Tailoring 3HV Fraction in Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) by Azotobacter vinelandii Through Oxygen and Carbon Limitation in Continuous Cultures
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strain, Culture Medium, and Inoculum Preparation
2.2. Chemostat Cultures
2.3. Analytical Methods
2.4. Determination of the OTR, CTR, and Estimation of the Specific Oxygen Uptake Rate and RQ
2.5. Measurements of the Intracellular NAD+, NADH, NADP+, and NADPH Concentrations
2.6. Carbon Balance
2.7. Estimation of Fermentation Parameters
2.8. Statistical Analysis
3. Results and Discussion
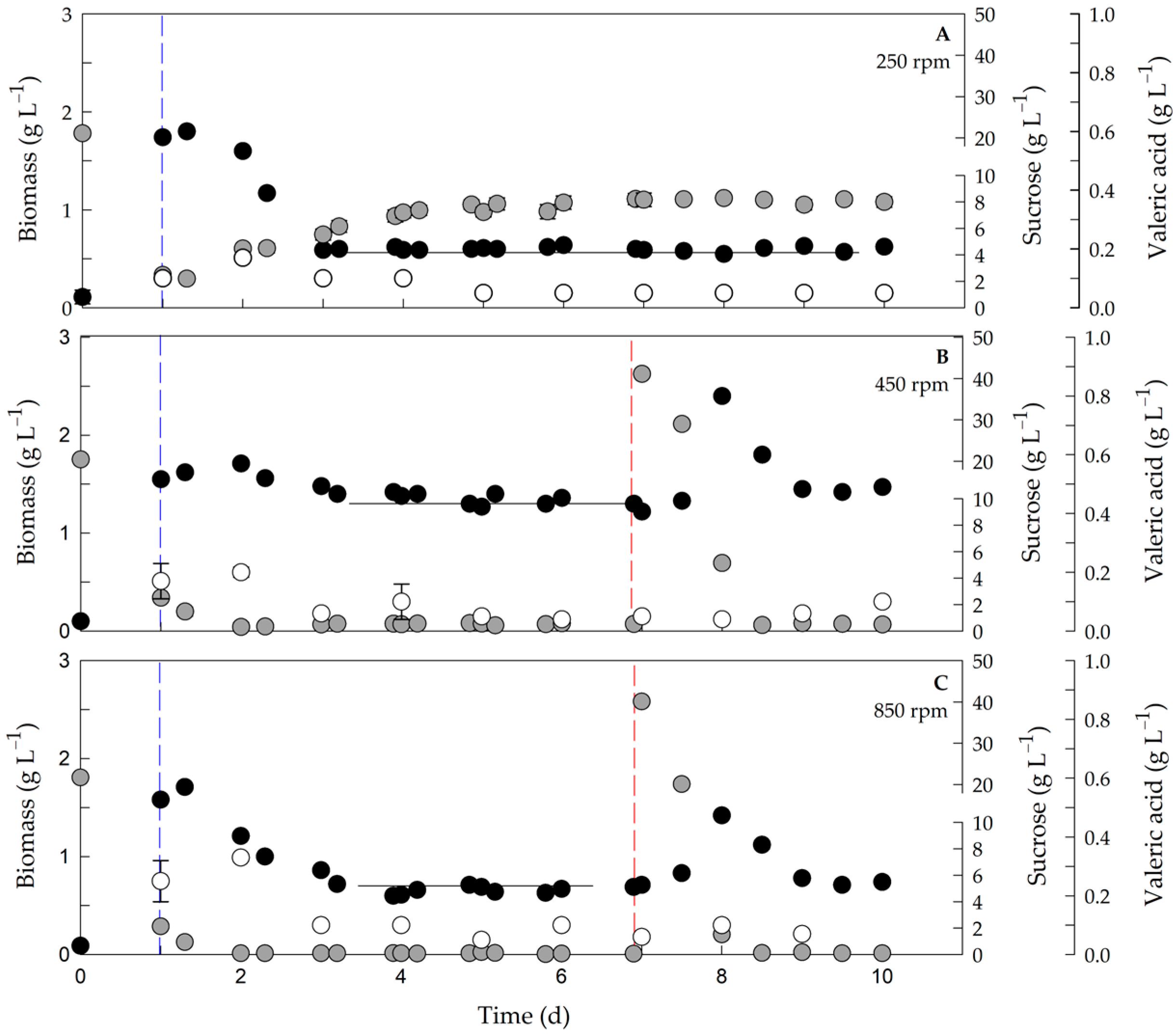
3.1. Nutritional Limitation and Respirometry Analyses in Chemostat Cultures of Azotobacter vinelandii OP
3.2. Influence of the qO2 on Biomass, P3HBV Production, the 3HV Fraction and the NAD(P)H/NAD(P)+ Ratio in the Steady State
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Agitation Rate | Biomass | P3HBV | P3HBV Accumulation (% w w−1) | P3HBV Composition (mol %) | |||
|---|---|---|---|---|---|---|---|
| (rpm) | (g L−1) | (g L−1) | 3HB | 3HV | PHBV | 3HB | 3HV |
| 250 | 1.59 ± 0.15 | 1.00 ± 0.11 | 40.85 ± 0.95 a | 22.23 ± 1.10 | 63.07 ± 1.77 a | 66.70 ± 2.78 a | 33.30 ± 2.78 a |
| 350 | 2.69 ± 0.07 | 1.68 ± 0.04 | 47.17 ± 1.61 | 15.56 ± 0.94 | 62.73 ± 1.60 a | 77.61 ± 1.24 b | 22.39 ± 1.24 b |
| 450 | 3.34 ± 0.19 | 2.09 ± 0.16 | 53.74 ± 1.23 | 8.42 ± 0.57 | 62.42 ± 1.30 a | 87.48 ± 1.23 | 12.52 ± 1.23 |
| 650 | 2.87 ± 0.16 | 1.52 ± 0.13 | 40.74 ± 1.56 a | 12.01 ± 1.69 a | 52.75 ± 2.36 | 77.49 ± 1.56 b | 22.51 ± 1.56 b |
| 750 | 1.24 ± 0.09 | 0.55 ± 0.02 | 32.10 ± 1.34 | 13.15 ± 0.63 a | 44.84 ± 1.62 | 73.35 ± 1.34 | 26.65 ± 1.34 |
| 850 | 1.01 ± 0.06 | 0.33 ± 0.02 | 20.95 ± 0.87 | 12.02 ± 0.51 a | 23.98 ± 1.12 | 63.60 ± 1.76 a | 36.40 ± 1.76 a |
References
- Dalton, B.; Bhagabati, P.; De Micco, J.; Padamati, R.B.; O’Connor, K. A review on biological synthesis of the biodegradable polymers polyhydroxyalkanoates and the development of multiple applications. Catalysts 2022, 12, 319. [Google Scholar] [CrossRef]
- Winnacker, M. Polyhydroxyalkanoates: Recent advances in their synthesis and applications. Eur. J. Lipid Sci. Technol. 2019, 121, 1900101–1900110. [Google Scholar] [CrossRef]
- Lee, G.N.; Na, J. Future of microbial polyesters. Microb. Cell Fact. 2013, 12, 54–58. [Google Scholar] [CrossRef]
