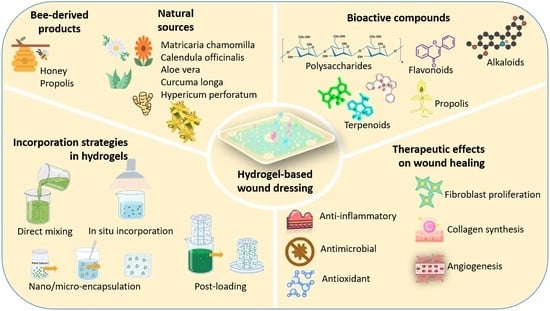
Strategies for Incorporating Natural Therapeutic Agents into Hydrogel Dressings: Innovations in Wound Healing
Abstract
1. Introduction
2. Natural Therapeutic Agents in Hydrogel Dressings for Wound Healing
2.1. Plant-Derived Natural Extracts
2.2. Bee-Derived Products
| Natural Extracts | Plant Source | Bioactive Compounds | Wound–Healing Properties | Wound-Healing Applications |
|---|---|---|---|---|
| Aloe vera [47] | Aloe vera | Mannose-rich polysaccharide (glucomannan), anthraquinones, vitamins B1/B6/ B12, gibberellin, growth hormones | Anti-inflammatory, antimicrobial, antioxidant, stimulates fibroblast proliferation, collagen synthesis, and angiogenesis | Enhances wound closure, hydration, and collagen deposition when incorporated into hydrogel matrices |
| Curcumin [48] | Curcuma longa (Turmeric) | Curcumin (polyphenolic compound) | Anti-inflammatory, antioxidant; promotes collagen deposition, wound contraction, and angiogenesis | Controlled release from hydrogels enhances healing efficacy |
| Neem [49] | Azadirachta indica | Nimbidin, terpenoids, flavonoids, azadirachtin | Antibacterial, antifungal, antiviral, anti-inflammatory; supports tissue regeneration | Promotes fibrocollagenic tissue formation and accelerates wound repair |
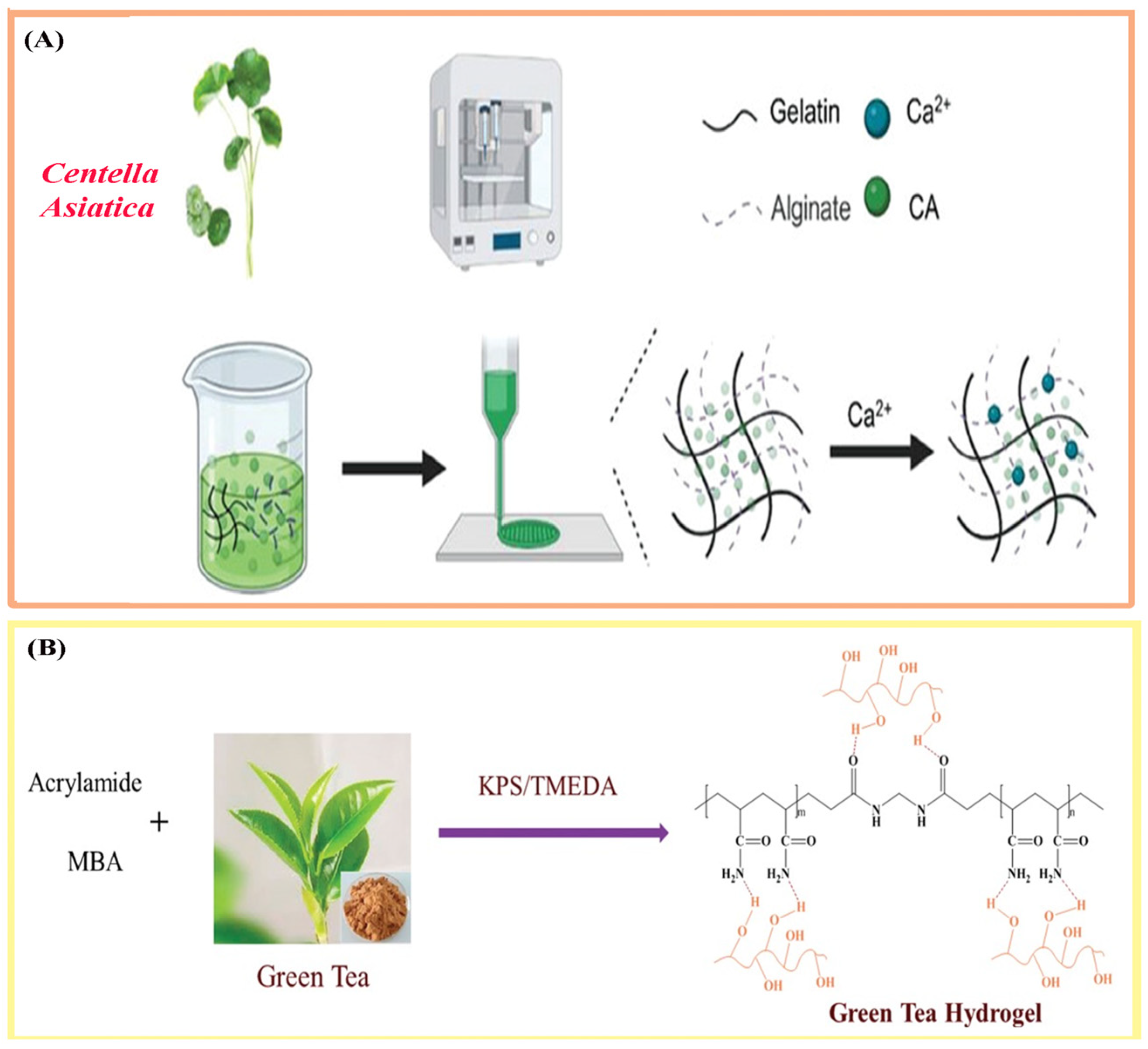
| Green tea [50] | Camellia sinensis | Epigallocatechin gallate (EGCG), catechins, polyphenols | Antimicrobial, antioxidant, anti-inflammatory; stimulates keratinocyte proliferation and skin regeneration | Provides antimicrobial and pro-regenerative effects in hydrogel formulations |
| Centella [51] | Centella Asiatica | Asiatic acid, madecassoside, triterpenoids | Anti-inflammatory, antimicrobial; promotes fibroblast proliferation, ECM and collagen synthesis, tissue regeneration | Facilitates dermal repair and skin regeneration in hydrogel systems |
| Calendula [52] | Calendula officinalis (marigold) | Flavonoids, saponins, triterpenes (faradiol monoesters), carotenoids, tannins | Anti-inflammatory, antioxidant, antimicrobial; enhances fibroblast proliferation and re-epithelialization | Exhibits anti-inflammatory and pro-healing effects in wound dressings |
| Chamomile [53] | Matricaria chamomilla | Bisabolol, chamazulene, apigenin, luteolin | Anti-inflammatory, antioxidant, antispasmodic; accelerates burn and wound healing | Provides anti-inflammatory and healing-enhancing activity in hydrogels |
| St. John’s Wort [50] | Hypericum perforatum | Hypericin, hyperforin, flavonoids | Antimicrobial, anti-inflammatory, antioxidant; supports tissue repair | Enhances antimicrobial protection and promotes tissue regeneration |
| Honey/ Propolis [54] | Honey, bee-derived products | Flavonoids, phenolic acids, organic acids, enzymes (glucose oxidase), vitamins | Broad-spectrum antimicrobial (bacteriostatic/bactericidal), anti-inflammatory, antioxidant; stimulates VEGF expression and fibroblast proliferation | Promotes wound closure and tissue repair through occlusive and bioactive effects |
3. Formulation and Synthesis Strategies of Hydrogels Incorporating Natural Therapeutic Agents
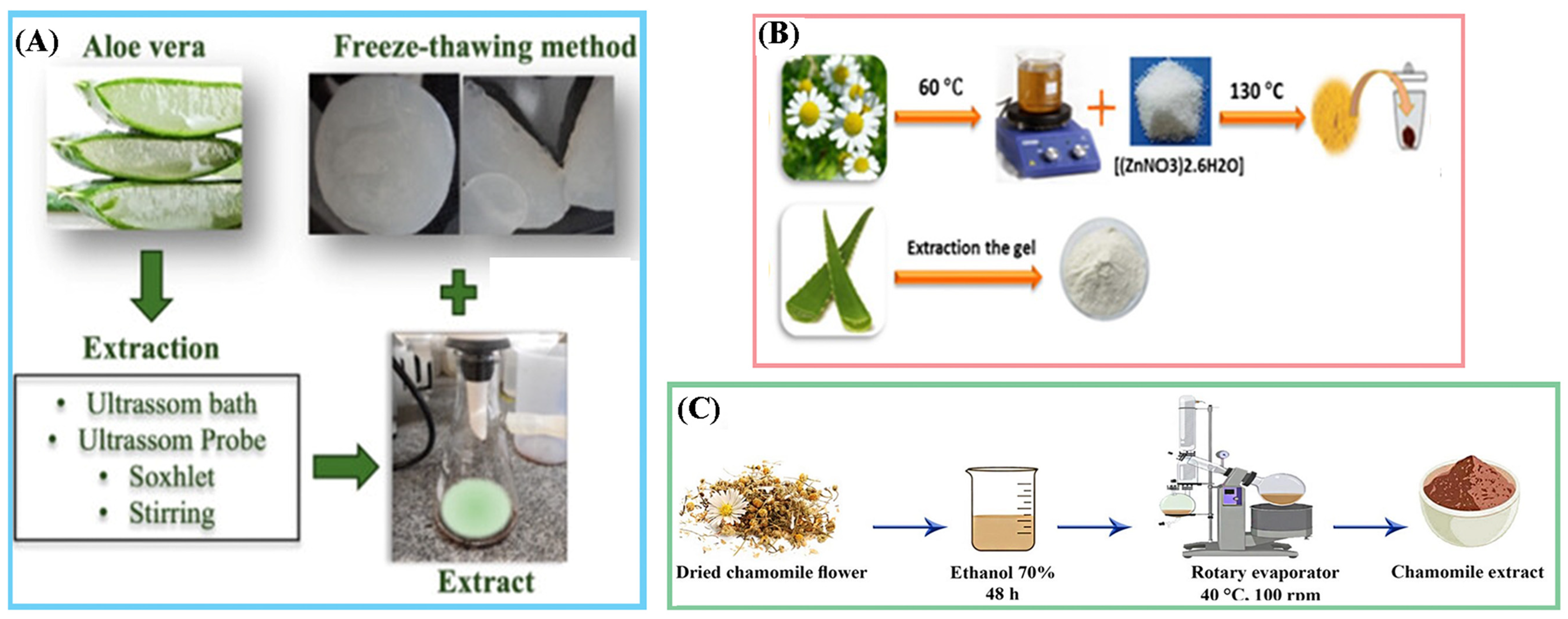
3.1. Extraction and Purification of Natural Therapeutic Agents
3.2. Incorporation Strategies for Natural Therapeutic Agents
3.2.1. Direct Mixing (Before Crosslinking)
3.2.2. In Situ Incorporation (During Gelation)
3.2.3. Post-Loading (After Gel Formation)
3.2.4. Nano/Microencapsulation
4. Biological Evaluation of Hydrogels Containing Natural Therapeutic Agents
4.1. Natural Therapeutic Extracts in Wound Repair: Experimental Insights from In Vitro and In Vivo Models
| Natural Agents | Type of Standardization | Polymeric Composition | Therapeutic & Biological Effects |
|---|---|---|---|
| Aloe vera | Powder extract (200× concentrate) [100] | 10% PVA–0.05% GO | Exhibits antibacterial activity (99.94% inhibition S. aureus); Enhances fibroblast viability (~295%), and demonstrates excellent cytocompatibility. |
| Powder extract [139] | 2% Alginate–0.01% COL–0.1% Gly | Promotes Hs27 fibroblast proliferation (up to 165%); Antibacterial activity (75–90% reduction) against S. aureus, E. coli, and P. aeruginosa; Enhanced wound healing potential. | |
| Powder extract [59] | 1% Carbopol 940–2% HEC | Demonstrates effective anti-inflammatory and skin regenerative properties. | |
| Powder extract (purity of 99.01%) [140] | 50% Gelatin–20% Agar–10% Gly | Non-cytotoxic to HDF and HaCaT cells; Promoted cell adhesion, migration, and wound closure; Exhibits anti-inflammatory activity. | |
| Powder extract [141] | 0.1% CS–0.1% dextran sulfate (DXS) | Exhibits antibacterial activity (≈50% inhibition of S. aureus and S. mutans); Shows antioxidant potential and improved wound-healing efficacy. | |
| Powder extract [108] | 2% Alginate–5% Gelatin | Demonstrates antibacterial activity against P. aeruginosa (inhibition zones ≈ 15.4 ± 3 mm for gelatin and 13.7 ± 2 mm for alginate hydrogels). | |
| Powder extract [109] | 5% PDMA–8% GelMA | Provides superior wound-healing and anti-inflammatory effects; Promotes tissue regeneration and exhibits excellent mechanical and adhesive properties. | |
| Calendula officinalis | Hydroalcoholic extract [142] | Carbomer 940–PG–TEA | Effectively manages 5-fluorouracil (FU)-associated hand–foot syndrome. |
| 70% EtOH macerated extract [68] | Carbopol 980NF–PEG400 | Non-biological performance. | |
| 70% EtOH macerated extract [143] | 2% CS–25% PEG400 | Exhibits antibacterial activity against P. aeruginosa; Synergistic effects when combined with other extracts. | |
| 3% Lyophilized hydroalcoholic extract [144] | CS–HPMC (2–3%) | Demonstrated antibacterial activity against S. aureus, P. acnes, E. coli. | |
| 10% Hydroglycolic macerated extract [70] | 1.5% SA–1% CMC | Non-cytotoxic effects to 3T3 cells, Promote cell proliferation; Supports cellular processes essential for skin repair. | |
| 50% EtOH macerated extract [145] | XG–PEG 8000 | Increases fibroblast migration; Promotes extensive re-epithelialization within 9 d without scarring or hair loss. | |
| (2–4)% Hydro-glycolic Extract [37] | (8–10)% PVA–(0.75–1.25% κ-CAR | No in vitro hemolysis; Swiss 3T3 albino fibroblasts cells viability above 95%; Increased wound closure after 48 h; Improvement in wound re-epithelialization, neovascularization and wound retraction. | |
| EtOH extract [122] | Okra mucilage–AMPS-EGDMA | Exhibits antibacterial activity against S. aureus and E. coli; Increased blood clotting index; Improves wound closure and overall healing. | |
| Aqueous extract [146] | (0.5–2.5)% CS–1%PVA | Strong antimicrobial activity against E. coli, S. aureus, and C. albicans; >80% cell viability in L929 fibroblasts; Potential for biomedical and wound healing applications. | |
| Centella asiatica | EtOH extract [123] | 1% HA–9% Dextran | Enhances fibroblast viability and migration at optimal 0.4 wt% CA concentration; Promoted wound closure without cytotoxic effects. |
| 90% MeOH extract [147] | 0.5% CS–1.25% Gelatin | Stimulates fibroblast proliferation (up to 142%); Antibacterial activity against P. acnes (26 mm inhibition zone, MIC = 150 µg/mL) and S. aureus; Non-cytotoxic and non-irritant, suitable for anti-acne and wound healing applications. | |
| Aqueous extract [78] | 2.5% SA–8% Gelatin | Accelerates wound healing and enhances neovascularization at the wound site; Increases epithelial thickness and hair follicle density; Effective in diabetic chronic-wound repair. | |
| 70% MeOH extract [34] | 3% CS | Antibacterial activity against S. aureus; Fibroblast migration and wound closure (73.4% at 24 h; 99.0% at 48 h); Synergistic wound-healing efficacy with good biocompatibility. | |
| 70% EtOH extract [124] | 3.6% Gelatin–3.6% MAAn | Antibacterial activity (inhibition zones 19–28 mm; MIC = 3.125–6.25 mg/mL) against Bacillus, E. coli, S. aureus, P. aeruginosa, Klebsiella, Streptococcus, and C. albicans; Non-toxic, biocompatible and promoted cell growth. | |
| 96% EtOH extract [111] | 30% CS–poly (β amino ester) | Achieves 99.5% wound closure within 24 h; Antibacterial activity against E. coli and S. aureus; Positive effect on L929 cell proliferation. | |
| Ultrasound-assisted extract (70% MeOH) [35] | 3% CS–2% Gelatin | Promotes fibroblast migration and wound closure (up to 99.9% after 36 h); Biocompatible, stimulates collagen synthesis and angiogenesis. | |
| 70% EtOH extract [125] | Gelatin–AA–AAm | HFF-2 cell viability; Significant antibacterial efficacy against multiple bacterial strains. | |
