Unidirectional Polyvinylidene/Copper-Impregnated Nanohydroxyapatite Composite Membrane Prepared by Electrospinning with Piezoelectricity and Biocompatibility for Potential Ligament Repair
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Preparations of Cu-nHA and PVDF/Cu-nHA Nanofibers
2.2.1. Cu-nHA Nanoparticles
2.2.2. Electrospun PVDF/Cu-nHA Nanofiber Preparation
2.3. Characterized Physiochemical Properties
2.3.1. Micromorphology and Fiber Diameter Measurement
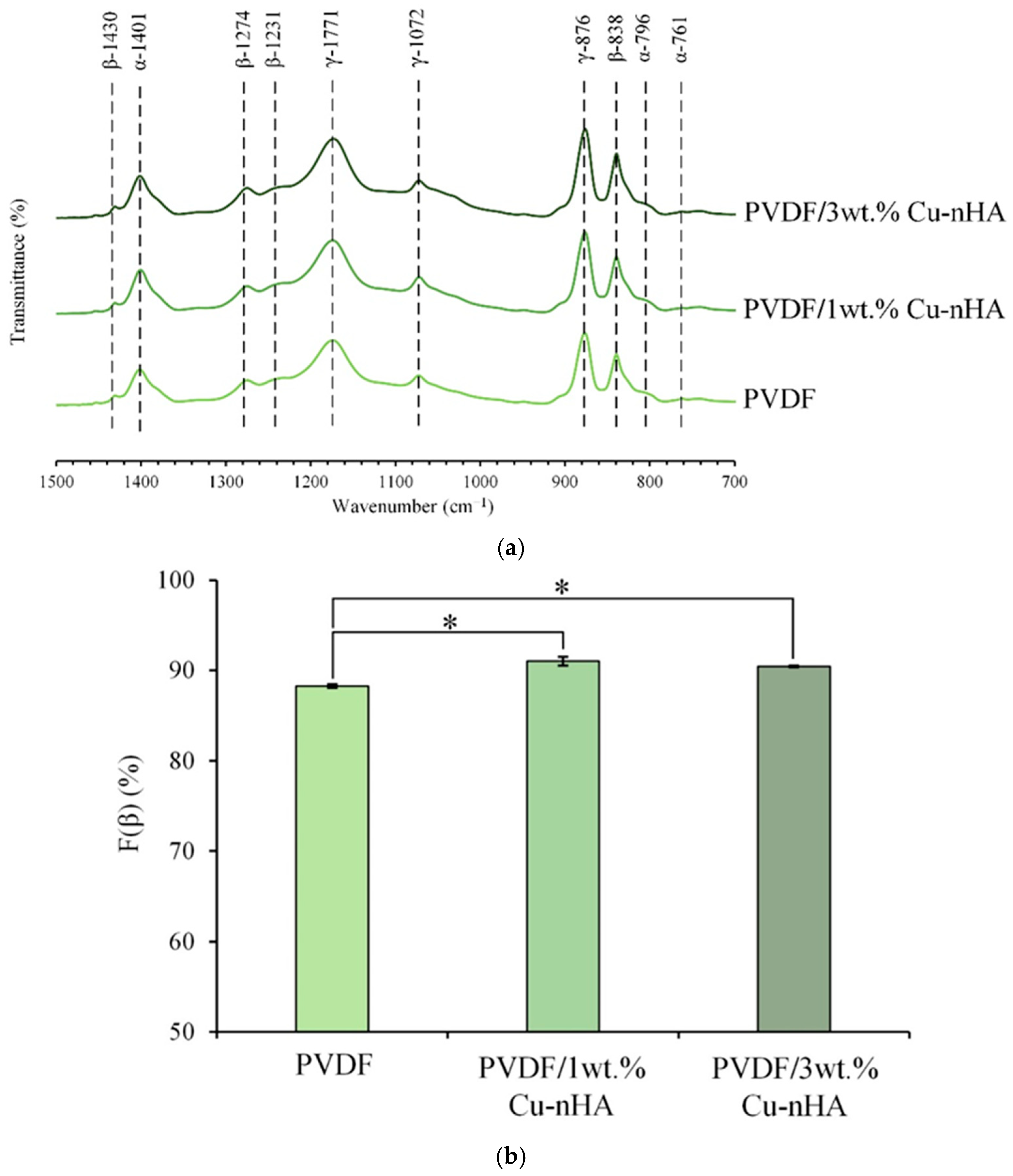
2.3.2. F(β) Index Calculation Through Infrared Spectra
2.3.3. Piezoelectric Response Under Dynamic Bending
2.3.4. Tensile Measurement
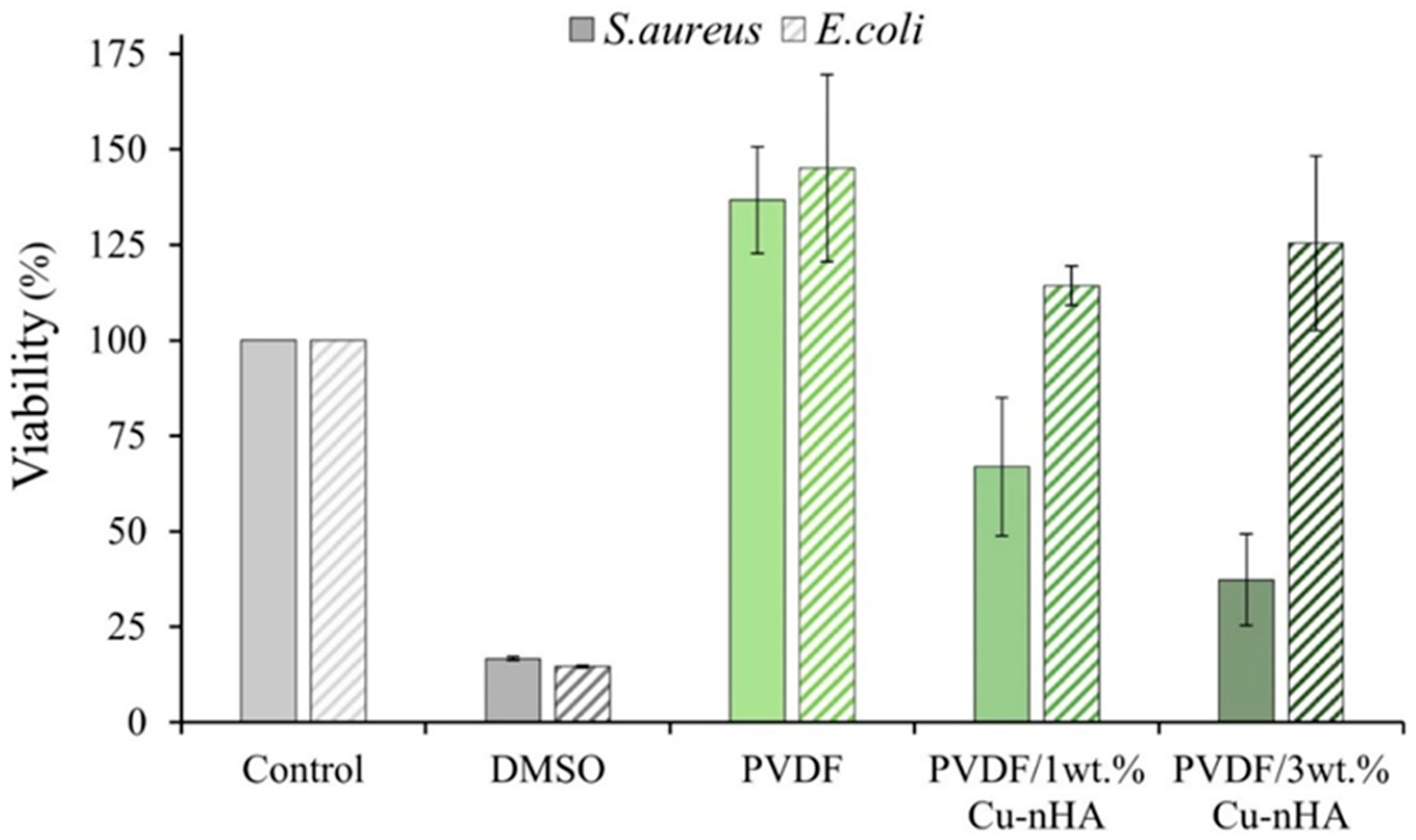
2.4. Antibacterial Tests
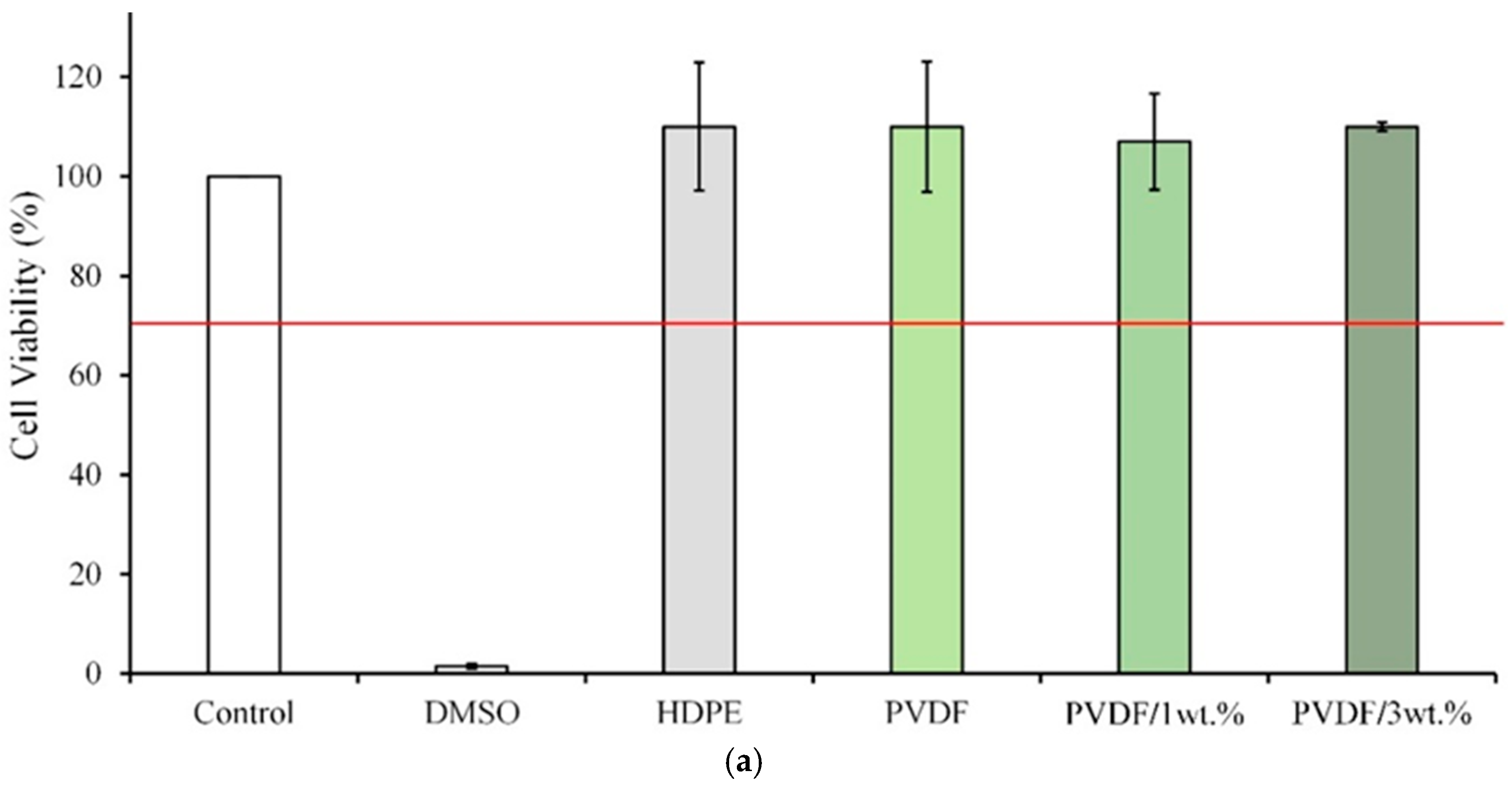
2.5. In Vitro Cytotoxicity Tests
2.6. Statistical Analysis
3. Results and Discussion
3.1. Microstructures and Average Fiber Diameter of Electrospun Membranes
3.2. IR Spectral and F(β) Index Analysis
3.3. Piezoelectric Voltage Generated by Electrospun Membranes Under Dynamic Bending
3.4. Tensile Testing of Membranes
3.5. Antibacterial Testing
3.6. Cytotoxicity Testing
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brennan, D.A.; Conte, A.A.; Kanski, G.; Turkula, S.; Hu, X.; Kleiner, M.T.; Beachley, V. Mechanical considerations for electrospun nanofibers in tendon and ligament repair. Adv. Healthc. Mater. 2018, 7, 1701277. [Google Scholar] [CrossRef] [PubMed]
