Artificial Neural Network Prediction of Mechanical Properties in Mycelium-Based Biocomposites
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Physical Properties of MBB
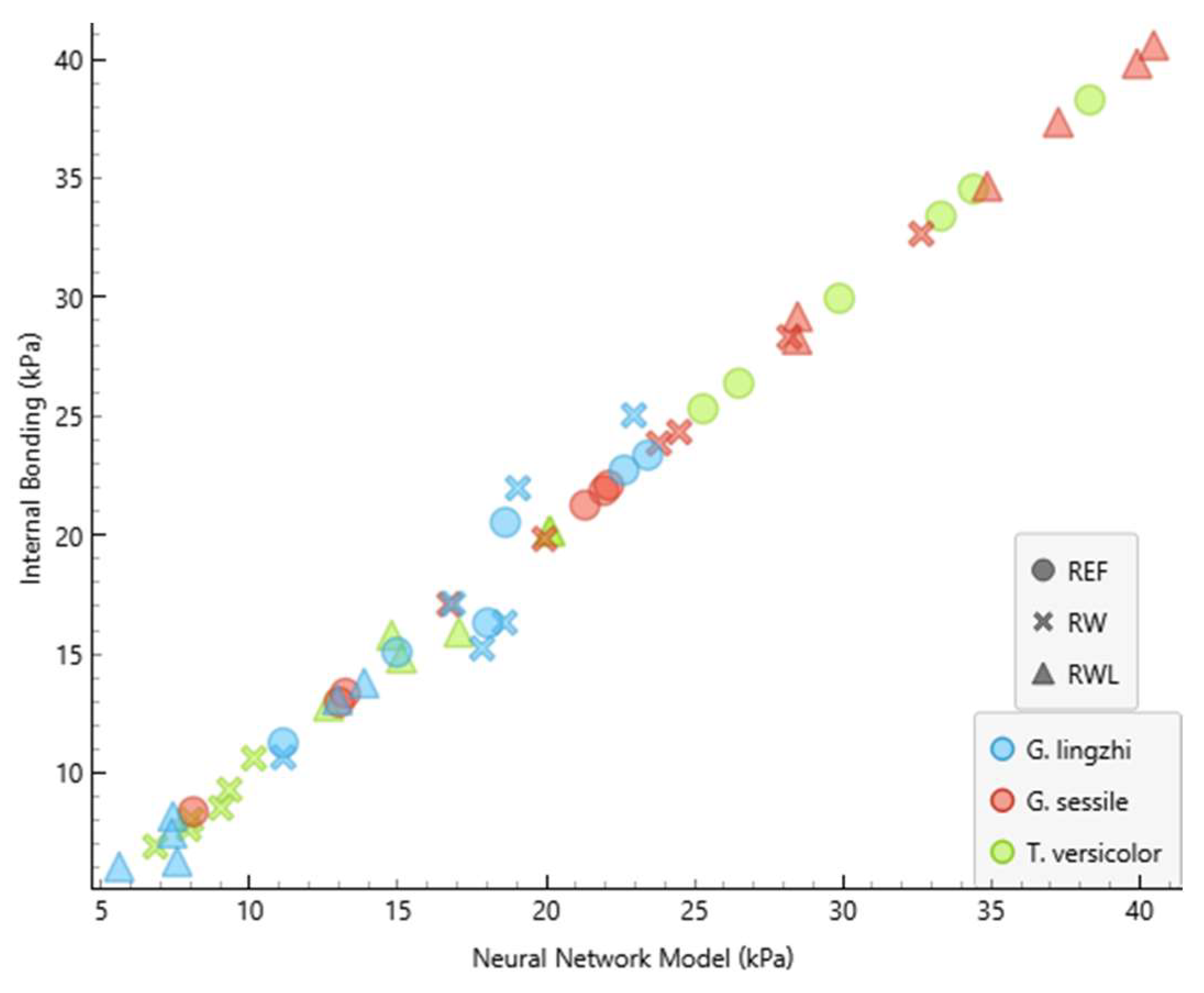
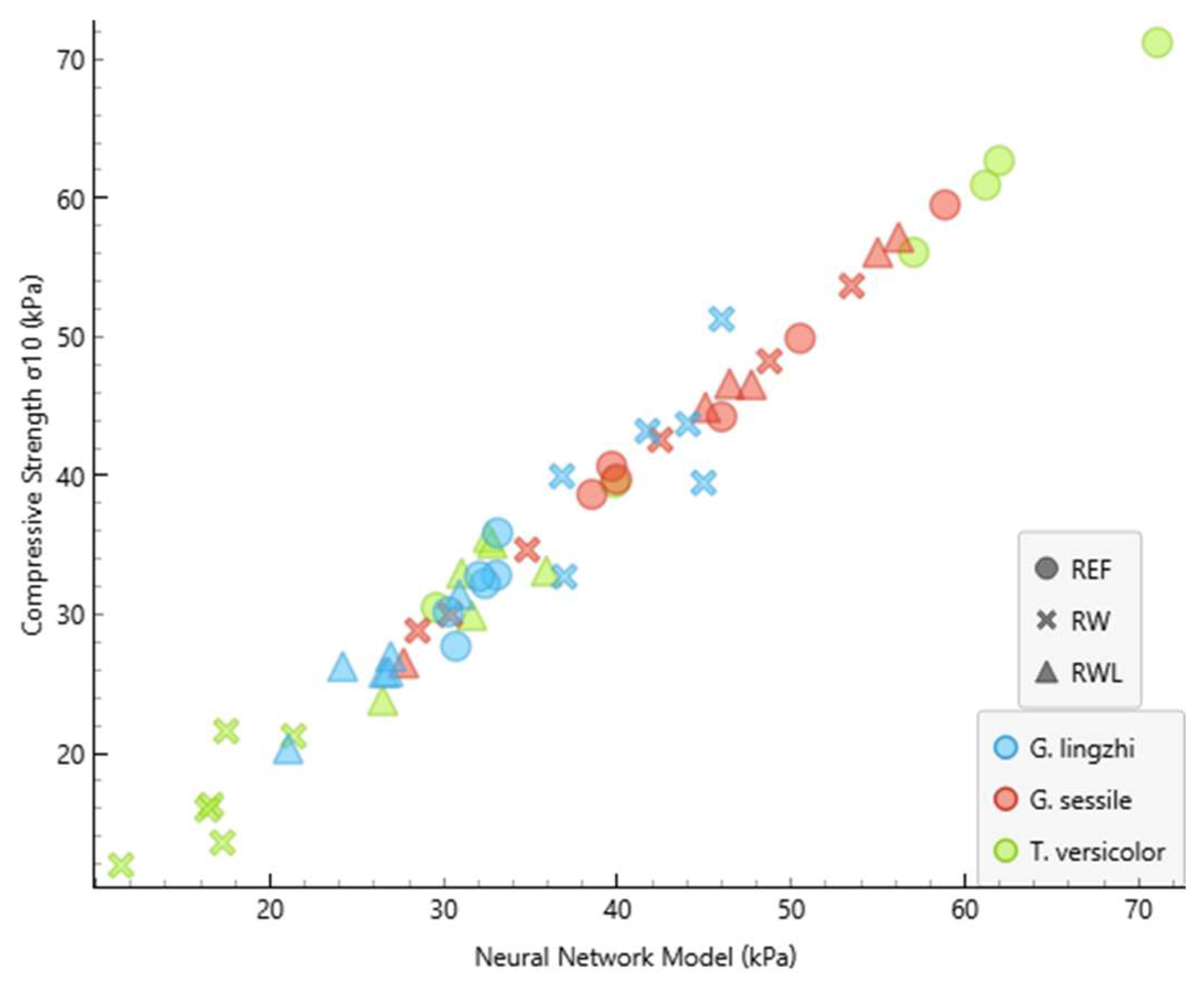
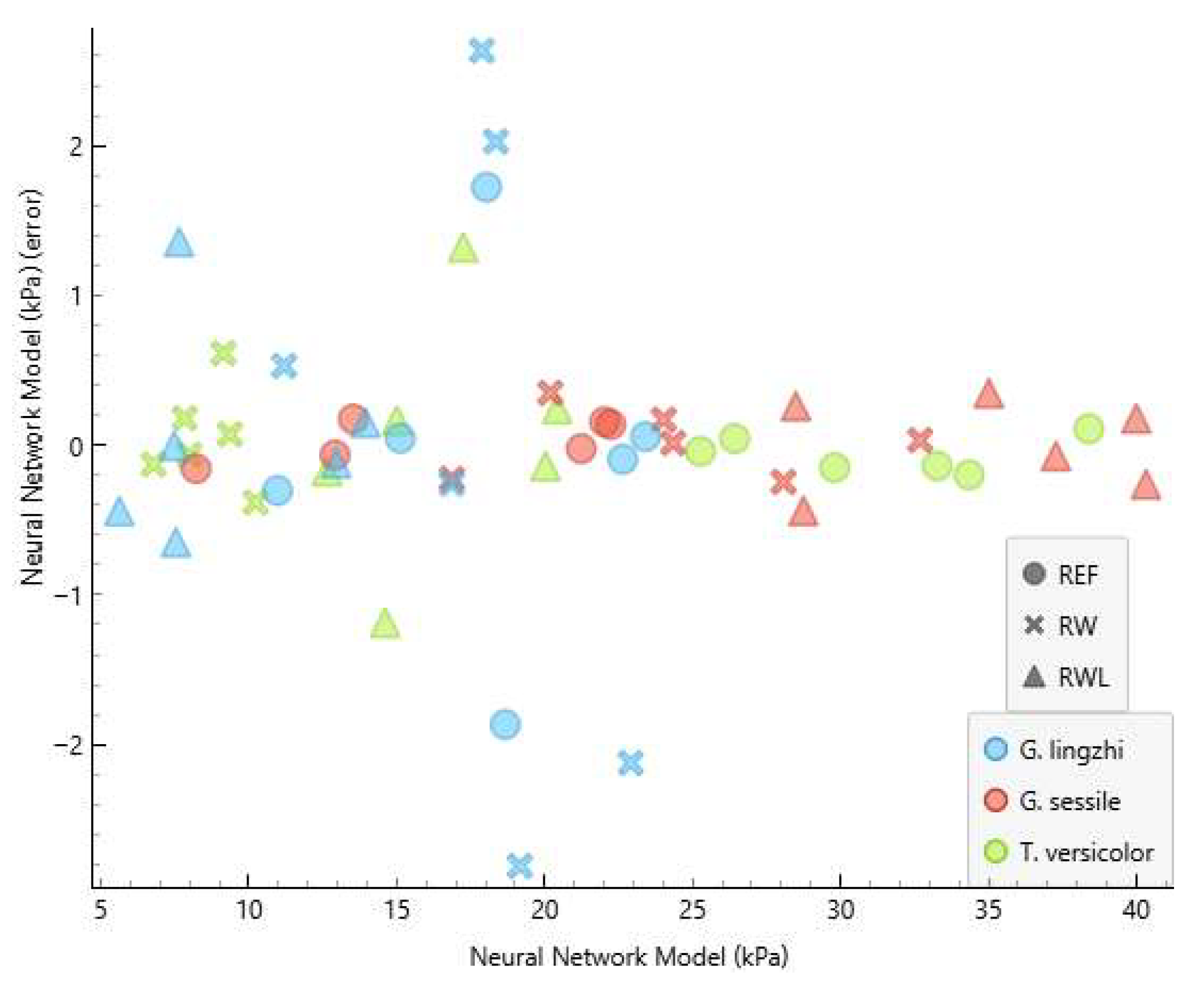
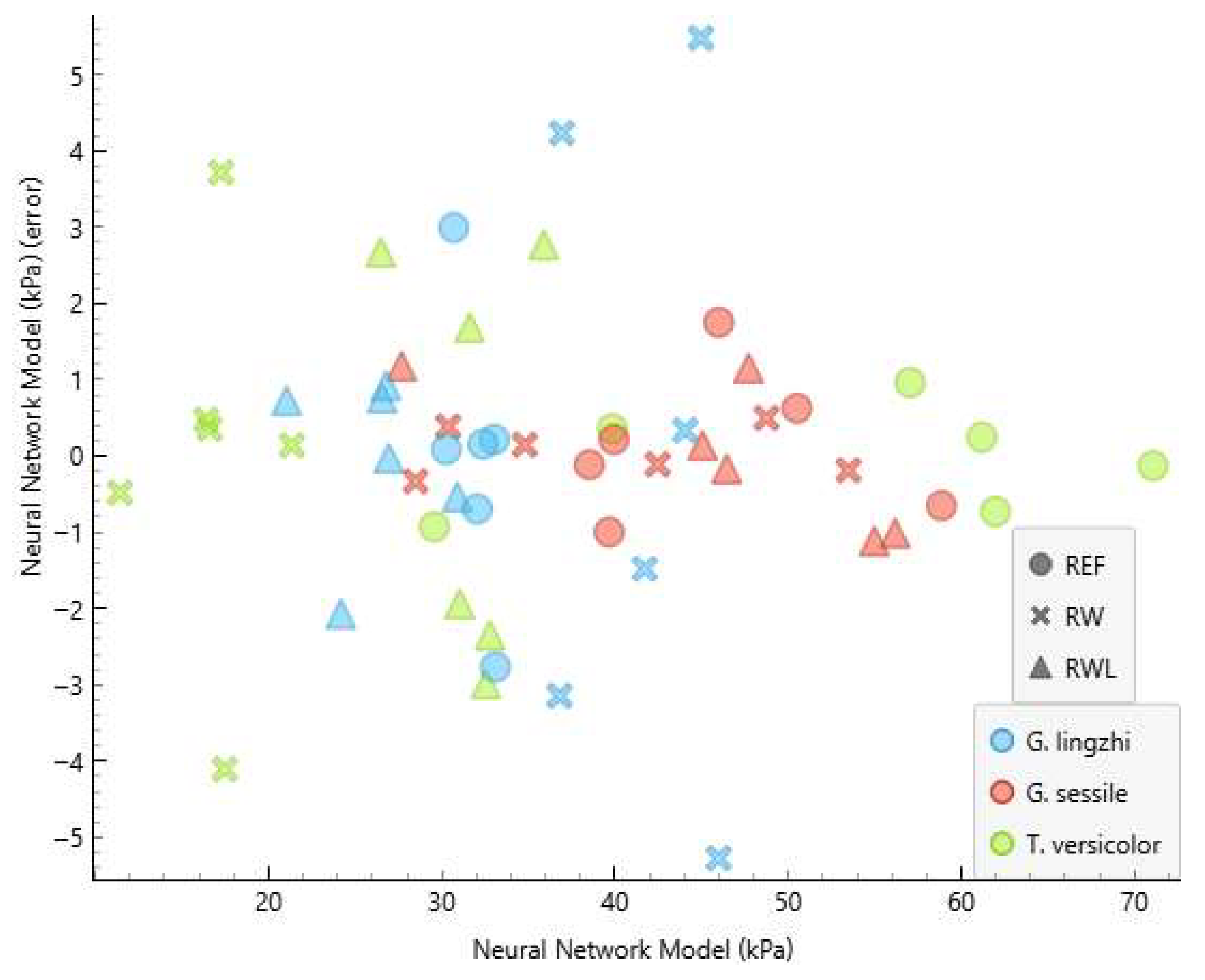
3.2. Model Performance
3.3. Residual Analysis
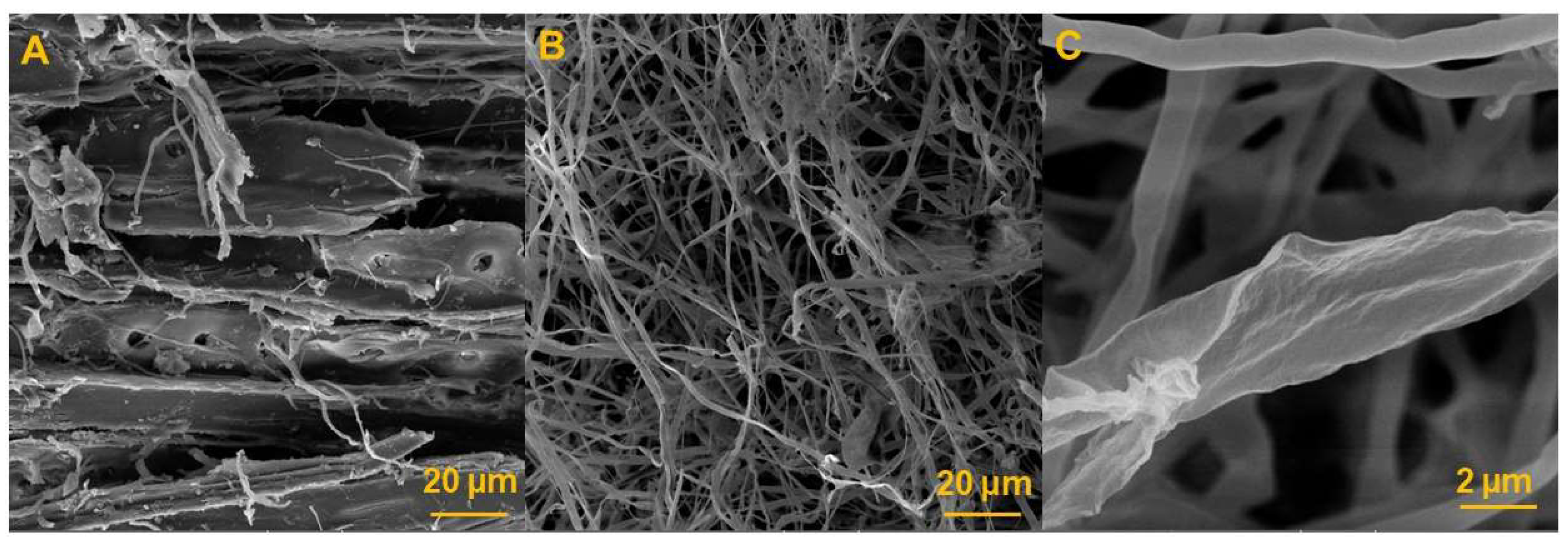
3.4. Microstructural Analysis
3.5. Limitations of This Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANN | artificial neural network |
| CS | compressive strength |
| IB | internal bonding |
| LC | lignocellulosic |
| MAE | mean absolute error |
| MAPE | mean absolute percentage error |
| MBB | mycelium-based biocomposite |
| MSE | mean square error |
| NNM | neural network model |
| SEM | scanning electron microscopy |
| R2 | coefficient of determination |
| RMSE | root mean square error |
References
- Dias, P.P.; Jayasinghe, L.B.; Waldmann, D. Investigation of Mycelium-Miscanthus Composites as Building Insulation Material. Results Mater. 2021, 10, 100189. [Google Scholar] [CrossRef]
- Muiruri, J.K.; Yeo, J.C.C.; Zhu, Q.; Ye, E.; Loh, X.J.; Li, Z. Sustainable Mycelium-Bound Biocomposites: Design Strategies, Materials Properties, and Emerging Applications. ACS Sustain. Chem. Eng. 2023, 11, 6801–6821. [Google Scholar] [CrossRef]