| Chamomile | Aqueous extract [148] | 0.5% TG–0.5% SA–0.5% BC | Antibacterial activity against Gram-positive and Gram-negative bacteria; Enhanced fibroblast cell viability and proliferation; Non-cytotoxicity and excellent biocompatibility. |
| Curcumin (Curcuma longa) | Powder extract [126] | 5% Gelatin– (0.5-1%) BC | Antibacterial activity (E. coli: 15 ± 0.5 mm; S. aureus: 19 ± 1 mm inhibition zones); Induced bacterial cell wall damage and death; Enhanced fibroblast proliferation and migration, leading to complete wound closure with 18 h; Antioxidant and anti-inflammatory effects. |
| 100% EtOH extract [135] | 1% TOCN–(5–10)% PVA | Promoted fibroblast proliferation and collagen deposition; Enhances wound contraction and tissue regeneration; Accelerates re-epithelization (≈81% closure after 2 weeks); Cell viability (~85%) and strong biocompatibility. | |
| Powder extract [149] | 4% SA–20.4% NIPAM | Accelerated wound contraction (≈96.5% closure at day 14) with complete re-epithelialization; Enhanced fibroblast proliferation, collagen deposition, and angiogenesis; Significantly reduces inflammation via NF-κB/TNF-α/IL-1 inhibition; Biocompatibility and regenerative effect. | |
| Powder extract [150] | 0.5% GG | Fibroblast proliferation (+45%) and collagen synthesis (+50% at 14 days); Enhanced cell migration (up to 100% vs. control) and re-epithelialization (73% closure at day 12); Antibacterial activity and excellent biocompatibility (≥80% cell viability). | |
| MeOH extract [136] | 2% Carbopol–0.25% COL | Wound-healing efficacy with 79.25% wound contraction by day 3 and complete closure by day 7, without scarring; Re-epithelialization (>70%) and collagen deposition; Anti-inflammatory effects via TLR4 and NF-κB inhibition. Excellent cytocompatibility and tissue regeneration. | |
| Powder extract [151] | 0.2% CS–4% PVA | Antibacterial activity against Streptococcus faecalis and E. coli (MIC = 2.60 ± 1.13 µg/mL and 1.30 ± 0.56 µg/mL, respectively). | |
| Powder extract [152] | 3% SF–3% Pluronic F127 | Cytocompatibility with fibroblast cells (no cytotoxicity after 1–7 days); Achieving up to 5-log reduction in bacterial growth; Provides potent bactericidal and bioactive properties. | |
| Powder extract [153] | 2% CS–2% Gelatin | Demonstrated >95% antibacterial efficiency against E. coli and S. aureus; Strong antioxidant performance. | |
| Powder extract [154] | 3.5% Ammonium alginate–3.5% PVA | Antioxidant activity (DPPH = 72.6%; ABTS = 98.50% after 6 h); Excellent biocompatibility (L0-2 cell viability >120% after 24 h) and hemocompatibility (hemolysis <5%). | |
| Powder extract [114] | 2.5% QCS | Excellent cytocompatibility with L929 fibroblasts (cell viability >100% after 5 days; Antioxidant capacity (DPPH scavenging 76.2%); Accelerated wound closure: 63.4% at day 3; 86.6% at day 10); Reduces inflammation and enhances granulation, collagen deposition, and angiogenesis. | |
| 80% EtOH extract [155] | 10% GelMA–5% SF | Provides 90.3% (S. aureus) and 87.9% (E. coli) reduction in colonies; inhibition zones of 30.9 ± 0.55 mm and 32.9 ± 0.49 mm; Maintains fibroblast viability >95% after 3 days; excellent hemocompatibility (hemolysis 1.18 ± 0.34%). | |
| 75% EtOH extract [156] | 5–10% GelMA | Excellent cytocompatibility with L929 fibroblasts; Strong antioxidant protection under oxidative stress, restoring H2O2-treated cell viability; Pronounced anti-inflammatory activity. | |
| Grape | 70% EtOH extract of white pomace [157] | 0.5% CS– 1% Alginate | Moderate antioxidant activity (1.016 ± 0.288 µmol/L); Antibacterial efficacy against S. aureus (~97% growth reduction) and non-toxic. |
| Draksha–Beeja powdered extract [158] | 5% Starch–5% Gelatin | Excellent cytocompatibility with >99% L929 fibroblast viability and strong cell proliferation; Highly hemocompatibility (hemolysis ≈ 1.5%) and regenerative potential. | |
| Green Tea | Powder extract [159] | 1% PVA–1% SA | Antibacterial activity, stronger against S. aureus than E. coli; Inhibition halos and OD600 reduction confirm effective suppression of bacterial proliferation; Excessive extract loading reduced antimicrobial efficiency. |
| Neem (Azadirachta indica) | Aqueous extract [160] | 0.25% N-succinyl chitosan–20% Pluronic F127 | Demonstrates dose-dependent antioxidant activity in DPPH assay (39% at 0.05 g/mL to 75% at 0.30 g/mL); Potential to reduce oxidative stress and inflammation in wound environments. |
| Powder extract [129] | 0.1% PCL–0.2% Kolliphor P188 | Pronounced antibacterial activity against S. typhi, E. coli, and S. aureus. | |
| St. John’s Wort | H. perforatum callus powdered extract [95] | 1% CS–1% SA | Demonstrates dose-dependent enhancement of fibroblast proliferation; Excellent cytocompatibility (≈96% viability), cell adhesion, spreading, and proliferation; Antibacterial activity against E. coli and K. pneumoniae. |
| 80% EtOH extract of H. perforatum callus [94] | 2% PV–1% CS– 1% Alginate | Excellent cytocompatibility with fibroblast viability around 99%; Enhances cell proliferation up to 150%; Promotes strong cell adhesion and accelerated wound closure (>99% healing within 14 days). | |
| Honey | Organic Manuka honey extract [161] | 4% CS–4% Gelatin | Exhibits strong antibacterial activity, inhibiting bacterial growth for up to 12 h; Accelerates wound healing via antimicrobial and exudate absorptive effects. |
| EtOH extract of Propolis [162] | 2% Carbopol 934–2% PG | Antibacterial activity against S. aureus and S. epidermidis; Excellent cytocompatibility with NIH 3T3 fibroblasts and anti-inflammatory effects; Promotes rapid wound contraction (>90% by day 14) and near-complete re-epithelialization (≈96% by day 28). | |
| 75% EtOH extract of propolis [130] | 4% κ-CAR | Antimicrobial activity against S. aureus, P. aeruginosa, and C. albicans; No inhibitory effect against A. niger. | |
| 70% EtOH macerated extract of propolis [163] | 10% PVA | Antimicrobial activity against S. mutans, E. coli, and C. albicans (inhibition zones up to 19 mm; MIC = 0.025–0.05 mg/mL); Excellent cytocompatibility (≥90% cell viability up to 125 μg/mL) and enhanced fibroblast adhesion. | |
| 70% EtOH macerated extract of propolis [92] | 7.2% AAm–0.5% MC | Strong antibacterial activity against S. aureus and P. aeruginosa; Moderate antifungal activity against C. albicans and C. tropicalis; Antioxidant potential. |
| Natural Agents | Type of Extraction | Polymeric Composition | Therapeutic/Biological Effects |
|---|---|---|---|
| Aloe vera | Fresh leaves (crude gel) [171] | 0.7% PVA | Enhances fibroblast proliferation; Exhibits strong wound healing potential. |
| Fresh leaves [101] | 3% PVA | Accelerated wound closure; Improves re-epithelialization and reduces inflammation. | |
| Fresh leaves [172] | 2% SF/2% PVP | Enhances cellular proliferation and migration; Reduces inflammation; Promotes granulation tissue formation and accelerated re-epithelialization. | |
| Fresh leaves [96] | 8% PVA–2% CS | Non-cytotoxic; Exhibits antibacterial activity and excellent wound dressing potential. | |
| Fresh leaves [173] | 12% PAN–1% TG | Significantly increases fibroblast viability; Demonstrates excellent cytocompatibility | |
| Calendula officinalis (Marigold) | Macerated extract [69] | 7.2% AAm–0.5% MC | Promotes tissue regeneration and accelerates the healing process. |
| Commercial Extract [102] | 2% CMC–5%PVA | Minimizes apoptosis in human dermal fibroblasts; Exhibits antimicrobial efficacy against S. aureus and E. coli. | |
| Centella asiatica | Macerated extract 95% EtOH [103] | 8% PVA–5% PEG | Non-irritant and biocompatible; Accelerates wound contraction and epithelialization; Promotes complete wound closure with thin epidermis formation by day 5. |
| Chamomile | Ultrasonic extract (water/ethanol 3:1) [73] | 1% TG | Exhibits antimicrobial activity (80% of E. coli, 90% of S. aureus, and 92% of C. albicans); Demonstrates anti-inflammatory and skin-protective potential. |
| Fresh leaves [72] | 15% AA–5% Cold/ Hot Starch | Non-cytotoxic (>70% cell viability); Promoted fibroblast proliferation and cell regeneration. | |
| Ethanolic extract (50%) [174] | 3% CS–2% Agarose | Antibacterial activity (inhibition zones: E. coli 7.5 mm, S. aureus 12.7 mm); Supports NIH 3T3 fibroblast adhesion, proliferation (~94% viability at day 7); Bioactive flavonoids and tannins contribute to antioxidant and wound-healing activity. | |
| Ethanolic extract (70%) [75] | 12% PAN–2% SA– 2% Gelatin | Antibacterial activity (23 ± 1 mm S. aureus, 12 ± 2 mm E. coli); Cytocompatible (>100% L929 viability); Enhances angiogenesis, collagen deposition, and wound closure (~85% after 28 days); Reduces inflammation and necrosis. | |