- Roldán, E.; Reeves, N.D.; Cooper, G.; Andrews, K. 2D and 3D PVA electrospun scaffold evaluation for ligament implant replacement: A mechanical testing, modelling and experimental biomechanics approach. Materialia 2024, 33, 102042. [Google Scholar] [CrossRef]
- Savić, L.; Augustyniak, E.M.; Kastensson, A.; Snelling, S.; Abhari, R.E.; Baldwin, M.; Price, A.; Jackson, W.; Carr, A.; Mouthuy, P.-A. Early development of a polycaprolactone electrospun augment for anterior cruciate ligament reconstruction. Mater. Sci. Eng. C 2021, 129, 112414. [Google Scholar] [CrossRef] [PubMed]
- Pauly, H.M.; Kelly, D.J.; Popat, K.C.; Trujillo, N.A.; Dunne, N.J.; McCarthy, H.O.; Haut Donahue, T.L. Mechanical properties and cellular response of novel electrospun nanofibers for ligament tissue engineering: Effects of orientation and geometry. J. Mech. Behav. Biomed. Mater. 2016, 61, 258–270. [Google Scholar] [CrossRef] [PubMed]
- Sensini, A.; Cristofolini, L. Biofabrication of Electrospun Scaffolds for the Regeneration of Tendons and Ligaments. Materials 2018, 11, 1963. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Shin, H.J.; Cho, I.H.; Kang, Y.-M.; Kim, I.A.; Park, K.-D.; Shin, J.-W. Nanofiber alignment and direction of mechanical strain affect the ECM production of human ACL fibroblast. Biomaterials 2005, 26, 1261–1270. [Google Scholar] [CrossRef] [PubMed]
