Aldehyde–Aminotriazole Condensation Products as Novel Corrosion Inhibitors for Mild Steel in Hydrochloric Acid
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Determination of Corrosion Rate and Inhibition Efficiency
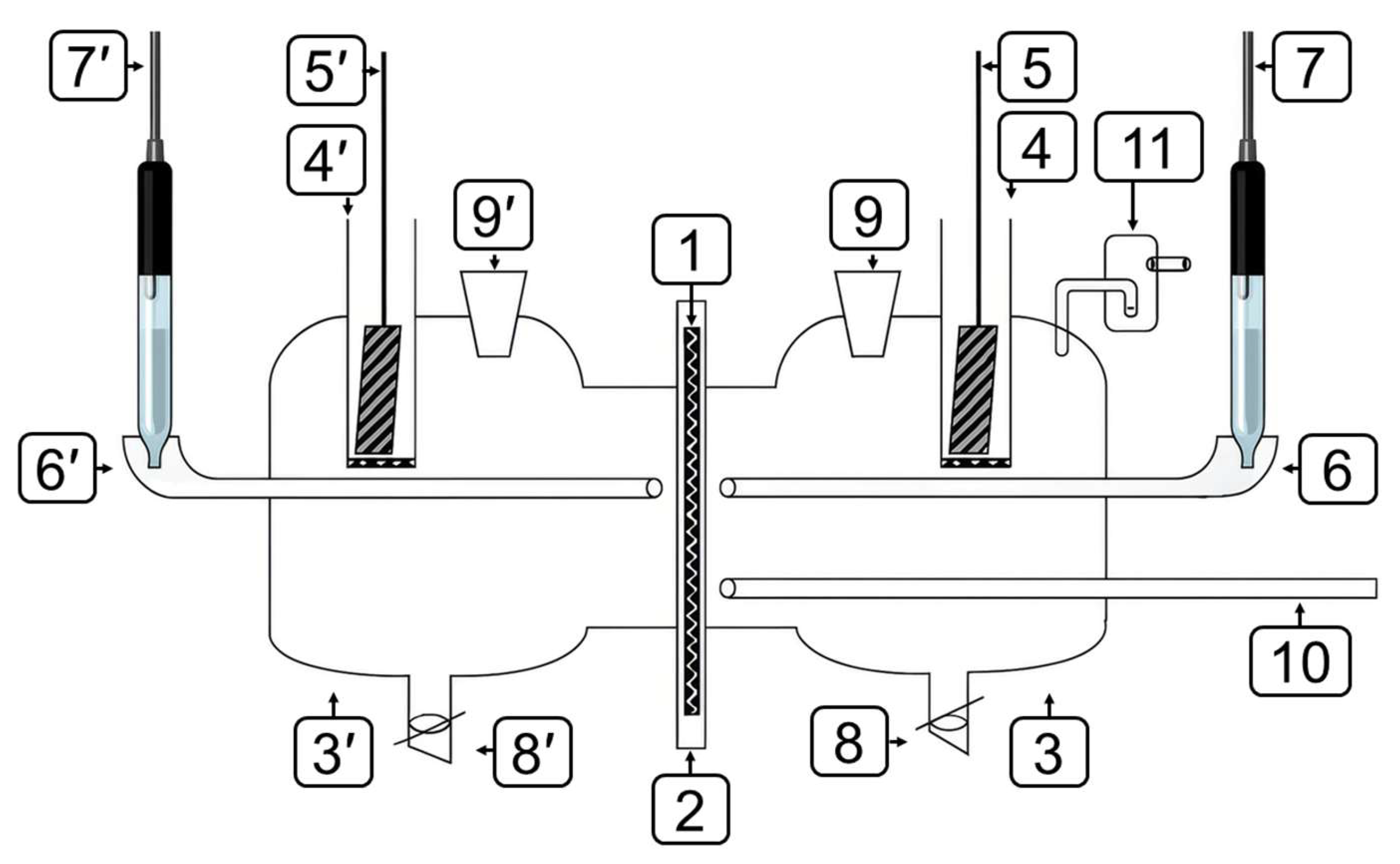
2.2.2. Vacuum Extraction Method
2.2.3. Cyclic Bending Tests
2.2.4. Voltammetry
2.2.5. Electrochemical Impedance Spectroscopy (EIS)
2.2.6. Bipolar Electrode Method
2.2.7. IPZ Analysis Method
2.2.8. AFM Method
2.2.9. XPS Method
2.2.10. Mass Spectrometry
2.2.11. Molecular Dynamics Simulations
2.2.12. Quantum-Chemical Calculations
3. Results and Discussion
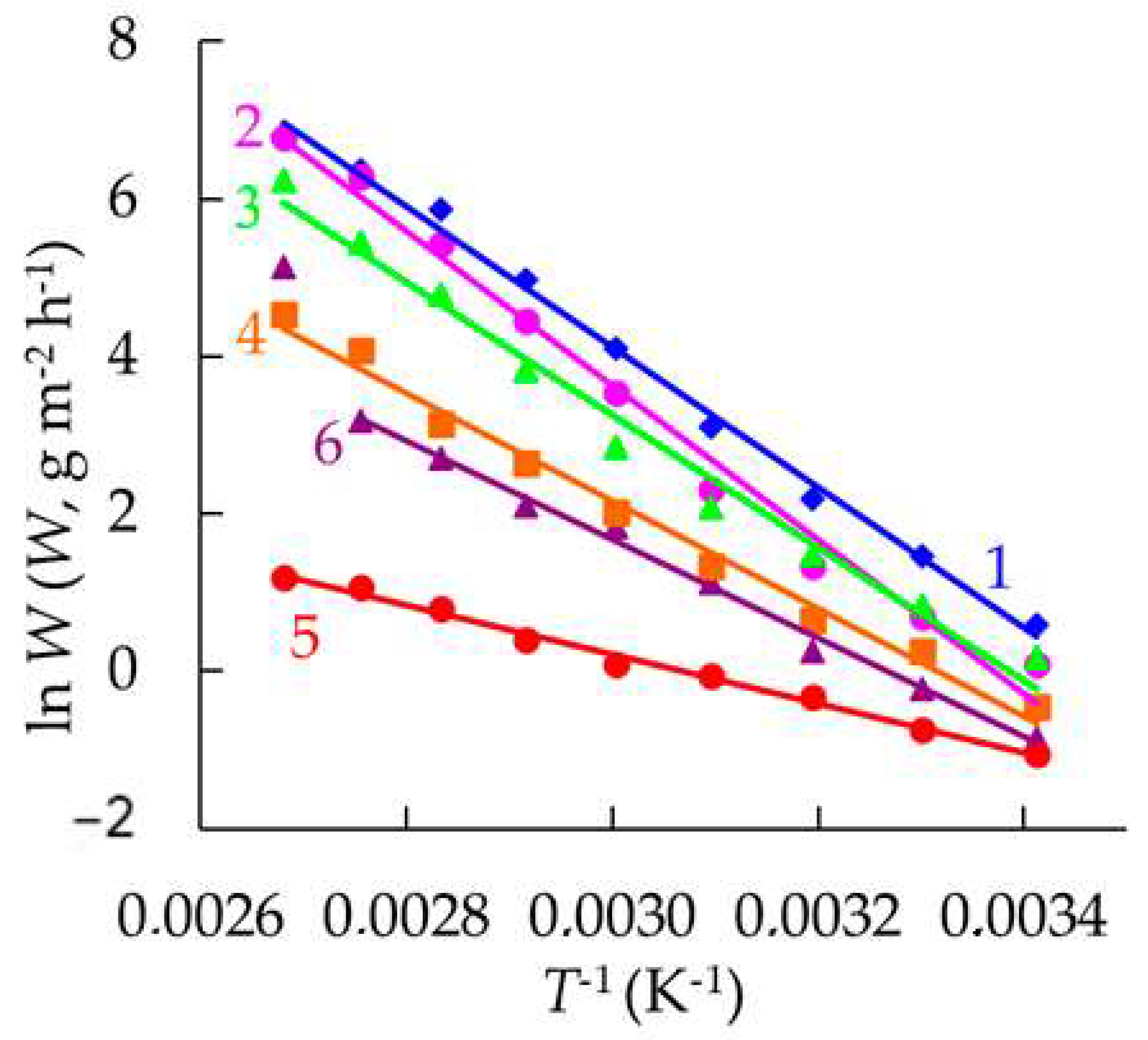
3.1. Effect of Aldehyde Condensation Products on Steel Corrosion in HCl Solution
3.2. Effect of CATA and CA on the Mechanical Properties of Steel and Its Hydrogen Uptake
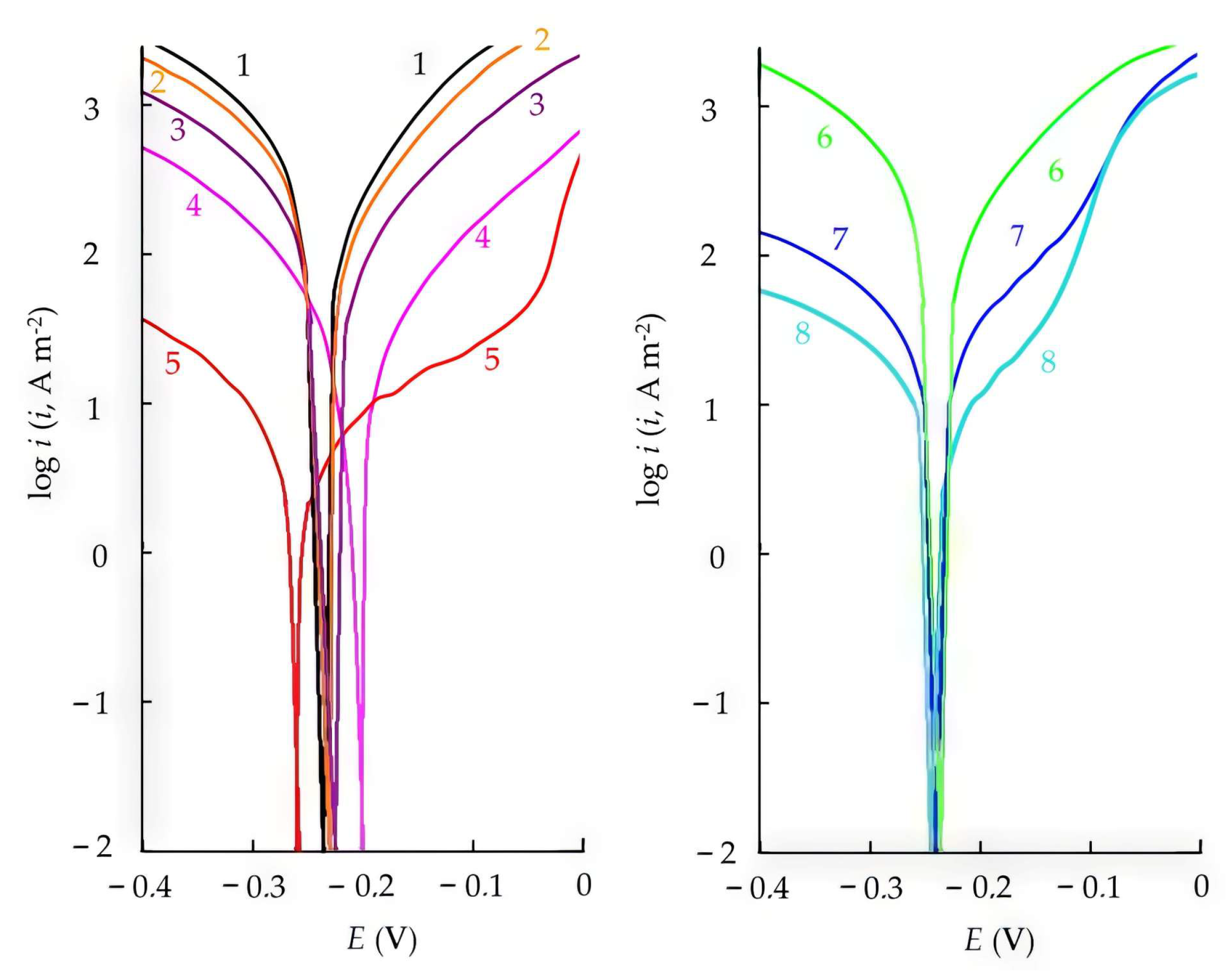
3.3. Effect of CATA on the Electrode Reactions of Steel
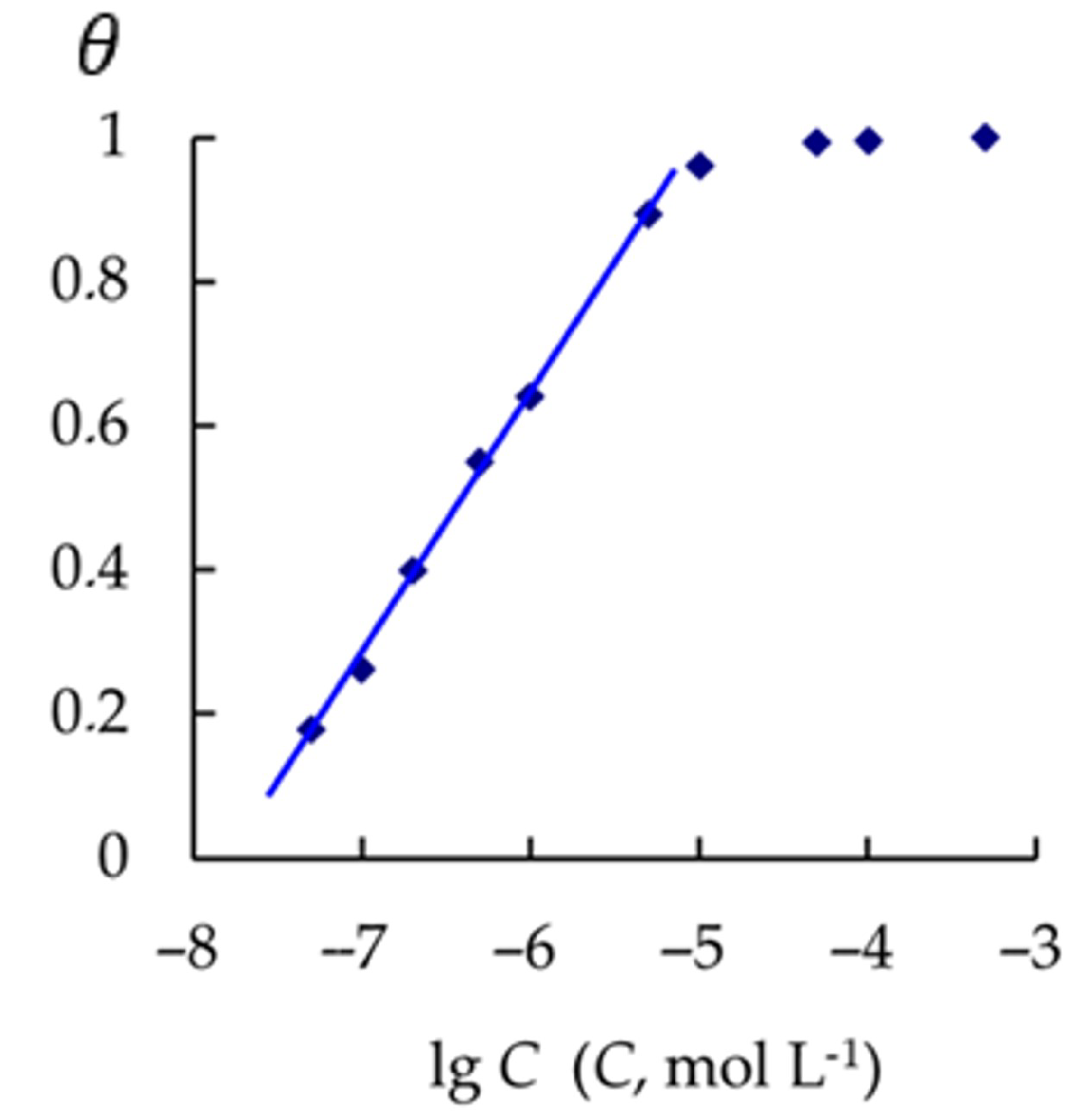
3.4. Adsorption of CATA on Steel
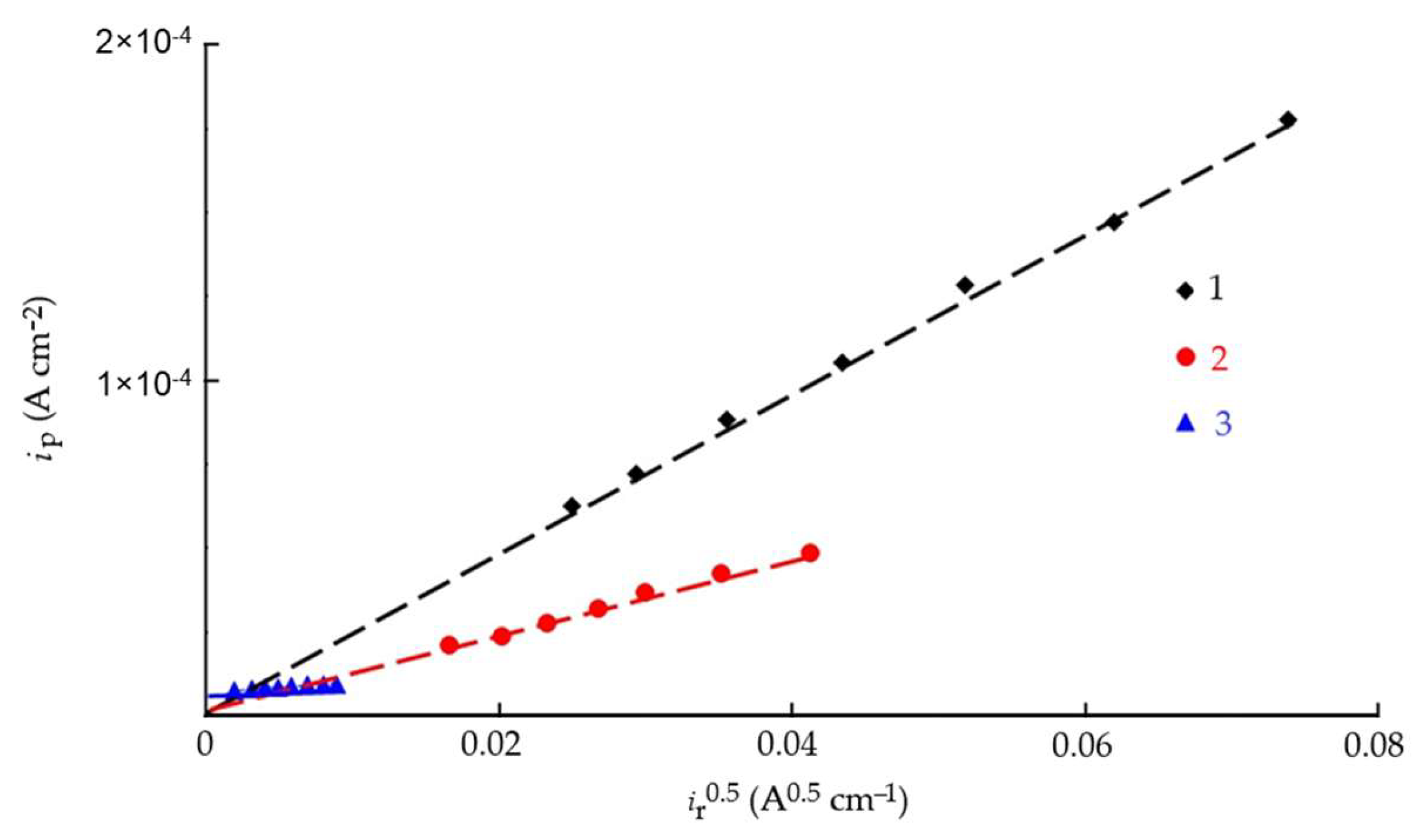
3.5. Kinetics of Cathodic Hydrogen Evolution and Hydrogen Penetration into Steel in the Presence of Corrosion Inhibitors
3.6. Calculation of the Kinetic Rate Constants of the Main Stages of Cathodic Hydrogen Evolution and Penetration into Steel
3.7. AFM Study of Steel Surface
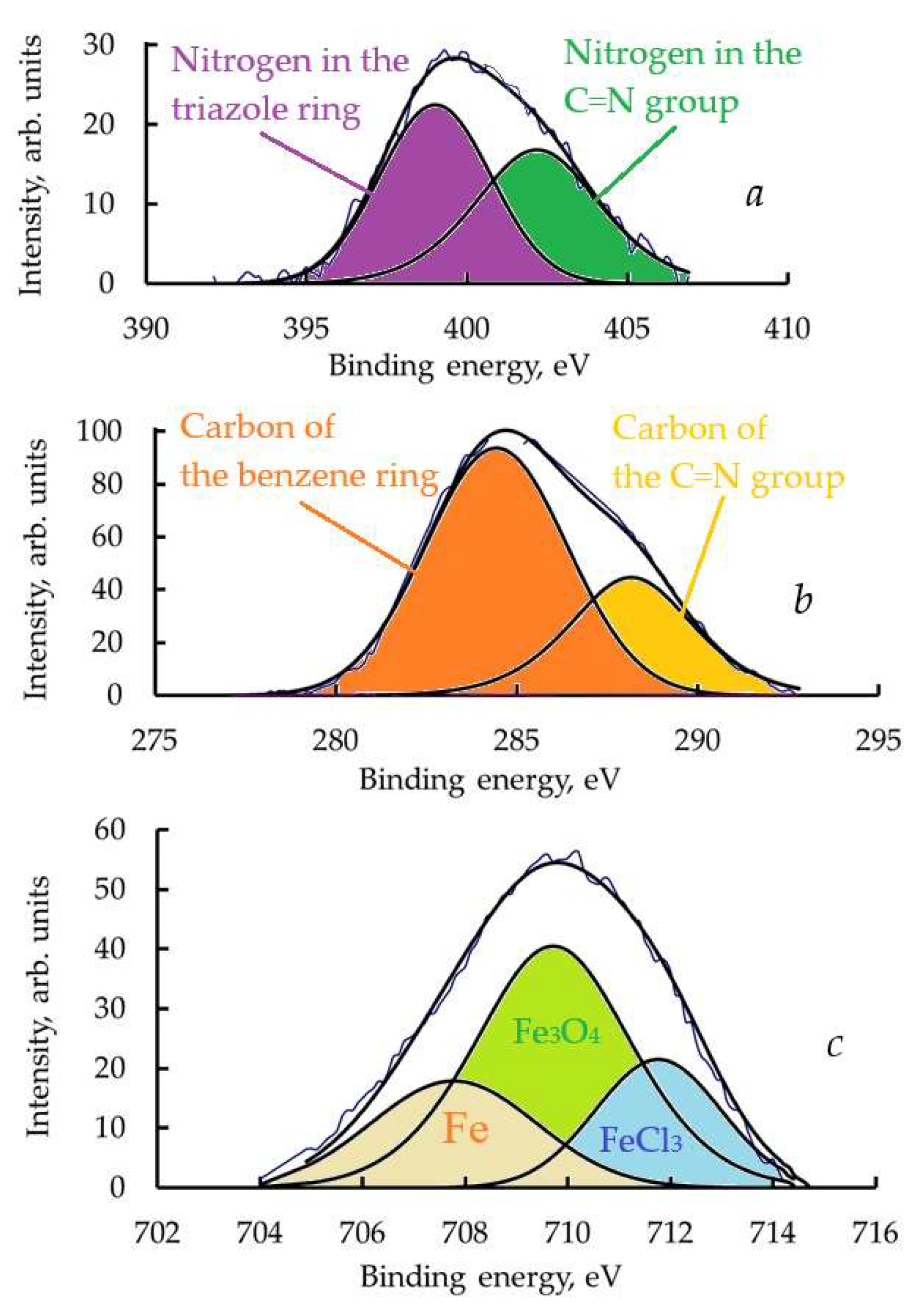
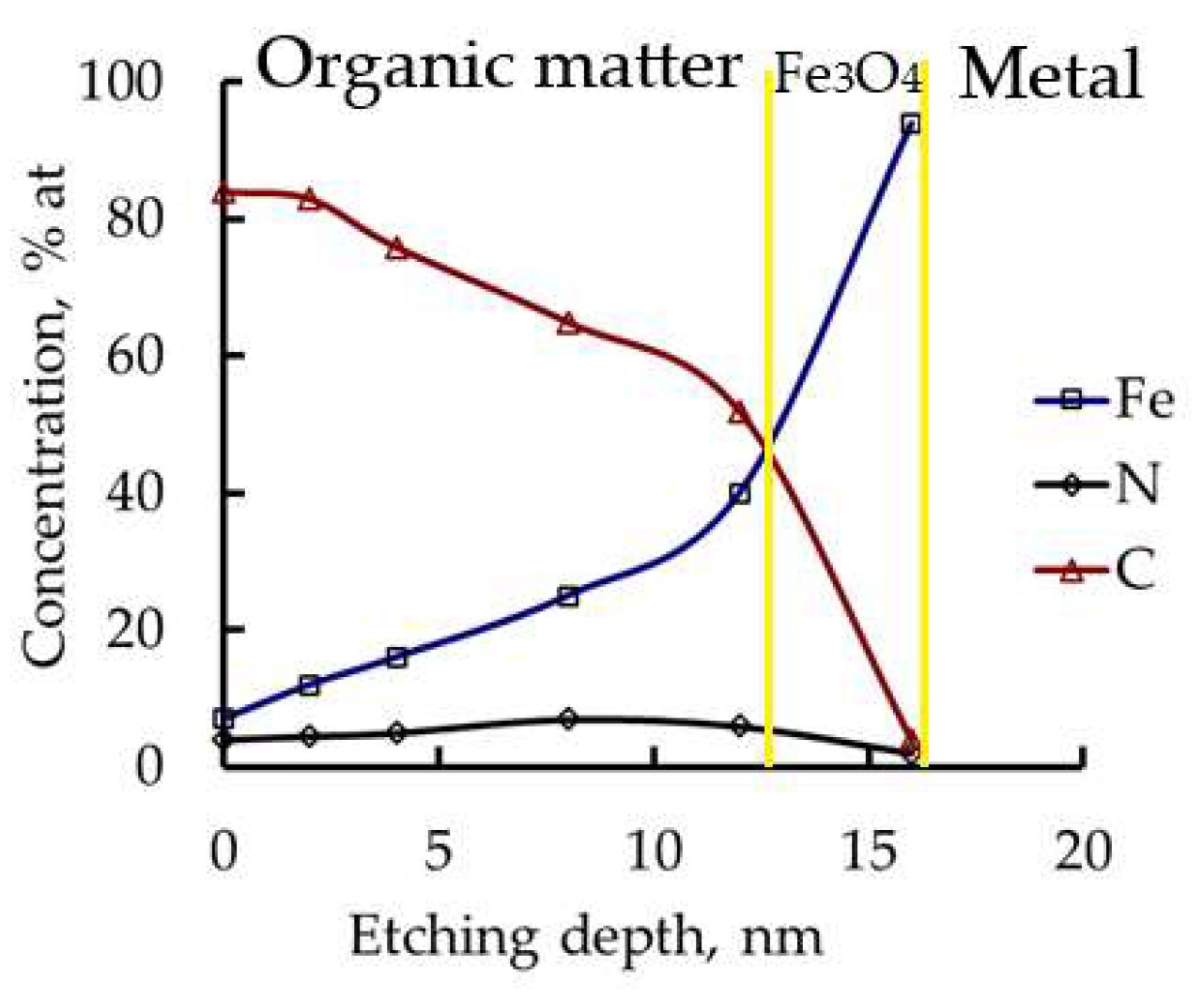
3.8. Layers Formed by CATA on Steel
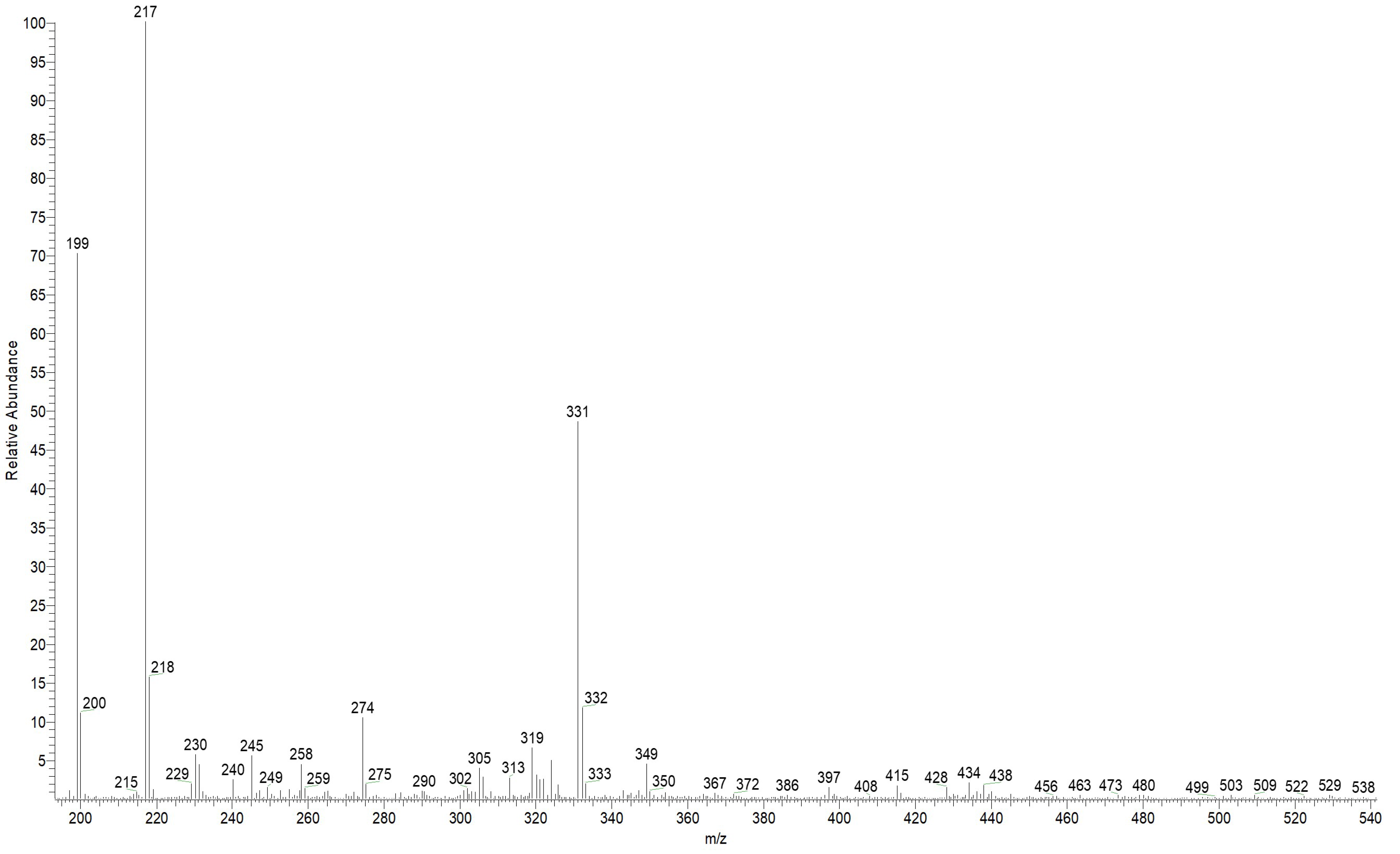
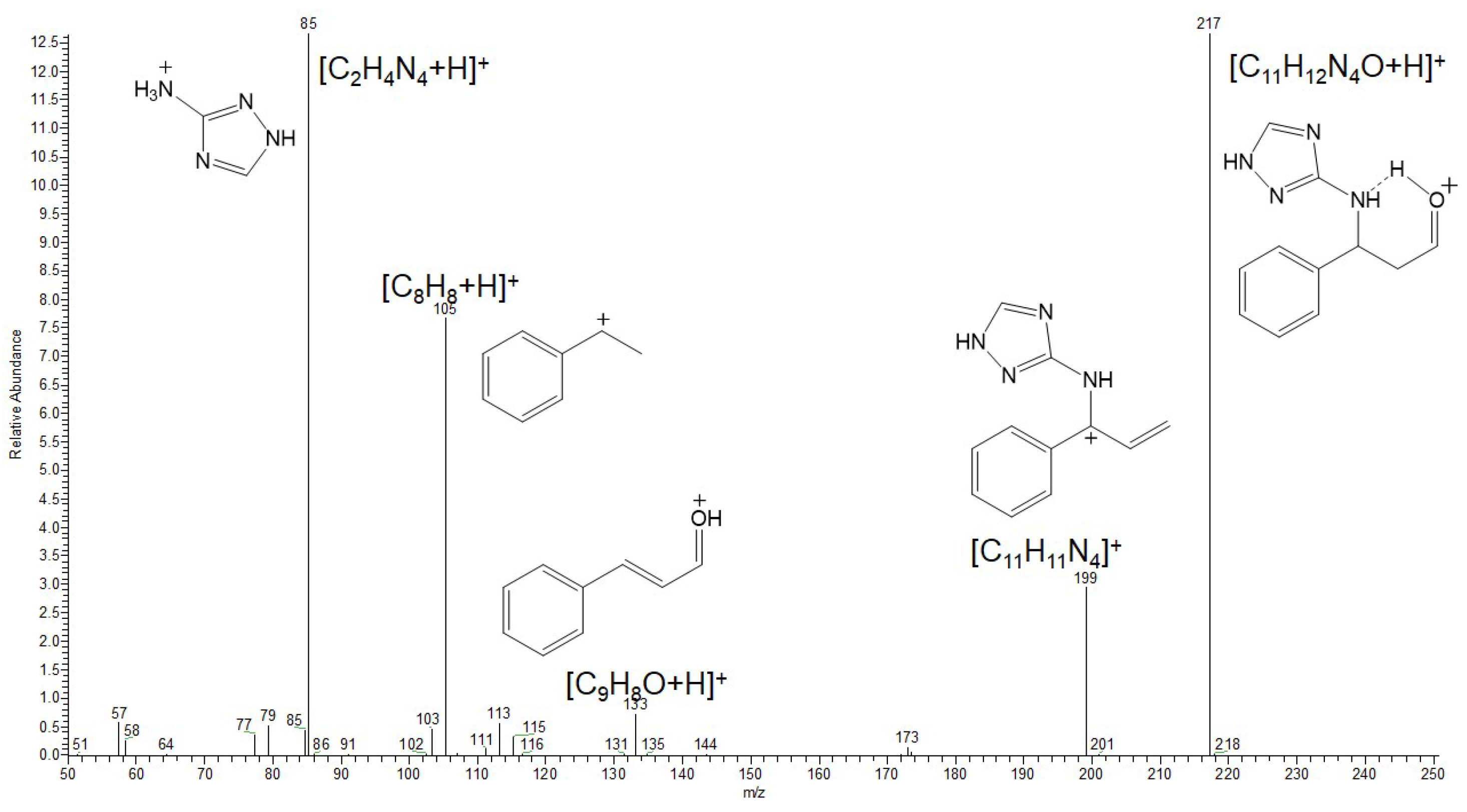
3.9. Analysis of the Condensation Products of Cinnamaldehyde with Amitrole
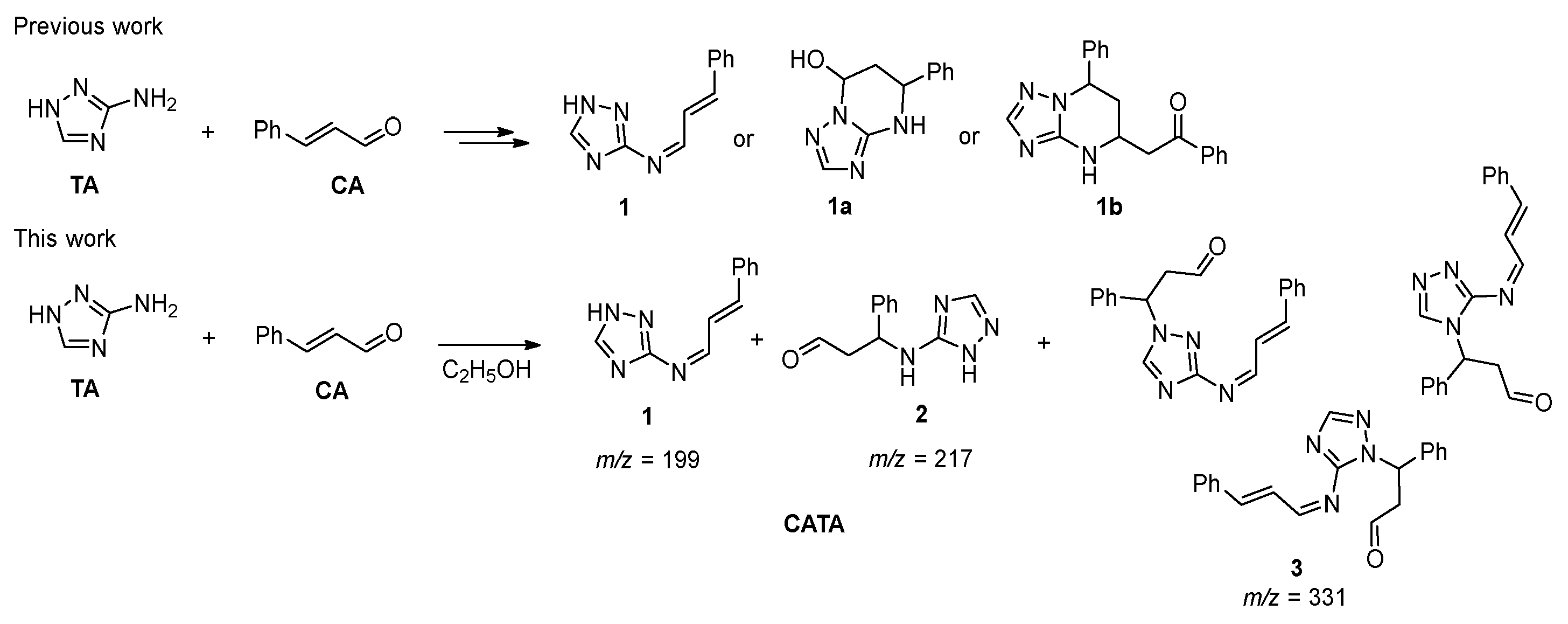
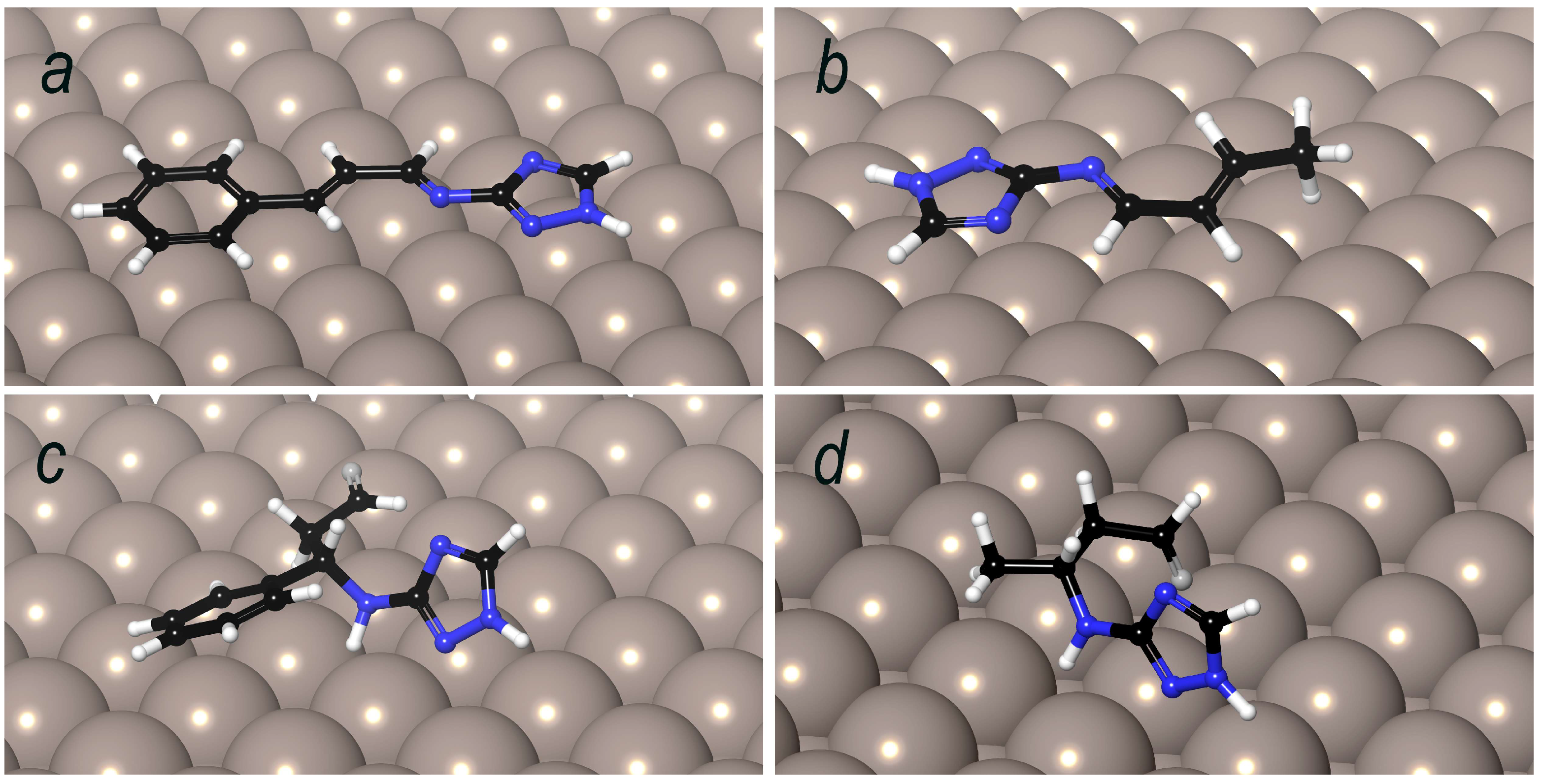
3.10. Molecular Dynamics of 1.2 and 1.4 Condensation Products on Metal Surface
3.11. Molecular Orbital Calculations for the 1.2- and 1.4-Addition Products of Cinnamaldehyde with Aminotriazole
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CA | cinnamaldehyde |
| BA | benzaldehyde |
| CrA | crotonaldehyde |
| TA | 1H-1,2,4-Triazol-3-amine (aminotriazole) |
| CATA | condensation products of cinnamaldehyde with aminotriazole |
| BATA | condensation products of benzaldehyde with aminotriazole |
| CrATA | condensation products of crotonaldehyde with aminotriazole |
