Advances in Membranes Based on PLA and Derivatives for Oil–Water Separation
Abstract
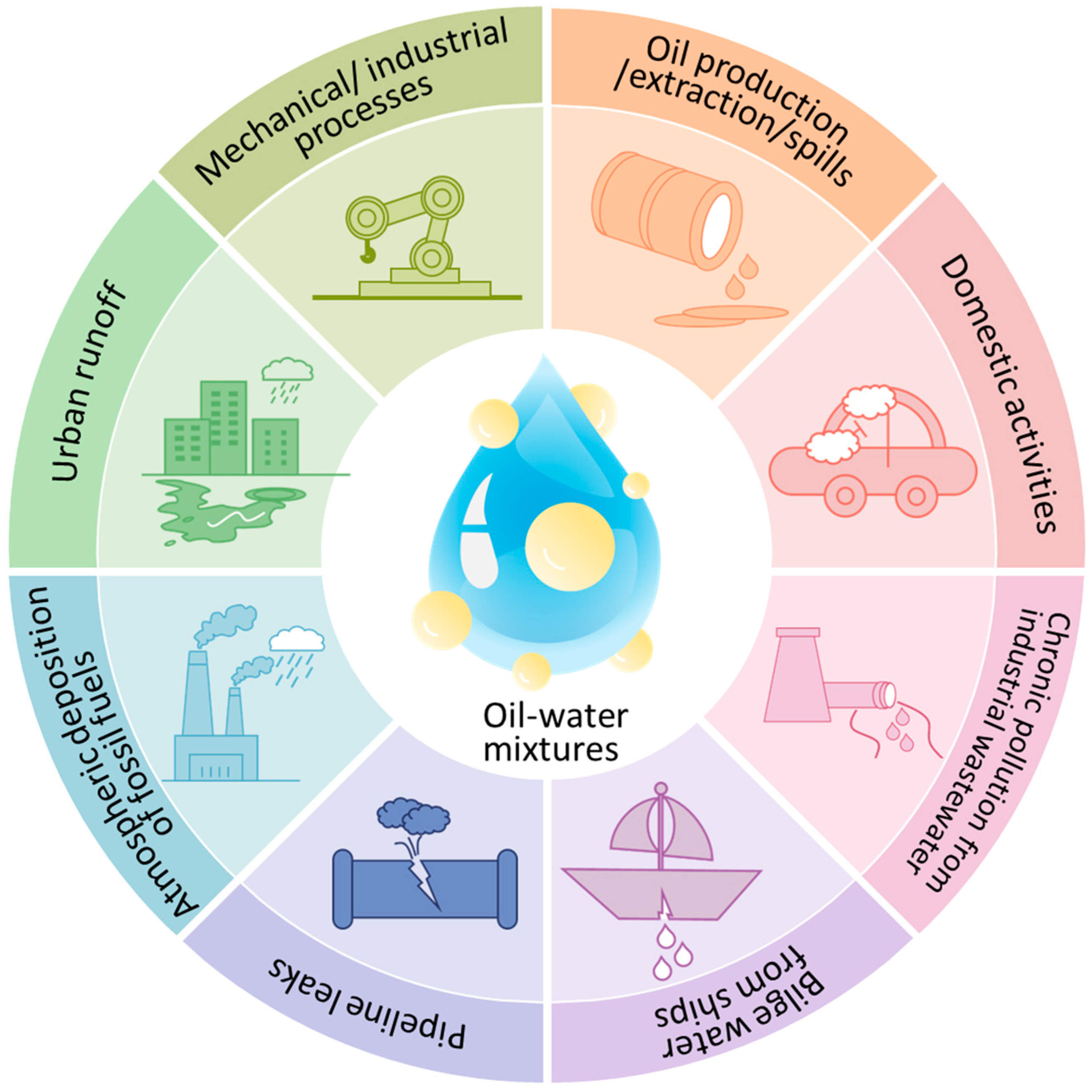
1. Introduction
2. Membranes for Oil–Water Separation
3. Polymeric Membranes
4. Preparation of Membranes Based on PLA and Derivatives
4.1. Fiber Formation Techniques
4.2. Surface Modification Techniques
4.3. Techniques for Enhancing Porosity
| Membrane/Additives | Modify Method | Pore Size | Water Contact Angle * (°) | Separation Efficiency (%) | Separation Flux (L/m2h) | Oil Takes /Absorption Capacity | Reuseful | Ref. |
|---|---|---|---|---|---|---|---|---|
| PLA | Immersed in dopamine aqueous solution/CNCs/Mixture: Hydrophobic > Hydrophilic | 223 μm | 121 | ~1000 | Water: 270% | [90] | ||
| PLA-PDA | 58.5 | ~3000 | Water: 778% | |||||
| PLA-PDA-CNCs | 128 μm | 0 | 99 | 3710 | Water: 1000% | 100 times: 98% | ||
| PLA | Immersed in dopamine aqueous solution/SiO2 nanoparticles/PS microspheres/Mixture: Hydrophobic > Superhydrophobic | 117 | [91] | |||||
| PLA-PDA | 23 | |||||||
| PLA-PDA-SiO2-PS | 152 | Hexane: ~9000; Toluene: ~10,500 Silicone Oil: ~11,500 Pump Oil: ~7000 Vegetable Oil: ~8500 | Hexane: ~25 g/g; Toluene: ~28 g/g Silicone Oil: ~32 g/g; Pump Oil: ~23 g/g Vegetable Oil: ~30 g/g; Soybean Oil: ~33 g/g | 10 times: 3–6° change | ||||
| PLA | Immersed in TiO2 suspension: Hydrophobic > Superhydrophobic | 97 | ESO: ~4 g/g; Peanut Oil: ~2 g/g Xylene: ~2 g/g | [93] | ||||
| PLA-TiO2 (55 °C/7 s) | 122 | ESO: ~5 g/g; Peanut Oil: ~3.5 g/g Xylene: ~2.5 g/g | ||||||
| PLA-TiO2 (65 °C/7 s) | 126 | ESO: ~4.9 g/g; Peanut Oil: ~4.5 g/g Xylene: ~2.5 g/g | ||||||
| PLA-TiO2 (75 °C/1 s) | 121 | |||||||
| PLA-TiO2 (75 °C/3 s) | No Wetting | |||||||
| PLA-TiO2 (75 °C/7 s) | No Wetting | ESO: ~4 g/g; Peanut Oil: ~3 g/g Xylene: ~2.5 g/g | 10 times: >99% | |||||
| PLA-TiO2 (75 °C/16 s) | 127 | |||||||
| PLA | Immersed in dioxane and water solvent: Hydrophobic > Superhydrophobic | 116 | [94] | |||||
| PLA–dioxane–water (12 wt.%) | ~135 | |||||||
| PLA–dioxane–water (16 wt.%) | ~141° | |||||||
| PLA–dioxane–water (20 wt.%) | 153 | N-hexane: 27; Cyclohexane: ~24 Ethanol: 17; CTC: 27 | 10 times: >97% | |||||
| PLA–dioxane–water (22 wt.%) | ~140 | |||||||
| PLA | MWCNTs and AC-FAS sprayed on membrane: Hydrophobic > Superhydrophobic | 140 | [92] | |||||
| PLA-MWCNTs (0.05 g) | ~142° | |||||||
| PLA-MWCNTs (0.08 g) | ~150 | |||||||
| PLA-MWCNTs (0.1 g) | ~158 | |||||||
| PLA-MWCNTs (0.15 g) | 161 | 99.5 | N-hexane: 59,713 Peanut Oil: ~3000 Engineering Oil: ~3000 | 10 times: >98.5% | ||||
| PLA-MWCNTs (0.18 g) | ~159 | |||||||
| PLA-MWCNTs (0.2 g) | ~159 | |||||||
| PLA | The PLA nanospheres were attached to PLA nonwoven membranes with the help of the chemical crosslinking of epoxy resin (ER) | 225 μm | 119.4 | [95] | ||||
| PLA–epoxy | 133.2 | |||||||
| PLA–epoxy–PLA nanoparticles | 140.8 | |||||||
| PLA nanoparticles (KH-560 treated) | 152.1 | 96 |

4.4. Phase Separation Techniques
4.4.1. Thermally Induced Phase Separation Method (TIPS)
4.4.2. Nonsolvent-Induced Phase Separation Method (NIPS)
4.4.3. Freeze Solidification Phase Separation Method (FSPS)
5. Challenges and Future Perspectives
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PVDF | Polyvinylidene fluoride |
| LDH | Layered double hydroxides |
| CO2 | Carbon dioxide |
| PP | Polypropylene |
| CNTs | Carbon nanotubes |
| PCL | Polycaprolactone |
| PSF | Poly sulfone |
| CNF | Cellulose nanofiber |
| PES | Polyether sulfone |
| WCA | Water contact angle |
| PLA | Polylactic acid |
| PBE | Propylene-based elastomer |
| 2D-NM | 2-Dimensional Nanomaterial |
| GO | Graphene oxide |
References
- Sen Gupta, R.; Samantaray, P.K.; Bose, S. Going beyond cellulose and chitosan: Synthetic biodegradable membranes for drinking water, wastewater, and oil–water remediation. ACS Omega 2023, 8, 24695–24717. [Google Scholar] [CrossRef]
- Li, X.; Jin, X.; Wu, Y.; Zhang, D.; Sun, F.; Ma, H.; Pugazhendhi, A.; Xia, C. A comprehensive review of lignocellulosic biomass derived materials for water/oil separation. Sci. Total Environ. 2023, 876, 162549. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Ras, R.H.; Tian, X. Antifouling membranes for oily wastewater treatment: Interplay between wetting and membrane fouling. Curr. Opin. Colloid Interface Sci. 2018, 36, 90–109. [Google Scholar] [CrossRef]
- Saravanan, A.; Kumar, P.S.; Jeevanantham, S.; Karishma, S.; Tajsabreen, B.; Yaashikaa, P.; Reshma, B. Effective water/wastewater treatment methodologies for toxic pollutants removal: Processes and applications towards sustainable development. Chemosphere 2021, 280, 130595. [Google Scholar] [CrossRef] [PubMed]
- Sathya, K.; Nagarajan, K.; Carlin Geor Malar, G.; Rajalakshmi, S.; Raja Lakshmi, P. A comprehensive review on comparison among effluent treatment methods and modern methods of treatment of industrial wastewater effluent from different sources. Appl. Water Sci. 2022, 12, 70. [Google Scholar] [CrossRef]
- Zulkefli, N.F.; Alias, N.H.; Jamaluddin, N.S.; Abdullah, N.; Abdul Manaf, S.F.; Othman, N.H.; Marpani, F.; Mat-Shayuti, M.S.; Kusworo, T.D. Recent mitigation strategies on membrane fouling for oily wastewater treatment. Membranes 2021, 12, 26. [Google Scholar] [CrossRef]
- Omar, R.A.; Talreja, N.; Ashfaq, M.; Chauhan, D. Two-Dimensional nanomaterial (2D-NMs)-based polymeric composite for Oil–Water separation: Strategies to improve Oil–Water separation. Sustainability 2023, 15, 10988. [Google Scholar] [CrossRef]
- Ng, L.Y.; Mohammad, A.W.; Leo, C.P.; Hilal, N. Polymeric membranes incorporated with metal/metal oxide nanoparticles: A comprehensive review. Desalination 2013, 308, 15–33. [Google Scholar] [CrossRef]
- Xiang, B.; Sun, Q.; Zhong, Q.; Mu, P.; Li, J. Current research situation and future prospect of superwetting smart oil/water separation materials. J. Mater. Chem. A 2022, 10, 20190–20217. [Google Scholar] [CrossRef]
- Sutrisna, P.D.; Kurnia, K.A.; Siagian, U.W.; Ismadji, S.; Wenten, I.G. Membrane fouling and fouling mitigation in oil–water separation: A review. J. Environ. Chem. Eng. 2022, 10, 107532. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, X.; Pu, X.; Shen, Z.; Xu, W.; Yang, J. Preparation Method and Application of Porous Poly(lactic acid) Membranes: A Review. Polymers 2024, 16, 1846. [Google Scholar] [CrossRef]
- Xiao, Y.; Xiao, F.; Ji, W.; Xia, L.; Li, L.; Chen, M.; Wang, H. Bioinspired Janus membrane of polyacrylonitrile/poly (vinylidene fluoride)@ poly (vinylidene fluoride)-methyltriethoxysilane for oil-water separation. J. Membr. Sci. 2023, 687, 122090. [Google Scholar] [CrossRef]
- Huang, Y.; Gancheva, T.; Favis, B.D.; Abidli, A.; Wang, J.; Park, C.B. Hydrophobic porous polypropylene with hierarchical structures for ultrafast and highly selective oil/water separation. ACS Appl. Mater. Interfaces 2021, 13, 16859–16868. [Google Scholar] [CrossRef]
- Pagidi, A.; Saranya, R.; Arthanareeswaran, G.; Ismail, A.; Matsuura, T. Enhanced oil–water separation using polysulfone membranes modified with polymeric additives. Desalination 2014, 344, 280–288. [Google Scholar] [CrossRef]
- Tavangar, T.; Zokaee Ashtiani, F.; Karimi, M. Morphological and performance evaluation of highly sulfonated polyethersulfone/polyethersulfone membrane for oil/water separation. J. Polym. Res. 2020, 27, 252. [Google Scholar] [CrossRef]
