Biomimetic Filler Strategy for Two-Step Universal Dental Adhesives Using PA–ACP/MSN: Effects on Wettability, Immediate Microtensile Bond Strength, and Cytocompatibility
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. MSN Synthesis
2.3. Amine Functionalization of the MSNs
2.4. PA–ACP Synthesis and Loading into AF-MSNs
2.5. Physicochemical Characterization of MSNs
2.6. Incorporation of PA–ACP/MSN into Two-Step Universal Adhesive Primers
2.7. Preliminary Studies
2.7.1. Wettability (Contact Angles) Study
2.7.2. Microtensile Bond Strength (μTBS) Study
2.8. Indirect MTT Cytotoxicity Assay
2.9. Statistical Analysis
3. Results
3.1. Physicochemical Characterization
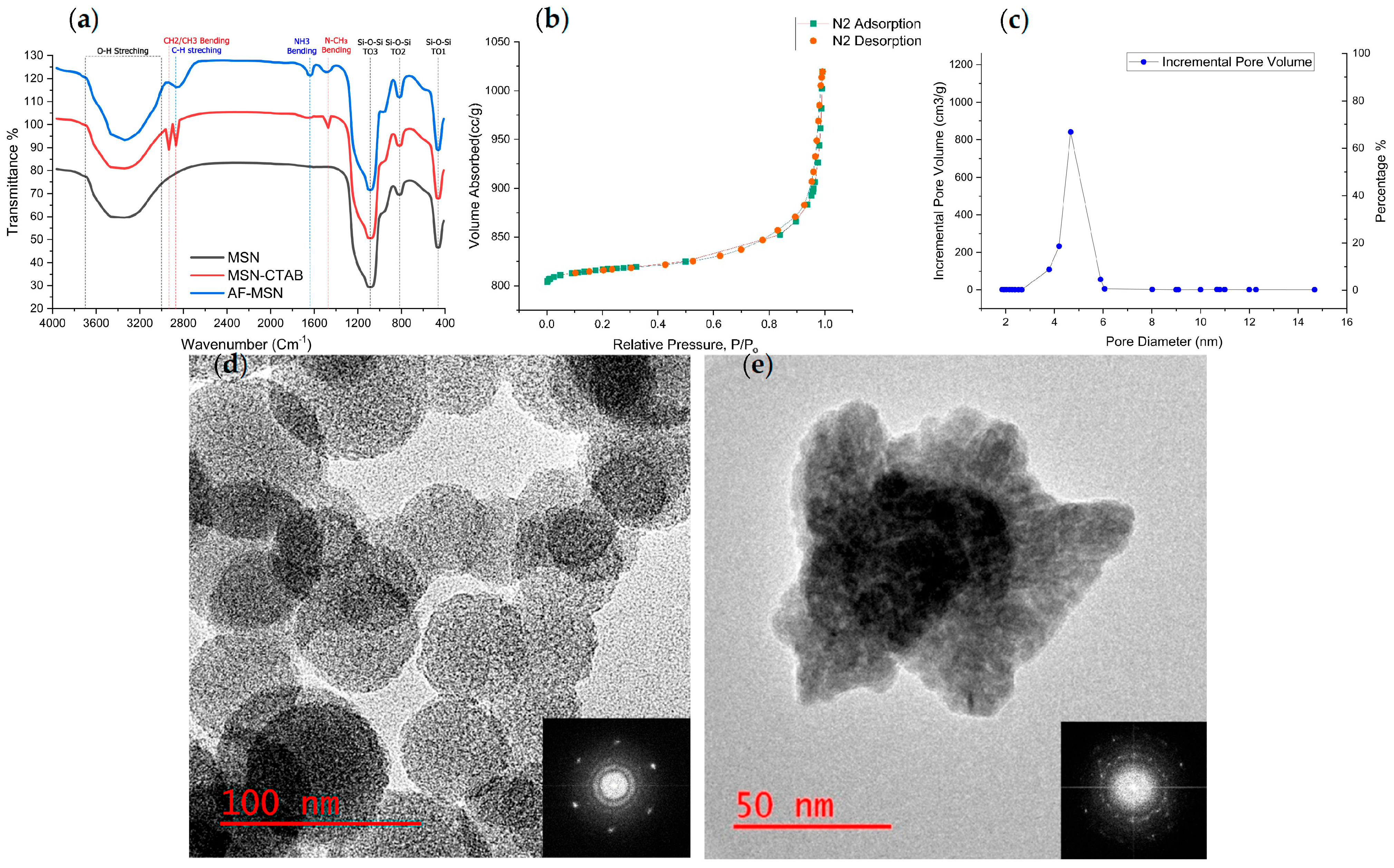
3.1.1. FTIR Spectroscopy
3.1.2. BET
3.1.3. HRTEM
3.2. Wettability (Contact Angle)
3.3. Microtensile Bond Strength (μTBS)
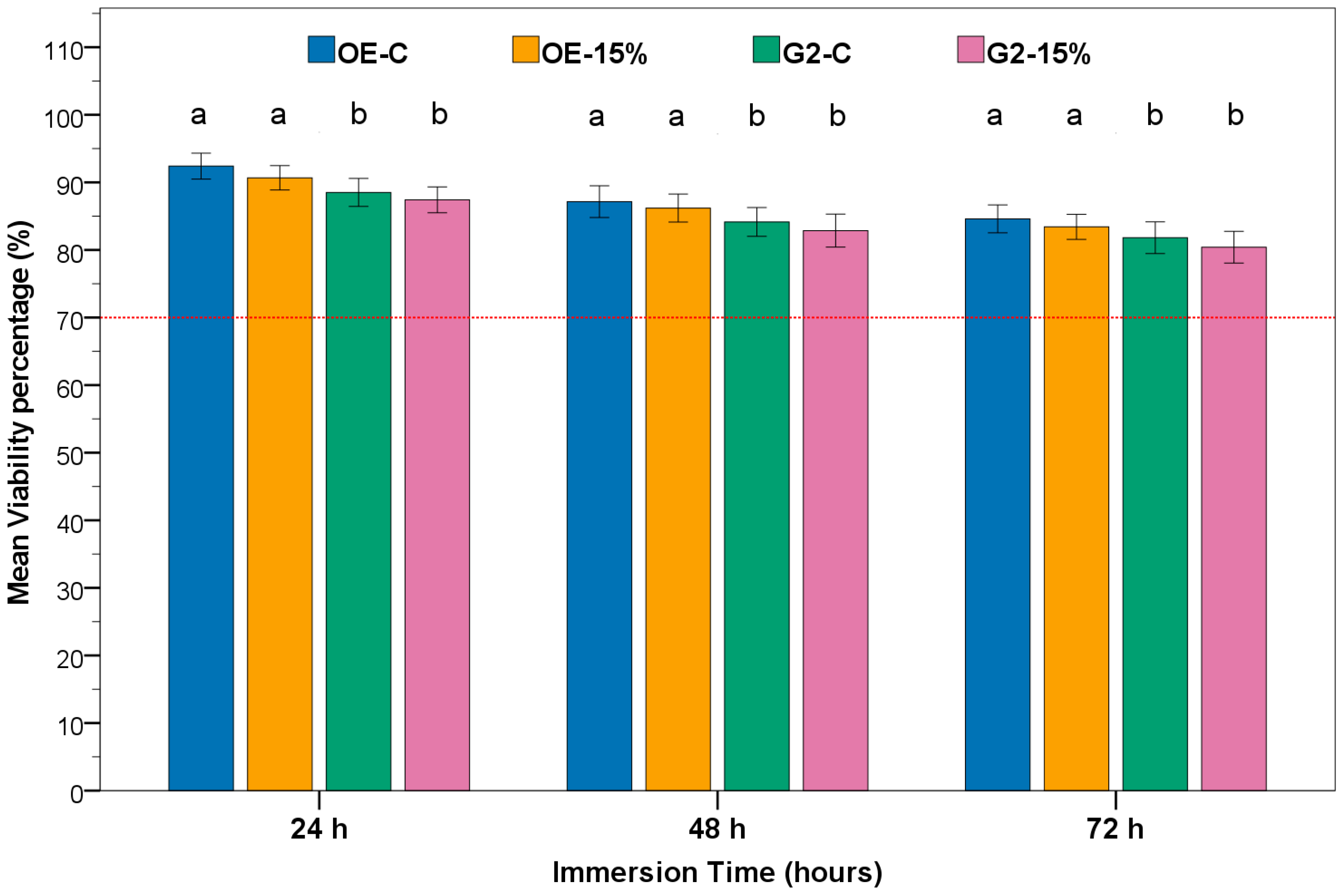
3.4. MTT Assay
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| G2 | G2 Bond Universal |
| OE | OptiBond eXTRa Universal |
| PA–ACP | Polyacrylic-acid-stabilized amorphous calcium phosphate |
| MSNs | Mesoporous silica nanoparticles |
| APTES | 3-Aminopropyltriethoxysilane |
| HPLFs | Human periodontal ligament fibroblasts |
| μTBS | Microtensile bond strength |
| BJH | Barrett–Joyner–Halenda |
| HRTEM | High-resolution transmission electron microscopy |
| FFT | Fast Fourier transform |
| FTIR | Fourier-transform infrared spectroscopy |
| BET | Brunauer–Emmett–Teller |
| 10-MDP | 10-methacryloyloxydecyl dihydrogen phosphate |
| GPDM | Glycerol phosphate dimethacrylate |
| 4-META | 4-methacryloxyethy trimellitic anhydride |
References
- Tjäderhane, L.; Nascimento, F.D.; Breschi, L.; Mazzoni, A.; Tersariol, I.L.; Geraldeli, S.; Tezvergil-Mutluay, A.; Carrilho, M.R.; Carvalho, R.M.; Tay, F.R. Optimizing dentin bond durability: Control of collagen degradation by matrix metalloproteinases and cysteine cathepsins. Dent. Mater. 2013, 29, 116–135. [Google Scholar] [CrossRef]
- Mokeem, L.S.; Garcia, I.M.; Melo, M.A. Degradation and failure phenomena at the dentin bonding interface. Biomedicines 2023, 11, 1256. [Google Scholar] [CrossRef]
- Al-Qrimli, A.F.; Al-Shammam, A.M.W. Comparative Evaluation of the Effect of Different Universal Adhesives and Bonding Techniques on the Marginal Gap of Class I Composite Restoration (a Sem Study). J. Baghdad Coll. Dent. 2016, 28, 34–42. [Google Scholar] [CrossRef]