| Curcumin (Curcuma longa) | Aqueous extract [175] | (1–2.5)% CS–5% PAAM | Enhances antibacterial efficiency and biocompatibility; Curcuminoids contribute to anti-inflammatory and wound-healing activity via microbial inhibition and tissue regeneration. |
| Green Tea | Fresh leaves [128] | 3% CS–10% PVA | Demonstrates concentration-dependent antioxidant activity (DPPH scavenging 20–80%; ABTS scavenging 2–50%). |
| Fresh leaves [82] | 2% CS–10% PVA | Antibacterial activity against E. coli and S. aureus; Non-cytotoxicity (L929 viability >70%); Promotes wound closure (~98–99% by day 12); Reduces inflammation and enhanced re-epithelialization. | |
| Neem (Azadirachta indica) | Fresh leaves [104] | 2% CS | Exhibits antibacterial activity against S. aureus; Enhances wound healing efficiency; Displays anti-inflammatory effects and promotes a moist wound environment. |
| Fresh leaves [25] | 1.25% GG–0.75% SF | Demonstrates moderate antibacterial activity (26.5 ± 0.9 mm inhibition zone); Free of microbial contamination |
4.2. Synergistic Effects of Natural Extracts in Hydrogel-Based Wound Dressings
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 5-FU | 5-Fluorouracil |
| AA | Acrylic acid |
| AAm | Acrylamide |
| AMPS | Acrylamido-2-methyl-1-propanesulfonic acid |
| BC | Bacterial cellulose |
| CA | Centella asiatica |
| CMC | Carboxymethylcellulose |
| COL | Collagen |
| CS | Chitosan |
| DMA | N,N-Dimethylacrylamide |
| DXS | Dextran sulfate |
| EGDMA EtOH | Ethylene glycol dimethacrylate Ethyl alcohol |
| GelMA | Gelatin methacryloyl |
| GG | Guar gum |
| Gly | Glycerol |
| GO | Graphene oxide |
| HA | Hyaluronic acid |
| HEC | Hydroxyethylcellulose |
| HP | Hypericum perforatum L. |
| HPMC | Hydroxypropyl methylcellulose |
| HUVECs | Human umbilical vein endothelial cells |
| MAAn | Methacrylic anhydride |
| MC MeOH | Methylcellulose Methyl alcohol |
| NIPAM | N-Isopropylacrylamide |
| PAAM | Polyacrylamide |
| PAN | Polyacrylonitrile |
| PCL | Poly-ε-caprolactone |
| PDMA | Poly(N,N-dimethylacrylamide) |
| PEG | Poly(ethylene glycol) |
| PEO | Polyethylene oxide |
| PG | Propylene glycol |
| PLA | Polylactic acid |
| PLGA | Poly(lactic-co-glycolic acid) |
| PVA | Polyvinyl alcohol |
| PVP | Polyvinylpyrrolidone |
| QCS | Quaternary aminated chitosan |
| SA | Sodium alginate |
| SF | Silk fibroin |
| TEA | Triethanolamine |
| TG | Tragacanth |
| TOCN | TEMPO oxidized cellulose nanofiber |
| XG | Xanthan gum |
| κ-CAR | Carrageenan |
References
- Cristea (Hohotă), A.-G.; Lisă, E.-L.; Iacob (Ciobotaru), S.; Dragostin, I.; Ștefan, C.S.; Fulga, I.; Anghel (Ștefan), A.M.; Dragan, M.; Morariu, I.D.; Dragostin, O.-M. Antimicrobial Smart Dressings for Combating Antibiotic Resistance in Wound Care. Pharmaceuticals 2025, 18, 825. [Google Scholar] [CrossRef] [PubMed]
- Herman, A.; Leska, A.; Wińska, P.; Herman, A.P. Plant Extracts as Modulators of the Wound Healing Process—Preliminary Study. Int. J. Mol. Sci. 2025, 26, 7490. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Gao, P.; He, F.; Zhang, C. Application of Alginate-Based Hydrogels in Hemostasis. Gels 2022, 8, 109. [Google Scholar] [CrossRef] [PubMed]
- Vitale, S.; Colanero, S.; Placidi, M.; Di Emidio, G.; Tatone, C.; Amicarelli, F.; D’Alessandro, A.M. Phytochemistry and Biological Activity of Medicinal Plants in Wound Healing: An Overview of Current Research. Molecules 2022, 27, 3566. [Google Scholar] [CrossRef]
- Revete, A.; Aparicio, A.; Cisterna, B.A.; Revete, J.; Luis, L.; Ibarra, E.; Segura González, E.A.; Molino, J.; Reginensi, D. Advancements in the Use of Hydrogels for Regenerative Medicine: Properties and Biomedical Applications. Int. J. Biomater. 2022, 2022, 3606765. [Google Scholar] [CrossRef]
- Kucinska-Lipka, J.; Gubanska, I.; Lewandowska, A.; Terebieniec, A.; Przybytek, A.; Cieśliński, H. Antibacterial Polyurethanes, Modified with Cinnamaldehyde, as Potential Materials for Fabrication of Wound Dressings. Polym. Bull. 2019, 76, 2725–2742. [Google Scholar] [CrossRef]
- Hassanzadeh-Tabrizi, S.A. Alginate Based Hemostatic Materials for Bleeding Management: A Review. Int. J. Biol. Macromol. 2024, 274, 133218. [Google Scholar] [CrossRef]
- Lopes, A.I.; Pintado, M.M.; Tavaria, F.K. Plant-Based Films and Hydrogels for Wound Healing. Microorganisms 2024, 12, 438. [Google Scholar] [CrossRef]
- Lyggitsou, G.; Barda, C.; Anagnostou, M.; Douros, A.; Statha, D.; Karampasi, C.; Papantonaki, A.I.; Svoliantopoulos, I.; Sfiniadakis, I.; Vitsos, A.; et al. Wound Healing Potential of Herbal Hydrogel Formulations of Cedrus Brevifolia Extracts in Mice. Gels 2024, 10, 750. [Google Scholar] [CrossRef]
- Gutterman, Y.; Chauser-Volfson, E. The Distribution of the Phenolic Metabolites Barbaloin, Aloeresin and Aloenin as a Peripheral Defense Strategy in the Succulent Leaf Parts of Aloe arborescens. Biochem. Syst. Ecol. 2000, 28, 825–838. [Google Scholar] [CrossRef]
- Hamman, J.H. Composition and Applications of Aloe vera Leaf Gel. Molecules 2008, 13, 1599–1616. [Google Scholar] [CrossRef]
- Hutter, J.A.; Salman, M.; Stavinoha, W.B.; Satsangi, N.; Williams, R.F.; Streeper, R.T.; Weintraub, S.T. Antiinflammatory C-Glucosyl Chromone from Aloe barbadensis. J. Nat. Prod. 1996, 59, 541–543. [Google Scholar] [CrossRef]
- Maan, A.A.; Nazir, A.; Khan, M.K.I.; Ahmad, T.; Zia, R.; Murid, M.; Abrar, M. The Therapeutic Properties and Applications of Aloe vera: A Review. J. Herb. Med. 2018, 12, 1–10. [Google Scholar] [CrossRef]
- Liu, C.; Cui, Y.; Pi, F.; Cheng, Y.; Guo, Y.; Qian, H. Extraction, Purification, Structural Characteristics, Biological Activities and Pharmacological Applications of Acemannan, a Polysaccharide from Aloe vera: A Review. Molecules 2019, 24, 1554. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Singh, R.L.; Raghubir, R. Antioxidant Status during Cutaneous Wound Healing in Immunocompromised Rats. Mol. Cell Biochem. 2002, 241, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Arafa, N.M.S.; Hummadi, H.M.A.; Badr, G.M. The Potential of Aloe vera Gel Utilization for Skin Wound Healing in Rats Based on GC–MS and HPLC Chemical Profile. J. Basic Appl. Zool. 2025, 86, 6. [Google Scholar] [CrossRef]
- Meza-Valle, K.Z.; Saucedo-Acuña, R.A.; Tovar-Carrillo, K.L.; Cuevas-González, J.C.; Zaragoza-Contreras, E.A.; Melgoza-Lozano, J. Characterization and Topical Study of Aloe vera Hydrogel on Wound-Healing Process. Polymers 2021, 13, 3958. [Google Scholar] [CrossRef]
- Movaffagh, J.; Khatib, M.; Fazly Bazzaz, B.S.; Taherzadeh, Z.; Hashemi, M.; Seyedian Moghaddam, A.; Tabatabaee, S.A.; Azizzadeh, M.; Jirofti, N. Evaluation of Wound-Healing Efficiency of a Functional Chitosan/Aloe vera Hydrogel on the Improvement of Re-Epithelialization in Full Thickness Wound Model of Rat. J. Tissue Viability 2022, 31, 649–656. [Google Scholar] [CrossRef]
- Kotha, R.R.; Luthria, D.L. Curcumin: Biological, Pharmaceutical, Nutraceutical, and Analytical Aspects. Molecules 2019, 24, 2930. [Google Scholar] [CrossRef]
- Kant, V.; Gopal, A.; Kumar, D.; Pathak, N.N.; Ram, M.; Jangir, B.L.; Tandan, S.K.; Kumar, D. Curcumin-Induced Angiogenesis Hastens Wound Healing in Diabetic Rats. J. Surg. Res. 2015, 193, 978–988. [Google Scholar] [CrossRef]
- Yen, Y.; Pu, C.; Liu, C.; Chen, Y.; Chen, Y.; Liang, C.; Hsieh, J.; Huang, H.; Chen, Y. Curcumin Accelerates Cutaneous Wound Healing via Multiple Biological Actions: The Involvement of TNF-α, MMP-9, A-SMA, and Collagen. Int. Wound J. 2018, 15, 605–617. [Google Scholar] [CrossRef]
- Dai, M.; Zheng, X.; Xu, X.; Kong, X.; Li, X.; Guo, G.; Luo, F.; Zhao, X.; Wei, Y.; Qian, Z. Chitosan-Alginate Sponge: Preparation and Application in Curcumin Delivery for Dermal Wound Healing in Rat. BioMed Res. Int. 2009, 2009, 595126. [Google Scholar] [CrossRef] [PubMed]
- Chundran, N.K.; Husen, I.R.; Rubianti, I. Effect of Neem Leaves Extract (Azadirachta indica) on Wound Healing. Althea Med. J. 2015, 2, 199–203. [Google Scholar] [CrossRef]
- Olorunsola, R.A.; Oke, F.; Bagbe, A.S.; Ayeyinbo, O.A. Evaluation of Wound Healing Potentials of Neem (Azadirachtaindica) Leaf Extract on Excision Wounds in Wistar Albino Rats. Niger. J. Anim. Prod. 2020, 45, 37–42. [Google Scholar] [CrossRef]
- Nasrine, A.; Narayana, S.; Gulzar Ahmed, M.; Sultana, R.; Noushida, N.; Raunak Salian, T.; Almuqbil, M.; Almadani, M.E.; Alshehri, A.; Alghamdi, A.; et al. Neem (Azadirachta indica) and Silk Fibroin Associated Hydrogel: Boon for Wound Healing Treatment Regimen. Saudi Pharm. J. 2023, 31, 101749. [Google Scholar] [CrossRef]