- Vatanpour, V.; Dehqan, A.; Paziresh, S.; Zinadini, S.; Zinatizadeh, A.A.; Koyuncu, I. Polylactic acid in the fabrication of separation membranes: A review. Sep. Purif. Technol. 2022, 296, 121433. [Google Scholar] [CrossRef]
- Bishai, M.; De, S.; Adhikari, B.; Banerjee, R. A comprehensive study on enhanced characteristics of modified polylactic acid based versatile biopolymer. Eur. Polym. J. 2014, 54, 52–61. [Google Scholar] [CrossRef]
- de Albuquerque, T.L.; Júnior, J.E.M.; de Queiroz, L.P.; Ricardo, A.D.S.; Rocha, M.V.P. Polylactic acid production from biotechnological routes: A review. Int. J. Biol. Macromol. 2021, 186, 933–951. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.S.; Elahee, G.F.; Fang, Y.; Yu, X.B.; Advincula, R.C.; Cao, C.C. Polylactic acid (PLA)-based multifunctional and biodegradable nanocomposites and their applications. Compos. Part B Eng. 2025, 306, 112842. [Google Scholar] [CrossRef]
- Wang, X.L.; Pan, Y.M.; Yuan, H.; Su, M.; Shao, C.G.; Liu, C.T.; Guo, Z.H.; Shen, C.Y.; Liu, X.H. Simple fabrication of superhydrophobic PLA with honeycomb-like structures for high-efficiency oil-water separation. Chin. Chem. Lett. 2020, 31, 365–368. [Google Scholar] [CrossRef]
- Mousa, H.M.; Fahmy, H.S.; Ali, G.A.M.; Abdelhamid, H.N.; Ateia, M. Membranes for Oil/Water Separation: A Review. Adv. Mater. Interfaces 2022, 9, 2200557. [Google Scholar] [CrossRef]
- Ghadhban, M.Y.; Rashid, K.T.; AbdulRazak, A.A.; Alsalhy, Q.F. Recent progress and future directions of membranes green polymers for oily wastewater treatment. Water Sci. Technol. 2023, 87, 57–82. [Google Scholar] [CrossRef]
- Ranakoti, L.; Gangil, B.; Mishra, S.K.; Singh, T.; Sharma, S.; Ilyas, R.A.; El-Khatib, S. Critical Review on Polylactic Acid: Properties, Structure, Processing, Biocomposites, and Nanocomposites. Materials 2022, 15, 4312. [Google Scholar] [CrossRef]
- Xing, R.Z.; Huang, R.L.; Qi, W.; Su, R.X.; He, Z.M. Three-dimensionally printed bioinspired superhydrophobic PLA membrane for oil-water separation. AIChE J. 2018, 64, 3700–3708. [Google Scholar] [CrossRef]
- Wu, M.M.; Mu, P.; Li, B.F.; Wang, Q.T.; Yang, Y.X.; Li, J. Pine powders-coated PVDF multifunctional membrane for highly efficient switchable oil/water emulsions separation and dyes adsorption. Sep. Purif. Technol. 2020, 248, 117028. [Google Scholar] [CrossRef]
- Hudaib, B.; Abu-Zurayk, R.; Eskhan, A.; Esaifan, M. PVDF/Polypyrrole Composite Ultrafiltration Membrane with Enhanced Hydrophilicity, Permeability, and Antifouling Properties for Efficient Crude Oil Wastewater Separation. Polymers 2025, 17, 2566. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.B.; Li, Y.; Yuan, Y.; Jiang, L.; Wu, H.T.; Dong, Y.M. Biomimetic hydrophilic modification of poly (vinylidene fluoride) membrane for efficient oil-in-water emulsions separation. Sep. Purif. Technol. 2024, 329, 125227. [Google Scholar] [CrossRef]
- Segneanu, A.E.; Herea, D.D.; Buema, G.; Bradu, I.A.; Cepan, M.; Grozescu, I. Advanced Aerogels for Water Remediation: Unraveling Their Potential in Fats, Oils, and Grease Sorption-A Comprehensive Review. Gels 2025, 11, 268. [Google Scholar] [CrossRef] [PubMed]
- Palanisamy, K.; Palanisamy, G.; Im, Y.M.; Thangarasu, S.; Phutela, U.G.; Oh, T.H. Recent Progress in Cellulose-Based Aerogels for Sustainable Oil-Water Separation Technologies. Polymers 2025, 17, 2723. [Google Scholar] [CrossRef]
- Sahini, M.G. Polylactic acid (PLA)-based materials: A review on the synthesis and drug delivery applications. Emergent Mater. 2023, 6, 1461–1479. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, T.; Zhang, D.; Sun, S.; Liu, J.; Li, B.; Shi, Z. The Preparation of Superhydrophobic Polylactic Acid Membrane with Adjustable Pore Size by Freeze Solidification Phase Separation Method for Oil-Water Separation. Molecules 2023, 28, 5590. [Google Scholar] [CrossRef]
- Yao, X.Y.; Hou, X.B.; Zhang, R.B. Flexible and mechanically robust polyimide foam membranes with adjustable structure for separation and recovery of oil-water emulsions and heavy oils. Polymer 2022, 250, 124889. [Google Scholar] [CrossRef]
- Baig, N.; Abdulazeez, I.; Aljundi, I.H. Low-pressure-driven special wettable graphene oxide-based membrane for efficient separation of water-in-oil emulsions. Npj Clean Water 2023, 6, 40. [Google Scholar] [CrossRef]
- Xin, Y.P.; Qi, B.; Wu, X.; Yang, C.; Li, B.F. Different types of membrane materials for oil-water separation: Status and challenges. Colloid Interface Sci. 2024, 59, 100772. [Google Scholar] [CrossRef]
- Zhai, T.L.; Du, Q.; Xu, S.; Wang, Y.; Zhang, C. Electrospun nanofibrous membrane of porous fluorine-containing triptycene-based polyimides for oil/water separation. RSC Adv. 2017, 7, 22548–22552. [Google Scholar] [CrossRef]
- Fang, W.; Liu, L.; Li, T.; Dang, Z.; Qiao, C.; Xu, J.; Wang, Y. Electrospun N-Substituted Polyurethane Membranes with Self-Healing Ability for Self-Cleaning and Oil/Water Separation. Chemistry 2016, 22, 878–883. [Google Scholar] [CrossRef]
- Asif, M.B.; Zhang, Z.H. Ceramic membrane technology for water and wastewater treatment: A critical review of performance, full-scale applications, membrane fouling and prospects. Chem. Eng. J. 2021, 418, 129481. [Google Scholar] [CrossRef]
- Gupta, S.; Tai, N.H. Carbon materials as oil sorbents: A review on the synthesis and performance. J. Mater. Chem. A 2016, 4, 1550–1565. [Google Scholar] [CrossRef]
- Yu, S.; Pang, H.; Huang, S.; Tang, H.; Wang, S.; Qiu, M.; Chen, Z.; Yang, H.; Song, G.; Fu, D.; et al. Recent advances in metal-organic framework membranes for water treatment: A review. Sci. Total Environ. 2021, 800, 149662. [Google Scholar] [CrossRef] [PubMed]
- Padaki, M.; Surya Murali, R.; Abdullah, M.S.; Misdan, N.; Moslehyani, A.; Kassim, M.A.; Hilal, N.; Ismail, A.F. Membrane technology enhancement in oil–water separation. A review. Desalination 2015, 357, 197–207. [Google Scholar] [CrossRef]
- Li, J.H.; Liu, Q.H.; He, J.M.; Zhao, Y.; Mu, L.H.; Liu, X.F.; Zhang, Y.; Sun, C.L.; Zhang, N.; Qu, M.N. A review of superwetting aerogel-based oil-water separation materials. Mater. Today Sustain. 2024, 26, 100741. [Google Scholar] [CrossRef]
- Zhang, Y.; Sam, E.K.; Liu, J.; Lv, X.M. Biomass-Based/Derived Value-Added Porous Absorbents for Oil/Water Separation. Waste Biomass Valoriz. 2023, 14, 3147–3168. [Google Scholar] [CrossRef]
- Akoumeh, R.; Idoudi, S.; El-Din, L.A.N.; Rekik, H.; Al-Ejji, M.; Ponnama, D.; Sharma, A.; Shamsabadi, A.A.; Alamgir, K.; Song, K.; et al. Advances in fabrication techniques and performance optimization of polymer membranes for enhanced industrial oil-water separation: A critical review. J. Environ. Chem. Eng. 2024, 12, 114411. [Google Scholar] [CrossRef]
- Han, P.; Yu, B.; Chen, Y.; Nie, G.; Wang, K.; Sun, X.; Li, X.; Shao, W.; Liu, F.; He, J. Electrospun Polyacrylonitrile-Fluorinated Polyurethane/Polysulfone Nanofiber Membranes for Oil–Water Separation. ACS Appl. Nano Mater. 2024, 7, 4336–4348. [Google Scholar] [CrossRef]
- Kumar, S.; Nandi, B.K.; Guria, C.; Mandal, A. Oil Removal from Produced Water by Ultrafiltration Using Polysulfone Membrane. Braz. J. Chem. Eng. 2017, 34, 583–596. [Google Scholar] [CrossRef]
- Dmitrieva, E.S.; Anokhina, T.S.; Novitsky, E.G.; Volkov, V.V.; Borisov, I.L.; Volkov, A.V. Polymeric Membranes for Oil-Water Separation: A Review. Polymers 2022, 14, 980. [Google Scholar] [CrossRef]