- Müller-Santos, M.; Koskimäki, J.; Silveira-Alves, L.P.; Maltempi de Souza, E.; Jendrossek, D.; Pirttilä, A.M. The protective role of PHB and its degradation products against stress situations in bacteria. FEMS Microbiol. Rev. 2021, 45, fuaa058. [Google Scholar] [CrossRef]
- Sehgal, R.; Gupta, R. Polyhydroxyalkanoate and its efficient production: An eco-friendly approach towards development. 3 Biotech 2020, 10, 549–563. [Google Scholar] [CrossRef]
- Mai, J.; Kockler, K.; Parisi, E.; Chan, C.M.; Pratt, S.; Laycock, B. Synthesis and physical properties of polyhydroxyalkanoate (PHA)-based block copolymers: A review. Int. J. Biol. Macromol. 2024, 263, 130204–130235. [Google Scholar] [CrossRef]
- García, A.; Aguirre, C.; Pérez, A.; Bahamonde, S.S.; Urtuvia, V.; Díaz-Barrera, A.; Peña, C. Recent trends in the production and recovery of bioplastics using polyhydroxyalkanoates copolymers. Microorganisms 2024, 12, 2135. [Google Scholar] [CrossRef]
- Bhola, S.; Arora, K.; Kulshrestha, S.; Mehariya, S.; Bhatia, R.K.; Kaur, P.; Kumar, P. Established and emerging producers of PHA: Redefining the possibility. Appl. Biochem. Biotechnol. 2021, 193, 3812–3854. [Google Scholar] [CrossRef]
- Diniz, M.S.d.F.; Mourão, M.M.; Xavier, L.P.; Santos, A.V. Recent Biotechnological Applications of Polyhydroxyalkanoates (PHA) in the Biomedical Sector—A Review. Polymers 2023, 15, 4405. [Google Scholar] [CrossRef]
- Khamplod, T.; Winterburn, J.B.; Cartmell, S.H. Electrospun poly(3-hydroxybutyrate-co-3-hydroxyvalerate) scaffolds—A step towards ligament repair applications. Sci. Technol. Adv. Mater. 2022, 23, 895–910. [Google Scholar] [CrossRef]
- Wang, S.; Chen, W.; Xiang, H.; Yang, J.; Zhou, Z.; Zhu, M. Modification and Potential Application of Short-Chain-Length Polyhydroxyalkanoate (SCL-PHA). Polymers 2016, 8, 273. [Google Scholar] [CrossRef]
- Jin, A.; Pérez, G.; Martínez de Ilarduya, A.; del Valle, L.J.; Puiggalí, J. Characterization and Biomedical Applications of Electrospun PHBV Scaffolds Derived from Organic Residues. Int. J. Mol. Sci. 2025, 26, 180. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Barrera, A.; Urtuvia, V.; Padilla-Cordova, C.; Peña, C. Poly(3-hydroxybutyrate) accumulation by Azotobacter vinelandii under different oxygen transfer strategies. J. Ind. Microbiol. Biotechnol. 2019, 46, 13–19. [Google Scholar] [CrossRef] [PubMed]
- García, A.; Pérez, D.; Castro, M.; Urtuvia, V.; Castillo, T.; Díaz-Barrera, A.; Espín, G.; Peña, C. Production and recovery of poly-3-hydroxybutyrate [P(3HB)] of ultra-high molecular weight using fed-batch cultures of Azotobacter vinelandii OPNA strain. J. Chem. Technol. Biotechnol. 2019, 94, 1853–1860. [Google Scholar] [CrossRef]
