Development and Characterization of Scented PLA-Based Biocomposites Reinforced with Spent Coffee Grounds and Lignin for FDM 3D Printing
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Biocomposites’ Characterization
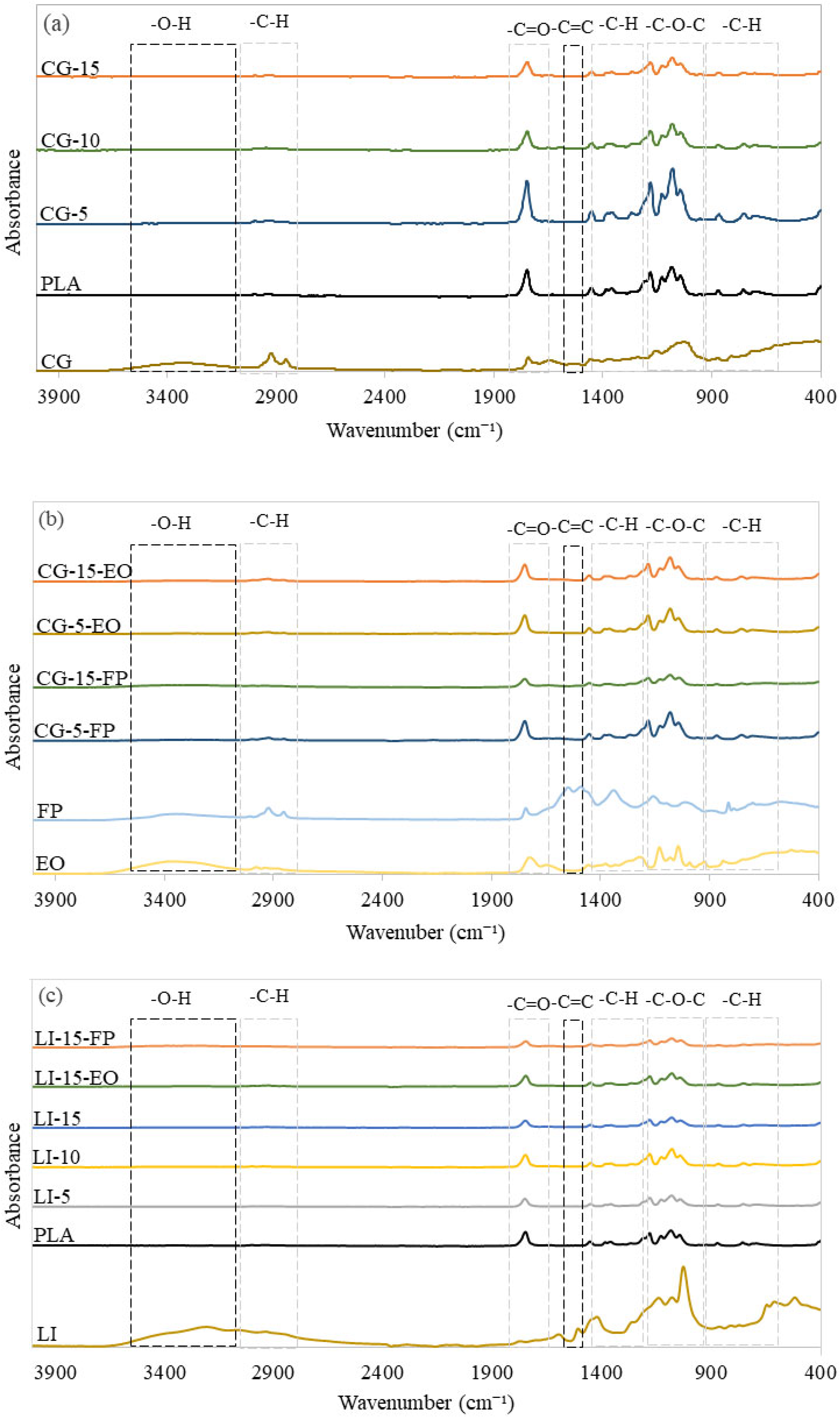
2.2.1. Surface Chemistry
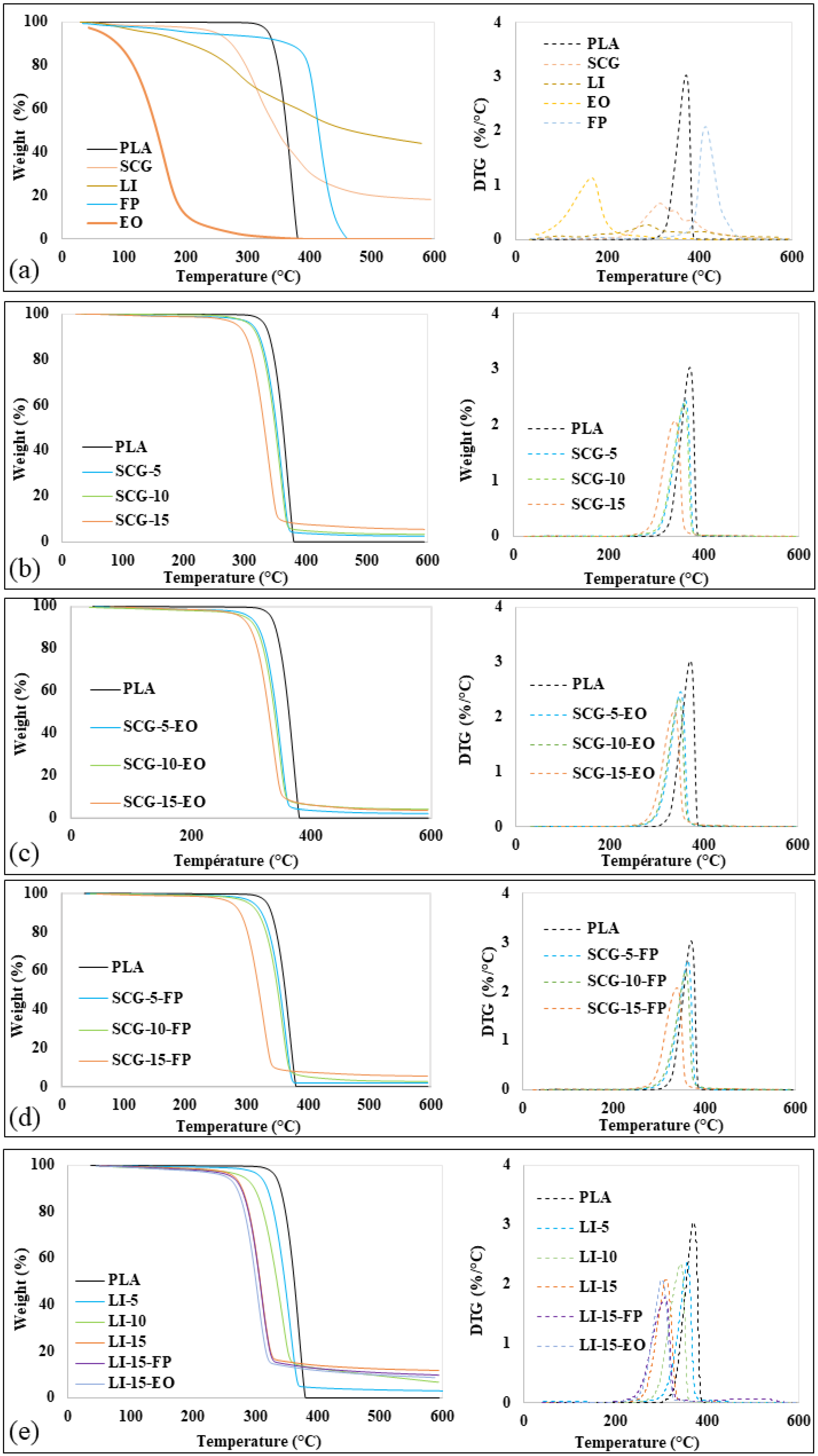
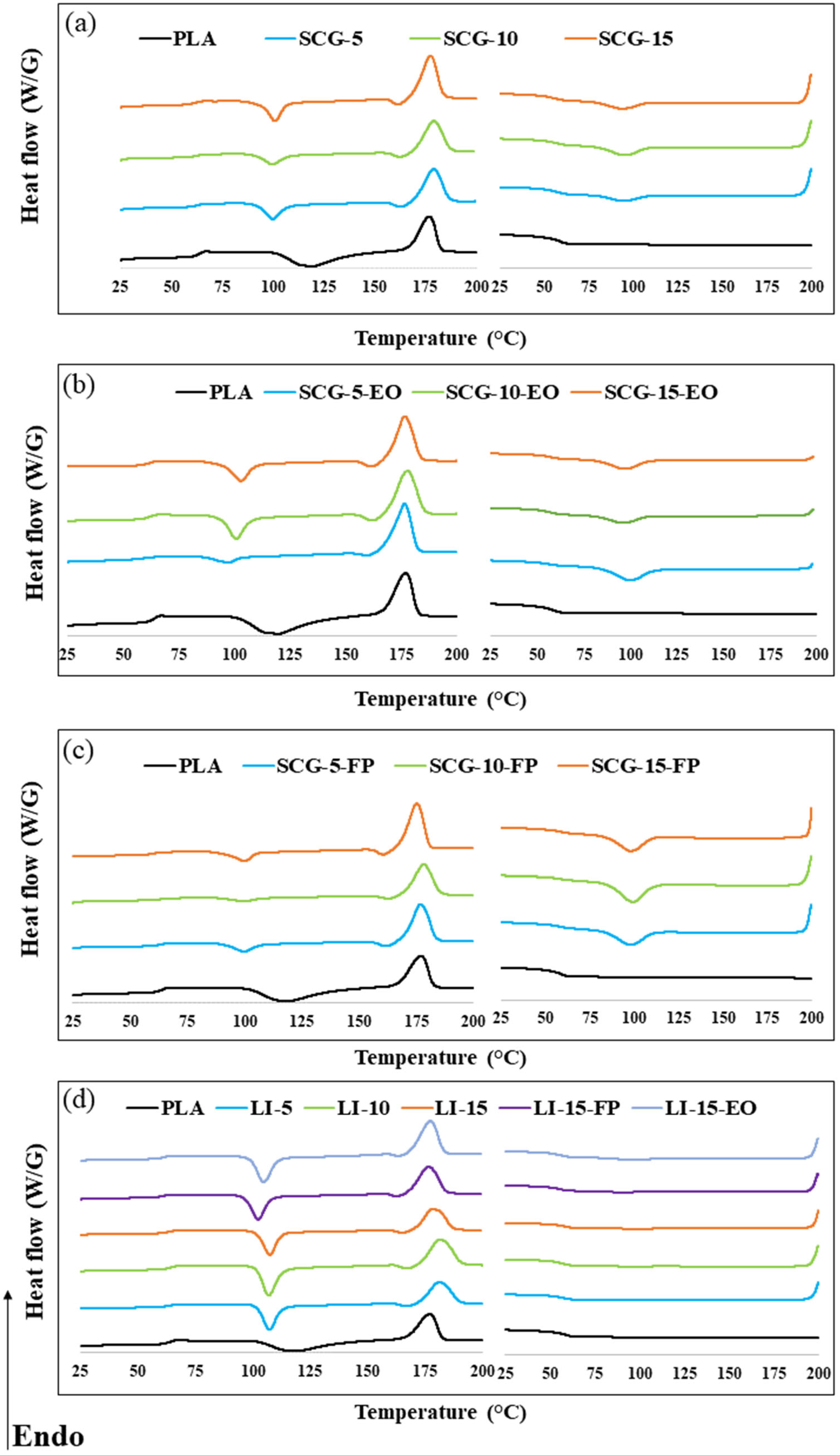
2.2.2. Thermal Properties
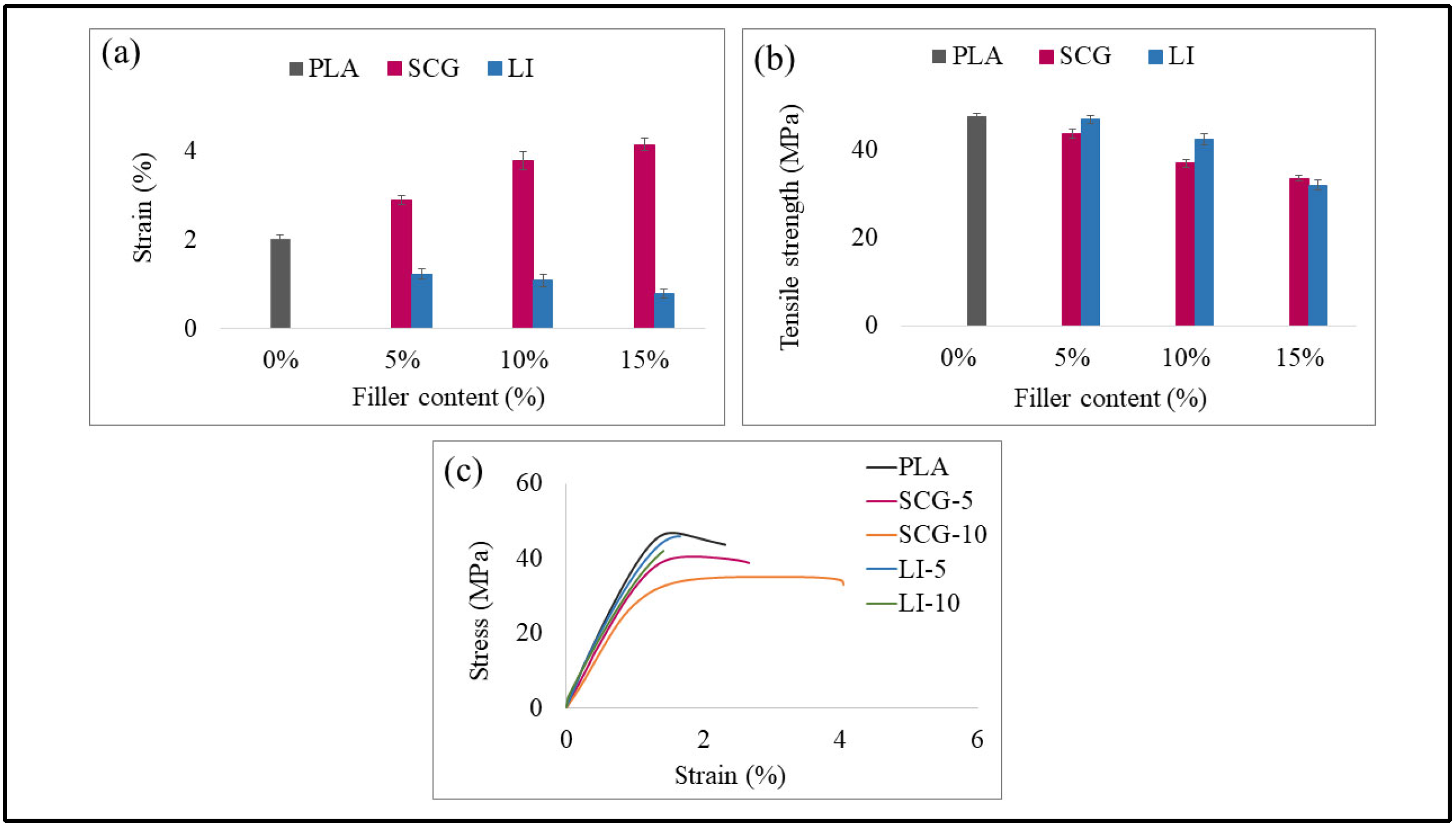
2.2.3. Mechanical Properties
2.2.4. Scanning Electron Microscopy (SEM)
2.2.5. Water Absorption Test (WA) and Thickness Swelling (TS)
3. Results
3.1. Surface Chemistry
3.2. Thermal Properties
3.3. Mechanical Properties
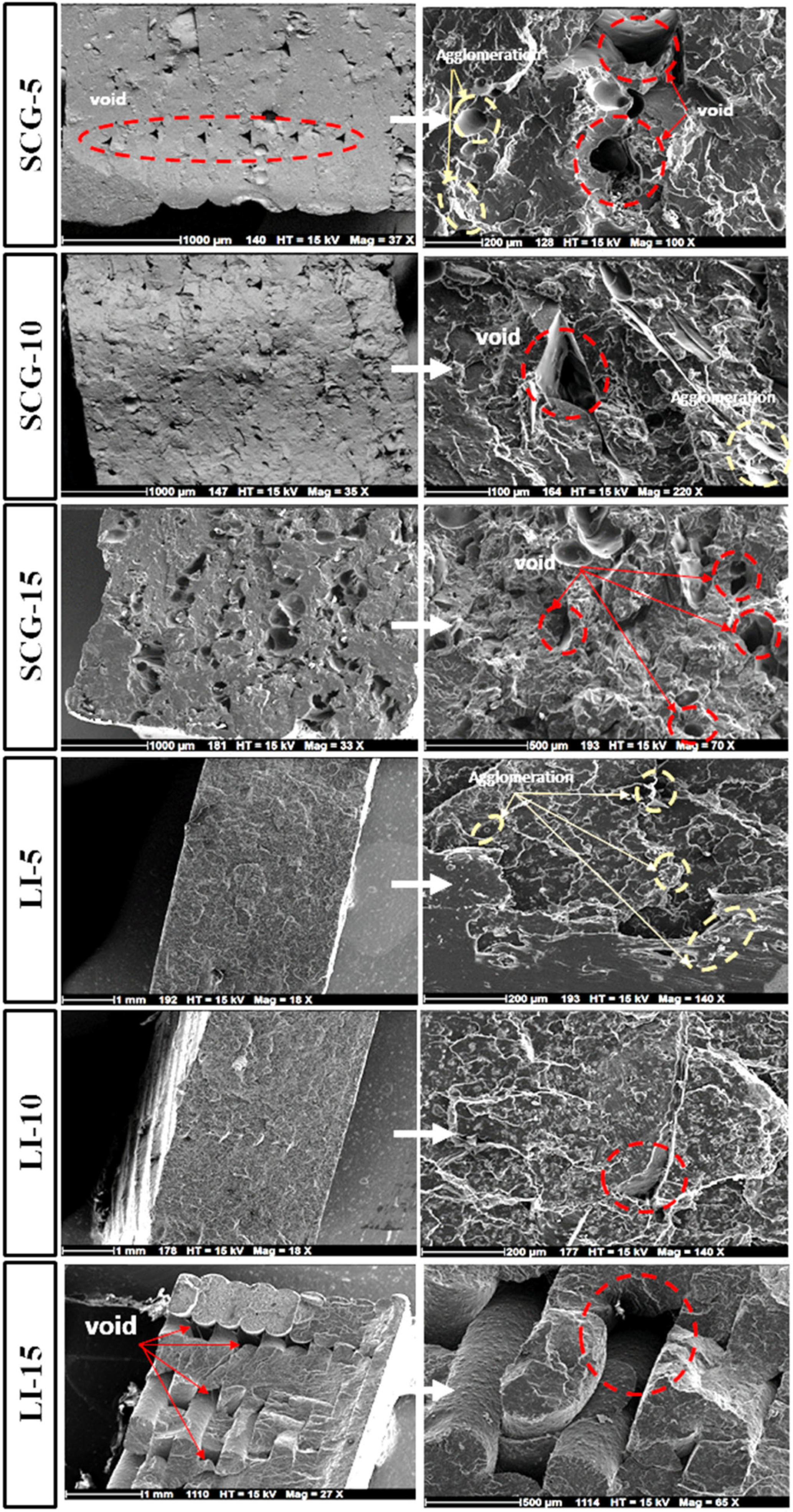
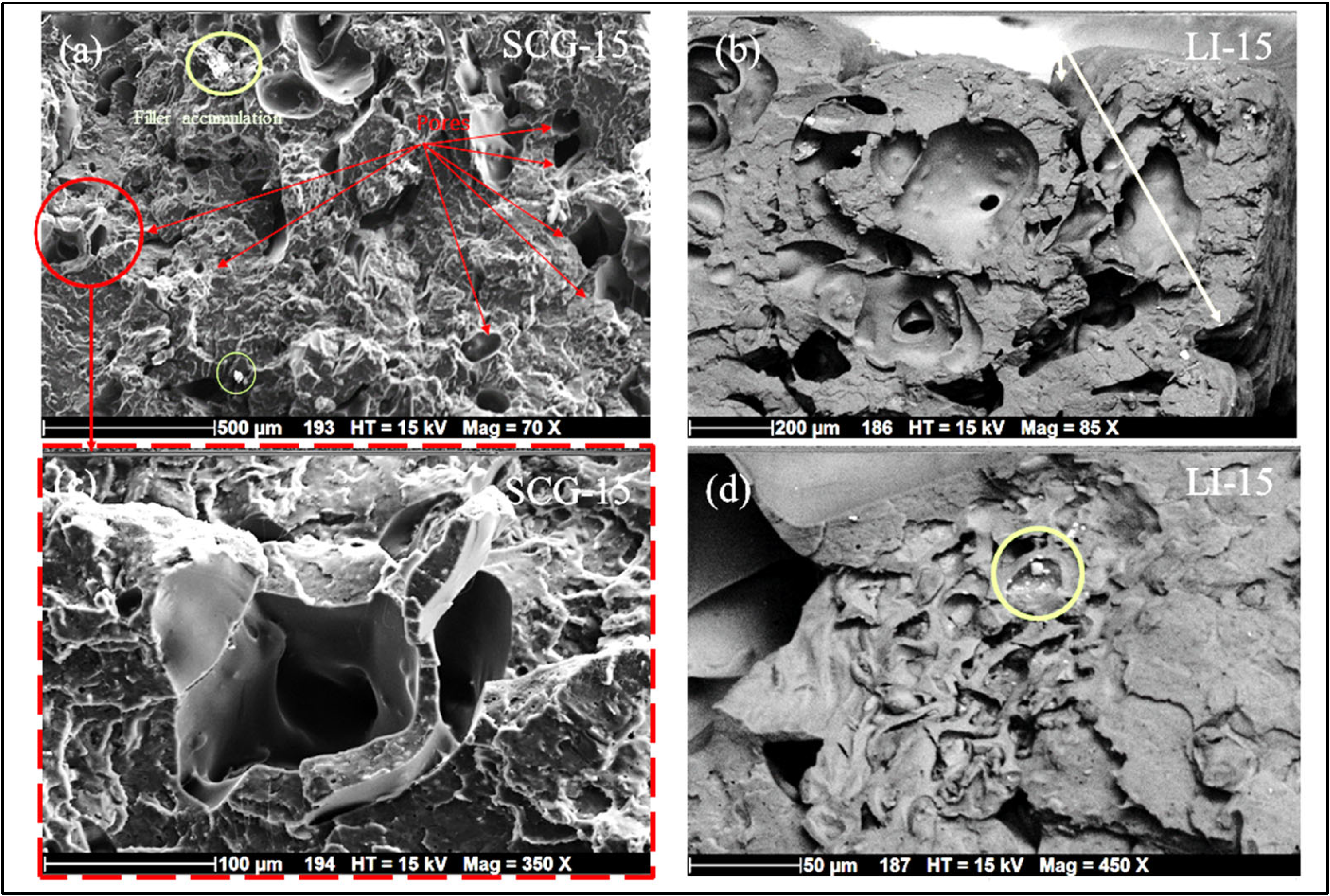
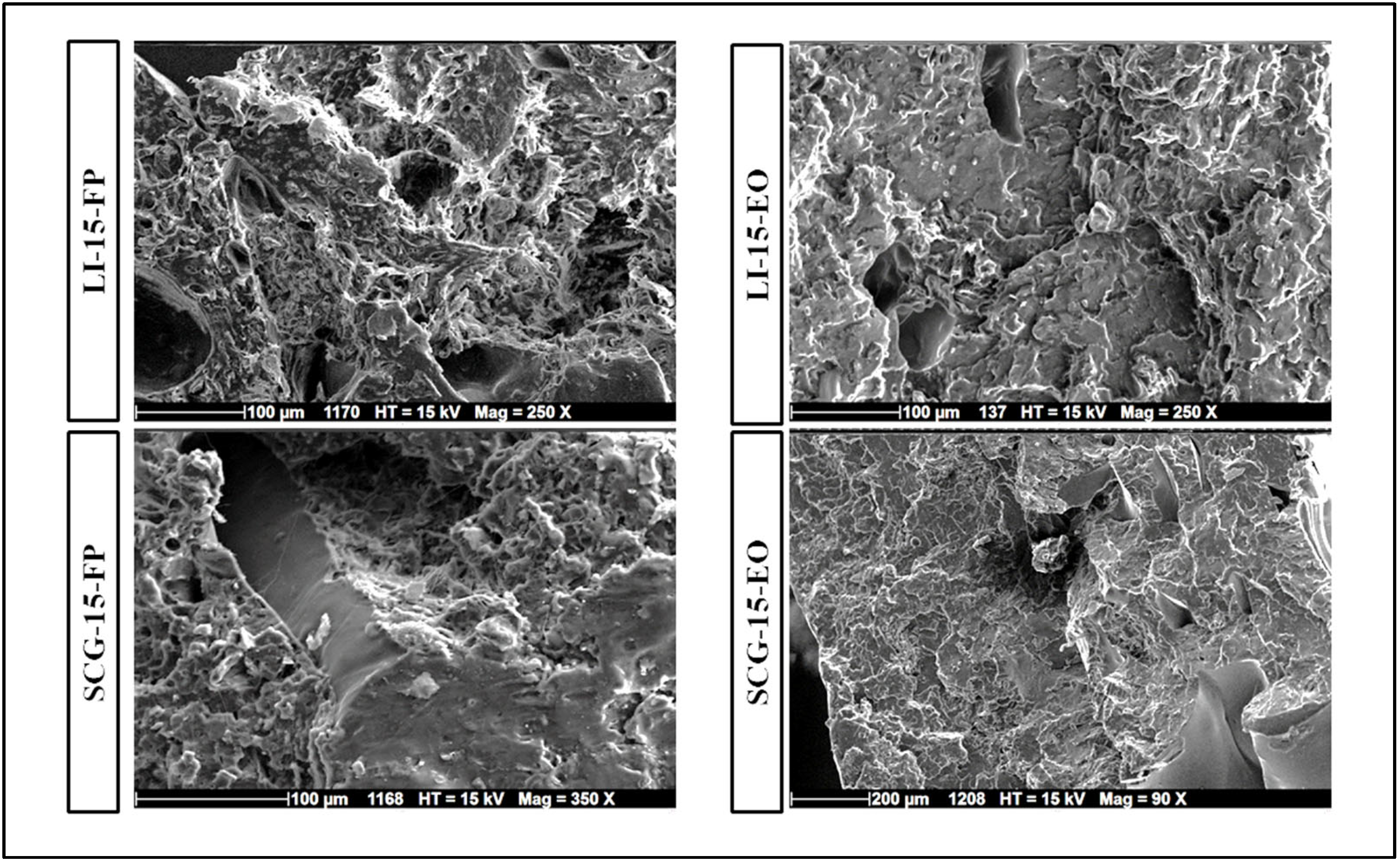
3.4. Biocomposites’ Microstructure
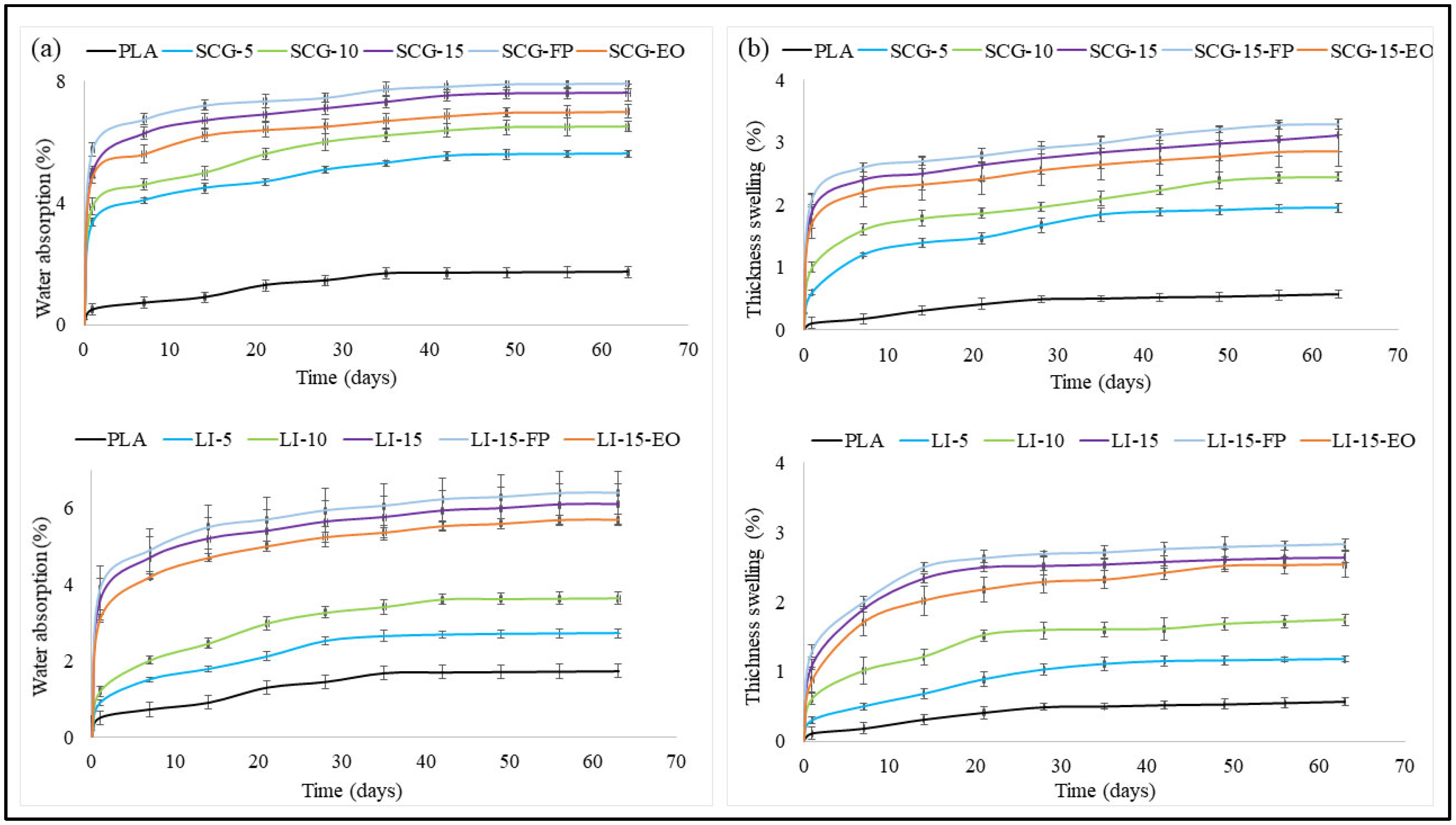
3.5. Water Absorption and Thickness Swelling
4. Practical Implications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BC | Biocomposite |
| EO | Essential oils |
| FDM | Fused Deposition Modeling |
| FP | Fragrance powders |
| LI | Lignin |
| PLA | Polylactic acid |
| SCG | Spent coffee grounds |
References
- Yousefi, M.A.; Rahmatabadi, D.; Baniassadi, M.; Bodaghi, M.; Baghani, M. 4D Printing of Multifunctional and Biodegradable PLA-PBAT-Fe3 O4 Nanocomposites with Supreme Mechanical and Shape Memory Properties. Macromol. Rapid Commun. 2025, 46, 2400661. [Google Scholar] [CrossRef]
- Mehrpouya, M.; Vosooghnia, A.; Dehghanghadikolaei, A.; Fotovvati, B. The benefits of additive manufacturing for sustainable design and production. In Sustainable Manufacturing; Elsevier: Amsterdam, The Netherlands, 2021; pp. 29–59. [Google Scholar] [CrossRef]
- Rajan, K.; Samykano, M.; Kadirgama, K.; Harun, W.S.W.; Rahman, M.M. Fused deposition modeling: Process, materials, parameters, properties, and applications. Int. J. Adv. Manuf. Technol. 2022, 120, 1531–1570. [Google Scholar] [CrossRef]