- Huh, Y.; Yu, S.; Huh, J.; Bang, J. Sustainable Superhydrophobic PVDF-Grafted Cellulose Membrane for Oil/Water Separation. ACS Appl. Polym. Mater. 2022, 4, 8441–8449. [Google Scholar] [CrossRef]
- Xie, T.; Zhang, Y.Y.; Zhang, X.; Huang, Y.X. Membrane surface engineering with hyperbranched polylysine for effective oil-water emulsion separation. J. Membr. Sci. 2025, 723, 123935. [Google Scholar] [CrossRef]
- Gu, M.H.; Zhang, J.; Wang, X.L.; Tao, H.J.; Ge, L.T. Formation of poly(vinylidene fluoride) (PVDF) membranes via thermally induced phase separation. Desalination 2006, 192, 160–167. [Google Scholar] [CrossRef]
- Tian, L.H.; Xing, Y.Q.; Gao, C.M.; Ji, S.F.; Gong, T.M.; Bai, Y.M.; Gan, F.H.; Wu, Z.L. Construction of metal polyphenol network of phytic acid-Graphene oxide PES ultrafiltration membranes achieving high-efficiency separation of oil-water emulsions. J. Environ. Chem. Eng. 2024, 12, 114529. [Google Scholar] [CrossRef]
- Cao, J.S.; Cheng, Z.Q.; Kang, L.J.; Zhang, Y.Y.; Zhao, X.D.; Zhao, S.Z.; Gao, B. Novel anti-fouling polyethersulfone/polyamide 66 membrane preparation for air filtration by electrospinning. Mater. Lett. 2017, 192, 12–16. [Google Scholar] [CrossRef]
- Cao, J.S.; Cheng, Z.Q.; Kang, L.J.; Chu, M.X.; Wu, D.; Li, M.T.; Xie, S.; Wen, R.C. Novel stellate poly(vinylidene fluoride)/polyethersulfone microsphere-nanofiber electrospun membrane with special wettability for oil/water separation. Mater. Lett. 2017, 207, 190–194. [Google Scholar] [CrossRef]
- Huang, J.X.; Tang, J.H.; Zhang, J.; Yang, L.; Zhang, M. Waste mask supported PES membranes for efficient separation of oil/water emulsion. Polym. Eng. Sci. 2024, 64, 3522–3529. [Google Scholar] [CrossRef]
- Li, C.; Ren, L.; Zhang, C.; Xu, W.; Liu, X. TiO2 Coated Polypropylene Membrane by Atomic Layer Deposition for Oil–Water Mixture Separation. Adv. Fiber Mater. 2021, 3, 138–146. [Google Scholar] [CrossRef]
- Ma, W.J.; Samal, S.K.; Liu, Z.C.; Xiong, R.H.; De Smedt, S.C.; Bhushan, B.; Zhang, Q.L.; Huang, C.B. Dual pH- and ammonia-vapor-responsive electrospun nanofibrous membranes for oil-water separations. J. Membr. Sci. 2017, 537, 128–139. [Google Scholar] [CrossRef]
- Yao, X.Y.; Hou, X.B.; Qi, G.C.; Zhang, R.B. Preparation of superhydrophobic polyimide fibrous membranes with controllable surface structure for high efficient oil-water emulsion and heavy oil separation. J. Environ. Chem. Eng. 2022, 10, 107470. [Google Scholar] [CrossRef]
- Long, Q.W.; Chen, J.X.; Wang, Z.; Zhang, Z.; Qi, G.X.; Liu, Z.Q. Vein-supported porous membranes with enhanced superhydrophilicity and mechanical strength for oil-water separation. Sep. Purif. Technol. 2021, 254, 117517. [Google Scholar] [CrossRef]
- Zhao, H.; He, Y.; Wang, Z.; Zhao, Y.; Sun, L. Mussel-Inspired Fabrication of PDA@PAN Electrospun Nanofibrous Membrane for Oil-in-Water Emulsion Separation. Nanomaterials 2021, 11, 3434. [Google Scholar] [CrossRef]
- Wang, S.; Pang, M.; Wen, Z. The structure and property of polyacrylonitrile-based microfiltration membranes for oil-water emulsion separation. J. Ind. Text. 2022, 51 (Suppl. 5), 8788S–8803S. [Google Scholar] [CrossRef]
- Cai, Y.W.; Chen, Y.; Wang, R.R.; Li, J.X.; Yang, H.; Li, Y.J.; Qu, D.N. Durable polyvinylpyrrolidone superhydrophilic modified ZIF-8 mesh membrane for gravitational oil-water separation and oil recovery. Colloids Surf. A Physicochem. Eng. Asp. 2024, 688, 133509. [Google Scholar] [CrossRef]
- Wang, C.F.; Wang, W.N.; Kuo, S.W.; Chiang, Y.W.; Hung, J.H.; Lee, K.J. Biocompatible Meshes with Appropriate Wettabilities for Underwater Oil Transportation/Collection and Highly Effective Oil/Water Separation. Langmuir 2018, 34, 11442–11448. [Google Scholar] [CrossRef]
- Yu, J.S.; Zhao, T.; Li, C.T.; Pan, H.W.; Tan, Z.Y.; Yang, H.L.; Zhang, H.L. Preparation and characterization of biodegradable polylactic acid/poly (butylene adipate-co-terephthalate) melt-blown nonwovens for oil-water separation. Colloid Polym. Sci. 2024, 302, 43–56. [Google Scholar] [CrossRef]
- Li, Z.Q.; Wang, M.L.; Li, Y.; Ren, J.M.; Pei, C.H. Effect of cellulose nanocrystals on bacterial cellulose hydrogel for oil-water separation. Sep. Purif. Technol. 2023, 304, 122349. [Google Scholar] [CrossRef]
- Zhao, X.Q.; Wahid, F.; Cui, J.X.; Wang, Y.Y.; Zhong, C. Cellulose-based special wetting materials for oil/water separation: A review. Int. J. Biol. Macromol. 2021, 185, 890–906. [Google Scholar] [CrossRef]
- Hu, M.X.; Niu, H.M.; Chen, X.L.; Zhan, H.B. Natural cellulose microfiltration membranes for oil/water nanoemulsions separation. Colloids Surf. A Physicochem. Eng. Asp. 2019, 564, 142–151. [Google Scholar] [CrossRef]
- Li, D.; Huang, X.; Huang, Y.; Yuan, J.; Huang, D.; Cheng, G.J.; Zhang, L.; Chang, C. Additive Printed All-Cellulose Membranes with Hierarchical Structure for Highly Efficient Separation of Oil/Water Nanoemulsions. ACS Appl. Mater. Interfaces 2019, 11, 44375–44382. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.H.; Yao, L.; Guo, Z.G. Biomass chitosan-based complexes with superwettability for oil-water separation. Mater. Today Chem. 2024, 40, 102265. [Google Scholar] [CrossRef]
- Cheng, X.; Li, T.; Yan, L.; Jiao, Y.; Zhang, Y.; Wang, K.; Cheng, Z.; Ma, J.; Shao, L. Biodegradable electrospinning superhydrophilic nanofiber membranes for ultrafast oil-water separation. Sci. Adv. 2023, 9, eadh8195. [Google Scholar] [CrossRef]
- Krasian, T.; Punyodom, W.; Worajittiphon, P. A hybrid of 2D materials (MoS2 and WS2) as an effective performance enhancer for poly(lactic acid) fibrous mats in oil adsorption and oil/water separation. Chem. Eng. J. 2019, 369, 563–575. [Google Scholar] [CrossRef]
- Torsello, M.; Ben-Zichri, S.; Pesenti, L.; Kunnath, S.M.; Samorí, C.; Pasteris, A.; Bacchelli, G.; Prishkolnik, N.; Ben-Nun, U.; Righi, S.; et al. Carbon dot/polylactic acid nanofibrous membranes for solar-mediated oil absorption/separation: Performance, environmental sustainability, ecotoxicity and reusability. Heliyon 2024, 10, e25417. [Google Scholar] [CrossRef]
- Mo, J.; Wang, Y.; Wang, J.; Zhao, J.; Ke, Y.; Han, S.; Gan, F.; Wang, L.; Ma, C. Hydrophobic/oleophilic polylactic acid electrospun fibrous membranes with the silicone semi-interpenetrated networks for oil–water separation. Polym. Biopolym. 2022, 57, 16048–16063. [Google Scholar]
- Yang, J.; Li, F.; Lu, G.; Lu, Y.; Song, C.; Zhou, R.; Wu, S. Electrospun Biodegradable Poly(L-lactic acid) Nanofiber Membranes as Highly Porous Oil Sorbent Nanomaterials. Nanomaterials 2022, 12, 2670. [Google Scholar] [CrossRef]
- Jiang, C.; Hua, M.; Mu, G.; Zhao, S.; Chen, H.; Yao, L.; Ge, J.; Zhang, L.; Pan, G. Superhydrophilic and underwater superoleophobic polylactide/cellulose diacetate composite nanofibrous membranes for effective oil-in-water emulsions separation. Sep. Purif. Technol. 2024, 348, 127806. [Google Scholar] [CrossRef]
- Wu, H.Z.; Geng, Q.; Li, Y.H.; Song, Y.Q.; Chu, J.Q.; Zhou, R.; Ning, X.; Dong, S.J.; Yuan, D. CuMOF-decorated biodegradable nanofibrous membrane: Facile fabrication, high-efficiency filtration/separation and effective antibacterial property. J. Ind. Eng. Chem. 2022, 114, 475–482. [Google Scholar] [CrossRef]
- Jeeshma, R.; Lakshmi, V.V.A.; James, A.; Stephen, R. Polyhedral Oligomeric Silsesquioxane Coated Electrospun Nanofibrous PLA Membranes: Properties and Application. J. Polym. Environ. 2024, 32, 5982–5993. [Google Scholar] [CrossRef]