- Spencer, P.; Ye, Q.; Park, J.; Topp, E.M.; Misra, A.; Marangos, O.; Wang, Y.; Bohaty, B.S.; Singh, V.; Sene, F. Adhesive/dentin interface: The weak link in the composite restoration. Ann. Biomed. Eng. 2010, 38, 1989–2003. [Google Scholar] [CrossRef]
- Amin, F.; Fareed, M.A.; Zafar, M.S.; Khurshid, Z.; Palma, P.J.; Kumar, N. Degradation and stabilization of resin-dentine interfaces in polymeric dental adhesives: An updated review. Coatings 2022, 12, 1094. [Google Scholar] [CrossRef]
- Spagnuolo, G. Bioactive dental materials: The current status. Materials 2022, 15, 2016. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, D.R.; Ibrahim, A.I.; Deb, S. Combating white spot lesions via incorporation of remineralizing/antibacterial additives into orthodontic adhesives: A review. J. Baghdad Coll. Dent. 2025, 37, 82–96. [Google Scholar] [CrossRef]
- Xu, H.H.; Moreau, J.L.; Sun, L.; Chow, L.C. Nanocomposite containing amorphous calcium phosphate nanoparticles for caries inhibition. Dent. Mater. 2011, 27, 762–769. [Google Scholar] [CrossRef]
- Ali, N.A.M.; Nissan, L.M.; Khamis, A.H.; Al-Taai, N. Enamel demineralization around orthodontic brackets bonded with new bioactive composite (in-vitro study). J. Baghdad Coll. Dent. 2024, 36, 54–62. [Google Scholar] [CrossRef]
- Sauro, S.; Osorio, R.; Watson, T.F.; Toledano, M. Therapeutic effects of novel resin bonding systems containing bioactive glasses on mineral-depleted areas within the bonded-dentine interface. J. Mater. Sci. Mater. Med. 2012, 23, 1521–1532. [Google Scholar] [CrossRef]
- Wang, Z.; Ouyang, Y.; Wu, Z.; Zhang, L.; Shao, C.; Fan, J.; Zhang, L.; Shi, Y.; Zhou, Z.; Pan, H. A novel fluorescent adhesive-assisted biomimetic mineralization. Nanoscale 2018, 10, 18980–18987. [Google Scholar] [CrossRef] [PubMed]
- Cölfen, H.; Mann, S. Higher-order organization by mesoscale self-assembly and transformation of hybrid nanostructures. Angew. Chem. Int. Ed. 2003, 42, 2350–2365. [Google Scholar] [CrossRef] [PubMed]
- Gower, L.B. Biomimetic model systems for investigating the amorphous precursor pathway and its role in biomineralization. Chem. Rev. 2008, 108, 4551–4627. [Google Scholar] [CrossRef] [PubMed]
- Dey, A.; Bomans, P.H.; Müller, F.A.; Will, J.; Frederik, P.M.; de With, G.; Sommerdijk, N.A. The role of prenucleation clusters in surface-induced calcium phosphate crystallization. Nat. Mater. 2010, 9, 1010–1014. [Google Scholar] [CrossRef]
- Hua, F.; Yan, J.; Zhao, S.; Yang, H.; He, H. In vitro remineralization of enamel white spot lesions with a carrier-based amorphous calcium phosphate delivery system. Clin. Oral Investig. 2020, 24, 2079–2089. [Google Scholar] [CrossRef]
