Electrochemical Oxidation Degradation of Methylene Blue Dye on 3D-Printed Anode Electrodes
Abstract
1. Introduction
2. Materials and Methods
2.1. Material
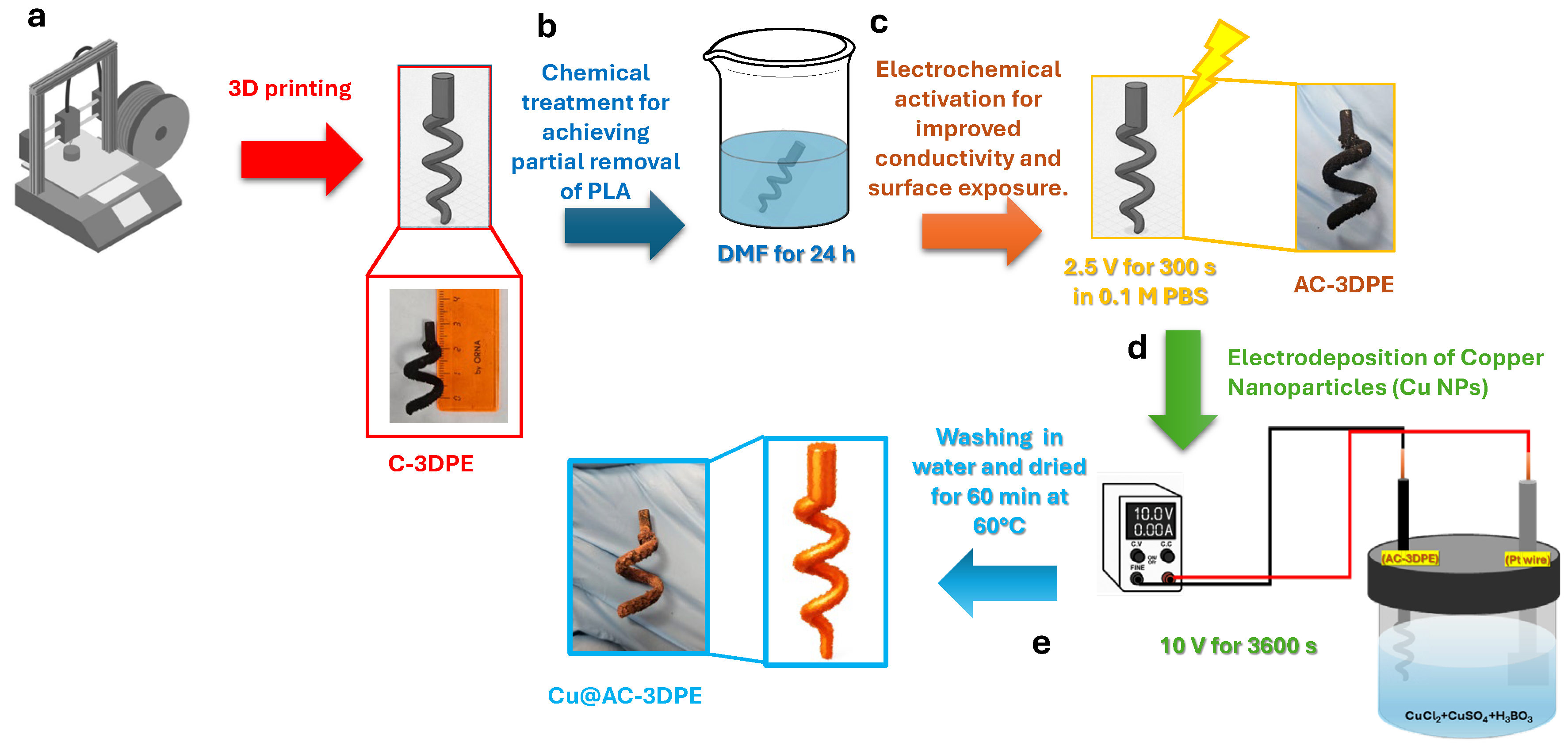
2.2. Electrode Fabrication
2.3. Electrodeposition of Copper Nanoparticles (Cu NPs) on AC-3DPE
2.4. Characterization of Modified Elecrodes
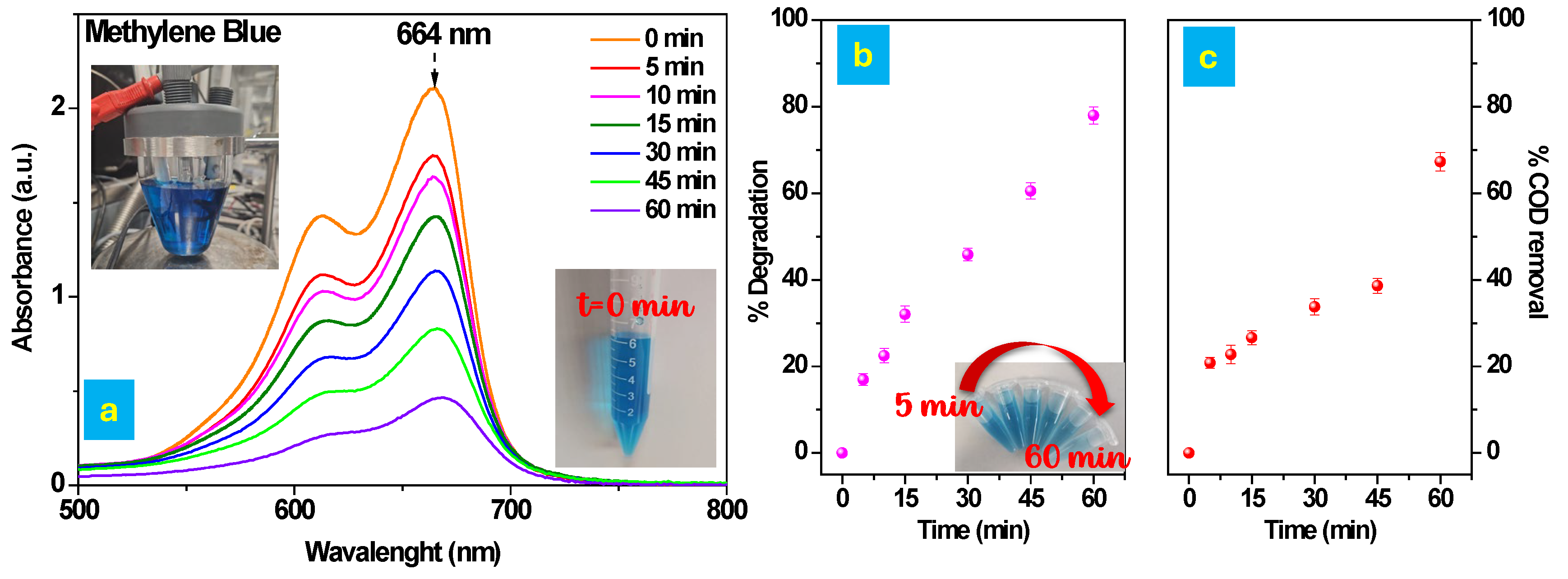
2.5. Electrochemical Degradation of MB
2.6. Analysis of Chemical Oxygen Demand
3. Results and Discussion
3.1. Stepwise Fabrication of Cu-Modified 3D-Printed Electrodes
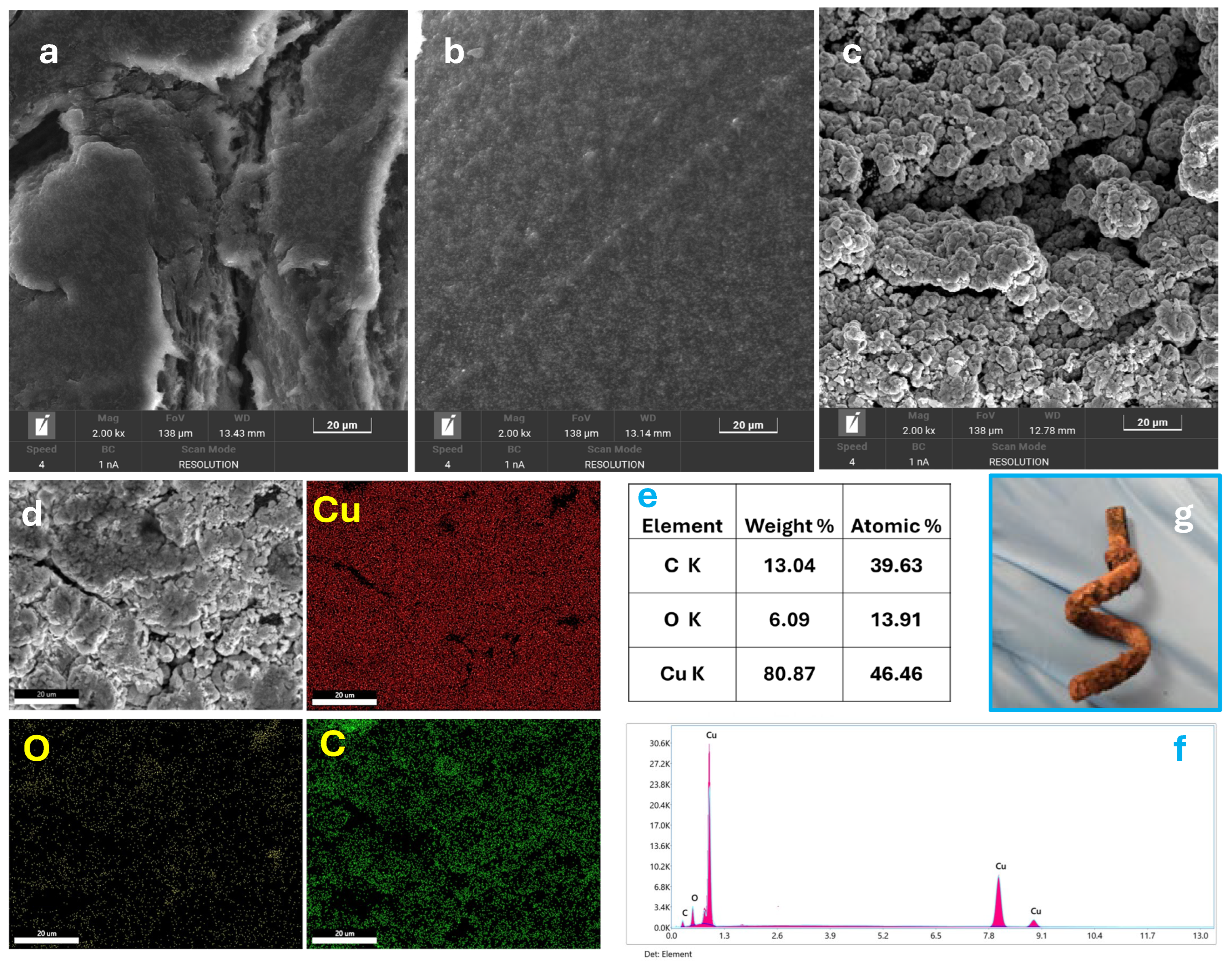
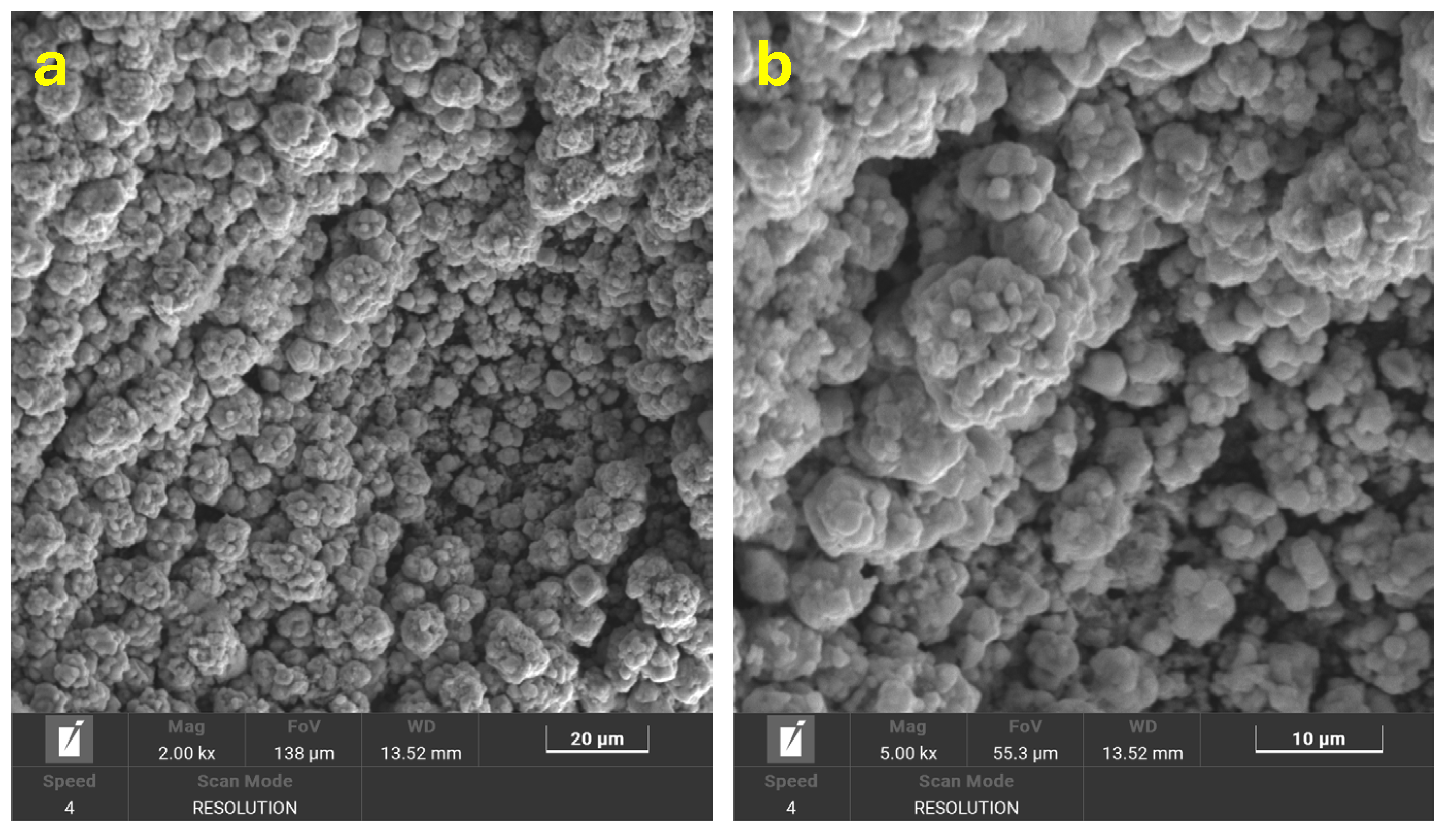
3.2. Characterization of Cu@AC-3DPE
3.3. Electrochemical Characteristics
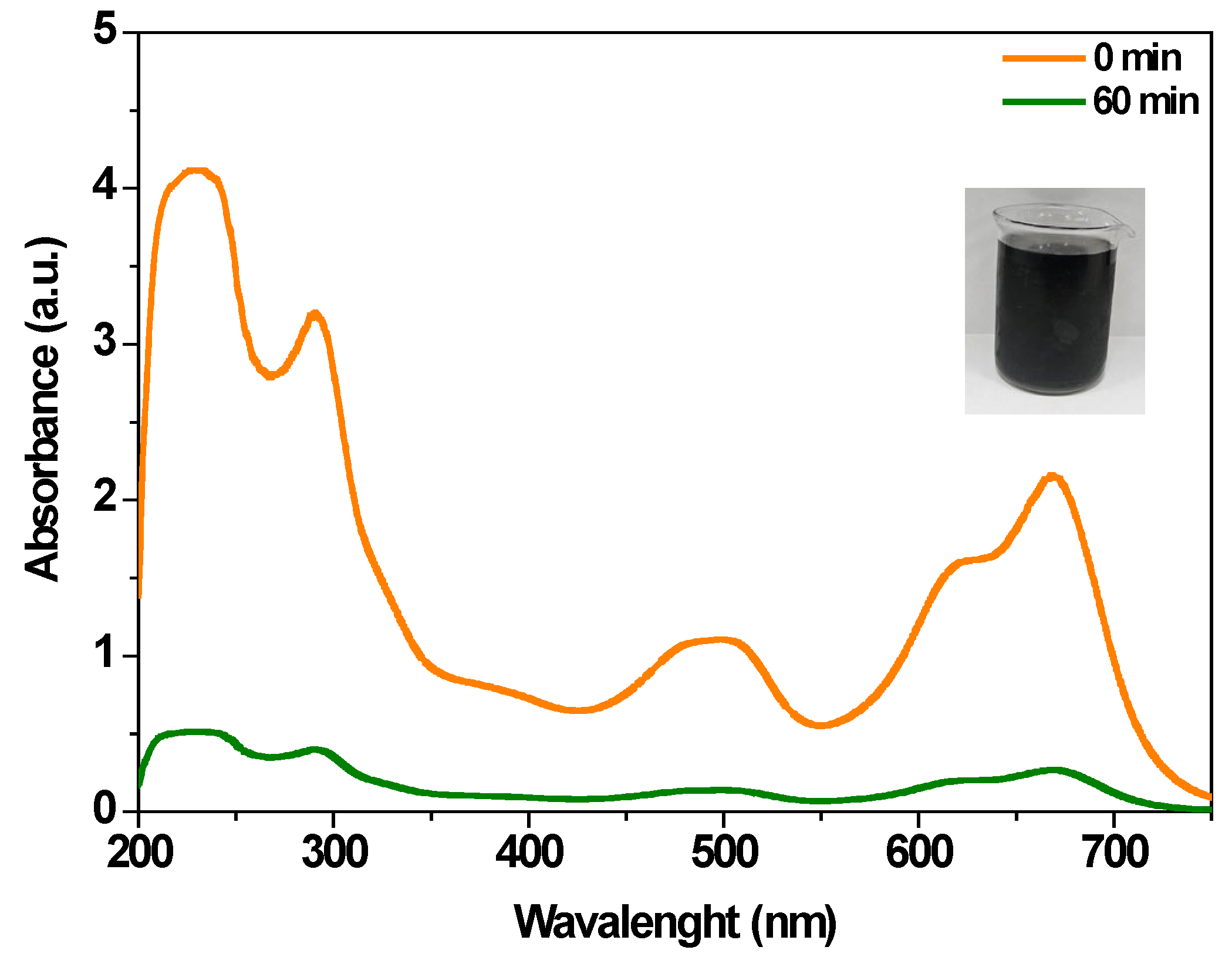
3.3.1. Electrochemical Oxidation of MB
Effect of NaCl Concentration
Effect of Applied Potential
Effect of pH
Reusability
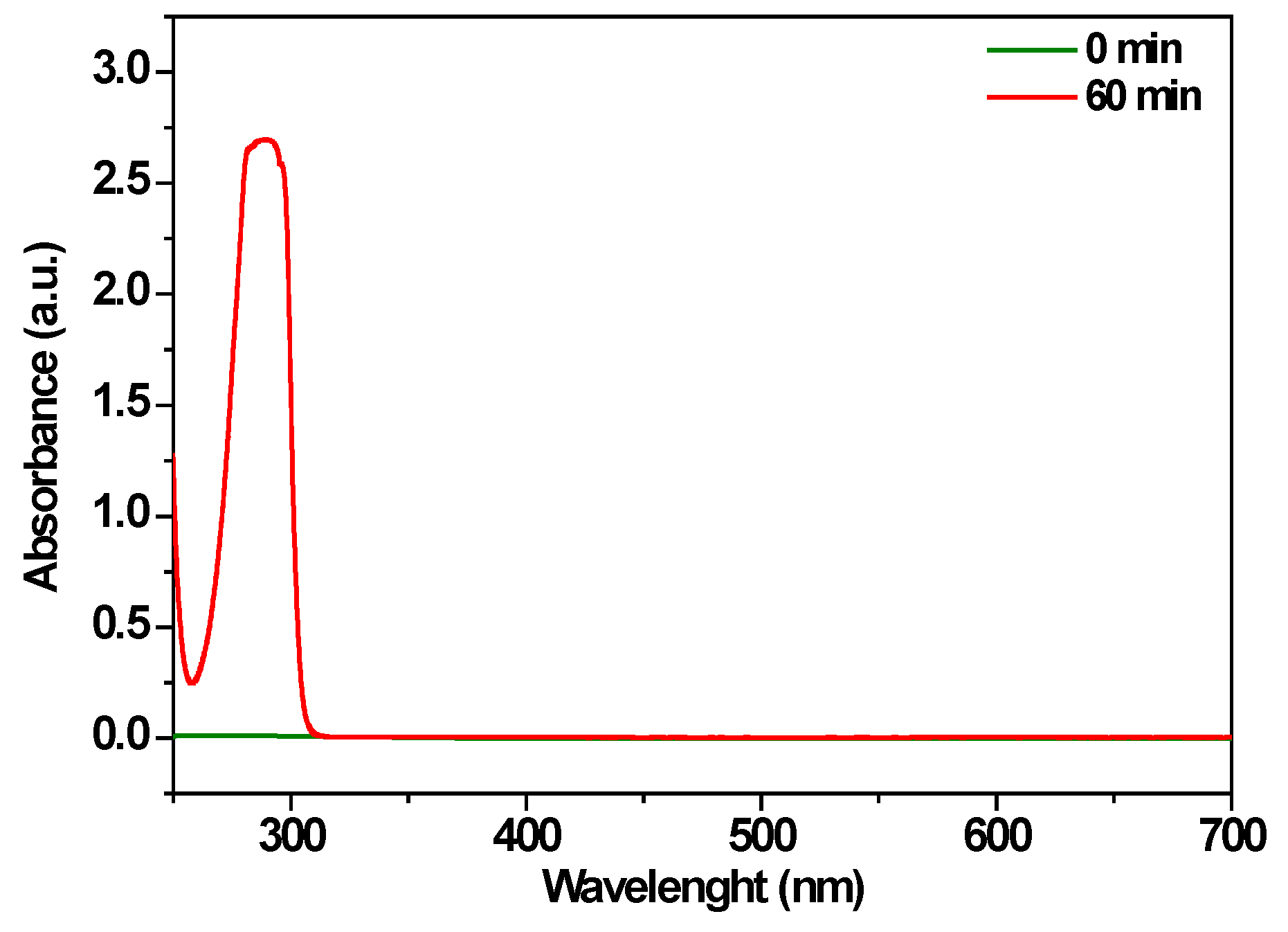
3.3.2. Degradation in Real Wastewater
3.3.3. Effect of 3D-Printed Electrode Geometry
3.4. Mechanism
3.5. Polymer-Based Electrodes for Pollutant Degradation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tong, Y.; Huang, Z.; Janssen, A.B.G.; Wishart, M.; He, W.; Wang, X.; Zhao, Y. Influence of social and environmental drivers on nutrient concentrations and ratios in lakes: A comparison between China and Europe. Water Res. 2022, 227, 119347. [Google Scholar] [CrossRef]
