1. Introduction
The use of polymer materials has quickly become one of the leading preferences when it comes to energy storage studies, a landslide shift in the manner in which researchers see the future of power generation, storage, and delivery in future devices. Their essential properties, structural versatility, molecular tunability, and ability to be multifunctional distinguish them from conventional inorganic materials and place them in a unique position to bridge long-standing energy gaps in batteries, supercapacitors, and solid-state systems [
1]. Polymeric frontiers’ vision of energy storage is based on their capability to combine molecular engineering with scalable device integration, overcoming limitations of the previous generation of materials and motivating holistic system innovation that meets real-world requirements, such as portable electronics and grid-scale renewables. The polymer materials used in energy storage have a huge spectrum, ranging from molecular design to device-level application. At the molecular scale, the chemist has an unprecedented control over the polymer structure and distribution of functional groups that can be subjected to specific engineering of electron and ion transport routes that are vital for energy harvesting and transfer [
2]. Non-rigid crystalline lattices, such as polymers, have the ability to be produced with custom degrees of flexibility, porosity, and conductivity that allow them to be used in thin-film batteries, bendable supercapacitors, and future-generation all-solid systems [
3]. This flexibility not only enables the development of a set of customizable energy devices but also leaves the door open to multifunctionality: an interplay between charge storage and self-healing, stretchability, and even a response to the environment. The promised multifunctionality is not just limited to performance improvement but also opens new possibilities of completely new types of devices that are tough, modular, and reconfigurable to new areas of application in the future.
The concept of molecular design in polymeric energy storage is based on a number of scientific requirements. The first is the problem of ion transport: inorganic solids may inhibit dynamic ion movement through the lattice constraints, but the polymers provide a chance to construct segmental dynamics and ion-conducting channels at the molecular level. Second, polymers are capable of accommodating various redox-active species that can include organic molecules, conducting segments, and dopant species, and perform synergistically in battery electrodes and electrode films in supercapacitors [
4]. Third, functional additives and nanoinclusions also increase the charge mobility, mechanical integrity, and processability, making a significant jump between the molecular structure and the performance at the device level. Finally, their ability to be functionalized easily and their capacity to produce green and low-energy products give them further sustainability benefits and make them appealing solutions to power sustainability in a carbon-reduced world. Polymeric frontiers are based on multifunctionality [
5].
Compared with traditional energy storage materials, which will often trade one property off against another of maximum capacitance versus flexibility or conductivity, polymers can be designed to simultaneously satisfy many of the desired characteristics. As an example, polymer nanocomposites consist of the combination of the strong electron transmission properties of carbon-based nanostructures and the stretchability and self-healing properties of organic matrices, resulting in the development of advanced supercapacitors that do not lose their performance even under the influence of mechanical forces or the surrounding environment. Comparably, solid-state battery polymers also come with the prospect of being both ionically conductive and thermally stable, therefore rendering their usage safe in a wide variety of scenarios [
6]. Physical, chemical, and biological functionalities integrated on the same polymer scaffold challenge conventional constraints and establish new standards for energy storage systems to be used in wearable, biomedical, and flexible electronic applications. Understanding what is lacking in traditional materials is important to emphasize the importance of polymers in the quest to store energy in the next generation [
7].
Inorganic substances, despite their crystalline perfection, are often brittle, hard to work with, and cannot be used in lightweight, portable, or flexible electronics. They are very hard and restrict the freedom of design to the extent of involving safety risks, such as dendrite axis or catastrophic collapse. Also, the traditional device architectures are ill-equipped to blend disparate functional needs like a high power density and environmental responsiveness, or the incorporation of a self-repair capability. Inorganic matrices do not have the processability or molecular-level customization required to support real system-level innovation, even when it is possible to make multifunctional composites [
8]. Polymers confront them directly: their synthetic versatility allows them to be modified on a molecular scale, their softness and stretchability create new application horizons, and their natural versatility creates opportunities to integrate mechanical, electrical, and ionic properties into one material platform. Such a distinct combination of characteristics puts them at the center of ground-shifting strategies in the development of high-energy storage technology [
9]. Recent advances in polymer science have triggered the technological path to the deployment of multifunctional devices, starting with the molecular design. Another important breakthrough that was recently announced with the use of polymer materials in battery development was presented by Shi et al. In this paper, a cubic-garnet (Li
7 La
3 Zr
2 O
12, LLZO) lithium-sulfur battery design based on a gel polymer buffer at the sulfur cathode/LLZO interface was demonstrated. A bilayer LLZO (dense/porous) solid electrolyte was used in the system and allowed a high sulfur loading (5.2 mg cm
−1) and an initial discharge capacity of 1542 mAh g
−1 and could be cycled at room temperature and with no external pressure at 80% capacity retention in 265 cycles. The novel polymer interface design was able to provide a solution to critical interfacial instability, which provided a base to stable, high-capacity, and commercially viable garnet lithium-sulfur batteries [
10].
This review starts with setting the principles of polymer materials as energy storage materials, which provide them with a distinct molecular tunability and multifunctionality that sets them apart from more traditional inorganic materials. More work has been performed on more sophisticated molecular engineering approaches where synthetic chemistry and computational chemistry are used to design polymers with desired charge transport and stability properties. Such development of general concepts into specific molecular design provides readers with a solid idea of the material basics of next-generation energy technologies.