- Garma, N.M.; Ibrahim, A.I. Development of a remineralizing calcium phosphate nanoparticle-containing self-etching system for orthodontic bonding. Clin. Oral Investig. 2023, 27, 1483–1497. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Y.; Feng, N. Mesoporous silica nanoparticles: Synthesis, classification, drug loading, pharmacokinetics, biocompatibility, and application in drug delivery. Expert Opin. Drug Deliv. 2019, 16, 219–237. [Google Scholar] [CrossRef]
- Florek, J.; Caillard, R.; Kleitz, F. Evaluation of mesoporous silica nanoparticles for oral drug delivery–current status and perspective of MSNs drug carriers. Nanoscale 2017, 9, 15252–15277. [Google Scholar] [CrossRef]
- Hussein, E.A.; Kareem, S.H. Mesoporous silica nanoparticles as a system for ciprofloxacin drug delivery; kinetic of adsorption and releasing. Baghdad Sci. J. 2021, 18, 15. [Google Scholar] [CrossRef]
- Choi, Y.; Sun, W.; Kim, Y.; Kim, I.-R.; Gong, M.-K.; Yoon, S.-Y.; Bae, M.-K.; Park, B.-S.; Park, S.-B.; Kim, Y.-I. Effects of Zn-doped mesoporous bioactive glass nanoparticles in etch-and-rinse adhesive on the microtensile bond strength. Nanomaterials 2020, 10, 1943. [Google Scholar] [CrossRef]
- Choi, A.; Yoo, K.-H.; Yoon, S.-Y.; Park, B.-S.; Kim, I.-R.; Kim, Y.-I. Anti-microbial and remineralizing properties of self-adhesive orthodontic resin containing mesoporous bioactive glass. Materials 2021, 14, 3550. [Google Scholar] [CrossRef] [PubMed]
- Alkhazaleh, A.; Elfagih, S.; Chakka, L.R.J.; Armstrong, S.R.; Comnick, C.L.; Qian, F.; Salem, A.K.; Guymon, C.A.; Haes, A.J.; Vidal, C.M. Development of proanthocyanidin-loaded mesoporous silica nanoparticles for improving dental adhesion. Mol. Pharm. 2022, 19, 4675–4684. [Google Scholar] [CrossRef] [PubMed]
- Möller, K.; Kobler, J.; Bein, T. Colloidal Suspensions of Nanometer-Sized Mesoporous Silica. Adv. Funct. Mater. 2007, 17, 605–612. [Google Scholar] [CrossRef]
- Kobler, J.; Möller, K.; Bein, T. Colloidal suspensions of functionalized mesoporous silica nanoparticles. ACS Nano 2008, 2, 791–799. [Google Scholar] [CrossRef]
- Kresge, a.C.; Leonowicz, M.E.; Roth, W.J.; Vartuli, J.; Beck, J. Ordered mesoporous molecular sieves synthesized by a liquid-crystal template mechanism. Nature 1992, 359, 710–712. [Google Scholar] [CrossRef]