- Li, X.H.; Zhang, S.L.; Li, J.Z.; Hu, X.Y.; Zhang, J.J.; Wei, Q.; Zhang, C.P.; Zhang, D.D.; Liu, Y. Bio-degradable fibrous membranes for oil/water separation by melt electrospinning. J. Appl. Polym. Sci. 2024, 141, e56018. [Google Scholar] [CrossRef]
- Zhang, D.; Jin, X.Z.; Huang, T.; Zhang, N.; Qi, X.D.; Yang, J.H.; Zhou, Z.W.; Wang, Y. Electrospun Fibrous Membranes with Dual-Scaled Porous Structure: Super Hydrophobicity, Super Lipophilicity, Excellent Water Adhesion, and Anti-Icing for Highly Efficient Oil Adsorption/Separation. ACS Appl. Mater. Interfaces 2019, 11, 5073–5083. [Google Scholar] [CrossRef] [PubMed]
- Li, K.X.; Feng, G.N.; Li, G.M.; Zhang, Z.B.; Xiang, J.H.; Jiao, F.; Chen, T.; Zhao, H.L. Structurally integrated janus polylactic acid fibrous membranes for oil-water separation. J. Water Process Eng. 2024, 68, 106525. [Google Scholar] [CrossRef]
- Liu, W.; Wu, X.; Liu, S.; Cheng, X.; Zhang, C. CNT@LDH functionalized poly(lactic acid) membranes with super oil–water separation and real-time press sensing properties. Polym. Compos. 2022, 43, 6548–6559. [Google Scholar] [CrossRef]
- Nugraha, M.W.; Wirzal, M.D.H.; Ali, F.; Roza, L.; Sambudi, N.S. Electrospun polylactic acid/tungsten oxide/amino-functionalized carbon quantum dots (PLA/WO/N-CQDs) fibers for oil/water separation and photocatalytic decolorization. J. Environ. Chem. Eng. 2021, 9, 106033. [Google Scholar] [CrossRef]
- Deng, Y.F.; Zhang, N.; Huang, T.; Lei, Y.Z.; Wang, Y. Constructing tubular/porous structures toward highly efficient oil/water separation in electrospun stereocomplex polylactide fibers via coaxial electrospinning technology. Appl. Surf. Sci. 2022, 573, 151619. [Google Scholar] [CrossRef]
- Guo, X.; Feng, C.; Huang, L.; Wang, H.; Liu, F.; Li, J. Superhydrophobic PBAT/PLA Fibrous Membrane with Excellent Mechanical Performance for Highly Efficient Oil–Water Separation. Fibers Polym. 2025, 26, 1479–1492. [Google Scholar] [CrossRef]
- Yu, W.T.; Song, X.F.; Wang, Y.H.; Zhang, L.K.; Liu, Y.; Liu, Y.H. Enhancing the oil/water separation efficiency of polylactic acid fiber membrane via polydimethylsiloxane-polycaprolactone copolymer. J. Environ. Chem. Eng. 2024, 12, 114738. [Google Scholar] [CrossRef]
- Xu, Q.Y.; Wang, Y.M. The Effects of Processing Parameter on Melt-Blown Filtration Materials. Adv. Mater. Res. 2013, 650, 78–84. [Google Scholar] [CrossRef]
- Li, H.; Zhang, H.; Hu, J.J.; Wang, G.F.; Cui, J.Q.; Zhang, Y.F.; Zhen, Q. Facile Preparation of Hydrophobic PLA/PBE Micro-Nanofiber Fabrics via the Melt-Blown Process for High-Efficacy Oil/Water Separation. Polymers 2022, 14, 1667. [Google Scholar] [CrossRef] [PubMed]
- Sayed, M.M.; Mousa, H.M.; El-Shazly, A.H.; Zkria, A.; Yoshitake, T.; ElKady, M. Novel post-heat treatment green biodegradable PLA@SiO2 nanocomposite membrane for water desalination. J. Environ. Chem. Eng. 2024, 12, 114378. [Google Scholar] [CrossRef]
- Scaffaro, R.; Lopresti, F.; Sutera, A.; Botta, L.; Fontana, R.M.; Gallo, G. Plasma modified PLA electrospun membranes for actinorhodin production intensification in Streptomyces coelicolor immobilized-cell cultivations. Colloids Surf. B Biointerfaces 2017, 157, 233–241. [Google Scholar] [CrossRef]
- Shi, J.; Zhang, L.; Xiao, P.; Huang, Y.; Chen, P.; Wang, X.; Gu, J.; Zhang, J.; Chen, T. Biodegradable PLA Nonwoven Fabric with Controllable Wettability for Efficient Water Purification and Photocatalysis Degradation. ACS Sustain. Chem. Eng. 2018, 6, 2445–2452. [Google Scholar] [CrossRef]
- Karthikeyan, A.; Girard, M.; Dumont, M.-J.; Chouinard, G.; Tavares, J.R. Surface Modification of Commercially Available PLA Polymer Mesh. Ind. Eng. Chem. Res. 2022, 61, 17297–17305. [Google Scholar] [CrossRef]
- Lu, J.; Wen, J.X.; Yu, Q.H.; Cui, C.F.; Su, J.J.; Han, J. Cellulose nanospheres coated polylactic acid nonwoven membranes for recyclable use in oil/water separation. Cellulose 2021, 28, 11417–11427. [Google Scholar] [CrossRef]
- Gu, J.; Xiao, P.; Chen, P.; Zhang, L.; Wang, H.; Dai, L.; Song, L.; Huang, Y.; Zhang, J.; Chen, T. Functionalization of Biodegradable PLA Nonwoven Fabric as Superoleophilic and Superhydrophobic Material for Efficient Oil Absorption and Oil/Water Separation. ACS Appl. Mater. Interfaces 2017, 9, 5968–5973. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.C.; Guo, J.; Xu, H.; Zhang, J.L.; Hu, N.N.; Liu, H.T. One-step fabrication of biodegradable superhydrophobic PLA fabric for continuous oil/water separation. Appl. Surf. Sci. 2022, 576, 151766. [Google Scholar] [CrossRef]
- Ren, D.; Guo, Z.; Song, W.; Guo, Z.; Liu, H.; Huang, M.; Liu, W. Modification of Poly(Lactic Acid) Non-Woven Fabric for Enhanced Oil–Water Separation. Fibers Polym. 2024, 25, 1727–1736. [Google Scholar] [CrossRef]
- Yuan, H.; Zhang, M.; Pan, Y.; Liu, C.; Shen, C.; Chen, Q.; Liu, X. Microspheres Modified with Superhydrophobic Non-Woven Fabric with High-Efficiency Oil–Water Separation: Controlled Water Content in PLA Solution. Macromol. Mater. Eng. 2022, 307, 2100919. [Google Scholar] [CrossRef]
- Lu, J.; Cui, C.F.; Yu, Q.H.; Su, J.J.; Han, J. Robustly superhydrophobic polylactic acid nonwoven membranes for efficient oil/water separation. J. Porous Mater. 2022, 29, 241–247. [Google Scholar] [CrossRef]
- Li, B.; Zhao, G.Q.; Wang, G.L.; Zhang, L.; Gong, J.; Shi, Z.L. Biodegradable PLA/PBS open-cell foam fabricated by supercritical CO foaming for selective oil-adsorption. Sep. Purif. Technol. 2021, 257, 117949. [Google Scholar] [CrossRef]
- Wang, S.; Yang, W.; Li, X.; Hu, Z.; Wang, B.; Li, M.; Dong, W. Preparation of high-expansion open-cell polylactic acid foam with superior oil-water separation performance. Int. J. Biol. Macromol. 2021, 193 Pt B, 1059–1067. [Google Scholar] [CrossRef]
- Li, D.; Zhang, S.; Zhao, Z.; Miao, Z.; Zhang, G.; Shi, X. High-Expansion Open-Cell Polylactide Foams Prepared by Microcellular Foaming Based on Stereocomplexation Mechanism with Outstanding Oil-Water Separation. Polymers 2023, 15, 1984. [Google Scholar] [CrossRef]
- Yuan, J.; Gao, X.; Chen, Y.; Zhao, L.; Hu, D. Green Fabrication of Biobased and Degradable Poly(lactic acid)/Poly(butylene succinate) Open-Cell Foams for Highly Efficient Oil–Water Separation with Ultrafast Degradation. ACS Sustain. Chem. Eng. 2024, 12, 18101–18113. [Google Scholar] [CrossRef]
- Zeng, Q.T.; Ma, P.M.; Lai, D.H.; Lai, X.J.; Zeng, X.R.; Li, H.Q. Superhydrophobic reduced graphene oxide@poly(lactic acid) foam with electrothermal effect for fast separation of viscous crude oil. J. Mater. Sci. 2021, 56, 11266–11277. [Google Scholar] [CrossRef]
- Cui, S.; Wu, M.; Xu, M.; Li, X.; Ren, Q.; Wang, L.; Zheng, W. Supercritical CO2 extrusion foaming of highly open-cell poly(lactic acid) foam with superior oil adsorption performance. Int. J. Biol. Macromol. 2024, 269 Pt 2, 132138. [Google Scholar] [CrossRef]
- Wang, X.; Pan, Y.; Liu, X.; Liu, H.; Li, N.; Liu, C.; Schubert, D.W.; Shen, C. Facile Fabrication of Superhydrophobic and Eco-Friendly Poly(lactic acid) Foam for Oil-Water Separation via Skin Peeling. ACS Appl. Mater. Interfaces 2019, 11, 14362–14367. [Google Scholar] [CrossRef] [PubMed]
- Hua, M.Q.; Chen, D.; Xu, Z.S.; Fang, Y.Q.; Song, Y.M. Fabrication of high-expansion, fully degradable polylactic acid-based foam with exponent oil/water separation. J. Appl. Polym. Sci. 2022, 139, e53234. [Google Scholar] [CrossRef]