- Full, S.M.; Delman, C.; Gluck, J.M.; Abdmaulen, R.; Shemin, R.J.; Heydarkhan-Hagvall, S. Effect of fiber orientation of collagen-based electrospun meshes on human fibroblasts for ligament tissue engineering applications. J. Biomed. Mater. Res. Part B Appl. Biomater. 2015, 103, 39–46. [Google Scholar] [CrossRef]
- Li, W.-M.; Zhang, H.; Dai, C.-M.; Miao, J.-J.; Fan, W.; Song, Q.-W.; Xia, L.-J.; Xu, W.-L. Enhanced flexoelectric and piezoelectric pairing of bent polyvinylidene fluoride (PVDF) membrane with two-dimensional (2D) stannic sulfide-bismuth oxychloride (SnS2-BiOCl) heterojunction. Nano Energy 2024, 130, 110104. [Google Scholar] [CrossRef]
- Chen, J.-X.; Li, J.-W.; Jiang, Z.-J.; Chiu, C.-W. Polymer-assisted dispersion of reduced graphene oxide in electrospun polyvinylidene fluoride nanofibers for enhanced piezoelectric monitoring of human body movement. Chem. Eng. J. 2024, 498, 155244. [Google Scholar] [CrossRef]
- Goonoo, N.; Bhaw-Luximon, A. Piezoelectric polymeric scaffold materials as biomechanical cellular stimuli to enhance tissue regeneration. Mater. Today Commun. 2022, 31, 103491. [Google Scholar] [CrossRef]
- Khare, D.; Basu, B.; Dubey, A.K. Electrical stimulation and piezoelectric biomaterials for bone tissue engineering applications. Biomaterials 2020, 258, 120280. [Google Scholar] [CrossRef]
- Zhang, L.; Li, S.; Zhu, Z.; Rui, G.; Du, B.; Chen, D.; Huang, Y.F.; Zhu, L. Recent progress on structure manipulation of poly (vinylidene fluoride)-based ferroelectric polymers for enhanced piezoelectricity and applications. Adv. Funct. Mater. 2023, 33, 2301302. [Google Scholar] [CrossRef]
