Production of Hydrogen-Rich Syngas via Biomass-Methane Co-Pyrolysis: Thermodynamic Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Related Reactions
2.3. Determination of Equilibrium Compositions
2.4. Determination of Energy Recovery Efficiency
3. Results
3.1. Gibbs Free Energy Variation in Key Reactions
3.2. Equilibrium Product Distribution
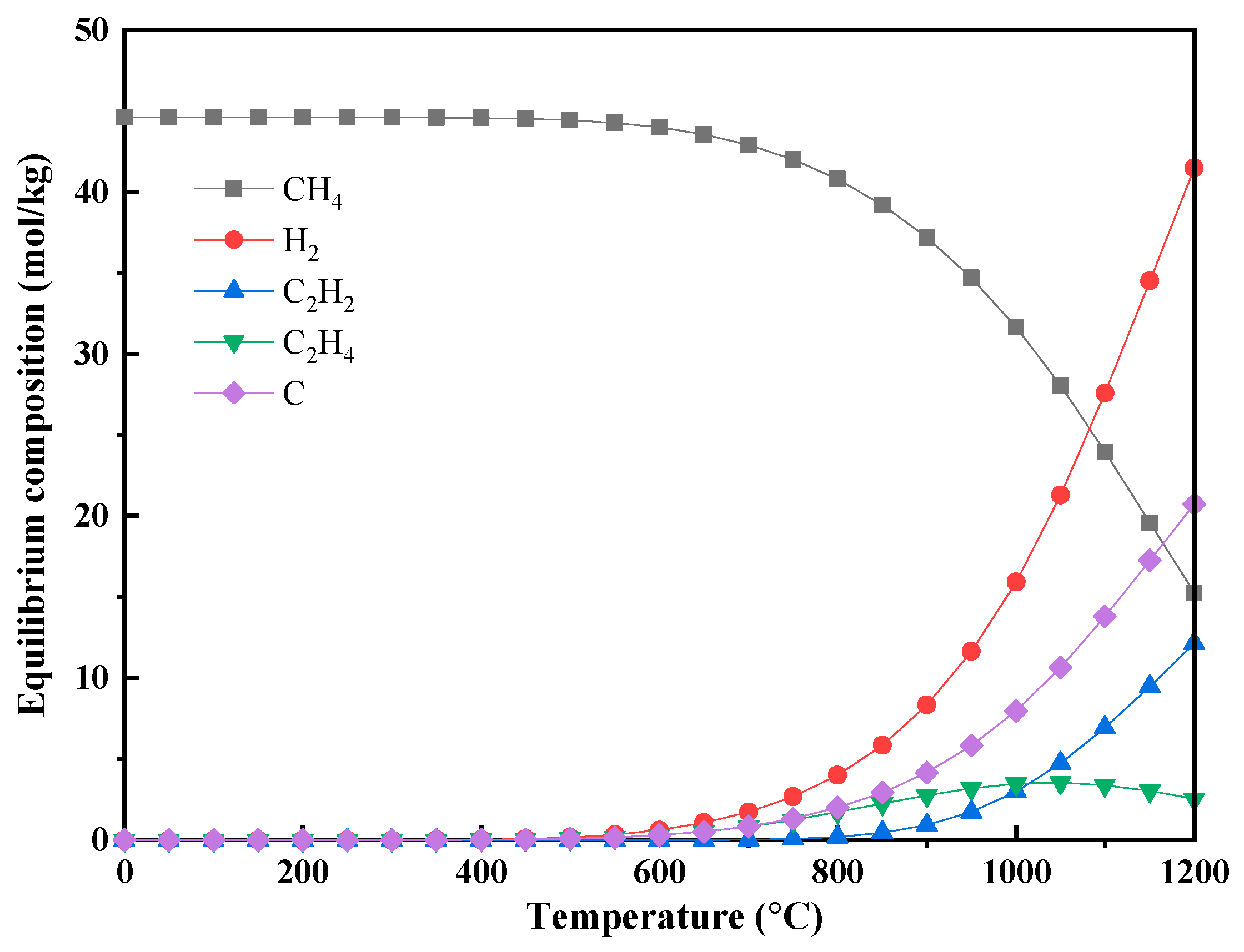
3.2.1. Pure Feedstock Pyrolysis
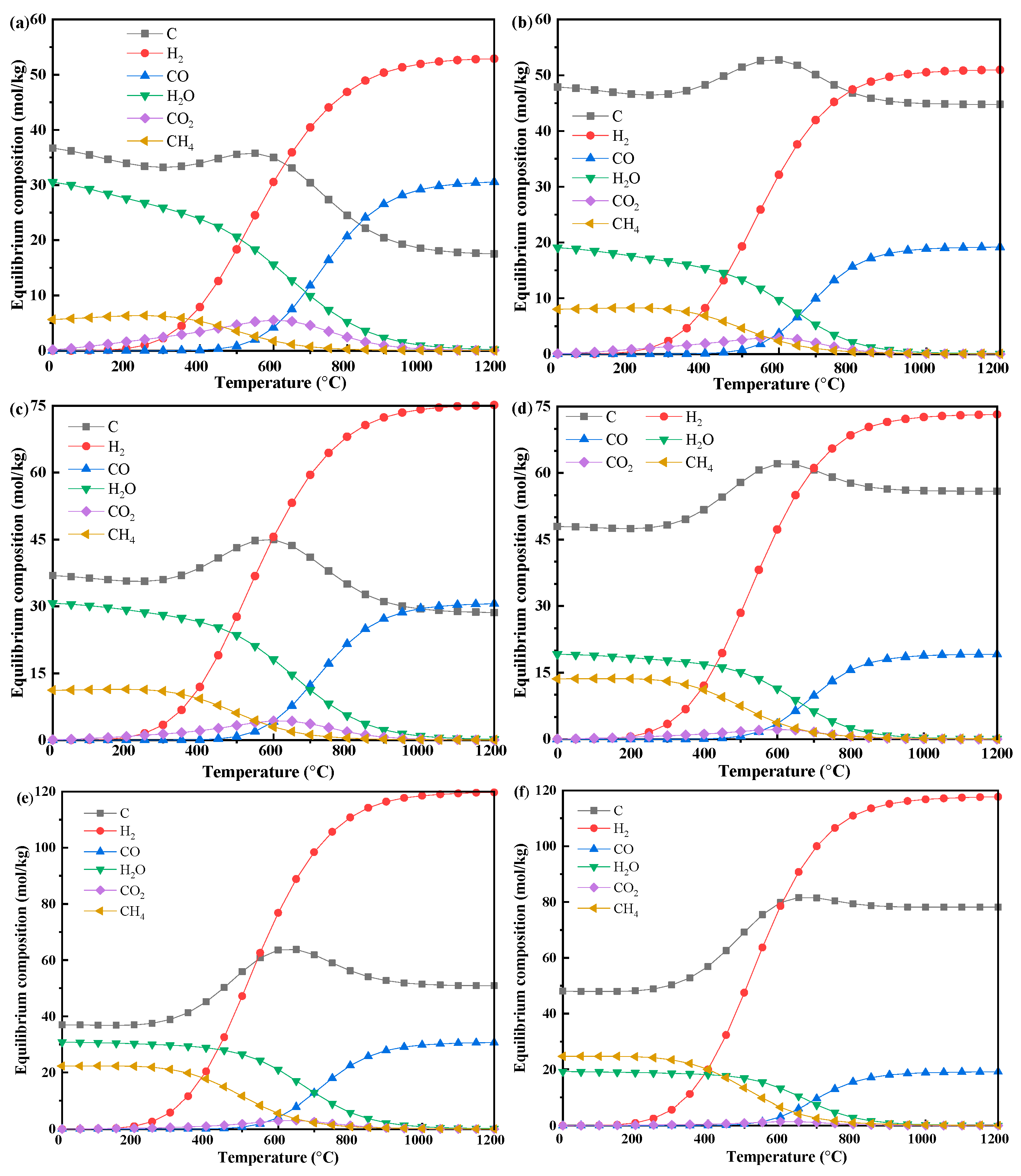
3.2.2. Biomass–Methane Co-Pyrolysis
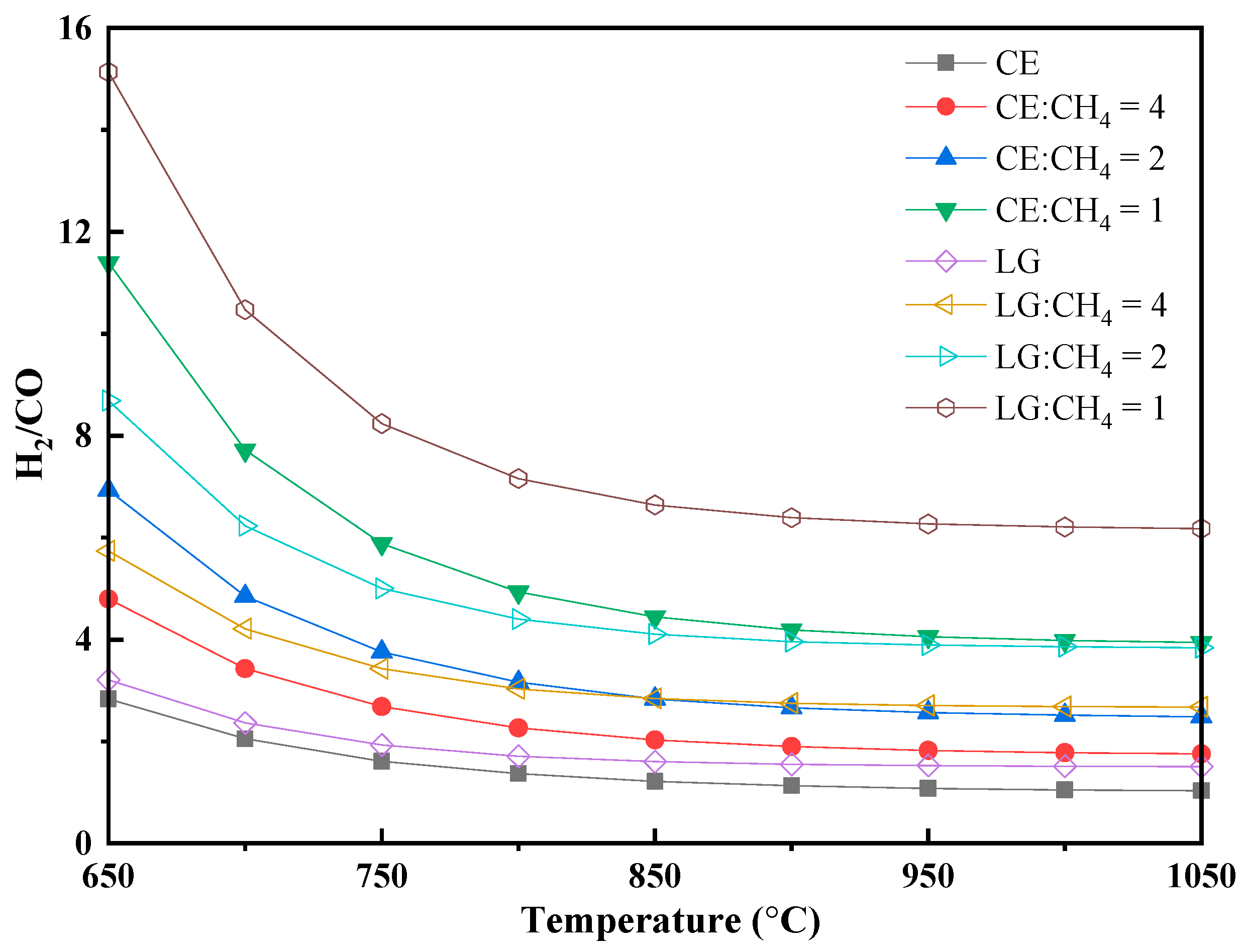
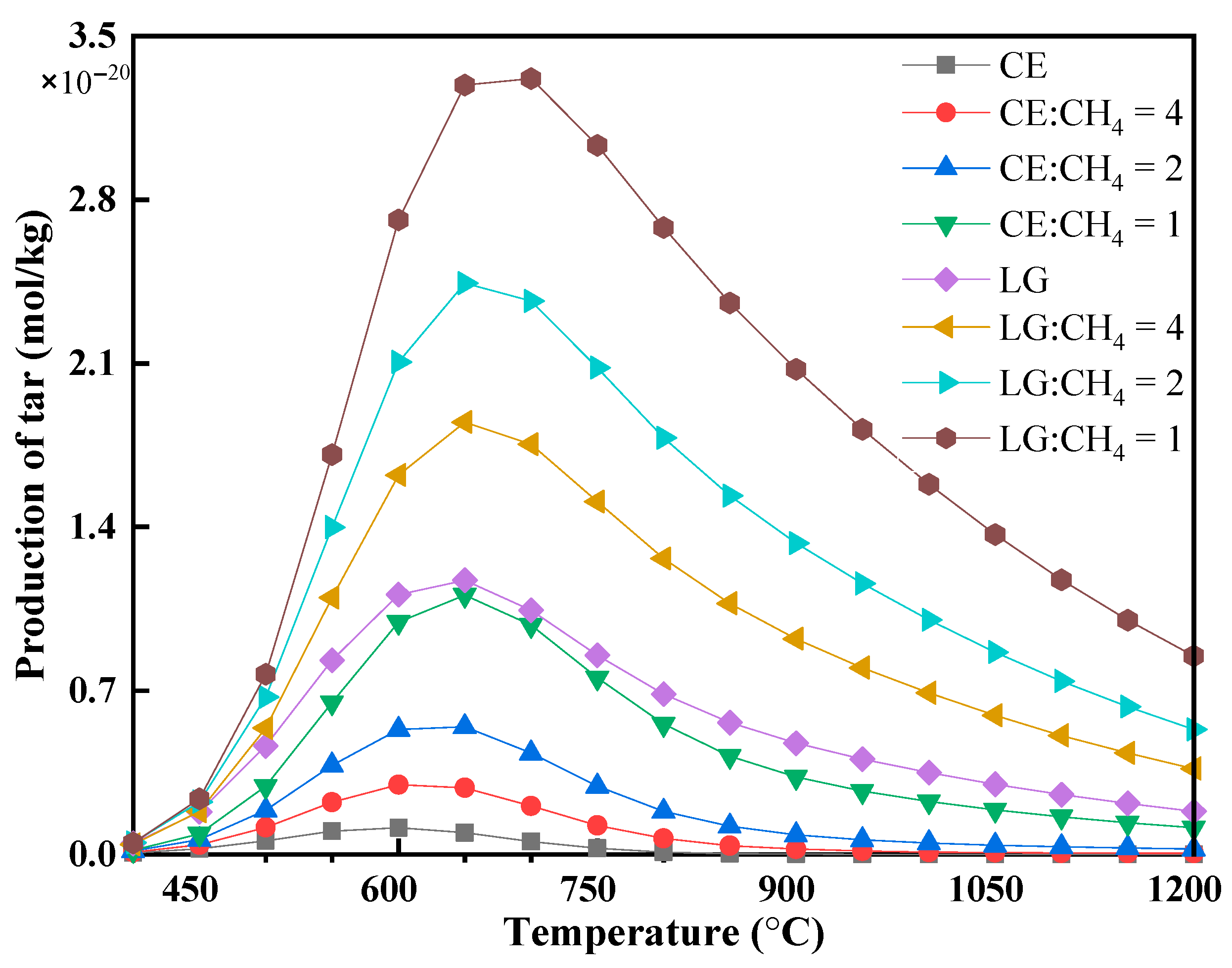
3.2.3. H2/CO Ratio and Tar Formation Characteristics
3.3. Energy Recovery Efficiency
4. Discussion
4.1. Thermodynamic Driving Forces and Reaction Pathways
4.2. Influence of Biomass Molecular Structure
4.3. Synergistic Mechanisms in Co-Pyrolysis
- (1)
- Methane enhances biomass conversion: At a 1:1 ratio, cellulose produces an additional 30.10 mol C/kg and 78.20 mol H2/kg, while lignin yields an extra 57.41 mol C/kg and 76.20 mol H2/kg compared to theoretical values from individual pyrolysis.
- (2)
- Biomass catalyzes methane cracking: Co-pyrolysis significantly boosts methane conversion, raising it from 24.90% for pure methane to over 53% with biomass present, nearly doubling the yield while maintaining high H2 selectivity (~67%).