- Singh, B.J.; Chakraborty, A.; Sehgal, R. A systematic review of industrial wastewater management: Evaluating challenges and enablers. J. Environ. Manag. 2023, 348, 119230. [Google Scholar] [CrossRef]
- Umoren, S.A.; Etim, U.J.; Israel, A.U. Adsorption of methylene blue from industrial effluent using poly (vinyl alcohol). J. Mater. Environ. Sci. 2013, 4, 75–86. [Google Scholar]
- Iuliano, M.; Ponticorvo, E.; Cirillo, C.; Sarno, M. A New Nanocomposite from Vesuvian Slope Pinecones for Azo-Dyes Removal. Ind. Eng. Chem. Res. 2022, 61, 1965–1976. [Google Scholar] [CrossRef]
- Iuliano, M.; Cirillo, C.; Astorga, E.N.; Sarno, M. A new nanocomposite as adsorbent and catalyst for enhanced removal of methylene blue. Surf. Interfaces 2024, 51, 104582. [Google Scholar] [CrossRef]
- Iuliano, M.; Ponticorvo, E.; Cirillo, C.; Adami, R.; Sarno, M. Catalytic hydrogenation of organic dyes by Ag and Au magnetic nanoparticles supported on nanocellulose from waste pistachio shells. Mol. Catal. 2023, 544, 113179. [Google Scholar] [CrossRef]
- Shindhal, T.; Rakholiya, P.; Varjani, S.; Pandey, A.; Ngo, H.H.; Guo, W.; Ng, H.Y.; Taherzadeh, M.J. A critical review on advances in the practices and perspectives for the treatment of dye industry wastewater. Bioengineered 2020, 12, 70–87. [Google Scholar] [CrossRef]
- Bhatia, D.; Sharma, N.R.; Singh, J.; Kanwar, R.S. Biological methods for textile dye removal from wastewater: A review. Crit. Rev. Environ. Sci. Technol. 2017, 47, 1836–1876. [Google Scholar] [CrossRef]
