Fluoride-Free MXene–Polymer Composites for Li-Metal and Li–S Batteries: Comparative Synthesis Methods, Integration Rules, Challenges, and Future Directions
Abstract
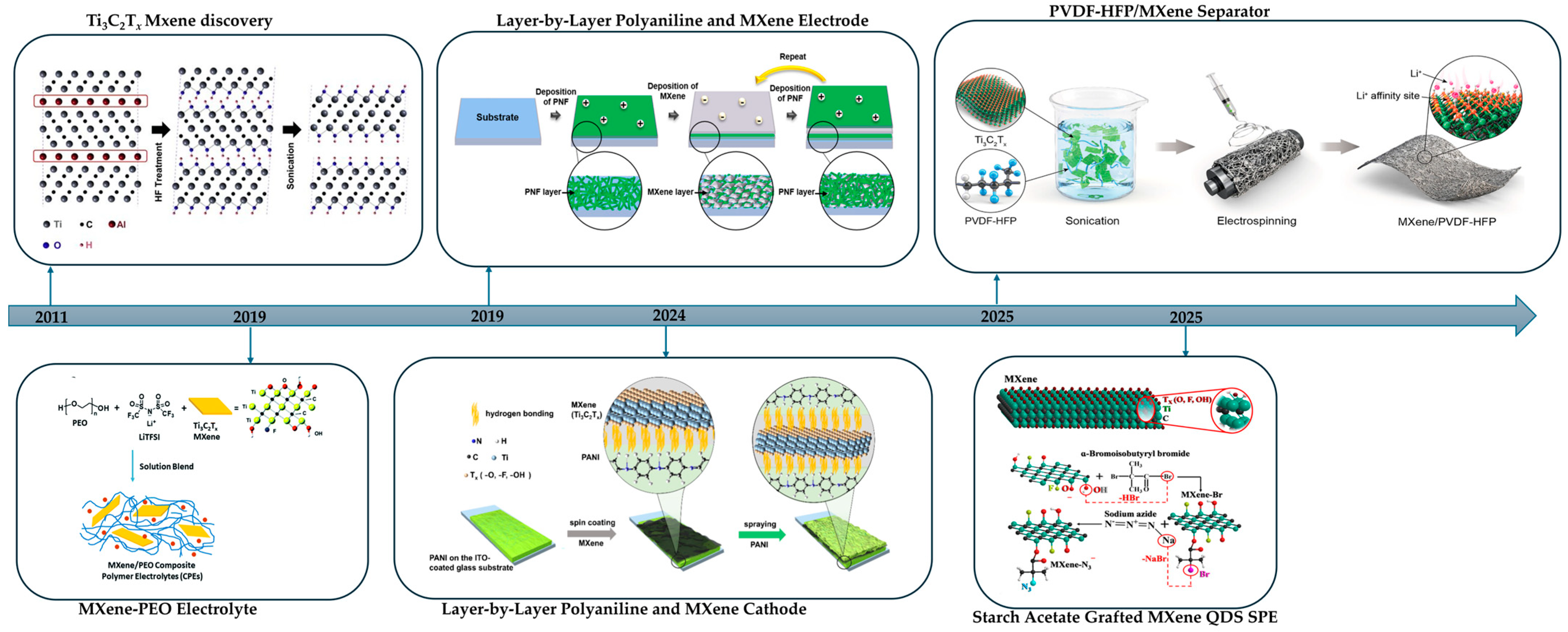
1. Introduction
2. MXenes: Structure, Synthesis, Function
2.1. HF-Based Synthesis of MXenes
2.2. Green Synthesis of MXenes
2.2.1. Hydrothermal Etching Methods
Alkali Etching Method
Acid Etching Method
Salt-Assisted Etching
Microwave–Hydrothermal Method
2.2.2. Electrochemical Etching Methods
2.2.3. Molten-Salt Etching and Derivatives
Lewis-Acid Molten-Salt Etching Method
Low-Temperature Hydrated Molten-Salt Etching
2.2.4. Iodine-Assisted, Non-Aqueous Etching
2.2.5. Photo-Fenton Soft-Chemistry Etching
2.2.6. Chemical Vapor Deposition–Bottom-Up MXene Growth
2.2.7. Mechanochemical Fluoride-Free Synthesis
3. MXene Property Pillars for Polymer LMB and Li–S Systems
3.1. Terminations and Wetting
3.2. Two-Dimensional Ion Pathways and Spacing
3.3. Interfacial Reactivity and Interphase Control
3.4. Mechanics and Heat Management
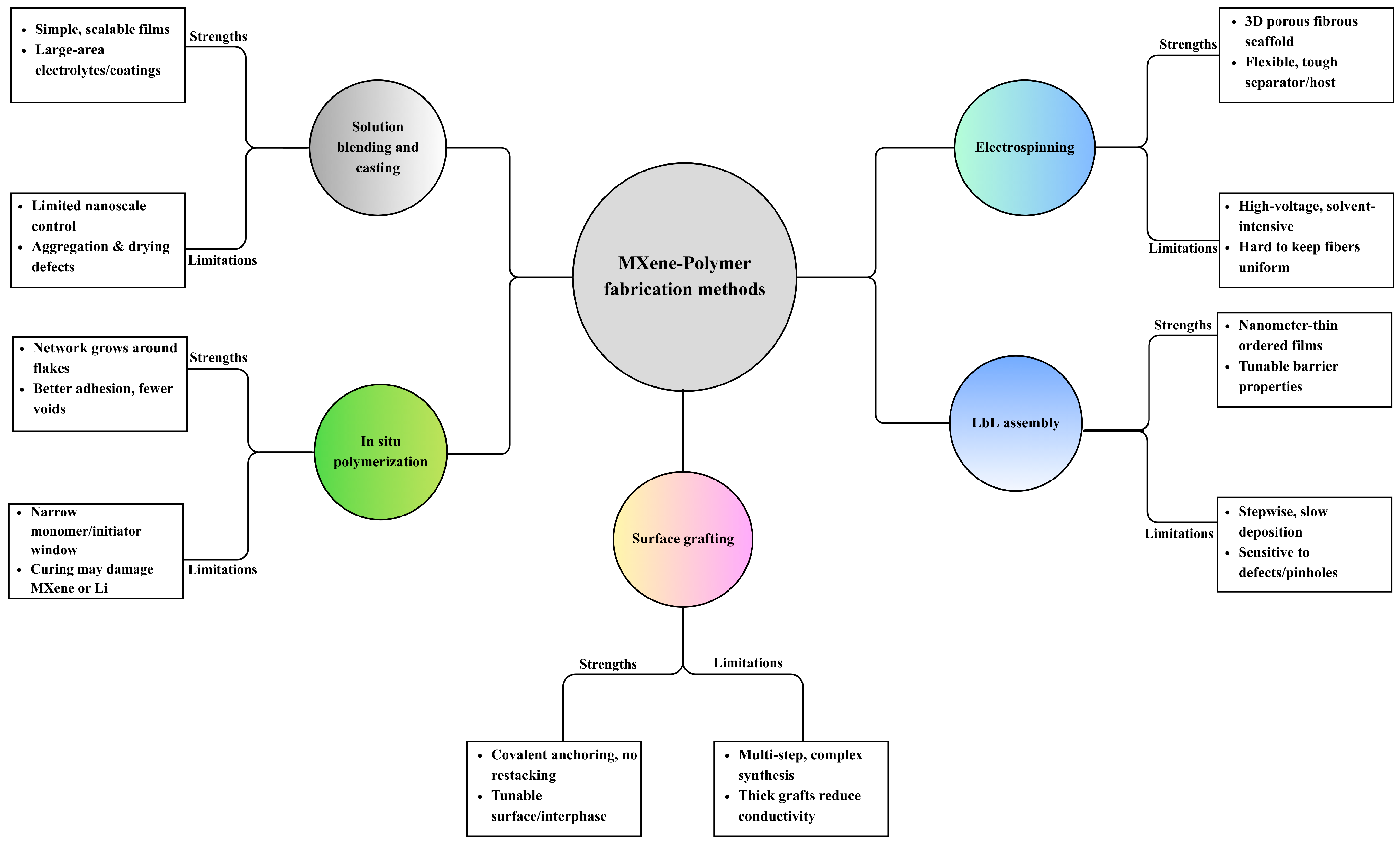
4. Synthetic Methods of MXene–Polymer Composites
4.1. Solution Blending and Film Casting
4.2. In Situ Polymerization
4.3. Surface Grafting (Grafting-to and Grafting-from)
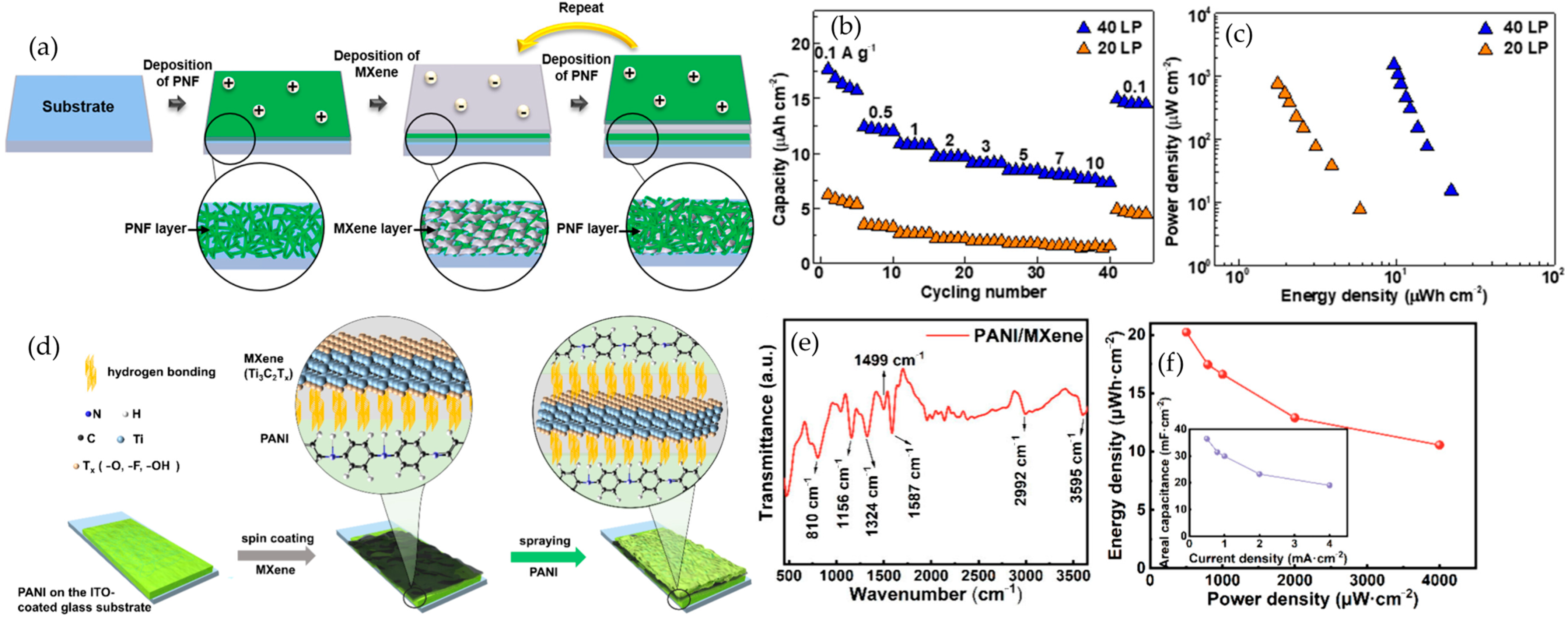
4.4. Layer-by-Layer (LbL) Assembly
4.5. Electrospinning
5. From Terminations to Composites: Design Rules for MXene–Polymer Systems
5.1. Purpose and a Route-to-Termination-to-Polymer Design Map
5.2. Evolution of Polymer Backbones in MXene-Based Battery Systems
5.3. Interfacial Chemistry and Polymer Matching
5.4. Processing and Microstructure That Preserve Chemistry
5.5. Compact Rules, Benchmarking, and a Failure-Mode Playbook
6. Application of MXene–Polymer Composites in Lithium Batteries: HF-Based MXene Focus
6.1. Lithium-Metal Batteries (LMBs)
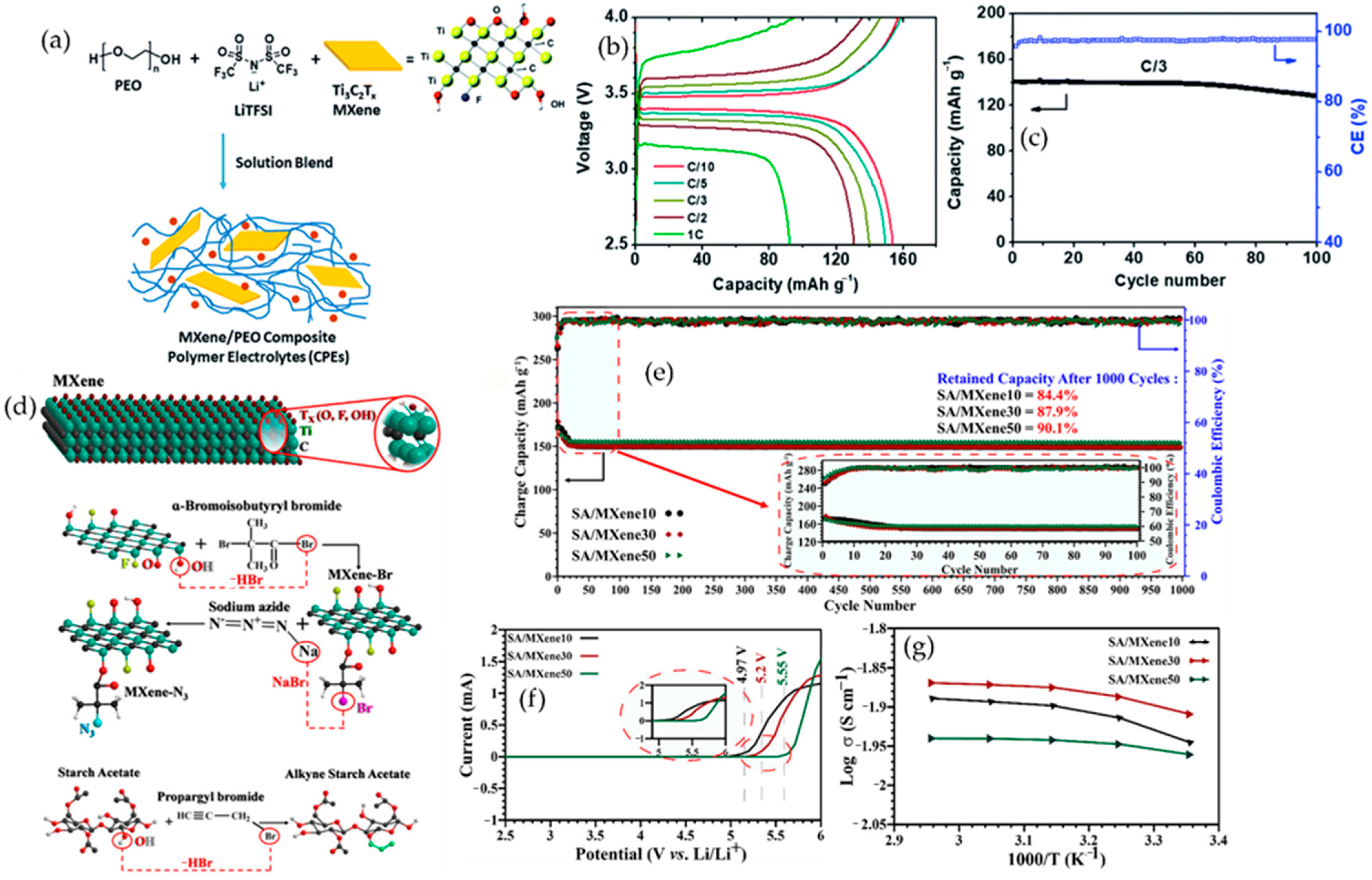
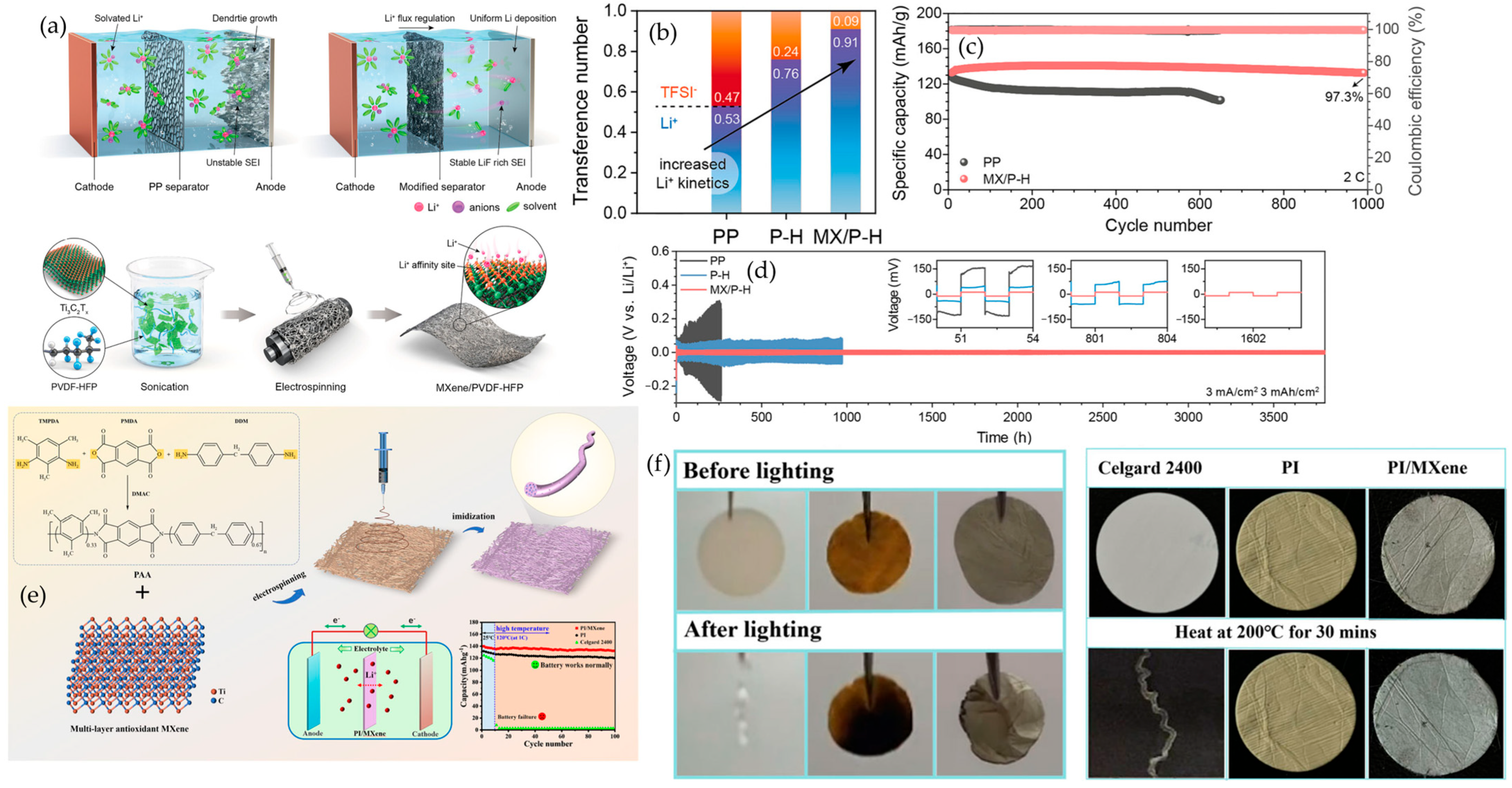
6.1.1. MXene–Polymer Electrolytes in Lithium-Metal Batteries
6.1.2. MXene–Polymer Separators in Lithium-Metal Batteries
6.1.3. MXene–Polymer-Programmed Solid Electrolytes Interphases (SEIs) in Lithium-Metal Batteries
6.1.4. MXene–Polymer Electrodes (Anode and Cathode) in LMBs
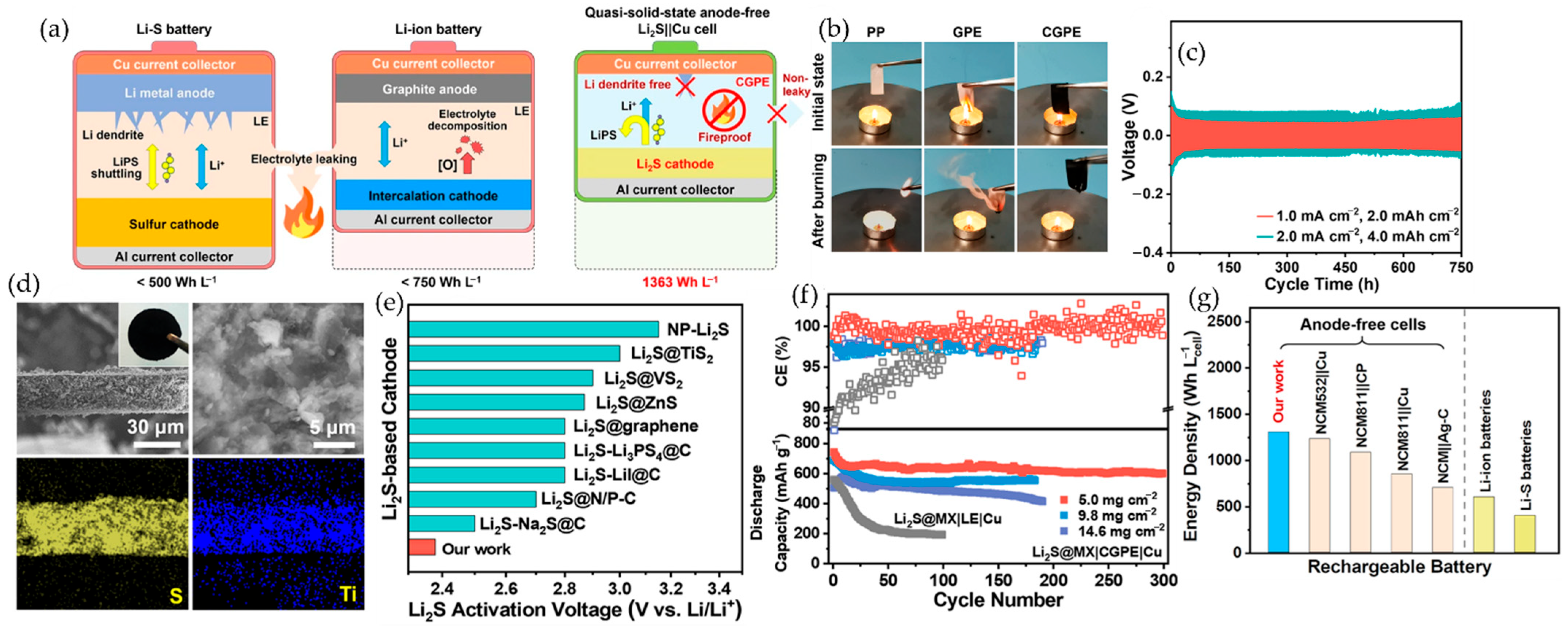
6.2. Lithium–Sulfur Batteries
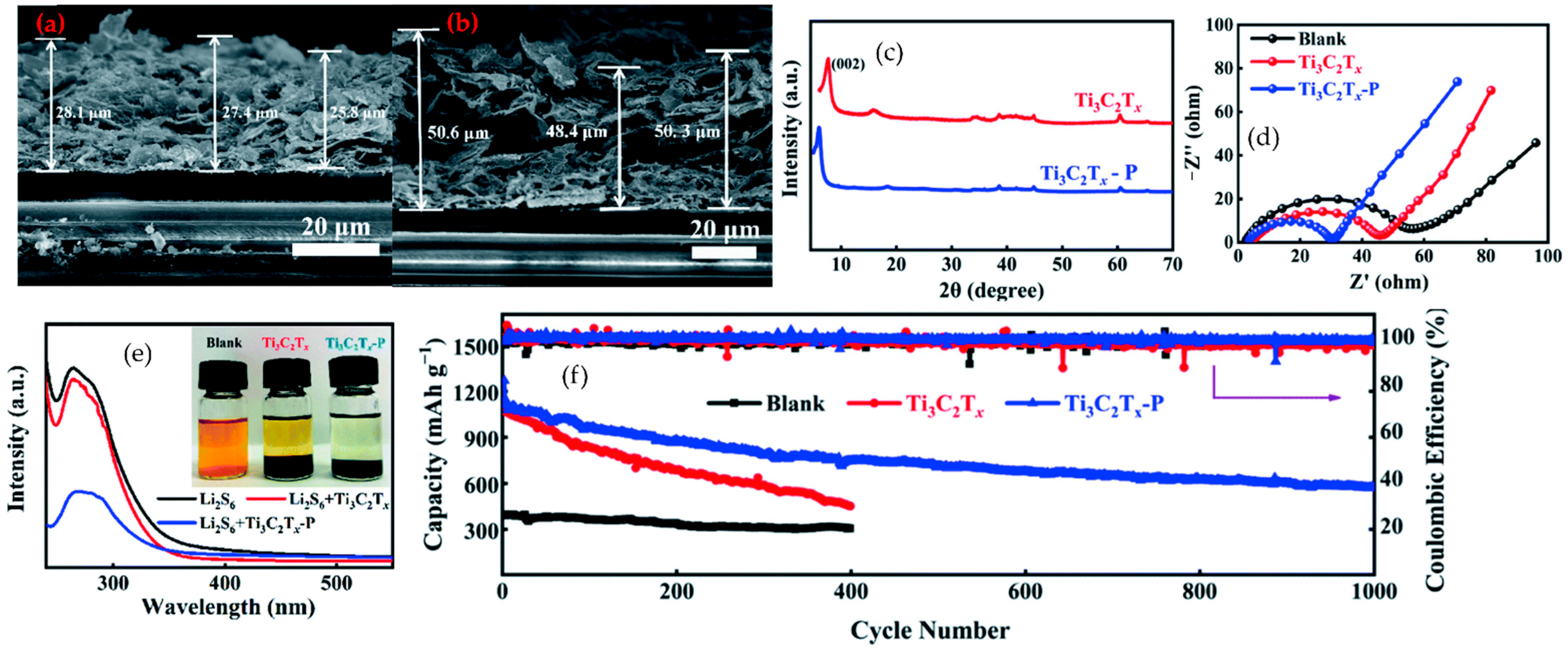
6.2.1. MXene–Polymer Separators in Lithium–Sulfur Batteries
6.2.2. MXene–Polymer Electrolytes in Lithium–Sulfur Batteries
6.2.3. MXene–Polymer Cathodes in Lithium–Sulfur Batteries
7. Green-Synthesized MXene–Polymer Composites for Lithium Batteries
7.1. Lithium-Metal Batteries
7.2. Lithium–Sulfur Batteries Li-S Batteries
8. Conclusions and Outlook for Green MXene–Polymer Composites in Li-Metal (LMB) and Li–S Batteries
8.1. Conlusions
8.2. Challenges and Practical Solutions
8.3. Future Outlook and Research Directions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Dunn, B.; Kamath, H.; Tarascon, J.M. Electrical Energy Storage for the Grid: A Battery of Choices. Science 2011, 334, 928–935. [Google Scholar] [CrossRef]
- Armand, M.; Tarascon, J.M. Building better batteries. Nature 2008, 451, 652–657. [Google Scholar] [CrossRef]
- Larcher, D.; Tarascon, J.M. Towards Greener and More Sustainable Batteries for Electrical Energy Storage. Nat. Chem. 2015, 7, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Goodenough, J.B.; Park, K.S. The Li-Ion Rechargeable Battery: A Perspective. J. Am. Chem. Soc. 2013, 135, 1167–1176. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Liu, Y.; Cui, Y. Reviving the Lithium Metal Anode for High-Energy Batteries. Nat. Nanotechnol. 2017, 12, 194–206. [Google Scholar] [CrossRef]
- Seh, Z.W.; Sun, Y.; Zhang, Q.; Cui, Y. Designing High-Energy Lithium–Sulfur Batteries. Chem. Soc. Rev. 2016, 45, 5605–5634. [Google Scholar] [CrossRef]
- Manthiram, A.; Fu, Y.; Chung, S.H.; Zu, C.; Su, Y.S. Rechargeable Lithium–Sulfur Batteries. Chem. Rev. 2014, 114, 11751–11787. [Google Scholar] [CrossRef]
- Zhou, J.; Dahlqvist, M.; Björk, J.; Rosen, J. Atomic Scale Design of MXenes and Their Parent Materials—From Theoretical and Experimental Perspectives. Chem. Rev. 2023, 123, 13291–13322. [Google Scholar] [CrossRef] [PubMed]
- Alhabeb, M.; Maleski, K.; Anasori, B.; Lelyukh, P.; Clark, L.; Sin, S.; Gogotsi, Y. Guidelines for Synthesis and Processing of Two-Dimensional Titanium Carbide (Ti3C2Tx MXene). Chem. Mater. 2017, 29, 7633–7644. [Google Scholar] [CrossRef]
- Naguib, M.; Kurtoglu, M.; Presser, V.; Lu, J.; Niu, J.; Heon, M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-Dimensional Nanocrystals Produced by Exfoliation of Ti3AlC2. Adv. Mater. 2011, 23, 4248–4253. [Google Scholar] [CrossRef]
- Le Truong, K.; Bae, J. Towards Fire-Safe Polymer Electrolytes for Lithium-Ion Batteries: Strategies for Electrolyte Design and Structural Design. Polymers 2025, 17, 2828. [Google Scholar] [CrossRef]
- Kumar, N.; Hoang, Q.-V.; Mohammad, B.; Kaja, K.R.; Nguyen, P.H.; Le-Van, Q.; Vien, V.; Linh, V.T.T.; Nguyen, P.K.T.; Ta, Q.T.H. Progress in Synthesis of Ti3C2Tx MXene-Based Nanostructures for Energy Harvesting and Storage: A Review. J. Sci. Adv. Mater. Devices 2025, 10, 101034. [Google Scholar] [CrossRef]
- Rahman, M.; Al Mamun, M.S. Future Prospects of MXenes: Synthesis, Functionalization, Properties, and Application in Field Effect Transistors. Nanoscale Adv. 2024, 6, 367–385. [Google Scholar] [CrossRef] [PubMed]
- Sinha, A.; Ma, K.; Zhao, H. 2D Ti3C2Tx Flakes Prepared by In-Situ HF Etchant for Simultaneous Screening of Carbamate Pesticides. J. Colloid Interface Sci. 2021, 590, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Bao, W.; Shen, H.; Zeng, G.; Zhang, Y.; Wang, Y.; Cui, D.; Xia, J.; Jing, K.; Liu, H.; Guo, C.; et al. Engineering the next Generation of MXenes: Challenges and Strategies for Scalable Production and Enhanced Performance. Nanoscale 2025, 17, 6204–6265. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S. Fluorine-Free MXenes: Recent Advances, Synthesis Strategies, and Mechanisms. Small 2024, 20, 2308225. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Li, J.; Wang, Y.; Han, W.; Xu, S.; Lu, M.; Zhang, W.; Li, H. Surface Terminations of MXene: Synthesis, Characterization, and Properties. Symmetry 2022, 14, 2232. [Google Scholar] [CrossRef]
- FitzPatrick, J.; Gogotsi, Y. MXene Polymer Composites: A Review. MRS Bull. 2025, 50, 1351–1363. [Google Scholar] [CrossRef]
- Ghidiu, M.; Lukatskaya, M.R.; Zhao, M.Q.; Gogotsi, Y.; Barsoum, M.W. Conductive Two-Dimensional Titanium Carbide “clay” with High Volumetric Capacitance. Nature 2014, 516, 78–81. [Google Scholar] [CrossRef]