References
- Glushchenko, V.N.; Silin, M.A. Neftepromyslovaya Khimiya. Vol. 4. Kislotnaya Obrabotka Skvazhyn (Oilfield Chemistry. Vol. 4. Matrix Acidizing); Mishchenko, I.T., Ed.; Intercontact Nauka: Moscow, Russia, 2010; 703p. (In Russian) [Google Scholar]
- Guo, B.; Liu, X.; Tan, X. Chapter 13. Acidizing. In Petroleum Production Engineering, 2nd ed.; Gulf Professional Publishing: Woburn, MA, USA, 2017; pp. 367–387. [Google Scholar] [CrossRef]
- Leong, V.H.; Hisham Ben, M. A preliminary screening and characterization of suitable acids for sandstone matrix acidizing technique: A comprehensive review. J. Pet. Explor. Prod. Technol. 2019, 9, 753–778. [Google Scholar] [CrossRef]
- Hong, L.V.; Ben Mahmud, H. A Comparative Study of Different Acids used for Sandstone Acid Stimulation: A Literature Review. IOP Conf. Ser. Mater. Sci. Eng. 2017, 217, 012018. [Google Scholar] [CrossRef]
- Shafiq, M.U.; Mahmud, H.B. Sandstone matrix acidizing knowledge and future development. J. Pet. Explor. Prod. Technol. 2017, 7, 1205–1216. [Google Scholar] [CrossRef]
- Schmitt, G. Application of Inhibitors for Acid Media. Br. Corros. J. 1984, 19, 165–176. [Google Scholar] [CrossRef]
- Finšgar, M.; Jackson, J. Application of corrosion inhibitors for steels in acidic media for the oil and gas industry: A review. Corros. Sci. 2014, 86, 17–41. [Google Scholar] [CrossRef]
- Ansari, K.R.; Chauhan, D.S.; Singh, A.; Saji, V.S.; Quraishi, M.A. Corrosion Inhibitors for Acidizing Process in Oil and Gas Sectors. In Corrosion Inhibitors in the Oil and Gas Industry; Saji, V.S., Umoren, S.A., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2020; pp. 153–176. [Google Scholar] [CrossRef]
- Singh, A.; Quraishi, M.A. Acidizing Corrosion Inhibitors: A Review. J. Mater. Environ. Sci. 2015, 6, 224–235. [Google Scholar]
- Askari, M.; Askari, M.; Aliofkhazraei, M.; Jafari, R.; Hamghalam, P.; Hajizadeh, A. Downhole corrosion inhibitors for oil and gas production—A review. Appl. Surf. Sci. Adv. 2021, 6, 100128. [Google Scholar] [CrossRef]
- Solomon, M.M.; Uzoma, I.E.; Olugbuyiro, J.A.O.; Ademosun, O.T. A censorious appraisal of the oil well acidizing corrosion inhibitors. J. Petrol. Sci. Eng. 2022, 215 Pt B, 110711. [Google Scholar] [CrossRef]
