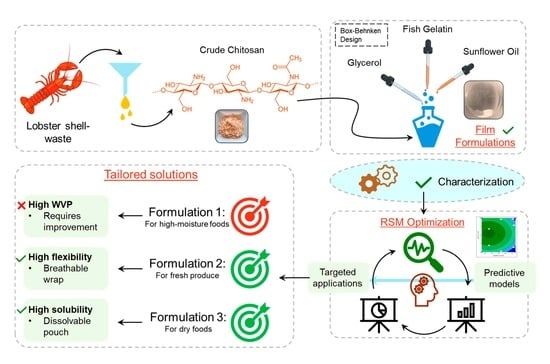
Development of Tailored Composite Biopolymer Film Formulations Using Minimally Refined Chitosan from American Lobster (Homarus americanus) Shell Waste for Different Food Packaging Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Experimental Design
| Run | Coded Variables | Uncoded Variables | ||||
|---|---|---|---|---|---|---|
| X1 | X2 | X3 | Ge (X1) (% w/w Polymer) | O (X2) (% w/w Polymer) | Gly (X3) (% w/w Polymer) | |
| 1 | −1 | −1 | 0 | 25 | 0 | 20 |
| 2 | +1 | −1 | 0 | 75 | 0 | 20 |
| 3 | −1 | +1 | 0 | 25 | 20 | 20 |
| 4 | +1 | +1 | 0 | 75 | 20 | 20 |
| 5 | −1 | 0 | −1 | 25 | 10 | 0 |
| 6 | +1 | 0 | −1 | 75 | 10 | 0 |
| 7 | −1 | 0 | +1 | 25 | 10 | 40 |
| 8 | +1 | 0 | +1 | 75 | 10 | 40 |
| 9 | 0 | −1 | −1 | 50 | 0 | 0 |
| 10 | 0 | +1 | −1 | 50 | 20 | 0 |
| 11 | 0 | −1 | +1 | 50 | 0 | 40 |
| 12 | 0 | +1 | +1 | 50 | 20 | 40 |
| 13 | 0 | 0 | 0 | 50 | 10 | 20 |
| 14 | 0 | 0 | 0 | 50 | 10 | 20 |
| 15 | 0 | 0 | 0 | 50 | 10 | 20 |
| 16 | 0 | 0 | 0 | 50 | 10 | 20 |
| 17 | 0 | 0 | 0 | 50 | 10 | 20 |
2.3. Preparation of the Films
2.4. Characterization of Prepared Films
2.4.1. Film Thickness (FT)
2.4.2. Equilibrated Moisture Content (EMC)
2.4.3. Degree of Swelling (DS) and Water Solubility (WS)
2.4.4. Water Vapor Permeability (WVP)
2.4.5. Surface Hydrophobicity
2.4.6. Film Opacity (OP)
2.4.7. Mechanical Properties
2.5. Simultaneous Optimization and Validation
2.6. Antimicrobial Testing
2.7. Statistical Analysis
3. Results and Discussion
3.1. RSM Model Fitting
3.2. Influence of Formulation Variables on Physical Properties
3.3. Influence of Formulation Variables on Water Vapour Permeability and Surface Hydrophobicity
3.4. Influence of Formulation Variables on Optical Properties
3.5. Influence of Formulation Variables on Mechanical Properties
3.6. Summary of Critical Film Formulation Trade-Offs
3.7. Optimization of Films and Validation of Regression Models for Specific Food Packaging Applications
3.8. Antimicrobial Activity of Optimized Films
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Plastics Europe, Plastics—The Fast Facts 2024. 2024. Available online: https://plasticseurope.org/wp-content/uploads/2024/11/PE_TheFacts_24_digital-1pager.pdf (accessed on 19 May 2025).
- Geyer, R. Production, use, and fate of synthetic polymers. In Plastic Waste and Recycling–Environemntal Impact, Societal Issues, Prevention, and Solution; Academic Press: Cambridge, MA, USA, 2020; pp. 13–32. [Google Scholar] [CrossRef]
- Barone, A.S.; Maragoni-Santos, C.; de Farias, P.M.; Cortat, C.M.G.; Maniglia, B.C.; Ongaratto, R.S.; Ferreira, S.; Fai, A.E.C. Rethinking single-use plastics: Innovations, polices, consumer awareness and market shaping biodegradable solutions in the packaging industry. Trends Food Sci. Technol. 2025, 158, 104906. [Google Scholar] [CrossRef]
- Arman Alim, A.A.; Mohammad Shirajuddin, S.S.; Anuar, F.H. A Review of Nonbiodegradable and Biodegradable Composites for Food Packaging Application. J. Chem. 2022, 2022, 7670819. [Google Scholar] [CrossRef]
- UNEP. From Pollution to Solution: A Global Assessment of Marine Litter and Plastic Pollution. 2021. Available online: https://wedocs.unep.org/bitstream/handle/20.500.11822/36965/POLSOLSum.pdf (accessed on 19 May 2025).