- Liu, F.; Zhou, A.; Chen, J.; Jia, J.; Zhou, W.; Wang, L.; Hu, Q. Preparation of Ti3C2 and Ti2C MXenes by Fluoride Salts Etching and Methane Adsorptive Properties. Appl. Surf. Sci. 2017, 416, 781–789. [Google Scholar] [CrossRef]
- Zhang, T.; Pan, L.; Tang, H.; Du, F.; Guo, Y.; Qiu, T.; Yang, J. Synthesis of Two-Dimensional Ti3C2Tx MXene Using HCl+LiF Etchant: Enhanced Exfoliation and Delamination. J. Alloys Compd. 2017, 695, 818–826. [Google Scholar] [CrossRef]
- Hope, M.A.; Forse, A.C.; Griffith, K.J.; Lukatskaya, M.R.; Ghidiu, M.; Gogotsi, Y.; Grey, C.P. NMR Reveals the Surface Functionalisation of Ti3C2 MXene. Phys. Chem. Chem. Phys. 2016, 18, 5099–5102. [Google Scholar] [CrossRef]
- Feng, A.; Yu, Y.; Wang, Y.; Jiang, F.; Yu, Y.; Mi, L.; Song, L. Two-Dimensional MXene Ti3C2 Produced by Exfoliation of Ti3AlC2. Mater. Des. 2017, 114, 161–166. [Google Scholar] [CrossRef]
- Halim, J.; Lukatskaya, M.R.; Cook, K.M.; Lu, J.; Smith, C.R.; Näslund, L.Å.; May, S.J.; Hultman, L.; Gogotsi, Y.; Eklund, P.; et al. Transparent Conductive Two-Dimensional Titanium Carbide Epitaxial Thin Films. Chem. Mater. 2014, 26, 2374–2381. [Google Scholar] [CrossRef]
- Li, T.; Yao, L.; Liu, Q.; Gu, J.; Luo, R.; Li, J.; Yan, X.; Wang, W.; Liu, P.; Chen, B.; et al. Fluorine-Free Synthesis of High-Purity Ti3C2Tx (T=OH, O) via Alkali Treatment. Angew. Chem. Int. Ed. 2018, 57, 6115–6119. [Google Scholar] [CrossRef]
- Li, Y.; Shao, H.; Lin, Z.; Lu, J.; Liu, L.; Duployer, B.; Persson, P.O.Å.; Eklund, P.; Hultman, L.; Li, M.; et al. A General Lewis Acidic Etching Route for Preparing MXenes with Enhanced Electrochemical Performance in Non-Aqueous Electrolyte. Nat. Mater. 2020, 19, 894–899. [Google Scholar] [CrossRef] [PubMed]
- Mim, M.; Habib, K.; Farabi, S.N.; Ali, S.A.; Zaed, M.A.; Younas, M.; Rahman, S. MXene: A Roadmap to Sustainable Energy Management, Synthesis Routes, Stabilization, and Economic Assessment. ACS Omega 2024, 9, 32350. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Han, W.Q. Recent Advances and Perspectives of Lewis Acidic Etching Route: An Emerging Preparation Strategy for MXenes. Nano-Micro Lett. 2023, 15, 68. [Google Scholar] [CrossRef] [PubMed]
- Khan, U.; Gao, B.; Kong, L.B.; Chen, Z.; Que, W. Green Synthesis of Fluorine-Free MXene via Hydrothermal Process: A Sustainable Approach for Proton Supercapacitor Electrodes. Electrochim. Acta 2024, 475, 143651. [Google Scholar] [CrossRef]
- Yoon, J.; Kim, S.; Park, K.H.; Lee, S.; Kim, S.J.; Lee, H.; Oh, T.; Koo, C.M. Biocompatible and Oxidation-Resistant Ti3C2Tx MXene with Halogen-Free Surface Terminations. Small Methods 2023, 7, e2201579. [Google Scholar] [CrossRef]
- Kulkarni, M.; Lalic, A.; Balu, R.; Zhang, H.; Dutta, N.K.; Roy Choudhury, N. Hydrothermal Synthesis of MXenes in Alkali Environment and Development of MXene/PEDOT:PSS Composite Electrodes for Supercapacitor Applications. MRS Adv. 2024, 9, 1310–1317. [Google Scholar] [CrossRef]
- Wang, C.; Shou, H.; Chen, S.; Wei, S.; Lin, Y.; Zhang, P.; Liu, Z.; Zhu, K.; Guo, X.; Wu, X.; et al. HCl-Based Hydrothermal Etching Strategy toward Fluoride-Free MXenes. Adv. Mater. 2021, 33, 2101015. [Google Scholar] [CrossRef]
- Khan, U.; Irshad, A.; Kong, L.B.; Que, W. Synthesis of Fluorine-Free Ti3C2Tx MXenes via Acidic Activation for Enhanced Electrochemical Applications. J. Alloys Compd. 2025, 1010, 177097. [Google Scholar] [CrossRef]
- Xie, K.; Wang, J.; Xu, K.; Wei, Z.; Zhang, M.; Zhang, J. In-Situ Synthesis of Fluorine-Free MXene/TiO2 Composite for High-Performance Supercapacitor. Arab. J. Chem. 2024, 17, 105551. [Google Scholar] [CrossRef]
- Mahabari, K.; Mohili, R.D.; Patel, M.; Jadhav, A.H.; Lee, K.; Chaudhari, N.K. HF-Free Microwave-Assisted Synthesis of MXene as an Electrocatalyst for Hydrogen Evolution in Alkaline Media. Nanoscale Adv. 2024, 6, 5388–5397. [Google Scholar] [CrossRef] [PubMed]
- Latif, F.E.A.; Khalid, M.; Numan, A.; Manaf, N.A.; Mubarak, N.M.; Zaharin, H.A.; Abdullah, E.C. Microwave-Assisted Hydrothermal Synthesis of Ti3C2Tx MXene: A Sustainable and Scalable Approach Using Alkaline Etchant. J. Mol. Struct. 2025, 1329, 141407. [Google Scholar] [CrossRef]
- Sun, W.; Shah, S.A.; Chen, Y.; Tan, Z.; Gao, H.; Habib, T.; Radovic, M.; Green, M.J. Electrochemical Etching of Ti2AlC to Ti2CTx (MXene) in Low-Concentration Hydrochloric Acid Solution. J. Mater. Chem. A 2017, 5, 21663–21668. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, P.; Wang, F.; Ricciardulli, A.G.; Lohe, M.R.; Blom, P.W.M.; Feng, X. Fluoride-Free Synthesis of Two-Dimensional Titanium Carbide (MXene) Using A Binary Aqueous System. Angew. Chem. Int. Ed. 2018, 57, 15491–15495. [Google Scholar] [CrossRef]
- Chen, J.; Chen, M.; Zhou, W.; Xu, X.; Liu, B.; Zhang, W.; Wong, C. Simplified Synthesis of Fluoride-Free Ti3C2Tx via Electrochemical Etching toward High-Performance Electrochemical Capacitors. ACS Nano 2022, 16, 2461–2470. [Google Scholar] [CrossRef]
- Malaki, M.; Varma, R.S. Wetting of MXenes and Beyond. Nanomicro Lett 2023, 15, 116. [Google Scholar] [CrossRef] [PubMed]
- Nouseen, S.; Pumera, M. Electrochemical Etching of MXenes: Mechanism, Challenges and Future Outlooks. J. Mater. Chem. A 2025, 13, 34055–34084. [Google Scholar] [CrossRef]
- Klement, W.J.N.; Savino, E.; Rooijmans, S.; Mulder, P.P.M.F.A.; Lynn, N.S.; Browne, W.R.; Verpoorte, E. Electrochemical Flow Reactors: Mass Transport, I.R. Drop, and Membrane-Free Performance with In-Line Analysis. ACS Electrochem. 2025, 1, 504–515. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Lu, J.; Luo, K.; Li, Y.; Chang, K.; Chen, K.; Zhou, J.; Rosen, J.; Hultman, L.; Eklund, P.; et al. Element Replacement Approach by Reaction with Lewis Acidic Molten Salts to Synthesize Nanolaminated MAX Phases and MXenes. J. Am. Chem. Soc. 2019, 141, 4730–4737. [Google Scholar] [CrossRef]
- Kamysbayev, V.; Filatov, A.S.; Hu, H.; Rui, X.; Lagunas, F.; Wang, D.; Klie, R.F.; Talapin, D.V. Covalent Surface Modifications and Superconductivity of Two-Dimensional Metal Carbide MXenes. Science 2020, 369, 979–983. [Google Scholar] [CrossRef]
- Zhang, T.; Shevchuk, K.; Wang, R.J.; Kim, H.; Hourani, J.; Gogotsi, Y. Delamination of Chlorine-Terminated MXene Produced Using Molten Salt Etching. Chem. Mater. 2024, 36, 1998–2006. [Google Scholar] [CrossRef]
- Shepelin, N.A.; Sherrell, P.C.; Skountzos, E.N.; Goudeli, E.; Zhang, J.; Lussini, V.C.; Imtiaz, B.; Usman, K.A.S.; Dicinoski, G.W.; Shapter, J.G.; et al. Interfacial Piezoelectric Polarization Locking in Printable Ti3C2Tx MXene-Fluoropolymer Composites. Nat. Commun. 2021, 12, 3171. [Google Scholar] [CrossRef]
- Shen, Y.; Liu, Y.; Sun, K.; Gu, T.; Wang, G.; Yang, Y.; Pang, J.; Zheng, Y.; Yang, X.; Chen, L. Zincophilic Ti3C2Cl2 MXene and Anti-Corrosive Cu NPs for Synergistically Regulated Deposition of Dendrite-Free Zn Metal Anode. J. Mater. Sci. Technol. 2024, 169, 137–147. [Google Scholar] [CrossRef]
