Flexible Intelligence on a Green Skeleton: Progress and Challenges of CNF-Enabled Multimodal Sensing Platforms
Abstract
1. Introduction
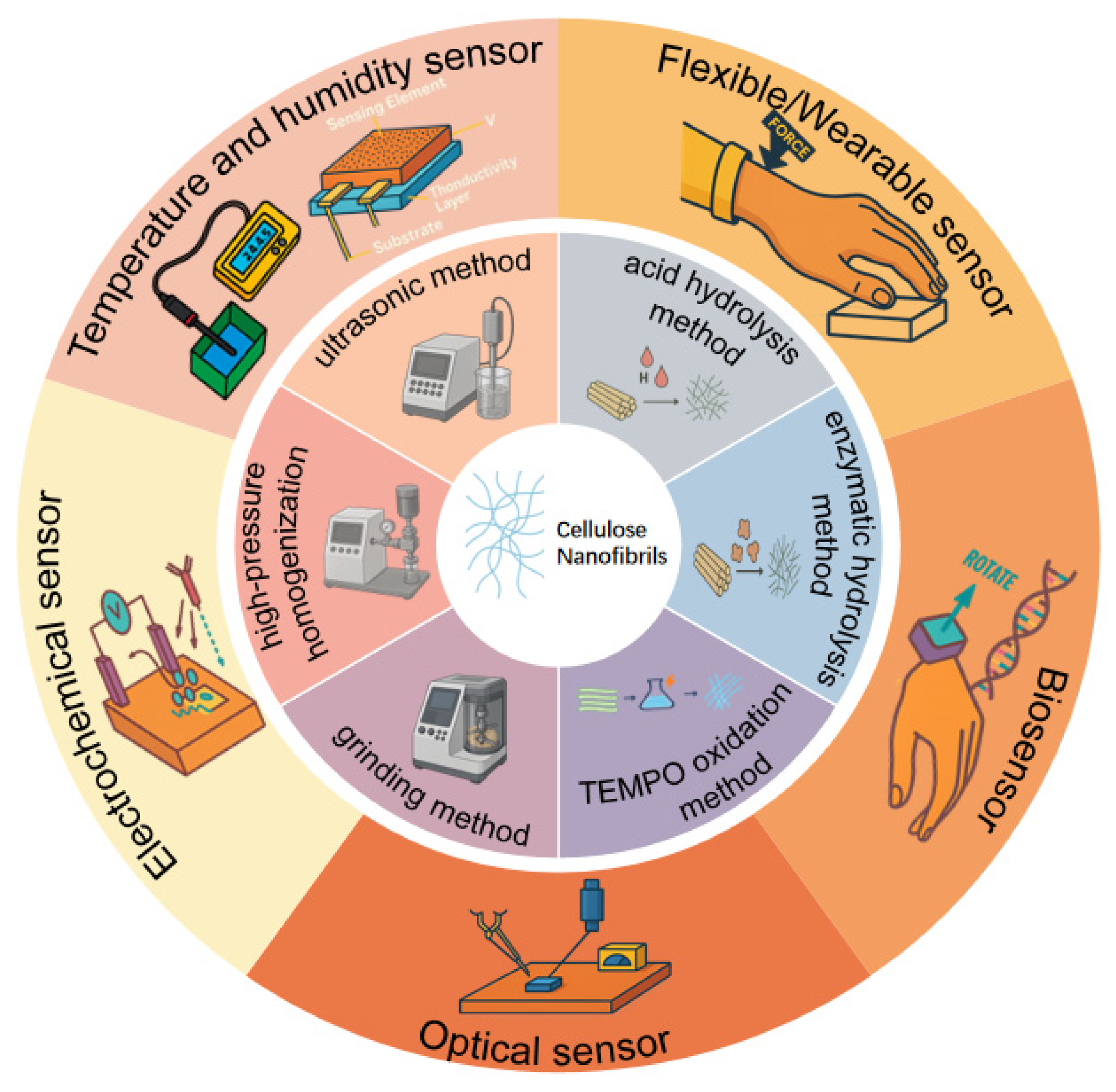
2. Synthesis and Preparation of CNFs
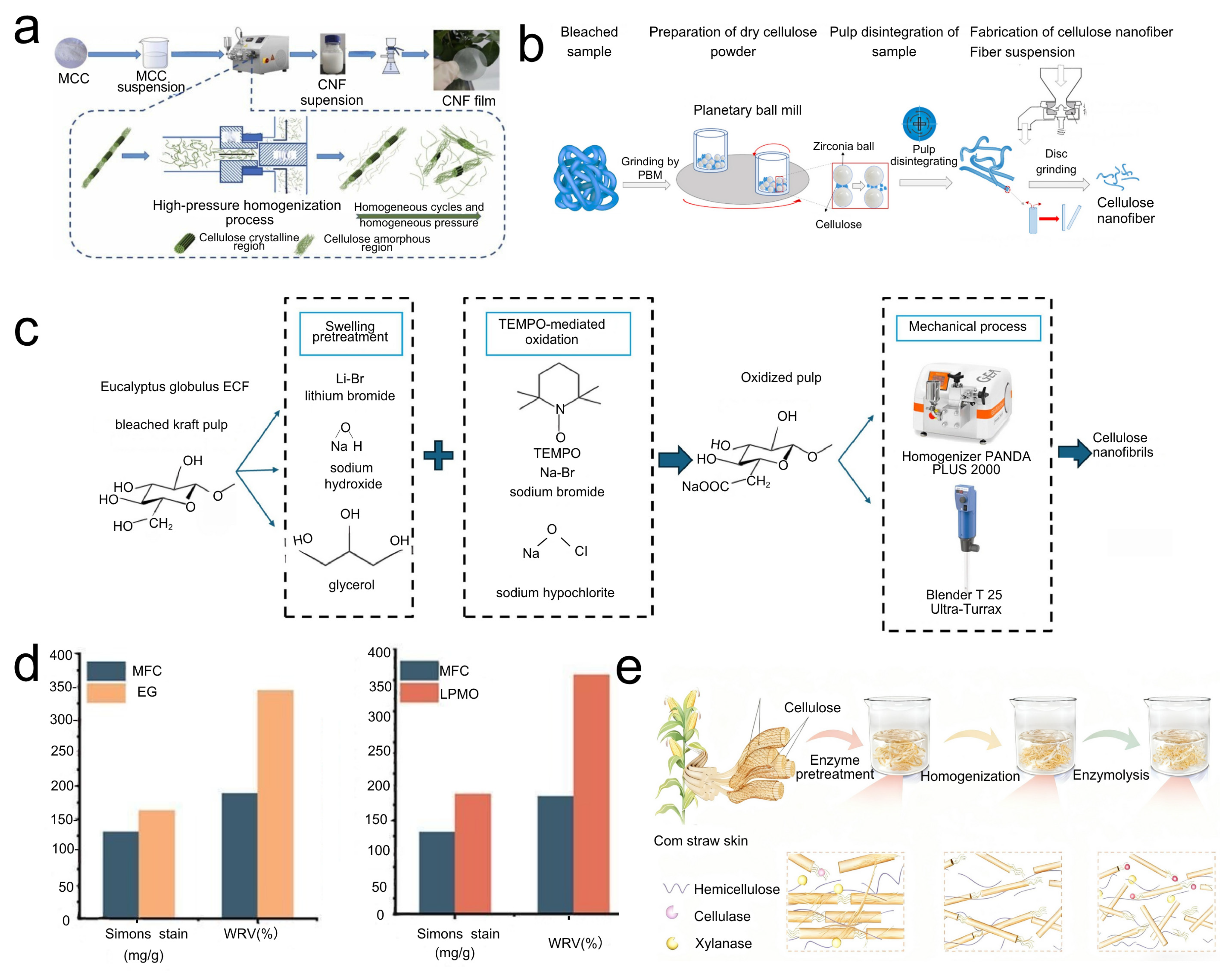
2.1. Mechanical Disintegration Methods
2.1.1. High-Pressure Homogenization (HPH)
2.1.2. Grinding and Slurry-Making Technologies
2.1.3. Ultrasonic Treatment
2.2. Chemical and Chemical–Mechanical Methods
2.2.1. TEMPO-Mediated Oxidation
2.2.2. Acid Hydrolysis for CNF Production (Distinct from CNC Production)
2.2.3. Enzymatic Hydrolysis
2.3. Other Promising Preparation Strategies
2.3.1. DES Pretreatment
2.3.2. Plasma Pretreatment
2.3.3. Microwave-Assisted Technology
3. Applications of CNFs in Advanced Sensing Platforms
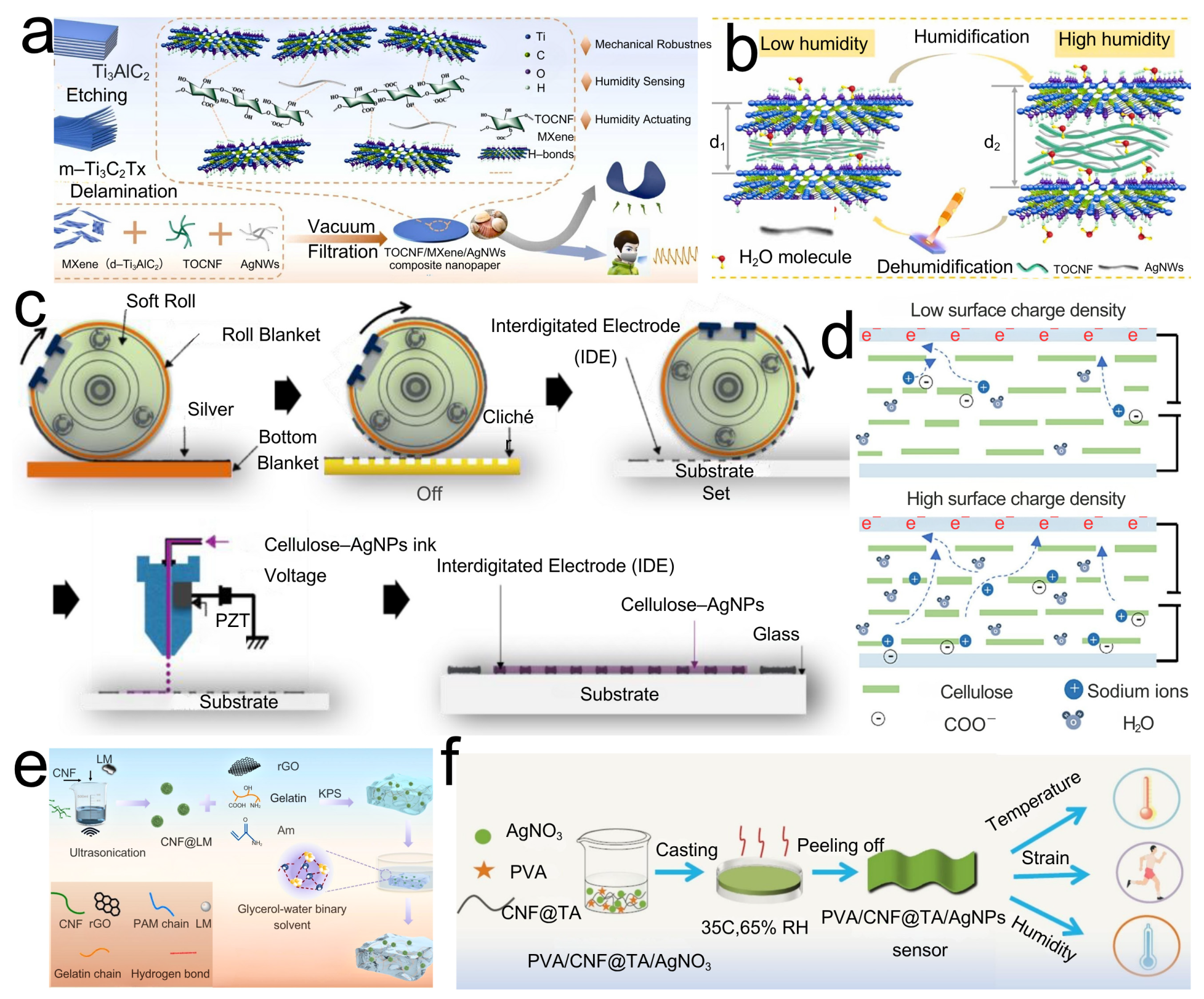
3.1. CNF-Based Temperature and Humidity Sensors
3.2. Flexible and Wearable Sensors Based on CNFs
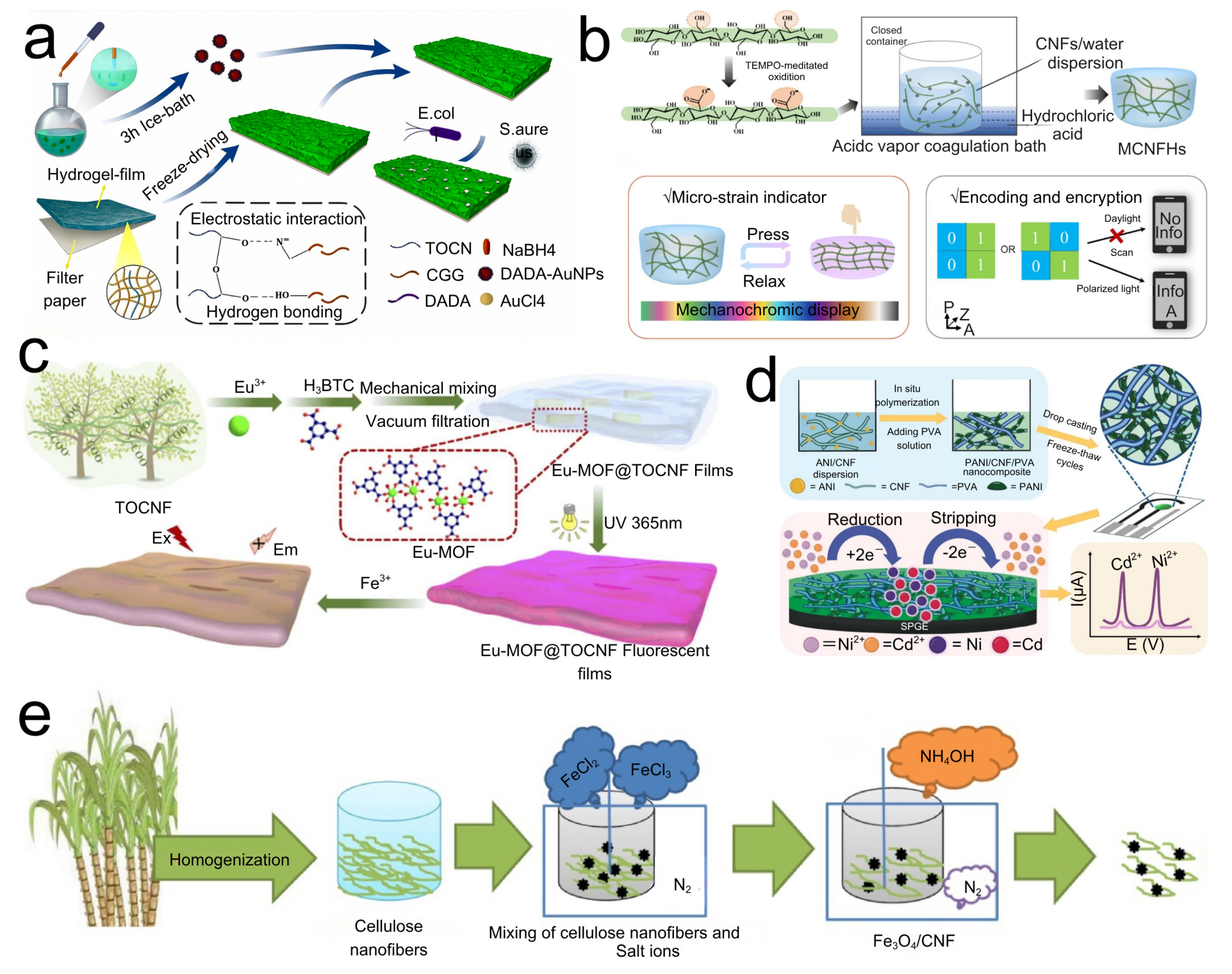
3.3. CNF-Based Biosensors
3.4. Optical Sensors Based on CNFs
3.5. CNF-Based Electrochemical Sensors
4. Future Research and Prospects
4.1. Overcoming Obstacles in Producing CNFs and Fabricating Sensors
4.2. Biocompatibility, Biodegradability, and Recyclability of CNF-Based Materials
4.3. Ways of Increasing Sensor Performance
4.4. Directions for Sustainable and Economically Viable CNF Sensing Technologies
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, Z.; Hu, Y.; Shi, G.; Zhuo, H.; Ali, M.A.; Jamróz, E.; Zhang, H.; Zhong, L.; Peng, X. Advanced Flexible Materials from Nanocellulose. Adv. Funct. Mater. 2023, 33, 2301514. [Google Scholar] [CrossRef]
- Klemm, D.; Kramer, F.; Moritz, S.; Lindström, T.; Ankerfors, M.; Gray, D.; Dorris, A. Nanocelluloses: A New Family of Nature-Based Materials. Angew. Chem. Int. Ed. 2011, 50, 5438–5466. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Du, H.; Yang, X.; Si, C. Recent Strategies in Preparation of Cellulose Nanocrystals and Cellulose Nanofibrils Derived from Raw Cellulose Materials. Int. J. Polym. Sci. 2018, 2018, 7923068. [Google Scholar] [CrossRef]
- Nguyen, H.V.D.; Crampton, A.C.L.A.; Schmidt, D.F.; Huber, T. Evaluating ultra-fine friction grinding for the continuous production of nanofibrillated cellulose. Carbohydr. Polym. Technol. Appl. 2025, 10, 100203. [Google Scholar] [CrossRef]
- Khurd, A.S. A systematic review of cellulosic material for green electronics devices. Carbohydr. Polym. Technol. Appl. 2022, 4, 100234. [Google Scholar] [CrossRef]
- Golmohammadi, H.; Morales-Narváez, E.; Naghdi, T.; Merkoçi, A. Nanocellulose in Sensing and Biosensing. Chem. Mater. 2017, 29, 5426–5446. [Google Scholar] [CrossRef]
- Xu, X.; Liu, F.; Jiang, L.; Zhu, J.Y.; Haagenson, D.; Wiesenborn, D.P. Cellulose Nanocrystals vs. Cellulose Nanofibrils: A Comparative Study on Their Microstructures and Effects as Polymer Reinforcing Agents. ACS Appl. Mater. Interfaces 2013, 5, 2999–3009. [Google Scholar] [CrossRef]
- Singh, S.; Bhardwaj, S.; Tiwari, P.; Dev, K.; Ghosh, K.; Maji, P.K. Recent advances in cellulose nanocrystals-based sensors: A review. Mater. Adv. 2024, 5, 2622–2654. [Google Scholar] [CrossRef]
- Alanazi, A.K. An Innovative Preparation, Characterization, and Optimization of Nanocellulose Fibers (NCF) Using Ultrasonic Waves. Polymers 2022, 14, 2040. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.; Li, F.; Chen, T.; Tang, Y. Green preparation of cellulose nanofibers via high-pressure homogenization and their film-forming properties. Ind. Crops Prod. 2023, 206, 118224. [Google Scholar] [CrossRef]
- Dehmani, Y.; Lainé, J.; Daouli, A.; Sellaoui, L.; Bonilla-Petriciolet, A.; Lamhasni, T.; Abouarnadasse, S.; Badawi, M. Unravelling the adsorption mechanism of phenol on zinc oxide at various coverages via statistical physics, artificial neural network modeling and ab initio molecular dynamics. Chem. Eng. J. 2023, 452, 140688. [Google Scholar] [CrossRef]
- Shi, X.; Wang, Z.; Liu, S.; Xia, Q.; Liu, Y.; Chen, W.; Yu, H.; Zhang, K. Scalable production of carboxylated cellulose nanofibres using a green and recyclable solvent. Nat. Sustain. 2024, 7, 315–325. [Google Scholar] [CrossRef]
