scholar] [CrossRef] [PubMed]
- Qi, M.; Zheng, C.; Wu, W.; Yu, G.; Wang, P. Exopolysaccharides from Marine Microbes: Source, Structure and Application. Mar. Drugs 2022, 20, 512. [Google Scholar] [CrossRef] [PubMed]
- Montuori, E.; de Pascale, D.; Lauritano, C. Recent Discoveries on Marine Organism Immunomodulatory Activities. Mar. Drugs 2022, 20, 422. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, B.; Ghosh, D.; Shamim, A.; Das, P.; Choudhuri, T.; Dey, S.; Mitra, A.K. Immunomodulatory Role of Microbial EPS in Food and Dairy Industry. In Extracellular Polysaccharides; Springer: Berlin/Heidelberg, Germany, 2025; pp. 243–281. [Google Scholar]
- Jiang, M.; Zhu, Z. The role of artificial intelligence algorithms in marine scientific research. Front. Mar. Sci. 2022, 9, 920994. [Google Scholar] [CrossRef]
- Rabiya, R.; Sen, R. Artificial intelligence driven advanced optimization strategy vis-à-vis response surface optimization of production medium: Bacterial exopolysaccharide production as a case-study. Biochem. Eng. J. 2022, 178, 108271. [Google Scholar] [CrossRef]
- Marimuthu, S.; Rajendran, K. Artificial neural network modeling and statistical optimization of medium components to enhance production of exopolysaccharide by Bacillus sp. EPS003. Prep. Biochem. Biotechnol. 2023, 53, 136–147. [Google Scholar] [CrossRef]
- Barot, A.; Das, K.; Patel, Y. Multi-approach optimization of exopolysaccharide production from Leuconostoc mesenteroides ABNFT-1 using statistical and AI-based model. Prep. Biochem. Biotechnol. 2025, 1–19. [Google Scholar] [CrossRef]
- Al Rawahi, A.M.; Zafar, M.; Khan, T.A.; Al Araimi, S.; Mahanty, B.; Behera, S.K. Genetic algorithm-optimized artificial neural network for multi-objective optimization of biomass and exopolysaccharide production by Haloferax mediterranei. Bioprocess Biosyst. Eng. 2025, 48, 785–798. [Google Scholar] [CrossRef]
- Desai, K.; Akolkar, S.; Badhe, Y.; Tambe, S.; Lele, S. Optimization of fermentation media for exopolysaccharide production from Lactobacillus plantarum using artificial intelligence-based techniques. Process Biochem. 2006, 41, 1842–1848. [Google Scholar] [CrossRef]
- Abdel-Monem, D.A.; Sabry, S.A.; Ghozlan, H.A.; Zaghloul, E.H. Preparation of novel marine Enterococcus faecium MSD8 exopolysaccharide ointment and In Vivo evaluation of its impact on cutaneous wound healing in male albino rats. Probiotics Antimicrob. Proteins 2025, 17, 963–975. [Google Scholar] [CrossRef]
- Nagaraj, A.; Subramaniyan, Y.; Surya, S.; Rekha, P.D. Burn wound healing abilities of a uronic acid containing exopolysaccharide produced by the marine bacterium Halomonas malpeensis YU-PRIM-29 T. Appl. Biochem. Biotechnol. 2024, 196, 8190–8213. [Google Scholar] [CrossRef]
- Sun, M.-L.; Zhao, F.; Chen, X.-L.; Zhang, X.-Y.; Zhang, Y.-Z.; Song, X.-Y.; Sun, C.-Y.; Yang, J. Promotion of Wound Healing and Prevention of Frostbite Injury in Rat Skin by Exopolysaccharide from the Arctic Marine Bacterium Polaribacter sp. SM1127. Mar. Drugs 2020, 18, 48. [Google Scholar] [CrossRef] [PubMed]