- Leung, L.K.; Su, Y.; Zhang, Z.; Chen, Z.-Y.; Huang, Y.; Chen, R. Theaflavins in Black Tea and Catechins in Green Tea Are Equally Effective Antioxidants. J. Nutr. 2001, 131, 2248–2251. [Google Scholar] [CrossRef]
- Reygaert, W.C. The Antimicrobial Possibilities of Green Tea. Front. Microbiol. 2014, 5, 434. [Google Scholar] [CrossRef]
- Lee, S.; Lee, K.-W. Protective Effect of (−)-Epigallocatechin Gallate against Advanced Glycation Endproducts-Induced Injury in Neuronal Cells. Biol. Pharm. Bull. 2007, 30, 1369–1373. [Google Scholar] [CrossRef]
- Kim, H.; Lee, J.; Kwon, B.J.; Lee, M.H.; Han, D.; Hyon, S.; Park, J. Promotion of Full-Thickness Wound Healing Using Epigallocatechin-3-O-Gallate/Poly (Lactic-CO-GLycolic Acid) Membrane as Temporary Wound Dressing. Artif. Organs 2014, 38, 411–417. [Google Scholar] [CrossRef]
- Chandrika, U.G.; Prasad Kumara, P.A.A.S. Chapter Four—Gotu Kola (Centella asiatica): Nutritional Properties and Plausible Health Benefits. In Advances in Food and Nutrition Research; Academic Press: Cambridge, MA, USA, 2015; Volume 76, pp. 125–157. [Google Scholar]
- Ratz-lyko, A.; Arct, J.; Pytkowska, K. Moisturizing and Antiinflammatory Properties of Cosmetic Formulations Containing Centella asiatica Extract. Indian J. Pharm. Sci. 2016, 78, 27. [Google Scholar] [CrossRef]
- Hamid, A.A.; Shah, Z.M.; Muse, R.; Mohamed, S. Characterisation of Antioxidative Activities of Various Extracts of Centella asiatica (L) Urban. Food Chem. 2002, 77, 465–469. [Google Scholar] [CrossRef]
- Witkowska, K.; Paczkowska-Walendowska, M.; Garbiec, E.; Cielecka-Piontek, J. Topical Application of Centella asiatica in Wound Healing: Recent Insights into Mechanisms and Clinical Efficacy. Pharmaceutics 2024, 16, 1252. [Google Scholar] [CrossRef] [PubMed]
- Witkowska, K.; Paczkowska-Walendowska, M.; Plech, T.; Szymanowska, D.; Michniak-Kohn, B.; Cielecka-Piontek, J. Chitosan-Based Hydrogels for Controlled Delivery of Asiaticoside-Rich Centella asiatica Extracts with Wound Healing Potential. Int. J. Mol. Sci. 2023, 24, 17229. [Google Scholar] [CrossRef] [PubMed]
- Witkowska, K.; Paczkowska-Walendowska, M.; Miklaszewski, A.; Plech, T.; Michniak-Kohn, B.; Swora-Cwynar, E.; Cielecka-Piontek, J. Development of 3D-Printed Chitosan-Based Hydrogels Rich in Centella asiatica Extract for Enhanced Wound Healing Applications. J. Drug Deliv. Sci. Technol. 2025, 111, 107143. [Google Scholar] [CrossRef]
- Cruceriu, D.; Balacescu, O.; Rakosy, E. Calendula officinalis: Potential Roles in Cancer Treatment and Palliative Care. Integr. Cancer Ther. 2018, 17, 1068–1078. [Google Scholar] [CrossRef]
- Rathod, L.; Bhowmick, S.; Patel, P.; Sawant, K. Calendula Flower Extract Loaded PVA Hydrogel Sheet for Wound Management: Optimization, Characterization and in-Vivo Study. J. Drug Deliv. Sci. Technol. 2022, 68, 103035. [Google Scholar] [CrossRef]
- Martins, M.D.; Marques, M.M.; Bussadori, S.K.; Martins, M.A.T.; Pavesi, V.C.S.; Mesquita-Ferrari, R.A.; Fernandes, K.P.S. Comparative Analysis between Chamomilla recutita and Corticosteroids on Wound Healing. An In Vitro and In Vivo Study. Phytother. Res. 2009, 23, 274–278. [Google Scholar] [CrossRef]
- Salehi, H.; Mehrasa, M.; Nasri-Nasrabadi, B.; Doostmohammadi, M.; Seyedebrahimi, R.; Davari, N.; Rafienia, M.; Hosseinabadi, M.E.; Agheb, M.; Siavash, M. Effects of Nanozeolite/Starch Thermoplastic Hydrogels on Wound Healing. J. Res. Med. Sci. 2017, 22, 110. [Google Scholar] [CrossRef]
- Yadollah-Damavandi, S.; Chavoshi-Nejad, M.; Jangholi, E.; Nekouyian, N.; Hosseini, S.; Seifaee, A.; Rafiee, S.; Karimi, H.; Ashkani-Esfahani, S.; Parsa, Y.; et al. Topical Hypericum perforatum Improves Tissue Regeneration in Full-Thickness Excisional Wounds in Diabetic Rat Model. Evid. Based Complement. Altern. Med. 2015, 2015, 245328. [Google Scholar] [CrossRef]
- Kurt, A.A.; Ibrahim, B.; Çınar, H.; Atsü, A.N.; Bursalıoğlu, E.O.; Bayır, İ.; Özmen, Ö.; Aslan, İ. Nanoemulsion Hydrogel Delivery System of Hypericum perforatum L.: In Silico Design, In Vitro Antimicrobial–Toxicological Profiling, and In Vivo Wound-Healing Evaluation. Gels 2025, 11, 431. [Google Scholar] [CrossRef]
- Ahmad, P.; Jaleel, C.A.; Salem, M.A.; Nabi, G.; Sharma, S. Roles of Enzymatic and Nonenzymatic Antioxidants in Plants during Abiotic Stress. Crit. Rev. Biotechnol. 2010, 30, 161–175. [Google Scholar] [CrossRef] [PubMed]
- Mandal, M.D.; Mandal, S. Honey: Its Medicinal Property and Antibacterial Activity. Asian Pac. J. Trop. Biomed. 2011, 1, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Abd Jalil, M.A.; Kasmuri, A.R.; Hadi, H. Stingless Bee Honey, the Natural Wound Healer: A Review. Skin. Pharmacol. Physiol. 2017, 30, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, A.; Rajput, M.; Barui, A.; Chatterjee, S.S.; Pal, N.K.; Chatterjee, J.; Mukherjee, R. Dual Cross-Linked Honey Coupled 3D Antimicrobial Alginate Hydrogels for Cutaneous Wound Healing. Mater. Sci. Eng. C 2020, 116, 111218. [Google Scholar] [CrossRef]
- El-Kased, R.F.; Amer, R.I.; Attia, D.; Elmazar, M.M. Honey-Based Hydrogel: In Vitro and Comparative In Vivo Evaluation for Burn Wound Healing. Sci. Rep. 2017, 7, 9692. [Google Scholar] [CrossRef]
- Rajesh, A.; Lone, S.A.; Ramasubburayan, R.; Sikkanthar, S.; Thajuddin, N.; Lee, S.-Y.; Kim, J.-W.; MubarakAli, D. A Systemic Review on Aloe vera Derived Natural Biomaterials for Wound Healing Applications. Biocatal. Agric. Biotechnol. 2023, 54, 102910. [Google Scholar] [CrossRef]
- Kumari, A.; Raina, N.; Wahi, A.; Goh, K.W.; Sharma, P.; Nagpal, R.; Jain, A.; Ming, L.C.; Gupta, M. Wound-Healing Effects of Curcumin and Its Nanoformulations: A Comprehensive Review. Pharmaceutics 2022, 14, 2288. [Google Scholar] [CrossRef]
- Cedillo-Cortezano, M.; Martinez-Cuevas, L.R.; López, J.A.M.; Barrera López, I.L.; Escutia-Perez, S.; Petricevich, V.L. Use of Medicinal Plants in the Process of Wound Healing: A Literature Review. Pharmaceuticals 2024, 17, 303. [Google Scholar] [CrossRef]
- Mssillou, I.; Bakour, M.; Slighoua, M.; Laaroussi, H.; Saghrouchni, H.; Ez-Zahra Amrati, F.; Lyoussi, B.; Derwich, E. Investigation on Wound Healing Effect of Mediterranean Medicinal Plants and Some Related Phenolic Compounds: A Review. J. Ethnopharmacol. 2022, 298, 115663. [Google Scholar] [CrossRef]
- Diniz, L.R.L.; Calado, L.L.; Duarte, A.B.S.; de Sousa, D.P. Centella asiatica and Its Metabolite Asiatic Acid: Wound Healing Effects and Therapeutic Potential. Metabolites 2023, 13, 276. [Google Scholar] [CrossRef]
- Ejiohuo, O.; Folami, S.; Maigoro, A.Y. Calendula in Modern Medicine: Advancements in Wound Healing and Drug Delivery Applications. Eur. J. Med. Chem. Rep. 2024, 12, 100199. [Google Scholar] [CrossRef]
- Dai, Y.-L.; Li, Y.; Wang, Q.; Niu, F.-J.; Li, K.-W.; Wang, Y.-Y.; Wang, J.; Zhou, C.-Z.; Gao, L.-N. Chamomile: A Review of Its Traditional Uses, Chemical Constituents, Pharmacological Activities and Quality Control Studies. Molecules 2022, 28, 133. [Google Scholar] [CrossRef] [PubMed]
- El-Sakhawy, M.; Salama, A.; Tohamy, H.-A.S. Applications of Propolis-Based Materials in Wound Healing. Arch. Dermatol. Res. 2023, 316, 61. [Google Scholar] [CrossRef] [PubMed]
- Jasso de Rodríguez, D.; Hernández-Castillo, D.; Rodríguez-García, R.; Angulo-Sánchez, J.L. Antifungal Activity In Vitro of Aloe vera Pulp and Liquid Fraction against Plant Pathogenic Fungi. Ind. Crops Prod. 2005, 21, 81–87. [Google Scholar] [CrossRef]
- Noori, A.S.; Mageed, N.F.; Abdalameer, N.K.; Mohammed, M.K.A.; Mazhir, S.N.; Ali, A.H.; Jaber, N.A.; Mohammed, S.H. The Histological Effect of Activated Aloe vera Extract by Microwave Plasma on Wound Healing. Chem. Phys. Lett. 2022, 807, 140112. [Google Scholar] [CrossRef]
- Kim, S.; Asnin, L.; Assefa, A.D.; Ko, E.Y.; Sharma, K.; Park, S.W. Extraction of Antioxidants from Aloe vera Leaf Gel: A Response Surface Methodology Study. Food Anal. Methods 2014, 7, 1804–1815. [Google Scholar] [CrossRef]
- Maneechan, W.; Khumfu, P.; Charoensit, P.; Tuanchai, A.; Ross, S.; Ross, G.M.; Ngoenkam, J.; Viyoch, J. Bioactive Hydrogel Scaffolds Integrating Chitosan, Silk Fibroin, and Aloe vera Extract for Enhanced Cartilage Tissue Regeneration. Polymers 2025, 17, 1409. [Google Scholar] [CrossRef]
- Jales, S.T.L.; Barbosa, R.d.M.; de Albuquerque, A.C.; Duarte, L.H.V.; da Silva, G.R.; Meirelles, L.M.A.; da Silva, T.M.S.; Alves, A.F.; Viseras, C.; Raffin, F.N.; et al. Development and Characterization of Aloe vera Mucilaginous-Based Hydrogels for Psoriasis Treatment. J. Compos. Sci. 2022, 6, 231. [Google Scholar] [CrossRef]