- Ma, H.; Cheng, Z.; Li, X.; Li, B.; Fu, Y.; Jiang, J. Advances and challenges of cellulose functional materials in sensors. J. Bioresour. Bioprod. 2023, 8, 15–32. [Google Scholar] [CrossRef]
- Shui, Y.-J.; Yao, W.-H.; Lin, J.-H.; Zhang, Y.; Yu, Y.; Wu, C.-S.; Zhang, X.; Tsou, C.-H. Enhancing Polyvinyl Alcohol Nanocomposites with Carboxy-Functionalized Graphene: An In-Depth Analysis of Mechanical, Barrier, Electrical, Antibacterial, and Chemical Properties. Polymers 2024, 16, 3415. [Google Scholar] [CrossRef]
- Dudnyk, Y.; Kulha, P.; Procházka, V.; Nyström, G.; Geiger, T. Printed circuit board substrates derived from lignocellulose nanofibrils for sustainable electronics applications. Sci. Rep. 2025, 15, 835. [Google Scholar] [CrossRef]
- Zhu, H.; Cheng, J.-H.; Ma, J.; Sun, D.-W. Deconstruction of pineapple peel cellulose based on Fe2+ assisted cold plasma pretreatment for cellulose nanofibrils preparation. Food Chem. 2023, 401, 134279. [Google Scholar] [CrossRef]
- Li, P.; Wang, Y.; Hou, Q.; Liu, H.; Lei, H.; Jian, B.; Li, X. Preparation of cellulose nanofibrils from okara by high pressure homogenization method using deep eutectic solvents. Cellulose 2020, 27, 2511–2520. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Y.; Wang, H.; Zhong, T. High-Consistency Mechano-Enzymatic Fibrillation of Bamboo Pulp Fibers to Produce Precursors for Highly Fibrillated Cellulose and Dense Films with Multifunctionalities. ACS Sustain. Chem. Eng. 2023, 11, 11924–11933. [Google Scholar] [CrossRef]
- Liu, S.-H. Molecular-level design of alternative media for energy-saving pilot-scale fibrillation of nanocellulose. Proc. Natl. Acad. Sci. USA 2024, 121, e2314641121. [Google Scholar] [CrossRef]
- Las-Casas, B.; Arantes, V. Endoglucanase pretreatment aids in isolating tailored-cellulose nanofibrils combining energy saving and high-performance packaging. Int. J. Biol. Macromol. 2023, 242, 124292. [Google Scholar] [CrossRef]
- Uranchimeg, K.; Jargalsaikhan, B.; Bor, A.; Yoon, K.; Choi, H. Comparative Study of the Morphology of Cellulose Nanofiber Fabricated Using Two Kinds of Grinding Method. Materials 2022, 15, 7270. [Google Scholar] [CrossRef]
- Bharimalla, A.K.; Deshmukh, S.P.; Patil, P.G.; Vigneshwaran, N. Energy Efficient Manufacturing of Nanocellulose by Chemo- and Bio-Mechanical Processes: A Review. World J. Nano Sci. Eng. 2015, 5, 204–212. [Google Scholar] [CrossRef]
- Sureshkumar, S.; Patel, D.B.; Varanasi, S. Low energy synthesis of crystalline cellulose nanofibers from Pennisetum hohenackeri by planetary ball milling. Carbohydr. Polym. Technol. Appl. 2025, 10, 100204. [Google Scholar] [CrossRef]
- Ding, Y.; Yu, X.; Zhang, Z.; Sun, W.; You, J.; He, N.; Zhou, G. Self-exfoliation of cellulose nanofibrils via one-pot pseudosolvent swelling/esterification for functional pellicular materials. Carbohydr. Polym. 2025, 361, 119967. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, L.; Khan, K.A.; Ullah, M.W.; Fan, Y.; Wang, Z. Deep eutectic solvents as a ‘multifunctional operating platform’ for nanocellulose production and modification: A review on sustainable composite advancements. Mater. Sci. Eng. R Rep. 2025, 165, 100876. [Google Scholar] [CrossRef]
- Sanchez-Salvador, J.L.; Campano, C.; Balea, A.; Tarrés, Q.; Delgado-Aguilar, M.; Mutjé, P.; Blanco, A.; Negro, C. Critical comparison of the properties of cellulose nanofibers produced from softwood and hardwood through enzymatic, chemical and mechanical processes. Int. J. Biol. Macromol. 2022, 205, 220–230. [Google Scholar] [CrossRef]
- Das, R.; Lindström, T.; Khan, M.; Rezaei, M.; Hsiao, B.S. Nanocellulose preparation from diverse plant feedstocks, processes, and chemical treatments: A review emphasizing non-woods. BioResources 2023, 19, 142–171. [Google Scholar] [CrossRef]