- Chen, P.F.; Bai, D.Y.; Tang, H.; Liu, H.L.; Wang, J.C.; Gao, G.Y.; Li, L. Polylactide aerogel with excellent comprehensive performances imparted by stereocomplex crystallization for efficient oil-water separation. Polymer 2022, 255, 125128. [Google Scholar] [CrossRef]
- Liu, H.L.; Chen, P.F.; Peng, Q.; Bai, D.Y. Ultralight, superhydrophobic and biodegradable stereocomplex polylactide aerogel with superior oil-water separation and thermal insulation performances. Ind. Crops Prod. 2024, 222, 119983. [Google Scholar] [CrossRef]
- Xu, J.; Seeger, S. Silicone nanofilament embedded, superhydrophobic polylactic acid composite aerogel. Chem. Eng. J. 2025, 507, 160208. [Google Scholar] [CrossRef]
- Qu, W.; Wang, Z.; Wang, X.; Wang, Z.; Yu, D.; Ji, D. High-hydrophobic ZIF-67@PLA honeycomb aerogel for efficient oil–water separation. Colloids Surf. A Physicochem. Eng. Asp. 2023, 658, 130768. [Google Scholar] [CrossRef]
- Fily, Y.; Marchetti, M.C. Athermal phase separation of self-propelled particles with no alignment. Phys. Rev. Lett. 2012, 108, 235702. [Google Scholar] [CrossRef]
- Lloyd, D.R.; Kim, S.S.; Kinzer, K.E. Microporous membrane formation via thermally-induced phase separation. II. Liquid—Liquid phase separation. J. Membr. Sci. 1991, 64, 1–11. [Google Scholar] [CrossRef]
- Guillen, G.R.; Pan, Y.; Li, M.; Hoek, E.M.V. Preparation and Characterization of Membranes Formed by Nonsolvent Induced Phase Separation: A Review. Ind. Eng. Chem. Res. 2011, 50, 3798–3817. [Google Scholar] [CrossRef]
- Fernandez-Rico, C.; Sai, T.; Sicher, A.; Style, R.W.; Dufresne, E.R. Putting the Squeeze on Phase Separation. JACS Au 2022, 2, 66–73. [Google Scholar] [CrossRef]
- Li, L.; Li, X.; Wu, H.; Chen, Y.; Zhang, J. Tailoring the cell morphology and characteristics of poly(lactic acid) foams via a stereocomplex crystallization-assisted phase separation strategy. Int. J. Biol. Macromol. 2025, 308 Pt 3, 142583. [Google Scholar] [CrossRef]
- Sun, X.; Wang, K.; Meng, Y.; Hu, K.; Huang, C.; He, Q.; Sun, J.; Bai, L.; Zhang, C.; Ma, Z. Fabrication of Poly(lactic acid)/Glycerol–Poly(ε-caprolactone)–Poly(d-lactic acid) Foam by Star Copolymer-Induce Stereocomplexed Microcrystalline Network for Oil–Water Separation. ACS Appl. Polym. Mater. 2025, 7, 331–342. [Google Scholar] [CrossRef]
- Rezabeigi, E.; Wood-Adams, P.M.; Drew, R.A.L. Production of porous polylactic acid monoliths via nonsolvent induced phase separation. Polymer 2014, 55, 6743–6753. [Google Scholar] [CrossRef]
- Dong, X.; Lu, D.; Harris, T.A.L.; Escobar, I.C. Polymers and Solvents Used in Membrane Fabrication: A Review Focusing on Sustainable Membrane Development. Membranes 2021, 11, 309. [Google Scholar] [CrossRef]
- Xin, Y.; Fujimoto, T.; Uyama, H. Facile fabrication of polycarbonate monolith by non-solvent induced phase separation method. Polymer 2012, 53, 2847–2853. [Google Scholar] [CrossRef]
- Ramachandran, J.; Thomas, S.P.; Thomas, S.; Stephen, R. Effect of annealing on the morphology and properties of poly(lactic acid)/polyhedral oligomeric silsesquioxane asymmetric porous membranes prepared through non-solvent induced phase separation and its application. Polym. Adv. Technol. 2024, 35, e6328. [Google Scholar] [CrossRef]
- Ma, Y.; Shao, T.; Niu, Q.; Jilili, Y.; Zhen, W. Superhydrophobic poly(lactic acid) membrane prepared with the induction of modified carbon dots for efficient separation of water-in-oil emulsions. Int. J. Biol. Macromol. 2024, 280 Pt 3, 136001. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Uyama, H.; Wang, X.; Sun, X. A green and facile approach to prepare polylactide/kapok monoliths for a sustainable and reusable oil sorbent. Colloid Polym. Sci. 2023, 301, 1261–1270. [Google Scholar] [CrossRef]
- Su, Y.; Zhao, Y.; Zheng, W.; Yu, H.; Liu, Y.; Xu, L. Asymmetric Sc-PLA Membrane with Multi-scale Microstructures: Wettability, Antifouling, and Oil-Water Separation. ACS Appl. Mater. Interfaces 2020, 12, 55520–55526. [Google Scholar] [CrossRef]
- Abuhantash, F.; Hegab, H.M.; Aljundi, I.H.; Hasan, S.W. Synergistic design of polylactic acid/functionalized multi-walled carbon nanotubes composite membrane for enhanced oil-water separation. J. Environ. Chem. Eng. 2023, 11, 111566. [Google Scholar] [CrossRef]
- Ghadhban, M.Y.; Rashid, K.T.; Abdulrazak, A.A.; Ibrahim, I.T.; Alsalhy, Q.F.; Shakor, Z.M.; Hamawand, I. Modification of Polylactide-poly (butylene adipate-co-terephthalate) (PLA/PBAT) Mixed-Matrix Membranes (MMMs) with Green Banana Peel Additives for Oil Wastewater Treatment. Water 2024, 16, 1040. [Google Scholar] [CrossRef]
- Deville, S. Freeze-Casting of Porous Biomaterials: Structure, Properties and Opportunities. Materials 2010, 3, 1913–1927. [Google Scholar] [CrossRef]
- Santos, L.N.R.M.; Silva, J.R.S.; Cartaxo, J.M.; Rodrigues, A.M.; Neves, G.A.; Menezes, R.R. Freeze-casting applied to ceramic materials: A short review of the influence of processing parameters. Cerâmica 2021, 67, 1–13. [Google Scholar] [CrossRef]

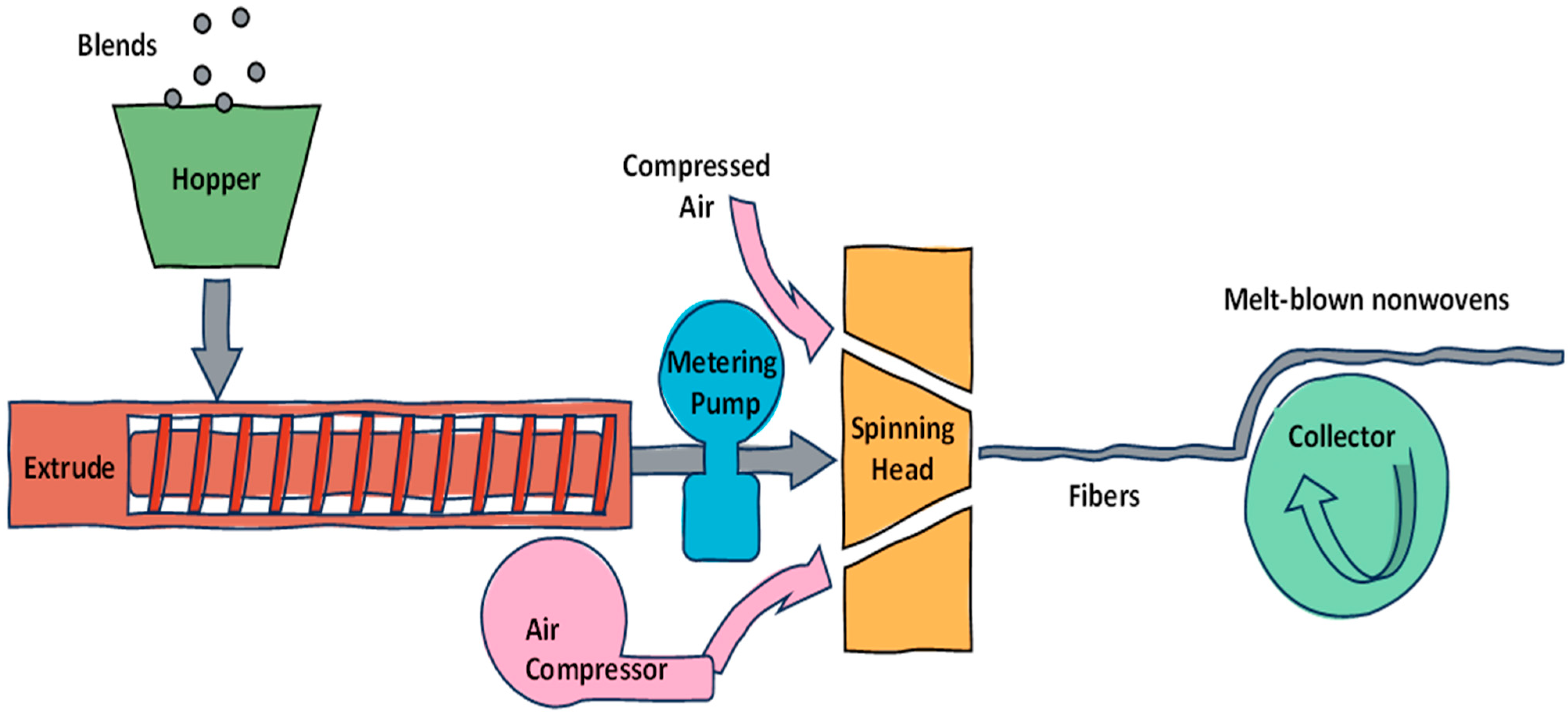


| Technology Term | Secondary Category | Tertiary Category | Typical Components | Advantages | Ref. |
|---|---|---|---|---|---|
| Membrane Materials | Inorganic Porous Materials | Ceramics | AlO3/ZrO2/TiO2/SiO2 | Thermally stable Chemically resistant | [37] |
| Carbon-based Materials | CNT/GO | High surface area Low density Excellent mechanical performance Chemically resistant Eco-friendly Large pore volume | [38] | ||
| Metal–Organic Framework Membranes (MOFs) | Highly crystalline solid compounds High surface area Adjustability of the structure Unique functionality caused by flexibility Rich in physical and chemical functions Easy modifiability | [39] | |||
| Organic Porous Materials | Polymeric Membranes | PVDF/PES/PSF/PP/PAN/ PLA/PE/PEG/PI/PTFE | High efficiency Emulsified and dispersed oil Small size Low energy requirements Cheap | [40] | |
| Aerogels/ Foams | Polymer-based aerogels Biomass-based aerogels Inorganic-based aerogels Carbon-based aerogels | Adjustability of the structure | [41] | ||
| Hybrid Materials | Polymer + SiO2/TiO2 nanoparticles Polymeric Membranes + GO/MOF coating Sponge + Fe3O4/GO coating | Integrating multiple advantages | |||
| Natural Porous Materials | Cellulose/CA/Chitosan/Sodium alginate | Easy processing Utilization Cheap High porosity | [42] | ||
| Polymer | Features | Ref. |
|---|---|---|
| Polysulfone (PSF) | High porosity, high flux and excellent mechanical properties Highly resistant to mineral acids, alkali, and electrolytes; resistant to oxidizing agents; resistant to surfactants and hydrocarbon oils; easily applicable for the conventional phase inversion processes; and able to modify the properties through blending with other polymers Hydrophobic, tendency to contaminate | [44,45,46] |
| Polyvinylidene difluoride (PVDF) | Hydrophilic Hydrophobic, low surface energy, outstanding chemical and thermal stability, environmentally sustainable and reusable. Forms isotropic, spherulitic microstructures that often include large macro voids | [47,48,49] |
| Polyether- sulfone (PES) | Hydrophobic, thermal stability and chemical resistance Small flux and poor mechanical properties less flexibility and expensive | [50,51,52] |
| Polypropylene (PP) | Exceptional chemical stability, filtration efficiency, and mechanical properties; large pore size Low cost, low water uptake, excellent mechanical robustness and excellent physical and chemical resistance | [53,54] |