4.4. Implications for Process Optimization and Challenges
4.5. Energetic Performance and Feedstock Selection
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CE | Cellulose |
| CGE | Cold gas efficiency |
| G | Guaiacyl lignin subunit |
| H | p-hydroxyphenyl |
| HDO | Hydrodeoxygenation |
| HHV | Higher heating value |
| LHV | Lower heating value |
| LG | Lignin (Used as a label in figures and tables) |
| MJ | Megajoule |
| PAHs | Polycyclic aromatic hydrocarbons |
| S | Syringyl lignin subunit |
| WGSR | Water–gas shift reaction |
References
- Korpeh, M.; Lotfollahi, A.; Moghimi, M.; Anvari-Moghaddam, A. Combustion optimization of various biomass types to hydrogen-rich syngas: Two-stage pyrolysis modeling, methane addition effects, and environmental impact assessment. Int. J. Hydrogen Energy 2025, 133, 458–471. [Google Scholar] [CrossRef]
- Rathi, N.; Das, T. Exploring biomass pyrolysis for sustainable hydrogen-rich gas production. Biomass Bioenergy 2025, 202, 108162. [Google Scholar] [CrossRef]
- Gholami, Z.; Gholami, F.; Šimek, J.; Svobodová, K.; Vakili, M. Hydrogen production for a decarbonized future: A review of production technologies. J. Ind. Eng. Chem. 2025. [Google Scholar] [CrossRef]
- Abedinia, O.; Shakibi, H.; Shokri, A.; Sobhani, B.; Sobhani, B.; Yari, M.; Bagheri, M. Optimization of a syngas-fueled SOFC-based multigeneration system: Enhanced performance with biomass and gasification agent selection. Renew. Sustain. Energy Rev. 2024, 199, 114460. [Google Scholar] [CrossRef]
- Rabea, K.; Michailos, S.; Hughes, K.J.; Ingham, D.; Pourkashanian, M. A new hydrogen production route through biomass gasification in a two-stage fixed bed reactor within the BECCS concept: A techno-economic and life cycle assessment study. Int. J. Hydrogen Energy 2025, 124, 140–152. [Google Scholar] [CrossRef]
- Silva, I.P.; Lima, R.M.A.; Silva, G.F.; Ruzene, D.S.; Silva, D.P. Thermodynamic equilibrium model based on stoichiometric method for biomass gasification: A review of model modifications. Renew. Sustain. Energy Rev. 2019, 114, 109305. [Google Scholar] [CrossRef]
- Lalsare, A.; Wang, Y.; Li, Q.; Sivri, A.; Vukmanovich, R.J.; Dumitrescu, C.E.; Hu, J. Hydrogen-rich syngas production through synergistic methane-activated catalytic biomass gasification. ACS Sustain. Chem. Eng. 2019, 7, 16060–16071. [Google Scholar] [CrossRef]
- Ren, J.; Cao, J.-P.; Zhao, X.-Y.; Yang, F.-L.; Wei, X.-Y. Recent advances in syngas production from biomass catalytic gasification: A critical review on reactors, catalysts, catalytic mechanisms and mathematical models. Renew. Sustain. Energy Rev. 2019, 116, 109426. [Google Scholar] [CrossRef]
- Chen, Y.; He, H.; Li, Y.; Liu, B.; Liu, X. Study on the regulation of Fischer-Tropsch synthesis catalytic performance by mixing oxides with iron-based catalysts. J. Fuel Chem. Technol. 2025, 53, 1212–1222. [Google Scholar] [CrossRef]
- Molefe, T.; Jiang, Y.; Magubane, A.; Mguni, L.; Sikeyi, L.; Jiang, C.; Liu, X.; Yao, Y. Hollow carbon spheres as catalyst support for Fischer-Tropsch synthesis: Synthesis techniques, optimization strategies, and future research. Fuel Process. Technol. 2025, 276, 108285. [Google Scholar] [CrossRef]