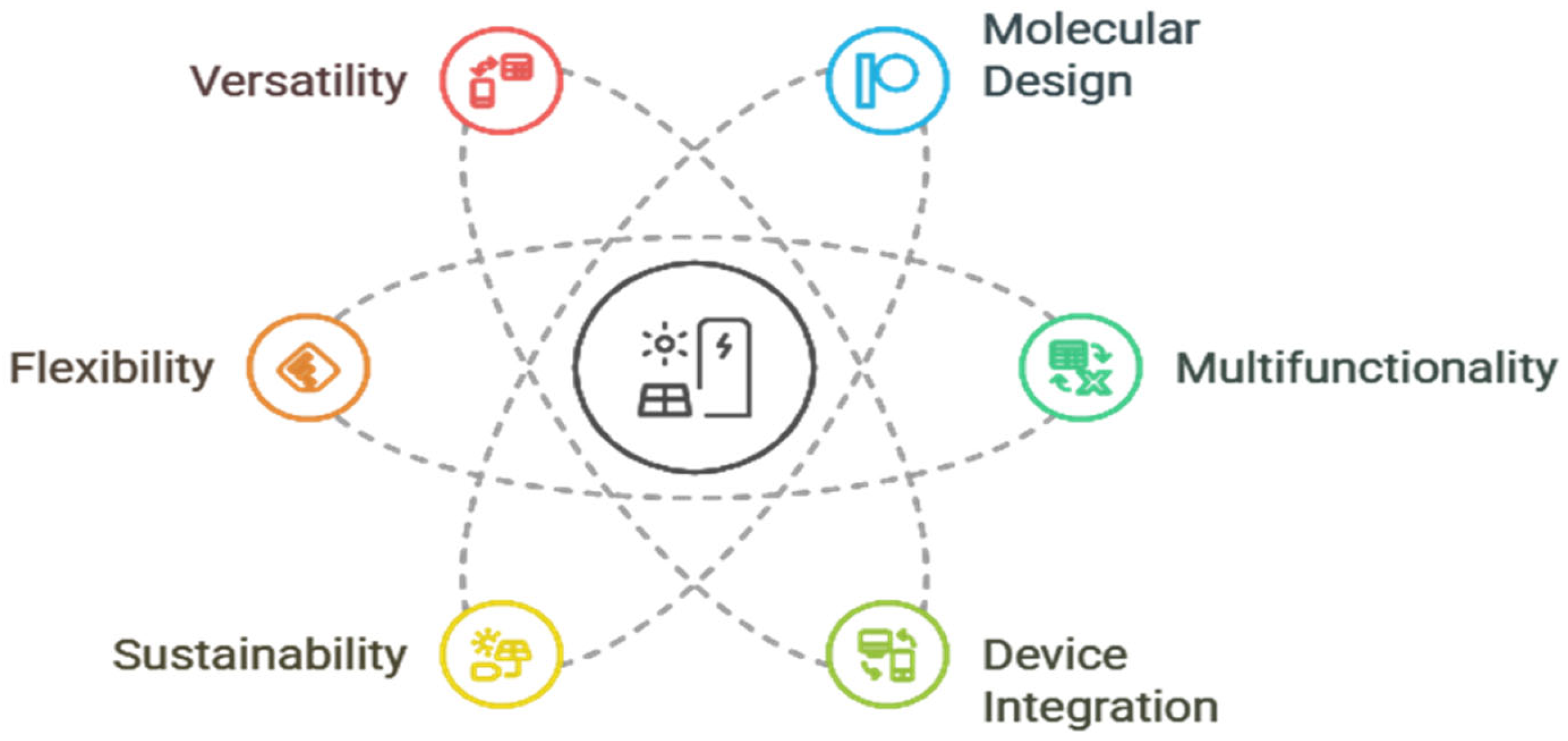
Figure 1 shows the key polymer properties play a crucial role in enhancing energy storage performance
2. Computer-Aided Molecular Engineering Innovations
AI-assisted property optimization and next-generation design strategies have enabled scientists to cross frontiers in materials. This capability to adjust the polymer composition in fine detail at the monomer, nanoparticle, and composite levels is directly translated to performance advantages in energy storage tools. In one example, carbon nanotubes or graphene sheet-based engineered conducting polymers have produced supercapacitors that have record-breaking capacitance and stability, and hybrid polymer electrolytes have made lithium metal an efficient and safe battery [
11]. Polymer systems have a particularly rich interfacial chemistry; in addition to being hosts to ion transport and redox reactions, polymer systems provide mechanical damping and structural support, which are important for cycling durability and robust operability. Regardless of such progress, there are still sharp gaps and unaddressed issues. Polymers have weaknesses in chemical stability during long-term service and can be degraded in high-voltage or high-temperature environments [
12]. The polymer material interface with other parts of the device, like the electrode electrolyte interface, could bring new complexities in terms of ion movements, electrochemical stability, and mechanical integrity.
The industrial application of fine-tuned polymer systems is a real problem with respect to scaling and reproducibility and requires interdisciplinary solutions in synthesis, process engineering, and computational models. The environmental problem of polymer manufacturing and disposal is also worth continuous consideration, which inspires further study of bio-derived and recyclable polymers that should meet performance and sustainability requirements [
13]. To fill these gaps, a complete research agenda is needed, integrating the state-of-the-art characterization, predictive modeling, and holistic engineering of devices to achieve the complete promise of polymeric frontiers in energy storage. In the scientific community, there is a growing agreement on the transformative role of polymers in energy storage, which is motivated by the fact that polymers permeate the dichotomies of the past. Polymers are flexible, tough, accessible, and high-performing, and are closing the gap between molecular complexity and simplicity of the device [
14].
Their development in batteries, supercapacitors, and solid-state systems signifies a paradigm shift in which materials science, synthetic chemistry, and electrochemical engineering converge to deliver not marginal benefits but systemic innovation. Now that researchers are going beyond the usual limits and establishing new methods of molecular design and system integration, the polymeric frontier will help to redefine the limits of energy storage and delivery, establishing the stage of an era of next-generation material-powered systems [
15]. To conclude, a scientific, dynamic, multidisciplinary, and radically transformative vision has been highlighted in the introduction of polymeric frontiers to next-generation energy storage. Moving beyond traditional constraints and tackling the root causes of the inherent limitations of conventional materials, polymers provide an avenue toward smarter, safer, and more adaptable energy systems through the application of molecular design principles, the embrace of multifunctionality, and confronting the fundamental limitations of traditional materials [
16]. Much work will be needed, though, to achieve long-term progress through continuous novelty, research cooperation, and fidelity, yet the trends and potential of polymer studies already signal a new phase that will bring together the gaps in theory and achievement in the pursuit of better energy technologies.
The need to develop new energy storage systems for an advanced energy storage system is increasing in line with the blistering development of portable electronics, renewable energy infrastructure, and battery technology in electric vehicles. This global energy shift and increasing environmental inquiries and constraints of traditional storage apparatuses have stimulated multidisciplinary research in seeking material platforms that can overcome the synergized challenges of performance, sustainability, and scalability [
17]. In this context, polymer materials have become a disruptive category with a distinct combination of molecular control, mechanical versatility, and multidimensional capabilities that have never been seen before in inorganic substrates. The frontiers of polymeric energy storage have a very interesting vision: to exploit the peculiarities of the organic molecules, such as their low weight, versatile structure, and compatibility with green synthesis procedures, to create materials and systems that should be better than the existing solutions in all the important criteria [
18].
The traditional batteries and supercapacitors that are, in many cases, premised on rigid inorganic crystals are also intrinsically limited by structural inflexibility, mass, and ecological footprint. Although their performance is admirable based on the capacity to store charges or deliver power, they are often undermined in the areas of safety, recyclability, and the capability to respond to new application areas like wearable biomedical devices or roll-to-roll technologies of flexible electronics [
19]. This discrepancy between the capabilities of the material and the needs of the system is a central justification of molecular design in contemporary energy storage. Unlike inorganic polymer systems, polymer systems can be designed and engineered at the level of the monomers themselves, allowing the chemical composition, topology, and microstructure to be controlled, unlike before. The optimization of charge transport pathways is made possible by the adjustment of charge transport pathways, electronic and ionic, within the matrix through the tailoring of backbone rigidity, side-chain functionality, and crosslinking density [
20]. Consequently, polymers can be engineered to have high ion-exchange functionality and selective redox, thermal, or mechanical stability, which is the main focus of next-generation batteries, solid-state devices, and high-rate supercapacitors.
Recent innovations have shown that conjugated polymers, biopolymers, and composite heterostructures may be used to deal with multiple modalities at the same time. As a case in point, n-type π-conjugated backbones can be engineered at the molecular level, which supports the use of high-rate lithium-ion and sodium-ion batteries [
21]. The polymer binders and anodic active materials can enable high efficiency, capacity maintenance, and rate sensitivity. Moreover, the scalable synthesis and low cost, as well as the introduction of electro-polymerizable additives and artificial solid electrolyte interfaces, increase the potential range and operational safety of the devices at the expense of their application [
22]. The inherent electronic conductivity and delocalized π-electron systems of π-conjugated polymers provide considerable benefits over the non-conductive binders used in prior studies (e.g., polyvinylidene fluoride, PVDF) in preserving electronic percolation, particularly at high areal loadings. Conventional binders are passive constituents, tending to induce compromised electron paths with an increase in the thickness of the electrode. Contrarily, π-conjugated systems, including polyaniline, polypyrrole, PEDOT and their analogs, create continuous, electronically conductive networks through the composite electrode, and facilitate rapid charge transport when the composite electrode is loaded with large amounts of mass. In addition, they can create smooth surfaces with active substances, which increase the connections between the particles and decrease internal resistance. Recent reports show that electrodes with pie-conjugated polymers as binders have an excellent retention capacity, rate and a low impedance at higher areal loadings than conventional binder systems with such strong percolative networks and synergistic electronic contributions from the polymer matrix itself [
23]. These features are not just scholarly innovations but are true developments toward more practical next-generation power systems capable of comfortably fitting new device architectures.