- Cui, M.; Yu, Y.; Zheng, Y. Effective Corrosion Inhibition of Carbon Steel in Hydrochloric Acid by Dopamine-Produced Carbon Dots. Polymers 2021, 13, 1923. [Google Scholar] [CrossRef]
- Yousif, Q.A.; Bedair, M.A.; Abuelela, A.M.; Ansari, A.; Malhotra, S.S.; Fadel, Z. High-performance corrosion inhibitors for carbon steel in hydrochloric acid: Electrochemical and DFT studies. RSC Adv. 2025, 15, 28666–28688. [Google Scholar] [CrossRef]
- Bondok, Z.Z.; Wahba, A.M.; Waly, M.M.; Salem, A.F.; Fouda, A.A.S. Synthesis and inhibitive characteristic of two benzothiazole derivatives towards the corrosion of carbon steel in hydrochloric acid solutions. Results Chem. 2025, 17, 102606. [Google Scholar] [CrossRef]
- Alfakeer, M.; Felaly, R.N.; Al-Juaid, S.S.; Seyam, D.F.; Mabrouk, E.M.; Abdallah, M. Synthesis and cyclic voltammetric studies of azo dye compounds derived from 1,5-dihydroxynaphthalene and their application as corrosion inhibitors for carbon steel in hydrochloric acid solution. Int. J. Electrochem. Sci. 2025, 20, 100892. [Google Scholar] [CrossRef]
- Timoudan, N.; Al-Gorair, A.S.; El Foujji, L.; Warad, I.; Safi, Z.; Dikici, B.; Benhiba, F.; El Kacem Qaiss, A.; Bouhfid, R.; Bentiss, F.; et al. Corrosion inhibition performance of benzimidazole derivatives for protection of carbon steel in hydrochloric acid solution. RSC Adv. 2024, 14, 30295–30316. [Google Scholar] [CrossRef] [PubMed]
- Hassan, H.M. Potentilla erecta extract as a green and eco-friendly corrosion inhibitor for 360 carbon steel in hydrochloric acid environment. Int. J. Electrochem. Sci. 2025, 20, 101028. [Google Scholar] [CrossRef]
- Mamudu, U.; Santos, J.H.; Umoren, S.A.; Alnarabiji, M.S.; Lim, R.C. Investigations of corrosion inhibition of ethanolic extract of Dillenia suffruticosa leaves as a green corrosion inhibitor of mild steel in hydrochloric acid medium. Corros. Commun. 2024, 15, 52–62. [Google Scholar] [CrossRef]
- Li, Y.; Guo, Z.; Zhi, H.; Qiang, Y.; Liu, X.; Zhang, Y.; Wan, Y.; Xiang, T.; Li, X. Experimental and computational exploration of the biodegradable platanus acerifolia leaf extract against mild steel corrosion in hydrochloric acid. J. Mater. Res. Technol. 2024, 30, 7830–7842. [Google Scholar] [CrossRef]