- Trabelsi, I.; Ktari, N.; Slima, S.B.; Triki, M.; Bardaa, S.; Mnif, H.; Salah, R.B. Evaluation of dermal wound healing activity and In Vitro antibacterial and antioxidant activities of a new exopolysaccharide produced by Lactobacillus sp. Ca6. Int. J. Biol. Macromol. 2017, 103, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Alharbi, M.A.; Shahlol, A.M.; Albureikan, M.O.I.; Johani, K.; Elsehrawy, M.G.; El-Nablaway, M.; Saleh, F.M.; Basyony10, A.; Zakai11, S.A.; Ghareeb12, A. Investigating the Multi-Targeted Pharmacological profile of an exopolysaccharide from Bacillus rugosus SYG20 via In Vitro evaluation of its antioxidant, Anti-inflammatory, Anti-diabetic, wound healing, and antimicrobial properties. Arch. Med. Sci. 2024. [Google Scholar] [CrossRef]
- Adegbolagun, T.I.; Odeniyi, O.A.; Odeniyi, M.A. Drug delivery applications and future prospects of microbial exopolysaccharides. Polym. Med. 2023, 53, 117–127. [Google Scholar] [CrossRef]
- Bellini, E.; Ciocci, M.; Savio, S.; Antonaroli, S.; Seliktar, D.; Melino, S.; Congestri, R. Trichormus variabilis (Cyanobacteria) Biomass: From the Nutraceutical Products to Novel EPS-Cell/Protein Carrier Systems. Mar. Drugs 2018, 16, 298. [Google Scholar] [CrossRef]
- Leite, J.P.; Mota, R.; Durão, J.; Neves, S.C.; Barrias, C.C.; Tamagnini, P.; Gales, L. Cyanobacterium-derived extracellular carbohydrate polymer for the controlled delivery of functional proteins. Macromol. Biosci. 2017, 17, 1600206. [Google Scholar] [CrossRef]
- Zykwinska, A.; Marquis, M.; Godin, M.; Marchand, L.; Sinquin, C.; Garnier, C.; Jonchère, C.; Chédeville, C.; Le Visage, C.; Guicheux, J.; et al. Microcarriers Based on Glycosaminoglycan-like Marine Exopolysaccharide for TGF-β1 Long-Term Protection. Mar. Drugs 2019, 17, 65. [Google Scholar] [CrossRef]
- Sengupta, S.; Dey, S. Microbial exo-polysaccharides (EPS): Role in agriculture and environment. Agric. Food 2019, 1, 4–8. [Google Scholar]
- Fadia, A.; Ibtissam, L.; Anass, W.; Laila, R.; Moustapha, A.; Imane, W. Comparison of the effect of two categories of Arthrospira platensis polysaccharides (exo and endopolysaccharides) on tomato growth: Effect on morphological, histological and biochemical plant growth traits. J. Appl. Phycol. 2023, 35, 1183–1192. [Google Scholar] [CrossRef]
- Van Camp, C.; Fraikin, C.; Claverie, E.; Onderwater, R.; Wattiez, R. Capsular polysaccharides and exopolysaccharides from Gloeothece verrucosa under various nitrogen regimes and their potential plant defence stimulation activity. Algal Res. 2022, 64, 102680. [Google Scholar] [CrossRef]
- Drira, M.; Elleuch, J.; Ben Hlima, H.; Hentati, F.; Gardarin, C.; Rihouey, C.; Le Cerf, D.; Michaud, P.; Abdelkafi, S.; Fendri, I. Optimization of Exopolysaccharides Production by Porphyridium sordidum and Their Potential to Induce Defense Responses in Arabidopsis thaliana against Fusarium oxysporum. Biomolecules 2021, 11, 282. [Google Scholar] [CrossRef]
- El Arroussi, H.; Benhima, R.; Elbaouchi, A.; Sijilmassi, B.; EL Mernissi, N.; Aafsar, A.; Meftah-Kadmiri, I.; Bendaou, N.; Smouni, A. Dunaliella salina exopolysaccharides: A promising biostimulant for salt stress tolerance in tomato (Solanum lycopersicum). J. Appl. Phycol. 2018, 30, 2929–2941. [Google Scholar] [CrossRef]