- Titchou, F.E.; Zazou, H.; Afanga, H.; Gaayda, J.E.; Akbour, R.A.; Lesage, G.; Rivallin, M.; Cretin, M.; Hamdani, M. Electrochemical oxidation treatment of Direct Red 23 aqueous solutions: Influence of the operating conditions. Sep. Sci. Technol. 2021, 57, 1501–1520. [Google Scholar] [CrossRef]
- Hamous, H.; Khenifi, A.; Bouberka, Z.; Derriche, Z. Electrochemical degradation of Orange G in K2SO4 and KCl medium. Environ. Eng. Res. 2020, 25, 571–578. [Google Scholar] [CrossRef]
- Rajoria, S.; Vashishtha, M.; Sangal, V.K. Treatment of electroplating industry wastewater: A review on the various techniques. Environ. Sci. Pollut. Res. 2022, 29, 72196–72246. [Google Scholar] [CrossRef]
- FLouafi, O. Brahmia Electrochemical oxidation process to degradation of aqueous solution dyes. Int. J. Adv. Chem. Eng. Biol. Sci. 2016, 3, 227–231. [Google Scholar] [CrossRef]
- Donaldson, J.D.; Grimes, S.M.; Yasri, N.G.; Wheals, B.; Parrick, J.; Errington, W.E. Anodic oxidation of the dye materials methylene blue, acid blue 25, reactive blue 2 and reactive blue 15 and the characterisation of novel intermediate compounds in the anodic oxidation of methylene blue. J. Chem. Technol. Biotechnol. 2002, 77, 756–760. [Google Scholar] [CrossRef]