- Yan, M.; Zhu, Y.; Huang, J.; Chen, H.; Deng, Y.; Chen, Y.; Wang, J.; Albina, J.M. Etching Mechanism of Ti3C2Cl2 MXene Phases by CuCl2-Lewis Molten Salt Method. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2024, 39, 863–868. [Google Scholar] [CrossRef]
- Pang, S.Y.; Io, W.F.; Wong, L.W.; Lao, X.; Bai, Q.; Chan, K.L.; Zhao, J.; Hao, J. Fluoride-Free Molten Salt Hydrate-Assisted Synthesis of MXene in Air Down to 150 °C. Adv. Funct. Mater. 2025, 35, 2504864. [Google Scholar] [CrossRef]
- Xu, J.; Li, T.; Yan, T.; Chao, J.; Wang, R. Dehydration Kinetics and Thermodynamics of Magnesium Chloride Hexahydrate for Thermal Energy Storage. Sol. Energy Mater. Sol. Cells 2021, 219, 110819. [Google Scholar] [CrossRef]
- Shi, H.; Zhang, P.; Liu, Z.; Park, S.; Lohe, M.R.; Wu, Y.; Nia, A.S.; Yang, S.; Feng, X.; Shi, H.; et al. Ambient-Stable Two-Dimensional Titanium Carbide (MXene) Enabled by Iodine Etching. Angew. Chem. Int. Ed. 2021, 60, 8689–8693. [Google Scholar] [CrossRef]
- Munir, M.A.; Khalid, S. Focused Review on the Synthesis of Titanium Carbide MXene via Fluorine-Free Methods for Lithium-Ion Batteries. Energy Fuels 2025, 39, 2889–2915. [Google Scholar] [CrossRef]
- Wang, X.; Wang, X.; Chen, J.; Zhao, Y.; Mao, Z.; Wang, D. Durable Sodium Battery Composed of Conductive Ti3C2Tx MXene Modified Gel Polymer Electrolyte. Solid State Ion. 2021, 365, 115655. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, F.; Huang, Y.; Li, S.; Li, C.; Wang, Z.; Xie, M. A Novel Gel Polymer Electrolyte Doped with MXene Enables Dendrite-Free Cycling for High-Performance Sodium Metal Batteries. J. Mater. Chem. A 2022, 10, 11553–11561. [Google Scholar] [CrossRef]
- Liang, L.; Niu, L.; Wu, T.; Zhou, D.; Xiao, Z. Fluorine-Free Fabrication of MXene via Photo-Fenton Approach for Advanced Lithium–Sulfur Batteries. ACS Nano 2022, 16, 7971–7981. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Jo, H.; Yun, J.; Lee, J.W.; Cho, J.; Kang, K.; Lim, H.D. Sustainable and Eco-Friendly Syntheses of Green MXenes for Advanced Battery Applications. Nano Converg. 2025, 12, 39. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhou, C.; Filatov, A.S.; Cho, W.; Lagunas, F.; Wang, M.; Vaikuntanathan, S.; Liu, C.; Klie, R.F.; Talapin, D.V. Direct Synthesis and Chemical Vapor Deposition of 2D Carbide and Nitride MXenes. Science 2023, 379, 1242–1247. [Google Scholar] [CrossRef] [PubMed]
- Xiang, M.; Shen, Z.; Zheng, J.; Song, M.; He, Q.; Yang, Y.; Zhu, J.; Geng, Y.; Yue, F.; Dong, Q.; et al. Gas-Phase Synthesis of Ti2CCl2 Enables an Efficient Catalyst for Lithium-Sulfur Batteries. Innov. 2024, 5, 100540. [Google Scholar] [CrossRef]
- Yue, F.; Xiang, M.; Zheng, J.; Zhu, J.; Wei, J.; Yang, P.; Shi, H.; Dong, Q.; Ding, W.; Chen, C.; et al. One-Step Gas-Phase Syntheses of Few-Layered Single-Phase Ti2NCl2 and Ti2CCl2 MXenes with High Stabilities. Nat. Commun. 2024, 15, 10334. [Google Scholar] [CrossRef]
- Xu, C.; Wang, L.; Liu, Z.; Chen, L.; Guo, J.; Kang, N.; Ma, X.L.; Cheng, H.M.; Ren, W. Large-Area High-Quality 2D Ultrathin Mo2C Superconducting Crystals. Nat. Mater. 2015, 14, 1135–1141. [Google Scholar] [CrossRef] [PubMed]
- Turker, F.; Caylan, O.R.; Mehmood, N.; Kasirga, T.S.; Sevik, C.; Cambaz Buke, G. CVD Synthesis and Characterization of Thin Mo2C Crystals. J. Am. Ceram. Soc. 2020, 103, 5586–5593. [Google Scholar] [CrossRef]
- Xue, N.; Li, X.; Zhang, M.; Han, L.; Liu, Y.; Tao, X. Chemical-Combined Ball-Milling Synthesis of Fluorine-Free Porous MXene for High-Performance Lithium Ion Batteries. ACS Appl. Energy Mater. 2020, 3, 10234–10241. [Google Scholar] [CrossRef]
- Iravani, S.; Zarepour, A.; Nazarzadeh Zare, E.; Makvandi, P.; Khosravi, A.; Varma, R.S.; Zarrabi, A. Advancements in MXenes and Mechanochemistry: Exploring New Horizons and Future Applications. Mater. Adv. 2024, 5, 8404–8418. [Google Scholar] [CrossRef]
- Ljubek, G.; Kralj, M.; Kraljić Roković, M. Fluorine-Free Mechanochemical Synthesis of MXene. Mater. Sci. Technol. 2023, 39, 1645–1649. [Google Scholar] [CrossRef]
- Naguib, M.; Mashtalir, O.; Carle, J.; Presser, V.; Lu, J.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-Dimensional Transition Metal Carbides. ACS Nano 2012, 6, 1322–1331. [Google Scholar] [CrossRef]
- Mashtalir, O.; Naguib, M.; Mochalin, V.N.; Dall’Agnese, Y.; Heon, M.; Barsoum, M.W.; Gogotsi, Y. Intercalation and Delamination of Layered Carbides and Carbonitrides. Nat. Commun. 2013, 4, 1716. [Google Scholar] [CrossRef]
- Cockreham, C.B.; Zhang, X.; Li, H.; Hammond-Pereira, E.; Sun, J.; Saunders, S.R.; Wang, Y.; Xu, H.; Wu, D. Inhibition of AlF3·3H2O Impurity Formation in Ti3C2Tx MXene Synthesis under a Unique CoFx/HCl Etching Environment. ACS Appl. Energy Mater. 2019, 2, 8145–8152. [Google Scholar] [CrossRef]
- Zhao, F.; Zhai, P.; Wei, Y.; Yang, Z.; Chen, Q.; Zuo, J.; Gu, X.; Gong, Y. Constructing Artificial SEI Layer on Lithiophilic MXene Surface for High-Performance Lithium Metal Anodes. Adv. Sci. 2022, 9, 2103930. [Google Scholar] [CrossRef]
- Wang, H.; Ning, M.; Sun, M.; Li, B.; Liang, Y.; Li, Z. Research Progress of Functional MXene in Inhibiting Lithium/Zinc Metal Battery Dendrites. RSC Adv. 2024, 14, 26837–26856. [Google Scholar] [CrossRef] [PubMed]
- Jagger, B.; Pasta, M. Solid Electrolyte Interphases in Lithium Metal Batteries. Joule 2023, 7, 2228–2244. [Google Scholar] [CrossRef]
- Marquez, K.P.; Sisican, K.M.D.; Ibabao, R.P.; Malenab, R.A.J.; Judicpa, M.A.N.; Henderson, L.; Zhang, J.; Usman, K.A.S.; Razal, J.M. Understanding the Chemical Degradation of Ti3C2Tx MXene Dispersions: A Chronological Analysis. Small Sci. 2024, 4, 2400150. [Google Scholar] [CrossRef] [PubMed]
- Yi, R.; Zhao, Y.; Liu, C.; Sun, Y.; Zhao, C.; Li, Y.; Yang, L.; Zhao, C. A Ti3C2Tx-Based Composite as Separator Coating for Stable Li-S Batteries. Nanomaterials 2022, 12, 3770. [Google Scholar] [CrossRef]
- Lee, D.K.; Chae, Y.; Yun, H.; Ahn, C.W.; Lee, J.W. CO2-Oxidized Ti3C2Tx-MXenes Components for Lithium-Sulfur Batteries: Suppressing the Shuttle Phenomenon through Physical and Chemical Adsorption. ACS Nano 2020, 14, 9744–9754. [Google Scholar] [CrossRef]
- Zhang, C.; Feng, J.; Guo, X.; Zhang, J.; Zhang, W.; Zhang, L.; Song, J.; Shao, G.; Wang, G. Blocking Polysulfide by Physical Confinement and Catalytic Conversion of SiO2@MXene for Li-S Battery. Appl. Phys. Lett. 2023, 122, 193901. [Google Scholar] [CrossRef]
- Yu, L.; Lu, L.; Zhou, X.; Xu, L. Current Understanding of the Wettability of MXenes. Adv. Mater. Interfaces 2023, 10, 2201818. [Google Scholar] [CrossRef]