- Jung, S.; Lee, J.; Moon, D.H.; Kim, K.-H.; Kwon, E.E. Upgrading biogas into syngas through dry reforming. Renew. Sustain. Energy Rev. 2021, 143, 110949. [Google Scholar] [CrossRef]
- Mishra, R.; Shu, C.-M.; Gollakota, A.R.K.; Pan, S.-Y. Unveiling the potential of pyrolysis-gasification for hydrogen-rich syngas production from biomass and plastic waste. Energy Convers. Manag. 2024, 321, 118997. [Google Scholar] [CrossRef]
- Palumbo, A.W.; Sorli, J.C.; Weimer, A.W. High temperature thermochemical processing of biomass and methane for high conversion and selectivity to H2-enriched syngas. Appl. Energy 2015, 157, 13–24. [Google Scholar] [CrossRef]
- Soomro, A.; Chen, S.; Ma, S.; Xu, C.; Sun, Z.; Xiang, W. Elucidation of syngas composition from catalytic steam gasification of lignin, cellulose, actual and simulated biomasses. Biomass Bioenergy 2018, 115, 210–222. [Google Scholar] [CrossRef]
- Gorensek, M.B.; Shukre, R.; Chen, C.-C. Development of a thermophysical properties model for flowsheet simulation of biomass pyrolysis processes. ACS Sustain. Chem. Eng. 2019, 7, 9017–9027. [Google Scholar] [CrossRef]
- Jung, H.S.; Kim, B.G.; Kwon, J.H.; Bae, J.W. Thermocatalytic technologies for syngas production from greenhouse gases and biomass-derived renewable oxygenates. Renew. Sustain. Energy Rev. 2025, 216, 115711. [Google Scholar] [CrossRef]
- Du, B.; Zhang, Z.; Grubner, S.; Yurkovich, J.T.; Palsson, B.O.; Zielinski, D.C. Temperature-dependent estimation of gibbs energies using an updated group-contribution method. Biophys. J. 2018, 114, 2691–2702. [Google Scholar] [CrossRef]
- Moreno, J.; Cobo, M.; Buendia, F.; Sánchez, N. Enhancing predictive models for steam gasification: A comparative study of stoichiometric, equilibrium, data-driven, and hybrid approaches. Renew. Sustain. Energy Rev. 2025, 210, 115151. [Google Scholar] [CrossRef]
- Kaydouh, M.-N.; El Hassan, N. Thermodynamic simulation of the co-gasification of biomass and plastic waste for hydrogen-rich syngas production. Results Eng. 2022, 16, 100771. [Google Scholar] [CrossRef]
- Plevan, M.; Geißler, T.; Abánades, A.; Mehravaran, K.; Rathnam, R.K.; Rubbia, C.; Salmieri, D.; Stoppel, L.; Stückrad, S.; Wetzel, T. Thermal cracking of methane in a liquid metal bubble column reactor: Experiments and kinetic analysis. Int. J. Hydrogen Energy 2015, 40, 8020–8033. [Google Scholar] [CrossRef]
- Zhang, J.; Li, X.; Chen, H.; Qi, M.; Zhang, G.; Hu, H.; Ma, X. Hydrogen production by catalytic methane decomposition: Carbon materials as catalysts or catalyst supports. Int. J. Hydrogen Energy 2017, 42, 19755–19775. [Google Scholar] [CrossRef]
- Ayodele, B.V.; Yazrul Bin Mohd Yassin, M.; Naim, R.; Abdullah, S. Hydrogen production by thermo-catalytic conversion of methane over lanthanum strontium cobalt ferrite (LSCF) and αAl2O3 supported Ni catalysts. J. Energy Inst. 2018, 92, 892–903. [Google Scholar] [CrossRef]
- Jyoti; Ashok, C.H.; Srilatha, K.; Patil, N.; Shilpa Chakra, C.H. Hydrogen production from methane decomposition using nano metal oxides. Mater. Today Proc. 2017, 4, 11679–11689. [Google Scholar] [CrossRef]