- Zhou, X.J.; Harmer, A.J.; Heinig, N.F.; Leung, K.T. Parametric study on electrochemical deposition of copper nanoparticles on an ultrathin polypyrrole film deposited on a gold film electrode. Langmuir 2004, 20, 5109–5113. [Google Scholar] [CrossRef] [PubMed]
- Ponticorvo, E.; Iuliano, M.; Cirillo, C.; Sarno, M. Selective C2 electrochemical synthesis from methane on modified alumina supporting single atom catalysts. Chem. Eng. J. 2022, 451, 139074. [Google Scholar] [CrossRef]
- Sarno, M.; Galvagno, S.; Scudieri, C.; Iovane, P.; Portofino, S.; Borriello, C.; Cirillo, C. Dopamine sensor in real sample based on thermal plasma silicon carbide nanopowders. J. Phys. Chem. Solids 2019, 131, 213–222. [Google Scholar] [CrossRef]
- Yang, J.; Jiang, L.-C.; Zhang, W.-D.; Gunasekaran, S. A highly sensitive non-enzymatic glucose sensor based on a simple two-step electrodeposition of cupric oxide (CuO) nanoparticles onto multi-walled carbon nanotube arrays. Talanta 2010, 83, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Sarno, M.; Rossi, G.; Cirillo, C.; Incarnato, L. Cold Wall Chemical vapor deposition Graphene-Based conductive tunable film barrier. Ind. Eng. Chem. Res. 2018, 57, 4895–4906. [Google Scholar] [CrossRef]
- Tedesco, G.C.; Moraes, P.B. Innovative design of a continuous flow photoelectrochemical reactor: Hydraulic design, CFD simulation and prototyping. J. Environ. Chem. Eng. 2021, 9, 105917. [Google Scholar] [CrossRef]