The basics of polymer molecular design are determined, and at this point, the discussion concentrates on the concept of multifunctionality. In this section, the way polymers are designed to satisfy the demands of the attributes of electrical conductivity, mechanical integrity, thermal stability, and self-healing characteristics is discussed for the development of flexible and durable energy storage devices.
3. Molecular Engineering of Advanced Polymer Systems
The molecular engineering of superior polymer systems to store energy is an interdisciplinary innovation in synthetic chemistry, computational modeling, and convergence at the interface of synthetic chemistry with functional materials science that can transform the limits of the field. The scientific community is also concentrating on the methods of engineering polymer backbones and side chains to maximally enhance the conductivity, stability, and multifunctional use in next-generation batteries, supercapacitors, and solid-state devices [
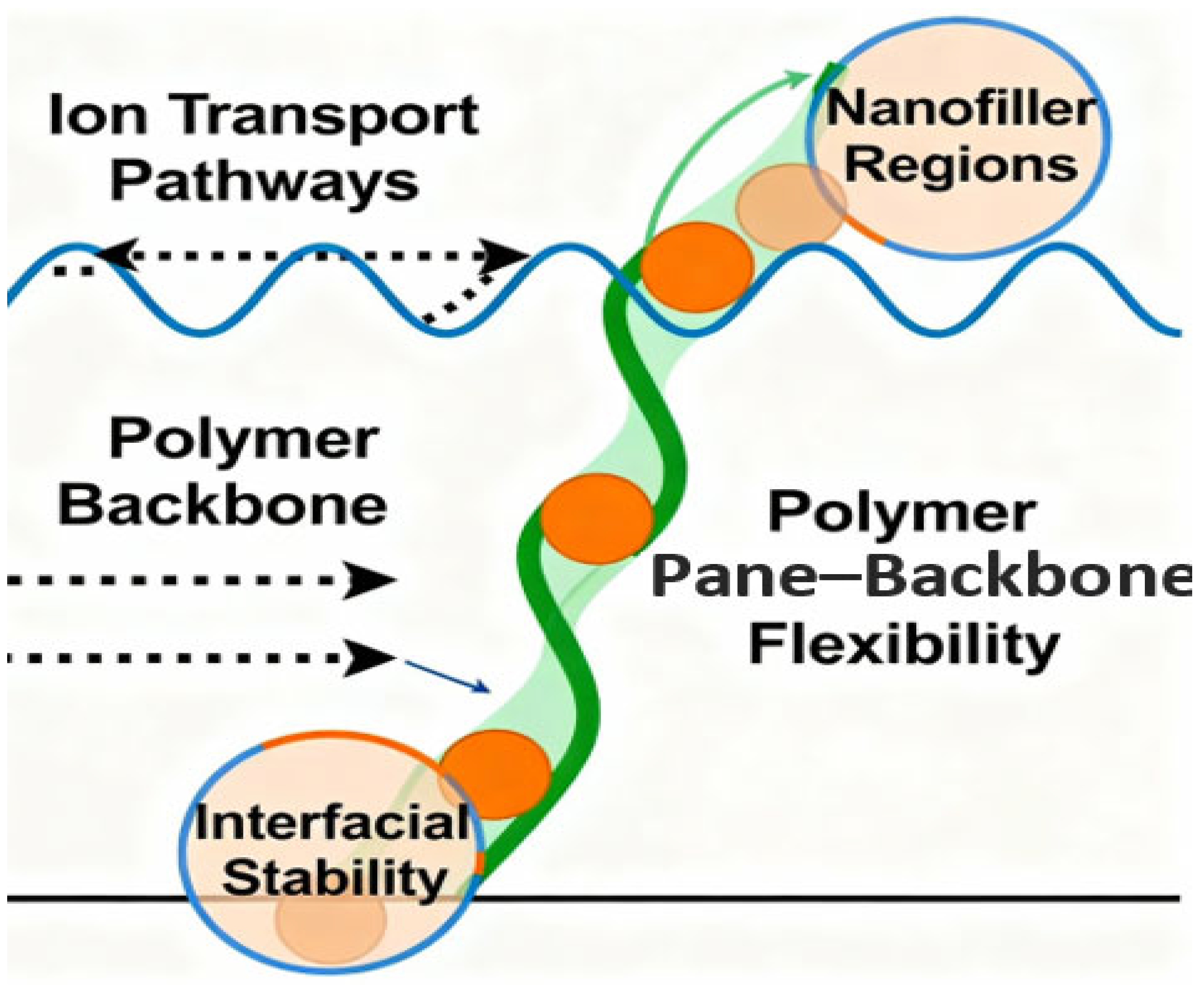
22]. The paradigm shift has gone beyond traditional empirical design and currently exploits AI-aided modeling, high-throughput computational strategies, and green synthetic methodologies, and is the age of rational material engineering, which used to be considered impossible. The modern methods of designing polymer molecules start with a realization that the backbone structure of a polymer defines its electronic characteristics, efficiency of transporting ions, and general stability under running conditions [
24] (
Figure 2).
Chemists can design polymers with predictable performance due to the precise synthetic control of monomer selection, sequence distribution, and chain architecture. An example is the addition of π-conjugated systems to polymer backbones to increase electron delocalization and redox activity, which is required in energy storage programs in lithium-ion batteries and supercapacitors [
25]. Customized side-chain capabilities, in turn, tune ionic conductivity, solubility, and interfacial adhesion, which, in solid-state electrolytes and flexible device matrices, is a decisive lever toward performance optimization. In addition to conventional wet chemistry, AI-aided and computational technologies have changed the speed at which polymers are discovered and optimized several times [
26]. The high-throughput in silico screening of candidate materials with optimal energy storage properties using machine learning (ML) models that are trained on large datasets of polymer structures, physical properties, and device performance metrics is made possible. They are algorithmic approaches that find latent relationships between molecular structures and emergent behaviors that can be used to steer synthesis systems to generate structures that can deliver increased ionic mobility, dielectric breakdown strength, and mechanical compliance [
27].
Transfer learning and multitask neural networks are ML-based methods that allow researchers to work with polymer systems that are too complex and multiscale. They not only optimize individual property domains but also contribute to the balancing of several, and usually conflicting, functional requirements. Importantly, computational screening based on data and stochastic breakdown simulations has aided in the prediction of dielectric breakdown processes and energy densities in polymer-based composites, and ML frameworks have been constructed that include variables (including dielectric constants, sizes, and contents of fillers) and their combinations to predict and experimentally validate the process [
28]. These high-throughput methodologies play a very important role in the rational design of polymer composites, in particular in areas where trial-and-error synthesis would have been resource-intensive or systematic. Simulation and laboratory results have supported these in silico paradigms, in which the gap between the theoretical design and experimental data has been reduced, to bring high-energy polymer capacitors and solid-state battery electrolytes closer to the goal of innovation. Another aspect of molecular engineering is the need to have a green synthesis process and a green source of materials [
29]. Recent studies focus on the design of bio-based polymers, i.e., the use of renewable monomers and environmentally friendly processing methods to minimize the carbon footprint and toxicological effects of new energy storage materials [
30]. The use of naturally occurring macromolecules, catalytic chain-growth processes, and recyclable polymer matrices has allowed the creation of friendly energy devices, which resonate with the growing concern of a circular material economy and regulation in high-end manufacturing industries.