- Perera, K.Y.; Jaiswal, A.K.; Jaiswal, S. Biopolymer-Based Sustainable Food Packaging Materials: Challenges, Solutions, and Applications. Foods 2023, 12, 2422. [Google Scholar] [CrossRef]
- Elsabee, M.Z. Chitosan-Based Edible Films. In Polysaccharides; Ramawat, K.G., Mérillon, J.-M., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 829–870. [Google Scholar] [CrossRef]
- Barik, M.; BhagyaRaj, G.V.S.; Dash, K.K.; Shams, R. A thorough evaluation of chitosan-based packaging film and coating for food product shelf-life extension. J. Agric. Food Res. 2024, 16, 101164. [Google Scholar] [CrossRef]
- Jain, A.; Mason, B.; Brooks, M.S.L. Sustainable development of chitosan-gelatin composite films for food packaging using crude chitosan extracted from American lobster (Homarus americanus) shell waste. Carbohydr. Polym. Technol. Appl. 2025, 10, 100788. [Google Scholar] [CrossRef]
- Kiehbadroudinezhad, M.; Hosseinzadeh-Bandbafha, H.; Varjani, S.; Wang, Y.; Peng, W.; Pan, J.; Aghbashlo, M.; Tabatabaei, M. Marine shell-based biorefinery: A sustainable solution for aquaculture waste valorization. Renew. Energy 2023, 206, 623–634. [Google Scholar] [CrossRef]
- Ansorena, M.R.; Marcovich, N.E.; Pereda, M. Food Biopackaging Based on Chitosan. In Handbook of Ecomaterials; Springer International Publishing: Cham, Switzerland, 2018; pp. 1–27. [Google Scholar] [CrossRef]
- Cazón, P.; Vázquez, M. Mechanical and barrier properties of chitosan combined with other components as food packaging film. Environ. Chem. Lett. 2020, 18, 257–267. [Google Scholar] [CrossRef]
- Aider, M. Chitosan application for active bio-based films production and potential in the food industry: Review. LWT Food Sci. Technol. 2010, 43, 837–842. [Google Scholar] [CrossRef]
- Tagrida, M.; Nilsuwan, K.; Gulzar, S.; Prodpran, T.; Benjakul, S. Fish gelatin/chitosan blend films incorporated with betel (Piper betle L.) leaf ethanolic extracts: Characteristics, antioxidant and antimicrobial properties. Food Hydrocoll. 2023, 137, 108316. [Google Scholar] [CrossRef]
- Inbaraj, B.S.; Sridhar, K.; Sharma, M.; Giannakas, A.; Fauzan, H.R.; Ningrum, A.; Supriyadi, S. Evaluation of a Fish Gelatin-Based Edible Film Incorporated with Ficus carica L. Leaf Extract as Active Packaging. Gels 2023, 9, 918. [Google Scholar] [CrossRef]
- Hosseini, S.F.; Rezaei, M.; Zandi, M.; Ghavi, F.F. Preparation and functional properties of fish gelatin-chitosan blend edible films. Food Chem. 2013, 136, 1490–1495. [Google Scholar] [CrossRef]
- Ziani, K.; Oses, J.; Coma, V.; Maté, J.I. Effect of the presence of glycerol and Tween 20 on the chemical and physical properties of films based on chitosan with different degree of deacetylation. LWT Food Sci. Technol. 2008, 41, 2159–2165. [Google Scholar] [CrossRef]
- Maria, V.D.; Bernal, C.; Francois, N.J. Development of Biodegradable Films Based on Chitosan/Glycerol Blends Suitable for Biomedical Applications. J. Tissue Sci. Eng. 2016, 7, 1–9. [Google Scholar] [CrossRef]
- Cerqueira, M.A.; Souza, B.W.S.; Teixeira, J.A.; Vicente, A.A. Effect of glycerol and corn oil on physicochemical properties of polysaccharide films—A comparative study. Food Hydrocoll. 2012, 27, 175–184. [Google Scholar] [CrossRef]
- Andrade, M.; Sanches-Silva, A.T.; Moghadas, H.C.; Chauhan, R.; Smith, J.S. Application of Plant Oils as Functional Additives in Edible Films and Coatings for Food Packaging: A Review. Foods 2024, 13, 997. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, C.; Abugoch, L.; Tapia, C. Quinoa protein–chitosan–sunflower oil edible film: Mechanical, barrier and structural properties. LWT Food Sci. Technol. 2013, 50, 531–537. [Google Scholar] [CrossRef]
- Pereda, M.; Amica, G.; Marcovich, N.E. Development and characterization of edible chitosan/olive oil emulsion films. Carbohydr. Polym. 2012, 87, 1318–1325. [Google Scholar] [CrossRef]
- Cerqueira, M.A.; Souza, B.W.S.; Teixeira, J.A.; Vicente, A.A. Effects of Interactions between the Constituents of Chitosan-Edible Films on Their Physical Properties. Food Bioprocess Technol. 2012, 5, 3181–3192. [Google Scholar] [CrossRef]
- Branam, C. Water-Resistant, Antimicrobial Edible Wrap for Food Preservation, Food Safety Magazine. 2017. Available online: https://www.food-safety.com/articles/5461-water-resistant-antimicrobial-edible-wrap-for-food-preservation (accessed on 4 June 2025).
- Demircan, B.; McClements, D.J.; Velioglu, Y.S. Next-Generation Edible Packaging: Development of Water-Soluble, Oil-Resistant, and Antioxidant-Loaded Pouches for Use in Noodle Sauces. Foods 2025, 14, 1061. [Google Scholar] [CrossRef] [PubMed]
- Azarifar, M.; Ghanbarzadeh, B.; Khiabani, M.S.; Akhondzadeh Basti, A.; Abdulkhani, A.; Noshirvani, N.; Hosseini, M. The optimization of gelatin-CMC based active films containing chitin nanofiber and Trachyspermum ammi essential oil by response surface methodology. Carbohydr. Polym. 2019, 208, 457–468. [Google Scholar] [CrossRef]
- Yarahmadi, A.; Dousti, B.; Karami-Khorramabadi, M.; Afkhami, H. Materials based on biodegradable polymers chitosan/gelatin: A review of potential applications. Front. Bioeng. Biotechnol. 2024, 12, 1397668. [Google Scholar] [CrossRef] [PubMed]
- Tomadoni, B.; Ponce, A.; Pereda, M.; Ansorena, M.R. Vanillin as a natural cross-linking agent in chitosan-based films: Optimizing formulation by response surface methodology. Polym. Test. 2019, 78, 105935. [Google Scholar] [CrossRef]
- Homez-Jara, A.; Daza, L.D.; Aguirre, D.M.; Muñoz, J.A.; Solanilla, J.F.; Váquiro, H.A. Characterization of chitosan edible films obtained with various polymer concentrations and drying temperatures. Int. J. Biol. Macromol. 2018, 113, 1233–1240. [Google Scholar] [CrossRef]
- ASTM E96/E96M-16; Standard Test Methods for Water Vapor Transmission of Materials. ASTM International: West Conshohocken, PA, USA, 2016. [CrossRef]
- Khouri, J. Chitosan Edible Films Crosslinked by Citric Acid, University of Waterloo. 2019. Available online: https://uwspace.uwaterloo.ca/handle/10012/14877 (accessed on 20 September 2021).