- Zhang, W.; Luo, X.-J.; Niu, L.-N.; Yang, H.-Y.; Yiu, C.K.; Wang, T.-D.; Zhou, L.-Q.; Mao, J.; Huang, C.; Pashley, D.H. Biomimetic intrafibrillar mineralization of type I collagen with intermediate precursors-loaded mesoporous carriers. Sci. Rep. 2015, 5, 11199. [Google Scholar] [CrossRef]
- Slowing, I.I.; Vivero-Escoto, J.L.; Wu, C.-W.; Lin, V.S.-Y. Mesoporous silica nanoparticles as controlled release drug delivery and gene transfection carriers. Adv. Drug Deliv. Rev. 2008, 60, 1278–1288. [Google Scholar] [CrossRef]
- Maleki, A.; Kettiger, H.; Schoubben, A.; Rosenholm, J.M.; Ambrogi, V.; Hamidi, M. Mesoporous silica materials: From physico-chemical properties to enhanced dissolution of poorly water-soluble drugs. J. Control. Release 2017, 262, 329–347. [Google Scholar] [CrossRef]
- Yang, H.-Y.; Niu, L.-N.; Sun, J.-L.; Huang, X.-Q.; Pei, D.-D.; Huang, C.; Tay, F.R. Biodegradable mesoporous delivery system for biomineralization precursors. Int. J. Nanomed. 2017, 12, 839–854. [Google Scholar] [CrossRef]
- Luo, X.-J.; Yang, H.-Y.; Niu, L.-N.; Mao, J.; Huang, C.; Pashley, D.H.; Tay, F.R. Translation of a solution-based biomineralization concept into a carrier-based delivery system via the use of expanded-pore mesoporous silica. Acta Biomater. 2016, 31, 378–387. [Google Scholar] [CrossRef]
- Gao, L.; Sun, J.; Li, Y.; Zhang, L. Bimodal mesoporous silicas functionalized with different level and species of the amino groups for adsorption and controlled release of aspirin. J. Nanosci. Nanotechnol. 2011, 11, 6690–6697. [Google Scholar] [CrossRef]
- Zong, C.; Wang, Y.; Jiang, S. Toward the understanding of poly (Acrylic Acid) on amorphous calcium phosphate mediated collagen intrafibrillar Mineralization: Surface adsorption versus bulk incorporation. J. Cryst. Growth 2023, 616, 127304. [Google Scholar] [CrossRef]
- Wang, J.; Chen, Y.; Li, L.; Sun, J.; Gu, X.; Xu, X.; Pan, H.; Tang, R. Remineralization of dentin collagen by meta-stabilized amorphous calcium phosphate. CrystEngComm 2013, 15, 6151–6158. [Google Scholar] [CrossRef]
- Qi, Y.; Ye, Z.; Fok, A.; Holmes, B.N.; Espanol, M.; Ginebra, M.-P.; Aparicio, C. Effects of molecular weight and concentration of poly (acrylic acid) on biomimetic mineralization of collagen. ACS Biomater. Sci. Eng. 2018, 4, 2758–2766. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.S.; Lu, G.; Whittaker, A.; Millar, G.; Zhu, H. Comprehensive study of surface chemistry of MCM-41 using 29Si CP/MAS NMR, FTIR, pyridine-TPD, and TGA. J. Phys. Chem. B 1997, 101, 6525–6531. [Google Scholar] [CrossRef]