- Taib, N.-A.A.B.; Rahman, M.R.; Huda, D.; Kuok, K.K.; Hamdan, S.; Bakri, M.K.B.; Julaihi, M.R.M.B.; Khan, A. A review on poly lactic acid (PLA) as a biodegradable polymer. Polym. Bull. 2023, 80, 1179–1213. [Google Scholar] [CrossRef]
- Tümer, E.H.; Erbil, H.Y. Extrusion-Based 3D Printing Applications of PLA Composites: A Review. Coatings 2021, 11, 390. [Google Scholar] [CrossRef]
- Zhai, S.; Liu, Q.; Zhao, Y.; Sun, H.; Yang, B.; Weng, Y. A Review: Research Progress in Modification of Poly (Lactic Acid) by Lignin and Cellulose. Polymers 2021, 13, 776. [Google Scholar] [CrossRef]
- Ilyas, R.; Zuhri, M.; Aisyah, H.; Asyraf, M.; Hassan, S.; Zainudin, E.; Sapuan, S.; Sharma, S.; Bangar, S.; Jumaidin, R.; et al. Natural Fiber-Reinforced Polylactic Acid, Polylactic Acid Blends and Their Composites for Advanced Applications. Polymers 2022, 14, 202. [Google Scholar] [CrossRef] [PubMed]
- Rajeshkumar, G.; Arvindh Seshadri, S.; Devnani, G.L.; Sanjay, M.R.; Siengchin, S.; Prakash Maran, J.; Al-Dhabi, N.A.; Karuppiah, P.; Mariadhas, V.A.; Sivarajasekar, N.; et al. Environment friendly, renewable and sustainable poly lactic acid (PLA) based natural fiber reinforced composites—A comprehensive review. J. Clean. Prod. 2021, 310, 127483. [Google Scholar] [CrossRef]
- Ahmad, M.N.; Ishak, M.R.; Mohammad Taha, M.; Mustapha, F.; Leman, Z. A Review of Natural Fiber-Based Filaments for 3D Printing: Filament Fabrication and Characterization. Materials 2023, 16, 4052. [Google Scholar] [CrossRef]
- Agaliotis, E.M.; Ake-Concha, B.D.; May-Pat, A.; Morales-Arias, J.P.; Bernal, C.; Valadez-Gonzalez, A.; Herrera-Franco, P.J.; Proust, G.; Koh-Dzul, J.F.; Carrillo, J.G.; et al. Tensile Behavior of 3D Printed Polylactic Acid (PLA) Based Composites Reinforced with Natural Fiber. Polymers 2022, 14, 3976. [Google Scholar] [CrossRef]
- Calì, M.; Pascoletti, G.; Gaeta, M.; Milazzo, G.; Ambu, R. A New Generation of Bio-Composite Thermoplastic Filaments for a More Sustainable Design of Parts Manufactured by FDM. Appl. Sci. 2020, 10, 5852. [Google Scholar] [CrossRef]