- Liu, X.; Gao, Y.; Guan, J.; Zhang, Q.; Lin, Y.; Shi, C.; Wang, Y.; Du, J.; Ma, N. Corrosion inhibition properties of spinach extract on Q235 steel in a hydrochloric acid medium. Arab. J. Chem. 2023, 16, 105066. [Google Scholar] [CrossRef]
- Feng, L.; Yao, H.; Ma, X.; Zhu, H.; Hu, Z. Effect of three penicillin-based as corrosion inhibitors on Q235 steel in hydrochloric acid. Int. J. Electrochem. Sci. 2023, 18, 100368. [Google Scholar] [CrossRef]
- Avdeev, Y.G. Protection of Steel in Solutions of Mineral Acids Using α,β-Unsaturated Aldehydes, Ketones, and Azomethines. Prot. Met. Phys. Chem. Surf. 2015, 51, 1140–1148. [Google Scholar] [CrossRef]
- Growcock, F.B.; Frenier, W.W. Kinetics of Steel Corrosion in Hydrochloric Acid Inhibited with trans-Cinnamaldehyde. J. Electrochem. Soc. 1988, 135, 817–822. [Google Scholar] [CrossRef]
- Cabello, G.; Funkhouser, G.P.; Cassidy, J.; Kiser, C.E.; Lane, J.; Cuesta, A. CO and trans-cinnamaldehyde as corrosion inhibitors of I825, L80-13Cr and N80 alloys in concentrated HCl solutions at high pressure and temperature. Electrochim. Acta 2013, 97, 1–9. [Google Scholar] [CrossRef]
- Growcock, F.B. Inhibition of Steel Corrosion in HCl by Derivatives of Cinnamaldehyde: Part I. Corrosion Inhibition Model. Corrosion 1989, 45, 1003–1007. [Google Scholar] [CrossRef]
- Growcock, F.B.; Frenier, W.W.; Andreozz, P.A. Inhibition of Steel Corrosion in HCl by Derivatives of Cinnamaldehyde: Part II. Structure-Activity Correlations. Corrosion 1989, 45, 1007–1015. [Google Scholar] [CrossRef]
- Zucchi, F.; Trabanelli, G.; Brunoro, G. Iron corrosion inhibition in hot 4 M HCl solution by t-cinnamaldehyde and its structure-related compounds. Corros. Sci. 1994, 36, 1683–1690. [Google Scholar] [CrossRef]
- Lazrak, J.; Ech-chihbi, E.; El Ibrahimi, B.; El Hajjaji, F.; Rais, Z.; Tachihante, M.; Taleb, M. Detailed DFT/MD simulation, surface analysis and electrochemical computer explorations of aldehyde derivatives for mild steel in 1.0 M HCl. Colloids Surf. A Physicochem. Eng. Asp. 2022, 632, 127822. [Google Scholar] [CrossRef]