- Zhao, D.; Huo, Q.; Feng, J.; Chmelka, B.F.; Stucky, G.D. Nonionic triblock and star diblock copolymer and oligomeric surfactant syntheses of highly ordered, hydrothermally stable, mesoporous silica structures. J. Am. Chem. Soc. 1998, 120, 6024–6036. [Google Scholar] [CrossRef]
- Vallet-Regi, M.; Rámila, A.; Del Real, R.; Pérez-Pariente, J. A new property of MCM-41: Drug delivery system. Chem. Mater. 2001, 13, 308–311. [Google Scholar] [CrossRef]
- Wei, S.; Wu, H.; Luo, X.-J. Biomineralization precursor carrier system based on carboxyl-functionalized large pore mesoporous silica nanoparticles. Curr. Med. Sci. 2020, 40, 155–167. [Google Scholar] [CrossRef]
- Chen, C.; Weir, M.D.; Cheng, L.; Lin, N.J.; Lin-Gibson, S.; Chow, L.C.; Zhou, X.; Xu, H.H. Antibacterial activity and ion release of bonding agent containing amorphous calcium phosphate nanoparticles. Dent. Mater. 2014, 30, 891–901. [Google Scholar] [CrossRef]
- Melo, M.A.S.; Cheng, L.; Zhang, K.; Weir, M.D.; Rodrigues, L.K.; Xu, H.H. Novel dental adhesives containing nanoparticles of silver and amorphous calcium phosphate. Dent. Mater. 2013, 29, 199–210. [Google Scholar] [CrossRef]
- Adabo, G.L.; dos Santos Cruz, C.A.; Fonseca, R.G.; Vaz, L.s.G. The volumetric fraction of inorganic particles and the flexural strength of composites for posterior teeth. J. Dent. 2003, 31, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Balkaya, H.; Demirbuğa, S. Evaluation of six different one-step universal adhesive systems in terms of dentin bond strength, adhesive interface characterization, surface tension, contact angle, degree of conversion and solvent evaporation after immediate and delayed use. J. Esthet. Restor. Dent. 2023, 35, 479–492. [Google Scholar] [CrossRef] [PubMed]
- Genari, B.; Leitune, V.C.B.; Jornada, D.S.; Aldrigui, B.R.; Pohlmann, A.R.; Guterres, S.S.; Samuel, S.M.W.; Collares, F.M. Effect on adhesion of a nanocapsules-loaded adhesive system. Braz. Oral Res. 2018, 32, e008. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, S.; Breschi, L.; Özcan, M.; Pfefferkorn, F.; Ferrari, M.; Van Meerbeek, B. Academy of Dental Materials guidance on in vitro testing of dental composite bonding effectiveness to dentin/enamel using micro-tensile bond strength (μTBS) approach. Dent. Mater. 2017, 33, 133–143. [Google Scholar] [CrossRef]
- ISO/TS 4640:2023; Dentistry—Test Methods for Tensile Bond Strength to Tooth Structure. International Organization for Standardization: Geneva, Switzerland, 2023.