- Yu, I.K.M.; Chan, O.Y.; Zhang, Q.; Wang, L.; Wong, K.-H.; Tsang, D.C.W. Upcycling of Spent Tea Leaves and Spent Coffee Grounds into Sustainable 3D-Printing Materials: Natural Plasticization and Low-Energy Fabrication. ACS Sustain. Chem. Eng. 2023, 11, 6230–6240. [Google Scholar] [CrossRef]
- Helaoui, S.; Koubaa, A.; Nouri, H.; Beauregard, M.; Guessasma, S. 3D printing of biodegradable biocomposites based on forest industrial residues by fused deposition modeling. Ind. Crops Prod. 2024, 222, 119799. [Google Scholar] [CrossRef]
- Karmee, S.K. A spent coffee grounds based biorefinery for the production of biofuels, biopolymers, antioxidants and biocomposites. Waste Manag. 2018, 72, 240–254. [Google Scholar] [CrossRef] [PubMed]
- Forcina, A.; Petrillo, A.; Travaglioni, M.; Di Chiara, S.; De Felice, F. A comparative life cycle assessment of different spent coffee ground reuse strategies and a sensitivity analysis for verifying the environmental convenience based on the location of sites. J. Clean. Prod. 2023, 385, 135727. [Google Scholar] [CrossRef]
- Mak, S.L.; Wu, M.Y.T.; Chak, W.Y.; Kwong, W.K.; Tang, W.F.; Li, C.H.; Lee, C.C.; Li, C.Y. A Review of the Feasibility of Producing Polylactic Acid (PLA) Polymers Using Spent Coffee Ground. Sustainability 2023, 15, 13498. [Google Scholar] [CrossRef]
- Ballesteros, L.F.; Teixeira, J.A.; Mussatto, S.I. Chemical, Functional, and Structural Properties of Spent Coffee Grounds and Coffee Silverskin. Food Bioprocess Technol. 2014, 7, 3493–3503. [Google Scholar] [CrossRef]
- Li, S.; Shi, C.; Sun, S.; Chan, H.; Lu, H.; Nilghaz, A.; Tian, J.; Cao, R. From brown to colored: Polylactic acid composite with micro/nano-structured white spent coffee grounds for three-dimensional printing. Int. J. Biol. Macromol. 2021, 174, 300–308. [Google Scholar] [CrossRef]