- Yang, H.; Wu, Y.; Ni, K.; Liu, T.; Ran, X.; Tan, X.; Gao, W.; Yang, Z.; Du, G.; Yang, L. Integration of Branched Amine and Dialdehyde Cellulose to Produce High-Performance Bio-Based Wood Adhesive. Int. J. Adhes. Adhes. 2023, 126, 103449. [Google Scholar] [CrossRef]
- Elsacker, E.; Vandelook, S.; Van Wylick, A.; Ruytinx, J.; De Laet, L.; Peeters, E. A Comprehensive Framework for the Production of Mycelium-Based Lignocellulosic Composites. Sci. Total Environ. 2020, 725, 138431. [Google Scholar] [CrossRef]
- Shen, S.C.; Lee, N.A.; Lockett, W.J.; Acuil, A.D.; Gazdus, H.B.; Spitzer, B.N.; Buehler, M.J. Robust Myco-Composites as a Platform for Versatile Hybrid-Living Structural Materials. arXiv 2023, arXiv:2305.12151. [Google Scholar] [CrossRef]
- Fricker, M.; Boddy, L.; Bebber, D. Network Organisation of Mycelial Fungi. In Biology of the Fungal Cell; Howard, R.J., Gow, N.A.R., Eds.; The Mycota; Springer: Berlin, Heidelberg, 2007; Volume 8, pp. 309–330. ISBN 978-3-540-70615-1. [Google Scholar]
- Attias, N.; Danai, O.; Abitbol, T.; Tarazi, E.; Ezov, N.; Pereman, I.; Grobman, Y.J. Mycelium Bio-Composites in Industrial Design and Architecture: Comparative Review and Experimental Analysis. J. Clean. Prod. 2020, 246, 119037. [Google Scholar] [CrossRef]
- Sydor, M.; Bonenberg, A.; Doczekalska, B.; Cofta, G. Mycelium-Based Composites in Art, Architecture, and Interior Design: A Review. Polymers 2021, 14, 145. [Google Scholar] [CrossRef]
