1. Introduction
In recent years, hydrogels have attracted significant attention in a wide range of applications, including tissue engineering, drug delivery, coatings, flexible electronics, and 3D printing, owing to their excellent flexibility, elasticity, high water content, and biocompatibility [
1]. Hydrogels are defined as three-dimensional polymer networks containing a large amount of water, typically formed through physical, chemical, or covalent crosslinking. Research on functional hydrogels has expanded in multiple directions, and one example is the development of anisotropic conductive hydrogels. However, while such studies have mainly focused on imparting electrical or mechanical properties, the present study aims to develop a novel hydrogel based on hyaluronic acid (HA), a major component of the extracellular matrix, with a specific emphasis on the retention and controlled release of growth factors.
Hyaluronic acid (HA) is a major component of the extracellular matrix, a linear polysaccharide consisting of
N-acetyl-D-glucosamine and D-glucuronic acid linked by β-glycosidic bonds to form repeating disaccharides with molecular weights ranging from 0.8 to 20,000 kDa [
2]. HA is a mucopolysaccharide present in various tissues in the body and has been used clinically as a building block of biomaterials for over 30 years because of its immunoneutrality [
3]. HA is a component involved in the migration and differentiation of cells. HA, which normally exists in vivo as a macromolecule >10
6 Da, is hydrolyzed by the enzyme hyaluronidase, which cleaves the β-1,4 and β-1,3 bonds. This half-life can range from a few hours to several days [
4]. High molecular weight HA exhibits anti-angiogenic and anti-inflammatory effects. On the other hand, low molecular weight HA (<3.5 × 10
4 Da) exhibits proinflammatory and angiogenic properties [
5,
6]. High molecular weight hyaluronan is a major component of the extracellular matrix (ECM). In contrast, low molecular weight hyaluronan is generated in vivo through the enzymatic action of hyaluronidase [
7], which is the main component of ECM. This low molecular weight HA is known to promote vascular endothelial cell proliferation and migration in vitro and angiogenesis in vivo [
7] HA is found in all tissues and fluids of animals and is particularly abundant in early embryos, where it forms a pericellular coat around most cells and functions as a signaling molecule that interacts with binding proteins to regulate cell adhesion, migration, and proliferation [
8]. HA is also found in the pericellular coats of most cells.
HA is also utilized as cross-linked gels. Chemical cross-linking is a stable cross-linking method that forms covalent bonds between functional groups. Chemical cross-linking can establish stable and irreversible hydrogel networks in the preparation of hydrogels for tissue engineering [
9]. Generally, hydrogels are used in a variety of medical applications, including skin substitutes, adhesives, matrices for drug delivery, and scaffolds for tissue engineering [
10]. For example, in 1992, Yui et al. evaluated the inflammation-responsive biodegradation of cross-linked HA gel. This suggested that cross-linked HA gels are promising as an inflammation-responsive degradable matrix for implantable drug delivery [
11]. In 2012, Schramm et al. prepared hydrogels by UV photocrosslinking HA and
N-vinylpyrrolidinone. This gel was suggested to be highly biocompatible and a potential alternative for long-term vitreous replacement [
12]. Wang et al. created a cross-linked biomaterial using genipin, a natural compound that is mesoarticulated from the fruit of the beech tree. This biomaterial has sufficient mechanical strength and can promote chondrogenic differentiation of seeded cells, making it suitable as a cartilage material [
9]. This cross-linked HA gel could result in a drug sustained release system [
12]. Addition reactions, condensation reactions, and radical polymerization continue to be developed for hyaluronan hydrogels in biomedical applications. Addition and condensation reactions include thiol-modified hyaluronan, haloacetate-modified hyaluronan, aldehyde-modified hyaluronan, and Huisgen cycloaddition. Hydrogels prepared using these methods have been shown to be effective in cell and molecule delivery, as well as cell proliferation. Radical polymerization includes reactions with anhydrous methacrylic acid, glycidyl methacrylate, and hydrolyzable hyaluronan. These are thought to have applications in cartilage tissue regeneration, cardiac repair, molecular delivery, and microdevices [
4].
Poly(ethylene glycol) (PEG) is a polymer compound composed of repeating ethylene oxide units. It is proposed from ethylene oxide, a three-membered ring ether, anionically ring-opened by alkali metals or hydroxides, and exists in a wide range of molecular weights from 200 to over 10,000 g/mol. It is a nonionic polymer with high biocompatibility. They also have a variety of structures, including PEGs with various functional groups at the ends and hyperbranched PEGs. It is approved and used by the U.S. Food and Drug Administration (FDA) in a variety of biochemical applications such as food, cosmetics, and formulations [
13,
14,
15,
16]. So far, hydrogels based on PEG, which has excellent biocompatibility, have been prepared and used as scaffold materials for 3D cell culture [
17]. The use of PEG as a scaffold material for three-dimensional cell culture has been studied.
Growth factors include epidermal growth factor family (EGF), transforming growth factor-beta family (TGF-beta), fibroblast growth factor family (FGF), vascular endothelial growth factor (VEGF), granulocyte macrophage colony stimulating factor (GM-CSF), platelet derived growth factor (PDGF), connective tissue growth factor (CTGF), interleukin family (IL), and tumor necrosis factor-α-family [
18]. In particular, growth factors that act on endothelial cells, smooth muscle cells, and pericytes, which are necessary for the formation of blood vessels, are called vascular growth-related growth factors. Fibroblast growth factor (FGF) was first extracted from bovine pituitary gland in 1973 and was able to be produced uniformly in 1983. The mammalian FGF consists of 22 species and is divided into seven subfamilies [
19].