- Yu, W.; Yuan, T.; Yao, Y.; Deng, Y.; Wang, X. PLA/Coffee Grounds Composite for 3D Printing and Its Properties. Forests 2023, 14, 367. [Google Scholar] [CrossRef]
- Paramatti, M.; Romani, A.; Pugliese, G.; Levi, M. PLA Feedstock Filled with Spent Coffee Grounds for New Product Applications with Large-Format Material Extrusion Additive Manufacturing. ACS Omega 2024, 9, 6423–6431. [Google Scholar] [CrossRef]
- Zhao, W.; Simmons, B.; Singh, S.; Ragauskas, A.; Cheng, G. From lignin association to nano-/micro-particle preparation: Extracting higher value of lignin. Green Chem. 2016, 18, 5693–5700. [Google Scholar] [CrossRef]
- Grossman, A.; Vermerris, W. Lignin-based polymers and nanomaterials. Curr. Opin. Biotechnol. 2019, 56, 112–120. [Google Scholar] [CrossRef]
- Tanase-Opedal, M.; Espinosa, E.; Rodríguez, A.; Chinga-Carrasco, G. Lignin: A Biopolymer from Forestry Biomass for Biocomposites and 3D Printing. Materials 2019, 12, 3006. [Google Scholar] [CrossRef]
- Mimini, V.; Sykacek, E.; Syed Hashim, S.N.A.; Holzweber, J.; Hettegger, H.; Fackler, K.; Potthast, A.; Mundigler, N.; Rosenau, T. Compatibility of Kraft Lignin, Organosolv Lignin and Lignosulfonate With PLA in 3D Printing. J. Wood Chem. Technol. 2019, 39, 14–30. [Google Scholar] [CrossRef]
- Wasti, S.; Triggs, E.; Farag, R.; Auad, M.; Adhikari, S.; Bajwa, D.; Li, M.; Ragauskas, A.J. Influence of plasticizers on thermal and mechanical properties of biocomposite filaments made from lignin and polylactic acid for 3D printing. Compos. Part B Eng. 2021, 205, 108483. [Google Scholar] [CrossRef]
- Zaidi, S.A.S.; Kwan, C.E.; Mohan, D.; Harun, S.; Luthfi, A.A.I.; Sajab, M.S. Evaluating the Stability of PLA-Lignin Filament Produced by Bench-Top Extruder for Sustainable 3D Printing. Materials 2023, 16, 1793. [Google Scholar] [CrossRef]
- Félix, J.S.; Domeño, C.; Nerín, C. Characterization of wood plastic composites made from landfill-derived plastic and sawdust: Volatile compounds and olfactometric analysis. Waste Manag. 2013, 33, 645–655. [Google Scholar] [CrossRef]