- Madusanka, C.; Udayanga, D.; Nilmini, R.; Rajapaksha, S.; Hewawasam, C.; Manamgoda, D.; Vasco-Correa, J. A Review of Recent Advances in Fungal Mycelium Based Composites. Discov. Mater. 2024, 4, 13. [Google Scholar] [CrossRef]
- Alaneme, K.K.; Anaele, J.U.; Oke, T.M.; Kareem, S.A.; Adediran, M.; Ajibuwa, O.A.; Anabaranze, Y.O. Mycelium Based Composites: A Review of Their Bio-Fabrication Procedures, Material Properties and Potential for Green Building and Construction Applications. Alex. Eng. J. 2023, 83, 234–250. [Google Scholar] [CrossRef]
- Butu, A.; Rodino, S.; Miu, B.A.; Butu, M. Mycelium-Based Materials for the Ecodesign of Bioeconomy. Dig. J. Nanomater. Biostructures 2020, 15, 1129–1140. [Google Scholar] [CrossRef]
- Javadian, A.; Le Ferrand, H.; Hebel, D.E.; Saeidi, N. Application of Mycelium-Bound Composite Materials in Construction Industry: A Short Review. SOJMSE 2020, 7, 1–9. [Google Scholar] [CrossRef]
- Vašatko, H.; Gosch, L.; Jauk, J.; Stavric, M. Basic Research of Material Properties of Mycelium-Based Composites. Biomimetics 2022, 7, 51. [Google Scholar] [CrossRef]
- Elsacker, E.; Vandelook, S.; Brancart, J.; Peeters, E.; De Laet, L. Mechanical, Physical and Chemical Characterisation of Mycelium-Based Composites with Different Types of Lignocellulosic Substrates. PLoS ONE 2019, 14, e0213954. [Google Scholar] [CrossRef]