- Bashipour, F.; Ghoreishi, S.M. Response Surface Optimization of Supercritical CO2 Extraction of α-Tocopherol from Gel and Skin of Aloe vera and Almond Leaves. J. Supercrit. Fluids 2014, 95, 348–354. [Google Scholar] [CrossRef]
- Rodrigues, C.L.; Tomoda, B.T.; Viganó, J.; Braga, A.R.C.; de Moraes, M.A.; Veggi, P.C. Production and Characterization of Silk Fibroin–Aloe vera Hydrogel: A Study on Extraction, Hydrogel Properties, and Release Mechanism. ACS Omega 2024, 9, 50515–50525. [Google Scholar] [CrossRef]
- Bashipour, F.; Ghoreishi, S.M. Experimental Optimization of Supercritical Extraction of β-Carotene from Aloe barbadensis Miller via Genetic Algorithm. J. Supercrit. Fluids 2012, 72, 312–319. [Google Scholar] [CrossRef]
- Hu, Q.; Hu, Y.; Xu, J. Free Radical-Scavenging Activity of Aloe vera (Aloe barbadensis Miller) Extracts by Supercritical Carbon Dioxide Extraction. Food Chem. 2005, 91, 85–90. [Google Scholar] [CrossRef]
- Ivanovic, J.; Zizovic, I.; Petrovic, S.; Skala, D. The Analysis of Different Processes of Extraction: Yield of Extracts Obtained from Aloe vera (Aloe barbadensis Miller) and Sweet Bay (Laurus nobilis L.) and the Exergy Analysis of Applied Processes. Chem. Ind. Chem. Eng. Q. 2009, 15, 271–278. [Google Scholar] [CrossRef]
- Tanaka, M.; Yamada, M.; Toida, T.; Iwatsuki, K. Safety Evaluation of Supercritical Carbon Dioxide Extract of Aloe vera Gel. J. Food Sci. 2012, 77, T2–T9. [Google Scholar] [CrossRef] [PubMed]
- Malavika, J.P.; Shobana, C.; Ragupathi, M.; Kumar, P.; Lee, Y.S.; Govarthanan, M.; Selvan, R.K. A Sustainable Green Synthesis of Functionalized Biocompatible Carbon Quantum Dots from Aloe barbadensis Miller and Its Multifunctional Applications. Environ. Res. 2021, 200, 111414. [Google Scholar] [CrossRef] [PubMed]
- Dinda, M.; Mazumdar, S.; Das, S.; Ganguly, D.; Dasgupta, U.B.; Dutta, A.; Jana, K.; Karmakar, P. The Water Fraction of Calendula officinalis Hydroethanol Extract Stimulates In Vitro and In Vivo Proliferation of Dermal Fibroblasts in Wound Healing. Phytother. Res. 2016, 30, 1696–1707. [Google Scholar] [CrossRef]
- Gavan, A.; Colobatiu, L.; Hanganu, D.; Bogdan, C.; Olah, N.; Achim, M.; Mirel, S. Development and Evaluation of Hydrogel Wound Dressings Loaded with Herbal Extracts. Processes 2022, 10, 242. [Google Scholar] [CrossRef]
- Ferreira, L.M.d.M.C.; Bandeira, E.d.S.; Gomes, M.F.; Lynch, D.G.; Bastos, G.N.T.; Silva-Júnior, J.O.C.; Ribeiro-Costa, R.M. Polyacrylamide Hydrogel Containing Calendula Extract as a Wound Healing Bandage: In Vivo Test. Int. J. Mol. Sci. 2023, 24, 3806. [Google Scholar] [CrossRef]
- Possa, G.d.O.K.; Chopek, S.; Pereira, A.V.; Koga, A.Y.; Oliveira, M.R.P.d.; Costa, M.D.M. Calendula Glycolic Extract Enhances Wound Healing of Alginate Hydrogel. Acta Cir. Bras. 2024, 39, e399724. [Google Scholar] [CrossRef]
- Nicolaus, C.; Junghanns, S.; Hartmann, A.; Murillo, R.; Ganzera, M.; Merfort, I. In Vitro Studies to Evaluate the Wound Healing Properties of Calendula officinalis Extracts. J. Ethnopharmacol. 2017, 196, 94–103. [Google Scholar] [CrossRef]
- Jamroży, M.; Głąb, M.; Kudłacik-Kramarczyk, S.; Drabczyk, A.; Gajda, P.; Tyliszczak, B. The Impact of the Matricaria chamomilla L. Extract, Starch Solution and the Photoinitiator on Physiochemical Properties of Acrylic Hydrogels. Materials 2022, 15, 2837. [Google Scholar] [CrossRef]
- Ghayempour, S.; Montazer, M. A Robust Friendly Nano-Encapsulated Plant Extract in Hydrogel Tragacanth Gum on Cotton Fabric through One Single Step in-Situ Synthesis and Fabrication. Cellulose 2016, 23, 2561–2572. [Google Scholar] [CrossRef]
- Sellappan, L.K.; Sanmugam, A.; Manoharan, S. Fabrication of Dual Layered Biocompatible Herbal Biopatch from Biological Waste for Skin—Tissue Regenerative Applications. Int. J. Biol. Macromol. 2021, 183, 1106–1118. [Google Scholar] [CrossRef]
- Moshfeghi, T.; Najmoddin, N.; Arkan, E.; Hosseinzadeh, L. A Multifunctional Polyacrylonitrile Fibers/Alginate-Based Hydrogel Loaded with Chamomile Extract and Silver Sulfadiazine for Full-Thickness Wound Healing. Int. J. Biol. Macromol. 2024, 279, 135425. [Google Scholar] [CrossRef] [PubMed]
- Azis, H.A.; Taher, M.; Ahmed, A.S.; Sulaiman, W.M.A.W.; Susanti, D.; Chowdhury, S.R.; Zakaria, Z.A. In Vitro and In Vivo Wound Healing Studies of Methanolic Fraction of Centella asiatica Extract. South Afr. J. Bot. 2017, 108, 163–174. [Google Scholar] [CrossRef]
- Kulawik-Pióro, A.; Osak, E.; Mendrycka, M.; Trześniewska-Ofiara, Z. Bigels as Novel Systems for the Delivery Active Compounds from Centella asiatica. Soft Mater. 2023, 21, 316–338. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Song, A.; Wang, H.; Wu, Y.; Chang, W.; Tian, B.; Xu, J.; Dai, H.; Ma, Q.; et al. A Printable Hydrogel Loaded with Medicinal Plant Extract for Promoting Wound Healing. Adv. Healthc. Mater. 2024, 13, e2303017. [Google Scholar] [CrossRef]
- Elgamily, H.M.; Safwat, E.M.; Youssef, A.M. Development of a Novel Agarose/Nano-Hydroxyapatite/Grape Seed Extract Hydrogel for Biomimetic Remineralization of Demineralized Human Enamel (An In-Vitro Study). Sci. Rep. 2025, 15, 26086. [Google Scholar] [CrossRef]
- Morales, C.; Roeckel, M.; Fernández, K. Microscopic Modeling of País Grape Seed Extract Absorption in the Small Intestine. AAPS PharmSciTech 2014, 15, 103–110. [Google Scholar] [CrossRef]
- Priya; Sharma, A.K.; Kaith, B.S.; Arora, S.; Simran; Bhagyashree. Synthesis of Gelatin and Green Tea Based Stretchable Self-Healing Material of Biomedical Importance. React. Funct. Polym. 2022, 172, 105188. [Google Scholar] [CrossRef]
- Aldakheel, F.; Mohsen, D.; El Sayed, M.; Alawam, K.; Binshaya, A.; Alduraywish, S. Silver Nanoparticles Loaded on Chitosan-g-PVA Hydrogel for the Wound-Healing Applications. Molecules 2023, 28, 3241. [Google Scholar] [CrossRef]
- Jena, A.; Sahoo, N.K.; Sahoo, P.K.; Mishra, S.; Rout, P.R.; Zhong, Y. Design of a Novel Green Synthesized ZVS/S-RGO -Deinococcus radiodurans R1 Chitosan Hydrogel Beads for Enhanced Recovery of Europium. J. Clean. Prod. 2025, 503, 145367. [Google Scholar] [CrossRef]
- Ravindra, S.; Murali Mohan, Y.; Narayana Reddy, N.; Mohana Raju, K. Fabrication of Antibacterial Cotton Fibres Loaded with Silver Nanoparticles via “Green. Approach”. Colloids Surf. A Physicochem. Eng. Asp. 2010, 367, 31–40. [Google Scholar] [CrossRef]
- Pal, P.; Syed, S.S.; Banat, F. Soxhlet Extraction of Neem Pigment to Synthesize Iron Oxide Nanoparticles and Its Catalytic and Adsorption Activity for Methylene Blue Removal. Bionanoscience 2017, 7, 546–553. [Google Scholar] [CrossRef]
- Polaquini, S.R.B.; Svidzinski, T.I.E.; Kemmelmeier, C.; Gasparetto, A. Effect of Aqueous Extract from Neem (Azadirachta indica A. Juss) on Hydrophobicity, Biofilm Formation and Adhesion in Composite Resin by Candida Albicans. Arch. Oral. Biol. 2006, 51, 482–490. [Google Scholar] [CrossRef]
- Seriana, I.; Akmal, M.; Darusman, D.; Wahyuni, S.; Khairan, K.; Sugito, S. Neem Leaf (Azadirachta indica A. Juss) Ethanolic Extract on the Liver and Kidney Function of Rats. Sci. World J. 2021, 2021, 7970424. [Google Scholar] [CrossRef]
- Nowak, A.; Muzykiewicz-Szymańska, A.; Perużyńska, M.; Kucharska, E.; Kucharski, Ł.; Jakubczyk, K.; Niedźwiedzka-Rystwej, P.; Stefanowicz-Hajduk, J.; Droździk, M.; Majtan, J. Assessment of In Vitro Skin Permeation and Accumulation of Phenolic Acids from Honey and Honey-Based Pharmaceutical Formulations. BMC Complement. Med. Ther. 2025, 25, 43. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, M.; Wang, T.; Chen, X.; Li, Q.; Zhao, X. Preparation of Aloe Polysaccharide/Honey/PVA Composite Hydrogel: Antibacterial Activity and Promoting Wound Healing. Int. J. Biol. Macromol. 2022, 211, 249–258. [Google Scholar] [CrossRef]
- Jansen-Alves, C.; Maia, D.S.V.; Krumreich, F.D.; Crizel-Cardoso, M.M.; Fioravante, J.B.; da Silva, W.P.; Borges, C.D.; Zambiazi, R.C. Propolis Microparticles Produced with Pea Protein: Characterization and Evaluation of Antioxidant and Antimicrobial Activities. Food Hydrocoll. 2019, 87, 703–711. [Google Scholar] [CrossRef]
- Amorim, J.D.P.; Nascimento, H.A.; Silva Junior, C.J.G.; Medeiros, A.D.M.; Silva, I.D.L.; Costa, A.F.S.; Vinhas, G.M.; Sarubbo, L.A. Obtainment of Bacterial Cellulose with Added Propolis Extract for Cosmetic Applications. Polym. Eng. Sci. 2022, 62, 565–575. [Google Scholar] [CrossRef]
- Ferreira, L.M.d.M.C.; Cruz, N.F.d.; Lynch, D.G.; Costa, P.F.d.; Salgado, C.G.; Silva-Júnior, J.O.C.; Rossi, A.; Ribeiro-Costa, R.M. Hydrogel Containing Propolis: Physical Characterization and Evaluation of Biological Activities for Potential Use in the Treatment of Skin Lesions. Pharmaceuticals 2024, 17, 1400. [Google Scholar] [CrossRef] [PubMed]