| Polyimide (PI) | Excellent mechanical strength, flexibility, and stability Excellent corrosion resistance and strong separation performance | [55,56] |
| Polyacrylonitrile (PAN) | High hydrophilic property, rich chemical modification spots, easy obtainment of the raw material and low cost Thermal stability Good stability in solvents, excellent mechanical and film-forming properties | [57,58,59] |
| Polyvinyl pyrrolidone (PVP) | Hydrophilic, abundant carbonyl functional groups, nontoxic and biocompatible Facility at forming complexes, strong adhesiveness, and resistance to thermal degradation in solution | [60,61] |
| Polylactic acid (PLA) | Derived from the fermentation of starch, it can be obtained from cellulose, kitchen waste, or fish waste as raw materials Excellent biodegradability Eventually completely degraded into CO2 and H2O, sustainable Limited oil adsorption capacity, low adsorption selectivity, and poor mechanical properties | [31,57,62] |
| Cellulose | Abundance, low cost, environmental protection, biodegradability, and sustainability Improved water retention, water absorption, antibacterial, and thermal properties, nontoxic, degraded by microorganisms into natural nontoxic compounds Low flux Hydrophilicity, high specific surface area, and high mechanical strength, pore size limitation of supporting membranes, restricted the formation of a uniform structure | [63,64,65,66] |
| Chitosan | Low cost, environment-friendly, biodegradable, and abundant sources, special wettability, poor chemical modification ability, and harsh modification conditions | [67] |
| Membrane/Additives | Fiber Diameter | Pore Size | Porosity (%) | Water Contact Angle * (°) | Oil Contact Angle (°) | Separation Efficiency (%) | Separation Flux (L/m2h) | Static Water Contact Angle Under Oil (°) | Oil Takes (g/g) /Absorption Capacity (g/g) | Ra (μm) | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| PLA | 1.2 μm | 81.2 | 96.1 | 0 | Motor oil: 7.75 ± 0.46 | 0.759 ± 0.252 | [69] | ||||
| PLA-MoS2(3 phr) | 911 ± 156 nm | 83.8 | 104.6 | Motor oil: 14.66 ± 0.92 | 0.264 ± 0.025 | ||||||
| PLA-WS2(2 phr) | 1447 ± 316 nm | 86.2 | 108.8 | Motor oil: 15.65 ± 1.24 | 0.351 ± 0.029 | ||||||
| PLA-MoS2-WS2 (1 phr/1 phr) | 1649 ± 406 nm | 88.2 | 121.4 | 94.68–97.17% | 390–762 | Motor oil: 22.45 ± 0.15 | 0.416 ± 0.071 | ||||
| PLA | 1.41 μm | 110.52 | >95% | Peanut oil: 31.09 Motor oil: 25.61 Silicone oil: 32.65 | [71] | ||||||
| PLA(1 g)–Vinyltrimethoxysilane(0.1 g) | 0.71 μm | >95% | Peanut oil: 60.20 | ||||||||
| PLA(1 g)–Vinyltrimethoxysilane(0.25 g) | |||||||||||
| PLA(1 g)–Vinyltrimethoxysilane(0.5 g) | 0.62 μm | Sunflower oil: 86.90 Motor oil: 52.11 Silicone oil: 64.42 | |||||||||
| PLA(1 g)–Aminopropyltrimethoxysilane (0.1 g) | 0.22 μm | 103.63 | 136.56 ** | Peanut oil: 24.09 Motor oil: 24.15 | |||||||
| PLA(1 g)–Aminopropyltrimethoxysilane (0.25 g) | 125.25 | ||||||||||
| PLA(1 g)–Aminopropyltrimethoxysilane (0.5 g) | 104.67 | 162.58 ** | |||||||||
| PLA | 770 ± 330 nm | Silicon oil: 20 times deadweight | [70] | ||||||||
| PLA–Carbon dot (3.5 w.%) | 740±420 nm | Higher than PLA | |||||||||
| PLA–Carbon dot (21.5 w.%) | 620±280 nm | Low than PLA | Silicon oil: 40 times deadweight | ||||||||
| PLA–Carbon dot (37.0 w.%) | 720±280 nm | Low than PLA | |||||||||
| PLA | ~700 nm | ~72% | 131.8 | [74] | |||||||
| PLA-CuMOF (1.5 w.%) | ~500 nm | 134.5 | |||||||||
| PLA-CuMOF (3 w.%) | ~480 nm | 138.1 | |||||||||
| PLA-CuMOF (4.5 w.%) | ~400 nm | ~83% | 140 | 99.47% | |||||||
| PLA-CuMOF (6 w.%) | ~500 nm | 136.5 | |||||||||
| PLA (8 wt./v%) | 237 nm | [75] | |||||||||
| PLA (10 wt./v%) | 489 nm | ||||||||||
| PLA (15 wt./v%) | 695 nm | 117.2 | 70% | Hexane: 351.64 ± 86.58 | Palm oil: 60.45 Used engine oil: 66.03 | ||||||
| PLA (20 wt./v%) | 1.129 μm | ||||||||||
| PLA (15 wt./v%)- POSS (5 wt./v%) | 983 nm | 121.4 | 75% | Hexane: 338.62 ± 65.18 | Palm oil: 51.10 Used engine oil: 58.78 | ||||||
| PLA (15 wt./v%)- POSS (5 wt./v%) coating | 132.9 | Hexane: 325.68 ± 56.00 | Palm oil: 48.86 Used engine oil: 49.85 | ||||||||
| PLA | 6.47 μm | 71.67% | 122 | 10 *** | 84.55% | Diesel oil: 11.63 Peanut oil: 9.77 | [76] | ||||
| PLA-CNF (2%) | ~4.2 μm | 73.81% | 90.39% | Diesel oil: ~13 Peanut oil: ~10 | |||||||
| PLA-CNF (4%) | ~3.7 μm | 74.10% | 91.63% | Diesel oil: ~13.5 Peanut oil: ~11 | |||||||
| PLA-CNF (6%) | ~3 μm | 74.47% | 92.26% | Diesel oil: ~14 Peanut oil: ~11.5 | |||||||
| PLA-CNF (8%) | 2.35 μm | 73.46% | 91.95% | Diesel oil: ~14.3 Peanut oil: ~12.5 | |||||||
| PLA-CNF (10%) | 3.16 μm | 76.29% | 133.1 | 93.54% | Diesel oil: ~14.5 Peanut oil: ~13.5 | ||||||
| PLA-NaCl (2%) | ~5.8 μm | 74.46% | 109.2 | 87.42% | Diesel oil: ~12.5 Peanut oil: ~10.4 | ||||||
| PLA-NaCl (4%) | ~4.6 μm | 76.05% | 88.97% | Diesel oil: ~15 Peanut oil: ~12 | |||||||
| PLA-NaCl (6%) | ~3.4 μm | 77.70% | 88.73% | Diesel oil: ~14.5 Peanut oil: ~11.7 | |||||||
| PLA-NaCl (8%) | ~2.5 μm | 72.23% | 90.29% | Diesel oil: ~15.2 Peanut oil: ~11.5 | |||||||
| PLA-NaCl (10%) | 1.69 μm | 79.90% | 119.2 | 90.14% | Diesel oil: ~15.2 Peanut oil: ~14 | ||||||
| PLA | 86.5 | 133 | 0 **** | 17.7–104.9 | [77] | ||||||
| PLA-γ-Fe2O3 (6 wt.%) | 91.2 | 23–268.6 | |||||||||
| PLA-γ-Fe2O3 (8 wt.%) | 92.2 | ||||||||||
| PLA-γ-Fe2O3 (10 wt.%) | 92 | 143.1 | 0 **** | ||||||||
| PLA-γ-Fe2O3 (12 wt.%) | 90.7 | ||||||||||
| PLA-γ-Fe2O3 (14 wt.%) | 90.3 | ||||||||||
| PLA–γ-Fe2O3 (10 wt.%)–Glycine (0.25 wt.%) | 90.3 | 137.7 | |||||||||
| PLA–γ-Fe2O3 (10 wt.%)–Glycine 0.5 wt.%) | 92 | 148 | |||||||||
| PLA–γ-Fe2O3 (10 wt.%)–Glycine (1 wt.%) | 92.4 | 144.4 | |||||||||
| PLA–γ-Fe2O3 (10 wt.%)–Glycine (1.5 wt.%) | 92.6 | 140.4 | |||||||||
| PLA | 120.7 | [78] | |||||||||
| PLA-CNT (0.25 wt.%) | |||||||||||
| PLA-CNT (0.5 wt.%) | 2.41 μm | ||||||||||
| PLA-CNT (0.75 wt.%) | 2.81 μm | ||||||||||
| PLA-CNT (1 wt.%) | 3.71 μm | 133.9 | 1.274 | ||||||||
| PLA (75 wt.%)-CDA (25 wt.%) | 1.59 μm | 0 | 0.864 | ||||||||
| PLA (60 wt.%)-CDA (40 wt.%) | 1.46 μm | ||||||||||
| PLA (50 wt.%)-CDA (50 wt.%) | 1.99 μm | ||||||||||
| PLA | 773 nm | 1430 nm | 96.96 | Soybean oil: 21 | [79] | ||||||
| PLA-CNT@LDH (1 wt.%) | 764 nm | 1100 nm | 103.37 | Soybean oil: 25 | |||||||
| PLA-CNT@LDH (3 wt.%) | 403 nm | 539 nm | 109.31 | Soybean oil: 28 | |||||||
| PLA-CNT@LDH (5 wt.%) | 192 nm | 414 nm | 113.27 | Soybean oil: 32 | |||||||
| PLA-ACNT@LDH (1 wt.%) | 226 nm | 599 nm | 105.11 | Soybean oil: 27 | |||||||
| PLA-ACNT@LDH (3 wt.%) | 160 nm | 361 nm | 110.68 | Soybean oil: 30 | |||||||
| PLA-ACNT@LDH (5 wt.%) | 158 nm | 337 nm | 114.01 | Soybean oil: 35 | |||||||
| PLA | 1.125 μm | 113.7 | 0 ***** | Heptane: ~5600 Hexane: ~5000 Hexadecane: ~2600 | N-heptane: 18.94 N-hexane: 19.11 Hexadecane: 18.54 | [80] | |||||
| PLA-WO3 | 2.946 μm | 121.81 | 0 ***** | Heptane: ~6500 Hexane: ~6000 Hexadecane: ~2800 | |||||||
| PLA-WO3-N-CQDs EDTA | 8.149 μm | 123.33 | 0 ***** | Heptane: ~6600 Hexane: ~7200 Hexadecane: ~2800 | |||||||
| PLA-WO3-N-CQDs EDA | 1.848 μm | 132.37 | 0 ***** | Heptane: ~11,500 Hexane: ~8200 Hexadecane: ~5800 | N-heptane: 27.90 N-hexane: 35.72 Hexadecane: 32.51 | 25.72 nm | |||||
| PLA-γ-Fe2O3 80/30/17 | 3.655 μm | 90.9 | 135.4 | 151.8 ****** | Castor oil: ~150; Motor oil: ~135 Silicon oil: ~133; Corn oil: ~130 Soybean oil: ~100; Olive oil: ~105 Sunflower oil: ~98 | [81] | |||||