- Kumar, D.; Muralidhar, K.V.; Jain, V.; Rai, B. Integrating experiments, DFT and characterization for comprehensive corrosion inhibition studies—A case for cinnamaldehyde as an excellent green inhibitor for steels in acidic media. Corros. Sci. 2022, 208, 110623. [Google Scholar] [CrossRef]
- Growcock, F.B.; Lopp, V.R. Film Formation on Steel in Cinnamaldehyde-Inhibited Hydrochloric Acid. Corrosion 1988, 44, 248–254. [Google Scholar] [CrossRef]
- Avdeev, Y.G.; Kuznetsov, Y.I.; Buryak, A.K. Inhibition of steel corrosion by unsaturated aldehydes in solutions of mineral acids. Corros. Sci. 2013, 69, 50–60. [Google Scholar] [CrossRef]
- Jiancun, G.; Yongji, W.; Salitanate; Li, F.; Hong, Y. Corrosion inhibition of α,β-unsaturated carbonyl compounds on steel in acid medium. Pet. Sci. 2009, 6, 201–207. [Google Scholar] [CrossRef]
- Frenier, W.W.; Growcock, F.B.; Lopp, V.R. α-Alkenylphenones—A New Class of Acid Corrosion Inhibitors. Corrosion 1988, 44, 590–598. [Google Scholar] [CrossRef]
- Growcock, F.B. Corrosion Kinetics of J55 Steel in Hydrochloric Acid Inhibited with Benzoyl Allyl Alcohol. Corrosion 1989, 45, 393–401. [Google Scholar] [CrossRef]
- Richmond, H. Preparation of Cinnamaldehyde. US Patent Application 2529186, 7 November 1950. [Google Scholar]
- Barceloux, D.G. Cinnamon (Cinnamomum Species). Dis. Mon. 2009, 55, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Haddi, K.; Faroni, L.R.A.; Oliveira, E.E. Cinnamon Oil. In Green Pesticides Handbook: Essential Oils for Pest Control; Nollet, L.M.L., Rathore, H.S., Eds.; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2017; pp. 117–150. [Google Scholar]
- Chauhan, D.S.; Mazumder, M.A.J.; Quraishi, M.A.; Ansari, K.R. Chitosan-cinnamaldehyde Schiff base: A bioinspired macromolecule as corrosion inhibitor for oil and gas industry. Int. J. Biol. Macromol. 2020, 158, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Ahamad, I.; Prasad, R.; Quraishi, M.A. Thermodynamic, electrochemical and quantum chemical investigation of some Schiff bases as corrosion inhibitors for mild steel in hydrochloric acid solutions. Corros. Sci. 2010, 52, 933–942. [Google Scholar] [CrossRef]
- Ansari, K.R.; Quraishi, M.A.; Singh, A. Schiff’s base of pyridyl substituted triazoles as new and effective corrosion inhibitors for mild steel in hydrochloric acid solution. Corros. Sci. 2014, 79, 5–15. [Google Scholar] [CrossRef]