- Castillo, T.; Flores, C.; Segura, D.; Espín, G.; Sanguino, J.; Cabrera, E.; Barreto, J.; Díaz-Barrera, A.; Peña, C. Production of polyhydroxybutyrate (PHB) of high and ultra-high molecular weight by Azotobacter vinelandii in batch and fed-batch cultures. J. Chem. Technol. Biotechnol. 2017, 92, 1809–1816. [Google Scholar] [CrossRef]
- Jackson, F.A.; Dawes, E.A. Regulation of the tricarboxylic acid cycle and poly-β-hydroxybutyrate metabolism in Azotobacter beijerinckii grown under nitrogen or oxygen limitation. J. Gen. Microbiol. 1976, 97, 303–312. [Google Scholar] [CrossRef]
- Madison, L.L.; Huisman, G.W. Metabolic engineering of poly(3-hydroxyalkanoates): From DNA to plastic. Microbiol. Mol. Biol. Rev. 1999, 63, 21–53. [Google Scholar] [CrossRef]
- Blunt, W.; Sparling, R.; Gapes, D.; Levin, D.; Cicek, N. The role of dissolved oxygen content as a modulator of microbial polyhydroxyalkanoate synthesis. World J. Microbiol. Biotechnol. 2018, 34, 106–119. [Google Scholar] [CrossRef]
- Amstutz, V.; Hanik, N.; Pott, J.; Utsunomia, C.; Zinn, M. Chapter Four—Tailored biosynthesis of polyhydroxyalkanoates in chemostat cultures. Methods Enzymol. 2019, 627, 99–123. [Google Scholar] [CrossRef]
- Sharma, P.K.; Fu, J.; Zhang, X.; Fristensky, B.; Sparling, R.; Levin, D.B. Genome features of Pseudomonas putida LS46, a novel polyhydroxyalkanoate producer and its comparison with other P. putida strains. AMB Expr. 2014, 4, 37–55. [Google Scholar] [CrossRef]
- Prieto, A.; Escapa, I.F.; Martínez, V.; Dinjaski, N.; Herencias, C.; De la Peña, F.; Tarazona, N.; Revelles, O. A holistic view of polyhydroxyalkanoate metabolism in Pseudomonas putida. Environ. Microbiol. 2016, 18, 341–357. [Google Scholar] [CrossRef]
- Page, W.J.; Manchak, J.; Rudy, B. Formation of poly(hydroxybutyrate-co-hydroxyvalerate) by Azotobacter vinelandii UWD. Appl. Environ. Microbiol. 1992, 58, 2866–2873. [Google Scholar] [CrossRef] [PubMed]
- Page, W.J.; Manchak, J. The role of β-oxidation of short-chain alkanaotes in polyhydroxyalkanoate copolymer synthesis in Azotobacter vinelandii UWD. Can. J. Microbiol. 1995, 41, 106–114. [Google Scholar] [CrossRef]
- Page, W.J.; Bhanthumnavin, N.; Manchak, J.; Ruman, M. Production of poly(β-hydroxybutyrate-β-hydroxyvalerate) copolymer from sugars by Azotobacter salinestris. Appl. Microbiol. Biotechnol. 1997, 48, 88–93. [Google Scholar] [CrossRef]