- ISO 10993–5: 2009; Biological Evaluation of Medical Devices—Part 5: Tests For In Vitro Cytotoxicity. International Organization for Standardization: Geneva, Switzerland, 2009; Volume 34.
- ISO 7405:2025; Dentistry—Evaluation of Biocompatibility of Medical Devices Used in Dentistry. International Organization for Standardization: Geneva, Switzerland, 2025; Edition 4.
- Alhashimi, R.A.; Mannocci, F.; Sauro, S. Bioactivity, cytocompatibility and thermal properties of experimental Bioglass-reinforced composites as potential root-canal filling materials. J. Mech. Behav. Biomed. Mater. 2017, 69, 355–361. [Google Scholar] [CrossRef]
- Catunda, R.-Q.; de Oliveira, E.-B.; da Silva, E.-C.; Brasil, V.-L.-M. Citotoxicity evaluation of three dental adhesives on vero cells in vitro. J. Clin. Exp. Dent. 2017, 9, e61. [Google Scholar] [CrossRef]
- Franz, A.; König, F.; Lucas, T.; Watts, D.C.; Schedle, A. Cytotoxic effects of dental bonding substances as a function of degree of conversion. Dent. Mater. 2009, 25, 232–239. [Google Scholar] [CrossRef]
- Sun, F.; Liu, Y.; Pan, Y.; Chen, M.; Meng, X. Cytotoxicity of Self-Adhesive Resin Cements on Human Periodontal Ligament Fibroblasts. BioMed Res. Int. 2018, 2018, 7823467. [Google Scholar] [CrossRef]
- Al-Labban, Y.R.; Alrubayee, M.; Zaidi, S.J.A.; Kazmi, S. Effects of Adding Tricalcium Silicate Nanoparticles to the Universal G2 Bond Adhesive as Self-Etch Mode on the Shear Bond Strength to the Orthodontic Bracket. Clin. Exp. Dent. Res. 2024, 10, e948. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Gregg, S.J.; Sing, K.S.W.; Salzberg, H. Adsorption surface area and porosity. J. Electrochem. Soc. 1967, 114, 279Ca. [Google Scholar] [CrossRef]
- Zhao, D.; Feng, J.; Huo, Q.; Melosh, N.; Fredrickson, G.H.; Chmelka, B.F.; Stucky, G.D. Triblock copolymer syntheses of mesoporous silica with periodic 50 to 300 angstrom pores. Science 1998, 279, 548–552. [Google Scholar] [CrossRef] [PubMed]
- Trewyn, B.G.; Slowing, I.I.; Giri, S.; Chen, H.-T.; Lin, V.S.-Y. Synthesis and functionalization of a mesoporous silica nanoparticle based on the sol–gel process and applications in controlled release. Acc. Chem. Res. 2007, 40, 846–853. [Google Scholar] [CrossRef] [PubMed]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The determination of pore volume and area distributions in porous substances. I. Computations from nitrogen isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- Xiong, L.; Du, X.; Shi, B.; Bi, J.; Kleitz, F.; Qiao, S.Z. Tunable stellate mesoporous silica nanoparticles for intracellular drug delivery. J. Mater. Chem. B 2015, 3, 1712–1721. [Google Scholar] [CrossRef]