- Jarzębski, M.; Smułek, W.; Baranowska, H.M.; Masewicz, Ł.; Kobus-Cisowska, J.; Ligaj, M.; Kaczorek, E. Characterization of St. John’s Wort (Hypericum perforatum L.) and the Impact of Filtration Process on Bioactive Extracts Incorporated into Carbohydrate-Based Hydrogels. Food Hydrocoll. 2020, 104, 105748. [Google Scholar] [CrossRef]
- Zivari-Ghader, T.; Shokouhi, B.; Kosari-Nasab, M.; Davaran, S.; Hamishehkar, H.; Farahpour, M.R.; Rashidi, M.; Mehrali, M. Hypericum perforatum Callus Extract-Loaded Composite Hydrogel with Diverse Bioactivities for Enhanced Wound Healing and Fibrosis Prevention. Small 2024, 20, e2407112. [Google Scholar] [CrossRef] [PubMed]
- Zivari-Ghader, T.; Hamishehkar, H.; Shokouhi, B.; Kosari-Nasab, M.; Farahpour, M.R.; Memar, M.Y.; Davaran, S.; Hanaee, J.; Rashidi, M.-R.; Mehrali, M. Chitosan-Alginate Hydrogel Enriched with Hypericum perforatum Callus Extract for Improved Wound Healing and Scar Inhibition. ACS Appl. Mater. Interfaces 2024, 16, 67344–67361. [Google Scholar] [CrossRef]
- Alvandi, H.; Rajati, H.; Naseriyeh, T.; Rahmatabadi, S.S.; Hosseinzadeh, L.; Arkan, E. Incorporation of Aloe vera and Green Synthesized ZnO Nanoparticles into the Chitosan/PVA Nanocomposite Hydrogel for Wound Dressing Application. Polym. Bull. 2024, 81, 4123–4148. [Google Scholar] [CrossRef]
- Baniasadi, H. State-of-the-Art in Natural Hydrogel-Based Wound Dressings: Design, Functionalization, and Fabrication Approaches. Adv. Colloid. Interface Sci. 2025, 342, 103527. [Google Scholar] [CrossRef]
- Luo, Z.; Wang, Y.; Xu, Y.; Wang, J.; Yu, Y. Modification and Crosslinking Strategies for Hyaluronic Acid-based Hydrogel Biomaterials. Smart Med. 2023, 2, e20230029. [Google Scholar] [CrossRef]
- Luo, Z.; Wang, Y.; Li, J.; Wang, J.; Yu, Y.; Zhao, Y. Tailoring Hyaluronic Acid Hydrogels for Biomedical Applications. Adv. Funct. Mater. 2023, 33, 2306554. [Google Scholar] [CrossRef]
- Hanif, W.; Hardiansyah, A.; Randy, A.; Asri, L.A.T.W. Physically Crosslinked PVA/Graphene-Based Materials/Aloe vera Hydrogel with Antibacterial Activity. RSC Adv. 2021, 11, 29029–29041. [Google Scholar] [CrossRef]
- Kenawy, E.-R.; El-Meligy, M.A.; Ghaly, Z.S.; Kenawy, M.E.; Kamoun, E.A. Novel Physically-Crosslinked Caffeine and Vitamin C-Loaded PVA/Aloe vera Hydrogel Membranes for Topical Wound Healing: Synthesis, Characterization and In-Vivo Wound Healing Tests. J. Polym. Environ. 2024, 32, 2140–2157. [Google Scholar] [CrossRef]
- Huang, W.-H.; Hung, C.-Y.; Chiang, P.-C.; Lee, H.; Lin, I.-T.; Lai, P.-C.; Chan, Y.-H.; Feng, S.-W. Physicochemical Characterization, Biocompatibility, and Antibacterial Properties of CMC/PVA/Calendula officinalis Films for Biomedical Applications. Polymers 2023, 15, 1454. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.S.; Taher, M.; Mandal, U.K.; Jaffri, J.M.; Susanti, D.; Mahmood, S.; Zakaria, Z.A. Pharmacological Properties of Centella asiatica Hydrogel in Accelerating Wound Healing in Rabbits. BMC Complement. Altern. Med. 2019, 19, 213. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, Y.; Raouf Malik, A.; Iqbal, T.; Hammad Aziz, M.; Ahmed, F.; Abolaban, F.A.; Mansoor Ali, S.; Ullah, H. Green Synthesis of ZnO and Ag-Doped ZnO Nanoparticles Using Azadirachta indica Leaves: Characterization and Their Potential Antibacterial, Antidiabetic, and Wound-Healing Activities. Mater. Lett. 2021, 305, 130671. [Google Scholar] [CrossRef]
- Saberian, M.; Seyedjafari, E.; Zargar, S.J.; Mahdavi, F.S.; Sanaei-rad, P. Fabrication and Characterization of Alginate/Chitosan Hydrogel Combined with Honey and Aloe vera for Wound Dressing Applications. J. Appl. Polym. Sci. 2021, 138, 51398. [Google Scholar] [CrossRef]
- Sabouri Moghadam, A.; Mirmohammad Meiguni, M.S.; Salami, M.; Askari, G.; Emam-Djomeh, Z.; Miran, M.; Buttar, H.S.; Brennan, C. Characterization of Physicochemical Properties of Mung Bean Protein Isolate and κ-Carrageenan Hydrogel as a Delivery System for Propolis Extract. Food Res. Int. 2024, 197, 115221. [Google Scholar] [CrossRef]
- Lu, P.; Ruan, D.; Huang, M.; Tian, M.; Zhu, K.; Gan, Z.; Xiao, Z. Harnessing the Potential of Hydrogels for Advanced Therapeutic Applications: Current Achievements and Future Directions. Signal Transduct. Target. Ther. 2024, 9, 166. [Google Scholar] [CrossRef]
- Shanmugha Mary, A.; Mani, A.; Ghosh, S.; Rajaram, K. Phage Embedded Gelatin, Alginate and Gelatin/Alginate-Starch Based Hydrogels for Topical Bactericidal Applications against Multi-Drug Resistant Pseudomonas aeruginosa. J. Indian Chem. Soc. 2024, 101, 101272. [Google Scholar] [CrossRef]
- Wang, X.; Yang, J.; Zhao, Q.; Xie, X.; Deng, F.; Wang, Z.; Jiang, K.; Li, X.; Liu, H.; Shi, Z.; et al. A Tissue-Adhesive, Mechanically Enhanced, Natural Aloe vera-Based Injectable Hydrogel for Wound Healing: Macrophage Mediation and Collagen Proliferation. Int. J. Biol. Macromol. 2024, 283, 137452. [Google Scholar] [CrossRef]
- Dey, A.; Bera, R.; Chakrabarty, D. Influence of Aloe vera on the Properties of N-Vinylpyrrolidone-Acrylamide Copolymer Hydrogel. Mater. Chem. Phys. 2015, 168, 168–179. [Google Scholar] [CrossRef]
- Rashedi, S.; Heydari, P.; Kharazi, A.Z.; Varshosaz, J.; Sheikholeslam, M. Chitosan/Poly (Β-amino Ester) Hydrogel by Controlled Release of Centella asiatica Promoted Wound Healing through Improved Collagen Expression and Antibacterial and Anti-inflammatory Properties. Polym. Eng. Sci. 2025, 65, 2418–2435. [Google Scholar] [CrossRef]
- Sampath Udeni Gunathilake, T.M.; Ching, Y.C.; Chuah, C.H.; Illias, H.A.; Ching, K.Y.; Singh, R.; Nai-Shang, L. Influence of a Nonionic Surfactant on Curcumin Delivery of Nanocellulose Reinforced Chitosan Hydrogel. Int. J. Biol. Macromol. 2018, 118, 1055–1064. [Google Scholar] [CrossRef]
- Alves, C.; Ribeiro, A.; Pinto, E.; Santos, J.; Soares, G. Exploring Z-Tyr-Phe-OH-Based Hydrogels Loaded with Curcumin for the Development of Dressings for Wound Healing. J. Drug Deliv. Sci. Technol. 2022, 73, 103484. [Google Scholar] [CrossRef]
- Bai, Q.; Hu, F.; Gou, S.; Gao, Q.; Wang, S.; Zhang, W.; Zhang, Y.; Lu, T. Curcumin-Loaded Chitosan-Based Hydrogels Accelerating S. Aureus-Infected Wound Healing. Int. J. Biol. Macromol. 2024, 259, 129111. [Google Scholar] [CrossRef]
- Gupta, S.; Ghoshal, G. Plant Protein Hydrogel as a Delivery System of Curcumin: Characterization and In Vitro Release Kinetics. Food Bioprod. Process. 2024, 143, 66–79. [Google Scholar] [CrossRef]
- Jayaramudu, T.; Raghavendra, G.M.; Varaprasad, K.; Raju, K.M.; Sadiku, E.R.; Kim, J. 5-Fluorouracil Encapsulated Magnetic Nanohydrogels for Drug-delivery Applications. J. Appl. Polym. Sci. 2016, 133, 43921. [Google Scholar] [CrossRef]
- Jayaramudu, T.; Varaprasad, K.; Sadiku, E.R.; Kim, H.C.; Kim, J. Preparation of Antibacterial Temperature-sensitive Silver-nanocomposite Hydrogels from N. -isopropylacrylamide with Green Tea. J. Appl. Polym. Sci. 2018, 135, 45739. [Google Scholar] [CrossRef]
- Abid Mustafa, M.; Rashid Hussain, H.; Akbar Khan, J.; Ahmad, N.; Bashir, S.; Asad, M.; Saeed Shah, H.; Ali Khan, A.; Malik, A.; Fatima, S.; et al. Development and In Vitro Characterization of Azadirachta indica Gum Grafted Polyacrylamide Based PH-Sensitive Hydrogels to Improve the Bioavailability of Lansoprazole. Chem. Biodivers. 2025, 22, e202401434. [Google Scholar] [CrossRef]
- Vigata, M.; Meinert, C.; Hutmacher, D.W.; Bock, N. Hydrogels as Drug Delivery Systems: A Review of Current Characterization and Evaluation Techniques. Pharmaceutics 2020, 12, 1188. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, A.H.; Farazin, A.; Mohammadimehr, M.; Naeimi, H. Fabrication and Characterization of Biopolymers with Antibacterial Nanoparticles and Calendula officinalis Flower Extract as an Active Ingredient for Modern Hydrogel Wound Dressings. Mater. Today Commun. 2022, 31, 103513. [Google Scholar] [CrossRef]
- Pelin, I.M.; Silion, M.; Popescu, I.; Rîmbu, C.M.; Fundueanu, G.; Constantin, M. Pullulan/Poly(Vinyl Alcohol) Hydrogels Loaded with Calendula officinalis Extract: Design and In Vitro Evaluation for Wound Healing Applications. Pharmaceutics 2023, 15, 1674. [Google Scholar] [CrossRef]
- Choudhary, V.; Malik, A. Enhanced Wound-Healing by Hydrogel from Okra Mucilage Grafted with Poly-2-Acrylamido-2-Methylpropane Sulfonic Acid (AMPS), a Stimuli-Responsive Polymer. Eur. Polym. J. 2025, 227, 113748. [Google Scholar] [CrossRef]