- Urtuvia, V.; Maturana, N.; Peña, C.; Díaz-Barrera, A. Accumulation of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) by Azotobacter vinelandii with different 3HV fraction in shake flasks and bioreactor. Bioprocess. Biosyst. Eng. 2020, 43, 1469–1478. [Google Scholar] [CrossRef]
- Singh, S.; Sithole, B.; Lekha, P.; Permaul, K.; Govinden, R. Optimization of cultivation medium and cyclic fed-batch fermentation strategy for enhanced polyhydroxyalkanoate production by Bacillus thuringiensis using a glucose-rich hydrolysate. Bioresour. Bioprocess. 2021, 8, 11–28. [Google Scholar] [CrossRef]
- Senior, P.J.; Beech, G.A.; Ritchie, G.A.; Dawes, E.A. The role of oxygen limitation in the formation of poly-β-hydroxybutyrate during batch and continuous culture of Azotobacter beijerinckii. Biochem. J. 1972, 128, 1193–1201. [Google Scholar] [CrossRef]
- Lillo, J.G.; Rodriguez-Valera, F. Effects of culture conditions on poly(beta-hydroxybutyric acid) production by Haloferax mediterranei. Appl. Environ. Microbiol. 1990, 56, 2517–2521. [Google Scholar] [CrossRef]
- Kocharin, K.; Nielsen, J. Specific growth rate and substrate dependent polyhydroxybutyrate production in Saccharomyces cerevisiae. AMB Express 2013, 3, 18–24. [Google Scholar] [CrossRef]
- Koller, M.; Muhr, A. Continuous production mode as a viable process-engineering tool for efficient poly(hydroxyalkanoate) (PHA) bio-production. Chem. Biochem. Eng. Q. 2014, 28, 65–77. [Google Scholar] [CrossRef]
- Koller, M.; Braunegg, G. Potential and prospects of continuous polyhydroxyalkanoate (PHA) production. Bioengineering 2015, 2, 94–121. [Google Scholar] [CrossRef]
- Ramsay, J.A.; Berger, E.; Ramsay, B.A.; Chavarie, C. Recovery of poly-3-hydroxyalpoic acid granules by a surfactant hypochlorite treatment. Biotechnol. Tech. 1990, 4, 221–226. [Google Scholar] [CrossRef]
- Yu, S.T.; Lin, C.C.; Too, J.R. PHBV production by Ralstonia eutropha in a continuous stirred tank reactor. Process Biochem. 2005, 40, 2729–2734. [Google Scholar] [CrossRef]
- Riis, V.; Mai, W. Gas chromatographic determination of poly-β-hydroxybutyric acid in microbial biomass after hydrochloric acid propanolysis. J. Chromatogr. A 1988, 445, 285–289. [Google Scholar] [CrossRef]
- Mendonça, T.T.; Gomez, J.G.C.; Buffoni, E.; Sánchez Rodriguez, R.J.; Schripsema, J.; Lopes, M.S.G.; Silva, L.F. Exploring the potential of Burkholderia sacchari to produce polyhydroxyalkanoates. J. Appl. Microbiol. 2014, 116, 815–829. [Google Scholar] [CrossRef]