- He, Y.; Luo, L.; Liang, S.; Long, M.; Xu, H. Amino-functionalized mesoporous silica nanoparticles as efficient carriers for anticancer drug delivery. J. Biomater. Appl. 2017, 32, 524–532. [Google Scholar] [CrossRef]
- Niu, L.-N.; Zhang, W.; Pashley, D.H.; Breschi, L.; Mao, J.; Chen, J.-H.; Tay, F.R. Biomimetic remineralization of dentin. Dent. Mater. 2014, 30, 77–96. [Google Scholar] [CrossRef]
- Pires-de, F.d.C.P.; Tonani-Torrieri, R.; Vivanco, R.G.; de Arruda, C.N.F.; Geraldeli, S.; Sinhoreti, M.A.C.; Roulet, J.-F. Effect of incorporation of bioactive glass-ceramic into self-etch adhesives. J. Adhes. Dent. 2022, 24, b2916451. [Google Scholar]
- Han, R.; Zhang, Z.; Zhao, Y.; Yan, L.; Tang, W.; Zhang, H. Preparation and performance evaluation of Zn-N-TiO2 nanoparticles-containing dental adhesive. Polym. Test. 2025, 147, 108814. [Google Scholar] [CrossRef]
- Yan, H.; Wang, S.; Han, L.; Peng, W.; Yi, L.; Guo, R.; Liu, S.; Yang, H.; Huang, C. Chlorhexidine-encapsulated mesoporous silica-modified dentin adhesive. J. Dent. 2018, 78, 83–90. [Google Scholar] [CrossRef]
- Melo, M.A.S.; Cheng, L.; Weir, M.D.; Hsia, R.C.; Rodrigues, L.K.; Xu, H.H. Novel dental adhesive containing antibacterial agents and calcium phosphate nanoparticles. J. Biomed. Mater. Res. Part B Appl. Biomater. 2013, 101, 620–629. [Google Scholar] [CrossRef]
- Yang, Y.; Ding, Y.; Fan, Y.; Ren, L.; Tang, X.; Meng, X. Application of silver nanoparticles in situ synthesized in dental adhesive resin. Int. J. Adhes. Adhes. 2021, 108, 102890. [Google Scholar] [CrossRef]
- Demirci, M.; Hiller, K.-A.; Bosl, C.; Galler, K.; Schmalz, G.; Schweikl, H. The induction of oxidative stress, cytotoxicity, and genotoxicity by dental adhesives. Dent. Mater. 2008, 24, 362–371. [Google Scholar] [CrossRef] [PubMed]
- Caldas, I.P.; Alves, G.G.; Barbosa, I.B.; Scelza, P.; de Noronha, F.; Scelza, M.Z. In vitro cytotoxicity of dental adhesives: A systematic review. Dent. Mater. 2019, 35, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Demirel, G.; Demirsoy, F.F.K.; Irmak, Ö. Cytotoxicity evaluation of eluates from universal adhesives by real-time cell analysis. Dent. Mater. J. 2020, 39, 815–824. [Google Scholar] [CrossRef]
- Cavallaro-Mota, F.D.; Esposo, G.N.; Kury, M.; Fronza, B.M.; Saraceni, C.H.C.; Andia, D.C.; Lima, A.F. Assessment of 10-MDP and GPDM monomers on viability and inflammatory response in human dental pulp stem cells. Dent. Mater. 2025, 41, 1–6. [Google Scholar] [CrossRef]
- Kim, E.C.; Park, H.; Lee, S.I.; Kim, S.Y. Effect of the acidic dental resin monomer 10-methacryloyloxydecyl dihydrogen phosphate on odontoblastic differentiation of human dental pulp cells. Basic Clin. Pharmacol. Toxicol. 2015, 117, 340–349. [Google Scholar] [CrossRef]