- Satpati, S.; Suhasaria, A.; Ghosal, S.; Saha, A.; Dey, S.; Sukul, D. Amino acid and cinnamaldehyde conjugated Schiff bases as proficient corrosion inhibitors for mild steel in 1 M HCl at higher temperature and prolonged exposure: Detailed electrochemical, adsorption and theoretical study. J. Mol. Liq. 2021, 324, 115077. [Google Scholar] [CrossRef]
- Avdeev, Y.G.; Nenasheva, T.A.; Luchkin, A.Y.; Marshakov, A.I.; Kuznetsov, Y.I. Complex Inhibitor Protection of Some Steels in Hydrochloric Acid Solutions by 1,2,4-Triazole Derivatives. Materials 2025, 18, 464. [Google Scholar] [CrossRef]
- Calow, A.D.J.; Carbo, J.J.; Cid, J.; Fernandez, E.; Whiting, A. Understanding α,β-Unsaturated Imine Formation from Amine Additions to α,β-Unsaturated Aldehydes and Ketones: An Analytical and Theoretical Investigation. J. Org. Chem. 2014, 79, 5163–5172. [Google Scholar] [CrossRef]
- Avdeev, Y.G.; Luchkin, A.Y.; Kuznetsov, Y.I. Adsorption of IFKhAN 92 Corrosion Inhibitor on Low Carbon Steel from Hydrochloric Acid Solution. Prot. Met. Phys. Chem. Surf. 2013, 49, 865–868. [Google Scholar] [CrossRef]
- Devanathan, M.A.V.; Stachurski, Z.J. Mechanism of Hydrogen Evolution on Iron in Acid Solutions by Determination of Permeation Rates. Electrochem. Soc. 1964, 111, 619–623. [Google Scholar] [CrossRef]
- Avdeev, Y.G.; Nenasheva, T.A.; Luchkin, A.Y.; Marshakov, A.I.; Kuznetsov, Y.I. Effect of Quaternary Ammonium Salts and 1,2,4-Triazole Derivatives on Hydrogen Absorption by Mild Steel in Hydrochloric Acid Solution. Materials 2022, 15, 6989. [Google Scholar] [CrossRef]
- Iyer, R.N.; Pickering, H.W.; Zamanzadeh, M. Analysis of Hydrogen Evolution and Entry into Metals for the Discharge-Recombination Process. J. Electrochem. Soc. 1989, 136, 2463–2472. [Google Scholar] [CrossRef]
- Marshakov, A.I.; Nenasheva, T.A. Effect of sorbed hydrogen on iron dissolution in the presence of tetraethylammonium cations. Prot. Met. Phys. Chem. Surf. 2002, 38, 556–562. [Google Scholar] [CrossRef]
- Necas, D.; Klapetek, P. Gwyddion Software. 3 November 2022. Available online: http://gwyddion.net/download.php#stable (accessed on 20 September 2025).