- Pereda, M.; Ponce, A.G.; Marcovich, N.E.; Ruseckaite, R.A.; Martucci, J.F. Chitosan-gelatin composites and bi-layer films with potential antimicrobial activity. Food Hydrocoll. 2011, 25, 1372–1381. [Google Scholar] [CrossRef]
- ASTM D882-18; Standard Test Method for Tensile Properties of Thin Plastic Sheeting. ASTM International: West Conshohocken, PA, USA, 2018. [CrossRef]
- Fernandez-Saiz, P.; Lagarón, J.M.; Ocio, M.J. Optimization of the film-forming and storage conditions of chitosan as an antimicrobial agent. J. Agric. Food Chem. 2009, 57, 3298–3307. [Google Scholar] [CrossRef]
- Park, S.-I.; Daeschel, M.A.; Zhao, Y. Functional Properties of Antimicrobial Lysozyme-Chitosan Composite Films. J. Food Sci. 2004, 69, M215–M221. [Google Scholar] [CrossRef]
- Makanjuola, S.; Enujiugha, V.; Omoba, O.; Sanni, D. Application of RSM and Multivariate Statistics in Predicting Antioxidant Property of Ethanolic Extracts of Tea-Ginger Blend. Eur. J. Med. Plants 2015, 6, 200–211. [Google Scholar] [CrossRef]
- Herrera-vázquez, S.E.; Dublán-garcía, O.; Arizmendi-cotero, D.; Gómez-oliván, L.M.; Islas-flores, H.; Hernández-navarro, M.D.; Ramírez-durán, N. Optimization of the Physical, Optical and Mechanical Properties of Composite Edible Films of Gelatin, Whey Protein and Chitosan. Molecules 2022, 27, 869. [Google Scholar] [CrossRef]
- Singh, T.P.; Chatli, M.K.; Sahoo, J. Development of chitosan based edible films: Process optimization using response surface methodology. J. Food Sci. Technol. 2015, 52, 2530–2543. [Google Scholar] [CrossRef]
- Mohan, S.; Unnikrishnan, T.G.; Dubey, U.; Ramesh, M.; Panneerselvam, K. Development and Characterization of Mustard Oil Incorporated Biodegradable Chitosan Films for Active Food Packaging Applications. J. Polym. Environ. 2023, 31, 2190–2203. [Google Scholar] [CrossRef]
- Benbettaïeb, N.; Kurek, M.; Bornaz, S.; Debeaufort, F. Barrier, structural and mechanical properties of bovine gelatin-chitosan blend films related to biopolymer interactions. J. Sci. Food Agric. 2014, 94, 2409–2419. [Google Scholar] [CrossRef]
- Yao, Y.; Ding, D.; Shao, H.; Peng, Q.; Huang, Y. Antibacterial Activity and Physical Properties of Fish Gelatin-Chitosan Edible Films Supplemented with D-Limonene. Int J Polym Sci 2017, 2017, 1837171. [Google Scholar] [CrossRef]
- Eranda, D.H.U.; Chaijan, M.; Panpipat, W.; Karnjanapratum, S.; Cerqueira, M.A.; Castro-Muñoz, R. Gelatin-chitosan interactions in edible films and coatings doped with plant extracts for biopreservation of fresh tuna fish products: A review. Int. J. Biol. Macromol. 2024, 280, 135661. [Google Scholar] [CrossRef]