- De Paula, F.C.; Kakazu, S.; De Paula, C.B.C.; De Almeida, A.F.; Gomez, J.G.C.; Conteiro, J. Burkholderia glumae MA13: A newly isolated bacterial strain suitable for polyhydroxyalkanoates production from crude glycerol. Biocatal. Agric. Biotechnol. 2019, 20, 101268–101278. [Google Scholar] [CrossRef]
- Díaz-Barrera, A.; Andler, R.; Martínez, I.; Peña, C. Poly-3-hydroxybutyrate production by Azotobacter vinelandii strains in batch cultures at different oxygen transfer rates. J. Chem. Technol. Biotechnol. 2016, 91, 1063–1071. [Google Scholar] [CrossRef]
- Jaenicke, L. Methods of Enzymatic Analysis. Hrsg. von H.-U. Bergmeyer, J. Bergmeyer und M. Grassl. 3. Aufl. Bd. VI. Metabolites I: Carbo- hydrates. XXIX, 701 S., DM 260; Bd. VII. Metabolites 11: Tri- and Dicarboxylic Acids, Purines, Pyrimidines and Derivatives, Coenzymes, Inorganic Compounds. XXVIII, 641 S., DM 325,–. VCH Verlagsgesellschaft, Weinheim-Deerfield Beach, Florida-Basel 1985. Chem. Unserer Zeit 1986, 20, 103. [Google Scholar] [CrossRef]
- Mohd Kamal, K.; Mahamad, M.H.; Abdul Rahim, N.; Hashim, Y.Z.H.; Abdullah Sani, M.S.; Azizan, K.A. Bacterial Metabolomics: Sample Preparation Methods. Biochem. Res. Int. 2022, 12, 9186536–9186550. [Google Scholar] [CrossRef]
- Díaz-Barrera, A.; Martínez, F.; Guevara Pezoa, F.; Acevedo, F. Evaluation of gene expression and alginate production in response to oxygen transfer in continuous culture of Azotobacter vinelandii. PLoS ONE 2014, 9, e105993. [Google Scholar] [CrossRef]
- García, A.; Ferrer, P.; Albiol, J.; Castillo, T.; Segura, D.; Peña, C. Metabolic flux analysis and the NAD(P)H/NAD(P)+ ratios in chemostat cultures of Azotobacter vinelandii. Microb. Cell Fact. 2018, 17, 10–23. [Google Scholar] [CrossRef]
- Castillo, T.; García, A.; Padilla-Córdova, C.; Díaz-Barrera, A.; Peña, C. Respiration in Azotobacter vinelandii and its relationship with the synthesis of biopolymers. Electron. J. Biotechnol. 2020, 48, 36–45. [Google Scholar] [CrossRef]
- Alleman, A.B.; Mus, F.; Peters, J.W. Metabolic model of the nitrogen-fixing obligate aerobe Azotobacter vinelandii predicts its adaptation to oxygen concentration and metal availability. MBio 2021, 12, e02593-21. [Google Scholar] [CrossRef] [PubMed]
- Bertsova, Y.V.; Bogachev, A.V.; Skulachev, V.P. Noncoupled NADH: Ubiquinone oxidoreductase of Azotobacter vinelandii is required for diazotrophic growth at high oxygen concentrations. J. Bacteriol. 2001, 183, 6869–6874. [Google Scholar] [CrossRef] [PubMed]
