Fully Biobased Biodegradable Elastomeric Polymer Blends Based on PHAs
Abstract
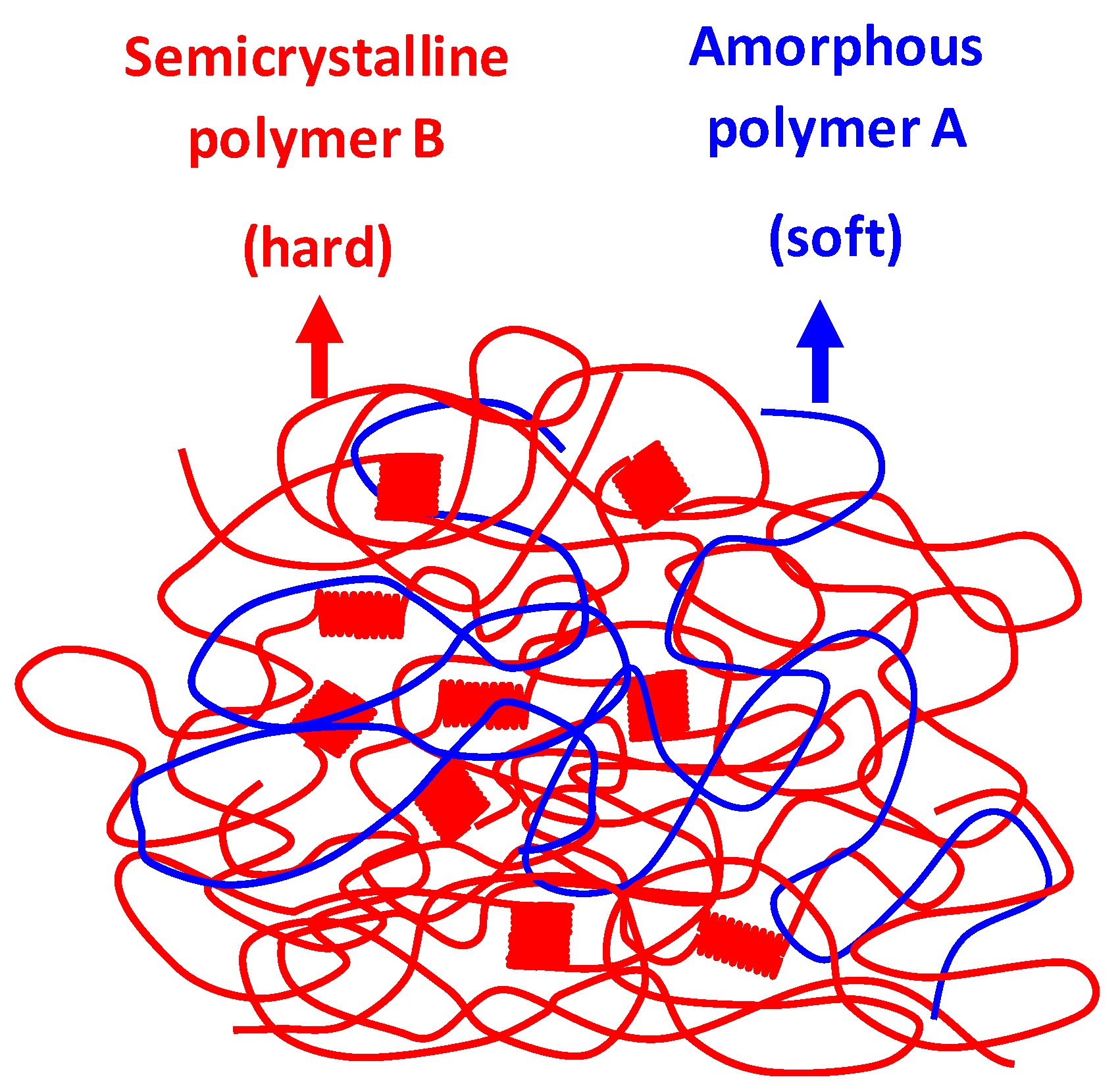
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Blend Preparation
2.2.2. Procedure for the Preparation of Monofilaments
2.2.3. Procedure for Drawing Monofilaments to the Maximum Drawing Ratio
- is maximum length of drawn sample
- initial length of sample before drawing
- Value = 1 means that sample was not possible to stretch.
2.2.4. Rheological Properties
2.2.5. Thermal Properties (DSC)
- Isothermal hold at −70 °C for 3 min;
- First heating cycle: −70 °C to +200 °C at a heating rate of 10 °C/min;
- Isothermal hold at +200 °C for 3 min;
- Cooling cycle: +200 °C to −70 °C at a cooling rate of 10 °C/min;
- Isothermal hold at −70 °C for 3 min;
- Second heating cycle: −70 °C to +200 °C at a heating rate of 10 °C/min.
2.2.6. Scanning Electron Microscopy (SEM)
2.2.7. Mechanical Properties Measurement
Tensile Test