- Pereda, M.; Aranguren, M.I.; Marcovich, N.E. Water vapor absorption and permeability of films based on chitosan and sodium caseinate. J. Appl. Polym. Sci. 2009, 111, 2777–2784. [Google Scholar] [CrossRef]
- Rodríguez-Núñez, J.R.; Madera-Santana, T.J.; Sánchez-Machado, D.I.; López-Cervantes, J.; Soto Valdez, H. Chitosan/Hydrophilic Plasticizer-Based Films: Preparation, Physicochemical and Antimicrobial Properties. J. Polym. Environ. 2014, 22, 41–51. [Google Scholar] [CrossRef]
- Brudzyńska, P.; Sionkowska, A. The Effects of Shellac and Glycerol on the Physicochemical Properties of Chitosan Films. Polymers 2025, 17, 1298. [Google Scholar] [CrossRef] [PubMed]
- Trung, T.S.; Thein-Han, W.W.; Qui, N.T.; Ng, C.-H.; Stevens, W.F. Functional characteristics of shrimp chitosan and its membranes as affected by the degree of deacetylation. Bioresour. Technol. 2006, 97, 659–663. [Google Scholar] [CrossRef]
- Yeddes, W.; Djebali, K.; Wannes, W.A.; Horchani-Naifer, K.; Hammami, M.; Younes, I.; Tounsi, M.S. Gelatin-chitosan-pectin films incorporated with rosemary essential oil: Optimized formulation using mixture design and response surface methodology. Int. J. Biol. Macromol. 2020, 154, 92–103. [Google Scholar] [CrossRef]
- Kowalczyk, D.; Kazimierczak, W.; Zięba, E.; Lis, M.; Wawrzkiewicz, M. Structural and Physicochemical Properties of Glycerol-Plasticized Edible Films Made from Pea Protein-Based Emulsions Containing Increasing Concentrations of Candelilla Wax or Oleic Acid. Molecules 2024, 29, 5998. [Google Scholar] [CrossRef]
- Pérez-Mateos, M.; Montero, P.; Gómez-Guillén, M.C. Formulation and stability of biodegradable films made from cod gelatin and sunflower oil blends. Food Hydrocoll. 2009, 23, 53–61. [Google Scholar] [CrossRef]
- Wulandari, D.; Erwanto, Y.; Pranoto, Y.; Rusman, R. The properties of edible film derived from bovine split hide gelatin with isolated soy protein using various levels of glycerol in the presence of transglutaminase. Bul. Peternak. 2017, 41, 319–327. [Google Scholar] [CrossRef]
- Hopkins, E.J.; Chang, C.; Lam, R.S.H.; Nickerson, M.T. Effects of flaxseed oil concentration on the performance of a soy protein isolate-based emulsion-type film. Food Res. Int. 2015, 67, 418–425. [Google Scholar] [CrossRef]
- Patel, S.; Srivastava, S.; Singh, M.R.; Singh, D. Preparation and optimization of chitosan-gelatin films for sustained delivery of lupeol for wound healing. Int. J. Biol. Macromol. 2018, 107, 1888–1897. [Google Scholar] [CrossRef] [PubMed]
- Asghar, L.; Sahar, A.; Khan, M.I.; Shahid, M. Fabrication and Characterization of Chitosan and Gelatin-Based Antimicrobial Films Incorporated with Different Essential Oils. Foods 2024, 13, 1796. [Google Scholar] [CrossRef] [PubMed]