- Luo, J.; Zhang, W.; Yuan, H.; Jin, C.; Zhang, L.; Huang, H.; Liang, C.; Xia, Y.; Zhang, J.; Gan, Y.; et al. Pillared Structure Design of MXene with Ultralarge Interlayer Spacing for High-Performance Lithium-Ion Capacitors. ACS Nano 2017, 11, 2459–2469. [Google Scholar] [CrossRef]
- Maughan, P.A.; Seymour, V.R.; Bernardo-Gavito, R.; Kelly, D.J.; Shao, S.; Tantisriyanurak, S.; Dawson, R.; Haigh, S.J.; Young, R.J.; Tapia-Ruiz, N.; et al. Porous Silica-Pillared MXenes with Controllable Interlayer Distances for Long-Life Na-Ion Batteries. Langmuir 2020, 36, 4370–4382. [Google Scholar] [CrossRef]
- Ren, C.E.; Hatzell, K.B.; Alhabeb, M.; Ling, Z.; Mahmoud, K.A.; Gogotsi, Y. Charge- and Size-Selective Ion Sieving Through Ti3C2Tx MXene Membranes. J. Phys. Chem. Lett. 2015, 6, 4026–4031. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Z.; Zhu, J.; Tian, M.; Zheng, S.; Wang, F.; Wang, X.; Wang, L. Ion Sieving by a Two-Dimensional Ti3C2Tx Alginate Lamellar Membrane with Stable Interlayer Spacing. Nat. Commun. 2020, 11, 3540. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Ji, Y.; Jiang, Q.; Xia, C. Molten-Salt Synthesized MXene for Catalytic Applications: A Review. Chem. Phys. Rev. 2024, 5, 031311. [Google Scholar] [CrossRef]
- Tao, F.Y.; Xie, D.; Diao, W.Y.; Liu, C.; Sun, H.Z.; Li, W.L.; Zhang, J.P.; Wu, X.L. Highly Lithiophilic Ti3C2Tx-Mxene Anchored on a Flexible Carbon Foam Scaffolds as the Basis for a Dendrite-Free Lithium Metal Anode. New Carbon Mater. 2023, 38, 765–775. [Google Scholar] [CrossRef]
- Ha, S.; Kim, D.; Lim, H.K.; Koo, C.M.; Kim, S.J.; Yun, Y.S. Lithiophilic MXene-Guided Lithium Metal Nucleation and Growth Behavior. Adv. Funct. Mater. 2021, 31, 2101261. [Google Scholar] [CrossRef]
- Liu, Y.; Zou, W.; Zhao, N.; Xu, J. Electrically Insulating PBO/MXene Film with Superior Thermal Conductivity, Mechanical Properties, Thermal Stability, and Flame Retardancy. Nat. Commun. 2023, 14, 5342. [Google Scholar] [CrossRef]
- Abdolhosseinzadeh, S.; Jiang, X.; Zhang, H.; Qiu, J.; Zhang, C. (John) Perspectives on Solution Processing of Two-Dimensional MXenes. Mater. Today 2021, 48, 214–240. [Google Scholar] [CrossRef]
- Ali, I.; Din, M.F.U.; Gu, Z.G. MXenes Thin Films: From Fabrication to Their Applications. Molecules 2022, 27, 4925. [Google Scholar] [CrossRef]
- Pan, Q.; Zheng, Y.; Kota, S.; Huang, W.; Wang, S.; Qi, H.; Kim, S.; Tu, Y.; Barsoum, M.W.; Li, C.Y. 2D MXene-Containing Polymer Electrolytes for All-Solid-State Lithium Metal Batteries. Nanoscale Adv. 2019, 1, 395–402. [Google Scholar] [CrossRef]
- Carey, M.; Barsoum, M.W. MXene Polymer Nanocomposites: A Review. Mater. Today Adv. 2021, 9, 100120. [Google Scholar] [CrossRef]
- Xia, B.; Wang, Z.; Wang, T.; Chen, S.; Wu, H.; Zhang, B.; Si, Y.; Chen, Z.; Li, B.W.; Kou, Z.; et al. Bridging Sheet Size Controls Densification of MXene Films for Robust Electromagnetic Interference Shielding. iScience 2022, 25, 105001. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Sun, X.; Zhang, H.; Yuan, C.; Wei, Y.; Li, J. Preparation Strategies and Applications of MXene-Polymer Composites: A Review. Macromol. Rapid Commun. 2021, 42, 2100324. [Google Scholar] [CrossRef]
- Adekoya, G.J.; Adekoya, O.C.; Sadiku, R.E.; Hamam, Y.; Ray, S.S. Applications of MXene-Containing Polypyrrole Nanocomposites in Electrochemical Energy Storage and Conversion. ACS Omega 2022, 7, 39498–39519. [Google Scholar] [CrossRef] [PubMed]
- Tian, M.; Gan, W.; Oh, E.S. MXene Clay (Ti2C)-Containing In Situ Polymerized Hollow Core–Shell Binder for Silicon-Based Anodes in Lithium-Ion Batteries. ACS Omega 2023, 8, 49302–49310. [Google Scholar] [CrossRef]
- Nazarlou, Z.; Hosseini, S.F.; Seyed Dorraji, M.S.; Rasoulifard, M.H.; Aydemir, U. Ti3C2 MXene/Polyaniline/Montmorillonite Nanostructures toward Solvent-Free Powder Coatings with Enhanced Corrosion Resistance and Mechanical Properties. ACS Appl. Nano Mater. 2023, 6, 8804–8818. [Google Scholar] [CrossRef]
- Iqbal, A.; Hong, J.; Ko, T.Y.; Koo, C.M. Improving Oxidation Stability of 2D MXenes: Synthesis, Storage Media, and Conditions. Nano Converg. 2021, 8, 9. [Google Scholar] [CrossRef] [PubMed]
- Soomro, R.A.; Zhang, P.; Fan, B.; Wei, Y.; Xu, B. Progression in the Oxidation Stability of MXenes. Nano-Micro Lett. 2023, 15, 108. [Google Scholar] [CrossRef] [PubMed]
- Chae, W.; Kim, B.; Ryoo, W.S.; Earmme, T. A Brief Review of Gel Polymer Electrolytes Using In Situ Polymerization for Lithium-Ion Polymer Batteries. Polymers 2023, 15, 803. [Google Scholar] [CrossRef]
- Liu, J.; Wu, Y.; Chen, Z.; Xu, Z.; Zhu, J.; Zhuang, X. In Situ Polymerization-Driven Quasi-Solid Electrolytes for Li-Metal Batteries. Mater. Chem. Front. 2025, 9, 1971–1996. [Google Scholar] [CrossRef]
- Huang, T.; Xiong, W.; Ye, X.; Huang, Z.; Feng, Y.; Liang, J.; Ye, S.; Huang, S.; Li, Y.; Ren, X.; et al. Constructing Robust Polymer/Two-Dimensional Ti3C2TX Solid-State Electrolyte Interphase via in-Situ Polymerization for High-Capacity Long-Life and Dendrite-Free Lithium Metal Anodes. J. Colloid Interface Sci. 2022, 628, 583–594. [Google Scholar] [CrossRef]
- Huang, Z.; Huang, T.; Ye, X.; Feng, X.; Yang, X.; Liang, J.; Ye, S.; Li, Y.; Ren, X.; Xiong, W.; et al. Constructing Methyl Methacrylate/MXene Artificial Solid-Electrolyte Interphase Layer for Lithium Metal Batteries with High Electrochemical Performance. Appl. Surf. Sci. 2022, 605, 154586. [Google Scholar] [CrossRef]
- Yao, W.; He, S.; Xue, Y.; Zhang, Q.; Wang, J.; He, M.; Xu, J.; Chen, C.; Xiao, X. V2CTx MXene Artificial Solid Electrolyte Interphases toward Dendrite-Free Lithium Metal Anodes. ACS Sustain. Chem. Eng. 2021, 9, 9961–9969. [Google Scholar] [CrossRef]
- Moussaei, M.; Babazadeh-Mamaqani, M.; Roghani-Mamaqani, H.; Haddadi-Asl, V.; Riazi, H. Polymer Grafting on MXene as a Versatile Nanoplatform: Synthesis and Applications. Coord. Chem. Rev. 2025, 544, 216989. [Google Scholar] [CrossRef]
- Mozafari, M.; Soroush, M. Surface Functionalization of MXenes. Mater. Adv. 2021, 2, 7277–7307. [Google Scholar] [CrossRef]
- Yousaf, T.; Areeb, A.; Murtaza, M.; Munir, A.; Khan, Y.; Waseem, A. Silane-Grafted MXene (Ti3C2TX) Membranes for Enhanced Water Purification Performance. ACS Omega 2022, 7, 19502–19512. [Google Scholar] [CrossRef]
- Jing, H.; Yeo, H.; Lyu, B.; Ryou, J.; Choi, S.; Park, J.H.; Lee, B.H.; Kim, Y.H.; Lee, S. Modulation of the Electronic Properties of MXene (Ti3C2Tx) via Surface-Covalent Functionalization with Diazonium. ACS Nano 2021, 15, 1388–1396. [Google Scholar] [CrossRef]
- Ji, J.; Zhao, L.; Shen, Y.; Liu, S.; Zhang, Y. Covalent Stabilization and Functionalization of MXene via Silylation Reactions with Improved Surface Properties. FlatChem 2019, 17, 100128. [Google Scholar] [CrossRef]