- Scarpa, D.; Iuliano, M.; Cirillo, C.; Iovane, P.; Borriello, C.; Portofino, S.; Ponticorvo, E.; Galvagno, S.; Sarno, M. Self-assembled monolayers of reduced graphene oxide for robust 3D-printed supercapacitors. Sci. Rep. 2024, 14, 14998. [Google Scholar] [CrossRef]
- Gnanasekaran, K.; Heijmans, T.; Van Bennekom, S.; Woldhuis, H.; Wijnia, S.; De With, G.; Friedrich, H. 3D printing of CNT- and graphene-based conductive polymer nanocomposites by fused deposition modeling. Appl. Mater. Today 2017, 9, 21–28. [Google Scholar] [CrossRef]
- Chen, Y.; Kirankumar, R.; Kao, C.; Chen, P. Electrodeposited Ag, Au, and AuAg nanoparticles on graphene oxide-modified screen-printed carbon electrodes for the voltammetric determination of free sulfide in alkaline solutions. Electrochim. Acta 2016, 205, 124–131. [Google Scholar] [CrossRef]
- Zhu, G.; Hou, Y.; Lu, J.; Zhang, H.; Zhuang, Z.; Baig, M.M.; Khan, M.Z.; Akram, M.A.; Dong, S.; Liu, P.; et al. MXene decorated 3D-printed carbon black-based electrodes for solid-state micro-supercapacitors. J. Mater. Chem. A 2023, 11, 25422–25428. [Google Scholar] [CrossRef]
- Abdalla, A.; Patel, B.A. 3D printed electrochemical sensors. Annu. Rev. Anal. Chem. 2021, 14, 47–63. [Google Scholar] [CrossRef]
- Browne, M.P.; Novotný, F.; Sofer, Z.; Pumera, M. 3D printed graphene electrodes’ electrochemical activation. ACS Appl. Mater. Interfaces 2018, 10, 40294–40301. [Google Scholar] [CrossRef]