| PLA-γ-Fe2O3 70/20/17 | 138.06 | 154.24 ****** | Castor oil: ~160; Motor oil: ~145 Silicon oil: ~140; Corn oil: ~125 Soybean oil: ~110; Olive oil: ~98 Sunflower oil: ~95 | ||||||||
| PLA-γ-Fe2O3 60/15/17 | 139.06 | 151.38 ****** | Castor oil: ~200; Motor oil: ~175 Silicon oil: ~180; Corn oil: ~130 Soybean oil: ~140; Olive oil: ~105 Sunflower oil: ~110 | ||||||||
| PLA-γ-Fe2O3 40/10/17 | 2.217 μm | 95.6 | 141.8 | N-hexane: 57,324.8 | 156.2 ****** | Castor oil:219; Motor oil: ~200 Silicon oil: ~190; Corn oil: ~145 Soybean oil: ~140; Olive oil: ~135 Sunflower oil: ~120 | |||||
| PLA | 312 nm | ~50 | ~120 | ~98.5 | Oil–water mixture: ~2400 Water-in-oil emulsion: ~320 | [82] | |||||
| PLA-PBAT (0.05 g) | ~360 nm | ~46 | ~125 | ~99 | Oil–water mixture: ~290 Water-in-oil emulsion: ~490 | ||||||
| PLA-PBAT (0.1 g) | ~415 nm | ~43 | ~130 | ~99.3 | Oil–water mixture: ~3100 Water-in-oil emulsion: ~500 | ||||||
| PLA-PBAT (0.15 g) | ~450 nm | ~38 | ~136 | ~99.5 | Oil–water mixture: ~3800 Water-in-oil emulsion: ~520 | ||||||
| PLA-PBAT (0.2 g) | 560 nm | ~35 | ~155 | ~99.6 | Oil–water mixture: ~6000 Water-in-oil emulsion: ~555 | ||||||
| PLA-PBAT (0.35 g) | ~550 nm | ~33 | ~145 | ~99.6 | Oil–water mixture: ~4300 Water-in-oil emulsion: ~400 | ||||||
| PLA-PBAT (0.3 g) | 621 nm | ~33 | ~142 | ~99.3 | Oil–water mixture: ~3800 Water-in-oil emulsion: ~400 | ||||||
| PLA | 89.91 | 131.76 | 96.7 | N-hexane: 1978 ± 35 CCl4: 1721 ± 141 | [83] | ||||||
| PLA-PDMS/PCL (5 wt.%) | 92.76 | ||||||||||
| PLA-PDMS/PCL (10 wt.%) | 93.35 | ||||||||||
| PLA-PDMS/PCL (15 wt.%) | 93.91 | 155.1 | 0 ******* | 99.3 | N-hexane: 3550 ± 50 CCl4: 3302 ± 181 | ||||||
| PLA-PDMS/PCL (20 wt.%) | 93.07 |
| Membrane/Additives | Fiber Size | Water Contact Angle (°) * | Separation Efficiency (%) | Oil Takes /Absorption Capacity | Ref. |
|---|---|---|---|---|---|
| PLA | ~5.5 μm | 122.3 | 79 | 4.2 g/g | [62] |
| PLA-PBAT (2 wt.%) | ~6.2 μm | 125.1 | 82 | 4.5 g/g | |
| PLA-PBAT (4 wt.%) | ~2.3 μm | 128.5 | 88 | 5.18 g/g | |
| PLA-PBAT (6 wt.%) | 130.8 | 91 | 4.9 g/g | ||
| PLA-PBAT (8 wt.%) | ~9.5 μm | 132.0 | 94 | 4.7 g/g | |
| PLA-PBAT (10 wt.%) | 133.2 | 96 | 4.5 g/g | ||
| PLA | ~5.7 μm | 125 | 4.64 g/g | [85] | |
| PLA-PBE (5 wt.%) | ~5.7 μm | 130 | 4.83 g/g | ||
| PLA-PBE (10 wt.%) | ~5.0 μm | 134 | 4.69 g/g | ||
| PLA-PBE (15 wt.%) | ~3.0 μm | 132 | 5.96 g/g | ||
| PLA-PBE (20 wt.%) | ~5.0 μm | 130 | 6.22 g/g |
| Membrane/Additives | Pore Size | Porosity (%) | Expansion Ratio | Density (g/cm3) | Water Contact Angle * (°) | Separation Efficiency (%) | Separation Flux (L/m2h) | Oil Takes/Absorption Capacity | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| PLLA | 28.26 μm | 3.03 | 0.42 | [98] | |||||
| PLLA-8-s-PDLA-13K-3% | CCl4: 13.36 g/g Silicone Oil: 7.97 g/g | ||||||||
| PLLA-8-s-PDLA-13K-5% | 20.52 μm | 14.45 | 0.09 | ||||||
| PLLA-8-s-PDLA-15K-5% | 27.95 μm | 8.63 | 0.15 | CCl4: 6.82 g/g Silicone Oil: 3.37 g/g | |||||
| PLLA-8-s-PDLA-39K-3% | 23.55 μm | 13.5 | 0.10 | ||||||
| PLLA-8-s-PDLA-39K-5% | 14.6 μm | 7.59 | 0.17 | CCl4: 5.7 g/g Silicone Oil: 3.56 g/g | |||||
| PLA | ~89 | ~0.046 | ~126 | [102] | |||||
| PLA–water (3 mL) | ~90 | ~0.0445 | ~135 | ||||||
| PLA–water (4 mL) | 90.66 | 0.04396 | ~140 | ||||||
| PLA–water (3 mL)/peeling | ~141 | ||||||||
| PLA–water (4 mL)/peeling | 151 | 12–31 times its own weight | |||||||
| PLA | 61.7 μm | ~36 | ~0.035 | 99.4 | Ethyl Acetate: ~7.0 g/g | [103] | |||
| PLA–Alkaline lignin | 59.7 μm | ~37 | ~0.034 | 108.5 | Ethyl Acetate: 4.3 g/g | ||||
| PLA–m-Alkaline lignin | 73.2 μm | 40.17 | 0.0303 | 130.0 | Ethyl Acetate: 12.44 g/g | ||||
| PLA | 197 μm | ~60 | 69 | ~126 | ~10 g/g | [99] | |||
| PLA-PBS (3 wt.%) | 177 μm | ~58 | ~128 | ~12 g/g | |||||
| PLA-PBS (6 wt.%) | 139.1 μm | 82.1 | ~54 | 52 | 139 | 24.5 g/g | |||
| PLA-PBS (9 wt.%) | 296.8 μm | ~48 | ~118 | 18.8 g/g | |||||
| PLA | 115 °C: 58.2 μm 110 °C: 48.6 μm 105 °C: 17.5 μm | 115 °C: 110 °C: 105 °C: | 115 °C: ~58 110 °C: ~46 105 °C: ~17 | 113 | CCl4: 12 (g/g)/135 min | General: 1.6–10.1 g/g | [96] | ||
| PLA-PBS (10 wt.%) | 115 °C: 54.7 μm 110 °C: 57.1 μm 105 °C: 45.5 μm | 115 °C: ~55 110 °C: ~57 105 °C: ~47 | |||||||
| PLA-PBS (20 wt.%) | 115 °C: 43.6 μm 110 °C: 50.6 μm 105 °C: 47.3 μm | 115 °C: 97.7 | 115 °C: 43.6 110 °C: ~48 105 °C: ~50 | 118 | CCl4: 20 (g/g)/15 min | CCl4: 21.9 C2Cl4: 19.4 Ethyl Acetate:18.0 Peanut Oil: 12.7 N-Octane: 11.7 Cyclohexane:10.1 Silicone Oil: 7.9 | |||
| PLA-PBS (30 wt.%) | 115 °C: 5.3 μm 110 °C: 30.7 μm 105 °C: 27.2 μm | 115 °C: ~8 110 °C: ~28 105 °C: ~25 | |||||||
| PLA | 10–50 μm | 135.8 | [100] | ||||||
| PLA-GO (1 cycle) | 142.9 | ||||||||
| PLA-GO (2 cycle) | 146.2 | ||||||||
| PLA-GO (3 cycle) | 147.5 | ||||||||
| PLA-GO (4 cycle) | 150.6 | >96 | N-hexane: 8.11 g/g; Diethyl Ether: 8.58 g/g Cyclohexane: 9.72 g/g; Toluene: 10.41 g/g Carbon Tetrachloride: 19.21 g/g Petroleum Ether: 7.79 g/g | ||||||
| PLA-GO (5 cycle) | 148.2 | ||||||||
| PLA | 114.3 | [101] | |||||||
| PLA-TPU (15 wt.%) | |||||||||
| PLA-TPU (30 wt.%) | |||||||||
| PLA-TPU (50 wt.%) | 124.7 | CCl4: 18.8 g/g; Ethyl Acetate: 11.8 g/g Silicone Oil: 8.5 g/g; Soybean Oil: 8.4 g/g cyclohexane: 6.4 g/g; gasoline: 7.7 g/g diesel: 7.2 g/g; corn oil: 4.7 g/g |
| Membrane/Additives | Density | Pore Size | Porosity (%) | Water Contact Angle * (°) | Separation Efficiency (%) | Separation Flux (L/m2h) | Oil Takes /Absorption Capacity | Xc (%) | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| PLA | 68.53 mg/cm3 | 66.11 μm | 94.0 | 137.6 | [106] | ||||
| PLA-H20 (2 wt.%)-SNFs (20 wt.%) | 12.63 μm | 150.2 | |||||||
| PLA-H20 (4 wt.%)-SNFs (20 wt.%) | 9.72 μm | 156.7 | |||||||
| PLA-H20 (6 wt.%)-SNFs (20 wt.%) | 7.78 μm | 164.4 | |||||||
| PLA-H20 (8 wt.%)-SNFs (20 wt.%) | 48.08 mg/cm3 | 5.95 μm | 95.58 | 174.6 | |||||
| PLA | 19.3 nm | 132 | [57] | ||||||
| PLA-ZIF-8 (0.5 wt.%) | 18.6 nm | 131 | |||||||
| PLA-ZIF-8 (1 wt.%) | 17.1 nm | 145 | Near to 100% | Heptane–Water: 13 ** Carbon Tetrachloride–Water: 20 ** Pentane–Water: 7.1 ** N-hexane–Water: 35 ** | Highest | ||||
| PLA-ZIF-8 (2 wt.%) | 18.4 nm | 135 | |||||||
| PLA-ZIF-8 (3 wt.%) | 19.1 nm | 133 | |||||||
| PLA | 7.1260 nm | 107 | [107] | ||||||
| PLA-ZIF-67 (1 wt.%) | 6.8410 nm | 112 | |||||||
| PLA-ZIF-67 (2 wt.%) | 8.1275 nm | 117 | |||||||
| PLA-ZIF-67 (3 wt.%) | 5.8450 nm | 132 | Near 100% | Petroleum Ether–Water: 71.66 *** Carb Tetrachloride–Water: 119.46 *** Heptane–Water: 59.66 *** Cyclohexane–Water: 32.56 *** Isooctane–Water: 51.18 *** | highest: 15–30 times its own weight | ||||
| PLA-ZIF-67 (4 wt.%) | 7.3395 nm | 121 | |||||||
| PLLA-PDLA | 21.1 nm | 140.1 | Ethanol: 38.5 | Anhydrous Ethanol: 19.7 g/g | 44.2 | [105] | |||
| PLLA-PDLA-PEO (10 wt.%) | 24.2 nm | 96.40% | 145.4 | Ethanol: 43.7 | 46.9 | ||||
| PLLA-PDLA-PEO (20 wt.%) | 22.4 nm | 96.80% | 149.5 | Ethanol: 53.2 | 46.7 | ||||
| PLLA-PDLA-PEO (30 wt.%) | 23.1 nm | 97.00% | 153.2 | Ethanol: 70.7 | 45.9 | ||||
| PLLA-PDLA-PEO (40 wt.%) | 27.7 nm | 97.60% | 156.8 | Ethanol: 99.5 | 44.5 | ||||
| PLLA-PDLA-PEO (50 wt.%) | 24.2 nm | 97.90% | 160.7 | Ethanol: 154.0 | Anhydrous Ethanol: 29.9 g/g 25 times its own weight | 44.6 | |||
| PLLA | 52.18 μm | 118.2 | 16.92 g/g | 40.46 | [104] | ||||
| PLLA-PLDA (10 wt.%) | 54.39 μm | 124.5 | 18.55 g/g | 33.17 | |||||
| PLLA-PLDA (20 wt.%) | 71.60 μm | 128.3 | 19.1 g/g | 17.45 | |||||