Hardness Testing
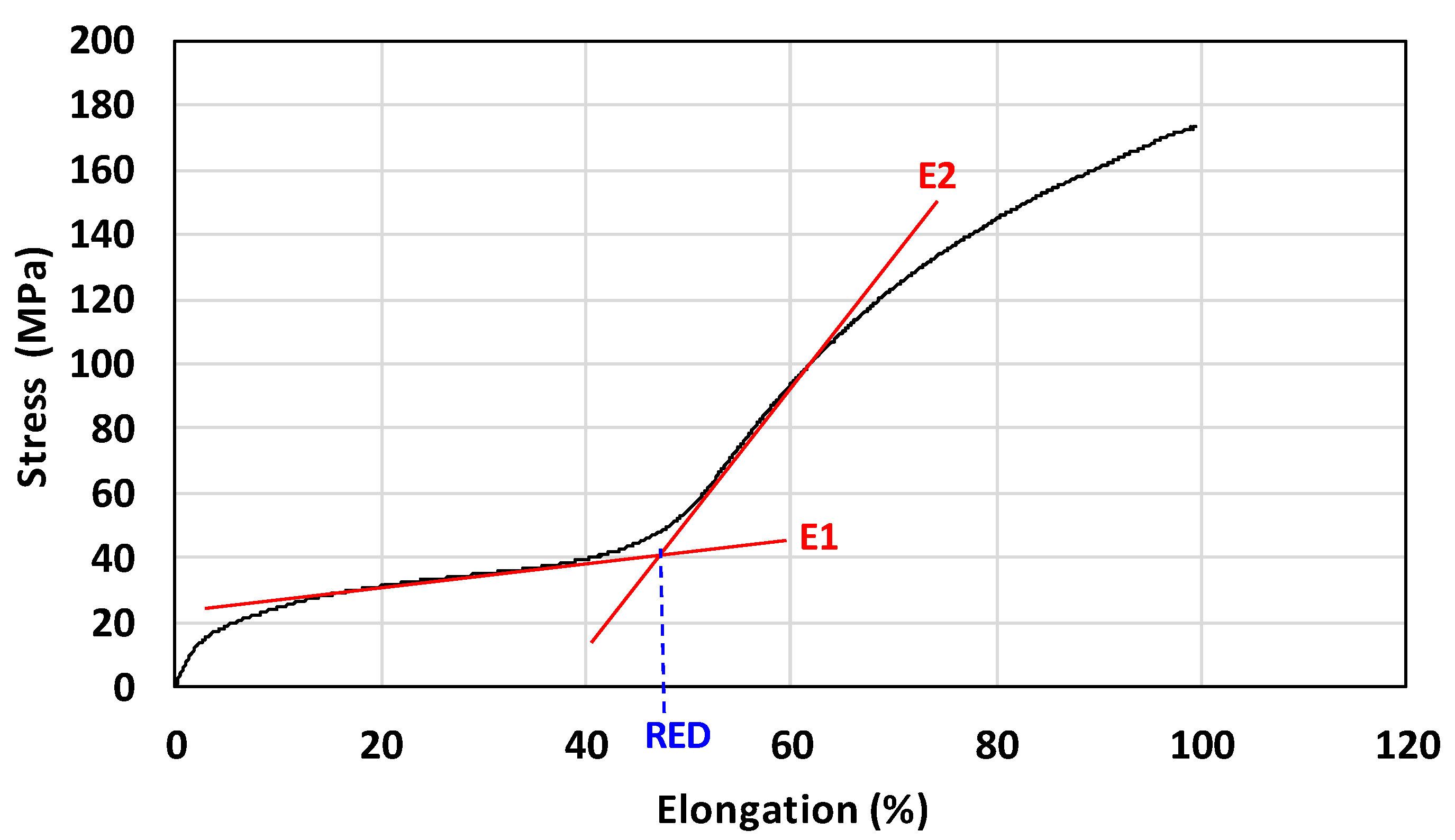
Determination of Elastically Reversible Deformation on Non-Stretched Specimen
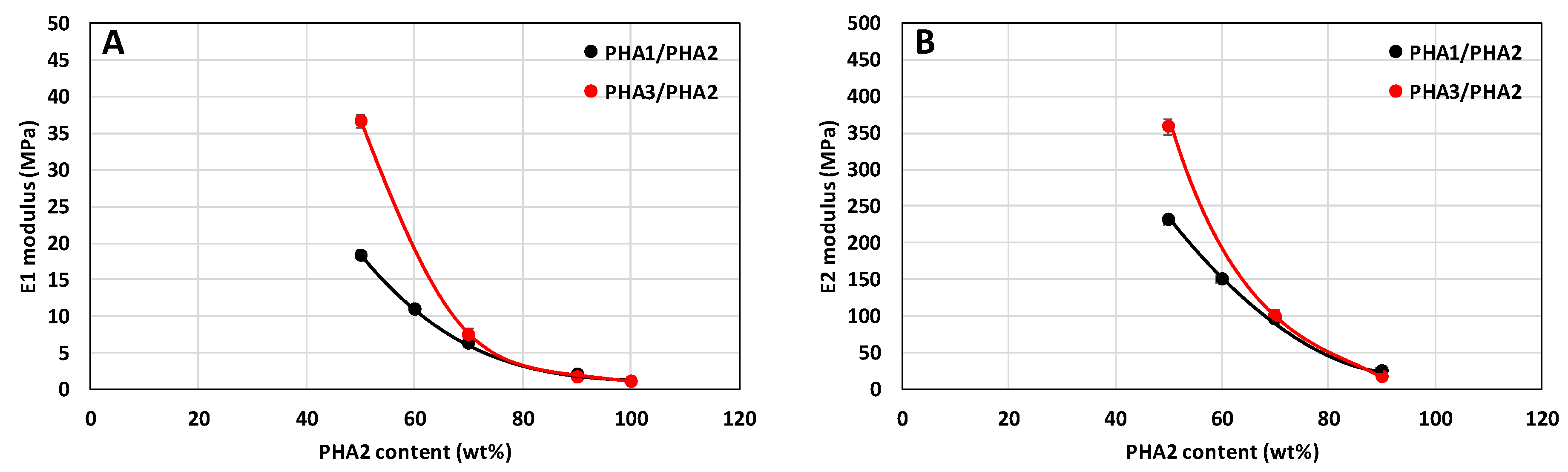
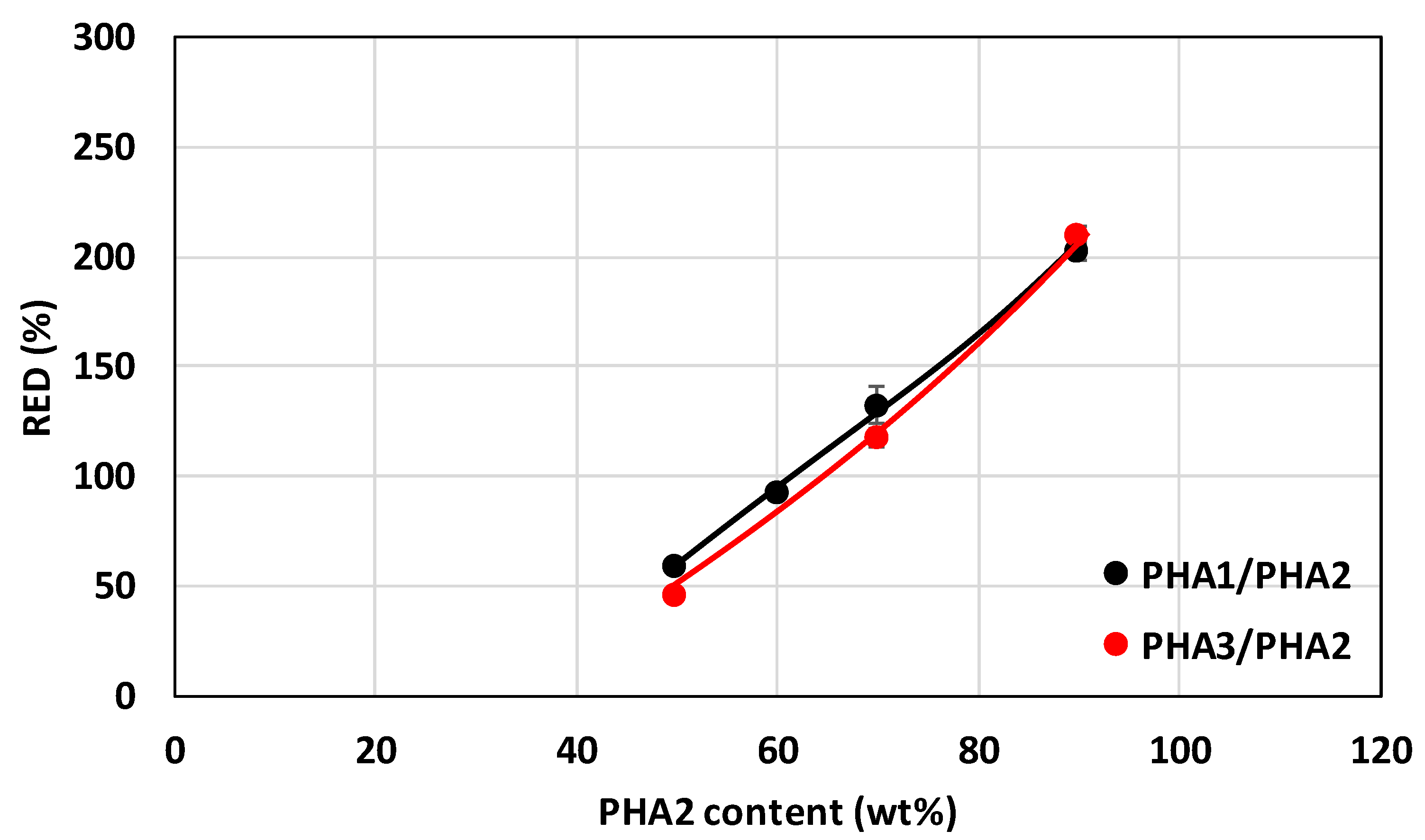
Determination of the Range of Elastically Reversible Deformation (RED) in Tension and E1 and E2 Moduli
Preparation of Test Specimens by Injection Molding
- Injection volume: 18 cm3;
- Injection speed: 10 cm3/s;
- Injection pressure: 1400 bar;
- Holding pressure: 1200 bar;
- Holding time: 10 s;
- Cooling time: 15 s;
- Mould temperature: 50 °C;
- Barrel temperature profile (from hopper to nozzle): 160–170–175–170–170 °C.
2.2.8. Determination of Surface Energy of Polymers
2.2.9. Wide-Angle X-Ray Scattering
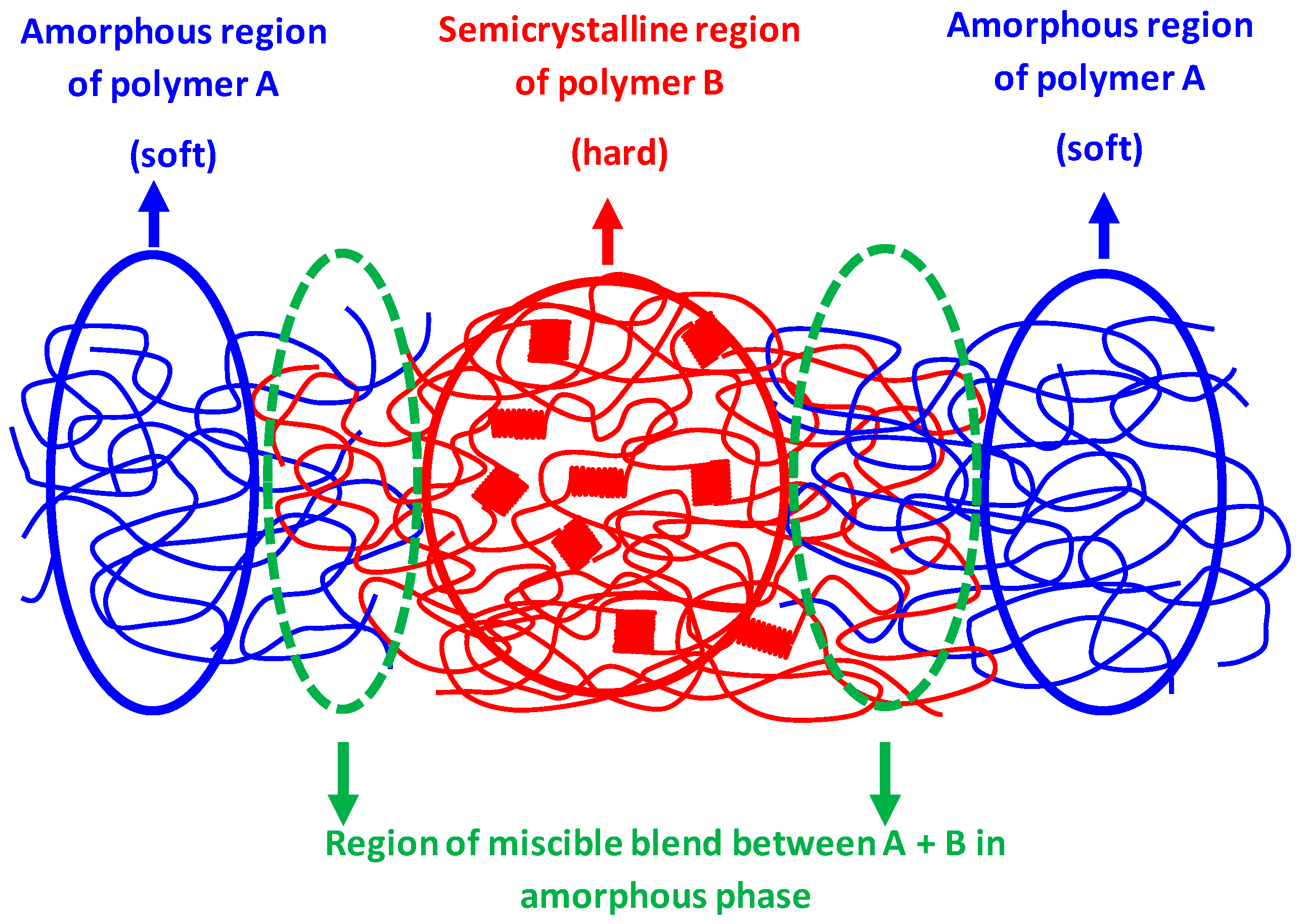
3. Results and Discussion
- (a)
- The blends must exhibit a two-phase morphological structure;
- (b)
- The polymers must demonstrate partial miscibility in the amorphous phase.