- Aiduang, W.; Kumla, J.; Srinuanpan, S.; Thamjaree, W.; Lumyong, S.; Suwannarach, N. Mechanical, Physical, and Chemical Properties of Mycelium-Based Composites Produced from Various Lignocellulosic Residues and Fungal Species. J. Fungi 2022, 8, 1125. [Google Scholar] [CrossRef]
- Vidholdová, Z.; Kormúthová, D.; Ždinský, J.I.; Lagaňa, R. Compressive Resistance of the Mycelium Composite. Ann. WULS SGGW For. Wood Technol. 2019, 107, 31–36. [Google Scholar] [CrossRef]
- Aiduang, W.; Chanthaluck, A.; Kumla, J.; Jatuwong, K.; Srinuanpan, S.; Waroonkun, T.; Oranratmanee, R.; Lumyong, S.; Suwannarach, N. Amazing Fungi for Eco-Friendly Composite Materials: A Comprehensive Review. J. Fungi 2022, 8, 842. [Google Scholar] [CrossRef]
- Soh, E.; Le Ferrand, H. Woodpile Structural Designs to Increase the Stiffness of Mycelium-Bound Composites. Mater. Des. 2023, 225, 111530. [Google Scholar] [CrossRef]
- Appels, F.V.W.; Camere, S.; Montalti, M.; Karana, E.; Jansen, K.M.B.; Dijksterhuis, J.; Krijgsheld, P.; Wösten, H.A.B. Fabrication Factors Influencing Mechanical, Moisture- and Water-Related Properties of Mycelium-Based Composites. Mater. Des. 2019, 161, 64–71. [Google Scholar] [CrossRef]
- Cerimi, K.; Akkaya, K.C.; Pohl, C.; Schmidt, B.; Neubauer, P. Fungi as Source for New Bio-Based Materials: A Patent Review. Fungal Biol. Biotechnol. 2019, 6, 17. [Google Scholar] [CrossRef]