- Nakagawa, K.; Saita, M.; Ikeda, T.; Hirota, M.; Park, W.; Lee, M.C.-I.; Ogawa, T. Biocompatibility of 4-META/MMA-TBB resin used as a dental luting agent. J. Prosthet. Dent. 2015, 114, 114–121. [Google Scholar] [CrossRef]
- Ersöz, B.; Aydin, N.; Oktay, E.A.; Çal, İ.K.; Karaoğlanoğlu, S. Effects of universal adhesives on dentin matrix proteins, matrix metalloproteinases and cytokine release of human pulp cells. Odontology 2025, 1–11. [Google Scholar] [CrossRef]
- Asefa, T.; Tao, Z. Biocompatibility of mesoporous silica nanoparticles. Chem. Res. Toxicol. 2012, 25, 2265–2284. [Google Scholar] [CrossRef]
- Tarn, D.; Ashley, C.E.; Xue, M.; Carnes, E.C.; Zink, J.I.; Brinker, C.J. Mesoporous silica nanoparticle nanocarriers: Biofunctionality and biocompatibility. Acc. Chem. Res. 2013, 46, 792–801. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.; Li, L.; Chen, D. Mesoporous silica nanoparticles: Synthesis, biocompatibility and drug delivery. Adv. Mater. 2012, 24, 1504–1534. [Google Scholar] [CrossRef]
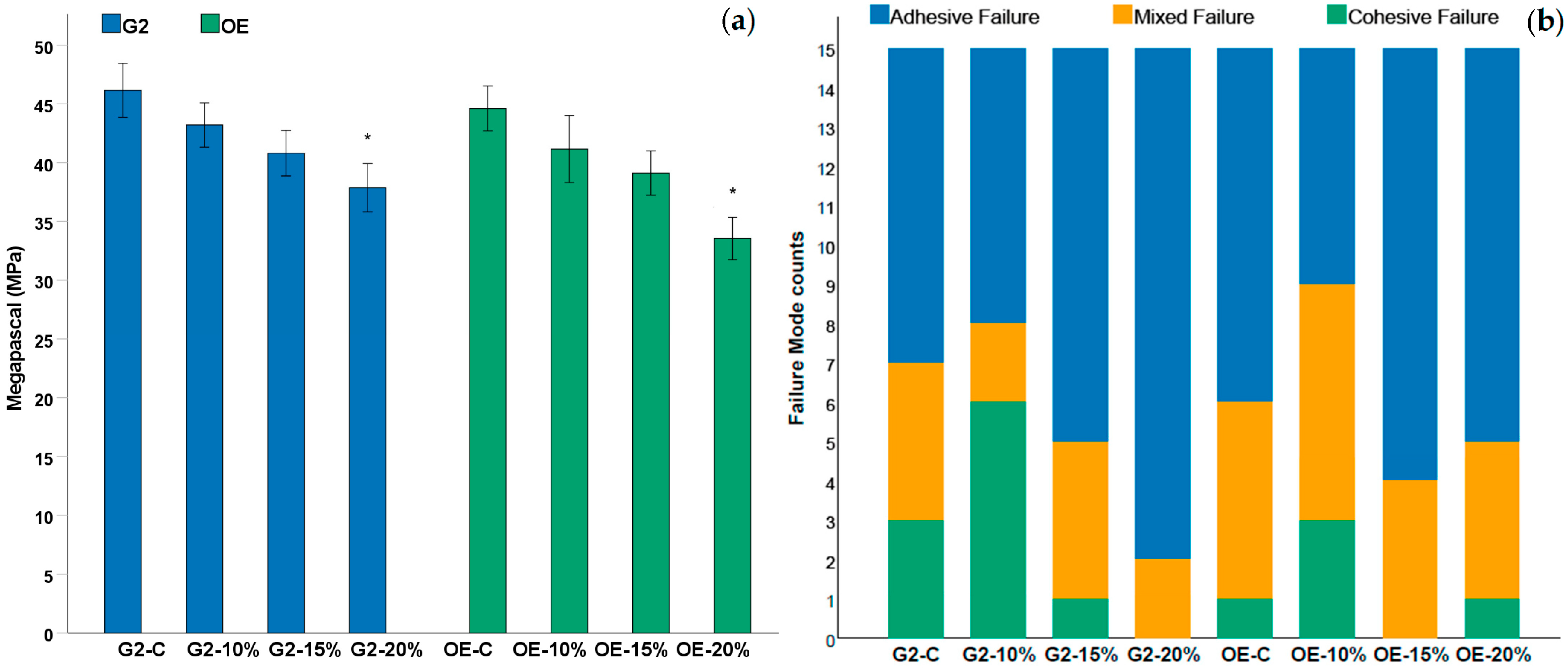
| Group | θ (°) ± SD | MPa ± SD (A/M/C) |
|---|---|---|
| G2-C | 20.6° ± 2.9° | 46.2 ± 6.9 (8/4/3) |
| G2-10% | 23.4° ± 2.6° | 43.2 ± 5.7 (7/2/6) |
| G2-15% | 27° ± 2° | 40.8 ± 5.8 (10/4/1) |
| G2-20% | 41.8° ± 3.4° * | 37.9 ± 6.2 (13/2/0) * |
| OE-C | 9.6° ± 1.9° | 44.6 ± 5.7 (9/5/1) |
| OE-10% | 12.7° ± 1.1° | 41.2 ± 8.6 (6/6/3) |
| OE-15% | 15.4° ± 3.3° | 39.1 ± 5.6 (11/4/0) |
| OE-20% | 24.3° ± 2.5° * | 33.6 ± 4.4 (10/4/1) * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alnakib, Y.; Majeed, M.A. Biomimetic Filler Strategy for Two-Step Universal Dental Adhesives Using PA–ACP/MSN: Effects on Wettability, Immediate Microtensile Bond Strength, and Cytocompatibility. Polymers 2025, 17, 2501. https://doi.org/10.3390/polym17182501
Alnakib Y, Majeed MA. Biomimetic Filler Strategy for Two-Step Universal Dental Adhesives Using PA–ACP/MSN: Effects on Wettability, Immediate Microtensile Bond Strength, and Cytocompatibility. Polymers. 2025; 17(18):2501. https://doi.org/10.3390/polym17182501
Chicago/Turabian StyleAlnakib, Yasir, and Manhal A. Majeed. 2025. "Biomimetic Filler Strategy for Two-Step Universal Dental Adhesives Using PA–ACP/MSN: Effects on Wettability, Immediate Microtensile Bond Strength, and Cytocompatibility" Polymers 17, no. 18: 2501. https://doi.org/10.3390/polym17182501
APA StyleAlnakib, Y., & Majeed, M. A. (2025). Biomimetic Filler Strategy for Two-Step Universal Dental Adhesives Using PA–ACP/MSN: Effects on Wettability, Immediate Microtensile Bond Strength, and Cytocompatibility. Polymers, 17(18), 2501. https://doi.org/10.3390/polym17182501