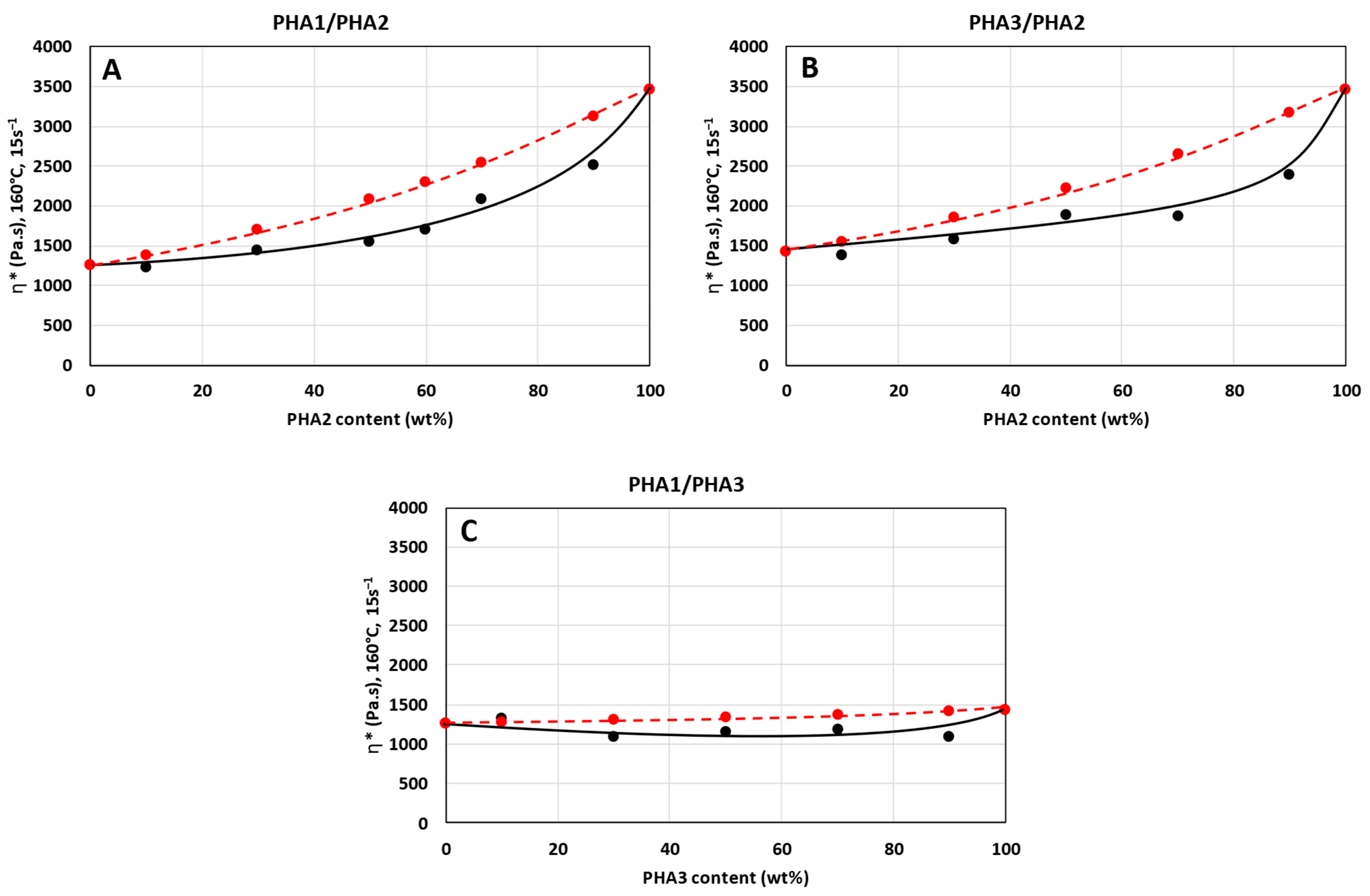
3.1. Rheology
- —viscosity of the polymer blend.
- —viscosity of component a.
- —viscosity of component b.
- , —volume fractions of components a and b.
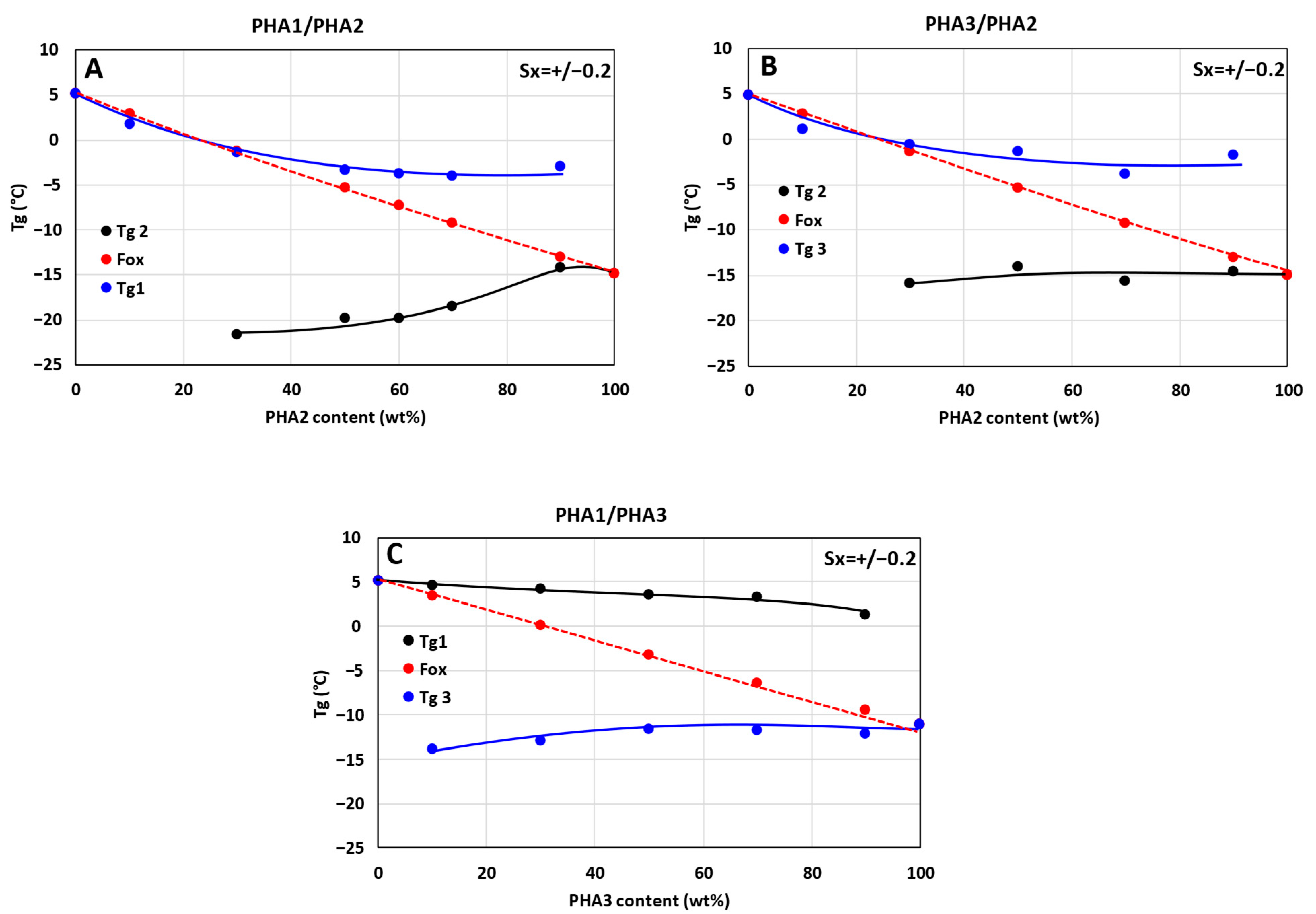
3.2. Differential Scanning Calorimetry
- w1, w2—weight fractions of the polymers.
- , —glass transition temperatures of the polymers in Kelvin.