Yoon et al. [
31] introduced an explainable ML model, which used optical absorbance spectra to make quick predictions of the electrical conductivity of doped conjugated polymers, and tested their predictions experimentally on brand new material systems. These case studies are examples of the practical combination of computational prediction and laboratory validation of modern polymer material development [
31]. The interpretation of ML models to predict the polyimide dielectric constant was performed by He et al. [
32]. The researchers synthesized three new polyimides based on the model, and the experimental results indicated that the deviation between the ML model and predictions was less than three percent, indicating the strength of the ML model in electronic materials discovery [
32]. Bradford et al. [
33] trained an Arrhenius-informed ML model to make predictions of the ionic conductivity of polymer electrolytes. The model was trained using a large set of experimental results and was shown to be very accurate in comparison with laboratory measurements in the case of numerous polymer structures, making it useful in a real-world situation of selecting electrolytes in solid polymers [
33]. In 2024, Lin et al. created a interpretable ML model for predicting polymer thermal conductivity (based on GBDT) using molecular features, which was validated in subsequent synthesis, and so it provides a physical understanding and reliable experimental validation [
34].
The integration of green chemistry concepts with molecular engineering is an important step toward responsible innovation that will guarantee the scalability of polymeric energy technologies in the future and their universal availability across the world. In spite of such developments, there are still significant gaps related to the logical design of polymers that are to be used in high-temperature, multifunctional, or fully bio-based applications. The lack of standardized datasets, small coverage of extreme-use cases, and gaps in mapping structure–property combinations prevent many machine learning models from achieving high levels of generalizability and predictive performance in special energy storage applications [
30]. The thermal and chemical stability of bio-based polymers is not yet everywhere, similar to that of petroleum-based analogs, and property integration at multiscale (i.e., high ionic conductivity and mechanical strength, thermal durability, etc.) is not always without trade-offs, which are not readily modellable in existing computational models. These loopholes are even made more complicated due to the difficulties in modeling complex interface phenomena, including the electrode-to-electrolyte compatibility and degradation routes in cyclic or extreme operating conditions [
35]. To overcome these weaknesses, scientists are spending time on constructing complete, high-quality databases, constructing interpretable ML models, and coming up with experimental guidelines to prove the models’ predictions and increase the size of structural exploration.
The use of convolutional neural networks to analyze nanoparticle fillers, reinforcement learning to optimize silicon nanoparticles and the polymer binder, and support vector machines to predict the lifetime of marine biodegradable composites are just a few examples of how molecular engineering and AI can work together to achieve discoveries in energy storage materials [
36]. Such collaboration not only allows the design of polymers with a specific functional profile but also empowers predictive maintenance, process control, and early failure intervention of deployed storage systems. Finally, the new approaches to synthetic control coupled with computational intelligence and green chemistry are revolutionizing the field of molecular engineering of innovative polymer systems to store energy [
37]. Controlled manipulation of the polymer backbone and side-chain designs, with inputs of machine learning and simulation, has widened the scope of accessible properties of ionic conductivity, mechanical flexibility, dielectric strength, and operational safety of flexible and multifunctional energy platforms. Although rational design still has some gaps, particularly in extreme environmental contexts and complete material sustainability, there is a steady improvement in the data collection, model formulation, and validation that are closing the gaps [
38]. The combination of AI-based molecular prediction, high-throughput simulation, and experimentally sound synthesis has become the new paradigm in the field, where the future of the next-generation energy storage systems is only bounded by imagination and cross-disciplinary cooperation.
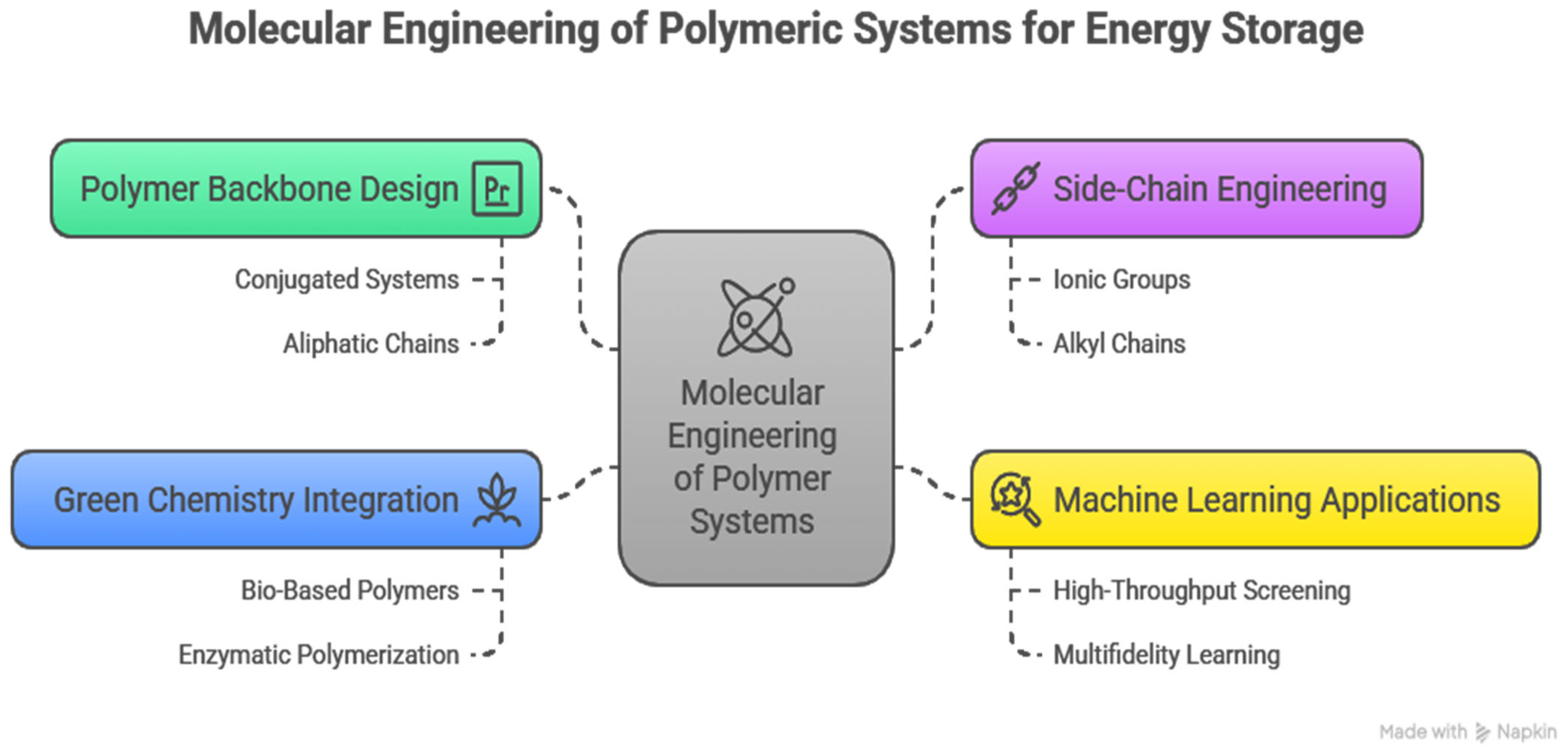
Innovative molecular engineering of novel polymeric energy storage systems is one of the emerging critical frontiers in materials science, synthesizing synthetic chemistry, computational innovation, and green methods to build better devices than are possible today. This part elaborates on approaches to designing polymer backbone and side-chain architectures to store energy, the applications of machine learning, new functionalization approaches, and challenges and gaps in design, with a concentration on scientific novelty and global understanding [
5]. The basis of molecular engineering is the specific control of the polymer backbone. The chemical structure of the backbone is the major determinant of the electronic properties of the polymer, mechanical stability, and ion mobility.