- Abánades, A.; Rathnam, R.K.; Geißler, T.; Heinzel, A.; Mehravaran, K.; Müller, G.; Plevan, M.; Rubbia, C.; Salmieri, D.; Stoppel, L.; et al. Development of methane decarbonisation based on liquid metal technology for CO2-free production of hydrogen. Int. J. Hydrogen. Energy 2016, 41, 8159–8167. [Google Scholar] [CrossRef]
- Srilatha, K.; Bhagawan, D.; Shiva Kumar, S.; Himabindu, V. Sustainable fuel production by thermocatalytic decomposition of methane—A review. S. Afr. J. Chem. Eng. 2017, 24, 156–167. [Google Scholar] [CrossRef]
- Ding, X.; Yan, L.; Guo, C.; Jia, D.; Guo, N.; Wang, L. Synergistic effects between lignin, cellulose and coal in the co-pyrolysis process of coal and cotton stalk. Molecules 2023, 28, 5708. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Wu, Z.; Chen, G. Catalytic gasification characteristics of cellulose, hemicellulose and lignin. Renew. Energy 2018, 121, 559–567. [Google Scholar] [CrossRef]
- Gogoi, M.; Konwar, K.; Bhuyan, N.; Borah, R.C.; Kalita, A.C.; Nath, H.P.; Saikia, N. Assessments of pyrolysis kinetics and mechanisms of biomass residues using thermogravimetry. Bioresour. Technol. Rep. 2018, 4, 40–49. [Google Scholar] [CrossRef]
- Zhao, J.; Xiuwen, W.; Hu, J.; Liu, Q.; Shen, D.; Xiao, R. Thermal degradation of softwood lignin and hardwood lignin by TG-FTIR and Py-GC/MS. Polym. Degrad. Stab. 2014, 108, 133–138. [Google Scholar] [CrossRef]
- Buentello-Montoya, D.A.; Duarte-Ruiz, C.A.; Maldonado-Escalante, J.F. Co-gasification of waste PET, PP and biomass for energy recovery: A thermodynamic model to assess the produced syngas quality. Energy 2023, 266, 126510. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, M.; Raheem, A.; Wang, F.; Wei, J.; Xu, D.; Song, X.; Bao, W.; Huang, A.; Zhang, S.; et al. Syngas production from biomass gasification: Influences of feedstock properties, reactor type, and reaction parameters. ACS Omega 2023, 8, 31620–31631. [Google Scholar] [CrossRef]
- Ellison, C.; Abdelsayed, V.; Smith, M.; Shekhawat, D. Comparative evaluation of microwave and conventional gasification of different coal types: Experimental reaction studies. Fuel 2022, 321, 124055. [Google Scholar] [CrossRef]
- Verissimo, G.L.; Leiroz, A.J.K.; Cruz, M.E. Influence of the pyrolysis and heterogeneous char reactions modeling in the simulation of sugarcane bagasse gasification in a bubbling fluidized bed reactor. Fuel 2020, 281, 118750. [Google Scholar] [CrossRef]
- Zhang, S.; Wu, M.; Bie, X.; Qian, Z.; Li, Q.; Zhang, Y.; Zhou, H. Deciphering interactions between biomass components during CO2 gasification: Insights from thermogravimetric behavior, gas production, and char reactivity. Fuel 2024, 371, 131974. [Google Scholar] [CrossRef]
- Ren, J.; Cao, J.-P.; Yang, F.-L.; Liu, Y.-L.; Tang, W.; Zhao, X.-Y. Understandings of catalyst deactivation and regeneration during biomass tar reforming: A crucial review. ACS Sustain. Chem. Eng. 2021, 9, 17186–17206. [Google Scholar] [CrossRef]
- Reizer, E.; Viskolcz, B.; Fiser, B. Formation and growth mechanisms of polycyclic aromatic hydrocarbons: A mini-review. Chemosphere 2022, 291, 132793. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.-J.; Jiang, H.; Yu, H.-Q. Development of biochar-based functional materials: Toward a sustainable platform carbon material. Chem. Rev. 2015, 115, 12251–12285. [Google Scholar] [CrossRef]