- dos Santos, P.L.; Katic, V.; Loureiro, H.C.; dos Santos, M.F.; dos Santos, D.P.; Formiga, A.L.; Bonacin, J.A. Enhanced performance of 3D printed graphene electrodes after electrochemical pre-treatment: Role of exposed graphene sheets. Sens. Actuators B Chem. 2018, 281, 837–848. [Google Scholar] [CrossRef]
- Kalinke, C.; Neumsteir, N.V.; Aparecido, G.D.O.; Ferraz, T.V.D.B.; dos Santos, P.L.; Janegitz, B.C.; Bonacin, J.A. Comparison of activation processes for 3D printed PLA-graphene electrodes: Electrochemical properties and application for sensing of dopamine. Analyst 2019, 145, 1207–1218. [Google Scholar] [CrossRef]
- Katseli, V.; Thomaidis, N.; Economou, A.; Kokkinos, C. Miniature 3D-printed integrated electrochemical cell for trace voltammetric Hg (II) determination. Sens. Actuators B Chem. 2020, 308, 127715. [Google Scholar] [CrossRef]
- Rocha, R.G.; Cardoso, R.M.; Zambiazi, P.J.; Castro, S.V.; Ferraz, T.V.; Aparecido, G.D.O.; Bonacin, J.A.; Munoz, R.A.; Richter, E.M. Production of 3D-printed disposable electrochemical sensors for glucose detection using a conductive filament modified with nickel microparticles. Anal. Chim. Acta 2020, 1132, 1–9. [Google Scholar] [CrossRef]
- Kumar, K.A.; Ghosh, K.; Alduhaish, O.; Pumera, M. Metal-plated 3D-printed electrode for electrochemical detection of carbohydrates. Electrochem. Commun. 2020, 120, 106827. [Google Scholar] [CrossRef]