- González-López, M.E.; Calva-Estrada, S.D.J.; Gradilla-Hernández, M.S.; Barajas-Álvarez, P. Current trends in biopolymers for food packaging: A review. Front. Sustain. Food Syst. 2023, 7, 1225371. [Google Scholar] [CrossRef]
- Rodríguez, M.; Osés, J.; Ziani, K.; Maté, J.I. Combined effect of plasticizers and surfactants on the physical properties of starch based edible films. Food Res. Int. 2006, 39, 840–846. [Google Scholar] [CrossRef]
- Galus, S. Functional properties of soy protein isolate edible films as affected by rapeseed oil concentration. Food Hydrocoll. 2018, 85, 233–241. [Google Scholar] [CrossRef]
- Xu, J.; Wei, R.; Jia, Z.; Song, R. Characteristics and bioactive functions of chitosan/gelatin-based film incorporated with ε-polylysine and astaxanthin extracts derived from by-products of shrimp (Litopenaeus vannamei). Food Hydrocoll. 2020, 100, 105436. [Google Scholar] [CrossRef]
- Basiak, E.; Lenart, A.; Debeaufort, F. How Glycerol and Water Contents Affect the Structural and Functional Properties of Starch-Based Edible Films. Polymers 2018, 10, 412. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Nguyen, T.-T.T.; Van Tran, T.; Van Tan, L.; Danh, L.T.; Than, V.T.; Antibacterial, D.O. Antioxidant, and UV-Barrier Chitosan Film Incorporated with Piper betle Linn Oil as Active Biodegradable Packaging Material. Coatings 2021, 11, 351. [Google Scholar] [CrossRef]
- Haghighi, H.; Biard, S.; Bigi, F.; De Leo, R.; Bedin, E.; Pfeifer, F.; Siesler, H.W.; Licciardello, F.; Pulvirenti, A. Comprehensive characterization of active chitosan-gelatin blend films enriched with different essential oils. Food Hydrocoll. 2019, 95, 33–42. [Google Scholar] [CrossRef]
- Rivero, S.; García, M.A.; Pinotti, A. Composite and bi-layer films based on gelatin and chitosan. J. Food Eng. 2009, 90, 531–539. [Google Scholar] [CrossRef]
- Jiménez, A.; Fabra, M.J.; Talens, P.; Chiralt, A. Effect of lipid self-association on the microstructure and physical properties of hydroxypropyl-methylcellulose edible films containing fatty acids. Carbohydr. Polym. 2010, 82, 585–593. [Google Scholar] [CrossRef]
- Bertan, L.C.; Tanada-Palmu, P.S.; Siani, A.C.; Grosso, C.R.F. Effect of fatty acids and ‘Brazilian elemi’ on composite films based on gelatin. Food Hydrocoll. 2005, 19, 73–82. [Google Scholar] [CrossRef]
- Klinkesorn, U. The Role of Chitosan in Emulsion Formation and Stabilization. Food Rev. Int. 2013, 29, 371–393. [Google Scholar] [CrossRef]
- Olam, M. Mechanical and Thermal Properties of HDPE/PET Microplastics, Applications, and Impact on Environment and Life. In Advances and Challenges in Microplastics; IntechOpen: London, UK, 2023. [Google Scholar] [CrossRef]