The mobility of charge carriers, redox reactivity, and thermal stability are directly dependent on different backbone chemistries that can be conjugated to zero-systems through aliphatic chains with polar functional groups. As an example, conjugated polymers having both single and double bonds are good sources of extended pi-electron delocalization, which mainly boosts electrical conductivity and allows redox reactions essential to battery electrodes and pseudocapacitive materials [
39]. Nonetheless, these polymers have to be processible and flexible, and therefore it is common that polymers undergo copolymerization with non-conjugated sections or that side chains that control both solubility and interchain separation are introduced. Equally important is the concept of side-chain engineering to control the performance of polymers. The side chains not only affect the solubility and processability but also the ion transport and mechanical characteristics [
40]. The introduction of ionic or polar pendant groups enhances ionic conduction, which is important in solid polymer electrolytes, and the ion exchange reaction is fast without the destruction of polymer stability. The presence of long alkyl side chains confers mechanical flexibility and may disrupt crystallinity to increase amorphous regions that are conducive to ion movement. Additionally, the side-chain architecture, such as length, branching, and density, can be tuned precisely, giving control of thermal and chemical stability, which are critical for polymers exposed to extreme electrochemical conditions or high temperatures [
41]. Synergistic backbone and side-chain design thereby forms multifunctional polymers to meet application-specific requirements.
Contemporary molecular engineering ceases to depend on the classical laboratory synthesis and chemical intuition. However, computational techniques, in particular, machine learning (ML), have turned polymer design into a science of data. ML algorithms, including graph neural networks, support vector machines, and Bayesian optimization, learn the relationships between the structures and properties of polymers with large-scale datasets to understand the complex structure–property relationship [
42]. It is a method that allows the testing of polymer candidates virtually within a few hours with predicted and high values in the parameters of conductivity, mechanical strength, thermal endurance, and electrochemical stability of the polymer, which are vital in energy storage systems. ML-assisted design involves several steps. The first step is to build detailed polymer databases with accurate models of molecular structures and related physicochemical properties. The second step is training predictive models on such data, capturing relationships, as well as predicting the behavior of polymers based on the desired behavior. The third step involves utilizing virtual screening and optimization algorithms to generate new polymer structures that match the desired behavior [
43].
Particularly, more recent progress in multifidelity learning enables the integration of experimental and computational measurements, and hence enables maximum accuracy of a model even when there is a small amount of high-quality experimental measurements. These computational methods have hastened discovery, especially of multifunctional polymers, wherein trial and error methods are strictly forbidden by the time requirements of classical methods. As an example, ML models have been used to predict the ideal filler materials and polymer matrix composition to improve the dielectric breakdown strength and energy density of polymer nanocomposites used in the production of flexible capacitors. With this development, microstructural motifs and side-chain functionalities can be identified, and they can work together to maximize charge storage without compromising flexibility or longevity [
44]. Moreover, ML enables a trade-off between conflicting properties, including the maximization of ionic conductivity and the maintenance of mechanical strength, which is a key factor of solid polymer electrolytes used in batteries. The molecular design is closely related to green synthesis and sustainability concerns. The new trend towards bio-based polymers based on renewable feeds demands novel molecular architectures that do not reduce but, in fact, improve performance and have a minimal environmental impact. They include the use of biodegradable moieties, enzymatic polymerization techniques, and low-toxicity solvents and catalysts [
45]. The special attention in regard to this is that such approaches demand a close molecular design in ensuring that biopolymer materials have similar ion transportation capacities, chemical stability, and mechanical qualities to the synthetic polymers.
Research in molecular engineering is emerging as a nascent field, but projections show that bio-derived polymers are poised to bridge the divide with promising electrochemical performance. Despite all these developments, there is still a lot left to be filled and a lot to be done, especially in the rational design of high-temperature and multifunctional polymer systems [
46]. Anisotropic temperature stability requires polymer backbones and side chains that are not thermoxidatively degradable, not ionically conductive, and maintain mechanical integrity. This makes the design of such materials complicated, with the improvement of one property affecting the other. The available state-of-the-art ML models are constrained by the lack of quality data on polymer behavior under extreme conditions, which limits their ability to make predictions. In addition, the chemical architecture and mesoscale morphology interactions that have a powerful impact on properties such as ion conduction paths and mechanical damping are not well represented in existing computational systems [
47].
The other important gap is connected to the creation and production of entirely bio-based polymers that offer multifunctional properties and can be compared to those based on petroleum. These polymers should incorporate biodegradability, electrochemical performance, and processability, which is a daunting trifecta that is yet to be achieved [
43]. A mechanistic understanding of matters and innovative methods and strategies at the molecular level, in association with AI-based design, are necessary to overcome these challenges, as they lead to a concept of polymers that meet the demands of the future energy environment and minimize the performance of the devices. The main strategies for molecular engineering of polymeric systems are illustrated in
Figure 3.