- Oelze, J. Respiratory protection of nitrogenase in Azotobacter species: Is a widely held hypothesis unequivocally supported by experimental evidence? FEMS Microbiol. Rev. 2000, 24, 321–333. [Google Scholar] [CrossRef]
- Egli, T. On multiple-nutrient-limited growth of microorganisms, with special reference to dual limitation by carbon and nitrogen substrates. Antonie Van Leeuwenhoek 1991, 60, 225–234. [Google Scholar] [CrossRef]
- Larsson, C.; von Stockar, U.; Marison, I.; Gustafsson, L. Growth and metabolism of Saccharomyces cerevisiae in chemostat cultures under carbon-, nitrogen-, or carbon- and nitrogen-limiting conditions. J. Bacteriol. 1993, 175, 4809–4816. [Google Scholar] [CrossRef]
- Durner, R.; Witholt, B.; Egli, T. Accumulation of poly [(R)-3-hydroxyalkanoates] in Pseudomonas oleovorans during growth with octanoate in continuous culture at different dilution rates. Appl. Environ. Microbiol. 2000, 66, 3408–3414. [Google Scholar] [CrossRef]
- Rajpurohit, H.; Eiteman, M.A. Nutrient-Limited Operational Strategies for the Microbial Production of Biochemicals. Microorganisms 2022, 10, 2226. [Google Scholar] [CrossRef]
- García-Ochoa, F.; Gomez, E.; Santos, V. Fluid dynamic conditions and oxygen availability effects on microbial cultures in STBR: An overview. Biochem. Eng. J. 2020, 164, 107803. [Google Scholar] [CrossRef]
- Urtuvia, V.; Ponce, B.; Andler, R.; Peña, C.; Diaz-Barrera, A. Extended batch cultures for poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV) production by Azotobacter vinelandii OP growing at different aeration rates. 3 Biotech 2022, 12, 304–316. [Google Scholar] [CrossRef]
- Kuhla, J.; Oelze, J. Dependency of growth yield, maintenance and Ks-values on the dissolved oxygen concentration in continuous cultures of Azotobacter vinelandii. Arch. Microbiol. 1988, 149, 509–514. [Google Scholar] [CrossRef]
- Contreras-Abara, P.; Castillo, T.; Ponce, B.; Urtuvia, V.; Peña, C.; Díaz-Barrera, A. Continuous bioproduction of alginate bacterial under nitrogen fixation and nonfixation conditions. Fermentation 2023, 9, 426. [Google Scholar] [CrossRef]
- Díaz-Barrera, A.; Soto, E.; Altamirano, C. Alginate production and alg8 gene expression by Azotobacter vinelandii in continuous cultures. J. Ind. Microbiol. Biotechnol. 2012, 39, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Parroquin-Gonzalez, M.; Winterburn, J. Continuous bioreactor production of polyhydroxyalkanoates in Haloferax mediterranei. Front. Bioeng. Biotechnol. 2023, 11, 1220271–1220281. [Google Scholar] [CrossRef] [PubMed]