- Sanchez-Salvador, J.L.; Blanco, A.; Duque, A.; Negro, M.J.; Manzanares, P.; Negro, C. Upscaling cellulose oxidation: Integrating TEMPO-mediated oxidation in a pilot-plant twin-screw extruder for cellulose nanofibril production. Carbohydr. Polym. Technol. Appl. 2024, 7, 100180. [Google Scholar] [CrossRef]
- Tang, C.; Chen, H.; Shi, Z.; Liu, X.; Liu, L.; Yu, J.; Fan, Y. Optimizing addition of NaClO in TEMPO-mediated oxidation of cellulose for less nanofiber degradation. Cellulose 2024, 31, 10785–10800. [Google Scholar] [CrossRef]
- Xu, H.; Balea, A.; Blanco, A.; Negro, C. Influence of swelling on the efficiency of TEMPO reaction, nanofibril production and characterization. Carbohydr. Polym. Technol. Appl. 2024, 8, 100221. [Google Scholar] [CrossRef]
- Miao, X.; Hua, W.; Li, Y.; Bian, F.; Xiao, T. Extraction of cellulose nanofibrils from pine sawdust by integrated chemical pretreatment. Heliyon 2024, 10, e06892. [Google Scholar] [CrossRef]
- Baş, Y.; Berglund, L.; Stevanic, J.S.; Scheepers, G.; Niittylä, T.; Oksman, K. Influence of TEMPO on preparation of softwood nanofibrils and their hydrogel network properties. Carbohydr. Polym. 2025, 348, 119785. [Google Scholar] [CrossRef]
- Guo, M.; Ede, J.D.; Sayes, C.M.; Shatkin, J.A.; Stark, N.; Hsieh, Y.-L. Regioselectively Carboxylated Cellulose Nanofibril Models from Dissolving Pulp: C6 via TEMPO Oxidation and C2,C3 via Periodate–Chlorite Oxidation. Nanomaterials 2024, 14, 1123. [Google Scholar] [CrossRef]
- Rostamabadi, H.; Bist, Y.; Kumar, Y.; Yildirim-Yalcin, M.; Ceyhan, T.; Falsafi, S.R. Cellulose nanofibers, nanocrystals, and bacterial nanocellulose: Fabrication, characterization, and their most recent applications. Future Postharvest Food 2024, 1, 5–33. [Google Scholar] [CrossRef]
- Hu, M.; Lv, X.; Wang, Y.; Zhang, Y.; Dai, H. Guideline for the extraction of nanocellulose from lignocellulosic feedstocks. Food Biomacromol. 2024, 1, 9–17. [Google Scholar] [CrossRef]
- Marques, A.P.S.; Almeida, R.O.; Pereira, L.F.R.; Carvalho, M.G.V.S.; Gamelas, J.A.F. Nanocelluloses and Their Applications in Conservation and Restoration of Historical Documents. Polymers 2024, 16, 4000. [Google Scholar] [CrossRef] [PubMed]
- Heidarian, P.; Behzad, T.; Karimi, K. Isolation and characterization of bagasse cellulose nanofibrils by optimized sulfur-free chemical delignification. Wood Sci. Technol. 2016, 50, 1071–1088. [Google Scholar] [CrossRef]
- Lan, K.; Wang, H.S.-H.; Lee, T.; de Assis, C.A.; Venditti, R.A.; Zhu, Y.; Yao, Y. A modeling framework to identify environmentally greener and lower-cost pathways of nanomaterials. Green Chem. 2024, 26, 3466–3478. [Google Scholar] [CrossRef]
- Tofanica, B.-M.; Mikhailidi, A.; Fortună, M.E.; Rotaru, R.; Ungureanu, O.C.; Ungureanu, E. Cellulose Nanomaterials: Characterization Methods, Isolation Techniques, and Strategies. Crystals 2025, 15, 1022. [Google Scholar] [CrossRef]
- Wu, J.; Yuan, Y.; Hua, Q.; Zou, T.; Wan, Z.; Bautista, G.F.; Rojas, O.; Renneckar, S.; Saddler, J. Comparing enzymatic post-treatments by endoglucanase (EG) and lytic polysaccharide monooxygenase (LPMO) on microfibrillated cellulose (MFC) to enhance cellulose film fabrication. Carbohydr. Polym. 2025, 349, 119825. [Google Scholar] [CrossRef]
- Ribeiro, R.S.A.; Pohlmann, B.C.; Calado, V.; Bojorge, N.; Pereira, N. Production of nanocellulose by enzymatic hydrolysis: Trends and challenges. Eng. Life Sci. 2019, 19, 279–291. [Google Scholar] [CrossRef]
- Ribeiro, R.S.A.; Bojorge, N.; Pereira, N. Statistical analysis of the crystallinity index of nanocellulose produced from Kraft pulp via controlled enzymatic hydrolysis. Biotechnol. Appl. Biochem. 2020, 67, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Ramírez Brenes, R.G.; Chaves, L.d.S.; Bojorge, N.; Pereira, N. Endo-Exoglucanase Synergism for Cellulose Nanofibril Production Assessment and Characterization. Molecules 2023, 28, 1388. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, M.A.; Barreiros, M.P.; Felgueiras, H.P.; Fangueiro, R.; Ferreira, D.P. Unlocking the potential of nanocellulose from textile waste: A pathway to nanocomposite applications. Cellulose 2024, 32, 29–57. [Google Scholar] [CrossRef]