- Courgneau, C.; Rusu, D.; Henneuse, C.; Ducruet, V.; Lacrampe, M.-F.; Krawczak, P. Characterisation of low-odour emissive polylactide/cellulose fibre biocomposites for car interior. Express Polym. Lett. 2013, 7, 787–804. [Google Scholar] [CrossRef]
- Barthod-Malat, B.; Hauguel, M.; Behlouli, K.; Grisel, M.; Savary, G. Influence of the Compression Molding Temperature on VOCs and Odors Produced from Natural Fiber Composite Materials. Coatings 2023, 13, 371. [Google Scholar] [CrossRef]
- Wei, M.; Pan, X.; Rong, L.; Dong, A.; He, Y.; Song, X.; Li, J. Polymer carriers for controlled fragrance release. Mater. Res. Express 2020, 7, 082001. [Google Scholar] [CrossRef]
- Xiao, Z.; Zhang, Y.; Zhu, G.; Niu, Y.; Xu, Z.; Zhu, J. Preparation of micro-encapsulated strawberry fragrance and its application in the aromatic wallpaper. Pol. J. Chem. Technol. 2017, 19, 89–94. [Google Scholar] [CrossRef]
- Liu, X.; Huang, Y.; Yang, Q.; Jiang, Z.; Feng, D. Preparation and Properties of a Fragrant Acrylonitrile–Butadiene–Styrene/Polycarbonate Alloy. Polym. Polym. Compos. 2009, 17, 41–45. [Google Scholar] [CrossRef]
- ASTM D3417; Standard Test Method for Enthalpies of Fusion and Crystallization of Polymers by Differential Scanning Calorimetry (DSC). ASTM International: West Conshohocken, PA, USA, 1999.
- ASTM D638; Standard Test Method for Tensile Properties of Plastics. ASTM International: West Conshohocken, PA, USA, 2022.
- ASTM D790; Standard Test Methods for Flexural Properties of Unreinforced and Reinforced Plastics and Electrical Insulating Materials. ASTM International: West Conshohocken, PA, USA, 2017.
- ASTM D256; Standard Test Methods for Determining the Izod Pendulum Impact Resistance of Plastics. ASTM International: West Conshohocken, PA, USA, 2010.
- ASTM D570-98; Standard Test Method for Water Absorption of Plastics. ASTM International: West Conshohocken, PA, USA, 1998.