It is important to maintain the stability of the activity of the bioactive substance to be delivered. Since growth factors are proteins, they have a higher-order structure, and if this higher-order structure is disrupted even slightly, the bioactivity will be inactivated. Therefore, as an approach to maintain stability, heparin has been the focus of attention when using cell growth factors, which represent bioactive substances. Another example of maintaining protein stability is the molecular crowding effect of PEG. Molecular crowding refers to a change in the properties of molecules in a solvent when a high concentration of a protein or other macromolecule is present, and this molecular crowding is observed in cells. Therefore, by adding a high concentration of PEG to a protein solution, a crowding environment is simulated and protein stability is maintained [
20]. The PEG solution is then added to the protein solution to simulate the crowding environment and maintain protein stability. Pike et al. reported in 2006 that a heparin-containing hyaluronic acid gel [
21]. In this report, heparin content enabled long-term storage and release while retaining in vivo activity. In 2013, Nguyen et al. reported a heparin-mimetic polymer conjugated with bFGF [
22]. The polymer was stable for 42 days and protected from degradation by heat, acids, and proteolytic enzymes, demonstrating retention of bioactive stability. These results indicate that controlled release and long-term storage are essential for drug delivery.
The easiest way to incorporate growth factors into hydrogels is to add them directly to the hydrogel. However, this method results in rapid burst release during the initial swelling phase. Therefore, controlling the release of growth factors over a long period of time is a challenge [
23]. In addition, biodegradable systems for controlled release drug delivery are currently being widely investigated because they do not require invasive techniques such as surgery. However, drug diffusion can lead to serious side effects if a different drug than the one intended to be delivered is released from the matrix. In 1996, Moriyama et al. reported that a heterogeneously structured hydrogel of degradable dextran matrices containing PEG as a drug reservoir had been developed for a novel design of implantable device for peptide delivery [
24]. In addition, the use of hyaluronic acid in place of the dextran matrix has been confirmed for controlled degradation and insulin release [
25]. However, the size and distribution of these PEG domains were not controlled. Therefore, they prepared hydrogels with PEG-grafted dextran, which enabled them to control PEG. Therefore, in 1999, Moriyama and colleagues prepared hydrogels using PEG-grafted hyaluronic acid to control insulin release [
25]. In particular, PEG and HA are known to form aqueous two-phase systems (ATPS), in which proteins preferentially partition into one of the phases [
25]. This property suggests that grafting PEG onto HA can provide the ability to control protein distribution and retention. Moreover, compared with other chemical modifications of HA (e.g., esterification or amidation), PEG grafting offers advantages such as easier reaction control, improved solubility, and enhanced structural stability as well as molecular crowding. Therefore, PEG grafting is considered an effective strategy for achieving stable incorporation and controlled release of growth factors in HA-based hydrogels. However, there is still no example of controlled release of cell growth factors such as basic FGF (bFGF) encapsulated in a gel.
In this study, we propose a new hydrogel using PEG-grafted hyaluronic acid and bFGF, a cell growth factor, and how it is released into the gel. In detail, we tried to clarify a relationship between changing the grafting rate of PEG and the release behavior of bFGF. We also evaluated whether there is a correlation between the grafting rate and the degradability of the hydrogel. It is suggested that angiogenesis is influenced by the local concentration and biological activity of bFGF. Moreover, while high molecular weight HA alone does not appear to induce angiogenesis, HA oligosaccharides have been reported to promote angiogenesis in synergy with a growth factor. In addition, it has been indicated that the degradation behavior and mechanical properties of HA hydrogels can be modulated by changes in molecular weight, crosslinking degree, and chemical modifications, which may affect angiogenic activity in vivo [
2,
3]. Therefore, the use of PEG-grafted HA may provide a promising strategy to regulate three important factors for angiogenesis: bFGF concentration, bFGF bioactivity, and HA degradation. Furthermore, we evaluated the physiological activity of bFGF by subcutaneously implanting bFGF-added hydrogel in mice and confirming its angiogenic effect.
2. Materials and Methods
2.1. Reagents
Hyaluronic acid (HA) (Mn: 230,000 g/mol) purchased from JNC Corporation, Tokyo, Japan Polyethylene Glycol (PEG) (Mn: 6000 g/mol), Deuterium oxide (D2O), Sodium chloride, Fluorescein isothiocyanate, Isomer I (FITC), Sodium carbonate and Ammonium chloride were purchased from Nacalai Tesque Corporation, Kyoto, Japan. α-Methyl-ω-aminopropoxy-polyoxyethylene (PEG-NH2) (Mn: 5205 g/mol), α-Aminopropyl-ω-aminopropoxy,-polyoxyethylene (PEG-BA) (Mn: 2121 g/mol) were purchased from NOF Co., Tokyo, Japan. 4-(4,6-Dimethoxy-1,3,5-triazin-2-yl)-4-methylmorpholinium chloride (DMT-MM), Potassium dihydrogen phosphate, potassium chloride, disodium hydrogenphosphate, sodium chloride, phosphate pH standard equimolal solution, phthalate pH standard solution, 2,4,6-trinitrobenzenesulfonic acid sodium salt dihydrate (TNBS), hydrochloric acid, hyaluronidase, from Ovine Testes (EC 3.2.1.35) were purchased from Wako Pure Chemical Industries, Osaka, Japan. Fiblast spray was purchased from Kaken Seiyaku, Hyogo, Japan.