- Lee, G.S.; Yun, T.; Kim, H.; Kim, I.H.; Choi, J.; Lee, S.H.; Lee, H.J.; Hwang, H.S.; Kim, J.G.; Kim, D.W.; et al. Mussel Inspired Highly Aligned Ti3C2Tx MXene Film with Synergistic Enhancement of Mechanical Strength and Ambient Stability. ACS Nano 2020, 14, 11722–11732. [Google Scholar] [CrossRef]
- Tao, N.; Zhang, D.; Li, X.; Lou, D.; Sun, X.; Wei, C.; Li, J.; Yang, J.; Liu, Y.N. Near-Infrared Light-Responsive Hydrogels via Peroxide-Decorated MXene-Initiated Polymerization. Chem. Sci. 2019, 10, 10765–10771. [Google Scholar] [CrossRef]
- Neal, N.N.; Arole, K.; Cao, H.; Kotasthane, V.; Xiang, S.; Ross, D.; Stevenson, P.R.; Radovic, M.; Green, M.J.; Lutkenhaus, J.L. Controlled Layer-by-Layer Assembly and Structured Coloration of Ti3C2Tz MXene/Polyelectrolyte Heterostructures. npj 2D Mater. Appl. 2024, 8, 76. [Google Scholar] [CrossRef]
- Echols, I.J.; An, H.; Zhao, X.; Prehn, E.M.; Tan, Z.; Radovic, M.; Green, M.J.; Lutkenhaus, J.L. PH-Response of Polycation/Ti3C2Tx MXene Layer-by-Layer Assemblies for Use as Resistive Sensors. Mol. Syst. Des. Eng. 2020, 5, 366–375. [Google Scholar] [CrossRef]
- Tian, W.; VahidMohammadi, A.; Wang, Z.; Ouyang, L.; Beidaghi, M.; Hamedi, M.M. Layer-by-Layer Self-Assembly of Pillared Two-Dimensional Multilayers. Nat. Commun. 2019, 10, 2558. [Google Scholar] [CrossRef] [PubMed]
- Lipton, J.; Weng, G.M.; Röhr, J.A.; Wang, H.; Taylor, A.D. Layer-by-Layer Assembly of Two-Dimensional Materials: Meticulous Control on the Nanoscale. Matter 2020, 2, 1148–1165. [Google Scholar] [CrossRef]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and Electrospun Nanofibers: Methods, Materials, and Applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.M.; Zhang, Y.Z.; Kotaki, M.; Ramakrishna, S. A Review on Polymer Nanofibers by Electrospinning and Their Applications in Nanocomposites. Compos. Sci. Technol. 2003, 63, 2223–2253. [Google Scholar] [CrossRef]
- Al-Abduljabbar, A.; Farooq, I. Electrospun Polymer Nanofibers: Processing, Properties, and Applications. Polymers 2022, 15, 65. [Google Scholar] [CrossRef]
- Robinson, A.J.; Pérez-Nava, A.; Ali, S.C.; González-Campos, J.B.; Holloway, J.L.; Cosgriff-Hernandez, E.M. Comparative Analysis of Fiber Alignment Methods in Electrospinning. Matter 2021, 4, 821–844. [Google Scholar] [CrossRef]
- Ahmadi Bonakdar, M.; Rodrigue, D. Electrospinning: Processes, Structures, and Materials. Macromolecules 2024, 4, 58–103. [Google Scholar] [CrossRef]
- He, J.; Yang, L.; Ruan, X.; Liu, Z.; Liao, K.; Duan, Q.; Zhan, Y. Electrospun PVDF-Based Polymers for Lithium-Ion Battery Separators: A Review. Polymers 2024, 16, 2895. [Google Scholar] [CrossRef]
- Sun, X.; Zhou, Y.; Li, D.; Zhao, K.; Wang, L.; Tan, P.; Dong, H.; Wang, Y.; Liang, J. A Review of Electrospun Separators for Lithium-Based Batteries: Progress and Application Prospects. Carbon Energy 2024, 6, e539. [Google Scholar] [CrossRef]
- Wu, Y.; Wei, W.; Feng, T.; Li, W.; Wang, X.; Wu, T.; Zhang, X. Electrospun MXene/Polyimide Nanofiber Composite Separator for Enhancing Thermal Stability and Ion Transport of Lithium-Ion Batteries. Front. Chem. 2025, 13, 1555323. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Liu, S.; Li, Z.; Ding, F.; Liu, J.; Wang, W.; Song, K.; Liu, T.; Hu, L. Synergistic Effect of Ti3C2Tx MXene/PAN Nanofiber and LLZTO Particles on High-Performance PEO-Based Solid Electrolyte for Lithium Metal Battery. J. Colloid Interface Sci. 2024, 668, 634–645. [Google Scholar] [CrossRef]
- Márquez, F. MXenes in Solid-State Batteries: Multifunctional Roles from Electrodes to Electrolytes and Interfacial Engineering. Batteries 2025, 11, 364. [Google Scholar] [CrossRef]
- Chen, L.; Du, Y.; Xie, Y.; Jia, G.; Zhu, Y.; Feng, D.; Meng, Y.; Mei, Y.; Xie, D. MXene-Enhanced PEGDA Crosslinked Quasi-Solid Electrolytes: A Flame-Retardant 3D Network for High-Performance Sodium-Ion Batteries. J. Mater. Chem. A 2025, 13, 25732–25748. [Google Scholar] [CrossRef]
- Cheng, L.; Tian, R.; Zhao, Y.; Wei, Z.; Pu, X.; Zhu, Y.L.; Zhang, D.; Du, F. Small Things Make a Big Difference: Conductive Cross-Linking Sodium Alginate@MXene Binder Enables High-Volumetric-Capacity and High-Mass-Loading Li-S Battery. Nano Lett. 2023, 23, 10538–10544. [Google Scholar] [CrossRef] [PubMed]
- Hadad, S.; Hamrahjoo, M.; Khezraqa, H.; Golshan, M.; Wang, Z.; Salami-Kalajahi, M. Starch Acetate Grafted to MXene Composite Surpasses Room Temperature Liquid Electrolyte Performance for All-Solid-State Lithium-Ion Batteries. Adv. Sci. 2025, 12, e03285. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Wang, J.; Zhong, L.; Qi, G.; Liu, F.; Pan, Y.; Yang, F.; Wang, X.; Li, J.; Li, L.; et al. Recent Advances on Cellulose-Based Solid Polymer Electrolytes. Ind. Chem. Mater. 2025, 3, 31–48. [Google Scholar] [CrossRef]
- Geng, K.; He, T.; Liu, R.; Dalapati, S.; Tan, K.T.; Li, Z.; Tao, S.; Gong, Y.; Jiang, Q.; Jiang, D. Covalent Organic Frameworks: Design, Synthesis, and Functions. Chem. Rev. 2020, 120, 8814–8933. [Google Scholar] [CrossRef]
- Nabeela, K.; Deka, R.; Abbas, Z.; Kumar, P.; Saraf, M.; Mobin, S.M. Covalent Organic Frameworks (COFs)/MXenes Heterostructures for Electrochemical Energy Storage. Cryst. Growth Des. 2023, 23, 3057–3078. [Google Scholar] [CrossRef]
- Pathak, D.D.; Neem, M.; Kumar, G.; Modak, B.; Neogi, S.; Mandal, B.P. Diamondoid Covalent Organic Framework-MXene Composite for Cathode Host in Lithium Sulfur Battery. J. Energy Storage 2025, 117, 116176. [Google Scholar] [CrossRef]
- Liu, H.; Sun, S.; Jin, B. Recent Progress in MXenes-Based Lithium-Sulfur Batteries. Energy Mater. 2024, 4, 400053. [Google Scholar] [CrossRef]
- Habib, T.; Zhao, X.; Shah, S.A.; Chen, Y.; Sun, W.; An, H.; Lutkenhaus, J.L.; Radovic, M.; Green, M.J. Oxidation Stability of Ti3C2Tx MXene Nanosheets in Solvents and Composite Films. npj 2D Mater. Appl. 2019, 3, 8. [Google Scholar] [CrossRef]
- Zhao, X.; Vashisth, A.; Prehn, E.; Sun, W.; Shah, S.A.; Habib, T.; Chen, Y.; Tan, Z.; Lutkenhaus, J.L.; Radovic, M.; et al. Antioxidants Unlock Shelf-Stable Ti3C2Tx (MXene) Nanosheet Dispersions. Matter 2019, 1, 513–526. [Google Scholar] [CrossRef]
- Huang, C.; Dontigny, M.; Zaghib, K.; Grant, P.S. Low-Tortuosity and Graded Lithium Ion Battery Cathodes by Ice Templating. J. Mater. Chem. A 2019, 7, 21421–21431. [Google Scholar] [CrossRef]
- He, F.; Ye, J.; Cao, Y.; Xiao, L.; Yang, H.; Ai, X. Coaxial Three-Layered Carbon/Sulfur/Polymer Nanofibers with High Sulfur Content and High Utilization for Lithium–Sulfur Batteries. ACS Appl. Mater. Interfaces 2017, 9, 11626–11633. [Google Scholar] [CrossRef]