- Zhang, Z.; Harrison, M.D.; Rackemann, D.W.; Doherty, W.O.S.; O’Hara, I.M. Organosolv pretreatment of plant biomass for enhanced enzymatic saccharification. Green Chem. 2016, 18, 360–381. [Google Scholar] [CrossRef]
- Nazrin, A.; Sapuan, S.M.; Zuhri, M.Y.M. Mechanical, Physical and Thermal Properties of Sugar Palm Nanocellulose Reinforced Thermoplastic Starch (TPS)/Poly (Lactic Acid) (PLA) Blend Bionanocomposites. Polymers 2020, 12, 2216. [Google Scholar] [CrossRef] [PubMed]
- Gamiz-Conde, A.K.; Burelo, M.; Franco-Urquiza, E.A.; Martínez-Franco, E.; Luna-Barcenas, G.; Bravo-Alfaro, D.A.; Treviño-Quintanilla, C.D. Development and properties of bio-based polymer composites using PLA and untreated agro-industrial residues. Polym. Test. 2024, 139, 108576. [Google Scholar] [CrossRef]
- Masssijaya, S.Y.; Lubis, M.A.R.; Nissa, R.C.; Nurhamiyah, Y.; Nugroho, P.; Antov, P.; Lee, S.-H.; Papadopoulos, A.N.; Kusumah, S.S.; Karlinasari, L. Utilization of Spent Coffee Grounds as a Sustainable Resource for the Synthesis of Bioplastic Composites with Polylactic Acid, Starch, and Sucrose. J. Compos. Sci. 2023, 7, 512. [Google Scholar] [CrossRef]
- Getachew, A.T.; Cho, Y.J.; Chun, B.S. Effect of pretreatments on isolation of bioactive polysaccharides from spent coffee grounds using subcritical water. Int. J. Biol. Macromol. 2018, 109, 711–719. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.I.; Martín-Sampedro, R.; Fillat, Ú.; Oliva, J.M.; Negro, M.J.; Ballesteros, M.; Eugenio, M.E.; Ibarra, D. Evaluating Lignin-Rich Residues from Biochemical Ethanol Production of Wheat Straw and Olive Tree Pruning by FTIR and 2D-NMR. Int. J. Polym. Sci. 2015, 2015, 314891. [Google Scholar] [CrossRef]
- Hong, S.-H.; Park, J.H.; Kim, O.Y.; Hwang, S.-H. Preparation of Chemically Modified Lignin-Reinforced PLA Biocomposites and Their 3D Printing Performance. Polymers 2021, 13, 667. [Google Scholar] [CrossRef]
- Guo, J.; Chen, X.; Wang, J.; He, Y.; Xie, H.; Zheng, Q. The Influence of Compatibility on the Structure and Properties of PLA/Lignin Biocomposites by Chemical Modification. Polymers 2019, 12, 56. [Google Scholar] [CrossRef]
- Spiridon, I.; Tanase, C.E. Design, characterization and preliminary biological evaluation of new lignin-PLA biocomposites. Int. J. Biol. Macromol. 2018, 114, 855–863. [Google Scholar] [CrossRef]
- Xiao, Z.; Hou, X.; Hwang, S.; Li, H. The biocomposites properties of compounded poly(lactic acid) with untreated and treated spent coffee grounds. J. Appl. Polym. Sci. 2022, 139, e53092. [Google Scholar] [CrossRef]
- Bounaas, K.; Bouzidi, N.; Daghbouche, Y.; Garrigues, S.; De La Guardia, M.; El Hattab, M. Essential oil counterfeit identification through middle infrared spectroscopy. Microchem. J. 2018, 139, 347–356. [Google Scholar] [CrossRef]
- Hernández-López, M.; Correa-Pacheco, Z.N.; Bautista-Baños, S.; Zavaleta-Avejar, L.; Benítez-Jiménez, J.J.; Sabino-Gutiérrez, M.A.; Ortega-Gudiño, P. Bio-based composite fibers from pine essential oil and PLA/PBAT polymer blend. Morphological, physicochemical, thermal and mechanical characterization. Mater. Chem. Phys. 2019, 234, 345–353. [Google Scholar] [CrossRef]
- Yang, J.; Ching, Y.C.; Chuah, C.H.; Liou, N.-S. Preparation and Characterization of Starch/Empty Fruit Bunch-Based Bioplastic Composites Reinforced with Epoxidized Oils. Polymers 2020, 13, 94. [Google Scholar] [CrossRef] [PubMed]
- Agatonovic-Kustrin, S.; Ristivojevic, P.; Gegechkori, V.; Litvinova, T.M.; Morton, D.W. Essential Oil Quality and Purity Evaluation via FT-IR Spectroscopy and Pattern Recognition Techniques. Appl. Sci. 2020, 10, 7294. [Google Scholar] [CrossRef]
- Stramarkou, M.; Oikonomopoulou, V.; Missirli, T.; Thanassoulia, I.; Krokida, M. Encapsulation of Rosemary Essential Oil into Biodegradable Polymers for Application in Crop Management. J. Polym. Environ. 2020, 28, 2161–2177. [Google Scholar] [CrossRef]
- Chu, Y.; Xu, T.; Gao, C.; Liu, X.; Zhang, N.; Feng, X.; Liu, X.; Shen, X.; Tang, X. Evaluations of physicochemical and biological properties of pullulan-based films incorporated with cinnamon essential oil and Tween 80. Int. J. Biol. Macromol. 2019, 122, 388–394. [Google Scholar] [CrossRef]
- Han, Y.; Shi, J.; Mao, L.; Wang, Z.; Zhang, L. Improvement of Compatibility and Mechanical Performances of PLA/PBAT Composites with Epoxidized Soybean Oil as Compatibilizer. Ind. Eng. Chem. Res. 2020, 59, 21779–21790. [Google Scholar] [CrossRef]
- Wang, J.; Minami, E.; Kawamoto, H. Thermal reactivity of hemicellulose and cellulose in cedar and beech wood cell walls. J. Wood Sci. 2020, 66, 41. [Google Scholar] [CrossRef]
- Baek, B.-S.; Park, J.-W.; Lee, B.-H.; Kim, H.-J. Development and Application of Green Composites: Using Coffee Ground and Bamboo Flour. J. Polym. Environ. 2013, 21, 702–709. [Google Scholar] [CrossRef]
- Garcia-Garcia, D.; Carbonell-Verdu, A.; Arrieta, M.P.; López-Martínez, J.; Samper, M.D. Improvement of PLA film ductility by plasticization with epoxidized karanja oil. Polym. Degrad. Stab. 2020, 179, 109259. [Google Scholar] [CrossRef]
- Bouftou, A.; Aghmih, K.; Lakhdar, F.; Abidi, N.; Gmouh, S.; Majid, S. Enhancing cellulose acetate film with green plasticizers for improved performance, biodegradability, and migration study into a food simulant. Meas. Food 2024, 15, 100180. [Google Scholar] [CrossRef]
- Pinheiro, L.D.S.M.; Sangoi, G.G.; Righi, N.C.; Vizzotto, B.S.; Ruiz, Y.P.M.; Galembeck, A.; Pavoski, G.; Espinosa, D.C.R.; Machado, A.K.; Da Silva, W.L. PLA/PCL polymer nanocomposite with silver and copper nanoparticles and lavender essential oil: Synthesis, characterization and application in tissue engineering. Surf. Interfaces 2024, 55, 105391. [Google Scholar] [CrossRef]
- Gkartzou, E.; Koumoulos, E.P.; Charitidis, C.A. Production and 3D printing processing of bio-based thermoplastic filament. Manuf. Rev. 2017, 4, 1. [Google Scholar] [CrossRef]
- Chang, Y.-C.; Chen, Y.; Ning, J.; Hao, C.; Rock, M.; Amer, M.; Feng, S.; Falahati, M.; Wang, L.-J.; Chen, R.K.; et al. No such thing as trash: A 3d-printable polymer composite composed of oil-extracted spent coffee grounds and polylactic acid with enhanced impact toughness. ACS Sustain. Chem. Eng. 2019, 7, 15304–15310. [Google Scholar] [CrossRef]
- Cacciotti, I.; Mori, S.; Cherubini, V.; Nanni, F. Eco-sustainable systems based on poly(lactic acid), diatomite and coffee grounds extract for food packaging. Int. J. Biol. Macromol. 2018, 112, 567–575. [Google Scholar] [CrossRef]
- Bakrani Balani, S.; Chabert, F.; Nassiet, V.; Cantarel, A. Influence of printing parameters on the stability of deposited beads in fused filament fabrication of poly(lactic) acid. Addit. Manuf. 2019, 25, 112–121. [Google Scholar] [CrossRef]
- Kuciel, S.; Mazur, K.; Hebda, M. The Influence of Wood and Basalt Fibres on Mechanical, Thermal and Hydrothermal Properties of PLA Composites. J. Polym. Environ. 2020, 28, 1204–1215. [Google Scholar] [CrossRef]
- Sheng, K.; Zhang, S.; Qian, S.; Fontanillo Lopez, C.A. High-toughness PLA/Bamboo cellulose nanowhiskers bionanocomposite strengthened with silylated ultrafine bamboo-char. Compos. Part B Eng. 2019, 165, 174–182. [Google Scholar] [CrossRef]