3.3. Mechanical Properties
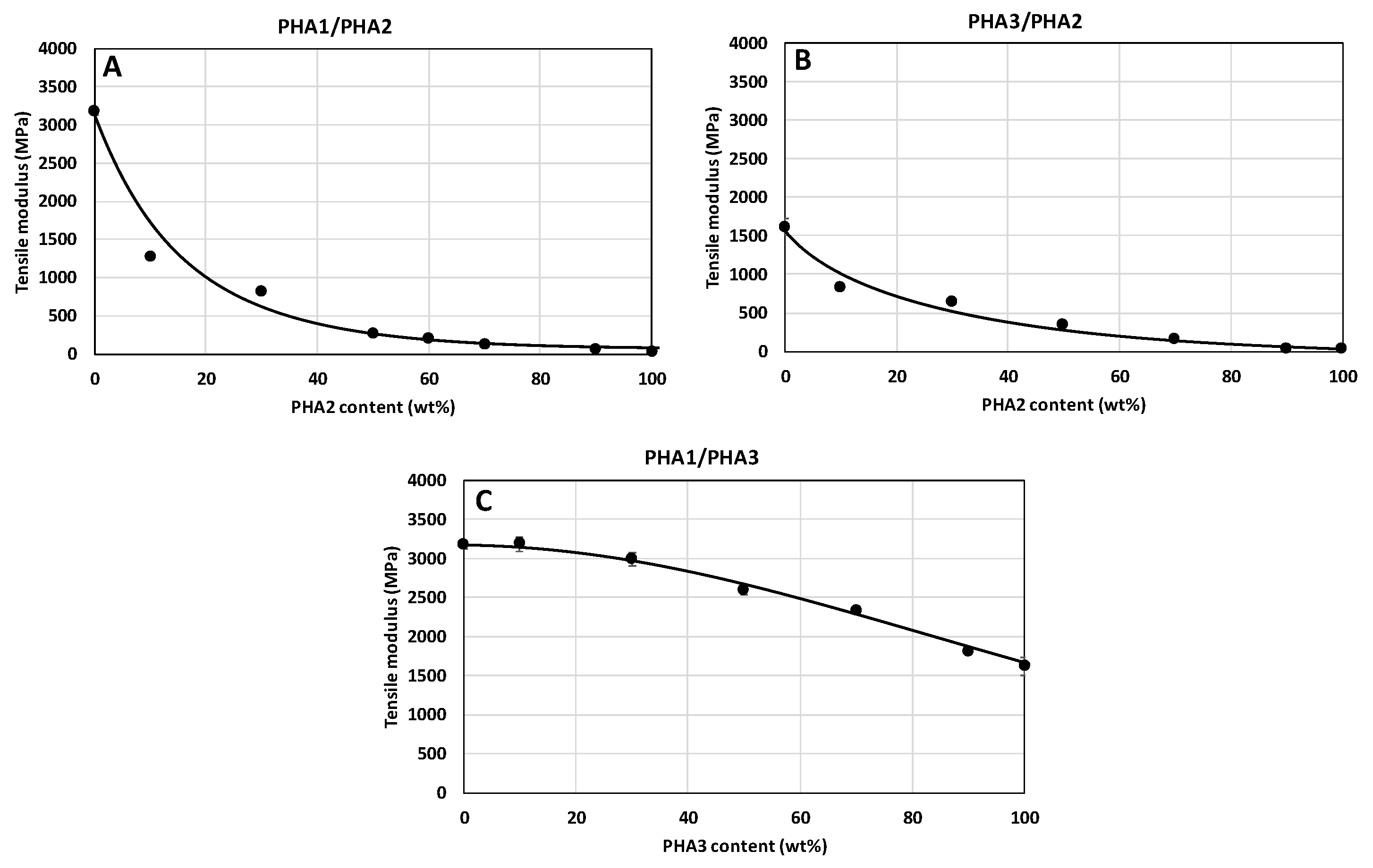
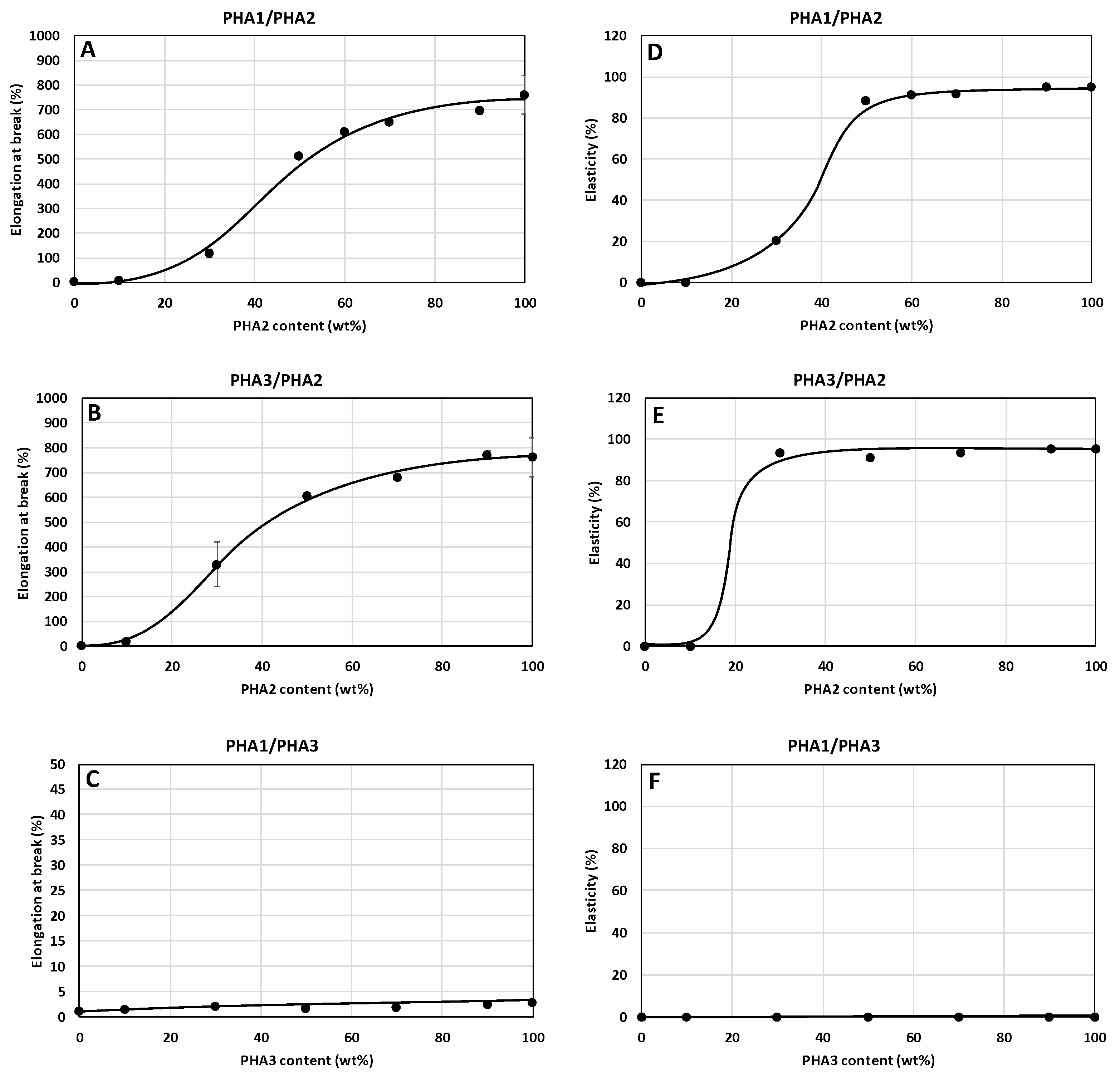
3.3.1. Mechanical Properties of Non-Stretched Samples
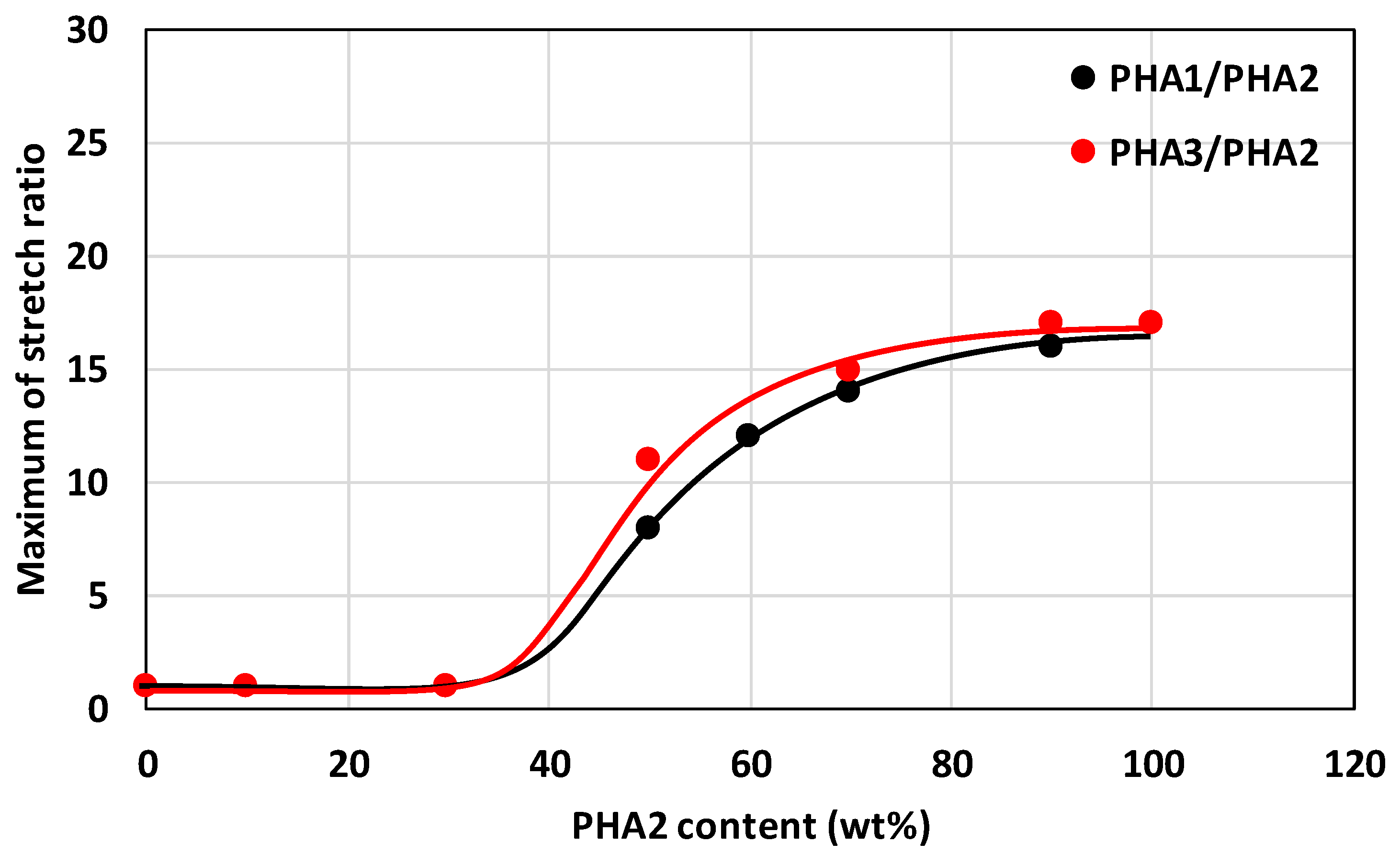
3.3.2. Mechanical Properties of Stretched Samples
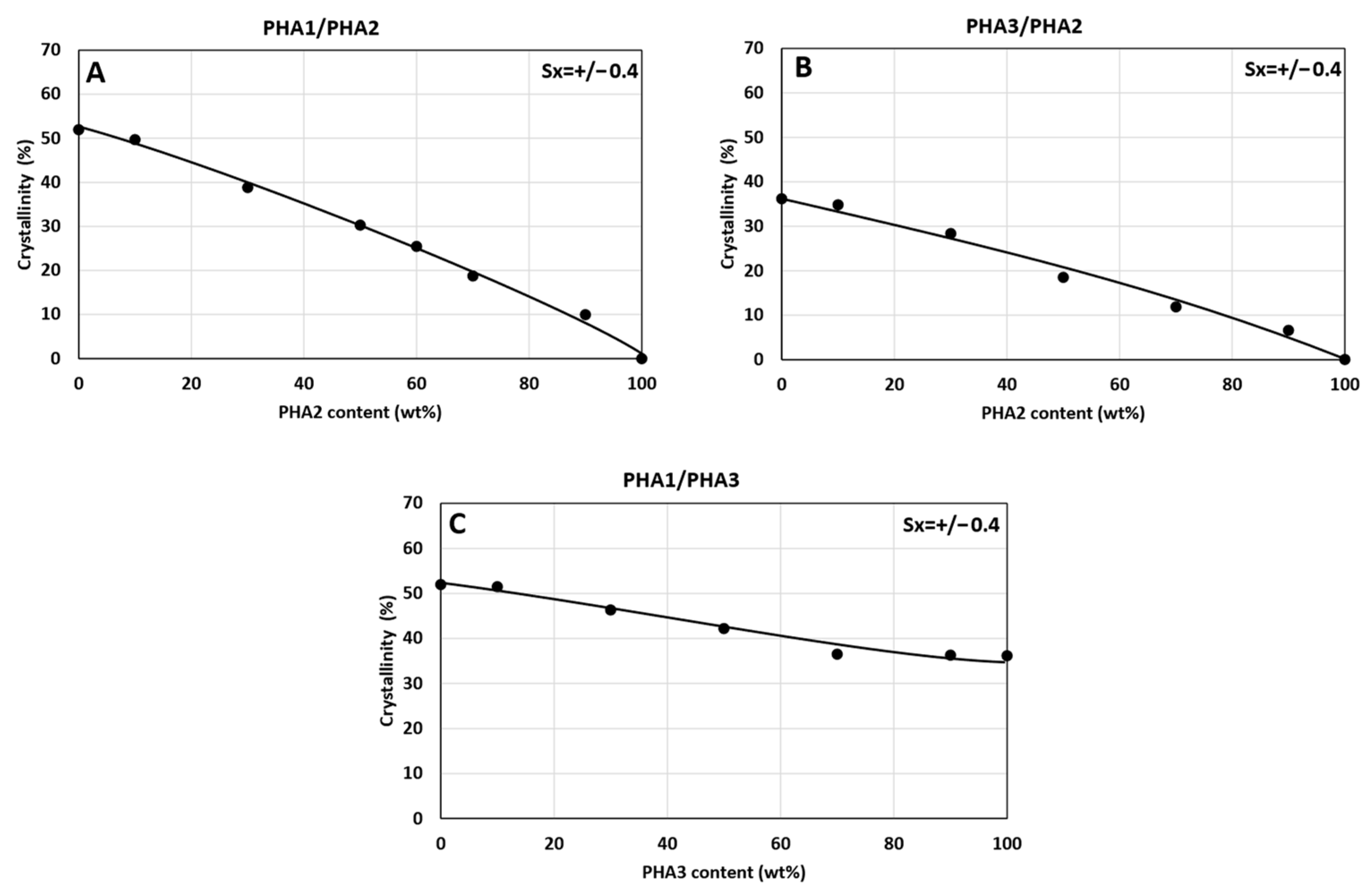
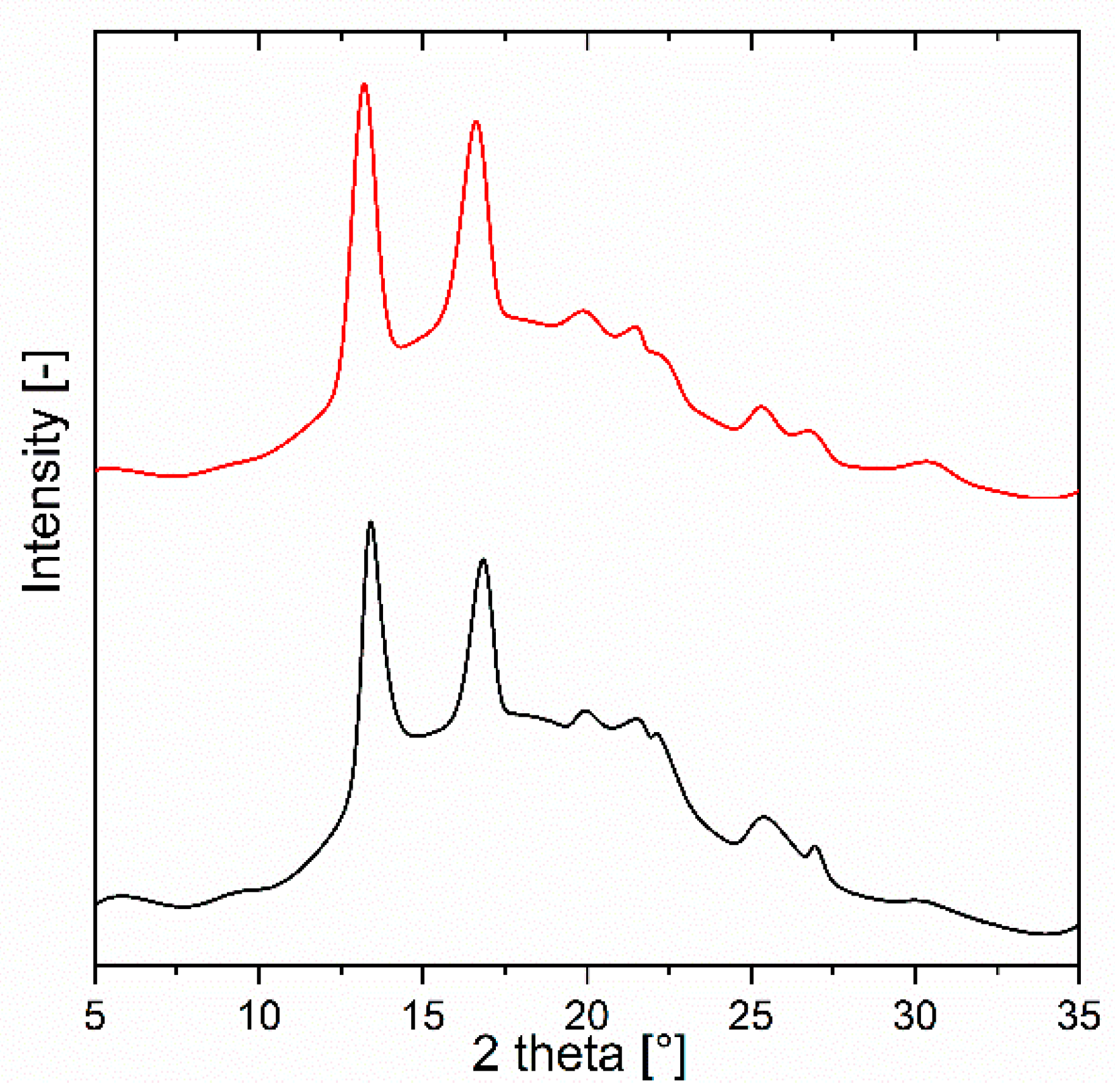
3.4. Wide-Angle X-Ray Scattering
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Osman, A.I.; Hosny, M.; Eltaweil, A.S.; Omar, S.; Elgarahy, A.M.; Farghali, M.; Yap, P.-S.; Wu, Y.-S.; Nagandran, S.; Batumalaie, K.; et al. Microplastic sources, formation, toxicity and remediation: A review. Environ. Chem. Lett. 2023, 21, 2129–2169. [Google Scholar] [CrossRef]
- Surendran, D.; Sakai, H.; Takagi, S.; Dimapilis, D.A. Tire-based microplastics: Composition, detection, and impacts of advanced oxidation processes in drinking water treatment. Sci. Total. Environ. 2025, 972, 179114. [Google Scholar] [CrossRef]
- Mayer, P.M.; Moran, K.D.; Miller, E.L.; Brander, S.M.; Harper, S.; Garcia-Jaramillo, M.; Carrasco-Navarro, V.; Ho, K.T.; Burgess, R.M.; Hampton, L.M.T.; et al. Where the rubber meets the road: Emerging environmental impacts of tire wear particles and their chemical cocktails. Sci. Total. Environ. 2024, 927, 171153. [Google Scholar] [CrossRef]
- Novakovic, K.; Thumbarathy, D.; Peeters, M.; Geoghegan, M.; Jefferies, J.G.; Hicks, C.; Manika, D.; Dai, S. Zero-waste circular economy of plastic packaging: The bottlenecks and a way forward. Sustain. Mater. Technol. 2023, 38, e00735. [Google Scholar] [CrossRef]
- Quattrosoldi, S.; Soccio, M.; Gazzano, M.; Lotti, N.; Munari, A. Fully biobased, elastomeric and compostable random copolyesters of poly(butylene succinate) containing Pripol 1009 moieties: Structure-property relationship. Polym. Degrad. Stab. 2020, 178, 109189. [Google Scholar] [CrossRef]