4. Multifunctionality: Beyond Single Performance Metrics
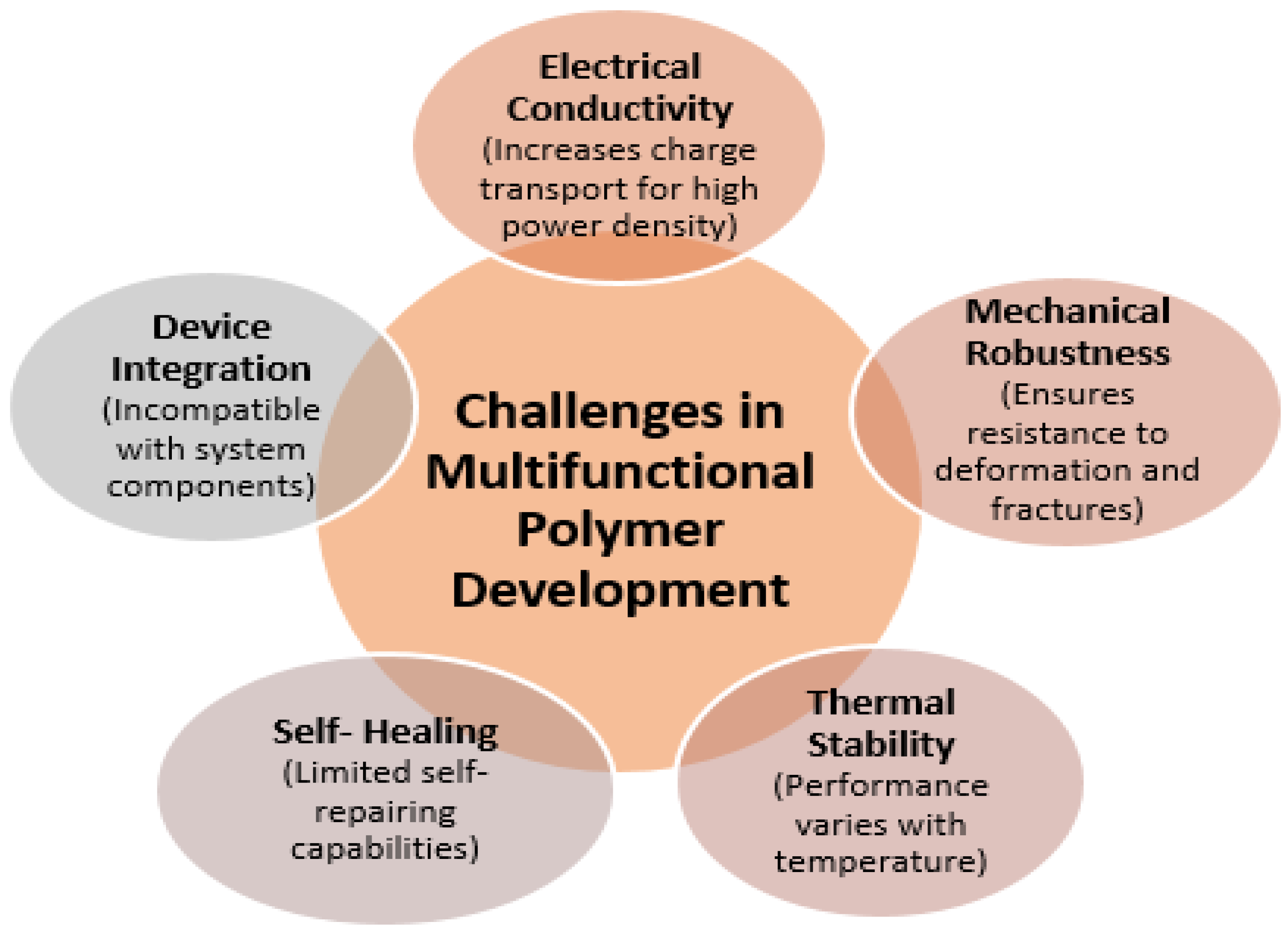
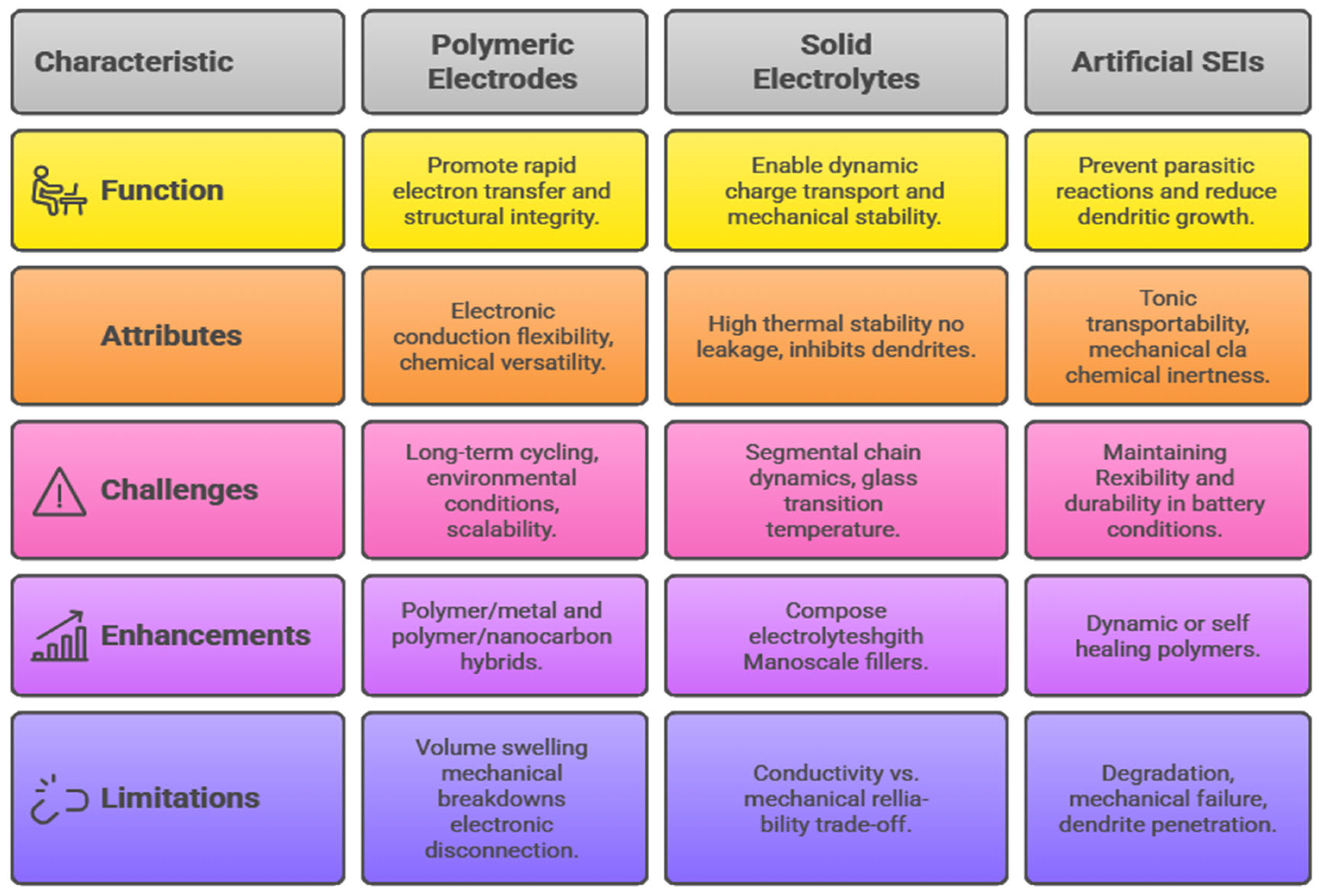
The multifunctionality of polymer materials as energy storage materials is transforming the paradigm of optimization of individual measures of performance, including conductivity or mechanical strength. The changing requirements for the next-generation energy apparatuses, especially batteries, supercapacitors, and flexible solid-state systems, require the incorporation of various functional characteristics into a single material platform. This integration entails the synthesis of electrical conductivity, mechanical durability, thermal stability, and advanced self-healing functionality, which are crucial for promoting the efficiency, durability, and safety of devices [
48]. The fact that these properties can be combined in one polymer material is a scientific breakthrough, enabling the creation of a device that can resist mechanical deformation, thermal stress, and long-term electrochemical cycling, broadening their application to the challenging demands of wearable electronics, as well as central energy storage in the grid.
Electrical conductivity underlines the existence of energy-storing polymers since it is primary in terms of transporting charge effectively to guarantee a high power density. Nonetheless, polymers have long had low conductivity compared to inorganic metals and ceramics. To address it, molecular engineering methods include conductive moieties like conjugated backbones and doping mechanisms, which boost the circulation of electrons and do not affect flexibility [
49]. At the same time, mechanical robustness is essential, which allows the polymers to resist fractures, fatigue, and deformation under repetitive loads, which is necessary especially when used in flexible and wearable devices that are therefore subjected to bending and stretching. Researchers develop materials that do not compromise mechanical strength to increase conductivity through molecular tailoring, such as crosslinking strategies, the incorporation of elastomeric segments, and creation of nanocomposites [
50]. This balance is needed because any device switching and charge–discharge processes are bound to cause both mechanical and electrochemical stresses. These properties are complemented by thermal stability, which ensures the integrity and performance of polymers at different operating temperatures. Energy equipment is commonly used under varying thermal conditions due to the exposure of the equipment to the environment or inherent heating of the equipment in Joule heating conditions during high-rate charging and discharging [
51].