- Yin, Z.; Yang, J.; Feng, Y.; Ma, Z.; Wang, J.; Liu, Q. Thermodynamics analysis of a biomass co-gasification based combined cooling, heating and power system. Renew. Energy 2025, 248, 123195. [Google Scholar] [CrossRef]
- Abbas, H.F.; Wan Daud, W.M.A. Hydrogen production by methane decomposition: A review. Int. J. Hydrogen Energy 2010, 35, 1160–1190. [Google Scholar] [CrossRef]
- Ashik, U.P.M.; Wan Daud, W.M.A.; Hayashi, J.I. A review on methane transformation to hydrogen and nanocarbon: Relevance of catalyst characteristics and experimental parameters on yield. Renew. Sustain. Energy Rev. 2017, 76, 743–767. [Google Scholar] [CrossRef]


| Formula | MW (g/mol) | ΔHf° (kJ/mol) | ΔSf° (J/K/mol) | HHV (kJ/mol) | LHV (kJ/mol) | Heat of Combustion (MJ/kg) | |
|---|---|---|---|---|---|---|---|
| Methane | CH4 | 16 | −74.81 | 186.3 | −891.0 | −802.0 | −56.0 |
| Cellulose | C6H10O5 | 162 | −1019.0 | 181.0 | −2624.4 | − | −16.2 |
| Lignin | C11H12O4 | 208 | −729.31 | 239.8 | −5096 | − | −24.5 |
| Oxygen | O2 | 32 | 0 | 205.2 | |||
| Water (liquid) | H2O (l) | 18 | −285.83 | 69.9 | |||
| Water (gas) | H2O (g) | 18 | −241.80 | 188.8 | |||
| Carbon dioxide | CO2 | 44 | −393.51 | 213.8 | |||
| Carbon monoxide | CO | 28 | −110.53 | 197.7 | −284.0 | −283.0 | −10.0 |
| Carbon | C | 12 | 0 | 5.7 | −394.0 | −110.5 | −33.0 |
| Hydrogen | H2 | 2 | 0 | 130.7 | −286.0 | −244.0 | −143.0 |
| Benzene | C6H6 | 78 | −82.93 | 269.31 | −3267.6 | −3129.6 | −41.8 |
| Biomass-to-Methane Ratio | Eout (MJ) | Eη (%) | ||||||
|---|---|---|---|---|---|---|---|---|
| CE a | LG a | CH4 b | 800 °C | 900 °C | 1000 °C | 800 °C | 900 °C | 1000 °C |
| 1 | 36.43 | 37.17 | 38.53 | 72.68 | 74.16 | 76.87 | ||
| 1 | 13.40 | 15.14 | 16.10 | 82.74 | 93.45 | 99.36 | ||
| 1 | 0.25 | 20.20 | 22.14 | 23.03 | 70.33 | 77.07 | 80.14 | |
| 1 | 0.5 | 26.94 | 28.95 | 29.78 | 65.30 | 70.16 | 72.16 | |
| 1 | 1 | 40.28 | 42.38 | 43.16 | 60.74 | 63.89 | 65.08 | |
| 1 | 15.13 | 15.86 | 16.08 | 61.77 | 64.71 | 65.62 | ||
| 1 | 0.25 | 23.63 | 24.25 | 24.54 | 63.81 | 65.49 | 66.28 | |
| 1 | 0.5 | 32.91 | 33.59 | 33.78 | 66.41 | 67.78 | 68.16 | |
| 1 | 1 | 51.00 | 52.42 | 52.91 | 68.34 | 70.25 | 70.90 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, H.; Wang, Z.; Kang, K.; Li, D. Production of Hydrogen-Rich Syngas via Biomass-Methane Co-Pyrolysis: Thermodynamic Analysis. Polymers 2025, 17, 2695. https://doi.org/10.3390/polym17192695
Guo H, Wang Z, Kang K, Li D. Production of Hydrogen-Rich Syngas via Biomass-Methane Co-Pyrolysis: Thermodynamic Analysis. Polymers. 2025; 17(19):2695. https://doi.org/10.3390/polym17192695
Chicago/Turabian StyleGuo, Haiyan, Zhiling Wang, Kang Kang, and Dongbing Li. 2025. "Production of Hydrogen-Rich Syngas via Biomass-Methane Co-Pyrolysis: Thermodynamic Analysis" Polymers 17, no. 19: 2695. https://doi.org/10.3390/polym17192695
APA StyleGuo, H., Wang, Z., Kang, K., & Li, D. (2025). Production of Hydrogen-Rich Syngas via Biomass-Methane Co-Pyrolysis: Thermodynamic Analysis. Polymers, 17(19), 2695. https://doi.org/10.3390/polym17192695