- de Araújo, L.G.S.; Rodrigues, T.H.S.; Rates, E.R.D.; Alencar, L.M.R.; Rosa, M.d.F.; Ponte Rocha, M.V. Production of Cellulose Nanoparticles from Cashew Apple Bagasse by Sequential Enzymatic Hydrolysis with an Ultrasonic Process and Its Application in Biofilm Packaging. ACS Omega 2024, 9, 50671–50684. [Google Scholar] [CrossRef]
- Yang, H.; Duan, Y.; Wang, Z.; Lu, D.; Xu, T.; Xie, H.; Gao, M.; Si, C. Eco-friendly production of cellulose nanocrystals from corn straw through combined enzyme pretreatment, mild homogenization, and enzymolysis. Ind. Crops Prod. 2025, 224, 119453. [Google Scholar] [CrossRef]
- Ghamari, M.; Suvish; Hwang See, C.; Yu, H.; Anitha, T.; Balamurugan, V.T.; Velusamy, S.; Hughes, D.; Sundaram, S. Nanocellulose Extraction from Biomass Waste: Unlocking Sustainable Pathways for Biomedical Applications. Chem. Rec. 2025, 25, 1420–1440. [Google Scholar] [CrossRef]
- Baraka, F.; Erdocia, X.; Velazco-Cabral, I.; Hernández-Ramos, F.; Dávila-Rodríguez, I.; Maugin, M.; Labidi, J. Impact of deep eutectic solvent pre-treatment on the extraction of cellulose nanofibers. Cellulose 2024, 31, 9645–9660. [Google Scholar] [CrossRef]
- Ruan, C.-Q.; Gustafsson, S.; Strømme, M.; Mihranyan, A.; Lindh, J. Cellulose Nanofibers Prepared via Pretreatment Based on Oxone® Oxidation. Molecules 2017, 22, 2092. [Google Scholar] [CrossRef]
- Wang, C.; Yuan, Z.; Wang, A.; Qu, J.; Fang, Z.; Wen, Y. Ultraviolet light enhanced sodium persulfate oxidation of cellulose to facilitate the preparation of cellulose nanofibers. Cellulose 2019, 27, 2041–2051. [Google Scholar] [CrossRef]
- Sanchez-Salvador, J.L.; Xu, H.; Balea, A.; Blanco, A.; Negro, C. Enhancement of the Production of TEMPO-Mediated Oxidation Cellulose Nanofibrils by Kneading. Int. J. Biol. Macromol. 2024, 261, 125012. [Google Scholar] [CrossRef]
- Ma, Z.; Duan, Y.; Deng, Y.; Quan, H.; Yang, X.; Li, H.; Ye, L.; Xu, B.; Yan, L. Deep Eutectic Solvent Assisted Preparation of Cellulose Nanofibers and Graphene Composite Films for Supercapacitors. RSC Sustain. 2023, 1, 1006–1015. [Google Scholar] [CrossRef]
- Atiqah, M.S.N.; Gopakumar, D.A.; Owolabi, F.A.T.; Pottathara, Y.B.; Rizal, S.; Aprilia, N.A.S.; Hermawan, D.; Paridah, M.T.; Thomas, S.; Abdul Khalil, H.P.S. Extraction of Cellulose Nanofibers via Eco-Friendly Supercritical Carbon Dioxide Treatment Followed by Mild Acid Hydrolysis and the Fabrication of Cellulose Nanopapers. Polymers 2019, 11, 1865. [Google Scholar] [CrossRef] [PubMed]
- Prasad, C.; Jeong, S.-G.; Won, J.S.; Ramanjaneyulu, S.; Sangaraju, S.; Kerru, N.; Choi, H.Y. Review on Recent Advances in Cellulose Nanofibril Based Hybrid Aerogels: Synthesis, Properties and Their Applications. Int. J. Biol. Macromol. 2024, 261, 124987. [Google Scholar] [CrossRef] [PubMed]
- Anari, E.S.; Soltanizadeh, N.; Fathi, M. The Potential of DBD Plasma Pretreatment for the Isolation of Micro- and Nano-Cellulose Fibers from the Walnut Shells. Carbohydr. Polym. 2024, 327, 121789. [Google Scholar] [CrossRef]
- Káčerová, S.; Muchová, M.; Doudová, H.; Münster, L.; Hanulíková, B.; Valášková, K.; Kašpárková, V.; Kuřitka, I.; Humpolíček, P.; Víchová, Z.; et al. Chitosan/Dialdehyde Cellulose Hydrogels with Covalently Anchored Polypyrrole: Novel Conductive, Antibacterial, Antioxidant, Immunomodulatory, and Anti-Inflammatory Materials. Carbohydr. Polym. 2024, 327, 121803. [Google Scholar] [CrossRef]
- Sadeghi-Shapourabadi, M.; Elkoun, S.; Robert, M. Microwave-Assisted Chemical Purification and Ultrasonication for Extraction of Nano-Fibrillated Cellulose from Potato Peel Waste. Macromolecules 2023, 3, 766–781. [Google Scholar] [CrossRef]
- Qian, J.; Tan, R.; Feng, M.; Shen, W.; Lv, D.; Song, W. Humidity Sensing Using Polymers: A Critical Review of Current Technologies and Emerging Trends. Chemosensors 2024, 12, 523. [Google Scholar] [CrossRef]
- Yang, L.; Yuan, Q.-Y.; Lou, C.-W.; Li, T.-T.; Lin, J.-H. Modified Nanocellulose Hydrogels and Applications in Sensing Fields. Gels 2025, 11, 105. [Google Scholar] [CrossRef]