2.2. Experimental Apparatus and Equipment
The differential scanning calorimeter (DSC) was an EXSTRA6000 DSC6200 Differential Scanning Calorimeter and its accompanying analysis software from Seiko Instrument Inc. The lyophilizer was an EYELA FD-1000 model from Tokyo Rikakikai Co., Ltd. (Tokyo, JAPAN). The dialysis tubes were Fast Gene Regenerate Cellulose Dialysis Tubing FG DM P4-MWCO 12,000–14,000 from Japan Genetics Corporation (Tokyo, JAPAN). Scanning electron microscope (SEM) used was JEOL’s JSM-6510 in the Soft Matter Interfacial Chemistry Laboratory (Tokyo, Japan). A V-730 Spectrophotometer from JASCO Corporation (Tokyo, Japan) was used for absorbance measurements. Perkin Elmer LS55 from PerkinElmer Japan G.K. (Yokohama, Japan) was used for fluorescence intensity measurements and FL WINLAB SOFTWARE (Ver. 4.00.02) for analysis software. A confocal laser scanning microscope (CLSM) was a FluoVIewTMFV1000 Confocal Microscope from Olympus, Inc. (Tokyo, JAPAN). Nuclear Magnetic Resonance (NMR) was a 300 Hz FT-NMR apparatus (JNM-LA300 FT NMR system, JEOL Ltd., Tokyo, Japan). Size-exclusion chromatography (SEC) system was comprised with a pump (PU-980, JASCO Corporation, Tokyo, Japan) equipped with a 7.5 × 300 mm SEC column (GF-310 HQ, Showa Denko K.K., Tokyo, Japan), a light scattering detector (miniDAWN TriStar, Wyatt Technology Corporation, Santa Barbara, CA, USA), and a refractive index detector (Shodex RI-101 detector, Showa Denko, Tokyo, Japan).
2.3. Synthesis of PEG-graft-HA
HA (142 mg) was dissolved in 20 mL of distilled water, and PEG-NH
2 was added at the specified amounts (the feed amounts and molar ratios are summarized in
Table 1). DMT-MM (400 mg) was then added and allowed to react at room temperature for 24 h (
Scheme 1). After the reaction was completed, the resulting mixture was dialyzed with distilled water for 2 days using a dialysis membrane (Spectra/Por
® 2, MWCO 12,000~14,000). After dialysis, the resulting solution was lyophilized for 24 h to obtain a white cotton-like solid. The obtained PEG-
graft-HAs were characterized by
1H-NMR and SEC-multi-angle light scattering (MALS) measurements. The SEC-MALS measurements were operated at 37 °C under a flow rate of 0.5 mL min
−1. D-PBS buffer was used as an eluent. The PEG grafting rate was quantified by DSC measurements (see;
Section 2.4 and
Section 2.5).
Yield: 87% 1H-NMR (300 MHz, D2O): (s, -NHCO-CH3, 3H), (t, -CONH-CH2CH2CH2O-), (s, -[CH2CH2O]m-CH3, 3H), (s, -[CH2CH2O]m-,)
2.4. Evaluation of PEG Incorporation Rate Using DSC
The DSC measurement conditions are shown in
Table S1. Samples were sealed in an aluminum pan and a reference αAlumina (5.48 mg) was used for the measurements under the following conditions: temperature range: −30 to 160 °C, temperature increase rate: 2 °C/min. All DSC measurements in this experiment were performed using the same method.
2.5. Calibration Curve for PEG Content
DSC measurements were performed on 5.0~7.5 mg of PEG-NH
2 in aluminum pans under the conditions of
Table S2. Calibration curves were generated from PEG-NH
2 weight fractions and enthalpies of melting (n = 3 trials).
2.6. Calculation of PEG Incorporation Rate of PEG-graft-HA by DSC Measurement
A predetermined amount of PEG-grafted-HA shown in
Table S3 was taken in an aluminum pan, and DSC measurements were performed under the conditions shown in
Table S1 (n = 3). The PEG incorporation rate was calculated from the measured enthalpy of melting of PEG using the calibration curve shown in
Figure S3.
2.7. Preparation and Characterization of HA, PEG-graft-HA Cross-Linked Gels
Conditions for gel preparation are shown in
Table 2. The 5 wt% aqueous solution of HA was prepared, and 2 mL was added dropwise to a 35 mm dish. PEG-BA was added, and after stirring to ensure complete dissolution, DMT-MM was added and allowed to stand for 12 h at room temperature. After standing, the cross-linked gel was cut into pieces 1.0 cm in diameter and 2.0 mm thick. The gel was then immersed in distilled water for 2 days to remove unreacted material, during which time the distilled water was replaced. After the dialysis was completed, the gel was lyophilized to obtain a white solid.