- Torres-Pedraza, A.J.; Salgado-Lugo, H.; Segura, D.; Díaz-Barrera, A.; Peña, C. Composition control of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) copolymerization by oxygen transfer rate (OTR) in Azotobacter vinelandii OPNA. J. Chem. Technol. Biotechnol. 2021, 96, 2782–2791. [Google Scholar] [CrossRef]
- Chen, G.Q.; Jiang, X.R. Engineering microorganisms for improving polyhydroxyalkanoate biosynthesis. Curr. Opin. Biotechnol. 2018, 53, 20–25. [Google Scholar] [CrossRef]
- Ferre-Guell, A.; Winterburn, J. Biosynthesis and Characterization of Polyhydroxyalkanoates with Controlled Composition and Microstructure. Biomacromolecules 2018, 12, 996–1005. [Google Scholar] [CrossRef]
- Aldor, I.S.; Kim, S.W.; Jones Prather, K.L.; Keasling, J.D. Metabolic engineering of a novel propionate-independent pathway for the production of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) in recombinant Salmonella enterica serovar typhimurium. Appl. Environ. Microbiol. 2002, 68, 3848–3854. [Google Scholar] [CrossRef]
- Shang, L.; Fei, Q.; Zhang, Y.H.; Wang, X.Z.; Fan, D.; Chang, H.N. Thermal Properties and Biodegradability Studies of Poly(3-hydroxybutyrate-co-3-hydroxyvalerate). J. Polym. Environ. 2012, 20, 23–28. [Google Scholar] [CrossRef]
- Alfano, S.; Doineau, E.; Perdrier, C.; Preziosi-Belloy, L.; Gontard, N.; Martinelli, A.; Grousseau, E.; Angellier-Coussy, H. Influence of the 3-Hydroxyvalerate Content on the Processability, Nucleating and Blending Ability of Poly(3-Hydroxybutyrate-co-3-hydroxyvalerate)-Based Materials. ACS Omega 2024, 9, 29360–29371. [Google Scholar] [CrossRef]
- Moungprayoon, A.; Lunprom, S.; Salakkam, A. High production of poly(hydroxybutyrate-co-hydroxyvalerate) with controllable 3-hydroxyvalerate content by Paracoccus sp. KKU01 using cassava processing wastes and propionic acid as feedstocks: Optimizing propionic acid feeding and kinetic analysis. Ind. Crop Prod. 2025, 234, 121559–121570. [Google Scholar] [CrossRef]
- Rivera-Briso, A.L.; Serrano-Aroca, Á. Poly(3-Hydroxybutyrate-co-3-Hydroxyvalerate): Enhancement Strategies for Advanced Applications. Polymers 2018, 10, 732. [Google Scholar] [CrossRef]