- Han, M.; Shen, W.; Tong, X.; Corriou, J.-P. Cellulose Nanofiber/MXene/AgNWs Composite Nanopaper with Mechanical Robustness for High-Performance Humidity Sensor and Smart Actuator. Sens. Actuators B Chem. 2024, 406, 136987. [Google Scholar] [CrossRef]
- Won, M.; Jung, M.; Kim, J.; Kim, D.-S. Fully Printed Cellulose Nanofiber–Ag Nanoparticle Composite for High-Performance Humidity Sensor. Nanomaterials 2024, 14, 987. [Google Scholar] [CrossRef]
- Zhu, J.; Zhu, P.; Zhu, Y.; Ye, Y.; Sun, X.; Zhang, Y.; Rojas, O.J.; Servati, P.; Jiang, F. Surface Charge Manipulation for Improved Humidity Sensing of TEMPO-Oxidized Cellulose Nanofibrils. Carbohydr. Polym. 2024, 335, 121345. [Google Scholar] [CrossRef]
- Zou, Y.; Liao, Z.; Zhang, R.; Song, S.; Yang, Y.; Xie, D.; Liu, X.; Wei, L.; Liu, Y.; Song, Y. Cellulose Nanofibers/Liquid Metal Hydrogels with High Tensile Strength, Environmental Adaptability and Electromagnetic Shielding for Temperature Monitoring and Strain Sensors. Carbohydr. Polym. 2025, 348, 121967. [Google Scholar] [CrossRef]
- Gong, W.; Liu, M.; Hu, B.; Fan, L.; Ye, D.; Xu, J. Room-Temperature and Recyclable Preparation of Cellulose Nanofibers Using Deep Eutectic Solvents for Multifunctional Sensor Applications. Int. J. Biol. Macromol. 2025, 296, 122543. [Google Scholar] [CrossRef]
- Norrrahim, M.N.F.; Knight, V.F.; Nurazzi, N.M.; Jenol, M.A.; Misenan, M.S.M.; Janudin, N.; Kasim, N.A.M.; Shukor, M.F.A.; Ilyas, R.A.; Asyraf, M.R.M.; et al. The Frontiers of Functionalized Nanocellulose-Based Composites and Their Application as Chemical Sensors. Polymers 2022, 14, 4456. [Google Scholar] [CrossRef]
- Li, J.; Yang, F.; Liu, D.; Han, S.; Li, J.; Sui, G. Graphene Composite Paper Synergized with Micro/Nanocellulose-Fiber and Silk Fibroin for Flexible Strain Sensor. Int. J. Biol. Macromol. 2023, 240, 124089. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wu, Z.; Alomar, T.S.; AlMasoud, N.; Liu, X.; Han, X.; Guo, N.; Weng, L.; Gao, J.; Algadi, H.; et al. Poly (Vinyl Alcohol)/Carboxylated Cellulose Nanofibers Composite Hydrogel Flexible Strain Sensors. Int. J. Biol. Macromol. 2025, 309, 123105. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Bi, X.; Zhou, B.; Yang, S.; Yu, C. Nanocellulose-Toughened Super-Stretchable Ionic Conductive Gel Fibers for Wearable Strain Sensors. Int. J. Biol. Macromol. 2025, 299, 122876. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Sun, K.; Li, D.; Lv, P.; Wang, Q.; Huang, F.; Wei, Q. Biosensor Based on Bacterial Cellulose-Au Nanoparticles Electrode Modified with Laccase for Hydroquinone Detection. Colloids Surf. A Physicochem. Eng. Asp. 2016, 509, 408–414. [Google Scholar] [CrossRef]
- Dai, L.; Xue, Y.; Tian, S.; He, P.; Xie, P.; Long, Z.; Fei, G.; Chen, Z. Paper Substrate Designed with TEMPO-Oxidized Cellulose Nanofibers/Cationic Guar Gum Hydrogel and Its Application in a Colorimetric Biosensor for Rapid Bacteria Detection. Int. J. Biol. Macromol. 2024, 274, 128156. [Google Scholar] [CrossRef]
- Hossain, L.; De Francesco, M.; Tedja, P.; Tanner, J.; Garnier, G. Nanocellulose Coated Paper Diagnostic to Measure Glucose Concentration in Human Blood. Front. Bioeng. Biotechnol. 2022, 10, 987654. [Google Scholar] [CrossRef]
- Kumar, V.; Rattan, G.; Tewatia, P.; Kaur, M.; Pathania, D.; Singhal, S.; Kaushik, A. Rice Straw Derived Cellulose Nanofibers Modified with L-Histidine for Ultra-Trace Fluorometric Assay of Cr(VI) and Hg(II) in Aqueous Medium. J. Clean. Prod. 2023, 391, 126789. [Google Scholar] [CrossRef]
- Yu, J.; Gong, S.; Xu, C.; Jiang, L.; Liu, J.; Wu, B.; Zhang, X.; Fan, Y.; Wang, Z. Designing Ultrasensitive Mechanochromic Pure Cellulose Nanofiber Hydrogels via Simple and Feasible Gas Phase Coagulation. Carbohydr. Polym. 2025, 362, 122345. [Google Scholar] [CrossRef]
- Wang, W.; Cao, H.; Fan, R.; Zhu, M.; Zhang, R.; Liu, P. In-Situ Growing Eu-MOF on TEMPO-Oxidized Cellulose Nanofibers to Form Fluorescent Films for Fe3+ Detection. Ind. Crops Prod. 2024, 222, 120156. [Google Scholar] [CrossRef]