- Yang, L.; Park, D.; Qin, Z. Material Function of Mycelium-Based Bio-Composite: A Review. Front. Mater. 2021, 8, 737377. [Google Scholar] [CrossRef]
- Hýsek, Š.; Daňková, M.; Jozífek, M.; Němec, M.; Wimmer, R. Mycelium-Based Biocomposites from Recycled Wood: Influence of Fungal Species on Properties of Biocomposites. In Proceedings of the Unleashing the Potential of Wood-Based Materials, Zagreb, Croatia, 7–8 December 2023; Volume 32. [Google Scholar]
- Ghazvinian, A.; Farrokhsiar, P.; Vieira, F.; Pecchia, J.; Gursoy, B. Mycelium-Based Bio-Composites For Architecture: Assessing the Effects of Cultivation Factors on Compressive Strength. In Blucher Design Proceedings; Editora Blucher: Porto, Portugal, 2019; pp. 505–514. [Google Scholar]
- Yang, L.; Qin, Z. Mycelium-Based Wood Composites for Light Weight and High Strength by Experiment and Machine Learning. Cell Rep. Phys. Sci. 2023, 4, 101424. [Google Scholar] [CrossRef]
- Voutetaki, M.E.; Mpalaskas, A.C. Natural Fiber-Reinforced Mycelium Composite for Innovative and Sustainable Construction Materials. Fibers 2024, 12, 57. [Google Scholar] [CrossRef]
- Němec, M.; Prokůpek, L.; Obst, V.; Pipíška, T.; Král, P.; Hýsek, Š. Novel Kraft-Lignin-Based Adhesives for the Production of Particleboards. Compos. Struct. 2024, 344, 118344. [Google Scholar] [CrossRef]
- ISO 16535:2019; Thermal Insulating Products for Building Applications—Determination of Long-Term Water Absorption by Immersion. NSAI: Dublin, Ireland, 2019.