- Wu, S.; Wang, F.; Wang, H.; Shen, C.; Yu, K. Meta-Analysis of Abiotic Conditions Affecting Exopolysaccharide Production in Cyanobacteria. Metabolites 2025, 15, 131. [Google Scholar] [CrossRef]
- Hu, X.; Fu, H.; Bao, M.; Zhang, X.; Liu, W.; Sun, X.; Pan, Y. Temperature mediates metabolism switching of Bacillus sp. ZT-1: Analysis of the properties and structure of exopolysaccharides. Microbiol. Res. 2021, 251, 126839. [Google Scholar] [CrossRef]
- Li, Z.; Liu, Y.; Zhou, T.; Cao, L.; Cai, Y.; Wang, Y.; Cui, X.; Yan, H.; Ruan, R.; Zhang, Q. Effects of Culture Conditions on the Performance of Arthrospira platensis and Its Production of Exopolysaccharides. Foods 2022, 11, 2020. [Google Scholar] [CrossRef]
- Bemal, S.; Anil, A.C. Effects of salinity on cellular growth and exopolysaccharide production of freshwater Synechococcus strain CCAP1405. J. Plankton Res. 2018, 40, 46–58. [Google Scholar] [CrossRef]
- Zuorro, A.; Lavecchia, R.; Moncada-Jacome, K.A.; García-Martínez, J.B.; Barajas-Solano, A.F. Light-Driven Optimization of Exopolysaccharide and Indole-3-Acetic Acid Production in Thermotolerant Cyanobacteria. Sci 2025, 7, 108. [Google Scholar] [CrossRef]





| EPS Name | Source Organism | Linkage Type | Monomer Composition | Key IR Signatures (cm−1) | Functional Properties | References |
|---|---|---|---|---|---|---|
| EPS B3-15 | Bacillus licheniformis B3-15 | α-(1→4) glycosidic linkages | Repeating disaccharide units with manno-pyranosidic configuration and low protein content; includes poly-γ-glutamic acid (γ-PGA) | Major EPS peaks: 3500–3200 (O–H/amide A), 1647 (C=O), 1542 (N–O from γ-PGA), 1200–950 (exopolysaccharide region), 872.7 (glycosidic bond) | Heavy metal adsorption | [26] |
| EPS5SH | Bacillus sp. H5 | α-(1→4)-Manp, α-(1→2)-Manp, α-(1→4,6)-Manp, β-terminal-Manp | Mannose 1.00, Glucosamine 0.02, Glucose 0.07, Galactose 0.02 | Major EPS peaks around 3400 (O–H), 2920 (C–H), 1640 (C=O), 1050–1150 (C–O–C, glycosidic) | Immunostimulatory mechanism activation | [27] |
| EPS | Saccharophagus degradans | Not specified | Glucose, Mannose, Galactose | Major EPS peaks around 1000–1200 (C–O, carbohydrates), 1637 (C=O, carboxyl), 1550 (amide II, protein), 1742/1262 (O-acetyl esters) | Carbon source and C/N ratio-dependent production | [28] |
| RSK CAS4 EPS | Bacillus thuringiensis RSK CAS4 | Not specified | Fructose, Galactose | –OH (3433 cm−1), C–H (2954, 2926 cm−1), C=O (1633 cm−1), C–O–C glycosidic linkages (1122 cm−1), and C–H bending (615 cm−1) | Antioxidant, antitumor activities | [29] |
| EPS | Enterobacter cloacae marine isolate B8 | glycosidic linkages (C–O–C) | Mainly glucose with trace mannose (Glc: Man ≈ 1:0.015) | Not specified | Antioxidant activity | [30] |
| EPS | Streptomyces carpaticus | C–O–C | Galactouronic acid, Glucose, Xylose, Galactose, Mannose, Fructose = 3:1:1:2:2:1 | Major peaks around 1380 (–COO–, sulfate ester), 1353 (monosaccharide sulfates), 836 (α-glycosidic bonds)—indicating an α-type acidic heteropolysaccharide | Anticancer-activity | [31] |
| K1T-9 | Neorhizobium urealyticum | Heteropolysaccharide | Galacturonic acid, Glucose | 3399 (OH), 2933.8 (C–H), 2099.8 (C≡C), 1644.3 (COO−), 1408 (C–O–H), 1200–1000 (glycosidic bonds/pyranoid ring), 617.2 (fingerprint) | Antioxidant activities | [32] |