- Vaněčková, E.; Bouša, M.; Shestivska, V.; Kubišta, J.; Moreno-García, P.; Broekmann, P.; Rahaman, M.; Zlámal, M.; Heyda, J.; Bernauer, M. Electrochemical reduction of carbon dioxide on 3D printed electrodes. Chem. Electro. Chem. 2021, 8, 2137. [Google Scholar] [CrossRef]
- Hüner, B.; Demir, N.; Kaya, M.F. Ni-Pt coating on graphene based 3D printed electrodes for hydrogen evolution reactions in alkaline media. Fuel 2023, 331, 125971. [Google Scholar] [CrossRef]
- Hüner, B.; Demir, N.; Kaya, M.F. Hydrogen evolution reaction performance of Ni–Co-coated graphene-based 3D printed electrodes. ACS Omega 2023, 8, 5958. [Google Scholar] [CrossRef]
- Huener, B.; Demir, N.; Kaya, M.F. Electrodeposition of NiCu bimetal on 3D printed electrodes for hydrogen evolution reactions in alkaline media. Int. J. Hydrogen Energy 2022, 47, 12136. [Google Scholar] [CrossRef]
- Iffelsberger, C.; Ng, S.; Pumera, M. Catalyst coating of 3D printed structures via electrochemical deposition: Case of the transition metal chalcogenide MoSx for hydrogen evolution reaction. Appl. Mater. Today 2020, 20, 100654. [Google Scholar] [CrossRef]
- Urbanova, V.; Plutnar, J.; Pumera, M. Atomic layer deposition of electrocatalytic layer of MoS2 onto metal-based 3D-printed electrode toward tailoring hydrogen evolution efficiency. Appl. Mater. Today 2021, 24, 101131. [Google Scholar] [CrossRef]