- Javidi, Z.; Hosseini, S.F.; Rezaei, M. Development of flexible bactericidal films based on poly(lactic acid) and essential oil and its effectiveness to reduce microbial growth of refrigerated rainbow trout. LWT Food Sci. Technol. 2016, 72, 251–260. [Google Scholar] [CrossRef]
- Gama, N.; Ferreira, A.; Evtuguin, D.V. New poly(lactic acid) composites produced from coffee beverage wastes. J. Appl. Polym. Sci. 2022, 139, 51434. [Google Scholar] [CrossRef]
- Ding, R.; Duan, Z.; Sun, Y.; Yuan, Q.; Tien, T.T.; Zúniga, M.G.; Oh, E.; Nam, J.-D.; Suhr, J. Enhancement of 3D printability and mechanical properties of polylactic acid/lignin biocomposites via interface engineering. Ind. Crops Prod. 2023, 194, 116286. [Google Scholar] [CrossRef]
- Spiridon, I.; Leluk, K.; Resmerita, A.M.; Darie, R.N. Evaluation of PLA–lignin bioplastics properties before and after accelerated weathering. Compos. Part B Eng. 2015, 69, 342–349. [Google Scholar] [CrossRef]
- Kumar Singla, R.; Maiti, S.N.; Ghosh, A.K. Crystallization, Morphological, and Mechanical Response of Poly(Lactic Acid)/Lignin-Based Biodegradable Composites. Polym. Plast. Technol. Eng. 2016, 55, 475–485. [Google Scholar] [CrossRef]
- Perinelli, D.R.; Palmieri, G.F.; Cespi, M.; Bonacucina, G. Encapsulation of Flavours and Fragrances into Polymeric Capsules and Cyclodextrins Inclusion Complexes: An Update. Molecules 2020, 25, 5878. [Google Scholar] [CrossRef]
- Bonilla, J.; Atarés, L.; Vargas, M.; Chiralt, A. Effect of essential oils and homogenization conditions on properties of chitosan-based films. Food Hydrocoll. 2012, 26, 9–16. [Google Scholar] [CrossRef]
- Balart, J.F.; Fombuena, V.; Fenollar, O.; Boronat, T.; Sánchez-Nacher, L. Processing and characterization of high environmental efficiency composites based on PLA and hazelnut shell flour (HSF) with biobased plasticizers derived from epoxidized linseed oil (ELO). Compos. Part B Eng. 2016, 86, 168–177. [Google Scholar] [CrossRef]
- Bajwa, D.; Eichers, M.; Shojaeiarani, J.; Kallmeyer, A. Influence of biobased plasticizers on 3D printed polylactic acid composites filled with sustainable biofiller. Ind. Crops Prod. 2021, 173, 114132. [Google Scholar] [CrossRef]
- Kartal, F.; Kaptan, A. Sustainable Reinforcement of PLA Composites with Waste Beech Sawdust for Enhanced 3D-Printing Performance. J. Mater. Eng. Perform. 2024, 34, 15248–15259. [Google Scholar] [CrossRef]
- De Bomfim, A.S.C.; De Oliveira, D.M.; Benini, K.C.C.D.C.; Cioffi, M.O.H.; Voorwald, H.J.C.; Rodrigue, D. Effect of spent coffee grounds on the crystallinity and viscoelastic behavior of polylactic acid composites. Polymers 2023, 15, 2719. [Google Scholar] [CrossRef]
- Langat, H.K.; Dimitrov, K.; Herzog, M.; Muchiri, P.; Keraita, J. Investigating the Thermal and Mechanical Performance of Polylactic Acid (PLA) Reinforced with cellulose, wood fibers and Copolymer. IOSR J. Polym. Text. Eng. 2017, 04, 25–32. [Google Scholar] [CrossRef]
- Soriano-Cuadrado, B.; Fontecha-Cámara, M.Á.; Mañas-Villar, M.; Delgado-Blanca, I.; Ramírez-Rodríguez, M.D. Mechanical, Thermal and Morphological Study of Bio-Based PLA Composites Reinforced with Lignin-Rich Agri-Food Wastes for Their Valorization in Industry. Polymers 2024, 16, 2462. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Lei, H.; Cai, H.; Han, X.; Lin, X.; Qian, M.; Zhao, Y.; Huo, E.; Villota, E.M.; Mateo, W. Improvement on the properties of microcrystalline cellulose/polylactic acid composites by using activated biochar. J. Clean. Prod. 2020, 252, 119898. [Google Scholar] [CrossRef]
- Pairon, M.S.; Ali, F.; Anuar, H.; Ahmad, F.; Suhr, J.; Mirghani, M.E.S. Reinforcement of Polylactic acid (PLA) bio-composite with lignin from oil palm empty fruit bunches (OPEFB) for 3D printing application. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1192, 012014. [Google Scholar] [CrossRef]
- Hsueh, M.-H.; Lai, C.-J.; Liu, K.-Y.; Chung, C.-F.; Wang, S.-H.; Pan, C.-Y.; Huang, W.-C.; Hsieh, C.-H.; Zeng, Y.-S. Effects of printing temperature and filling percentage on the mechanical behavior of fused deposition molding technology components for 3D printing. Polymers 2021, 13, 2910. [Google Scholar] [CrossRef]
- Sehhat, M.H.; Mahdianikhotbesara, A.; Yadegari, F. Impact of temperature and material variation on mechanical properties of parts fabricated with fused deposition modeling (FDM) additive manufacturing. Int. J. Adv. Manuf. Technol. 2022, 120, 4791–4801. [Google Scholar] [CrossRef]
- Yang, T.-C. Effect of Extrusion Temperature on the Physico-Mechanical Properties of Unidirectional Wood Fiber-Reinforced Polylactic Acid Composite (WFRPC) Components Using Fused Deposition Modeling. Polymers 2018, 10, 976. [Google Scholar] [CrossRef]
- Abouzaid, K.; Guessasma, S.; Belhabib, S.; Bassir, D.; Chouaf, A. Printability of co-polyester using fused deposition modelling and related mechanical performance. Eur. Polym. J. 2018, 108, 262–273. [Google Scholar] [CrossRef]
- Loskot, J.; Jezbera, D.; Loskot, R.; Bušovský, D.; Barylski, A.; Glowka, K.; Duda, P.; Aniołek, K.; Voglová, K.; Zubko, M. Influence of print speed on the microstructure, morphology, and mechanical properties of 3D-printed PETG products. Polym. Test. 2023, 123, 108055. [Google Scholar] [CrossRef]
- Guessasma, S.; Belhabib, S.; Nouri, H. Understanding the microstructural role of bio-sourced 3D printed structures on the tensile performance. Polym. Test. 2019, 77, 105924. [Google Scholar] [CrossRef]
- Guessasma, S.; Belhabib, S.; Nouri, H. Microstructure and Mechanical Performance of 3D Printed Wood-PLA/PHA Using Fused Deposition Modelling: Effect of Printing Temperature. Polymers 2019, 11, 1778. [Google Scholar] [CrossRef]
- Rognoli, V.; Ayala Garcia, C. Material activism. New hybrid scenarios between design and technology. Cuad. Cent. Estud. Diseño Comun. 2019, 70, 105–115. [Google Scholar] [CrossRef]
- Ferri, J.M.; Garcia-Garcia, D.; Sánchez-Nacher, L.; Fenollar, O.; Balart, R. The effect of maleinized linseed oil (MLO) on mechanical performance of poly(lactic acid)-thermoplastic starch (PLA-TPS) blends. Carbohydr. Polym. 2016, 147, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Lage-Rivera, S.; Ares-Pernas, A.; Dopico-García, M.S.; Covas, J.; Abad, M. Comparing lignin and spent coffee grounds as bio-fillers in PLA 3D-printable filaments. Polym. Compos. 2024, 45, 14566–14579. [Google Scholar] [CrossRef]
- Radhakrishnan, S.; Khan, A.; Dwivedi, S.P.; Sharma, B.; Gupta, S.; Gupta, P.; Chaudhary, V. Studies on mechanical, thermal, and water immersion of plant and animal wastage nanofiller–based bio-fiber-reinforced composites. Biomass Convers. Biorefinery 2024, 14, 29591–29612. [Google Scholar] [CrossRef]
- Ainin, F.N.; Azaman, M.D.; Abdul Majid, M.S.; Ridzuan, M.J.M. The influence of water absorption on the mechanical performance of 3D-printed sandwich composite structures made from PLA-based materials under quasi-static loading conditions. Polym. Compos. 2024, 46, 6221–6240. [Google Scholar] [CrossRef]
- Mukoroh, P.F.; Gouda, F.; Skrifvars, M.; Ramamoorthy, S.K. Influence of the Manufacturing Method (3D Printing and Injection Molding) on Water Absorption and Mechanical and Thermal Properties of Polymer Composites Based on Poly(lactic acid). Polymers 2024, 16, 1619. [Google Scholar] [CrossRef]