Thermally stable backbones, flame-retardant side chains, and crosslinked networks in polymers allow the polymers to withstand higher temperatures without losing their conductivity and mechanical properties. This property makes the devices safer and more resilient to counterbalance the disaster failure modes associated with material breakdown. In addition, the use of self-healing features brings in a paradigm shift in longevity improvement [
52]. Dynamic covalent bonds or reversible supramolecular interactions used as polymers can heal microcracks or chemical damage independently and thus increase the life of devices and minimize their maintenance expenses. This capability is especially appreciated in the novel wearable or implantable energy storage systems where service is restricted. Polymeric nanocomposites are more positively multifunctional with the addition of two-dimensional (2D) and three-dimensional (3D) nanomaterials, including graphene, metal–organic frameworks (MOFs), and MXenes, which have shown synergies in various fields [
53].
Graphene, an outstanding electrical conductor, has mechanical strength and a specific surface area, and can act as a conductive scaffold besides enhancing structural strengthening at the nanoscale. Its incorporation in polymer matrices leads to high capacitance of the composite, cyclic stability, and mechanical durability. MOFs also provide porosity and adjustable chemistry, which increases the rates of ion and electrolyte accessibility that are essential for a high rate of charge storage [
54]. The more recent group of 2D transition metal carbides and nitrides, called MXenes, has integrated layered structures with metallic conductivity as well as hydrophilic surfaces, leading to efficient ion diffusion and high electrode–electrolyte interactions. The incorporation of MXenes has shown an exceptional enhancement of electrochemical performance, such as increased capacitance, charge–discharge rates, and cycle life, and, at the same time, anti-corrosion and self-passivating properties [
55]. This type of multifunctionality of nanocomposites is revolutionary in actual devices. In the case of batteries and supercapacitors, the polymer matrices supported by these new nanomaterials connect conduction orders and mechanical strength, which polymers or fillers do not have.
In the case of flexible solid-state systems, such composites exhibit mechanical compliance and do not reduce ionic or electrical conductivity. In addition, these polymer nanocomposites with multifunctional characteristics enable environmental resilience against humidity, temperature changes, and mechanical deformations that are essential for business viability. The composite method further allows functional gradient customization, with various parts of a device playing different functions such as mechanical support, ion transport, or charge collection, being a philosophy of materials-by-design. In spite of these developments, significant gaps and issues still exist, especially with respect to modular trade-offs of multifunctionality [
56]. The process of combining several properties in one material may be associated with complicated interdependencies, where an improvement in one of the properties may adversely affect another. As an example, mechanical strength can be enhanced by increasing the density of crosslinks, which can lower ionic mobility and conductivity. In the same way, the addition of high levels of nanofillers to increase conductivity may affect the processability and the flexibility of the polymer as well as form interfacial flaws, which compromise reliability.
Multifunctional polymer systems also often have challenges in integration at the device level because of their incompatibility with other components of the system in terms of mechanical or thermal behavior, resulting in stress buildup and possible points of failure (
Figure 4). These trade-offs require a strict knowledge of structure–property–process interaction at several length scales, between molecules and device structures. It is urgently required to have frameworks that systematize the optimization of the balance between conflicting functional properties, taking advantage of advanced characterization techniques (including in situ spectroscopy and tomography) and predictive modelling [
57]. Moreover, the creation of standardized testing procedures to measure multifunctionality as a whole will speed up the process of screening and optimization of material. The other difficulty is the scalability and reproducibility of multifunctional polymer composites.
The production of manufacturing processes that guarantee homogeneous nanomaterial dispersions and unified polymer deformation is essential to convert the prototypes that are working in the laboratory into commercially viable products. Fluctuations in material batches/processing conditions will cause inconsistency in the performance of the device, which will interfere with industrial adoption [
58]. The development of manufacturing methods, including controlled 3D printing, electrospinning, and roll-to-roll coating, is promising, but it needs to be optimized further to be used with multifunctional polymer systems.
Having insights into the multifunctional nature of polymer materials, this section discusses how these materials can be incorporated into real-life energy storage solutions. The following transition emphases the translation of molecular and multifunctional properties into the key components of batteries, supercapacitors, and solid-state systems, which reflects the evolution of matter to the device.
6. Emerging Frontiers: AI, Computational Chemistry, and Data-Driven Discovery
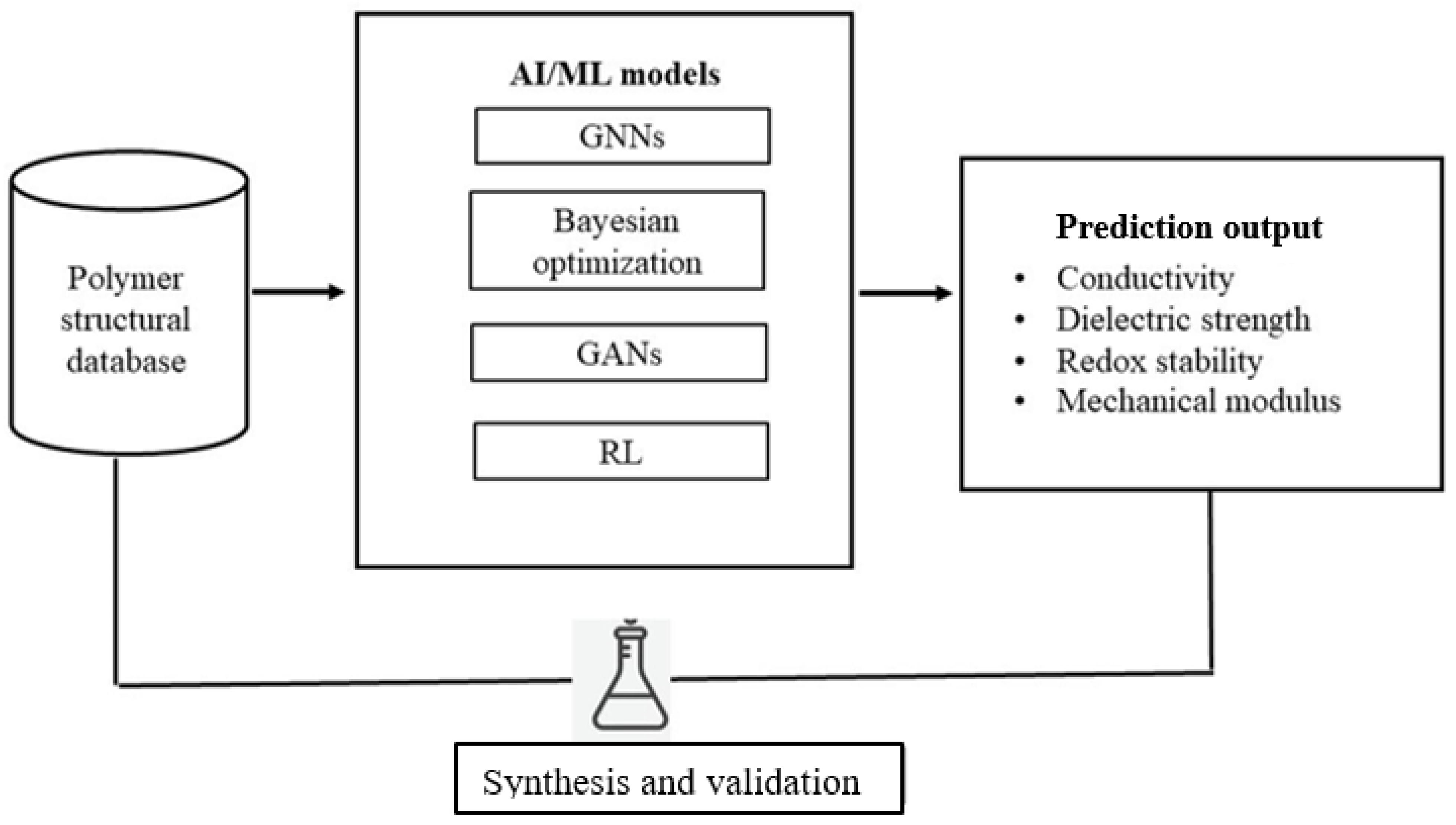
In the complicated environment of polymer design and device integration, there has emerged a paradigm shift in the use of computational tools and especially artificial intelligence (AI) and machine learning (ML). These tools provide a systematic analysis of large amounts of structure–property–performance data that can be used to predict and optimize polymer functionalities, which otherwise would be unattainable through trial and error using only experimental data alone. Machine learning (ML) and artificial intelligence (AI) are no longer considered as computational aids, but rather as the driving forces of polymer innovation. It is these techniques that are now used to push the process of rational discovery, prediction, and validation to shrink experimental timescales once measured in years to weeks. In polymer science, AI algorithms can identify structure–property–performance relationships using huge datasets that are made up of molecular descriptors, synthesis parameters, and device outputs. As an example, to predict ionic conductivity and dielectric constants, polymer graphs can be directly transformed into graph neural networks (GNNs) and transformer-based models and be accurately predicted [
84]. In the meantime, monomer combinations that maximize conductivity in solid electrolyte stability trade-offs are autonomously suggested by reinforcement learning (RL) frameworks.