- Rodríguez-Cendal, A.I.; Gómez-Seoane, I.; de Toro-Santos, F.J.; Fuentes-Boquete, I.M.; Señarís-Rodríguez, J.; Díaz-Prado, S.M. Biomedical Applications of the Biopolymer Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV): Drug Encapsulation and Scaffold Fabrication. Int. J. Mol. Sci. 2023, 24, 11674. [Google Scholar] [CrossRef]


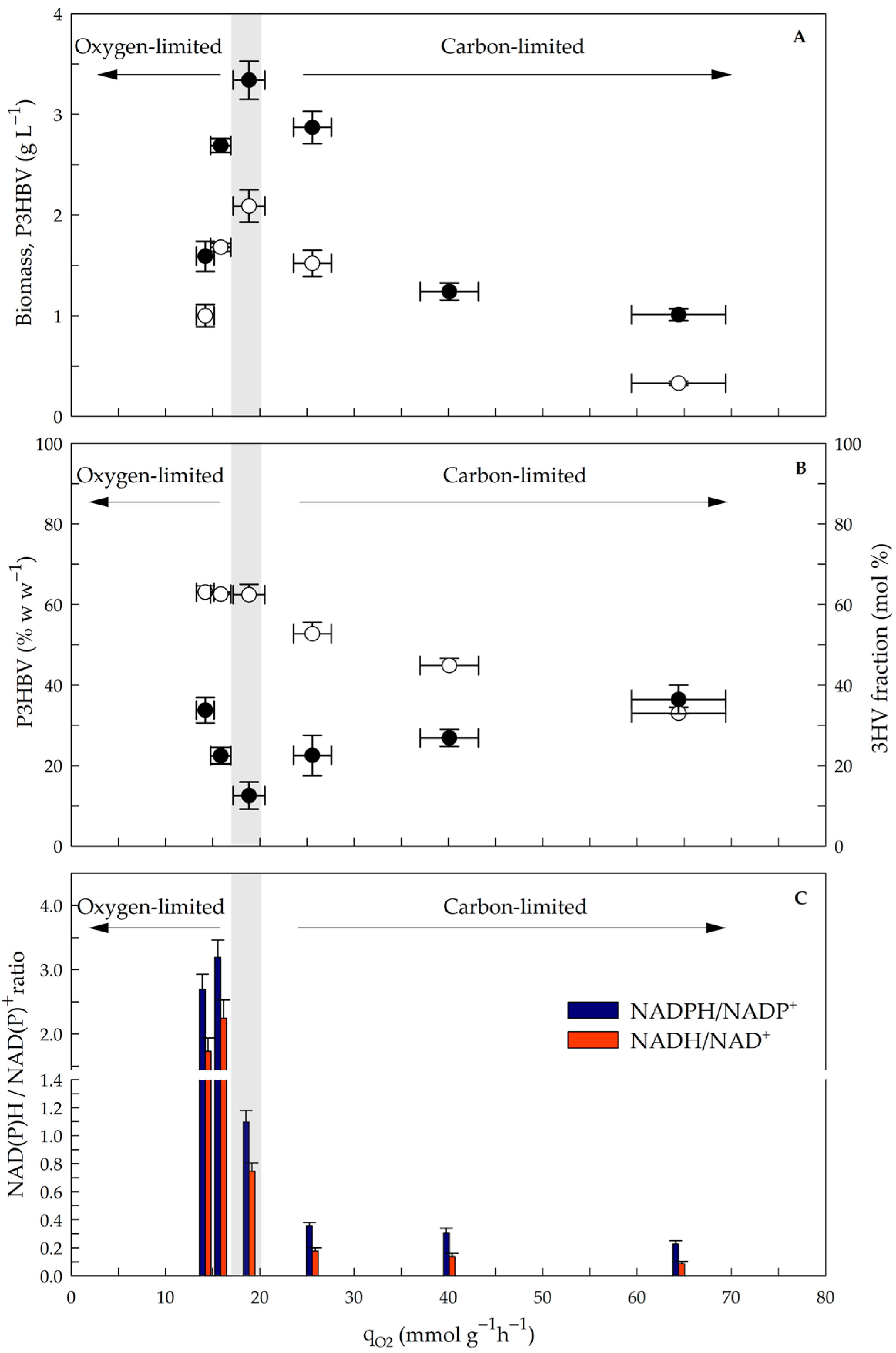
| Oxygen-Limited | Dual Limitation | Carbon-Limited | ||||
|---|---|---|---|---|---|---|
| qO2 (mmol g−1 h−1) | 14.2 | 15.9 | 18.9 | 25.6 | 40.1 | 64.4 |
| Biomass (%) | 6.5 | 9.0 | 8.4 | 9.0 | 4.4 | 4.4 |
| P3HBV (%) | 13.1 | 14.8 | 16.6 | 12.1 | 4.3 | 2.2 |
| CO2 (%) | 26.8 | 42.4 | 51.3 | 78.7 | 88.9 | 93.8 |
| Carbon recovered (%) | 46.4 | 66.2 | 76.4 | 99.8 | 97.7 | 100.4 |
| Oxygen-Limited | Dual Limitation | Carbon-Limited | ||||
|---|---|---|---|---|---|---|
| qO2 (mmol g−1 h−1) | 14.2 | 15.9 | 18.9 | 25.6 | 40.1 | 64.4 |
| YX/S (g g−1) | 0.131 ± 0.014 a | 0.174 ± 0.003 b | 0.165 ± 0.006 b | 0.137 ± 0.007 a | 0.059 ± 0.001 | 0.049 ± 0.003 |
| YP3HBV/S (g g−1) | 0.083 ± 0.009 a | 0.108 ± 0.002 b | 0.100 ± 0.008 b | 0.072 ± 0.006 a | 0.026 ± 0.001 | 0.016 ± 0.001 |
| QP (g L−1 h−1) | 0.040 ± 0.004 | 0.067 ± 0.002 c | 0.083 ± 0.006 | 0.061 ± 0.005 c | 0.022 ± 0.001 | 0.013 ± 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez, A.; García, A.; Urtuvia, V.; Peña, C.; Díaz-Barrera, A. Tailoring 3HV Fraction in Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) by Azotobacter vinelandii Through Oxygen and Carbon Limitation in Continuous Cultures. Polymers 2025, 17, 2578. https://doi.org/10.3390/polym17192578
Pérez A, García A, Urtuvia V, Peña C, Díaz-Barrera A. Tailoring 3HV Fraction in Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) by Azotobacter vinelandii Through Oxygen and Carbon Limitation in Continuous Cultures. Polymers. 2025; 17(19):2578. https://doi.org/10.3390/polym17192578
Chicago/Turabian StylePérez, Andrés, Andrés García, Viviana Urtuvia, Carlos Peña, and Alvaro Díaz-Barrera. 2025. "Tailoring 3HV Fraction in Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) by Azotobacter vinelandii Through Oxygen and Carbon Limitation in Continuous Cultures" Polymers 17, no. 19: 2578. https://doi.org/10.3390/polym17192578
APA StylePérez, A., García, A., Urtuvia, V., Peña, C., & Díaz-Barrera, A. (2025). Tailoring 3HV Fraction in Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) by Azotobacter vinelandii Through Oxygen and Carbon Limitation in Continuous Cultures. Polymers, 17(19), 2578. https://doi.org/10.3390/polym17192578