- Rahman, R.; Mustapa, N.R. Water Absorption Properties of Natural Fibres Reinforced PLA Bio-Composite. In Biocomposite Materials; Hameed Sultan, M.T., Majid, M.S.A., Jamir, M.R.M., Azmi, A.I., Saba, N., Eds.; Composites Science and Technology; Springer: Singapore, 2021; pp. 251–271. [Google Scholar] [CrossRef]
- Da Silva, A.P.; Pereira, M.D.P.; Passador, F.R.; Montagna, L.S. PLA/coffee grounds composites: A study of photodegradation and biodegradation in soil. Macromol. Symp. 2020, 394, 2000091. [Google Scholar] [CrossRef]
- Lee, J.-S.; Lee, E.; Han, J. Enhancement of the water-resistance properties of an edible film prepared from mung bean starch via the incorporation of sunflower seed oil. Sci. Rep. 2020, 10, 13622. [Google Scholar] [CrossRef]
- Trongchuen, K.; Ounkaew, A.; Kasemsiri, P.; Hiziroglu, S.; Mongkolthanaruk, W.; Wannasutta, R.; Pongsa, U.; Chindaprasirt, P. Bioactive Starch Foam Composite Enriched With Natural Antioxidants from Spent Coffee Ground and Essential Oil. Starch Stärke 2018, 70, 1700238. [Google Scholar] [CrossRef]
- Gómez-Contreras, P.; Figueroa-Lopez, K.J.; Hernández-Fernández, J.; Cortés Rodríguez, M.; Ortega-Toro, R. Effect of Different Essential Oils on the Properties of Edible Coatings Based on Yam (Dioscorea rotundata L.) Starch and Its Application in Strawberry (Fragaria vesca L.) Preservation. Appl. Sci. 2021, 11, 11057. [Google Scholar] [CrossRef]
- Vianna, T.C.; Marinho, C.O.; Marangoni Júnior, L.; Ibrahim, S.A.; Vieira, R.P. Essential oils as additives in active starch-based food packaging films: A review. Int. J. Biol. Macromol. 2021, 182, 1803–1819. [Google Scholar] [CrossRef]

| Designation | Weight Content (wt%) | ||||
|---|---|---|---|---|---|
| PLA | SCG | LI | FP | EO | |
| PLA | 100 | - | - | - | - |
| SCG-5 | 95 | 5 | - | - | - |
| SCG-10 | 90 | 10 | - | - | - |
| SCG-15 | 85 | 15 | - | - | - |
| SCG-5-FP | 95 | 5 | - | 3 | - |
| SCG-10-FP | 90 | 10 | - | 3 | - |
| SCG-15-FP | 85 | 15 | - | 3 | - |
| SCG-5-EO | 95 | 5 | - | - | 3 |
| SCG-10-EO | 90 | 10 | - | - | 3 |
| SCG-15-EO | 85 | 15 | - | - | 3 |
| LI-5 | 95 | - | 5 | - | - |
| LI-10 | 90 | - | 10 | - | - |
| LI-15 | 85 | - | 15 | - | - |
| LI-15-FP | 85 | - | 15 | 3 | - |
| LI-15-EO | 85 | - | 15 | - | 3 |
| Extrusion Parameters | |
|---|---|
| Extrusion speed | 3.5–3.9 RPM |
| Fan speed | 30% |
| Filament diameter | 1.75 mm |
| Feed zone temperature | 170 °C |
| Melting zone temperature | 180 °C |
| Mixing zone temperature | 175 °C |
| Die temperature | 195 °C |
| Formulation | Carbon Residue (%) | ||||
|---|---|---|---|---|---|
| SCG | 274.54 | 248.56 | 296.66 | 314.01 | 18.30 |
| LI | 212.99 | 142.52 | 269.67 | 245.57 | 44.15 |
| FP | 394.46 | 212.37 | 397.42 | 314.24 | 0.02 |
| EO | 125.66 | 65.04 | 114.09 | 190.31 | 0 |
| PLA | 342.88 | 332.58 | 346.63 | 370.88 | 00 |
| SCG-5 | 333.07 | 313.08 | 334.89 | 360.79 | 2.5 |
| SCG-10 | 329.41 | 309.53 | 332.25 | 358.40 | 3.3 |
| SCG-15 | 309.13 | 288.16 | 313.72 | 339.36 | 5.49 |
| SCG-5-FP | 336.42 | 311.92 | 336.72 | 363.62 | 1.9 |
| SCG-10-FP | 330.95 | 303.74 | 331.86 | 358.94 | 2.6 |
| SCG-15-FP | 304.04 | 276.08 | 301.63 | 324.35 | 4.08 |
| SCG-5-EO | 323.13 | 297.83 | 324.29 | 350.10 | 2.1 |
| SCG-10-EO | 319.30 | 290.48 | 320.45 | 346.75 | 3.91 |
| SCG-15-EO | 306.86 | 283.18 | 310.66 | 336.63 | 3.68 |
| LI-5 | 328.31 | 301.58 | 332.81 | 364.31 | 4.32 |
| LI-10 | 312.09 | 293.91 | 314.89 | 341.93 | 6.76 |
| LI-15 | 285.10 | 267.18 | 290.23 | 310.12 | 11.76 |
| LI-15-FP | 283.05 | 263.13 | 286.94 | 289.94 | 10.03 |
| LI-15-EO | 279.98 | 258.64 | 282.41 | 282.12 | 8.54 |
| Formulation | (J/g) | (J/g) | ||||||
|---|---|---|---|---|---|---|---|---|
| PLA | 64.3 | 119.97 | 34.77 | 177.02 | 3.28 | 33.63 | - | - |
| SCG-5 | 63.71 | 99.93 | 15.2 | 179.18 | 31.75 | 35.93 | 93.27 | 5.02 |
| SCG-10 | 63.5 | 99.89 | 11.12 | 179.04 | 30.37 | 36.28 | 94.19 | 8.13 |
| SCG-15 | 62.17 | 101.07 | 18.02 | 178.58 | 30.03 | 37.98 | 94.74 | 7.96 |
| SCG-5-FP | 62.49 | 100.07 | 10.29 | 176.96 | 32.8 | 37.12 | 97.06 | 14.3 |
| SCG-10-FP | 62.6 | 100.61 | 4.76 | 178.49 | 30.69 | 36.66 | 98.61 | 17.79 |
| SCG-15-FP | 61.58 | 99.92 | 10.25 | 175.22 | 33.67 | 42.59 | 97.63 | 14.36 |
| SCG-5-EO | 61.42 | 99.63 | 5.85 | 176.45 | 33.82 | 38.27 | 99.11 | 12.11 |
| SCG-10-EO | 61.71 | 100.79 | 18.75 | 177.93 | 34.15 | 40.80 | 95.24 | 7.84 |
| SCG-15-EO | 61.08 | 101.01 | 17.62 | 176.61 | 34.38 | 43.49 | 96.1 | 9.19 |
| LI-5 | 63.43 | 107.3 | 25.27 | 180.46 | 29.91 | 33.85 | - | - |
| LI-10 | 63.85 | 106.89 | 28.93 | 181.74 | 29.48 | 35.22 | - | - |
| LI-15 | 62.99 | 107.36 | 23.14 | 178.73 | 27.77 | 35.12 | - | - |
| LI-15-FP | 62.48 | 102.02 | 20.41 | 177.14 | 29.01 | 36.69 | - | - |
| LI-15-EO | 62.12 | 105.14 | 20.15 | 177.75 | 32.14 | 40.65 | - | - |
| Tensile | Flexural | Impact | |||||
|---|---|---|---|---|---|---|---|
| Formulation | Tensile Modulus (GPa) | Tensile Strength (MPa) | Strain (%) | Flexural Modulus (GPa) | Flexural Strength (MPa) | Elongation at Break (%) | Impact Strength (KJ/m2) |
| PLA | 4.73 | 47.57 | 2.01 | 3.12 | 90.34 | 5.16 | 15.47 |
| SCG-5 | 3.77 | 43.79 | 2.88 | 2.84 | 73.75 | 5.39 | 16.28 |
| SCG-10 | 3.32 | 37.03 | 3.78 | 2.33 | 48.07 | 6.55 | 12.17 |
| SCG-15 | 3.09 | 33.62 | 4.14 | 1.95 | 37.04 | 5.32 | 10.01 |
| SCG-5-FP | 3.26 | 25.44 | 7.02 | 2.66 | 48.58 | 8.28 | 16.04 |
| SCG-10-FP | 3.07 | 21.95 | 5.32 | 2.08 | 42.05 | 6.56 | 12.29 |
| SCG-15-FP | 2.68 | 19.81 | 5.06 | 1.81 | 36.34 | 5.49 | 9.55 |
| SCG-5-EO | 3.5 | 26.01 | 5.13 | 2.35 | 50.19 | 8.91 | 15.88 |
| SCG-10-EO | 3.21 | 22.68 | 4.99 | 1.89 | 45.12 | 7.44 | 10.95 |
| SCG-15-EO | 3.06 | 20.89 | 4.2 | 1.74 | 31.44 | 5.78 | 9.61 |
| LI-5 | 5.19 | 46.03 | 1.23 | 3.89 | 81.89 | 3.84 | 13.51 |
| LI-10 | 5.04 | 42.49 | 1.09 | 3.59 | 78.59 | 3.33 | 12.43 |
| LI-15 | 4.11 | 31.08 | 0.78 | 2.98 | 65.98 | 2.91 | 10.38 |
| LI-15-FP | 3.25 | 22.28 | 0.88 | 2.62 | 51.42 | 3.06 | 7.03 |
| LI-15-EO | 3.72 | 21.73 | 0.81 | 2.28 | 55.58 | 3.12 | 6.74 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siala, Z.; Koubaa, A.; Guessasma, S.; Stephant, N.; Elloumi, A.; Beauregard, M. Development and Characterization of Scented PLA-Based Biocomposites Reinforced with Spent Coffee Grounds and Lignin for FDM 3D Printing. Polymers 2025, 17, 2836. https://doi.org/10.3390/polym17212836
Siala Z, Koubaa A, Guessasma S, Stephant N, Elloumi A, Beauregard M. Development and Characterization of Scented PLA-Based Biocomposites Reinforced with Spent Coffee Grounds and Lignin for FDM 3D Printing. Polymers. 2025; 17(21):2836. https://doi.org/10.3390/polym17212836
Chicago/Turabian StyleSiala, Zeineb, Ahmed Koubaa, Sofiane Guessasma, Nicolas Stephant, Ahmed Elloumi, and Martin Beauregard. 2025. "Development and Characterization of Scented PLA-Based Biocomposites Reinforced with Spent Coffee Grounds and Lignin for FDM 3D Printing" Polymers 17, no. 21: 2836. https://doi.org/10.3390/polym17212836
APA StyleSiala, Z., Koubaa, A., Guessasma, S., Stephant, N., Elloumi, A., & Beauregard, M. (2025). Development and Characterization of Scented PLA-Based Biocomposites Reinforced with Spent Coffee Grounds and Lignin for FDM 3D Printing. Polymers, 17(21), 2836. https://doi.org/10.3390/polym17212836