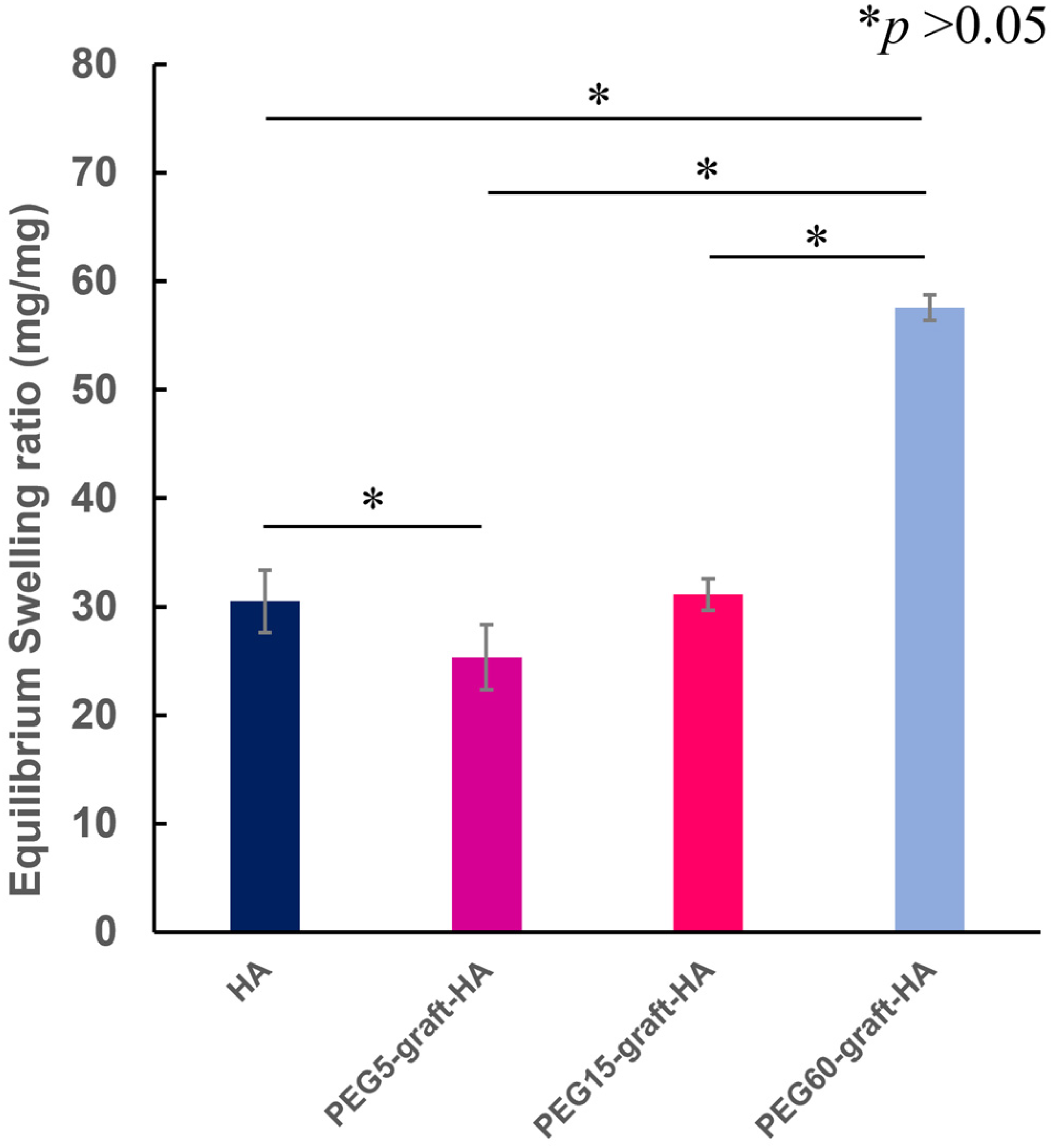
The weight of the cross-linked gel after immersion in distilled water for 2 days (
Wwet) and the dry weight after lyophilization (
Wdry) were determined by weighing. These values were then used to calculate the swelling ratio (or water content) using the following Formula (1) (n = 3).
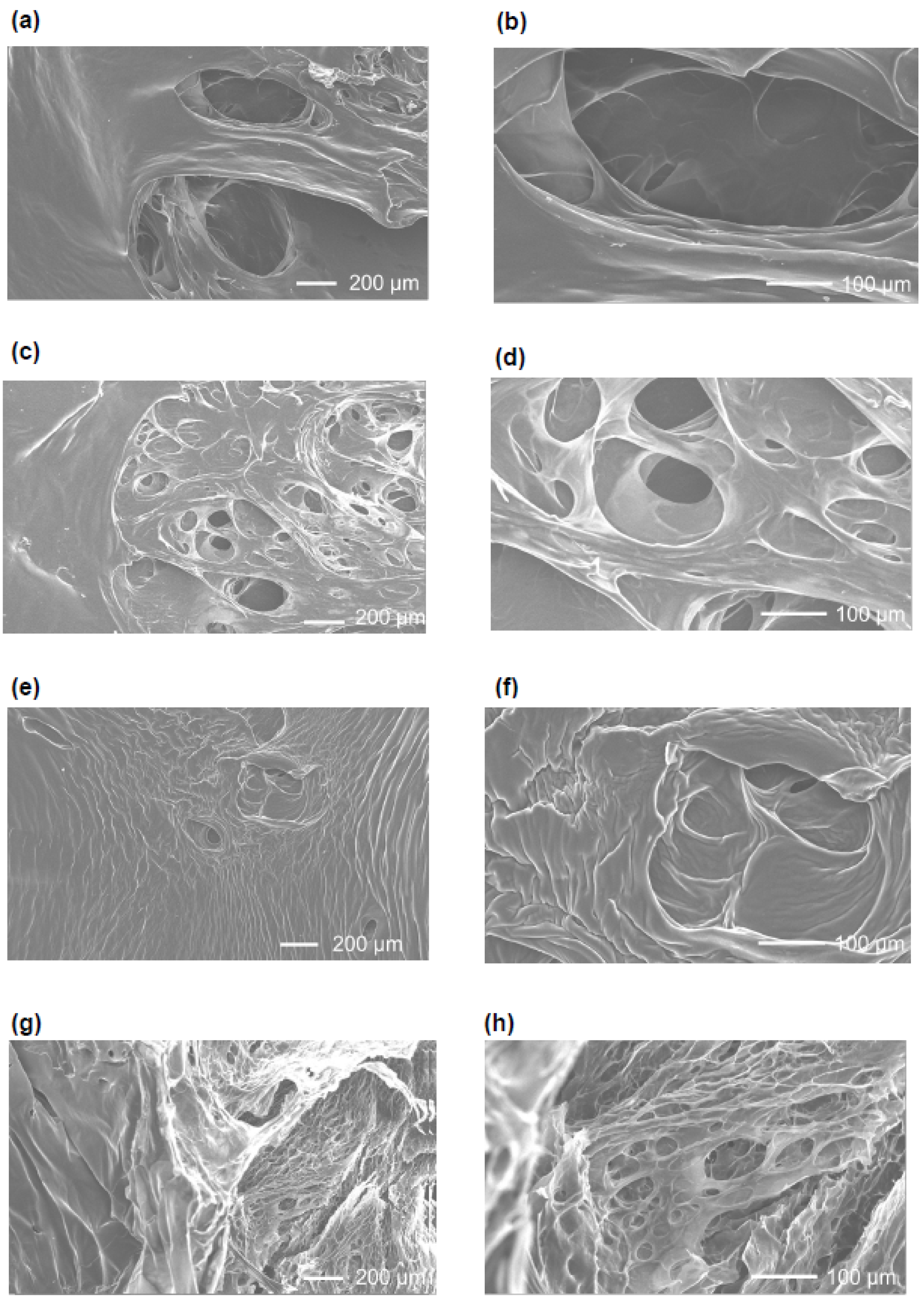
Observation of the gel after freeze-drying was performed by scanning electron microscopy (SEM). After freeze-drying, the cross-linked gel was cut into approximately 3.0 mm squares and adhered to a cover glass with pre-stretched carbon tape, and then platinum deposition was performed. SEM observation was performed at an acceleration voltage of 20 kV.