| EPS-B3-15 | Bacillus licheniformis B3-15 | α-(1→4) glycosidic linkages | Mannose, Glucose | glycosidic linkages, pyranosidic monosaccharides, OH stretching (3500–3000 cm−1), C=O at 1665 cm−1, and CH at 2061 cm−1 | Thermostable up to 78.5 °C | [33] |
| EPS-BFB-6S | Pseudomonas sihuiensis BFB-6S | α/β-glycosidic | Mannose, Glucose, Fructose | Amide: C–N, C–C, C=O, –NH bending; polysaccharide peaks dominant | Metabolic modulation under high pCO2, Stress adaptation | [34] |
| EPS-CDR-SL 7Cii | Rhodobacter johrii | 1,6 α-D-Glcp, 1,4 β-D-Glcp, 1,3 β-D-GlcA, 1,3 β-D-Galp, 1,6 β-D-Galf, 3 α-L-Rhmp | Glucose: Glucuronic acid: Rhamnose: Galactose = 3:1.5:0.25:0.25 | Major Peaks at 3398 cm−1—OH (hydroxyl), 1735 cm−1—diacyl ester/glucuronic acid, 1608 and 1414 cm−1—COO− (carboxyl), 1041 cm−1—C–O–C glycosidic bonds | Bio-emulsifier applications | [35] |
| Marine Microorganism | Production Method | Key Factors | Results/Properties | EPS Yield | References |
|---|---|---|---|---|---|
| Streptomyces vinaceusdrappus strain AMG31 | Submerged fermentation | Acidic EPS, High uronic acid content: 39.77%, High sulfate content: 18.8%, Monosaccharide ratio: Arabinose: Glucose: Galacturonic acid = 0.5: 2:2 | Antioxidant, Anti-inflammatory, and Enzyme-inhibitory activity | 10.6 g/L | [42] |
| Actinobacterium Streptomyces violaceus MM72 | submerged fermentation | Molecular weight of 8.96 × 105 Da | Strong antioxidant activity, including DPPH radical scavenging, superoxide scavenging, and metal chelation. Exhibited moderate lipid peroxidation inhibition and reducing power, suggesting potential for natural antioxidant applications | Not specified | [43] |
| Bacillus subtilis strain AG4, | fractionation and precipitation processes | Sulphated β-glycosidic heteropolysaccharide; molecular weight 1.48 × 104 g/mol; monosaccharide composition: glucose, rhamnose, and arabinose (5:1:3) | High crystallinity and porosity, strong antioxidant activity, cytotoxicity against multiple cancer cell lines, anti-inflammatory effects (LOX, COX-2, membrane stabilization), and acetylcholinesterase inhibition | 8.12 g/L | [44] |
| Bacillus cereus | Not specified | Monosaccharide composition: glucose, galacturonic acid, and arabinose (2.0:0.8:1.0), uronic acid content 28.7%, no sulfate | Strong antioxidant activity (DPPH IC50 ≈ 500 µg/mL; H2O2 IC50 ≈ 1500 µg/mL); cytotoxicity against T-24, MCF-7, and PC-3 cancer cells; potent anti-inflammatory activity (LOX IC50 ≈ 12.9 µg/mL; COX-2 IC50 ≈ 29.6 µg/mL); antibacterial activity against MRSA and coagulase-negative staphylococci | 7.95 g/L | [45] |
| Bacillus velezensis AG6 | Not specified | Monosaccharide composition: xylose, galactose, and galacturonic acid in a molar ratio of 2:0.5:2 | Strong antioxidant activity in DPPH, H2O2, and ABTS assays, showing dose- and time-dependent inhibition (91.3%, 80.2%, and 75.3% at 1500 μg/mL), inhibition of six cancer cell lines, anti-inflammatory effects via LOX and COX-2 inhibition; antimicrobial and antibiofilm activities | 5.79 g/L | [46] |