- Damaraju, S.M.; Wu, S.; Jaffe, M.; Arinzeh, T.L. Structural changes in PVDF fibers due to electrospinning and its effect on biological function. Biomed. Mater. 2013, 8, 045007. [Google Scholar] [CrossRef] [PubMed]
- Kalimuldina, G.; Turdakyn, N.; Abay, I.; Medeubayev, A.; Nurpeissova, A.; Adair, D.; Bakenov, Z. A Review of Piezoelectric PVDF Film by Electrospinning and Its Applications. Sensors 2020, 20, 5214. [Google Scholar] [CrossRef] [PubMed]
- Szewczyk, P.K.; Metwally, S.; Karbowniczek, J.E.; Marzec, M.M.; Stodolak-Zych, E.; Gruszczyński, A.; Bernasik, A.; Stachewicz, U. Surface-Potential-Controlled Cell Proliferation and Collagen Mineralization on Electrospun Polyvinylidene Fluoride (PVDF) Fiber Scaffolds for Bone Regeneration. ACS Biomater. Sci. Eng. 2019, 5, 582–593. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, S.; Gopal, B. Copper substituted hydroxyapatite and fluorapatite: Synthesis, characterization and antimicrobial properties. Ceram. Int. 2014, 40, 15655–15662. [Google Scholar] [CrossRef]
- Du, M.; Chen, J.; Liu, K.; Xing, H.; Song, C. Recent advances in biomedical engineering of nano-hydroxyapatite including dentistry, cancer treatment and bone repair. Compos. B Eng. 2021, 215, 108790. [Google Scholar] [CrossRef]