The weight of the gel after 6 days of immersion in 12 mM PBS solution (pH 7.4) was determined as
Ws. The swelling ratio was determined from Equation (2):
The reaction rate of the crosslinker PEG-BA was determined from the quantitative method for amino groups using TNBS. In order to make a calibration curve, various concentration of PEG-BA was prepared (4.0 mL). To this solution, 2.0 mL of 0.1 wt% TNBS solution was added, and the solution was kept in a refrigerator for 1 day (see
Table S4). Then, 300 μL of 2 M HCl solution was added to the solution to terminate the reaction, and the absorbance at 345 nm was measured by a spectrophotometer to obtain a calibration curve (
Scheme 2). After measuring the volume of dialysis water of the crosslinked gels with distilled water, 4.0 mL of each dialysis water was weighed out and mixed with 2.0 mL of 0.1 wt% TNBS solution and left in a refrigerator for 1 day. 300 μL of 2M HCl solution was added to complete the reaction. The absorbance at 345 nm was measured using a spectrophotometer, and the amount of unreacted PEG-BA material in the dialysis water was calculated using the calibration curve obtained to calculate the crosslinker incorporation rate.
Autograph measurement and cross-linked gel measurement conditions were shown in
Tables S5 and S6, respectively. The prepared crosslinked gels were cut into cylindrical shapes with a diameter of 1.0 cm and a thickness of 2.0 mm. The thickness (
t0) and volume (
V0) of the crosslinked gel before swelling were measured. After further swelling in 12 mM PBS solution (pH 7.4) until swelling equilibrium was reached, the thickness (
ts) and volume (
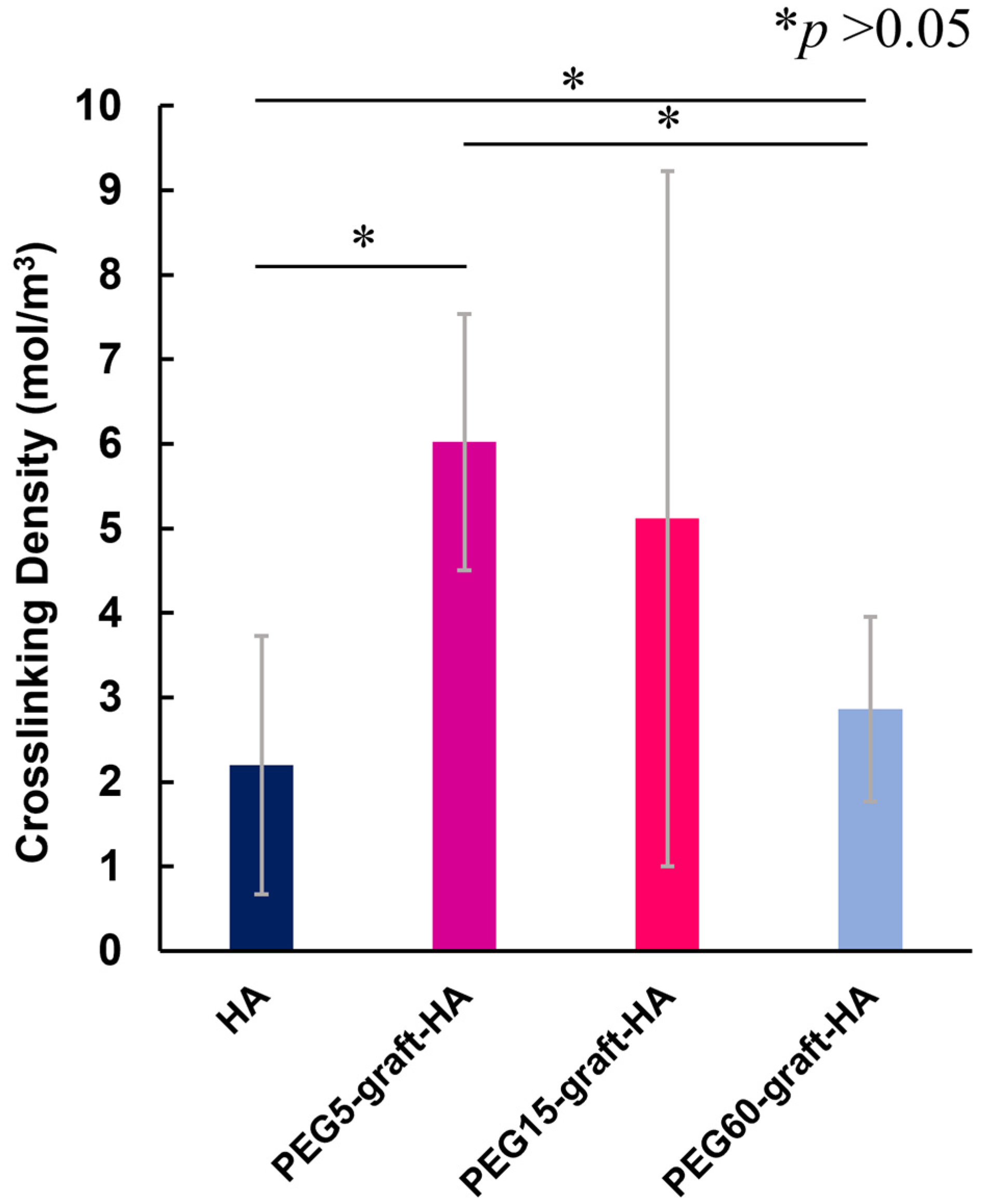
Vs) of the cross-linked gel were measured. After wiping off the water on the surface and inserting the crosslinked gel between fixed plates, autographs were taken at a compression speed of 1.0 mm/min to measure the stress. The cross-linking density of the crosslinked gel was calculated by applying the results obtained to the theoretical formula for rubber elasticity shown in Equation (3) below (n = 5) [
26]
: Stress
: Gas constant
: Temperature
: Cross-linking density
: Inverse of the degree of swelling
: Expansion/contraction ratio ()
2.8. Evaluation of Enzymatic Degradation of PEG-graft-HA Cross-Linked Gels
The sample conditions are shown in