- EN 319:1993; Particleboards and Fibreboards—Determination of Tensile Strength Perpendicular to the Plane of the Board. CEN: Bruxelles, Belgium, 1993.
- EN 826; Thermal Insulation Products for Building Applications—Determination of Compression Behavior. CEN: Bruxelles, Belgium, 2013.
- Klímek, P.; Wimmer, R. Alternative Raw Materials for Bio-Based Composite. In Proceedings of the International Conference “Wood Science and Engineering in the Third Millennium”, Brasov, Romania, 2–4 November 2017. [Google Scholar]
- Kirsch, A.; Ostendorf, K.; Euring, M. Improvements in the Production of Wood Fiber Insulation Boards Using Hot-Air/Hot-Steam Process. Eur. J. Wood Prod. 2018, 76, 1233–1240. [Google Scholar] [CrossRef]
- Hýsek, Š.; Neuberger, P.; Sikora, A.; Schönfelder, O.; Ditommaso, G. Waste Utilization: Insulation Panel from Recycled Polyurethane Particles and Wheat Husks. Materials 2019, 12, 3075. [Google Scholar] [CrossRef]
- Eroğlu, M.A.; Altun, S.; Ciritcioğlu, H.H. Modeling of Mechanical Properties of Wood-Polymer Composites with Artificial Neural Networks. BioResources 2024, 19, 4468–4485. [Google Scholar] [CrossRef]
- Gama, N.V.; Soares, B.; Freire, C.S.R.; Silva, R.; Neto, C.P.; Barros-Timmons, A.; Ferreira, A. Bio-Based Polyurethane Foams toward Applications beyond Thermal Insulation. Mater. Des. 2015, 76, 77–85. [Google Scholar] [CrossRef]
- Jones, M.; Mautner, A.; Luenco, S.; Bismarck, A.; John, S. Engineered Mycelium Composite Construction Materials from Fungal Biorefineries: A Critical Review. Mater. Des. 2020, 187, 108397. [Google Scholar] [CrossRef]


| Fungus | Substrate | N | Water Uptake (%) | Thermal Conductivity (W·m−1·K−1) | Volumetric Heat Capacity (MJ·K−1·m−3) | Thermal Diffusivity (m2·s−1·10−6) |
|---|---|---|---|---|---|---|
| G. lingzhi | RW | 6 | 153 (8) | 0.0825 (0.0008) | 0.2347 (0.0099) | 0.3519 (0.0124) |
| G. lingzhi | RWL | 6 | 111 (12) | 0.0821 (0.0007) | 0.2175 (0.0083) | 0.3779 (0.0118) |
| G. lingzhi | REF | 6 | 109 (21) | 0.0833 (0.0005) | 0.2152 (0.0127) | 0.3881 (0.0260) |
| G. sessile | RW | 6 | 128 (27) | 0.0836 (0.0009) | 0.2407 (0.0203) | 0.3497 (0.0343) |
| G. sessile | RWL | 6 | 77 (11) | 0.0824 (0.0011) | 0.2428 (0.0142) | 0.3400 (0.0159) |
| G. sessile | REF | 6 | 123 (10) | 0.0849 (0.0031) | 0.2598 (0.0201) | 0.3276 (0.0131) |
| T. versicolor | RW | 6 | 168 (20) | 0.0785 (0.0027) | 0.2047 (0.0170) | 0.3850 (0.0204) |
| T. versicolor | RWL | 6 | 136 (5) | 0.0783 (0.0010) | 0.2044 (0.0106) | 0.3836 (0.0159) |
| T. versicolor | REF | 6 | 108 (7) | 0.0751 (0.0041) | 0.1605 (0.0080) | 0.4693 (0.0373) |
| Fungus | Substrate | Internal Bonding (kPa) | NNM Internal Bonding (kPa) | NNM Error (kPa) |
|---|---|---|---|---|
| G. lingzhi | RW | 25.0 | 23.0 | −2.079 |
| G. lingzhi | RW | 16.3 | 18.6 | 2.275 |
| G. lingzhi | RW | 10.7 | 11.1 | 0.474 |
| G. lingzhi | RW | 17.1 | 16.9 | −0.268 |
| G. lingzhi | RW | 15.2 | 17.8 | 2.597 |
| G. lingzhi | RW | 22.0 | 19.0 | −2.927 |
| G. lingzhi | RWL | 8.2 | 7.4 | −0.764 |
| G. lingzhi | RWL | 6.3 | 7.6 | 1.276 |
| G. lingzhi | RWL | 13.8 | 13.9 | 0.077 |