- Kuśnieruk, S.; Wojnarowicz, J.; Chodara, A.; Chudoba, T.; Gierlotka, S.; Lojkowski, W. Influence of hydrothermal synthesis parameters on the properties of hydroxyapatite nanoparticles. Beilstein J. Nanotechnol. 2016, 7, 1586–1601. [Google Scholar] [CrossRef] [PubMed]
- Rahmati, M.; Mills, D.K.; Urbanska, A.M.; Saeb, M.R.; Venugopal, J.R.; Ramakrishna, S.; Mozafari, M. Electrospinning for tissue engineering applications. Prog. Mater. Sci. 2021, 117, 100721. [Google Scholar] [CrossRef]
- De Lima, I.R.; Alves, G.G.; Soriano, C.A.; Campaneli, A.P.; Gasparoto, T.H.; Schnaider Ramos, E.; De Sena, L.Á.; Rossi, A.M.; Granjeiro, J.M. Understanding the impact of divalent cation substitution on hydroxyapatite: An in vitro multiparametric study on biocompatibility. J. Biomed. Mater. Res. A 2011, 98, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Layek, R.K.; Samanta, S.; Chatterjee, D.P.; Nandi, A.K. Physical and mechanical properties of poly(methyl methacrylate)-functionalized graphene/poly(vinylidine fluoride) nanocomposites: Piezoelectric β polymorph formation. Polymer 2010, 51, 5846–5856. [Google Scholar] [CrossRef]
- Wu, C.M.; Chou, M.H.; Zeng, W.Y. Piezoelectric response of aligned electrospun polyvinylidene fluoride/ carbon nanotube nanofibrous membranes. Nanomaterials 2018, 8, 420. [Google Scholar] [CrossRef] [PubMed]
- ASTM D882-18; Standard Test Method for Tensile Properties of Thin Plastic Sheeting. ASTM International: West Conshohocken, PA, USA, 2018.