Table S7. 12 mM PBS solution (pH 7.5) was used to swell the cross-linked gels for about 6 days and allowed to stand until swelling equilibrium was reached. The average thickness, surface area, and weight of the swollen cross-linked gels were then measured. 100 U/mL hyaluronidase solution was prepared in 12 mM PBS solution (pH 7.5). 5 mL of hyaluronidase solution (100 U/mL) was immersed in the cross-linked gel that had reached swelling equilibrium, and then the gel was shaken at 37 °C, 50 rpm. After each time elapsed, the crosslinked gel was shaken at 37 °C and 50 rpm. After each time, the cross-linked gels were removed from the hyaluronidase solution, the water in the cross-linked gels was lightly wiped off, and the weight was measured (n = 2, 3 trials). The hyaluronidase solution was changed every other day.
Based on the weight data obtained above, each was normalized according to the following Equation (4) to evaluate whether the degradation behavior of the cross-linked gel was surface degradation.
: Weight of gel after t hours of immersion in hyaluronidase solution
: Total weight of gel
: Time when the cross-linked gel is completely decomposed.
The remaining percentage of crosslinked gel was calculated according to Formula (5) to evaluate the degradability of the crosslinked gel.
: Weight of gel after t hours of immersion in hyaluronidase solution
: Weight of gel before soaking in hyaluronidase solution
2.9. Synthesis of FITC-labeld bFGF (FITC-bFGF)
To 500 μg of bFGF, 0.50 mL of distilled water was added and stirred. At this time, the mixture was still cloudy, but then 1 mL of 0.1 M Na2 CO3 (pH 11.54) was added to confirm complete dissolution. At this time, pH 12 was simultaneously confirmed. To this solution, 100 μL of 1 mg/mL FITC solution (in DMSO) was gently added in 10 μL drops. At this time, the DMSO and water were vortexed as needed to ensure adequate mixing. After 24 h of reaction in a refrigerator (at 4 °C), 1 mL of 5 M NH4Cl was added dropwise to ensure that pH 7 was reached. After stirring for another 2 h, the reaction was terminated. Then, gel filtration chromatography (Sephadex 25, expansion solvent: PBS(-)) was used to remove unreacted FITC to obtain the product. The solution of the product was lyophilized to remove water, and a yellow cottony solid was recovered. Since this synthesis was performed twice, the amount of FITC bound to each was calculated. A 20 mg/mL solution of the product was prepared and the amount of FITC bound was calculated by fluorescence intensity measurement (excitation wavelength: 488 nm, fluorescence wavelength: 530 nm).
2.10. Loading of FITC-bFGF into HA and PEG-graft-HA Hydrogels
FITC-bFGF was dissolved in PBS(-) to be 7500 ng/mL. Twenty or 6.7 μL of this solution was added to the HA hydrogel (150 or 50 ng/gel) and a leakage test was performed in 15 mL of PBS(-) after soaking in a refrigerator for 1 day.