Virtual screening with high throughput using ML has already been used to discover candidate backbones with optimized ion transport pathways of lithium polymer electrolytes and predict polymer chain motifs that enable a high dielectric strength. Simultaneously, generative adversarial networks (GANs) and Bayesian optimization algorithms develop new polymer structures with desired mechanical capabilities and redox potentials. Such predictions with AI are then verified with automated synthesis as well as in situ characterization and generate a closed-loop design pipeline, which continuously enhances model fidelity [
85].
ML algorithms can help to screen molecular candidates, optimize the processing parameters, as well as clarify the degradation pathways, thereby reducing the innovation cycles and improving reproducibility [
84]. As an example, AI-based molecular dynamics simulations offer a more faithful prediction of the ion migration pathways in solid polymer electrolytes to inform the molecular engineering of polymers with greater ionic conduction and mechanical strength. Equally, generative models synthesize polymer structures with enhanced mechanical flexibility to be worn and at the same time balance electrochemical performance needs. The discovery made through data discovery improves the current knowledge about the degradation mechanisms and mechanical breakdown, allowing predictive maintenance and lifetime optimization of flexible energy devices. However, these computing boundaries are significantly limited [
85].
The accessibility of a high-quality, full dataset is a major bottleneck that limits model accuracy and applicability between polymer classes and application conditions. Most datasets are not characterized using standardized characterization protocols, and, therefore, inter-laboratory comparisons are challenging. The disparity between the atomic scale models and the macroscopic level in the operation of devices poses more computational issues that demand multiscale modeling paradigms [
27]. The development of the explainability and interpretability of AI models is essential to user trust and regulatory acceptance of AI models, particularly safety-oriented biomedical devices. Schematic representation of a data-driven workflow illustrating machine learning model prediction, synthesis, and validation of polymer properties (
Figure 6), alongside an overview of polymer systems used in supercapacitors and next-generation energy storage (
Table 1).
In the complicated environment of polymer design and device integration, computational chemistry, artificial intelligence (AI), and machine learning (ML) have led to a paradigm shift. These are used to quantitatively examine large amounts of structure–property–performance data, thereby predicting and optimizing polymer functions that were previously unattainable through purely experimental trial-and-error methodology. ML algorithms can help to filter molecular candidates, optimize processing parameters, clarify degradation pathways, reduce the duration of innovation, and improve reproducibility [
40]. As an illustration, a molecular dynamics simulation using AI is more likely to predict ion transport pathways in solid polymer electrolytes in a more faithful manner, informing the molecular engineering of polymers with an increased ionic conductivity and mechanical strength.
Equally, generative models produce polymer scaffolds characterized by optimized mechanical dexterity to wearable tasks and trade-offs in electrochemical functioning. This is because data-driven discovery is increasing knowledge of degradation kinetics and mechanical failure modes to compute predictive maintenance and lifetime optimization of flexible energy devices. There are, however, significant limitations to these computational frontiers. Access to large, high-quality datasets is also a major bottleneck and limits the accuracy and transferability of the models across types of polymers and to different application settings. A large number of data sets do not have standardized characterization protocols, and thus an inter-laboratory comparison is not possible. Overcoming the discrepancies between small-scale simulations and the large-scale behavior of a device is an additional computational problem that needs multiscale modeling frameworks [
82]. The adaptability of AI models in the interpretation and explainability of AI models is essential for gaining user confidence and regulatory approval, particularly in safety-sensitive biomedical technologies.
Figure 7 represents the synergistic role of polymer material and computational tools in advancing energy storage innovation.
8. Future Perspectives, Research Gaps, and Innovation Roadmap
Polymer materials have the potential to revolutionize energy storage in the future, with the ability to be multifunctional, scalable, and embedded into a variety of platforms in devices. Their development is guided toward the facilitation of high power and energy densities, reliability, and cost-effectiveness. This is possible by designing hybrid polymer architectures consisting of conductivity, stability, and the ability to be mechanically flexible to provide greater functionality in batteries, supercapacitors, and solid-state systems without hurting manufacturability. One major issue is the need to have a balance between high charge–discharge rates and energy storage over the long term. Developments in controlled polymer molecular design and conductive nanomaterial composites are a possibility, although the optimization of ionic/electronic transport, interfacial stability, and polymer morphology at various scales is still important. The other issue is long-term reliability because polymers are subject to chemical degradation, mechanical fatigue, and interfacial failure when used in repeated cycling. Innovations are being made in developing self-healing polymers, mechanically compliant environments, and stable dynamic interfaces that do not allow capacity loss and structural disintegration.
Hybrid systems that incorporate organic polymers with inorganic fillers or nanostructures can improve on the limitations of individual materials by combining the flexibility of polymers and ionic conductivity of ceramics. New technologies in polymer chemistry, along with dynamic covalent bonding, supramolecular assembly, and sequence-controlled polymerization, allow the development of networks with responsive ion transport, mechanical strength, and flexibility in solid-state devices. Future developments involve interdisciplinary work that involves chemistry, materials science, computational modeling, and engineering. The predictive tools for structure–property–performance relationships will require advanced characterization methods and machine learning software for quick discovery and optimization. Another important aspect is sustainability through environmentally friendly synthesis, recyclability, and life cycle analysis. The polymeric energy storage innovation roadmap, in general, focuses on multifunctionality, durability, scalable manufacturing, and sustainable design. These issues can be mitigated through the integration of science and technologies that have enabled polymer-based systems to form the backbone of the future generation of energy storage that is high-performance and environmentally friendly.