- Noori, A.; Hoseinpour, M.; Kolivand, S.; Lotfibakhshaiesh, N.; Ebrahimi-Barough, S.; Ai, J.; Azami, M. Exploring the various effects of Cu doping in hydroxyapatite nanoparticle. Sci. Rep. 2024, 14, 3421. [Google Scholar] [CrossRef]
- Gregorio, R., Jr.; Cestari, M. Effect of crystallization temperature on the crystalline phase content and morphology of poly (vinylidene fluoride). J. Polym. Sci. B Polym. Phys. 1994, 32, 859–870. [Google Scholar] [CrossRef]
- Chen, W.-C.; Huang, B.-Y.; Huang, S.-M.; Liu, S.-M.; Chang, K.-C.; Ko, C.-L.; Lin, C.-L. In vitro evaluation of electrospun polyvinylidene fluoride hybrid nanoparticles as direct piezoelectric membranes for guided bone regeneration. Biomater. Adv. 2023, 144, 213228. [Google Scholar] [CrossRef] [PubMed]
- International Organization for Standardization. Biological Evaluation of Medical Devices—Part 5: Tests for in Vitro Cytotoxicity; International Organization for Standardization: Geneve, Switzerland, 2009. [Google Scholar]
- Zhou, K.; Feng, B.; Wang, W.; Jiang, Y.; Zhang, W.; Zhou, G.; Jiang, T.; Cao, Y.; Liu, W. Nanoscaled and microscaled parallel topography promotes tenogenic differentiation of ASC and neotendon formation in vitro. Int. J. Nanomed. 2018, 13, 3867–3881. [Google Scholar] [CrossRef] [PubMed]
- Cirillo, V.; Guarino, V.; Alvarez-Perez, M.A.; Marrese, M.; Ambrosio, L. Optimization of fully aligned bioactive electrospun fibers for “in vitro” nerve guidance. J. Mater. Sci. Mater. Med. 2014, 25, 2323–2332. [Google Scholar] [CrossRef]
- Bai, Y.; Liu, Y.; Lv, H.; Shi, H.; Zhou, W.; Liu, Y.; Yu, D.-G. Processes of Electrospun Polyvinylidene Fluoride-Based Nanofibers, Their Piezoelectric Properties, and Several Fantastic Applications. Polymers 2022, 14, 4311. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Lv, J.; Yu, S. Piezoelectric Properties of As-Spun Poly (vinylidene Fluoride)/Multi-Walled Carbon Nanotube/Zinc Oxide Nanoparticle (PVDF/MWCNT/ZnO) Nanofibrous Films. Polymers 2024, 16, 2483. [Google Scholar] [CrossRef]
- Wu, Y.; Zou, J.; Tang, K.; Xia, Y.; Wang, X.; Song, L.; Wang, J.; Wang, K.; Wang, Z. From electricity to vitality: The emerging use of piezoelectric materials in tissue regeneration. Burn. Trauma 2024, 12, tkae013. [Google Scholar] [CrossRef] [PubMed]
- Tandon, B.; Kamble, P.; Olsson, R.T.; Blaker, J.J.; Cartmell, S.H. Fabrication and Characterisation of Stimuli Responsive Piezoelectric PVDF and Hydroxyapatite-Filled PVDF Fibrous Membranes. Molecules 2019, 24, 1903. [Google Scholar] [CrossRef]
- dos Santos, G.G.; Malherbi, M.S.; de Souza, N.S.; César, G.B.; Tominaga, T.T.; Miyahara, R.Y.; de Mendonça, P.D.; Faria, D.R.; Rosso, J.M.; Freitas, V.F.; et al. 4th Generation Biomaterials Based on PVDF-Hydroxyapatite Composites Produced by Electrospinning: Processing and Characterization. Polymers 2022, 14, 4190. [Google Scholar] [CrossRef]
- Sultana, A.; Ghosh, S.K.; Sencadas, V.; Zheng, T.; Higgins, M.J.; Middya, T.R.; Mandal, D. Human skin interactive self-powered wearable piezoelectric bio-e-skin by electrospun poly-l-lactic acid nanofibers for non-invasive physiological signal monitoring. J. Mater. Chem. B 2017, 5, 7352–7359. [Google Scholar] [CrossRef] [PubMed]
- Tai, Y.; Yang, S.; Yu, S.; Banerjee, A.; Myung, N.V.; Nam, J. Modulation of piezoelectric properties in electrospun PLLA nanofibers for application-specific self-powered stem cell culture platforms. Nano Energy 2021, 89, 106444. [Google Scholar] [CrossRef]