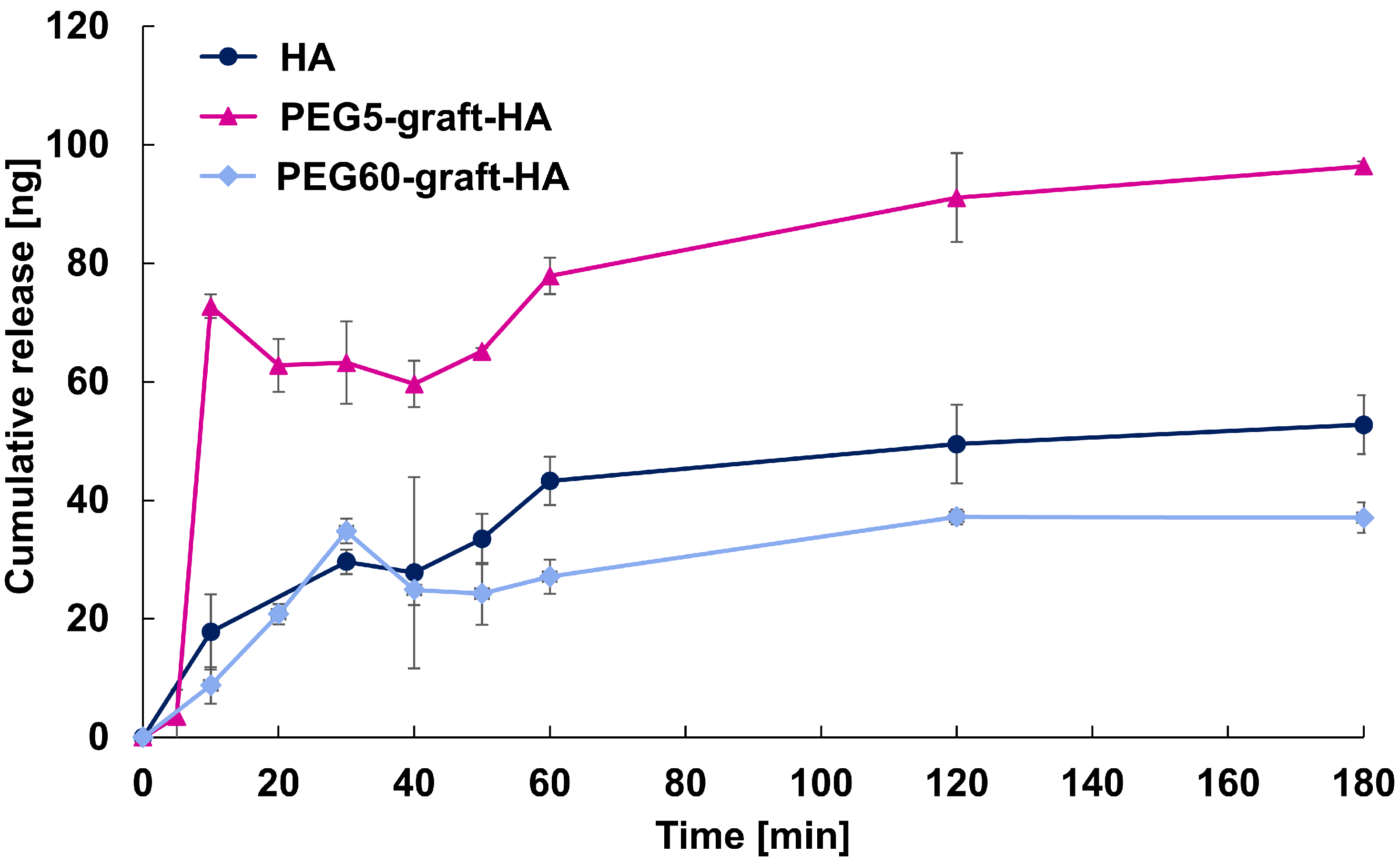
2.11. Release Studies of FITC-bFGF Using HA and PEG-graft-HA Hydrogels
The procedure was performed in a 50 mL centrifuge tube with 15 mL of PBS(-) solution in a thermostatic chamber at 37 °C. Sampling times were 5, 10, 20, 30, 40, 50, 60, 120, and 180 min. Every hour, 0.70 mL of sample was squeezed out and the same volume of PBS(-) was added. The accumulated emission rate was calculated by measuring the fluorescence intensity (excitation wavelength: 488 nm, fluorescence wavelength: 530 nm) of 100 μL of the sample solution dropped onto a 96 well plate at n = 6. The cumulative emission rate was calculated using n = 4, omitting the maximum and minimum values from n = 6.
2.12. Evaluation of Angiogenesis in Mouse Dorsal Subcutaneous Tissue Using Hydrogels with Sustained bFGF Release
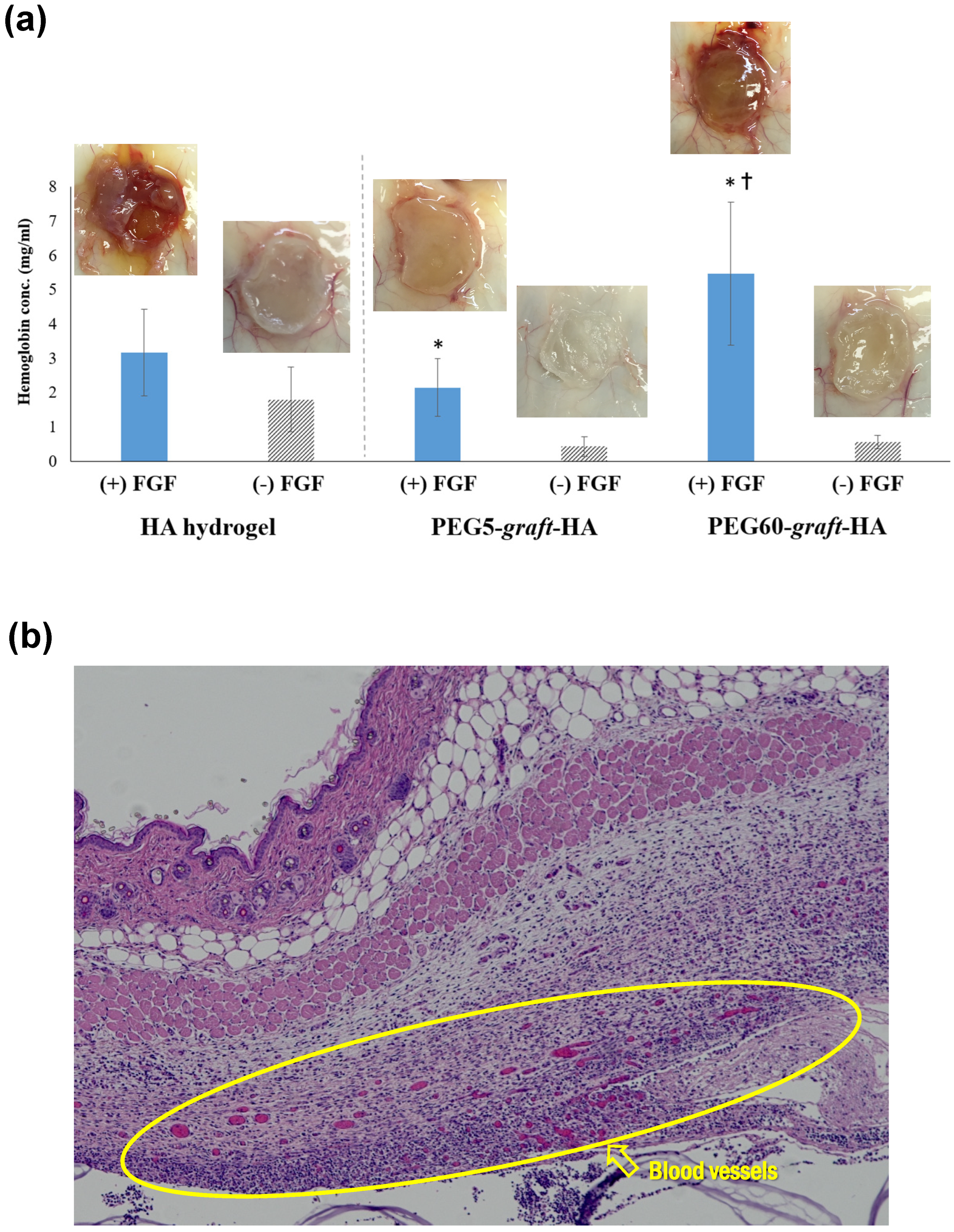
HA hydrogel, PEG5-graft-HA hydrogel, and PEG60-graft-HA hydrogel were used. One hundred μg of bFGF was added to these hydrogels. Animal experiments were conducted in full compliance with the “Guidelines for Animal Experiments at Kyoto University” and the relevant regulations of the Institute for Frontier Life and Medical Sciences, with utmost consideration for animal welfare. The research protocol was reviewed and approved by the Animal Welfare and Ethical Review Body (AWERB) at Kyoto University (Approval ID: F-148-22, 22 August 2015). Mice were housed under specific pathogen-free conditions with free access to food and water and monitored regularly for health and well-being. Humane endpoints were predefined, and mice showing severe suffering or >20% weight loss were euthanized using CO2 inhalation followed by cervical dislocation, in accordance with institutional protocols. Specifically, ddY mice (female, 6 weeks old, total 9 animals, n = 3 for each hydrogel) were anesthetized with Nembutal, and a small incision (approx. 5 mm) was made in the skin on their backs. Hydrogels impregnated with 100 µg of bFGF were implanted subcutaneously. One week after implantation, the mice were euthanized with a lethal dose of anesthetic, and the subcutaneous tissue (2 × 2 cm2) from the implantation site was harvested. Hemoglobin was then extracted from the harvested tissue by immersing it in a 17 mM Tris-HCl buffer (pH 7.6) containing 0.75% ammonium chloride at 4 °C for 24 h. The extracted hemoglobin was quantified using the Hemoglobin B-Test Wako kit (Wako).
The harvested subcutaneous tissue was fixed by immersing it in 4% paraformaldehyde/phosphate-buffered saline (pH 7.4) at 4 °C for 12 h. The solution was then replaced with 30% sucrose/phosphate-buffered saline, and the tissue was embedded using a Frozen Tissue Section Embedding Agent (OCT Compound, Sakura Finetek Japan, Tokyo, Japan). Frozen sections (approx. 5 µm thick) were prepared from the embedded tissue using a cryomicrotome (CM1950, Leica Microsystems, Tokyo, Japan) and stained with hematoxylin and eosin (H&E).