- Wagner, C.D.; Davis, L.E.; Zeller, M.V.; Taylor, J.A.; Raymond, R.H.; Gale, L.H. Empirical atomic sensitivity factors for quantitative analysis by electron spectroscopy for chemical analysis. Surf. Interface Anal. 1981, 3, 211–225. [Google Scholar] [CrossRef]
- Shirley, D.A. High-Resolution X-Ray Photoemission Spectrum of the Valence Bands of Gold. Phys. Rev. B 1972, 5, 4709–4713. [Google Scholar] [CrossRef]
- Lin, T.-C.; Sesadri, G.; Kelber, J.A. A consistent method for quantitative XPS peak analysis of thin oxide films on clean polycrystalline iron surfaces. Appl. Surf. Sci. 1997, 119, 83–92. [Google Scholar] [CrossRef]
- Graat, P.C.J.; Somers, M.A.J. Simultaneous determination of composition and thickness of thin iron-oxide films from XPS Fe2p spectra. Appl. Surf. Sci. 1996, 100–101, 36–40. [Google Scholar] [CrossRef]
- Powell, C.J.; Conny, J.M. Evaluation of uncertainties in X-ray photoelectron spectroscopy intensities associated with different methods and procedures for background subtraction. II. Spectra for unmonochromated Al and Mg X-rays. Surf. Interface Anal. 2009, 41, 804–813. [Google Scholar] [CrossRef]
- Mohai, M.; Bertoti, I. Calculation of overlayer thickness on curved surfaces based on XPS intensities. Surf. Interface Anal. 2004, 36, 805–808. [Google Scholar] [CrossRef]
- Scofield, J.H. Hartree-Slater Subshell Photoionization Cross-sections at 1254 and 1487 eV. J. Electron Spectrosc. Relat. Phenom. 1976, 8, 129–137. [Google Scholar] [CrossRef]
- Cumpson, P.J.; Seah, M.P. Elastic Scattering Correction in AES and XPS. II. Estimating Attenuation Lengths and Conditions Required for their Valid Use on Overlayer/Substrate Experiments. Surf. Interface Anal. 1997, 25, 430–446. [Google Scholar] [CrossRef]
- Ivanov, E.S. Ingibitory Korrozii Metallov v Kislykh Sredakh (Inhibitors of Metal Corrosion in Acid Media); Metallurgiya: Moscow, Russia, 1986; 175p. (In Russian) [Google Scholar]
- Kokalj, A. Corrosion inhibitors: Physisorbed or chemisorbed? Corros. Sci. 2022, 196, 109939. [Google Scholar] [CrossRef]
- Popov, B.N.; Lee, J.-W.; Djukic, M.B. Chapter 7—Hydrogen Permeation and Hydrogen-Induced Cracking. In Handbook of Environmental Degradation of Materials, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 133–162. [Google Scholar] [CrossRef]
- Reshetnikov, S.M. Ingibitory Kislotnoy Korrozii Metallov (Inhibitors of Acid Corrosion of Metals); Chemistry: Leningrad, Russia, 1986; 144p. (In Russian) [Google Scholar]
- Afanas’ev, B.N.; Skobochkina, Y.P.; Serdyukova, G.G. Physicochemical Bases of the Action of Corrosion Inhibitors; UdGu: Moscow, Russia, 1990; 20p. (In Russian) [Google Scholar]
- Kiuchi, K.; Mc Lellan, R.B. The solubility and diffusivity of hydrogen in well-annealed and deformed iron. Acta Metall. 1983, 31, 961–984. [Google Scholar] [CrossRef]
- Avdeev, Y.G.; Nenasheva, T.A.; Luchkin, A.Y.; Marshakov, A.I.; Kuznetsov, Y.I. Thin Films of a Complex Polymer Compound for the Inhibition of Iron Alloy Corrosion in a H3PO4 Solution. Polymers 2023, 15, 4280. [Google Scholar] [CrossRef]
- Lipson, V.V.; Karnozhitskaya, T.M.; Shishkina, S.V.; Shishkin, O.V.; Turov, A.V. Reactions of 3-Amino-1,2,4-Triazoles with Cinnamic Aldehydes. Russ. Chem. Bull. 2009, 58, 1441–1444. [Google Scholar] [CrossRef]
- Zemlyanaya, N.I.; Karnozhitskaya, T.M.; Musatov, V.I.; Konovalova, I.S.; Shishkina, S.V.; Lipson, V.V. Synthesis and Chemical Transformations of 5-Alkyl(Phenyl)-4,5,6,7-Tetrahydro[1,2,4]Triazolo[1,5-a]Pyrimidin-7-Oles. Russ. J. Org. Chem. 2018, 54, 1241–1249. [Google Scholar] [CrossRef]
- Kokalj, A. On the Alleged Importance of the Molecular Electron-Donating Ability and the HOMO–LUMO Gap in Corrosion Inhibition Studies. Corros. Sci. 2021, 180, 109016. [Google Scholar] [CrossRef]





| № | Inhibitor | Corrosive Environment | Steel | Z, % | Ref. |
|---|---|---|---|---|---|
| 1 | Nitrogen doped carbon dots synthesized using a dopamine (400 ppm) | 1 M HCl (25–55 °C) | Q235 carbon steel | 86.2–92.9 | [12] |
| 2 | (S,E)-2-((1-(4-Minophenyl)ethylidene)amino) (3-(4-hydroxyphenyl)propanoic acid) (10 mM) | 1 M HCl (30 °C) | Carbon steel | 90.4 | [13] |
| 3 | 2-Cyano-N-(6-methylbenzo[d]thiazol-2-yl)-3-(4-nitrophenyl) acrylamide (0.1 mM) | 2 M HCl (25–45 °C) | Carbon steel | 73.9–85.8 | [14] |
| 4 | bis-Azo dyes derived from 1,5-dihydroxynaphthalene (1 mM) | 1 M HCl (24 °C) | Carbon steel | 78.2–97.1 | [15] |
| 5 | 1-Dodecyl-2-((dodecylthio)methyl)-1Hbenzimidazole (1 mM) | 1 M HCl (30–60 °C) | Carbon steel | 71.1–95.4 | [16] |
| 6 | Potentilla erecta (Tormentil) extract (300 ppm) | 1 M HCl (25–55 °C) | 360 carbon steel | 90.2–92.7 | [17] |
| 7 | Dillenia suffruticosa leaves extract (1 g L−1) | 1 M HCl (25 °C) | Mild steel | 91.3 | [18] |
| 8 | Platanus acerifolia leaf extract (0.4 g L−1) | 1 M HCl (25–45 °C) | Q235 carbon steel | 83.8–88.9 | [19] |
| 9 | Spinach extract (0.3 g L−1) | 1 M HCl (20–50 °C) | Q235 carbon steel | 88.6–95.3 | [20] |
| 10 | Sodium carbenicillin, sodium ampicillin, and sodium sulbactam (8 mM) | 1 M HCl (25–40 °C) | Q235 carbon steel | 49.1–96.8 | [21] |
| № | Inhibitor | Molecular Formula | Molar Mass (g mol−1) | Structural Formula | Label |
|---|---|---|---|---|---|
| 1 | cinnamaldehyde | C9H8O | 132 | 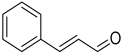 | CA |
| 2 | benzaldehyde | C7H6O | 106 | 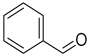 | BA |
| 3 | crotonaldehyde | C4H6O | 70 |  | CrA |
| 4 | 1H-1,2,4-Triazol-3-amine | C2H4N4 | 84 | 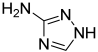 | TA |
| Inhibitor | Δms, W, Z | T (°C) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 20 | 30 | 40 | 50 | 60 | 70 | 80 | 90 | 100 | ||
| – | Δms | 3.6 | 8.6 | 18 | 45 | 120 | 290 | 700 | 1170 | 1830 |
| W | 1.8 | 4.3 | 9.0 | 23 | 60 | 145 | 350 | 590 | 920 | |