- Butler, D.L.; Guan, Y.; Kay, M.D.; Cummings, J.F.; Feder, S.M.; Levy, M.S. Location-dependent variations in the material properties of the anterior cruciate ligament. J. Biomech. 1992, 25, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Ayres, C.; Bowlin, G.L.; Henderson, S.C.; Taylor, L.; Shultz, J.; Alexander, J.; Telemeco, T.A.; Simpson, D.G. Modulation of anisotropy in electrospun tissue-engineering scaffolds: Analysis of fiber alignment by the fast Fourier transform. Biomaterials 2006, 27, 5524–5534. [Google Scholar] [CrossRef]
- Liu, Z.; Cai, M.; Zhang, X.; Yu, X.; Wang, S.; Wan, X.; Wang, Z.L.; Li, L. Cell-traction-triggered on-demand electrical stimulation for neuron-like differentiation. Adv. Mater. 2021, 33, 2106317. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Shi, C.; Jin, F.; Yang, F.; Gu, L.; Wang, T.; Dong, W.; Feng, Z.-Q. Cell activity modulation and its specific function maintenance by bioinspired electromechanical nanogenerator. Sci. Adv. 2021, 7, eabh2350. [Google Scholar] [CrossRef] [PubMed]
- Xia, G.; Song, B.; Fang, J. Electrical Stimulation Enabled via Electrospun Piezoelectric Polymeric Nanofibers for Tissue Regeneration. Research 2022, 2022, 9896274. [Google Scholar] [CrossRef]
- Barbosa, F.; Garrudo, F.F.F.; Alberte, P.S.; Resina, L.; Carvalho, M.S.; Jain, A.; Marques, A.C.; Estrany, F.; Rawson, F.J.; Aléman, C.; et al. Hydroxyapatite-filled osteoinductive and piezoelectric nanofibers for bone tissue engineering. Sci. Technol. Adv. Mater. 2023, 24, 2242242. [Google Scholar] [CrossRef] [PubMed]
- Tofail, S.A.M.; Gandhi, A.A.; Gregor, M.; Bauer, J. Electrical properties of hydroxyapatite. Pure Appl. Chem. 2015, 87, 221–229. [Google Scholar] [CrossRef]





| Designated Group | Average Fiber Diameter (nm) |
|---|---|
| PVDF | 645 ± 282 |
| PVDF/1 wt.% Cu-nHA | 605 ± 202 |
| PVDF/3 wt.% Cu-nHA | 554 ± 126 |
| Groups | Piezoelectric Value (V/g·m−2) |
|---|---|
| PVDF | 18.98 ± 1.18 |
| PVDF/1 wt.% Cu-nHA | 17.46 ± 0.55 |
| PVDF/3 wt.% Cu-nHA | 25.02 ± 0.68 |
| Groups | Ultimate Tensile Stress (MPa) | Strain After Rupture (%) |
|---|---|---|
| PVDF | 7.60 ± 1.67 | 16.06 ± 2.92 |
| PVDF/1 wt.% Cu-nHA | 13.28 ± 2.84 | 29.71 ± 12.24 |
| PVDF/3 wt.% Cu-nHA | 14.16 ± 2.75 | 31.33 ± 7.26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, C.-H.; Chen, W.-C.; Yang, W.-C.; Yang, S.-C.; Liu, S.-M.; Chen, Y.-S.; Chen, J.-C. Unidirectional Polyvinylidene/Copper-Impregnated Nanohydroxyapatite Composite Membrane Prepared by Electrospinning with Piezoelectricity and Biocompatibility for Potential Ligament Repair. Polymers 2025, 17, 185. https://doi.org/10.3390/polym17020185
Cheng C-H, Chen W-C, Yang W-C, Yang S-C, Liu S-M, Chen Y-S, Chen J-C. Unidirectional Polyvinylidene/Copper-Impregnated Nanohydroxyapatite Composite Membrane Prepared by Electrospinning with Piezoelectricity and Biocompatibility for Potential Ligament Repair. Polymers. 2025; 17(2):185. https://doi.org/10.3390/polym17020185
Chicago/Turabian StyleCheng, Chih-Hsin, Wen-Cheng Chen, Wen-Chieh Yang, Sen-Chi Yang, Shih-Ming Liu, Ya-Shun Chen, and Jian-Chih Chen. 2025. "Unidirectional Polyvinylidene/Copper-Impregnated Nanohydroxyapatite Composite Membrane Prepared by Electrospinning with Piezoelectricity and Biocompatibility for Potential Ligament Repair" Polymers 17, no. 2: 185. https://doi.org/10.3390/polym17020185
APA StyleCheng, C.-H., Chen, W.-C., Yang, W.-C., Yang, S.-C., Liu, S.-M., Chen, Y.-S., & Chen, J.-C. (2025). Unidirectional Polyvinylidene/Copper-Impregnated Nanohydroxyapatite Composite Membrane Prepared by Electrospinning with Piezoelectricity and Biocompatibility for Potential Ligament Repair. Polymers, 17(2), 185. https://doi.org/10.3390/polym17020185