- Dong, Y.; Zheng, S.; Qin, J.; Zhao, X.; Shi, H.; Wang, X.; Chen, J.; Wu, Z.S. All-MXene-Based Integrated Electrode Constructed by Ti3C2 Nanoribbon Framework Host and Nanosheet Interlayer for High-Energy-Density Li–S Batteries. ACS Nano 2018, 12, 2381–2388. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, A.V.; Rosen, J.; Gogotsi, Y. The World of Two-Dimensional Carbides and Nitrides (MXenes). Science 2021, 372, eabf1581. [Google Scholar] [CrossRef]
- Qian, Z.; Qiu, Z.D.; Wang, R.Q.; Wei, M.T.; Fei, A.M.; Hu, Z.Y.; Mohamed, H.S.H.; Chen, L.H.; Li, Y.; Su, B.L. Ionic Liquid-Grafted MXene Composite Polymer Electrolytes for High-Performance Solid-State Batteries. Chem. Eng. J. 2025, 514, 163121. [Google Scholar] [CrossRef]
- Xu, H.; Liu, S.; Li, Z.; Ding, F.; Wang, T.; Liu, T.; Wang, W.; Song, K.; Liu, J.; Hu, L. Ti3C2Tx MXene Enhanced PEO/SN-Based Solid Electrolyte for High-Performance Li Metal Battery. J. Mater. Sci. Technol. 2025, 219, 101–112. [Google Scholar] [CrossRef]
- Xiang, H.; Zhang, F.; Zou, B.; Hou, Q.; Cheng, C.; Lu, M.; Wang, X.; Ping, W.; Sun, Y.; Song, X. High-Performance Lithium Batteries Achieved by Electrospun MXene-Enhanced Cation-Selective Membranes. J. Membr. Sci. 2024, 704, 122867. [Google Scholar] [CrossRef]
- Kim, S.; Lee, J.; Choi, S.; Woo, S.; Hong, S.; Kim, H.; Kim, J.; Lee, D. Regulating Solvation Dynamics and Lithium Plating via Ti3C2Tx-Engineered PVDF-HFP Separators. Adv. Energy Mater. 2025, 15, e03091. [Google Scholar] [CrossRef]
- Kim, S.; Lee, S.; Ryu, H.S.; Jo, H.; Yun, J.; Mun, S.; Park, S.; Kim, K.; Lim, H.D. Fluorine-Aligned Functional MXene Enabling Unusual Bead-like Li Growth for Anode-Less Li-Metal Batteries. Chem. Eng. J. 2025, 513, 162294. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Z.; Chen, D.; Ma, F.; Huang, J.; Wang, Y.; Wang, L.; Wu, Y.; Chen, Y. A Dual-Protective MXene/COF Artificial Interface for Dendrite-Free and Stable Lithium Metal Anodes. Adv. Funct. Mater. 2025, 35, 2505390. [Google Scholar] [CrossRef]
- Shovon, O.G.; Wong, F.E.Y.; Niu, J. Designing Lithiophilic Lithium Metal Surface by a Hybrid Covalent Organic Framework and MXene Coating. Small 2025, 21, 2501769. [Google Scholar] [CrossRef]
- Shi, C.; Huang, J.; Cen, Z.; Yi, T.; Liu, S.; Fu, R. MXene-Based Polymer Brushes Decorated with Small-Sized Ag Nanoparticles Enabled High-Performance Lithium Host for Stable Lithium Metal Battery. Carbon 2024, 217, 118616. [Google Scholar] [CrossRef]
- Wang, C.Y.; Zheng, Z.J.; Feng, Y.Q.; Ye, H.; Cao, F.F.; Guo, Z.P. Topological Design of Ultrastrong MXene Paper Hosted Li Enables Ultrathin and Fully Flexible Lithium Metal Batteries. Nano Energy 2020, 74, 104817. [Google Scholar] [CrossRef]
- Yun, J.; Echols, I.; Flouda, P.; Wang, S.; Easley, A.; Zhao, X.; Tan, Z.; Prehn, E.; Zi, G.; Radovic, M.; et al. Layer-by-Layer Assembly of Polyaniline Nanofibers and MXene Thin-Film Electrodes for Electrochemical Energy Storage. ACS Appl. Mater. Interfaces 2019, 11, 47929–47938. [Google Scholar] [CrossRef]
- Lu, D.; Li, J.; Zhang, D.; Li, L.; Tong, Z.; Ji, H.; Wang, J.; Chi, C.; Qu, H.Y. Layer-by-Layer-Assembled Polyaniline/MXene Thin Film and Device for Improved Electrochromic and Energy Storage Capabilities. ACS Appl. Polym. Mater. 2024, 6, 12492–12502. [Google Scholar] [CrossRef]
- Li, F.; Mei, S.; Ye, X.; Yuan, H.; Li, X.; Tan, J.; Zhao, X.; Wu, T.; Chen, X.; Wu, F.; et al. Enhancing Lithium–Sulfur Battery Performance with MXene: Specialized Structures and Innovative Designs. Adv. Sci. 2024, 11, 2404328. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Liu, Y.; Zhao, F.; Li, J.; Yang, H.; Wang, Y.; He, Y. Multifunction of MXene in Lithium–Sulfur Batteries: A Review. Energy Fuels 2024, 38, 13837–13857. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, C.; He, W.; Zhang, C.; Zhou, L.; Wang, G.; Wei, W. MXene and MXene-Based Materials for Lithium-Sulfur Batteries. Prog. Nat. Sci. Mater. Int. 2021, 31, 501–513. [Google Scholar] [CrossRef]
- Wang, J.; Zhai, P.; Zhao, T.; Li, M.; Yang, Z.; Zhang, H.; Huang, J. Laminar MXene-Nafion-Modified Separator with Highly Inhibited Shuttle Effect for Long-Life Lithium–Sulfur Batteries. Electrochim. Acta 2019, 320, 134558. [Google Scholar] [CrossRef]
- Li, J.; Jin, Q.; Yin, F.; Zhu, C.; Zhang, X.; Zhang, Z. Effect of Ti3C2Tx–PEDOT:PSS Modified-Separators on the Electrochemical Performance of Li–S Batteries. RSC Adv. 2020, 10, 40276–40283. [Google Scholar] [CrossRef]
- Li, Y.; Li, M.; Zhu, Y.C.; Song, S.; Li, S.N.; Aarons, J.; Tang, L.C.; Bae, J. Polysulfide-Inhibiting, Thermotolerant and Nonflammable Separators Enabled by DNA Co-Assembled CNT/MXene Networks for Stable High-Safety Li–S Batteries. Compos. Part B Eng. 2023, 251, 110465. [Google Scholar] [CrossRef]
- Liu, Y.; Meng, X.; Wang, Z.; Qiu, J. Development of Quasi-Solid-State Anode-Free High-Energy Lithium Sulfide-Based Batteries. Nat. Commun. 2022, 13, 4415. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, H.; Cui, Y.; Da, H.; Wu, H.; Cai, Y.; Zhang, S. Constructing Robust Cathode/Li Interfaces and Intensifying Ion Transport Kinetics for PEO-Based Solid-State Lithium-Sulfur Batteries. Chem. Eng. J. 2023, 454, 140385. [Google Scholar] [CrossRef]
- Liu, S.M.; Chen, M.X.; Xie, Y.; Liu, D.H.; Zheng, J.F.; Xiong, X.; Jiang, H.; Wang, L.C.; Luo, H.; Han, K. Nb2CTx MXene Boosting PEO Polymer Electrolyte for All-Solid-State Li-S Batteries: Two Birds with One Stone Strategy to Enhance Li+ Conductivity and Polysulfide Adsorptivity. Rare Met. 2023, 42, 2562–2576. [Google Scholar] [CrossRef]
- Yao, Y.; Feng, W.; Chen, M.; Zhong, X.; Wu, X.; Zhang, H.; Yu, Y.; Yao, Y.; Chen, M.; Zhong, X.; et al. Boosting the Electrochemical Performance of Li–S Batteries with a Dual Polysulfides Confinement Strategy. Small 2018, 14, 1802516. [Google Scholar] [CrossRef]
- Yan, F.; Lu, L.; Ye, C.; Chen, Q.; Kumar, S.; Li, W.; Hemmatpour, H.; Spyrou, K.; de Graaf, S.; Stuart, M.C.A.; et al. Nitrogen-Doped Ti3C2Tx Coated with a Molecularly Imprinted Polymer as Efficient Cathode Material for Lithium-Sulfur Batteries. Electrochim. Acta 2025, 520, 145851. [Google Scholar] [CrossRef]
- Cao, Y.; Jia, Y.; Meng, X.; Fan, X.; Zhang, J.; Zhou, J.; Matoga, D.; Bielawski, C.W.; Geng, J. Covalently Grafting Conjugated Porous Polymers to MXene Offers a Two-Dimensional Sandwich-Structured Electrocatalytic Sulfur Host for Lithium–Sulfur Batteries. Chem. Eng. J. 2022, 446, 137365. [Google Scholar] [CrossRef]
- Yang, C.; Yu, Z.; Jian, C.; Li, T.; Tian, L.; Liu, H. Molten Salt Etched Ti3C2Tx MXene for Ameliorated Electrochemical Performances of Lithium-Sulfur Batteries. J. Mater. Sci. Mater. Electron. 2023, 34, 718. [Google Scholar] [CrossRef]