- Quan, Z.; Xue, F.; Li, H.; Chen, Z.; Zhu, H.; He, H. Design of a Biomimetic Cellulose Nanofibre-Based Double Information Encryption Sensor for Fingerprint Imaging. Carbohydr. Polym. 2023, 302, 120456. [Google Scholar] [CrossRef] [PubMed]
- Deroco, P.B.; Rocha-Filho, R.C.; Fatibello-Filho, O. A New and Simple Method for the Simultaneous Determination of Amoxicillin and Nimesulide Using Carbon Black Within a Dihexadecylphosphate Film as Electrochemical Sensor. Talanta 2018, 179, 115–123. [Google Scholar] [CrossRef]
- Liu, C.; Weber, S.; Peng, R.; Wu, L.; Zhang, W.-s.; Luppa, P.B.; Popp, J.; Cialla-May, D. Toward SERS-Based Therapeutic Drug Monitoring in Clinical Settings: Recent Developments and Trends. TrAC Trends Anal. Chem. 2023, 164, 112034. [Google Scholar] [CrossRef]
- Rkik, M.; Brahim, M.B.; Samet, Y. Electrochemical Determination of Levofloxacin Antibiotic in Biological Samples Using Boron Doped Diamond Electrode. J. Electroanal. Chem. 2017, 794, 175–181. [Google Scholar] [CrossRef]
- Dong, L.X.; Dong, Z.P.; Dong, C.X.; Zhang, W.; Li, X.L.; Ma, J.T. Ni@Pd Core–Shell Nanoparticles Modified Fibrous Silica Nanospheres as Highly Efficient and Recoverable Catalyst for Reduction of 4-Nitrophenol and Hydrodechlorination of 4-Chlorophenol. Appl. Catal. B Environ. 2015, 162, 372–380. [Google Scholar] [CrossRef]
- Wang, B.; Okoth, O.K.; Yan, K.; Zhang, J. A Highly Selective Electrochemical Sensor for 4-Chlorophenol Determination Based on Molecularly Imprinted Polymer and PDDA-Functionalized Graphene. Sens. Actuators B Chem. 2016, 236, 294–303. [Google Scholar] [CrossRef]
- Deng, Z.; Wu, Z.; Alizadeh, M.; Zhang, H.; Chen, Y.; Karaman, C. Electrochemical Monitoring of 4-Chlorophenol as a Water Pollutant via Carbon Paste Electrode Amplified with Fe3O4 Incorporated Cellulose Nanofibers (CNF). Environ. Res. 2023, 219, 115089. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, X.; Du, T.; Liu, L.; Webley, P.A.; Li, G.K. Hydrogen Production from Low Pressure Coke Oven Gas by Vacuum Pressure Swing Adsorption. Chem. Eng. J. 2023, 472, 145089. [Google Scholar] [CrossRef]
- Wang, J.; Yu, P.; Kan, K.; Lv, H.; Liu, Z.; Sun, B.; Bai, X.; Chen, J.; Zhang, Y.; Shi, K. Efficient Ultra-Trace Electrochemical Detection of Cd2+, Pb2+ and Hg2+ Based on Hierarchical Porous S-Doped C3N4 Tube Bundles/Graphene Nanosheets Composite. Chem. Eng. J. 2021, 420, 129789. [Google Scholar] [CrossRef]
- Boonkrong, P.; Promphet, N.; Rodthongkum, N.; Puthongkham, P. Polyaniline/Cellulose Nanofiber/Polyvinyl Alcohol Porous Conductive Hydrogel-Modified Graphene Electrode for Simultaneous Detection of Cadmium and Nickel in Wastewater. Microchem. J. 2025, 210, 108056. [Google Scholar] [CrossRef]
- Das, J.; Mishra, H.N. Electrochemical Biosensor for Monitoring Fish Spoilage Based on Nanocellulose as Enzyme Immobilization Matrix. J. Food Meas. Charact. 2023, 17, 3827–3844. [Google Scholar] [CrossRef]
- Hou, Z.; Zhang, Y.; Li, M.; Wang, S. A Cellulose Nanofiber Capacitive Humidity Sensor with High Sensitivity and Fast Recovery Characteristics. Sensors 2022, 22, 464. [Google Scholar] [CrossRef]
- Kumari, P.; Kumar, A.; Yadav, A.; Gupta, G.; Gupta, G.; Shivagan, D.D.; Bapna, K. Chitosan-Based Highly Sensitive Viable Humidity Sensor for Human Health Monitoring. ACS Omega 2023, 8, 39511–39522. [Google Scholar] [CrossRef]
- Chen, Q.; Liu, D.; Huang, X.-H.; Yao, Y.; Mao, K.-L. Impedance Analysis of Chitin Nanofibers Integrated Bulk Acoustic Wave Humidity Sensor with Asymmetric Electrode Configuration. Nanomaterials 2022, 12, 3035. [Google Scholar] [CrossRef]
- Wen, D.-L.; Liu, Y.; Zhang, S.; Zhao, H. Silk Fibroin/Ag Nanowire-Based Multifunctional Sensor for Wearable Self-Powered Wireless Multi-Sensing Microsystems. Nano Energy 2023, 113, 108569. [Google Scholar] [CrossRef]
- Hassan, R.Y.A.; Fathy, R.M. Novel Paper-Based Potentiometric Combined Sensors Using Coumarin Derivatives Modified with Vanadium Pentoxide Nanoparticles for the Selective Determination of Trace Levels of Lead Ions. Microchim. Acta 2024, 191, 427. [Google Scholar] [CrossRef]