2.13. Statistical Analysis
Comparisons between two different groups were performed using Student’s t-test. Statistical differences among means were considered significant when p < 0.05. As for the data of the hemoglobin concentration, sample means were compared using the one-way ANOVA test. In the case of a significant difference between the means, multiple comparisons of the means were carried out using Fisher PLSD. Differences for which p < 0.05 was considered to be statistically significant. All the analysis was conducted using SPSS Statistics 21 (IBM Corp., Armonk, NY, USA).
4. Conclusions
In this study, we successfully developed PEG-grafted HA hydrogels with varying PEG grafting ratios to address the challenge of burst release of growth factors. Our findings demonstrate a strong correlation between the PEG grafting ratio and the physicochemical properties of the gels. The PEG60-graft-HA hydrogel, with its higher swelling capacity and more rapid, bulk-like degradation, showed an ability to achieve sustained release of bFGF. This sustained release, in turn, resulted in a significant promotion of angiogenesis in a mouse subcutaneous model.
While we did not find a direct correlation between cross-linking density and swelling ratio, likely due to measurement limitations with the highly swollen gels, the overall data points to a key conclusion: the introduction of a high ratio of PEG grafts creates a unique gel structure that can effectively modulate the release kinetics of encapsulated proteins. This controlled, sustained release is crucial for maintaining the bioactivity of growth factors like bFGF, which is likely due to aqueous two-phase partition of bFGF into PEG region, and maximizing their therapeutic effect in vivo. Our results suggest strong potential for applications in regenerative medicine, particularly in vascular tissue engineering. However, several translational challenges remain, including:
Stability of bFGF: While PEG grafting improves release control, long-term stability in vivo needs further improvement.
Immunogenicity: Both bFGF and the hydrogel components may pose immunological risks that require thorough evaluation.
Scalability: The synthesis and loading processes must be optimized for consistent large-scale manufacturing.
In the future, this material holds strong potential for clinical applications, particularly in improving therapeutic outcomes for diabetic foot ulcers, which affect approximately 18.6 million individuals worldwide each year. By accelerating wound healing and reducing the risk of amputation, this approach could offer significant social and medical benefits.