| Pseudomonas sp. strain AHG22 | Cultured in broth solution | Potent DPPH-scavenging profile indicated by an IC50 of 46.99 μg/mL | Moderate anti-inflammatory activity through 5-LOX and COX-2 inhibition; antidiabetic, anti-obesity, neuroprotective, antibiofilm, and broad-spectrum antibacterial properties with MBC/MIC ≤ 2 | 6.98 g/L | [47] |
| Kocuria sp. strain AG5 | Cultivation/fermentation | Molecular weight of approximately 4.9 × 104 g/mol; composed primarily of glucose, galacturonic acid, arabinose, and xylose | Potent antioxidant activity reaching 98% at 2000 µg/mL, notable anti-inflammatory effects with 5-LOX IC50 = 15.39 µg/mL and COX-2 IC50 = 28.06 µg/mL, cytotoxicity against cancer cell lines, hemolysis suppression, moderate acetylcholine esterase inhibition | 6.84 g/L | [48] |
| Bacillus subtilis strain SH1 | one-factor-at-a-time experiments and Response Surface Methodology (RSM) | Multifunctional bioactivity under different concentrations to determine dose-dependent effects | Antibacterial activity against S. faecalis, significant antitumor effects on MCF-7, HCT-116, and HepG2 cells, antiviral activity at 500 µg/mL | 33.8 g/L | [49] |
| Bacillus amyloliquefaciens 3MS 2017 | Not Specified | Explored multifunctional bioactivity, including antioxidant mechanisms (ROS scavenging, metal chelation), selective COX inhibition, and anticancer effects. In vitro studies were conducted on MCF7, PC3, and EAC cells, and in vivo evaluation in EAC-bearing models (oral, 200 mg/kg) | Exhibited strong antioxidant activity via ROS scavenging and metal chelation, weak reducing power, selectively inhibited COX-2, showed high in vitro anticancer activity against MCF7 (65.2% death; IC50 = 70 μg/mL), reduced PC3 and EAC cell viability, and significantly slowed EAC progression in vivo | Not Specified | [50] |
| Bacillus subtilis MKU SERB2 | Response Surface Methodology (RSM) | 11.5 g/L sucrose, 3.5 g/L yeast extract, 3.0 g/L peptone, 2.5 g/L CaCl2. No detectable hemolytic or lymphocyte toxicity, indicating safe biomedical applicability | EPS exhibited strong antioxidant activity, moderate anticoagulant potential | 617.81 μg/mL | [51] |
| Lactiplantibacillus plantarum C7 | MRS broth cultivation | No molecular weight specified, Prebiotic score 0.043, Carbohydrate content 3.679 g eq glucose/L | Antioxidant, antibacterial, and enzyme inhibition activities | 0.2 to 0.9 g/L | [52] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aslam, B.; Khalid, M.H.; Aljasir, S.F. Marine Microbial Exopolysaccharides (EPSs): Untapped Bio-Reserves. Polymers 2025, 17, 3249. https://doi.org/10.3390/polym17243249
Aslam B, Khalid MH, Aljasir SF. Marine Microbial Exopolysaccharides (EPSs): Untapped Bio-Reserves. Polymers. 2025; 17(24):3249. https://doi.org/10.3390/polym17243249
Chicago/Turabian StyleAslam, Bilal, Muhammad Hassan Khalid, and Sulaiman F. Aljasir. 2025. "Marine Microbial Exopolysaccharides (EPSs): Untapped Bio-Reserves" Polymers 17, no. 24: 3249. https://doi.org/10.3390/polym17243249
APA StyleAslam, B., Khalid, M. H., & Aljasir, S. F. (2025). Marine Microbial Exopolysaccharides (EPSs): Untapped Bio-Reserves. Polymers, 17(24), 3249. https://doi.org/10.3390/polym17243249