- Pham, N.T.H. Characterization of Low-Density Polyethylene and LDPE-Based/Ethylene-Vinyl Acetate with Medium Content of Vinyl Acetate. Polymers 2021, 13, 2352. [Google Scholar] [CrossRef] [PubMed]
- Vargas, M.; Albors, A.; Chiralt, A. Application of chitosan-sunflower oil edible films to pork meat hamburgers. Ital. Oral. Surg. 2011, 1, 39–43. [Google Scholar] [CrossRef]
- Vargas, M.; Albors, A.; Chiralt, A.; González-Martínez, C. Characterization of chitosan–oleic acid composite films. Food Hydrocoll. 2009, 23, 536–547. [Google Scholar] [CrossRef]
- Batista, J.T.S.; Araújo, C.S.; Joele, M.R.S.P.; Silva, J.O.C.; Lourenço, L.F.H. Study of the effect of the chitosan use on the properties of biodegradable films of myofibrillar proteins of fish residues using response surface methodology. Food Packag. Shelf Life 2019, 20, 100306. [Google Scholar] [CrossRef]
- Handayasari, F.; Suyatma, N.E.; Nurjanah, S. Physiochemical and antibacterial analysis of gelatin–chitosan edible film with the addition of nitrite and garlic essential oil by response surface methodology. J. Food Process Preserv. 2019, 43, e14265. [Google Scholar] [CrossRef]
- Jridi, M.; Hajji, S.; Ayed, H.B.; Lassoued, I.; Mbarek, A.; Kammoun, M.; Souissi, N.; Nasri, M. Physical, structural, antioxidant and antimicrobial properties of gelatin-chitosan composite edible films. Int. J. Biol. Macromol. 2014, 67, 373–379. [Google Scholar] [CrossRef]
- Sandri, G.; Miele, D.; Faccendini, A.; Bonferoni, M.C.; Rossi, S.; Grisoli, P.; Taglietti, A.; Ruggeri, M.; Bruni, G.; Vigani, B.; et al. Chitosan/Glycosaminoglycan Scaffolds: The Role of Silver Nanoparticles to Control Microbial Infections in Wound Healing. Polymers 2019, 11, 1207. [Google Scholar] [CrossRef] [PubMed]
- Rivero, S.; Damonte, L.; García, M.A.; Pinotti, A. An Insight into the Role of Glycerol in Chitosan Films. Food Biophys. 2016, 11, 117–127. [Google Scholar] [CrossRef]
- Malinowska-Pańczyk, E.; Staroszczyk, H.; Gottfried, K.; Kołodziejska, I.; Wojtasz-Pająk, A. Antimicrobial properties of chitosan solutions, chitosan films and gelatin-chitosan films. Polimery 2015, 60, 735–741. [Google Scholar] [CrossRef]
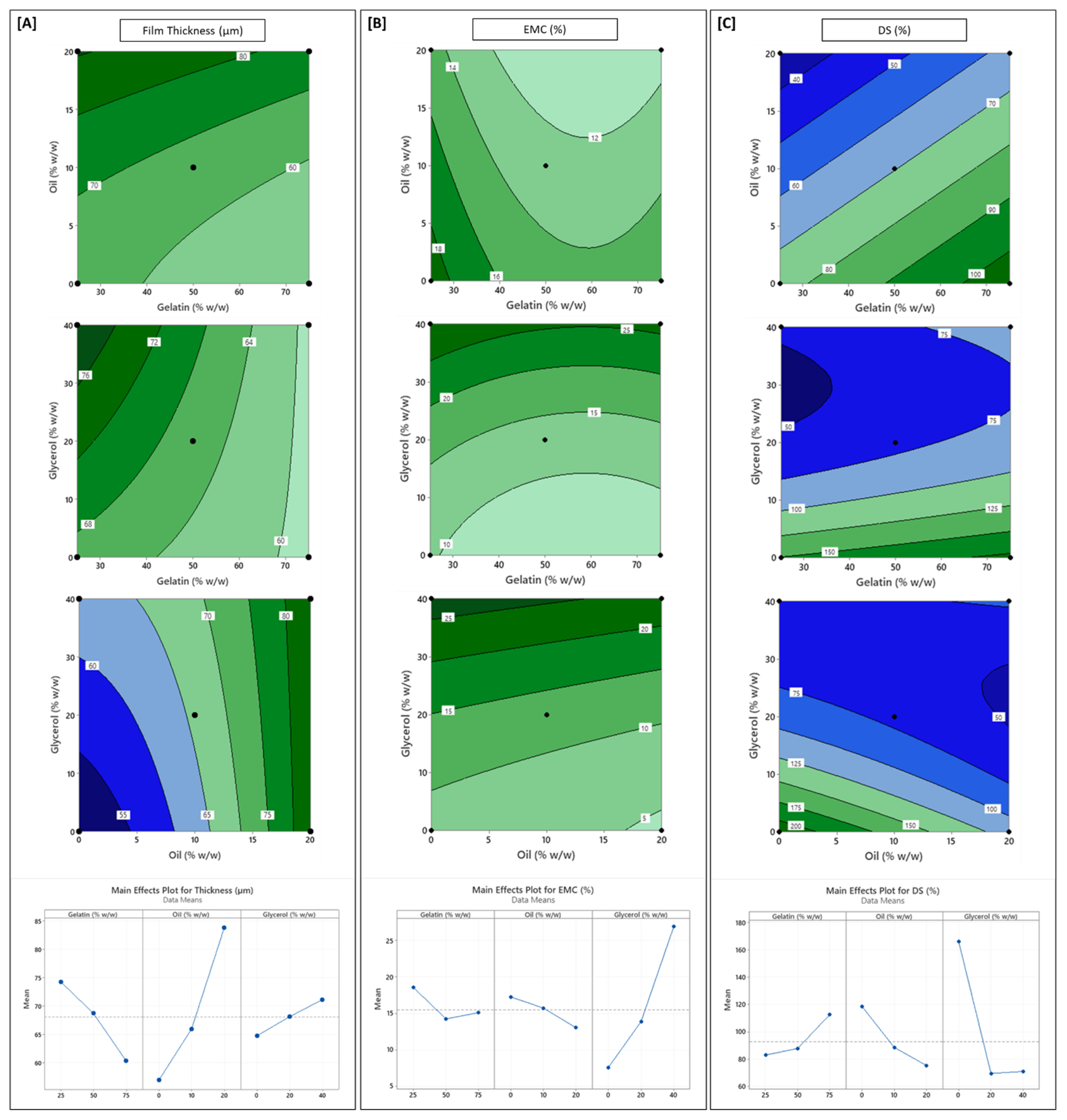
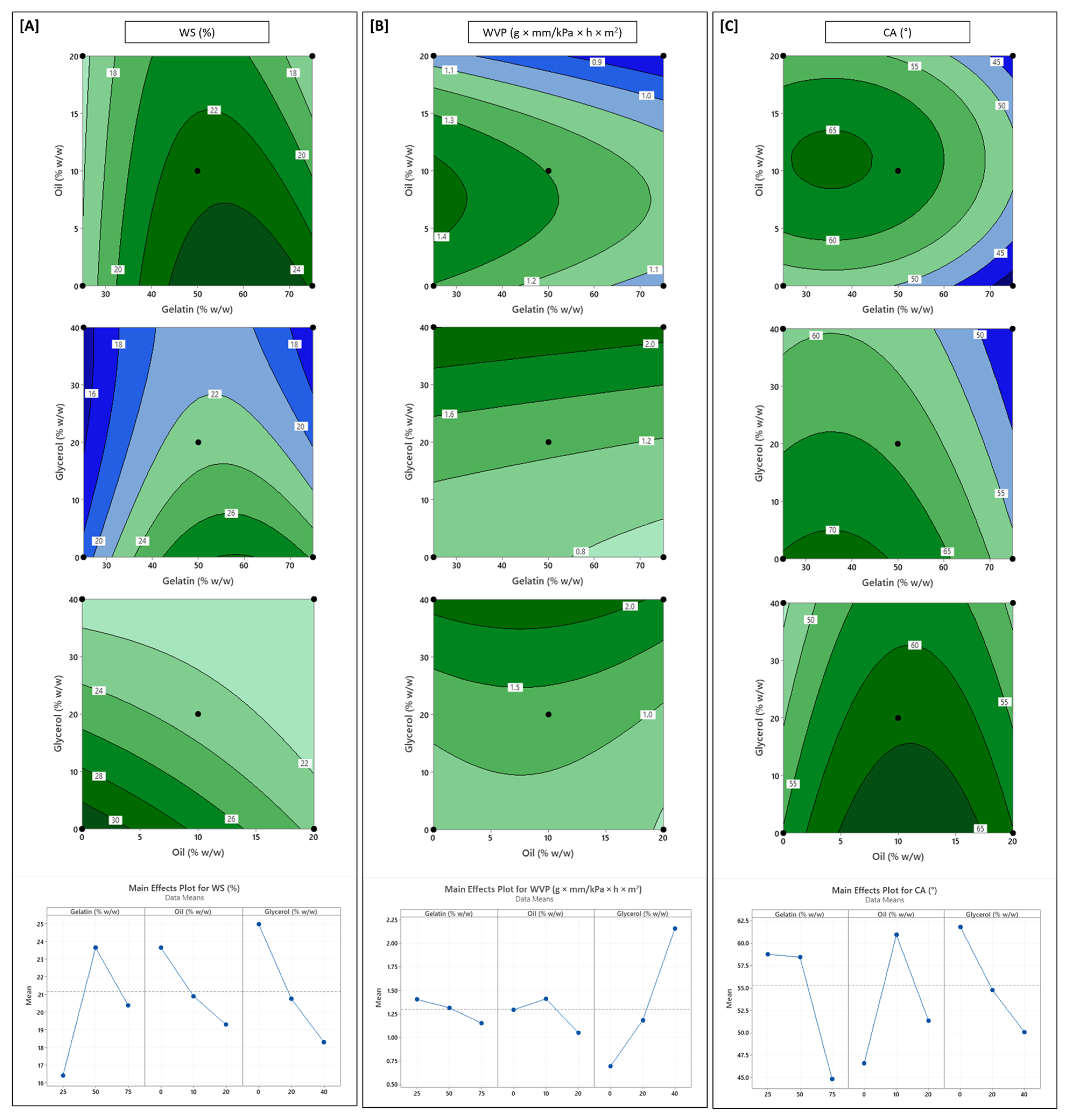
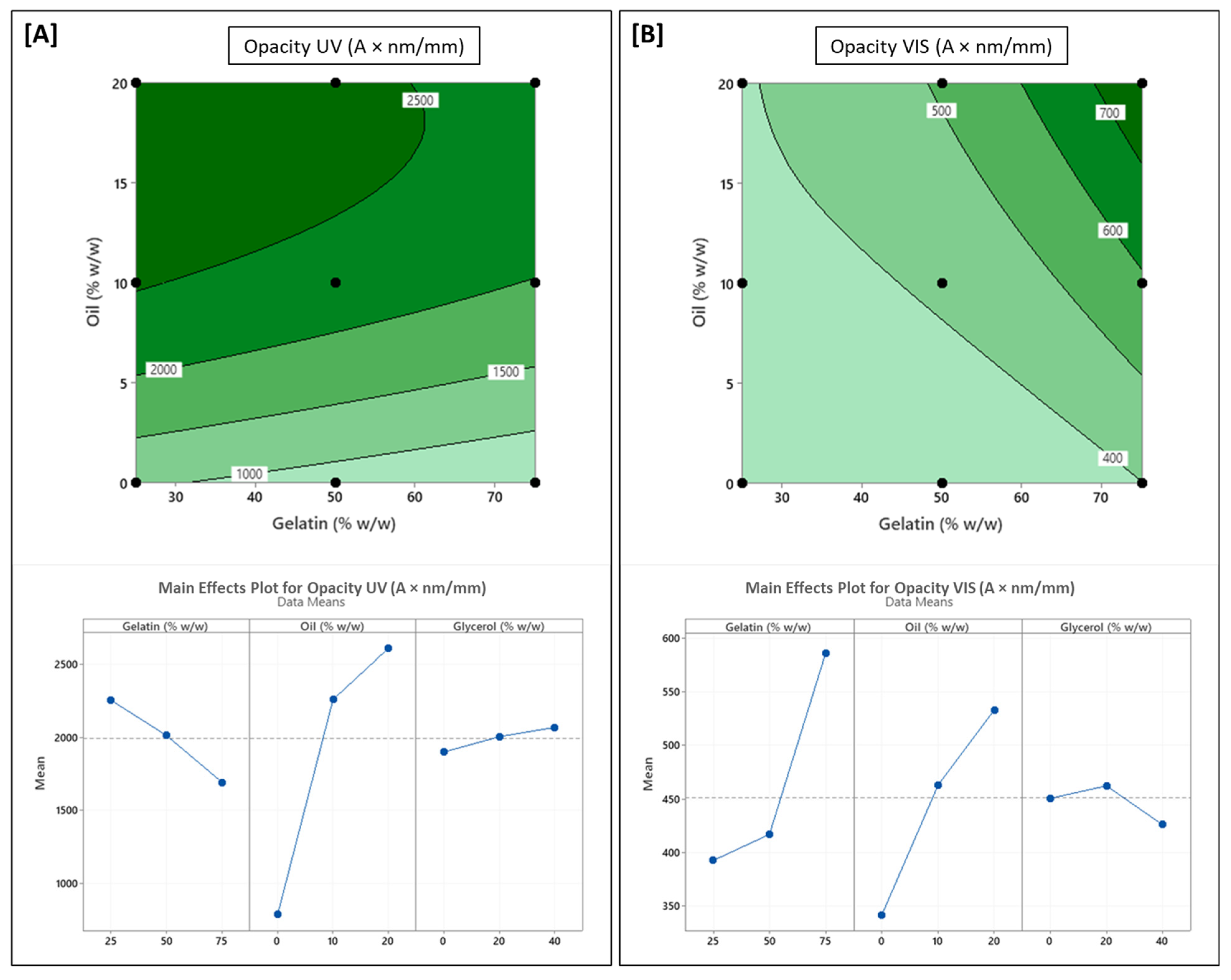
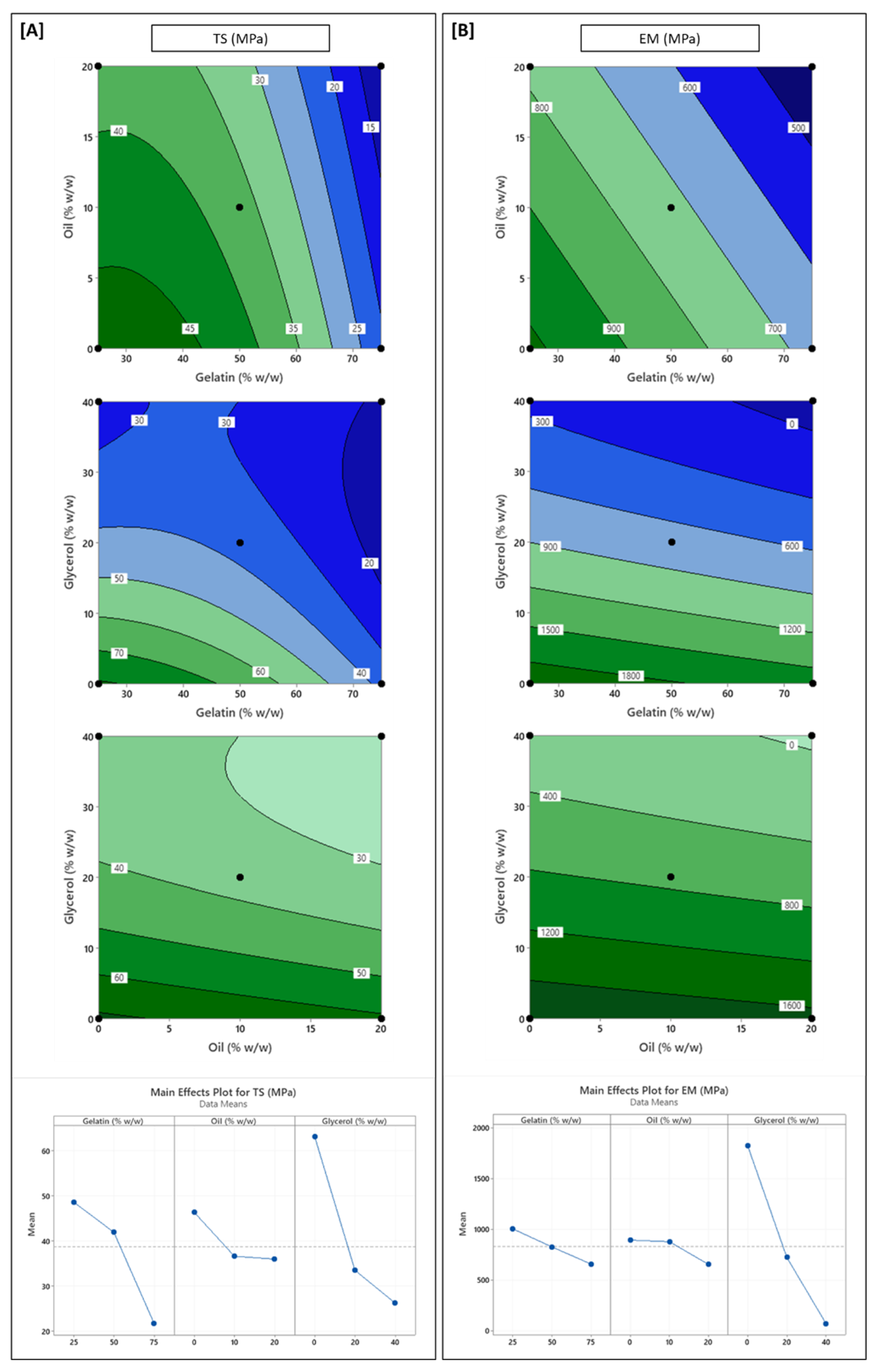
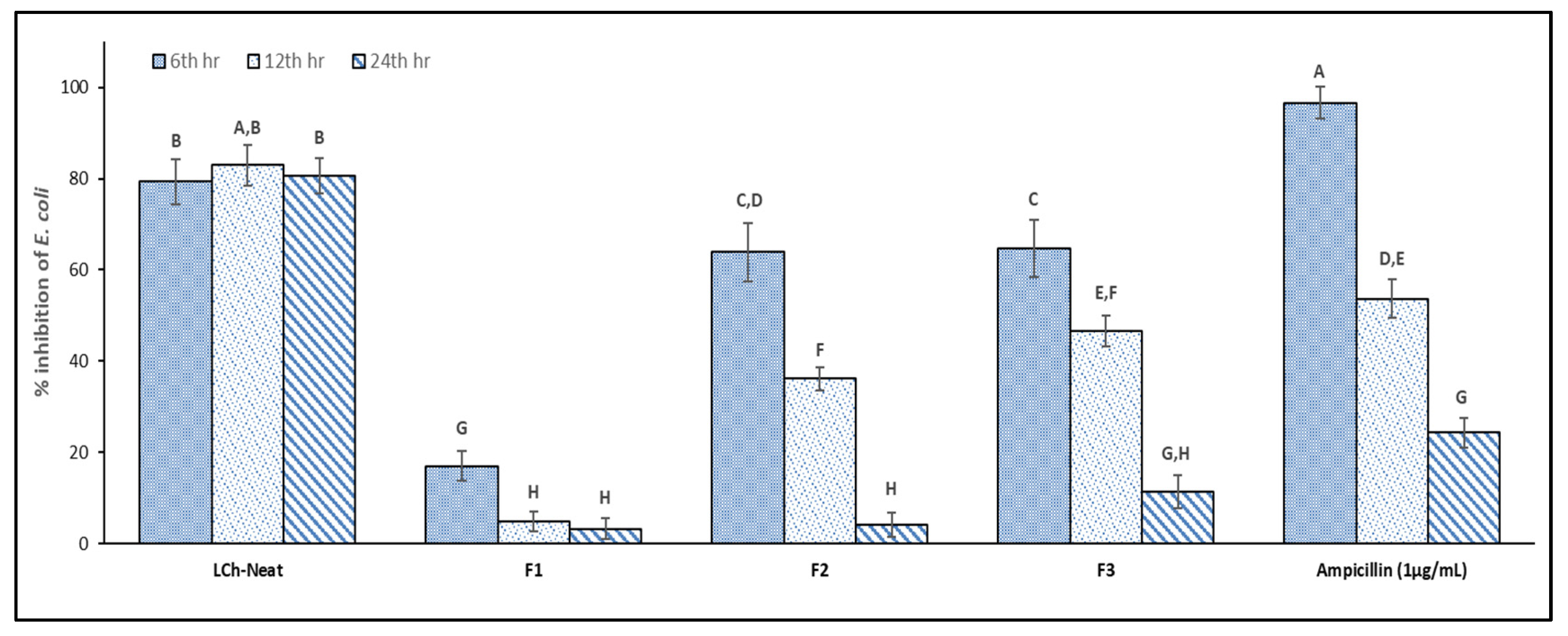
| Source | DF # | FT | EMC | DS | WS | OPUV | OPVIS | TS | % EAB | EM | WVP | CA |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Full Model | 9 | 48.26 ** | 41.19 ** | 16.11 * | 48.94 ** | 9.15 * | 40.79 ** | 37.91 ** | 18.65 ** | 68.88 ** | 29.75 ** | 16.61 * |
| Linear | 3 | 132.04 ** | 112.43 ** | 30.23 ** | 73.06 ** | 19.09 * | 102.41 ** | 89.61 ** | 54.22 ** | 197.59 ** | 78.90 ** | 22.55 * |