- Van Lam, D.; Nguyen, V.H.; Yoo, H.; Dung, D.T.; Syed, S.A.; Ha, J.; Oh, W.; Lee, S.M.; Oh, I.K. Dry Synthesis of Sulfur-Terminated MXene as Multifunctional Catalyst for Stable Lithium–Sulfur Batteries. Small 2025, 21, 2411668. [Google Scholar] [CrossRef]
- Wang, H.; de Kogel, A.; Wang, Z.; Zou, R.; Wang, X. Strategies of Tailoring 2D MXenes for Enhancing Sulfur-Based Battery Performance. Chem. Eng. J. 2025, 506, 159924. [Google Scholar] [CrossRef]
- Oyehan, T.A.; Salami, B.A.; Abdulrasheed, A.A.; Hambali, H.U.; Gbadamosi, A.; Valsami-Jones, E.; Saleh, T.A. MXenes: Synthesis, Properties, and Applications for Sustainable Energy and Environment. Appl. Mater. Today 2023, 35, 101993. [Google Scholar] [CrossRef]
- Shuck, C.E.; Ventura-Martinez, K.; Goad, A.; Uzun, S.; Shekhirev, M.; Gogotsi, Y. Safe Synthesis of MAX and MXene: Guidelines to Reduce Risk during Synthesis. ACS Chem. Health Saf. 2021, 28, 326–338. [Google Scholar] [CrossRef]
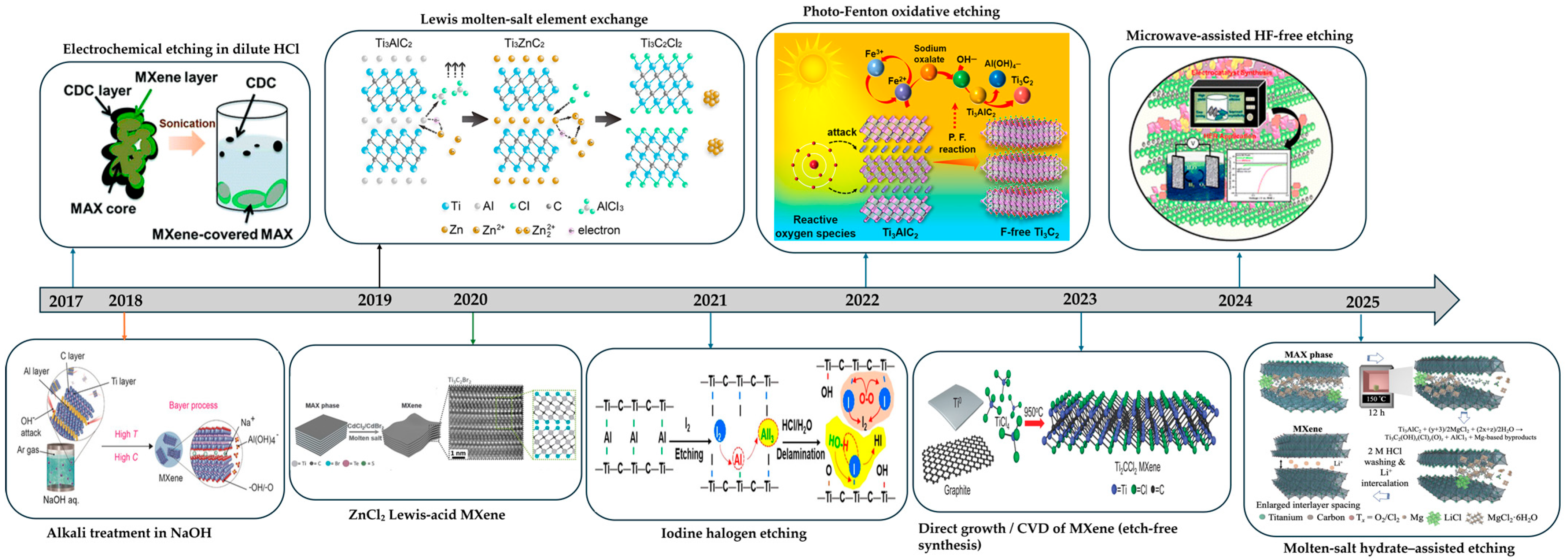
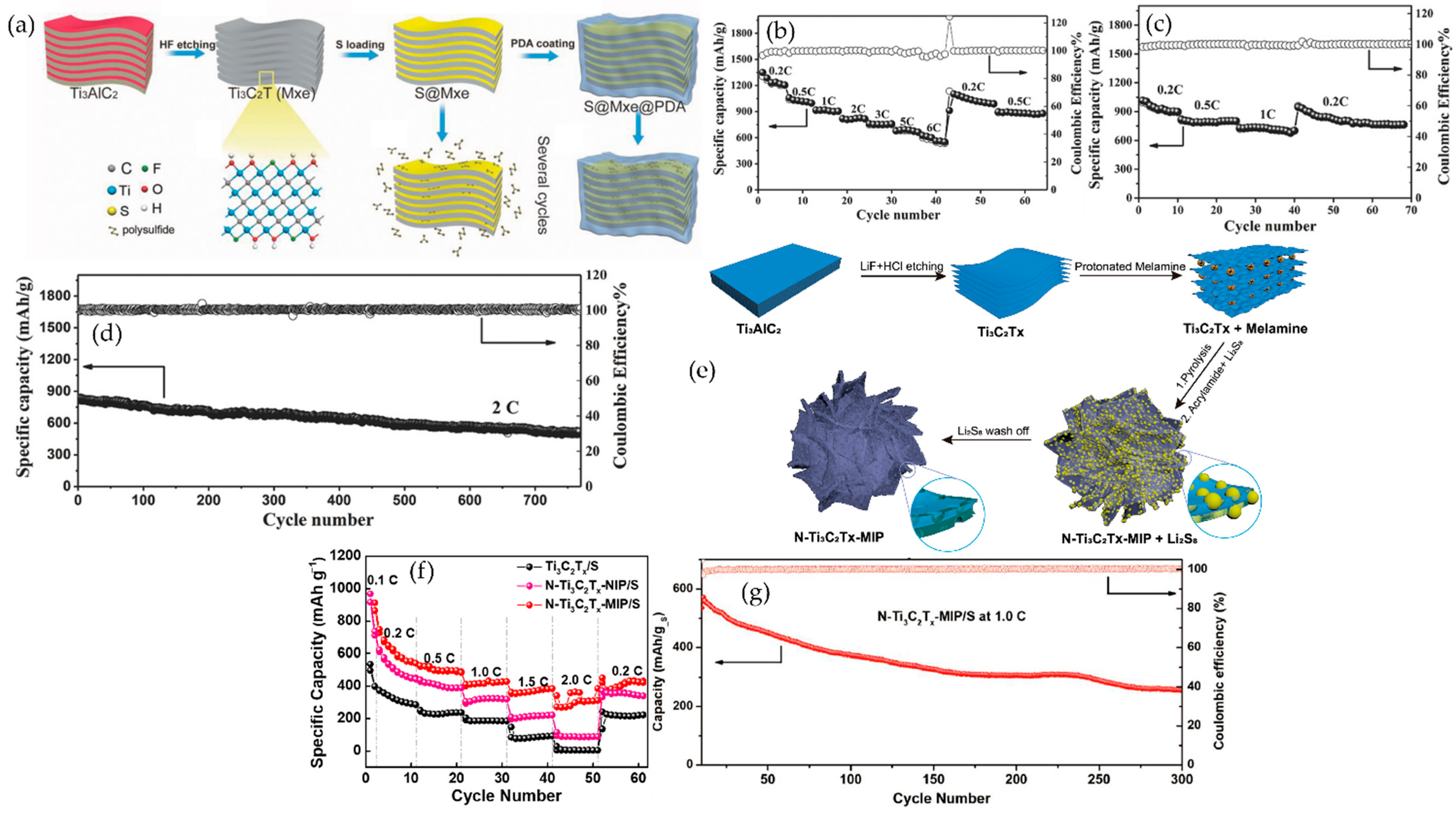
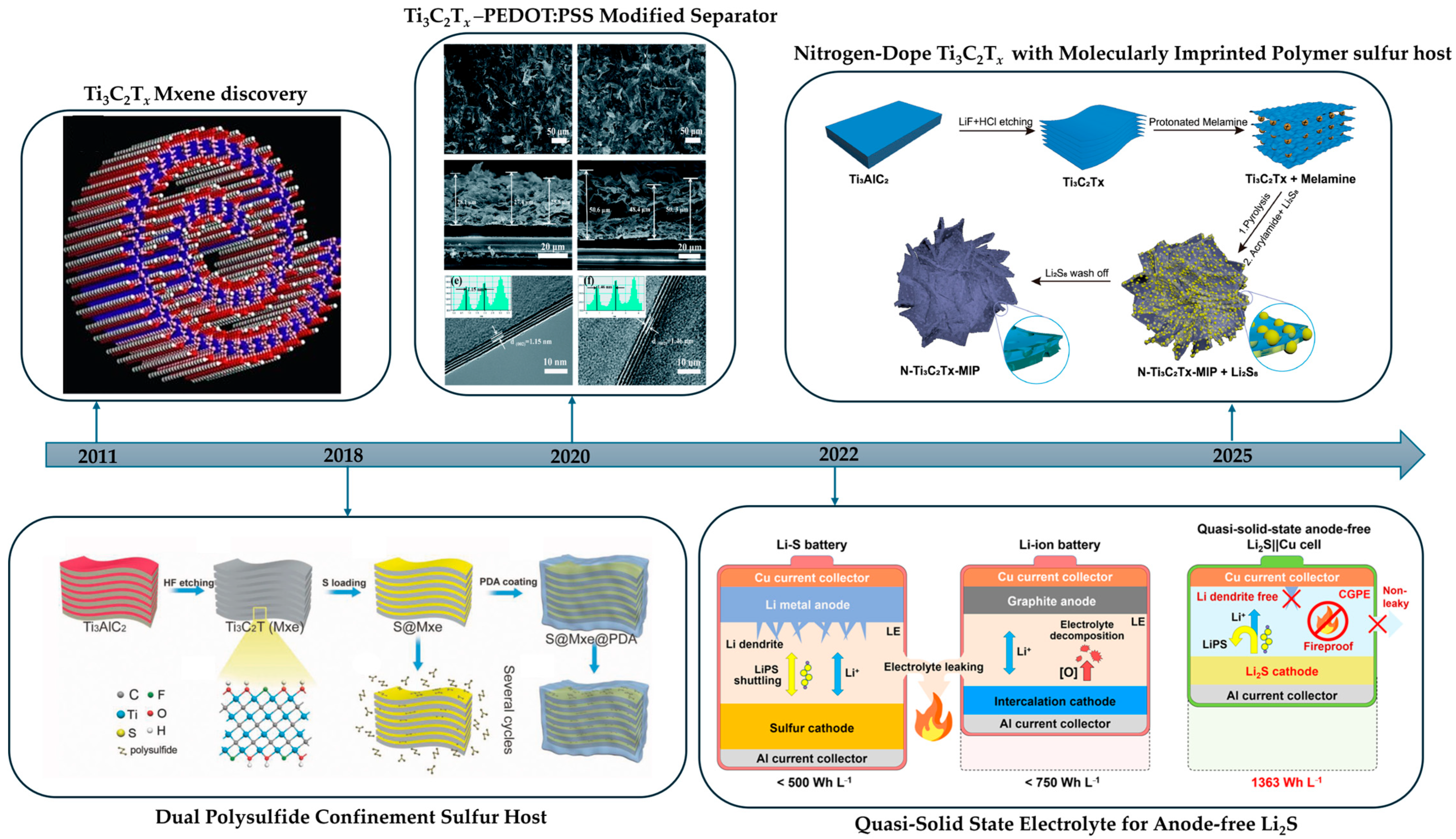
| Method | Typical Etching Window (Concentration, T, Time) | Key Features | Advantages | Disadvantages | Surface Terminations | Refs |
|---|---|---|---|---|---|---|
| Direct HF | 20–50 wt% HF, 25 °C, 12–48 h | Concentrated HF at room temperature removes Al fast and delaminates after DMSO or TBAOH, yielding F-bearing Ti3C2Tx. | Very fast and simple with a large know-how base. | Uses hazardous HF, gives F-rich surfaces, and risks AlF3·3H2O. | Mostly F with −OH/−O mixed. | [10,65,66] |
| In situ HF | 6–9 M HCl with LiF, 25–40 °C, 12–48 h | LiF with HCl generates HF while Li+ and water pre-intercalate, giving clay-like MXene that delaminates easily and remains F-bearing. | Easier handling than neat HF and delaminates easily at scale. | Still fluorine-bearing and needs careful washing to avoid AlF3·3H2O. | −F/−OH/−O mixed, usually less F than neat HF. | [19,21,67] |
| Bifluoride etching | Concentrated bifluoride solution (NH4HF2, KHF2), 25–60 °C, 24–72 h | Bifluoride salts buffer HF at 25–60 °C and expand the galleries, providing controllable etching that is still F-bearing. | Buffered HF broadens the window and often increases interlayer spacing. | Etching is slower and fluorine remains to manage. | −F/−OH/−O mixed from buffered HF. | [23,24] |
| Alkali hydrothermal etching | 27–30 M NaOH (or KOH), 180–280 °C, 12–48 h (autoclave) | Concentrated NaOH under hydrothermal conditions leaches Al and creates fluoride-free O or OH terminated MXene. | Fluoride-free with −O/−OH-rich, hydrophilic surfaces suited to water processing. | Requires strict control to avoid titanates and safe handling of hydrogen. | Predominantly −O/−OH, fluoride-free. | [25,29,30,31] |
| Acidic hydrothermal | 6–12 M acid (HCl), 160–200 °C, 12–48 h (autoclave) | HCl in an autoclave complexes the A layer and yields fluoride-free MXene with mixed Cl and O or OH terminations. | Fluoride-free with tunable Cl and O terminations using simple reagents. | Demands corrosion-proof hardware and thorough chloride removal. | Mixed −Cl with −O/−OH. | [32,33] |
| Salt-assisted alkali etching | Concentrated NaOH with salt or mild oxidant, 270 °C, 12 h (autoclave) | NaOH with a mild oxidant accelerates leaching and forms a thin TiO2 spacing layer, producing fluoride-free O or OH surfaces. | Faster kinetics and a thin TiO2 skin that reduces restacking. | Over-oxidation can occur and chloride must be rinsed well. | −O/−OH with a thin TiO2 interfacial layer. | [34] |
| Microwave–hydrothermal | Concentrated NaOH, 160–220 °C, 30–45 min (microwave-assisted autoclave) | Microwave heating speeds alkaline hydrothermal etching near 180 °C and narrows thickness while staying fluoride-free. | Short dwell time and narrow thickness distribution without fluorides. | Needs microwave-rated reactors with uniform fields and pressure control. | −O/−OH from alkaline media. | [35,36] |
| Electrochemical etching | 1 M NH4Cl + 0.2 M TMAOH, ~5 V, room temperature, ~5 h (electrochemical cell) | Chloride electrolytes under applied bias remove Al and hydroxide writes O or OH terminations with widened galleries, fluoride-free. | Fluoride-free with tunable terminations and large few-layer flakes. | Over-etching can form a CDC skin and scale-up is engineering heavy. | −O/−OH with optional −Cl depending on electrolyte. | [37,38,39] |
| Lewis-acid molten-salt | Lewis-acidic molten salts (ZnCl2/CuCl2), 550–800 °C, ~1.5–3 h (sealed ampoule) | Molten Lewis salts at high temperature replace Al and halogenate the surface, giving halogen-terminated MXene without HF. | HF-free with programmable Cl, Br, or I terminations and high conductivity. | Runs at high temperature and can cause melt corrosion and residues. | Designed −Cl/−Br/−I halogen terminations. | [26,44,45] |
| Low-temperature hydrated molten-salt | LiCl/MgCl2·6H2O (molten salt hydrate), 150 °C, ≈10–12 h (muffle furnace in air) | LiCl and MgCl2·6H2O near 150 °C in air create a semi-molten shield that etches gently and delaminates spontaneously with mixed Cl and O or OH terminations. | Mild, air-operable conditions with spontaneous delamination and polymer-friendly surfaces. | The window is narrow, yields are moderate, and an acid cleanup is needed. | Mixed −Cl and −O/−OH. | [49] |
| Iodine-assisted non-aqueous etching | I2 in anhydrous CH3CN (Ti3AlC2:I2 ≈ 1:3), 100 °C, ~4 days (sealed, halogen-assisted etching in organic solvent) | I2 in dry solvent forms an I-terminated intermediate that converts to −O or −OH during work-up and remains fluoride-free. | Fluoride-free route that gives oxygen-rich, stable, and conductive films. | Requires dry handling and a post-exchange and delamination step. | I-terminated intermediate that converts to −O/−OH after work-up. | [51] |
| Photo-Fenton soft-chemistry etching | Aqueous Na2C2O4/Fe3+ photo-Fenton solution (pH = 3, Na2C2O4:Fe3+ = 3:1) with added H2O2, room temperature, ~10 h under UV–vis irradiation (batch reactor). | Light-driven Fe and H2O2 generate radicals that remove Al under mild acidity and produce fluoride-free O or OH terminations. | Green, low-temperature chemistry that yields O/OH-rich MXene. | Residual iron and oxidants must be removed and TiO2 growth must be limited. | Predominantly −O/−OH, fluoride-free. | [55] |
| Chemical vapor deposition | Metal and halide precursors (e.g., Ti/TiCl4, TiCl3 or Mo/CH4), 650–1100 °C, 0.5–3 h (gas-phase CVD in quartz tube furnace) | Gas-phase growth yields Ti2CCl2 or Ti2NCl2 films with halogen terminations at wafer scale without HF. | Wafer-scale films with very low sheet resistance and precise control. | The thermal budget is high and transfer or activation is required. | As-grown −Cl on Ti2CCl2/Ti2NCl2. | [57,58,59] |