- Rogovina, S.; Lomakin, S.; Usachev, S.; Yakhina, A.; Zhorina, L.; Berlin, A. Thermal Behavior of Biodegradable Compositions of Polylactide and Poly(3-hydroxybutyrate) with Chitosan and the Effect of UV Radiation on Their Structure. Appl. Sci. 2023, 13, 3920. [Google Scholar] [CrossRef]
- Cai, K.; Liu, X.; Ma, X.; Zhang, J.; Tu, S.; Feng, J. Preparation of biodegradable PLA/PBAT blends with balanced toughness and strength by dynamic vulcanization process. Polymer 2023, 291, 126587. [Google Scholar] [CrossRef]
- Fernandes, M.; Salvador, A.F.; Vicente, A.A. Biodegradation of PHB/PBAT films and isolation of novel PBAT biodegraders from soil microbiomes. Chemosphere 2024, 362, 142696. [Google Scholar] [CrossRef] [PubMed]
- Fredi, G.; Dorigato, A. Compatibilization of biopolymer blends: A review. Adv. Ind. Eng. Polym. Res. 2024, 7, 373–404. [Google Scholar] [CrossRef]
- Matta, A.; Rao, R.U.; Suman, K.; Rambabu, V. Preparation and Characterization of Biodegradable PLA/PCL Polymeric Blends. Procedia Mater. Sci. 2014, 6, 1266–1270. [Google Scholar] [CrossRef]
- Eryildiz, M.; Karakus, A.; Eksi, M.A. Development and Characterization of PLA/PCL Blend Filaments and 3D Printed Scaffolds. J. Mater. Eng. Perform. 2025, 34, 14043–14054. [Google Scholar] [CrossRef]
- Gundlapalli, M.; Ganesan, S. Polyhydroxyalkanoates (PHAs): Key Challenges in production and sustainable strategies for cost reduction within a circular economy framework. Results Eng. 2025, 26, 105345. [Google Scholar] [CrossRef]