| Ge (X1) | 1 | 80.51 ** | 10.01 * | 6.79 * | 43.47 ** | 28.74 * | 26.56 * | 88.63 ** | 3.19 | 22.22 * | 6.56 * | 37.14 ** |
| O (X2) | 1 | 298.81 ** | 14.77 * | 14.45 * | 52.40 ** | 28.08 * | 278.28 ** | 13.24 * | 0.27 | 10.56 * | 6.30 * | 6.33 * |
| Gly (X3) | 1 | 16.81 * | 312.49 ** | 69.44 ** | 123.32 ** | 0.46 | 2.39 | 166.96 ** | 159.18 ** | 559.99 ** | 223.82 ** | 26.19 ** |
| Square | 3 | 6.49 * | 10.58 * | 13.49 * | 57.65 ** | 3.69 | 18.67 * | 17.18 * | 0.05 | 8.23 * | 9.20 * | 24.74 ** |
| Ge * Ge | 1 | 2.33 | 10.54 * | 0.8 | 166.97 ** | 9.20 * | 0.01 | 15.06 * | 0.09 | 0.01 | 0.29 | 15.07 * |
| O * O | 1 | 17.75 * | 1.46 | 0.45 | 3.7 | 1.28 | 55.68 ** | 4.9 | 0.02 | 5.24 | 13.93 * | 55.34 ** |
| Gly * Gly | 1 | 0.1 | 19.00 * | 37.84 ** | 7.36 * | 1.14 | 0.02 | 33.31 * | 0.06 | 20.46 * | 14.83 * | 1.88 |
| Interaction | 3 | 6.26 * | 0.56 | 4.60 * | 16.09 * | 4.66 * | 1.3 | 6.95 * | 1.69 | 0.82 | 1.16 | 2.55 |
| Ge * O | 1 | 3.12 | 0.02 | 0.02 | 16.48 * | 13.24 * | 0.11 | 2.01 | 0.01 | 0.02 | 0.43 | 1.01 |
| Ge * Gly | 1 | 8.31 * | 0.2 | 0.56 | 11.38 * | 0.07 | 0.26 | 17.94 * | 4.16 | 0.84 | 0.02 | 4.34 |
| O * Gly | 1 | 7.35 * | 1.49 | 13.21 * | 20.42 * | 0.66 | 3.53 | 0.91 | 0.92 | 1.61 | 3.02 | 2.31 |
| Lack of Fit | 3 | 0.83 | 1.93 | 5.04 | 0.68 | 1.74 | 0.32 | 3.04 | 5.23 | 0.78 | 2.05 | 1.67 |
| Response Parameters | Formulation 1 | Formulation 2 | Formulation 3 |
|---|---|---|---|
| Target | Target | Target | |
| FT (µm) | NO | NO | NO |
| EMC (%) | NO | NO | NO |
| DS (%) | Minimize | NO | Maximize |
| WS (%) | Minimize | NO | Maximize |
| OPVIS (A × nm/mm) | Minimize | Minimize | NO |
| OPUV (A × nm/mm) | Maximize | Maximize | NO |
| TS (MPa) | Range (35–45) | Range (35–45) | Range (35–45) |
| EAB (%) | Range (35–45) | Range (45–55) | Range (35–45) |
| EM (MPa) | Range (800–1000) | Range (800–1000) | Range (800–1000) |
| WVP (g × mm/kPa × h × m2) | Minimize | Minimize | Minimize |
| CA (°) | Maximize | NO | NO |
| Ge (X1) (% w/w polymer) | 59.34 | 43.76 | 59.69 |
| O (X2) (% w/w polymer) | 11.31 | 15.42 | 0 |
| Gly (X3) (% w/w polymer) | 14.94 | 17.41 | 11.55 |
| Desirability (D) | 0.81 | 0.84 | 0.77 |
| Response Parameters | Formulation 1 | Formulation 2 | Formulation 3 | |||
|---|---|---|---|---|---|---|
| Predicted Value and Range at 95% CI * | Experimental Value | Predicted Value and Range at 95% CI * | Experimental Value | Predicted Value and Range at 95% CI * | Experimental Value | |
| FT (µm) | 64.79 (62.75–66.84) | 66.87 ± 4.61 | 76.01 (74.14–77.87) | 74.53 ± 5.37 | 52.12 (48.89–55.34) | 51.12 ± 7.69 |
| EMC (%) | 10.01 (8.74–11.25) | 12.25 ± 1.36 | 11.06 (9.65–12.48) | 13.12 ± 0.92 | 11.11 (9.42–12.79) | 11.40 ± 1.45 |