| G. lingzhi | RWL | 13.1 | 13.0 | −0.119 |
| G. lingzhi | RWL | 6.1 | 5.6 | −0.453 |
| G. lingzhi | RWL | 7.5 | 7.4 | −0.102 |
| G. lingzhi | REF | 23.4 | 23.4 | 0.065 |
| G. lingzhi | REF | 11.3 | 11.1 | −0.140 |
| G. lingzhi | REF | 20.5 | 18.6 | −1.910 |
| G. lingzhi | REF | 22.7 | 22.6 | −0.107 |
| G. lingzhi | REF | 16.3 | 18.0 | 1.719 |
| G. lingzhi | REF | 15.1 | 15.0 | −0.106 |
| G. sessile | RW | 24.3 | 24.5 | 0.156 |
| G. sessile | RW | 17.1 | 16.7 | −0.355 |
| G. sessile | RW | 19.8 | 19.9 | 0.102 |
| G. sessile | RW | 23.9 | 23.8 | −0.055 |
| G. sessile | RW | 28.3 | 28.2 | −0.119 |
| G. sessile | RW | 32.7 | 32.6 | −0.011 |
| G. sessile | RWL | 28.2 | 28.4 | 0.221 |
| G. sessile | RWL | 40.6 | 40.5 | −0.105 |
| G. sessile | RWL | 37.4 | 37.3 | −0.087 |
| G. sessile | RWL | 39.8 | 39.9 | 0.102 |
| G. sessile | RWL | 29.2 | 28.5 | −0.712 |
| G. sessile | RWL | 34.7 | 34.9 | 0.204 |
| G. sessile | REF | 13.0 | 13.0 | 0.049 |
| G. sessile | REF | 22.1 | 22.1 | 0.013 |
| G. sessile | REF | 21.9 | 21.9 | 0.075 |
| G. sessile | REF | 13.4 | 13.2 | −0.128 |
| G. sessile | REF | 8.4 | 8.1 | −0.276 |
| G. sessile | REF | 21.3 | 21.3 | 0.052 |
| T. versicolor | RW | 8.5 | 9.0 | 0.502 |
| T. versicolor | RW | 10.6 | 10.1 | −0.474 |
| T. versicolor | RW | 6.9 | 6.8 | −0.082 |
| T. versicolor | RW | 8.1 | 8.0 | −0.018 |
| T. versicolor | RW | 9.3 | 9.3 | 0.028 |
| T. versicolor | RW | 7.7 | 7.9 | 0.290 |
| T. versicolor | RWL | 15.9 | 17.1 | 1.133 |
| T. versicolor | RWL | 15.8 | 14.8 | −1.004 |
| T. versicolor | RWL | 14.8 | 15.1 | 0.283 |
| T. versicolor | RWL | 20.2 | 20.1 | −0.077 |
| T. versicolor | RWL | 12.8 | 12.7 | −0.157 |
| T. versicolor | RWL | 20.2 | 20.1 | −0.040 |
| T. versicolor | REF | 33.4 | 33.3 | −0.096 |
| T. versicolor | REF | 29.9 | 29.9 | −0.069 |
| T. versicolor | REF | 38.3 | 38.3 | 0.044 |
| T. versicolor | REF | 25.3 | 25.3 | −0.023 |
| T. versicolor | REF | 34.5 | 34.4 | −0.139 |
| T. versicolor | REF | 26.4 | 26.5 | 0.112 |
| Fungus | Substrate | Compressive Strength (kPa) | NNM Compressive Strength (kPa) | NNM Error (kPa) |
|---|---|---|---|---|
| G. lingzhi | RW | 51.3 | 46.0 | −5.276 |
| G. lingzhi | RW | 39.5 | 45.0 | 5.480 |
| G. lingzhi | RW | 43.7 | 44.1 | 0.336 |
| G. lingzhi | RW | 43.2 | 41.7 | −1.480 |
| G. lingzhi | RW | 40.0 | 36.8 | −3.146 |
| G. lingzhi | RW | 32.7 | 36.9 | 4.231 |
| G. lingzhi | RWL | 20.3 | 21.0 | 0.725 |
| G. lingzhi | RWL | 26.3 | 24.2 | −2.069 |
| G. lingzhi | RWL | 31.4 | 30.9 | −0.536 |
| G. lingzhi | RWL | 25.9 | 26.8 | 0.921 |
| G. lingzhi | RWL | 25.8 | 26.6 | 0.760 |
| G. lingzhi | RWL | 27.0 | 26.9 | −0.028 |
| G. lingzhi | REF | 32.8 | 33.0 | 0.215 |
| G. lingzhi | REF | 32.2 | 32.4 | 0.155 |
| G. lingzhi | REF | 27.7 | 30.7 | 2.996 |
| G. lingzhi | REF | 32.7 | 32.1 | −0.693 |
| G. lingzhi | REF | 35.9 | 33.1 | −2.767 |
| G. lingzhi | REF | 30.2 | 30.3 | 0.082 |
| G. sessile | RW | 42.6 | 42.5 | −0.111 |
| G. sessile | RW | 48.3 | 48.8 | 0.496 |
| G. sessile | RW | 30.0 | 30.4 | 0.382 |
| G. sessile | RW | 28.8 | 28.5 | −0.334 |
| G. sessile | RW | 53.7 | 53.5 | −0.191 |