- Kovalcik, A.; Smilek, J.; Machovsky, M.; Kalina, M.; Enev, V.; Dugova, H.; Cernekova, N.; Kovacova, M.; Spitalsky, Z. Properties and structure of poly(3-hydroxybutyrate-co-4-hydroxybutyrate) filaments for fused deposition modelling. Int. J. Biol. Macromol. 2021, 183, 880–889. [Google Scholar] [CrossRef]
- Wang, Y.-W.; Mo, W.; Yao, H.; Wu, Q.; Chen, J.; Chen, G.-Q. Biodegradation studies of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate). Polym. Degrad. Stab. 2004, 85, 815–821. [Google Scholar] [CrossRef]
- Luo, R.; Xu, K.; Chen, G. Study of miscibility, crystallization, mechanical properties, and thermal stability of blends of poly(3-hydroxybutyrate) and poly(3-hydroxybutyrate-co-4-hydroxybutyrate). J. Appl. Polym. Sci. 2007, 105, 3402–3408. [Google Scholar] [CrossRef]
- Brutman, J.P.; Schneiderman, D.K.; Hillmyer, M.A.; Hoe, G.X.D. Methods for Making Renewable and Chemically Recyclable Crosslinked Polyester Elastomers. US10808084B2, 20 October 2020. Available online: https://patents.google.com/patent/US10808084B2/en (accessed on 24 September 2025).
- Croom, A.K.; Lerum, R.V.; Gormley, J. Bio-Based and Biodegradable Elastomer for Cosmetic and Personal Care. US11534389B2, 27 December 2022. Available online: https://patents.google.com/patent/US11534389B2/en (accessed on 24 September 2025).
- Krishnaswamy, R.K.; Walsem, J.V.; Peoples, O.P.; Shabtai, Y.; Padwa, A.R. Biobased Rubber Modified Biodegradable Polymer Blends. US10113060B2, 30 October 2018. Available online: https://patents.google.com/patent/US10113060B2/en (accessed on 24 September 2025).
- Venkatraman, S.; Abadie, M.; Widjaja, L.K.; Llpik, V. Biodegradable Thermoplastic Elastomers. US8716410B2, 6 May 2014. Available online: https://patents.google.com/patent/US8716410B2/en?oq=Biodegradable+thermoplastic+elastomers+us+8%2c716%2c410+b2 (accessed on 24 September 2025).