| Aspect | Bio-Based Sensor Platforms (e.g., CNFs, Bacterial Cellulose, Chitosan-/Silk-Derived Matrices) | Synthetic Sensor Platforms (e.g., PI/PET/PDMS/PVDF Polymers; Si/Metal-Oxide Microsystems) | Design Implications |
|---|---|---|---|
| Feedstock and sustainability | Renewable biomass; green pretreatments possible (enzymes, DESs); potential for recyclable media and lower embodied burden. | Predominantly petroleum-derived polymers and energy-intensive inorganics. | Bio-based routes reduce e-waste footprint and align with circularity goals; synthetics benefit from mature commodity supply chains. |
| Intrinsic biocompatibility | Generally high; CNFs and related biopolymers are well-tolerated and biodegradable. | Variable; many require passivation/encapsulation for bio-contact. | Bio-based scaffolds suit wearables/biomedical interfaces; synthetics require additional surface engineering. |
| Baseline electrical behavior | Insulating backbones; performance arises from ionic/proton transport and percolating conductive networks (MXenes, AgNWs, graphene, liquid metals) engineered within CNFs. | Either insulating (needs fillers) or intrinsically functional (e.g., piezoelectric PVDF; semiconducting oxides). | CNFs leverage surface-charge regulation and network topology to fine-tune sensitivity/linearity; synthetics may offer built-in functions but often at e-waste cost. |
| Processing and patterning | Aqueous processing; film casting, vacuum filtration, gelation; compatible with printing/roll-to-roll and in situ growth of active phases. | Wide process window from solution casting to photolithography and microfabrication. | CNFs enable low-temperature, solvent-lean routes and direct integration on paper/plastic; synthetics excel in high-temperature microfabrication. |
| Mechanical profile | High specific strength; compliant films/aerogels/hydrogels; dynamic/dual-network crosslinking dissipates energy while preserving signal fidelity under strain. | Broad: elastomers (PDMS) to high-Tg films (PI); robust thermal profiles. | CNF architectures can match flexibility while offering tunable mechanics via hydrogen bonds/ionic/metal coordination. |
| Environmental stability | Hydrophilic; prone to humidity-driven swelling and signal drift without encapsulation/chemical stabilization. | Typically better moisture/thermal stability out of the box. | CNFs demand encapsulation, hydrophobization, or covalent filler interfaces for long-term stability; synthetics suit harsher ambient conditions. |
| Modalities demonstrated | Humidity, temperature, strain/pressure, optical, electrochemical, biosensing with sub-second humidity response and nanomolar electroanalysis achievable in CNF composites. | Broad coverage across the same modalities; extensive legacy in micro- and opto-electronics. | CNFs are no longer a “green substrate” only—they are active, tunable platforms across modalities when networks and interfaces are co-designed. |
| Representative device performance (from this review) | Humidity: <1 s response (CNF/MXene/AgNWs); printed CNF–AgNPs humidity with fast recovery; strain/temperature hydrogels functional at sub-zero; electrochemical detection to nM–µM regimes. | Not focus of this review; included here for context. | CNF composites already meet or approach the state of the art for many use cases while offering sustainability dividends. |
| End of life | Biodegradable/recyclable; opportunity to curb e-waste (≈62 Mt in 2022) with degradable/compostable form factors. | Persistent; recycling varies by polymer; inorganic residues remain. | CNFs enable green, disposable, or recyclable sensors for distributed IoT and packaging. |
| Key risks/mitigations | Batch variability, humidity sensitivity, filler aggregation; mitigate via standardized CNF synthesis, encapsulation, covalent filler bonding. | Long-established QC; environmental burden at the end of life. | A clear materials–process–architecture playbook exists to harden CNF devices for deployment. |