| Mechanochemical | 0.25 M LiCl + 1 M TMAOH aqueous etchant, ball-milled with Ti3AlC2 at 400 rpm (15 min reverse rotation), room temperature (chemical-combined ball-milling, fluorine-free porous Ti3C2). | Near-dry ball-milling with base and salt couples −OH etching with defect-assisted delamination and gives fluoride-free O or OH terminations. | Solvent-lean and scalable with high-area −O/−OH- rich flakes. | Contamination and oxidation can occur and yields and terminations vary. | Predominantly −O/−OH with defect-rich edges. | [62,64] |
| Pillars | Must Report | Measurements | Link to Device Metrics | Practical and Green Controls |
|---|---|---|---|---|
| Terminations and wetting | Termination ensemble by XPS, surface energy and contact angles, solvent system noted as NMP-free or not | XPS with fitting notes, advancing and receding angles at controlled humidity, zeta potential for dispersion stability | Interfacial impedance for Li-facing layers, ESR for Li–S electrodes, electrolyte uptake and wetting time | Limit oxidation during dispersion, shorten water dwell time, use antioxidants when compatible, match O- or OH-rich MXene to ether or nitrile matrices, match Cl- and O-rich MXene to fluoropolymers or ionogels |
| 2D ion pathways and spacing | d(002) and its humidity dependence, flake size and thickness distributions, alignment or tortuosity indicator | XRD or SAXS with humidity control, temperature-dependent impedance for activation energy, microscopy for alignment and porosity | Ionic conductivity at stated thickness, CCD and Li+ flux uniformity in LMB, rate retention at matched thickness in Li–S | Remove interlayer water after casting, use gentle intercalants or pillaring to prevent restacking, pair green MXene with short aqueous steps, use low-polarity binders for molten-salt MXene |
| Interfacial reactivity and interphase control | Nucleation overpotential, interfacial resistance evolution, CE protocol, post-cycling chemistry and morphology | Galvanostatic CE tests with defined current and areal capacity, EIS before and after cycling, XPS or ToF-SIMS and SEM or TEM | CCD and overpotential in LMB, shuttle suppression and areal utilization in Li–S, stable impedance under rate changes | Match terminations to polymer and salt, prefer fluoride-free routes when performance is comparable so LiF arises from controlled salt breakdown, document electrolyte and binder to separate route effects from formulation |
| Mechanics and heat management | Storage modulus or tensile metrics on the same films, in-plane or through-film thermal conductivity, electronic conductivity at intended loading | DMA or tensile testing, laser-flash or steady-state thermal conductivity, four-probe conductivity | Shape stability and resistance to filament penetration in LMB, temperature rise at power in Li–S and LMB, long-cycle retention at matched power | Keep electronic networks below percolation in electrolyte-rich regions, use aqueous or alcohol processing for green MXene to preserve aspect ratio, consider PVDF latex to avoid NMP, align or grade platelets to boost modulus and heat spreading at low loading |
| Cross-cutting moderators | Electronic percolation restraint, preservation of surface chemistry | Four-probe conductivity at loading of interest, oxidation and humidity indicators over storage time | Self-discharge and parameter drift with storage and humidity | Inert storage, humidity control, short wet-processing steps, log time from synthesis to casting |
| Method | Process Type | Interface Chemistry | Morphology Control | Mechanical Compatibility | Scalability | Common Pitfalls |
|---|---|---|---|---|---|---|
| Solution blending and casting | Wet mixing then cast or coat | Mainly physical interactions, hydrogen bonding, van der Waals | Film or coating thickness tuned by solids and shear, some flake alignment by coating | Moderate, depends on dispersion and loading | High roll to roll slot, die blade or spray coating straightforward, solvent recovery needed | Restacking, brittle film at high loading, uneven thickness |
| In situ polymerization | Polymerization in presence of MXene thermal or UV | Stronger interfacial contact chains form near flakes, possible covalent links | Dense networks around flakes, good embedment | High, good load transfer and cohesion | Moderate batch or inline curing, feasible chemistry and oxygen sensitivity can limit throughput | MXene oxidation, residual monomer, oxygen sensitivity, shrinkage |
| Surface grafting-to or -from | Covalent functionalization and chain attachment | Covalent brushes or tethers on MXene | Brush layers prevent restacking, interface tailored | Excellent, very strong adhesion | Low to moderate multistep wet chemistry, washing and control of graft density, slow for large area | Multistep complexity, low throughput, over-grafting reduces conductivity |
| Layer-by-layer assembly | Alternating deposition of oppositely charged species | Electrostatic hydrogen bonding, secondary interactions | Nanometer-level thickness, highly ordered laminar stacks | Good but can be brittle through-thickness | Low cyclic dipping or spray LbL is slow, automation helps but still limited | Slow cycles, rinse defects, substrate dependence |
| Electrospinning | High voltage fiber formation from polymer MXene dope | Physical embedment, affinity driven | 3D porous nonwoven fiber alignment via collector | High, flexible and tough in plane | Moderate multi-needle or needleless setups give m2 scale solvent handling and safety required | Jet clogging, solvent hazards, thickness control, MXene aggregation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khang, T.L.; Bae, J. Fluoride-Free MXene–Polymer Composites for Li-Metal and Li–S Batteries: Comparative Synthesis Methods, Integration Rules, Challenges, and Future Directions. Polymers 2025, 17, 3109. https://doi.org/10.3390/polym17233109
Khang TL, Bae J. Fluoride-Free MXene–Polymer Composites for Li-Metal and Li–S Batteries: Comparative Synthesis Methods, Integration Rules, Challenges, and Future Directions. Polymers. 2025; 17(23):3109. https://doi.org/10.3390/polym17233109
Chicago/Turabian StyleKhang, Truong Le, and Joonho Bae. 2025. "Fluoride-Free MXene–Polymer Composites for Li-Metal and Li–S Batteries: Comparative Synthesis Methods, Integration Rules, Challenges, and Future Directions" Polymers 17, no. 23: 3109. https://doi.org/10.3390/polym17233109
APA StyleKhang, T. L., & Bae, J. (2025). Fluoride-Free MXene–Polymer Composites for Li-Metal and Li–S Batteries: Comparative Synthesis Methods, Integration Rules, Challenges, and Future Directions. Polymers, 17(23), 3109. https://doi.org/10.3390/polym17233109