- Zhou, C.; Wei, Z.; Jin, C.; Wang, Y.; Yu, Y.; Leng, X.; Li, Y. Fully biobased thermoplastic elastomers: Synthesis of highly branched linear comb poly(β-myrcene)-graft-poly(l-lactide) copolymers with tunable mechanical properties. Polymer 2018, 138, 57–64. [Google Scholar] [CrossRef]
- Abidin, A.Z.; Zango, Z.U.; Akhdar, H.; Aldaghri, O.; Ibnaouf, K.H.; Sulieman, A.; Lim, J.W.; Ng, H.-S.; Khoo, K.S. Sustainable grafting of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) and natural rubber into a new thermoplastic elastomer film. Polymer 2023, 289, 126476. [Google Scholar] [CrossRef]
- Ning, N.; Li, S.; Wu, H.; Tian, H.; Yao, P.; Hu, G.-H.; Tian, M.; Zhang, L. Preparation, microstructure, and microstructure-properties relationship of thermoplastic vulcanizates (TPVs): A review. Prog. Polym. Sci. 2018, 79, 61–97. [Google Scholar] [CrossRef]
- Zheng, M.; Zhang, S.; Chen, Y.; Wu, Q.; Li, Q.; Wang, S. Structure evolution of bio-based PLA/ENR thermoplastic vulcanizates during dynamic vulcanization processing. Polym. Test. 2020, 82, 106324. [Google Scholar] [CrossRef]
- Si, W.-J.; Yuan, W.-Q.; Li, Y.-D.; Chen, Y.-K.; Zeng, J.-B. Tailoring toughness of fully biobased poly(lactic acid)/natural rubber blends through dynamic vulcanization. Polym. Test. 2018, 65, 249–255. [Google Scholar] [CrossRef]
- Li, D.-F.; Zhao, X.; Jia, Y.-W.; He, L.; Wang, X.-L.; Wang, Y.-Z. Dual effect of dynamic vulcanization of biobased unsaturated polyester: Simultaneously enhance the toughness and fire safety of Poly(lactic acid). Compos. Part B Eng. 2019, 175, 107069. [Google Scholar] [CrossRef]
- Shou, T.; Dong, Q.; Yin, D.; Hu, S.; Zhao, X.; Zhang, L. Super-tough polylactic acid blends via tunable dynamic vulcanization of biobased polyurethanes. Compos. Part B Eng. 2024, 276, 111363. [Google Scholar] [CrossRef]
- Kang, H.; Hu, X.; Li, M.; Zhang, L.; Wu, Y.; Ning, N.; Tian, M. Novel biobased thermoplastic elastomer consisting of synthetic polyester elastomer and polylactide by in situ dynamical crosslinking method. RSC Adv. 2015, 5, 23498–23507. [Google Scholar] [CrossRef]
- Banerjee, S.S.; Bhowmick, A.K. Dynamic vulcanization of novel nanostructured polyamide 6/fluoroelastomer thermoplastic elastomeric blends with special reference to morphology, physical properties and degree of vulcanization. Polymer 2015, 57, 105–116. [Google Scholar] [CrossRef]
- Qin, S.-X.; Yu, C.-X.; Chen, X.-Y.; Zhou, H.-P.; Zhao, L.-F. Fully Biodegradable Poly(lactic acid)/Poly(propylene carbonate) Shape Memory Materials with Low Recovery Temperature Based on in situ Compatibilization by Dicumyl Peroxide. Chin. J. Polym. Sci. 2018, 36, 783–790. [Google Scholar] [CrossRef]
- Valerio, O.; Misra, M.; Mohanty, A.K. Sustainable biobased blends of poly(lactic acid) (PLA) and poly(glycerol succinate-co-maleate) (PGSMA) with balanced performance prepared by dynamic vulcanization. RSC Adv. 2017, 7, 38594–38603. [Google Scholar] [CrossRef]
- Choi, J.-H.; Woo, J.-J.; Kim, I. Sustainable Polycaprolactone Polyol-Based Thermoplastic Poly(ester ester) Elastomers Showing Superior Mechanical Properties and Biodegradability. Polymers 2023, 15, 3209. [Google Scholar] [CrossRef] [PubMed]
- Tejani, J.G. Thermoplastic Elastomers: Emerging Trends and Applications in Rubber Manufacturing. Glob. Discl. Econ. Bus. 2017, 6, 133–144. [Google Scholar] [CrossRef]
- Pebax® Elastomer Family|Arkema High Performance Polymers. Available online: https://hpp.arkema.com/en/product-families/pebax-elastomer-family/ (accessed on 24 September 2025).
- Sheth, J.P.; Xu, J.; Wilkes, G.L. Solid state structure–property behavior of semicrystalline poly(ether-block-amide) PEBAX® thermoplastic elastomers. Polymer 2003, 44, 743–756. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Liu, A.; Xu, C.; Zhang, C.; Yu, J.; Yuan, R.; Li, F. Poly(trimethylene terephthalate-b-poly(trimethylene ether) glycol) copolymers: From bio-based thermoplastic elastomers to elastic fibers for apparel. Eur. Polym. J. 2025, 225, 113706. [Google Scholar] [CrossRef]
- Koo, J.M.; Kang, J.; Shin, S.-H.; Jegal, J.; Gil Cha, H.; Choy, S.; Hakkarainen, M.; Park, J.; Oh, D.X.; Hwang, S.Y. Biobased thermoplastic elastomer with seamless 3D-Printability and superior mechanical properties empowered by in-situ polymerization in the presence of nanocellulose. Compos. Sci. Technol. 2020, 185, 107885. [Google Scholar] [CrossRef]
- Ďurfina, M.; Babaei, N.; Vanovčanová, Z.; Feranc, J.; Horváth, V.; Vašková, I.; Kruželák, J.; Tomanová, K.; Plavec, R. Bio-Based Polyhydroxyalkanoate (PHA) Blends for 3D Printing: Rheological, Mechanical, Biocompatibility, and Biodegradation Properties. Polymers 2025, 17, 1477. [Google Scholar] [CrossRef]
- Kalita, U.; Parameswaran, B.; Singha, N. Thermoplastic Elastomers, ResearchGate. Available online: https://www.researchgate.net/publication/367022965_Thermoplastic_Elastomers (accessed on 24 September 2025).
- ISO 527-1:2019; Plastics—Determination of Tensile Properties. ISO: Geneva, Switzerland, 2019. Available online: https://www.iso.org/standard/527-1 (accessed on 25 September 2025).
- ISO 868:2003; Plastics and Ebonite—Determination of Indentation Hardness by Means of a Durometer (Shore Hardness). ISO: Geneva, Switzerland, 2003. Available online: https://www.iso.org/standard/34804.html (accessed on 25 September 2025).
- Liu, H.; Ma, J.; Zhu, C.; Xu, J.; Gong, J. Miscibility and Crystallization Behavior of Poly (Ethylene Terephthalate)/Phosphate Glass Hybrids. J. Macromol. Sci. Part B 2016, 55, 1039–1050. [Google Scholar] [CrossRef]
- Jańczuk, B.; Wójcik, W.; Zdziennicka, A. Determination of the Components of the Surface Tension of Some Liquids from Interfacial Liquid-Liquid Tension Measurements. J. Colloid Interface Sci. 1993, 157, 384–393. [Google Scholar] [CrossRef]
- Koning, C.; Van Duin, M.; Pagnoulle, C.; Jerome, R. Strategies for compatibilization of polymer blends. Prog. Polym. Sci. 1998, 23, 707–757. [Google Scholar] [CrossRef]
- Liu, C.; Wang, J.; He, J. Rheological and thermal properties of m-LLDPE blends with m-HDPE and LDPE. Polymer 2002, 43, 3811–3818. [Google Scholar] [CrossRef]
- Charon, G.; Peixinho, J.; Michely, L.; Guinault, A.; Langlois, V. Rosin natural terpenes as processing aid for polyhydroxyalkanoate: Thermal, mechanical, and viscoelastic properties. J. Appl. Polym. Sci. 2022, 139, e53052. [Google Scholar] [CrossRef]