| Sensor Type | Material System/Composition | Structural Form | Detected Performance | References |
|---|---|---|---|---|
| Humidity sensor | TOCNF/MXene/AgNWs | Composite nanopaper | The RH range is wide, with a reversible response and a recovery time of 185 s under NIR. | [60] |
| Humidity sensor | CNF/AgNPs | Inkjet-printing film | 35–70% RH; high sensitivity response 4 s/recovery 43 s. | [61] |
| Humidity sensor | TOCNFs (surface charge regulation) | Thin-film type | 15–75% RH; strong linearity, sensitivity 44.5% (%RH). | [62] |
| Humidity sensor | CNF/Liquid Metal/Gelatin/Graphene Oxide | Antifreeze hydrogel | Response 210 ms/recovery 150 ms. | [63] |
| Temperature and pressure sensor | PVA/CNF@TA/AgNPs | Multifunctional flexible membrane | High sensitivity (GF ≈ 46.42), low detection limit (<1%), response time of 80 milliseconds; temperature coefficient change rate (TCR ≈ 29.84/°C). | [64] |
| Pressure sensor | Micro/Nano Cellulose/Silk Fibroin/Graphene Paper (flexibility/strength) | Composite paper | High sensitivity (GF up to 2.31), wide detection range, and excellent stability. | [66] |
| Pressure sensor | Carboxylated CNF/Tannic Acid/PVA/Al3+ | Hydrogel | After electrode spacing optimization, it exhibited a high sensitivity of 0.0654 log(ΔR/%RH), with response and recovery times of approximately 4 s and 34 s, respectively. | [67] |
| Pressure sensor | TOCNF/Polyacrylamide/LiCl/Glycerol | Dual-network hydrogel | Sensitivity (GF value up to 8.6), response time approximately 290 ms. | [68] |
| Biosensor | TOCNF/CGG/DADA-AuNPs | Paper-based | The test can detect 10 CFU/mL within 15 min. | [70] |
| Biosensor | TOCNF/Glucose Oxidase/Phenol Red | Hydrogel | Detect glucose concentration of 7–13 mM at 440 °C. | [71] |
| Optical sensor | L-histidine-modified CNFs | Fluorescent sensing probe | Detectable Cr, Hg; low detection limit. | [72] |
| Optical sensor | TEMPO-CNF/HCl | Aerogel | Visible structural color changes can be induced by an external force as low as 0.05 N; the response time is approximately 0.3 s. | [73] |
| Optical sensor | TOCNF/Eu-MOF | Thin-film type | Response time within 30 s; detection can reach 1.27 μM. | [74] |
| Optical sensor | ACNF/DIES | Thin-film type | Linear fluorescence response in the pH range of 3–11. | [75] |
| Electrochemical sensor | Fe3O4/CNF/CPE | Carbon paste electrode | Accurate detection is achieved within a linear range (e.g., 0.01–100 μM), with a detection limit as low as the nanomolar level (~6.3 nM), and a response time of just a few seconds. | [81] |
| Electrochemical sensor | PANI/CNF/PVA/SPGE | Hydrogel | Exhibits a good linear response relationship to Cd2+ and Ni2+ with extremely low detection limits (0.24 μg/L and 0.31 μg/L, respectively). | [84] |
| Electrochemical sensor | CNF/GCE/XO | Hydrogel film | The linear detection range is 3–50 μM, with a detection limit of 47.96 nM (approximately 4.8 × 10−8 M), and a sensitivity as high as 5281 μA mM−1 cm−1; the response time is between 4 and 10 s. | [85] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Huo, G.; Xu, G.; Yu, D.; Liu, S.; Wang, Q. Flexible Intelligence on a Green Skeleton: Progress and Challenges of CNF-Enabled Multimodal Sensing Platforms. Polymers 2025, 17, 2941. https://doi.org/10.3390/polym17212941
Wang H, Huo G, Xu G, Yu D, Liu S, Wang Q. Flexible Intelligence on a Green Skeleton: Progress and Challenges of CNF-Enabled Multimodal Sensing Platforms. Polymers. 2025; 17(21):2941. https://doi.org/10.3390/polym17212941
Chicago/Turabian StyleWang, Hemiao, Guanlin Huo, Guijuan Xu, Dehai Yu, Shanshan Liu, and Qiang Wang. 2025. "Flexible Intelligence on a Green Skeleton: Progress and Challenges of CNF-Enabled Multimodal Sensing Platforms" Polymers 17, no. 21: 2941. https://doi.org/10.3390/polym17212941
APA StyleWang, H., Huo, G., Xu, G., Yu, D., Liu, S., & Wang, Q. (2025). Flexible Intelligence on a Green Skeleton: Progress and Challenges of CNF-Enabled Multimodal Sensing Platforms. Polymers, 17(21), 2941. https://doi.org/10.3390/polym17212941