| G. sessile | RW | 34.7 | 34.8 | 0.144 |
| G. sessile | RWL | 57.2 | 56.2 | −1.008 |
| G. sessile | RWL | 46.6 | 47.7 | 1.153 |
| G. sessile | RWL | 56.1 | 55.0 | −1.112 |
| G. sessile | RWL | 26.5 | 27.7 | 1.179 |
| G. sessile | RWL | 46.6 | 46.5 | −0.167 |
| G. sessile | RWL | 44.9 | 45.1 | 0.138 |
| G. sessile | REF | 44.2 | 46.0 | 1.752 |
| G. sessile | REF | 39.7 | 39.9 | 0.211 |
| G. sessile | REF | 49.9 | 50.5 | 0.623 |
| G. sessile | REF | 38.6 | 38.5 | −0.116 |
| G. sessile | REF | 40.7 | 39.7 | −0.997 |
| G. sessile | REF | 59.5 | 58.9 | −0.654 |
| T. versicolor | RW | 16.3 | 16.6 | 0.358 |
| T. versicolor | RW | 11.9 | 11.4 | −0.481 |
| T. versicolor | RW | 13.6 | 17.3 | 3.714 |
| T. versicolor | RW | 21.6 | 17.5 | −4.106 |
| T. versicolor | RW | 21.2 | 21.4 | 0.141 |
| T. versicolor | RW | 15.9 | 16.4 | 0.475 |
| T. versicolor | RWL | 23.8 | 26.5 | 2.673 |
| T. versicolor | RWL | 35.5 | 32.6 | −2.989 |
| T. versicolor | RWL | 33.1 | 35.9 | 2.772 |
| T. versicolor | RWL | 35.1 | 32.8 | −2.350 |
| T. versicolor | RWL | 29.9 | 31.6 | 1.685 |
| T. versicolor | RWL | 33.0 | 31.0 | −1.940 |
| T. versicolor | REF | 39.5 | 39.9 | 0.356 |
| T. versicolor | REF | 71.2 | 71.1 | −0.129 |
| T. versicolor | REF | 62.7 | 62.0 | −0.728 |
| T. versicolor | REF | 60.9 | 61.2 | 0.244 |
| T. versicolor | REF | 30.5 | 29.6 | −0.927 |
| T. versicolor | REF | 56.1 | 57.1 | 0.962 |
| Metrics Based on Training Data | MSE (kPa2) | RMSE (kPa) | MAE (kPa) | MAPE (%) | R2 (-) |
|---|---|---|---|---|---|
| Internal bonding | 0.706 | 0.840 | 0.460 | 3.137 | 0.992 |
| Compressive strength | 3.553 | 1.885 | 1.291 | 4.191 | 0.979 |
| Metrics Based on Cross-Validation | MSE (kPa2) | RMSE (kPa) | MAE (kPa) | MAPE (%) | R2 (-) |
| Internal bonding | 204.397 | 14.297 | 9.341 | 46.304 | −1.273 |
| Compressive strength | 673.462 | 25.951 | 19.781 | 55.694 | −2.892 |
| RReliefF | Fungus | Substrate | Water Uptake | Thermal Diffusivity | Volumetric Heat Capacity | Thermal Conductivity |
|---|---|---|---|---|---|---|
| Internal bonding | 0.605 | 0.570 | 0.234 | 0.216 | 0.198 | 0.131 |
| Compressive strength | 0.601 | 0.563 | 0.226 | 0.217 | 0.208 | 0.157 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hýsek, Š.; Jozífek, M.; Petržela, B.; Němec, M. Artificial Neural Network Prediction of Mechanical Properties in Mycelium-Based Biocomposites. Polymers 2025, 17, 2506. https://doi.org/10.3390/polym17182506
Hýsek Š, Jozífek M, Petržela B, Němec M. Artificial Neural Network Prediction of Mechanical Properties in Mycelium-Based Biocomposites. Polymers. 2025; 17(18):2506. https://doi.org/10.3390/polym17182506
Chicago/Turabian StyleHýsek, Štěpán, Miroslav Jozífek, Benjamín Petržela, and Miroslav Němec. 2025. "Artificial Neural Network Prediction of Mechanical Properties in Mycelium-Based Biocomposites" Polymers 17, no. 18: 2506. https://doi.org/10.3390/polym17182506
APA StyleHýsek, Š., Jozífek, M., Petržela, B., & Němec, M. (2025). Artificial Neural Network Prediction of Mechanical Properties in Mycelium-Based Biocomposites. Polymers, 17(18), 2506. https://doi.org/10.3390/polym17182506