- Hong, G.S.; Choi, J.Y.; Suh, J.S.; Lim, J.O.; Choi, J.H. Development of a Natural Matrix Hybrid Hydrogel Patch and Evaluation of Its Efficacy against Atopic Dermatitis. Appl. Sci. 2020, 10, 8759. [Google Scholar] [CrossRef]
- Hezari, S.; Olad, A.; Dilmaghani, A. Investigation of Antibacterial Properties and Sustained Release of Centella asiatica Extract from Fe-MOF-Reinforced Gelatin-Based Hydrogels. Polym. Bull. 2024, 81, 14701–14725. [Google Scholar] [CrossRef]
- Hezari, S.; Olad, A.; Dilmaghani, A. Development of Gelatin and Modified Gelatin Hydrogels Incorporated with Aluminum-Based Metal–Organic Frameworks as a Potential Wound Dressing. Polym. Bull. 2025, 82, 11947–11981. [Google Scholar] [CrossRef]
- Khamrai, M.; Banerjee, S.L.; Paul, S.; Samanta, S.; Kundu, P.P. Curcumin Entrapped Gelatin/Ionically Modified Bacterial Cellulose Based Self-Healable Hydrogel Film: An Eco-Friendly Sustainable Synthesis Method of Wound Healing Patch. Int. J. Biol. Macromol. 2019, 122, 940–953. [Google Scholar] [CrossRef]
- Luo, C.; Wei, N.; Sun, X.; Luo, F. Fabrication of Self-healable, Conductive, and Ultra-strong Hydrogel from Polyvinyl Alcohol and Grape Seed–Extracted Polymer. J. Appl. Polym. Sci. 2020, 137, 49118. [Google Scholar] [CrossRef]
- Chuysinuan, P.; Chunshom, N.; Kotcharat, P.; Thanyacharoen, T.; Techasakul, S.; Ummartyotin, S. The Encapsulation of Green Tea Extract in Cyclodextrin and Loading into Chitosan-Based Composites: Controlled-Release Behavior and Antioxidant Properties. J. Polym. Environ. 2021, 29, 2628–2638. [Google Scholar] [CrossRef]
- Aminu, N.; Alfred-Ugbenbo, D.; Moradeke, O.; Audu Mumuni, M.; Muhammad Umar, N.; Tanko, N.; Raghavulu Bitra, V.; Tshepo Moshapa, F.; Monkgogi, T.; Siok-Yee, C. Nanogel Drug Delivery System Loaded with Azadirachta indica A. Juss. (Neem) for Potential Treatment of Wound Infection: Development and Characterization. Beni Suef Univ. J. Basic. Appl. Sci. 2025, 14, 67. [Google Scholar] [CrossRef]
- Sharaf, S.; El-Naggar, M.E. Wound Dressing Properties of Cationized Cotton Fabric Treated with Carrageenan/Cyclodextrin Hydrogel Loaded with Honey Bee Propolis Extract. Int. J. Biol. Macromol. 2019, 133, 583–591. [Google Scholar] [CrossRef]
- Eakwaropas, P.; Ngawhirunpat, T.; Rojanarata, T.; Patrojanasophon, P.; Opanasopit, P.; Nuntharatanapong, N. Formulation and Optimal Design of Dioscorea bulbifera and Honey-Loaded Gantrez®/Xyloglucan Hydrogel as Wound Healing Patches. Pharmaceutics 2022, 14, 1302. [Google Scholar] [CrossRef]
- Yang, S.; Wang, F.; Han, H.; Santos, H.A.; Zhang, Y.; Zhang, H.; Wei, J.; Cai, Z. Fabricated Technology of Biomedical Micro-Nano Hydrogel. Biomed. Technol. 2023, 2, 31–48. [Google Scholar] [CrossRef]
- Julie, B.-A.; John, R.; Natalia, M.-C. Microstructural, Bioactive, and Wound-Healing Properties of Chitosan-Based Dressings with Encapsulated Aloe vera and Curcuma longa Extracts. Mater. Today Commun. 2025, 47, 113047. [Google Scholar] [CrossRef]
- Ameli, S.; Nourani, M.; Bakhshi, N.; Salemi, B.; Assadpour, E.; Jafari, S.M. Alginate-Gelatin Composite Hydrogels for Encapsulating Aloe vera Extract; Optimization, Characterization, and Release Kinetics. Carbohydr. Polym. Technol. Appl. 2025, 9, 100717. [Google Scholar] [CrossRef]
- Shefa, A.A.; Sultana, T.; Park, M.K.; Lee, S.Y.; Gwon, J.-G.; Lee, B.-T. Curcumin Incorporation into an Oxidized Cellulose Nanofiber-Polyvinyl Alcohol Hydrogel System Promotes Wound Healing. Mater. Des. 2020, 186, 108313. [Google Scholar] [CrossRef]
- Cardoso-Daodu, I.M.; Ilomuanya, M.O.; Azubuike, C.P. Development of Curcumin-Loaded Liposomes in Lysine–Collagen Hydrogel for Surgical Wound Healing. Beni Suef Univ. J. Basic. Appl. Sci. 2022, 11, 100. [Google Scholar] [CrossRef]
- Kour, P.; Afzal, S.; Gani, A.; Zargar, M.I.; Nabi Tak, U.; Rashid, S.; Dar, A.A. Effect of Nanoemulsion-Loaded Hybrid Biopolymeric Hydrogel Beads on the Release Kinetics, Antioxidant Potential and Antibacterial Activity of Encapsulated Curcumin. Food Chem. 2022, 376, 131925. [Google Scholar] [CrossRef]
- Mahdian, M.; Akbari Asrari, S.; Ahmadi, M.; Madrakian, T.; Rezvani Jalal, N.; Afkhami, A.; Moradi, M.; Gholami, L. Dual Stimuli-Responsive Gelatin-Based Hydrogel for PH and Temperature-Sensitive Delivery of Curcumin Anticancer Drug. J. Drug Deliv. Sci. Technol. 2023, 84, 104537. [Google Scholar] [CrossRef]
- Preda, P.; Enciu, A.-M.; Adiaconita, B.; Mihalache, I.; Craciun, G.; Boldeiu, A.; Aricov, L.; Romanitan, C.; Stan, D.; Marculescu, C.; et al. New Amorphous Hydrogels with Proliferative Properties as Potential Tools in Wound Healing. Gels 2022, 8, 604. [Google Scholar] [CrossRef]
- Garcia-Orue, I.; Santos-Vizcaino, E.; Uranga, J.; de la Caba, K.; Guerrero, P.; Igartua, M.; Hernandez, R.M. Agar/Gelatin Hydro-Film Containing EGF and Aloe vera for Effective Wound Healing. J. Mater. Chem. B 2023, 11, 6896–6910. [Google Scholar] [CrossRef]
- Darwish, M.M.; Elneklawi, M.S.; Mohamad, E.A. Aloe vera Coated Dextran Sulfate/Chitosan Nanoparticles (Aloe vera @ DS/CS) Encapsulating Eucalyptus Essential Oil with Antibacterial Potent Property. J. Biomater. Sci. Polym. Ed. 2023, 34, 810–827. [Google Scholar] [CrossRef]
- Karimi, E.; Gharib, B.; Rostami, N.; Navidpour, L.; Afshar, M. Clinical Efficacy of a Topical Polyherbal Formulation in the Management of Fluorouracil-Associated Hand-Foot Syndrome. J. Herb. Med. 2019, 17, 100270. [Google Scholar] [CrossRef]
- Colobatiu, L.; Gavan, A.; Mocan, A.; Bogdan, C.; Mirel, S.; Tomuta, I. Development of Bioactive Compounds-Loaded Chitosan Films by Using a QbD Approach—A Novel and Potential Wound Dressing Material. React. Funct. Polym. 2019, 138, 46–54. [Google Scholar] [CrossRef]
- Chanaj-Kaczmarek, J.; Paczkowska, M.; Osmałek, T.; Kaproń, B.; Plech, T.; Szymanowska, D.; Karaźniewicz-Łada, M.; Kobus-Cisowska, J.; Cielecka-Piontek, J. Hydrogel Delivery System Containing Calendulae Flos Lyophilized Extract with Chitosan as a Supporting Strategy for Wound Healing Applications. Pharmaceutics 2020, 12, 634. [Google Scholar] [CrossRef] [PubMed]
- Saha, I.; Roy, S.; Das, D.; Das, S.; Karmakar, P. Topical Effect of Polyherbal Flowers Extract on Xanthan Gum Hydrogel Patch—Induced Wound Healing Activity in Human Cell Lines and Male BALB/c Mice. Biomed. Mater. 2023, 18, 035016. [Google Scholar] [CrossRef] [PubMed]
- Mathew, D.; Thomas, B.; Soumya, P.T.; Sudheep, N.M. Development of Chitosan Based Hydrogels with Marigold Flower Extract: An Innovative, Low Cost, Biodegradable and Antimicrobial Solution for Enhanced Wound Healing Applications. Results Surf. Interfaces 2025, 20, 100602. [Google Scholar] [CrossRef]
- Kuo, C.-W.; Chiu, Y.-F.; Wu, M.-H.; Li, M.-H.; Wu, C.-N.; Chen, W.-S.; Huang, C.-H. Gelatin/Chitosan Bilayer Patches Loaded with Cortex Phellodendron amurense/Centella asiatica Extracts for Anti-Acne Application. Polymers 2021, 13, 579. [Google Scholar] [CrossRef]
- Tohidi, A.; Montazer, M.; Mianehro, A.; Rad, M.M. Biocompatible Polysaccharide-Based Wound Dressing Comprising Cellulose Fabric Treated with Gum Tragacanth, Alginate, Bacterial Cellulose, and Chamomile Extracts. Starch-Stärke 2024, 76, 2300117. [Google Scholar] [CrossRef]
- Zakerikhoob, M.; Abbasi, S.; Yousefi, G.; Mokhtari, M.; Noorbakhsh, M.S. Curcumin-Incorporated Crosslinked Sodium Alginate-g-Poly (N-Isopropyl Acrylamide) Thermo-Responsive Hydrogel as an in-Situ Forming Injectable Dressing for Wound Healing: In Vitro Characterization and In Vivo Evaluation. Carbohydr. Polym. 2021, 271, 118434. [Google Scholar] [CrossRef]
- Bhubhanil, S.; Talodthaisong, C.; Khongkow, M.; Namdee, K.; Wongchitrat, P.; Yingmema, W.; Hutchison, J.A.; Lapmanee, S.; Kulchat, S. Enhanced Wound Healing Properties of Guar Gum/Curcumin-Stabilized Silver Nanoparticle Hydrogels. Sci. Rep. 2021, 11, 21836. [Google Scholar] [CrossRef]
- Chopra, H.; Bibi, S.; Mohanta, Y.K.; Kumar Mohanta, T.; Kumar, S.; Singh, I.; Saad Khan, M.; Ranjan Rauta, P.; Alshammari, A.; Alharbi, M.; et al. In Vitro and In Silico Characterization of Curcumin-Loaded Chitosan–PVA Hydrogels: Antimicrobial and Potential Wound Healing Activity. Gels 2023, 9, 394. [Google Scholar] [CrossRef]
- Khodaei, A.; Johari, N.; Jahanmard, F.; Cecotto, L.; Khosravimelal, S.; Madaah Hosseini, H.R.; Bagheri, R.; Samadikuchaksaraei, A.; Amin Yavari, S. Particulate 3D Hydrogels of Silk Fibroin-Pluronic to Deliver Curcumin for Infection-Free Wound Healing. Biomimetics 2024, 9, 483. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Duan, A.; Shen, L.; Liu, Q.; Wang, F.; Liu, Y. Preparation and Application of Curcumin Loaded with Citric Acid Crosslinked Chitosan-Gelatin Hydrogels. Int. J. Biol. Macromol. 2024, 264, 130801. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Zhong, H.; Tang, W.; Wen, F.; Lv, Y.; Huang, X.; Luo, J.; Li, P. Multiple Response Behaviors of Curcumin-Loaded Ammonium Alginate/Polyvinyl Alcohol Hydrogel and Its Application. Biomass Convers. Biorefin 2024, 14, 16121–16139. [Google Scholar] [CrossRef]