| TA | Δms | 2.2 | 4.0 | 7.6 | 20 | 68 | 170 | 450 | 1060 | 1770 |
| W | 1.1 | 2 | 3.8 | 10 | 34 | 85 | 230 | 530 | 880 | |
| Z | 38.9 | 53.5 | 57.8 | 55.6 | 43.3 | 41.4 | 35.7 | 9.4 | 3.3 | |
| CA | Δms | 0.86 | 1.6 | 2.6 | 6.2 | 12 | 16 | 30 | 49 | 340 |
| W | 0.43 | 0.8 | 1.3 | 3.1 | 6 | 8 | 15 | 24.5 | 170 | |
| Z | 76.1 | 81.4 | 85.6 | 86.2 | 90.0 | 94.5 | 95.7 | 95.8 | 81.4 | |
| CATA | Δms | 0.70 | 0.96 | 1.4 | 1.9 | 2.2 | 3.0 | 4.4 | 5.8 | 6.6 |
| W | 0.35 | 0.48 | 0.70 | 0.95 | 1.1 | 1.5 | 2.2 | 2.9 | 3.3 | |
| Z | 80.6 | 88.8 | 92.2 | 95.8 | 98.2 | 90.0 | 99.4 | 99.5 | 99.6 | |
| CA + TA | Δms | 0.20 | 0.54 | 0.84 | 2.0 | 5.2 | 9.8 | 12 | 18 | 130 |
| W | 0.10 | 0.27 | 0.42 | 1.0 | 2.6 | 4.9 | 6 | 9 | 65 | |
| Z | 94.4 | 93.7 | 95.3 | 95.5 | 95.7 | 96.6 | 98.3 | 98.5 | 92.9 | |
| CrA | Δms | 3.4 | 8.2 | 10 | 16 | 30 | 53 | 170 | 690 | 1290 |
| W | 1.7 | 4.1 | 5.0 | 8.0 | 15 | 26.5 | 85 | 345 | 645 | |
| Z | 5.6 | 4.7 | 44.4 | 64.4 | 75.0 | 81.7 | 75.7 | 41.0 | 29.5 | |
| CrATA | Δms | 1.3 | 2.6 | 3.8 | 7.6 | 15 | 27 | 45 | 120 | 190 |
| W | 0.65 | 1.3 | 1.9 | 3.8 | 7.5 | 13.5 | 22.5 | 60 | 95 | |
| Z | 63.9 | 69.8 | 78.9 | 83.1 | 87.5 | 90.7 | 93.6 | 89.7 | 89.6 | |
| CrA + TA | Δms | 2.8 | 8.0 | 10 | 13 | 26 | 45 | 100 | 430 | 750 |
| W | 1.4 | 4.0 | 5.0 | 6.5 | 13 | 22.5 | 50 | 215 | 375 | |
| Z | 22.2 | 7.0 | 44.4 | 71.4 | 78.3 | 84.5 | 85.7 | 63.2 | 59.0 | |
| BA | Δms | 4.0 | 8.0 | 15 | 26 | 63 | 140 | 430 | 990 | 1630 |
| W | 2.0 | 4.0 | 7.5 | 13 | 31.5 | 70 | 215 | 495 | 815 | |
| Z | –11.1 | 7.0 | 16.7 | 42.2 | 47.5 | 51.7 | 38.6 | 15.4 | 10.9 | |
| BATA | Δms | 2.4 | 5.0 | 8.8 | 16 | 34 | 92 | 240 | 460 | 1020 |
| W | 1.2 | 2.5 | 4.4 | 8 | 17 | 46 | 120 | 230 | 510 | |
| Z | 33.3 | 41.9 | 51.1 | 64.4 | 71.7 | 68.3 | 65.7 | 60.7 | 44.3 | |
| BA + TA | Δms | 2.2 | 4.8 | 4.6 | 18 | 42 | 100 | 260 | 600 | 1200 |
| W | 1.1 | 2.4 | 2.3 | 9 | 21 | 50 | 130 | 300 | 600 | |
| Z | 38.9 | 44.2 | 74.4 | 60.0 | 65.0 | 65.5 | 62.9 | 48.7 | 34.4 | |
| Inhibitor | - | TA | BATA | CrATA | CATA | CA |
|---|---|---|---|---|---|---|
| Eac, kJ mol−1 | 74 | 81 | 70 | 57 | 26 | 52 |
| Inhibitor | Temperature, °C | W, g m−2 h−1 | Vs(H2), mL (100 g of Metal)−1 | π, % |
|---|---|---|---|---|
| - | 25 | 11.4 | 4.62 | - * |
| 10 mM CA | 25 | 1.3 | 0.75 | 100 |
| 10 mM CATA | 25 | 0.73 | 0.60 | 100 |
| - | 60 | 48.7 | 2.56 | - |
| 10 mM CA | 60 | 12.2 | 0.36 | 52 |
| 10 mM CATA | 60 | 2.2 | 0.33 | 100 |
| Inhibitor | Ecor, V | bc | ba * | ic | ia | Zc | Za |
|---|---|---|---|---|---|---|---|
| 2M HCl | |||||||
| - | −0.235 | 0.130 | 0.060 | 866 | 872 | - | - |
| 0.03 mM CATA | −0.230 | 0.135 | 0.065 | 708 | 636 | 18.2 | 27.1 |
| 0.1 mM CATA | −0.225 | 0.135 | 0.070 | 382 | 266 | 55.9 | 69.5 |
| 1.0 mM CATA | −0.200 | 0.140 | 0.090 | 162 | 59.4 | 81.3 | 93.2 |
| 10.0 mM CATA | −0.260 | 0.180 | id ** | 8.7 | 15.0 | 99.0 | 98.3 |
| 10 mM TA | −0.235 | 0.140 | 0.065 | 605 | 588 | 30.1 | 32.6 |
| 10 mM CA | −0.240 | 0.180 | 0.120 | 56.1 | 84.5 | 93.5 | 90.3 |
| 10 mM CA + 10 mM TA | −0.245 | 0.180 | 0.100 | 24.6 | 30.5 | 97.2 | 96.5 |
| Solution | k1,i, mol cm−2 s−1 | k, cm3 mol−1 | kr, mol cm−2 s−1 | θH × 100 | , mol cm−3 | , % |
|---|---|---|---|---|---|---|
| 2 M HCl | 9.73 × 10−9 | 3.5 × 105 | 7.5 × 10−6 | 3.43 | 1.0 × 10−7 | |
| 2 M HCl + 10 mM CrATA | 2.18 × 10−9 | 2.1 × 106 | 8.3 × 10−7 | 3.57 | 1.8 × 10−8 | 81.6 |
| Solution | k1,i, mol cm−2 s−1 | k, cm3 mol−1 | kr, mol cm−2 s−1 | θH × 100 | , mol cm−3 | , % |
|---|---|---|---|---|---|---|
| 2 M HCl | 7.17 × 10−8 | 3.5 × 105 | 7.5 × 10−6 | 9.38 | 3.17 × 10−7 | - |
| 2 M HCl + 10 mM CrATA | 9.64 × 10−9 | 2.1 × 106 | 8.3 × 10−7 | 7.61 | 3.73 × 10−8 | 88.2 |
| Treatment | Micrograph 600 × 400 μm | Topography 30 × 30 μm | Root Mean Square Roughness, nm | Work Function, eV |
|---|---|---|---|---|
| Blank |  | 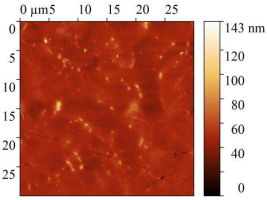 | 5.25 ± 1 | 4.96 |
| 2 M HCl |  | 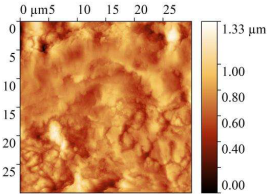 | 141.9 ± 10 | 4.73 |
| 2 M HCl + CrATA |  |  | 63.59 ± 5 | 5.9 |
| 2 M HCl + CATA |  | 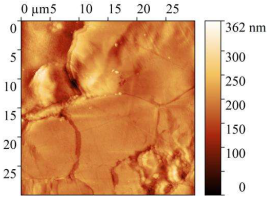 | 31.32 ± 3 | 5.63 |
| T, °C | C, % | N, % | O, % | Cl, % | Fe, % | Thickness, nm |
|---|---|---|---|---|---|---|
| 20 | 32.4 | 11.3 | 9.1 | 5.2 | 21.3 | 2 ± 0.2 |
| 60 | 42.6 | 9.2 | 12.4 | 3.4 | 17.2 | 6 ± 0.5 |
| 95 | 63.9 | 5.4 | 8.4 | 2.1 | 12.3 | 12 ± 0.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bazanov, D.R.; Avdeev, Y.G.; Nenasheva, T.A.; Luchkin, A.Y.; Mazur, D.M.; Makarychev, Y.B.; Andreeva, T.E.; Marshakov, A.I.; Kuznetsov, Y.I. Aldehyde–Aminotriazole Condensation Products as Novel Corrosion Inhibitors for Mild Steel in Hydrochloric Acid. Polymers 2025, 17, 2761. https://doi.org/10.3390/polym17202761
Bazanov DR, Avdeev YG, Nenasheva TA, Luchkin AY, Mazur DM, Makarychev YB, Andreeva TE, Marshakov AI, Kuznetsov YI. Aldehyde–Aminotriazole Condensation Products as Novel Corrosion Inhibitors for Mild Steel in Hydrochloric Acid. Polymers. 2025; 17(20):2761. https://doi.org/10.3390/polym17202761
Chicago/Turabian StyleBazanov, Daniil R., Yaroslav G. Avdeev, Tatyana A. Nenasheva, Andrey Yu. Luchkin, Dmitrii M. Mazur, Yury B. Makarychev, Tatiana E. Andreeva, Andrey I. Marshakov, and Yurii I. Kuznetsov. 2025. "Aldehyde–Aminotriazole Condensation Products as Novel Corrosion Inhibitors for Mild Steel in Hydrochloric Acid" Polymers 17, no. 20: 2761. https://doi.org/10.3390/polym17202761
APA StyleBazanov, D. R., Avdeev, Y. G., Nenasheva, T. A., Luchkin, A. Y., Mazur, D. M., Makarychev, Y. B., Andreeva, T. E., Marshakov, A. I., & Kuznetsov, Y. I. (2025). Aldehyde–Aminotriazole Condensation Products as Novel Corrosion Inhibitors for Mild Steel in Hydrochloric Acid. Polymers, 17(20), 2761. https://doi.org/10.3390/polym17202761