| DS (%) | 86.53 (75.23–97.83) | 83.51 ± 5.47 | 59.14 (46.58–71.71) | 61.06 ± 2.04 | 138.18 (121.03–155.34) | 157.21 ± 4.89 |
| WS (%) | 23.74 (22.85–24.61) | 23.02 ± 1.81 | 21.61 (20.61–22.62) | 19.98 ± 1.27 | 28.59 (27.24–29.95) | 30.87 ± 3.35 |
| OPVIS (A × nm/mm) | 480.46 (446.35–514.58) | 498.80 ± 23.89 | 436.74 (396.32–477.17) | 438.59 ± 9.49 | 333.78 (279.62–387.95) | 297.00 ± 7.73 |
| OPUV (A × nm/mm) | 2262.4 (2142.3–2382.5) | 2176.1 ± 39.2 | 2656.0 (2543.9–2768.2) | 2683.9 ± 53.6 | 679.6 (504.9–854.3) | 593.9 ± 28.3 |
| TS (MPa) | 34.63 (30.39–38.86) | 33.08 ± 2.41 | 39.69 (34.94–44.44) | 41.01 ± 3.12 | 44.07 (38.36–49.79) | 48.79 ± 5.59 |
| EAB (%) | 40.94 (36.01–45.88) | 41.39 ± 10.05 | 45.77 (41.05–50.48) | 48.71 ± 13.74 | 34.32 (28.88–39.76) | 27.12 ± 6.34 |
| EM (MPa) | 880.4 (791.1–969.7) | 863.5 ± 51.2 | 821.1 (722.0–920.1) | 798.6 ± 43.5 | 1187.5 (1062.7–1312.4) | 1259.6 ± 69.1 |
| WVP (g × mm/kPa × h × m2) | 1.06 (0.94–1.18) | 1.05 ± 0.03 | 1.09 (0.98–1.21) | 1.11 ± 0.04 | 0.86 (0.68–1.02) | 0.79 ± 0.06 |
| CA (°) | 61.8 (58.3–65.2) | 58.9 ± 2.5 | 63.6 (60.2–66.9) | 61.8 ± 1.9 | 48.5 (43.6–53.4) | 45.6 ± 1.6 |
| Samples | Log (CFU/mL) |
|---|---|
| LDPE | 10.11 ± 0.18 A |
| LCh-Neat | 6.20 ± 0.19 C |
| F1 | 10.03 ± 0.11 A,B |
| F2 | 9.97 ± 0.15 A,B |
| F3 | 9.66 ± 0.07 A,B |
| Ampicillin (1 µg/mL) | 9.53 ± 0.09 B |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jain, A.; Mason, B.; Brooks, M.S.-L. Development of Tailored Composite Biopolymer Film Formulations Using Minimally Refined Chitosan from American Lobster (Homarus americanus) Shell Waste for Different Food Packaging Applications. Polymers 2025, 17, 3132. https://doi.org/10.3390/polym17233132
Jain A, Mason B, Brooks MS-L. Development of Tailored Composite Biopolymer Film Formulations Using Minimally Refined Chitosan from American Lobster (Homarus americanus) Shell Waste for Different Food Packaging Applications. Polymers. 2025; 17(23):3132. https://doi.org/10.3390/polym17233132
Chicago/Turabian StyleJain, Abhinav, Beth Mason, and Marianne Su-Ling Brooks. 2025. "Development of Tailored Composite Biopolymer Film Formulations Using Minimally Refined Chitosan from American Lobster (Homarus americanus) Shell Waste for Different Food Packaging Applications" Polymers 17, no. 23: 3132. https://doi.org/10.3390/polym17233132
APA StyleJain, A., Mason, B., & Brooks, M. S.-L. (2025). Development of Tailored Composite Biopolymer Film Formulations Using Minimally Refined Chitosan from American Lobster (Homarus americanus) Shell Waste for Different Food Packaging Applications. Polymers, 17(23), 3132. https://doi.org/10.3390/polym17233132