- Kannan, P.R.; Kumar, C.S.; Zhao, R.; Iqbal, M.Z.; Li, Y.; Kong, X. Dual-Functional Hydrogel with Curcumin-Loaded GelMA and Silk Fibroin for Wound Healing: Characterization and In Vitro Evaluation. Mater. Today Commun. 2025, 44, 112014. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, J.; Liu, Y.; Sun, S.; Wang, K. Multi-Functional Dressing with Curcumin Displays Anti-Inflammatory, Antioxidant, Angiogenic, and Collagen Regeneration Effects in Diabetic Wound Healing. J. Mater. Sci. 2025, 60, 6217–6234. [Google Scholar] [CrossRef]
- Teixeira, L.S.; Sousa, M.; Massano, F.; Borges, A. Exploring Grape Pomace Extracts for the Formulation of New Bioactive Multifunctional Chitosan/Alginate-Based Hydrogels for Wound Healing Applications. Food Biosci. 2024, 62, 105073. [Google Scholar] [CrossRef]
- Das, P.; Chakravarty, T.; Roy, A.J.; Manna, S.; Nandi, S.K.; Basak, P. Sustainable Development of Draksha- Beeja Extract Loaded Gelatin and Starch-Based Green and Biodegradable Mats for Potential Tissue Engineering Applications. Sustain. Chem. Pharm. 2023, 34, 101134. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, F.; Zhao, R.; Wang, C.; Hu, K.; Sun, Y.; Politis, C.; Shavandi, A.; Nie, L. Polyvinyl Alcohol/Sodium Alginate Hydrogels Incorporated with Silver Nanoclusters via Green Tea Extract for Antibacterial Applications. Des. Monomers Polym. 2020, 23, 118–133. [Google Scholar] [CrossRef]
- Rungrod, A.; Makarasen, A.; Patnin, S.; Techasakul, S.; Somsunan, R. Design and Development of a Sprayable Hydrogel Based on Thermo/PH Dual-Responsive Polymer Incorporating Azadirachta indica (Neem) Extract for Wound Dressing Applications. Polymers 2025, 17, 2157. [Google Scholar] [CrossRef]
- Abd El-Malek, F.F.; Yousef, A.S.; El-Assar, S.A. Hydrogel Film Loaded with New Formula from Manuka Honey for Treatment of Chronic Wound Infections. J. Glob. Antimicrob. Resist. 2017, 11, 171–176. [Google Scholar] [CrossRef]
- Kim, J.; Lee, C.-M. Transdermal Hydrogel Composed of Polyacrylic Acid Containing Propolis for Wound Healing in a Rat Model. Macromol. Res. 2018, 26, 1219–1224. [Google Scholar] [CrossRef]
- Saleh, S.; Salama, A.; Ali, A.M.; Saleh, A.K.; Elhady, B.A.; Tolba, E. Egyptian Propolis Extract for Functionalization of Cellulose Nanofiber/Poly(Vinyl Alcohol) Porous Hydrogel along with Characterization and Biological Applications. Sci. Rep. 2023, 13, 7739. [Google Scholar] [CrossRef] [PubMed]
- Cao-Luu, N.-H.; Nguyen, T.-V.; Luong, H.-V.-T.; Dang, H.-G.; Pham, H.-G. Engineered Polyvinyl Alcohol/Chitosan/Carrageenan Nanofibrous Membrane Loaded with Aloe vera for Accelerating Third-Degree Burn Wound Healing. Int. J. Biol. Macromol. 2025, 311, 143880. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, V.; Sharma, S.; Malik, A.; Shrivastav, A.; Shukla, P.K. In Vivo Study of Modified Okra Mucilage/Acrylic Acid Hydrogels Loaded with Ethanolic Extracts of Calendula officinalis for Wound Healing Application. Adv. Pharmacol. Pharm. 2025, 13, 426–437. [Google Scholar] [CrossRef]
- Le, T.T.N.; Nguyen, T.K.N.; Nguyen, V.M.; Dao, T.C.M.; Nguyen, H.B.C.; Dang, C.T.; Le, T.B.C.; Nguyen, T.K.L.; Nguyen, P.T.T.; Dang, L.H.N.; et al. Development and Characterization of a Hydrogel Containing Curcumin-Loaded Nanoemulsion for Enhanced In Vitro Antibacteria and In Vivo Wound Healing. Molecules 2023, 28, 6433. [Google Scholar] [CrossRef]
- Cai, X.; He, Y.; Cai, L.; Zhan, J.; Li, Q.; Zhong, S.; Hou, H.; Wang, W.; Qiu, X. An Injectable Elastic Hydrogel Crosslinked with Curcumin–Gelatin Nanoparticles as a Multifunctional Dressing for the Rapid Repair of Bacterially Infected Wounds. Biomater. Sci. 2023, 11, 3227–3240. [Google Scholar] [CrossRef]
- Fan, X.; Huang, J.; Zhang, W.; Su, Z.; Li, J.; Wu, Z.; Zhang, P. A Multifunctional, Tough, Stretchable, and Transparent Curcumin Hydrogel with Potent Antimicrobial, Antioxidative, Anti-Inflammatory, and Angiogenesis Capabilities for Diabetic Wound Healing. ACS Appl. Mater. Interfaces 2024, 16, 9749–9767. [Google Scholar] [CrossRef]
- Ma, H.; Zhou, Q.; Chang, J.; Wu, C. Grape Seed-Inspired Smart Hydrogel Scaffolds for Melanoma Therapy and Wound Healing. ACS Nano 2019, 13, 4302–4311. [Google Scholar] [CrossRef]
- Kurt, A.A.; Ibrahim, B.; Cinar, H.; Ozmen, O. Enhanced Therapeutic Potential of Hypericum perforatum L.: A Comprehensive In Vitro and In Vivo Evaluation of Nanoparticle-Hydrogel Formulations. J. Nanotechnol. 2025, 2025, 6232038. [Google Scholar] [CrossRef]
- Hajian, M.; Mahmoodi, M.; Imani, R. In Vitro Assessment of Poly (Vinyl Alcohol) Film Incorporating Aloe vera for Potential Application as a Wound Dressing. J. Macromol. Sci. Part B 2017, 56, 435–450. [Google Scholar] [CrossRef]
- Bhar, B.; Chakraborty, B.; Nandi, S.K.; Mandal, B.B. Silk-Based Phyto-Hydrogel Formulation Expedites Key Events of Wound Healing in Full-Thickness Skin Defect Model. Int. J. Biol. Macromol. 2022, 203, 623–637. [Google Scholar] [CrossRef]
- Alvandi, H.; Jaymand, M.; Eskandari, M.; Aghaz, F.; Hosseinzadeh, L.; Heydari, M.; Arkan, E. A Sandwich Electrospun Nanofibers/Tragacanth Hydrogel Composite Containing Aloe vera Extract and Silver Sulfadiazine as a Wound Dressing. Polym. Bull. 2023, 80, 11235–11248. [Google Scholar] [CrossRef]
- Manoharan, S.; Balakrishnan, P.; Sellappan, L.K. Fabrication of Highly Flexible Biopolymeric Chitosan/Agarose Based Bioscaffold with Matricaria recutita Herbal Extract for Antimicrobial Wound Dressing Applications. Int. J. Biol. Macromol. 2024, 281, 136195. [Google Scholar] [CrossRef]
- Ferfera-Harrar, H.; Berdous, D.; Benhalima, T. Hydrogel Nanocomposites Based on Chitosan-g-Polyacrylamide and Silver Nanoparticles Synthesized Using Curcuma longa for Antibacterial Applications. Polym. Bull. 2018, 75, 2819–2846. [Google Scholar] [CrossRef]
- Jessy Mercy, D.; Thirumalai, A.; Udayakumar, S.; Deepika, B.; Janani, G.; Girigoswami, A.; Girigoswami, K. Enhancing Wound Healing with Nanohydrogel-Entrapped Plant Extracts and Nanosilver: An In Vitro Investigation. Molecules 2024, 29, 5004. [Google Scholar] [CrossRef] [PubMed]
- Oryan, A.; Alemzadeh, E.; Mohammadi, A.A.; Moshiri, A. Healing Potential of Injectable Aloe vera Hydrogel Loaded by Adipose-Derived Stem Cell in Skin Tissue-Engineering in a Rat Burn Wound Model. Cell Tissue Res. 2019, 377, 215–227. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Mittal, P.; Yadav, A.; Mishra, A.K.; Hazari, P.P.; Sharma, R.K. Sustained Activity of Stimuli-Responsive Curcumin and Acemannan Based Hydrogel Patches in Wound Healing. ACS Appl. Bio Mater. 2022, 5, 598–609. [Google Scholar] [CrossRef]
- Liao, H.T.; Lai, Y.-T.; Kuo, C.-Y.; Chen, J.-P. A Bioactive Multi-Functional Heparin-Grafted Aligned Poly(Lactide-Co-Glycolide)/Curcumin Nanofiber Membrane to Accelerate Diabetic Wound Healing. Mater. Sci. Eng. C 2021, 120, 111689. [Google Scholar] [CrossRef]
- Lacatusu, I.; Badea, G.; Popescu, M.; Bordei, N.; Istrati, D.; Moldovan, L.; Seciu, A.M.; Panteli, M.I.; Rasit, I.; Badea, N. Marigold Extract, Azelaic Acid and Black Caraway Oil into Lipid Nanocarriers Provides a Strong Anti-Inflammatory Effect In Vivo. Ind. Crops Prod. 2017, 109, 141–150. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Călina, I.C.; Scărișoreanu, A.; Demeter, M.; Mănăilă, E.; Crăciun, G. Strategies for Incorporating Natural Therapeutic Agents into Hydrogel Dressings: Innovations in Wound Healing. Polymers 2025, 17, 3105. https://doi.org/10.3390/polym17233105
Călina IC, Scărișoreanu A, Demeter M, Mănăilă E, Crăciun G. Strategies for Incorporating Natural Therapeutic Agents into Hydrogel Dressings: Innovations in Wound Healing. Polymers. 2025; 17(23):3105. https://doi.org/10.3390/polym17233105
Chicago/Turabian StyleCălina, Ion Cosmin, Anca Scărișoreanu, Maria Demeter, Elena Mănăilă, and Gabriela Crăciun. 2025. "Strategies for Incorporating Natural Therapeutic Agents into Hydrogel Dressings: Innovations in Wound Healing" Polymers 17, no. 23: 3105. https://doi.org/10.3390/polym17233105
APA StyleCălina, I. C., Scărișoreanu, A., Demeter, M., Mănăilă, E., & Crăciun, G. (2025). Strategies for Incorporating Natural Therapeutic Agents into Hydrogel Dressings: Innovations in Wound Healing. Polymers, 17(23), 3105. https://doi.org/10.3390/polym17233105