| PLLA-PLDA (30 wt.%) | 54.54 μm | 131.1 | 20.15 g/g | 8.10 | |||||
| PLLA-PLDA (40 wt.%) | 37.92 μm | 136.7 | 20.53 g/g | 1.08 | |||||
| PLLA-PLDA (50 wt.%) | 28.11 μm | 140.1 | 20.94 g/g | 0 |
| Membrane/Additives | TIPS Method | Pore Size | Porosity (%) | Water Contact Angle * (°) | Oil Takes /Absorption Capacity | Xc (%) | Ref. |
|---|---|---|---|---|---|---|---|
| PLA | At −4 °C for 12 h, −80 °C for 72 h at 10 Pa | 80.34 m2/g | ~125 | [20] | |||
| PLA-H2O (2 mL) | ~130 | ||||||
| PLA-H2O (3 mL) | ~132 | ||||||
| PLA-H2O (4 mL) | 94.6 m2/g | 158 | Isooctane: ~15 g/g; Ethyl Acetate: ~20 g/g Acetone: ~12 g/g; Ethyl Alcohol: ~17 g/g P-xylene: ~16 g/g; Trie Thylamine: ~14 g/g Pump Oil: ~20 g/g; Engine Oil: ~15 g/g Phenixin: ~28 g/g; N-hexane: ~12 g/g Xylene: ~13.5 g/g; Cyclohexane: ~14 g/g | ||||
| PLLA/freeze | At −20 °C for 24 h | 50–100 μm | 111.4 | ~1.5 g/g | ~5 | [112] | |
| PLLA-PDLA (10 wt.%)/freeze | 30 μm | 116.2 | ~1.6 g/g | ~20 | |||
| PLLA-PDLA (30 wt.%)/freeze | 117.7 | ~1.7 g/g | ~45 | ||||
| PLLA-PDLA (50 wt.%)/freeze | 119.7 | ~1.8 g/g | ~57 | ||||
| PLLA/gelation | At 20 °C for 24 h, followed by freezing At −20 °C, followed freeze-drying | 14.5 μm | 66.9 | 98.3 | ~1.4 g/g | ~5 | |
| PLLA-PDLA (10 wt.%)/gelation | 9.3 μm, | 106.8 | ~1.5 g/g | ~20 | |||
| PLLA-PDLA (30 wt.%)/gelation | 4.0 μm | 128.0 | ~2.4 g/g | ~58 | |||
| PLLA-PDLA (50 wt.%)/gelation | 3.3 μm | >80 | 137.2 | ~2.5 g/g | ~62 | ||
| PDLA (50 chain lengths)-GLY | At −18 °C for 12 h, −80 °C for 24 h at 10 Pa | [113] | |||||
| PDLA (70 chain lengths)-GLY-PCL | 10–60 μm | 94.01 | 145.9 | 13.0 g/g | 21.22 | ||
| PDLA (50 chain lengths)-GLY-PCL | 92.86 | 142.2 | 12.8 g/g | 23.66 | |||
| PDLA (30 chain lengths)-GLY-PCL | 89.14 | 135.5 | 12.1 g/g | 35.9 |
| Membrane/Additives | NIPS Method | Pore Size | Porosity (%) | Water Contact Angle * (°) | Separation Efficiency (%) | Separation Flux (LMH/bar) | Oil Takes /Absorption Capacity | Xc (%) | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| PLA | Immersed in the coagulation bath filled with distilled water at ambient temperature | 1.04 μm | 26.43 | Castor Oil: 2.96 g/g Engine Oil: ~3.0 g/g | 21.16 | [117] | |||
| PLA-POSS (1 wt.%) | 0.82 μm | 17.52 | Castor Oil: 3.59 g/g Engine Oil: ~3.1 g/g | 27.59 | |||||
| PLA-POSS (3 wt.%) | 0.41 μm | 17.02 | Castor Oil: ~3.56 g/g Engine Oil: 3.05 g/g | 22.52 | |||||
| PLA/annealing | 0.81 μm | 15.94 | Castor Oil: 1.31 g/g Engine Oil: ~2.0 g/g | 84.54 | |||||
| PLA-POSS (1 wt.%)/annealing | 0.17 μm | 14.71 | Castor Oil: ~1.85 g/g Engine Oil: ~2.35 g/g | 42.12 | |||||
| PLA-POSS (3 wt.%)/annealing | 0.21 μm | 13.37 | Castor Oil: ~2.3 g/g Engine Oil: 1.81 g/g | 61.69 | |||||
| PLA | 1. Placed on the ice, and an equal volume of C2H5OH was slowly dripped in; 2. Placed in a container containing saturated CuSO4 (98% relative humidity) | 28.35 μm | 122 | 73.29 | N-Hexane: 7562.8 Kerosene: ~5000 | N-Hexane: ~0.95 g/g Kerosene: ~1.2 g/g | 38.25 | [118] | |
| PLA-Cys-CDs (1 wt.%) | 24.81 μm | 132 | N-Hexane: ~6800 Kerosene: ~4500 | N-Hexane: ~1.65 g/g Kerosene: ~2.2 g/g | 39.48 | ||||
| PLA-Cys-CDs (3 wt.%) | 23.74 μm | 140 | N-Hexane: ~6300 Kerosene: ~4000 | N-Hexane: ~1.55 g/g Kerosene: ~1.6 g/g | 42.75 | ||||
| PLA-Cys-CDs (5 wt.%) | 20.33 μm | 159 | >99.9 | N-Hexane: ~4900 Kerosene: 3157.4 | N-Hexane: ~1.45 g/g Kerosene: ~1.5 g/g | 47.86 | |||
| PLA-Cys-CDs (7 wt.%) | 18.32 μm | 151 | 99.98 | N-Hexane: ~5200 Kerosene: ~3500 | N-Hexane: ~1.2 g/g Kerosene: ~1.3 g/g | 46.12 | |||
| PLA (7 wt.%) | A certain amount of n-hexane was added dropwise as nonsolvent | 86.10 | ~6.1 g/g | [119] | |||||
| PLA (10 wt.%) | 76.60 | ~3.7 g/g | |||||||
| PLA (13 wt.%) | 83 | 128 | 5 μL/4 s | ~4.5 g/g | |||||
| PLA (15 wt.%) | 80.80 | ~4.1 g/g | |||||||
| PLA (13 wt.%)–Kapok (0.10 wt.%) | 0.09 m3/g | ~5.5 g/g | |||||||
| PLA (13 wt.%)–Kapok (0.18 wt.%) | 0.077 m3/g | 141 | 5 μL/0.68 s | ~6 g/g | |||||
| PLA (13 wt.%)–Kapok (0.27 wt.%) | 0.066 m3/g | ~7 g/g | |||||||
| PLA (7 wt.%)–Kapok (0.34 wt.%) | 0.079 m3/g | ~7.5 g/g | |||||||
| PLA (7 wt.%)–Kapok (0.34 wt.%)–NaCl | 0.248 m3/g | 17.34 g/g | |||||||
| PLLA-PLDA/400 μm | A coagulation bath containing deionized water | 109 | 33.66 | [120] | |||||
| PLLA-PLDA/500 μm | 129.5 | 32.78 | |||||||
| PLLA-PLDA/600 μm | 142.9 | 34.26 | |||||||
| PLLA-PLDA/600 μm (Peeling) | 151.9 | Cyclohexane: ~2 s Tetrachloromethane: ~4.3 s Pump Oil: ~3.8 s; Vegetable Oil: ~3.7 s | 34.26 | ||||||
| PLA | Submerged in a DI water coagulation bath for 24 h | 65 | 91 | 94.9 | Emulsion: 155 ** | [121] | |||
| PLA-PVP-MWCNT | ~78 | 85 | 95.1 | ||||||
| PLA-PVP-f-MWCNTs (0.5 wt.%) | ~77 | 79 | 100 | Emulsion: 281 ** | |||||
| PLA-PVP-f-MWCNTs (1 wt.%) | ~80 | <79 | 98.9 | Water: 485 Emulsion: 260 ** | |||||
| PLA-PVP-f-MWCNTs (2 wt.%) | 82 | 72 | 99.2 | Water: 370 | |||||
| PLA-PBAT (4 wt.%) | Immersed in water to facilitate its natural detachment from the glass surface | 23 nm | 50 | 73.71 | Pure Water: 62 Oil–water: 28.6 *** | [122] | |||
| PLA–PBAT–Banana Peel (0.0125 wt.%) | 25 nm | 58 | 50.8 | 93 | |||||
| PLA–PBAT–Banana Peel (0.025 wt.%) | ~27.5 nm | 61 | 41.3 | 93.3 | |||||
| PLA–PBAT–Banana Peel (0.05 wt.%) | 29 nm | 63 | 38.99 | 95.2 | Pure Water: 52.655 oil–water: 40 *** | ||||
| PLA–PBAT–Banana Peel (0.1 wt.%) | ~26 nm | 57 | 51.88 | 89.5 | Pure Water: 32.32 oil–water: 19.8 *** |
| Membrane/Additives | FSPS Method | Pore Size | Porosity (%) | Water Contact Angle * (°) | Separation Efficiency (%) | Separation Flux (L/m2h) | Oil Takes /AbsorptionCapacity | Ra | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| PLA | Casting | 25.86 μm | 51.01 | 117.80 | 23.4 nm | [31] | |||
| PLA | In low temperature (below the freezing point of DiOX) | 0.46 μm | 77.60 | 141.67 | 96.4 | 2923 | 78.4 nm | ||
| PLA (H20) | 1. In low temperature (below the freezing point of DiOX and DCM) 2. Solution impregnation | 145.73 | 177 nm | ||||||
| PLA (acetic acid) | 145.07 | 171 nm | |||||||
| PLA (H20:acetic acid = 1: 1) | 151.00 | 99.7 | 16,084 | n-hexane: 2.54 ± 0.55 g/g; petroleum ether: 2.93 ± 0.21 g/g ethyl acetate: 4.72 ± 0.28 g/g; methylbenzene: 5.37 ± 0.14 g/g | 338 nm | ||||
| PLA (DiOX: DCM = 10:0) | In low temperature (below the freezing point of DiOX and DCM) | 0.54 μm | 2923 | ||||||
| PLA (DiOX: DCM = 9:1) | 0.98 μm | 6266 | |||||||
| PLA (DiOX: DCM = 4:1) | 1.32 μm | 95.1 | 8287 | ||||||
| PLA (DiOX: DCM = 1:1) | 0.35 μm | 4278 | |||||||
| PLA (DiOX: DCM = 0:10) | 379 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, W.; Verma, A.; Martin, O.; Sharma, G.; García-Peñas, A. Advances in Membranes Based on PLA and Derivatives for Oil–Water Separation. Polymers 2025, 17, 3135. https://doi.org/10.3390/polym17233135
Liang W, Verma A, Martin O, Sharma G, García-Peñas A. Advances in Membranes Based on PLA and Derivatives for Oil–Water Separation. Polymers. 2025; 17(23):3135. https://doi.org/10.3390/polym17233135
Chicago/Turabian StyleLiang, Weijun, Akshay Verma, Olga Martin, Gaurav Sharma, and Alberto García-Peñas. 2025. "Advances in Membranes Based on PLA and Derivatives for Oil–Water Separation" Polymers 17, no. 23: 3135. https://doi.org/10.3390/polym17233135
APA StyleLiang, W., Verma, A., Martin, O., Sharma, G., & García-Peñas, A. (2025). Advances in Membranes Based on PLA and Derivatives for Oil–Water Separation. Polymers, 17(23), 3135. https://doi.org/10.3390/polym17233135