- Bugnicourt, E.; Cinelli, P.; Lazzeri, A.; Alvarez, V. Polyhydroxyalkanoate (PHA): Review of synthesis, characteristics, processing and potential applications in packaging. Express Polym. Lett. 2014, 8, 791–808. [Google Scholar] [CrossRef]
- Brostow, W.; Chiu, R.; Kalogeras, I.M.; Vassilikou-Dova, A. Prediction of glass transition temperatures: Binary blends and copolymers. Mater. Lett. 2008, 62, 3152–3155. [Google Scholar] [CrossRef]
- Sudesh, K.; Abe, H.; Doi, Y. Synthesis, structure and properties of polyhydroxyalkanoates: Biological polyesters. Prog. Polym. Sci. 2000, 25, 1503–1555. [Google Scholar] [CrossRef]
- Barham, P.J.; Keller, A. The relationship between microstructure and mode of fracture in polyhydroxybutyrate. J. Polym. Sci. Polym. Phys. Ed. 1986, 24, 69–77. [Google Scholar] [CrossRef]
- El-Hadi, A.; Schnabel, R.; Straube, E.; Müller, G.; Henning, S. Correlation between degree of crystallinity, morphology, glass temperature, mechanical properties and biodegradation of poly (3-hydroxyalkanoate) PHAs and their blends. Polym. Test. 2002, 21, 665–674. [Google Scholar] [CrossRef]
- Doi, Y.; Kitamura, S.; Abe, H. Microbial Synthesis and Characterization of Poly(3-hydroxybutyrate-co-3-hydroxyhexanoate). Macromolecules 1995, 28, 4822–4828. [Google Scholar] [CrossRef]
- Volova, T.G.; Uspenskaya, M.V.; Kiselev, E.G.; Sukovatyi, A.G.; Zhila, N.O.; Vasiliev, A.D.; Shishatskaya, E.I. Effect of Monomers of 3-Hydroxyhexanoate on Properties of Copolymers Poly(3-Hydroxybutyrate-co 3-Hydroxyhexanoate). Polymers 2023, 15, 2890. [Google Scholar] [CrossRef]
- Furutate, S.; Kamoi, J.; Nomura, C.T.; Taguchi, S.; Abe, H.; Tsuge, T. Superior thermal stability and fast crystallization behavior of a novel, biodegradable α-methylated bacterial polyester. NPG Asia Mater. 2021, 13, 31. [Google Scholar] [CrossRef]
- Majka, T.M.; Raftopoulos, K.N.; Hebda, E.; Szeligowski, A.; Zastawny, O.; Guzik, M.; Pielichowski, K. PHB+aPHA Blends: From Polymer Bacterial Synthesis through Blend Preparation to Final Processing by Extrusion for Sustainable Materials Design. Materials 2024, 17, 3105. [Google Scholar] [CrossRef] [PubMed]
- Feijoo, P.; Samaniego-Aguilar, K.; Sánchez-Safont, E.; Torres-Giner, S.; Lagaron, J.M.; Gamez-Perez, J.; Cabedo, L. Development and Characterization of Fully Renewable and Biodegradable Polyhydroxyalkanoate Blends with Improved Thermoformability. Polymers 2022, 14, 2527. [Google Scholar] [CrossRef] [PubMed]
- Kabe, T.; Sato, T.; Kasuya, K.-I.; Hikima, T.; Takata, M.; Iwata, T. Transition of spherulite morphology in a crystalline/crystalline binary blend of biodegradable microbial polyesters. Polymer 2014, 55, 271–277. [Google Scholar] [CrossRef]
- Wen, X.; Lu, X.; Peng, Q.; Zhu, F.; Zheng, N. Crystallization behaviors and morphology of biodegradable poly(3-hydroxybutyrate-co-4-hydroxybutyrate). J. Therm. Anal. Calorim. 2012, 109, 959–966. [Google Scholar] [CrossRef]
- Yu, F.; Nakamura, N.; Inoue, Y. Comonomer-unit compositional distribution and its effect on thermal and crystallization behavior of bacterial poly(3-hydroxybutyrate-co-3-hydroxyhexanoate). Polymer 2010, 51, 4408–4418. [Google Scholar] [CrossRef]
- Eesaee, M.; Ghassemi, P.; Nguyen, D.D.; Thomas, S.; Elkoun, S.; Nguyen-Tri, P. Morphology and crystallization behaviour of polyhydroxyalkanoates-based blends and composites: A review. Biochem. Eng. J. 2022, 187, 108588. [Google Scholar] [CrossRef]
- Phongtamrug, S.; Tashiro, K. X-ray Crystal Structure Analysis of Poly(3-hydroxybutyrate) β-Form and the Proposition of a Mechanism of the Stress-Induced α-to-β Phase Transition. Macromolecules 2019, 52, 2995–3009. [Google Scholar] [CrossRef]
- Mottin, A.C.; Ayres, E.; Oréfice, R.L.; Câmara, J.J.D. What Changes in Poly(3-Hydroxybutyrate) (PHB) When Processed as Electrospun Nanofibers or Thermo-Compression Molded Film? Mater. Res. 2016, 19, 57–66. [Google Scholar] [CrossRef]
- Panaitescu, D.M.; Frone, A.N.; Chiulan, I.; Nicolae, C.A.; Trusca, R.; Ghiurea, M.; Gabor, A.R.; Mihailescu, M.; Casarica, A.; Lupescu, I. Role of bacterial cellulose and poly (3-hydroxyhexanoate-co-3-hydroxyoctanoate) in poly (3-hydroxybutyrate) blends and composites. Cellulose 2018, 25, 5569–5591. [Google Scholar] [CrossRef]

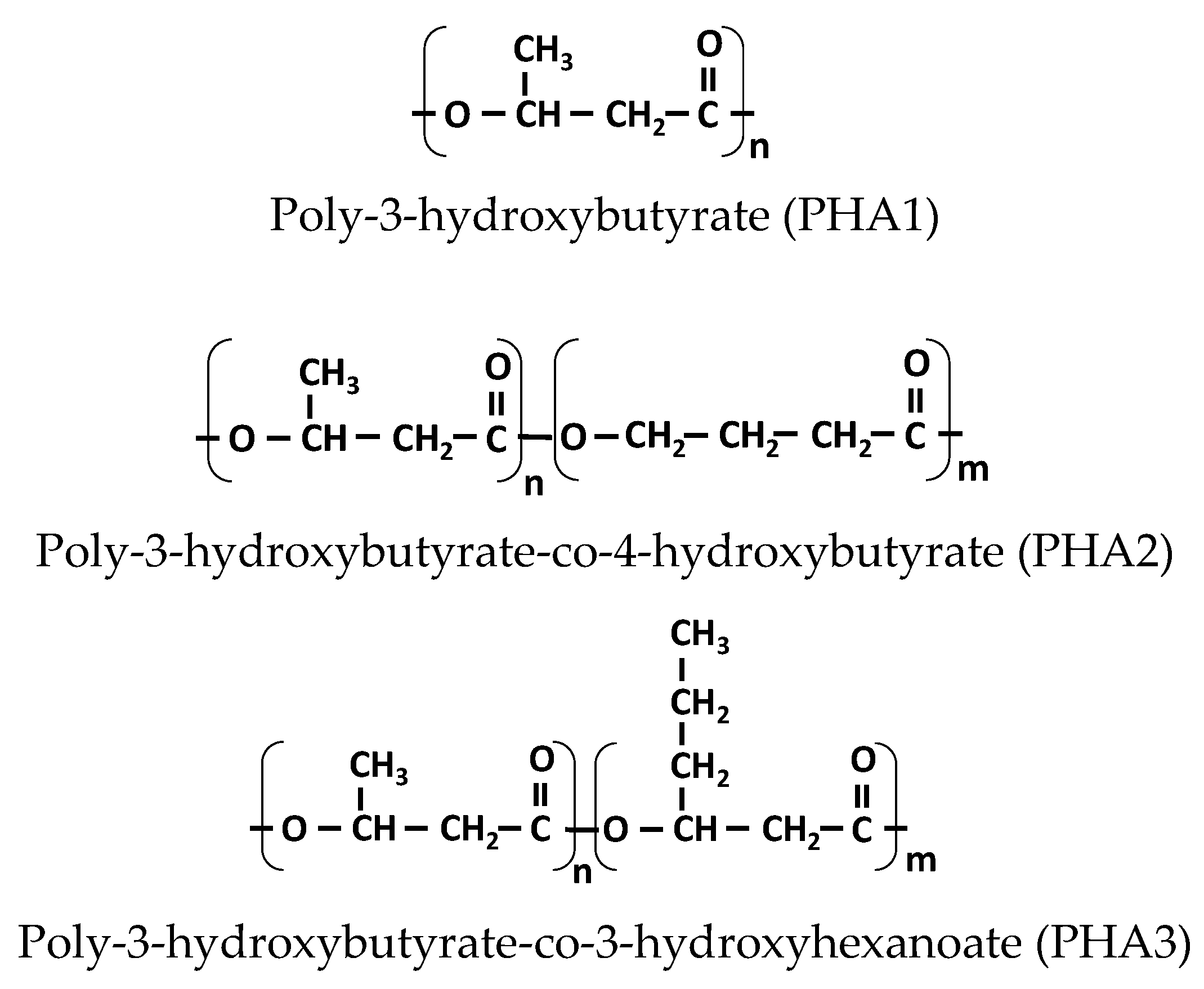





| Sample | PHA1 (wt%) | PHA2 (wt%) | PHA3 (wt%) |
|---|---|---|---|
| B1 | 100 | - | 0 |
| B2 | 90 | - | 10 |
| B3 | 70 | - | 30 |
| B4 | 50 | - | 50 |
| B5 | 30 | - | 70 |
| B6 | 10 | - | 90 |
| B7 | 0 | - | 100 |
| B8 | - | 100 | 0 |
| B9 | - | 90 | 10 |
| B10 | - | 70 | 30 |
| B11 | - | 50 | 50 |
| B12 | - | 30 | 70 |
| B13 | - | 10 | 90 |
| B7 | - | 0 | 100 |
| B1 | 100 | 0 | - |
| B14 | 90 | 10 | - |
| B15 | 70 | 30 | - |
| B16 | 50 | 50 | - |
| B17 | 40 | 60 | - |
| B18 | 30 | 70 | - |
| B19 | 10 | 90 | - |
| B8 | 0 | 100 | - |
| Polymer or Polymer Pair | Surface Tension (mN/m) | Interfacial Tension mN/m | |
|---|---|---|---|
| Dispersive | Polar | ||
| PHA1 | 41.33 | 8.79 | - |
| PHA2 | 40.5 | 8.45 | - |
| PHA3 | 39.58 | 7.42 | - |
| PHA1/PHA2 | - | - | 0.015 |
| PHA3/PHA2 | - | - | 0.077 |
| PHA1/PHA3 | - | - | 2.103 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alexy, P.; Horváth, V.; Plavec, R.; Vanovčanová, Z.; Tomanová, K.; Ďurfina, M.; Fogašová, M.; Omaníková, L.; Hlaváčiková, S.; Kramárová, Z.; et al. Fully Biobased Biodegradable Elastomeric Polymer Blends Based on PHAs. Polymers 2025, 17, 2811. https://doi.org/10.3390/polym17212811
Alexy P, Horváth V, Plavec R, Vanovčanová Z, Tomanová K, Ďurfina M, Fogašová M, Omaníková L, Hlaváčiková S, Kramárová Z, et al. Fully Biobased Biodegradable Elastomeric Polymer Blends Based on PHAs. Polymers. 2025; 17(21):2811. https://doi.org/10.3390/polym17212811
Chicago/Turabian StyleAlexy, Pavol, Vojtech Horváth, Roderik Plavec, Zuzana Vanovčanová, Katarína Tomanová, Michal Ďurfina, Mária Fogašová, Leona Omaníková, Slávka Hlaváčiková, Zuzana Kramárová, and et al. 2025. "Fully Biobased Biodegradable Elastomeric Polymer Blends Based on PHAs" Polymers 17, no. 21: 2811. https://doi.org/10.3390/polym17212811
APA StyleAlexy, P., Horváth, V., Plavec, R., Vanovčanová, Z., Tomanová, K., Ďurfina, M., Fogašová, M., Omaníková, L., Hlaváčiková, S., Kramárová, Z., Navrátilová, J., Komínek, V., Jaška, D., & Feranc, J. (2025). Fully Biobased Biodegradable Elastomeric Polymer Blends Based on PHAs. Polymers, 17(21), 2811. https://doi.org/10.3390/polym17212811