- Akshay Kumar, K.P.; Ghosh, K.; Alduhaish, O.; Pumera, M. Dip-coating of MXene and transition metal dichalcogenides on 3D-printed nanocarbon electrodes for the hydrogen evolution reaction. Electrochem. Commun. 2021, 122, 106890. [Google Scholar] [CrossRef]
- Zhong, L.; Liao, M.; Ou, J.; Yang, Y.; Wen, J.; Jiang, Y.; Yang, H.; Dai, X.; Wang, L. Gold particles modified 3D printed carbon black nanonetwork electrode for improving the detection sensitivity of dopamine. Microchem. J. 2024, 201, 110630. [Google Scholar] [CrossRef]
- Alves, A.O.; de Faria, L.V.; Caldas, N.M.; Batista, A.G.; Nascimento, S.F.L.D.; Danho, B.E.; Peixoto, D.A.; Nossol, E.; Rocha, D.P.; Semaan, F.S.; et al. 3D-printed carbon black/polylactic acid electrode modified with silver particles: A powerful alternative and cost-effective sensor for nitrate sensing in real water samples. J. Solid State Electrochem. 2024, 29, 1217–1225. [Google Scholar] [CrossRef]
- Marzo, A.M.L.; Mayorga-Martinez, C.C.; Pumera, M. 3D-printed graphene direct electron transfer enzyme biosensors. Biosens. Bioelectron. 2019, 151, 111980. [Google Scholar] [CrossRef]
- Browne, M.P.; Redondo, E.; Pumera, M. 3D printing for electrochemical energy applications. Chem. Rev. 2020, 120, 2783–2810. [Google Scholar] [CrossRef]
- Weber, C.J.; Strom, N.E.; Simoska, O. Electrochemical deposition of gold nanoparticles on carbon ultramicroelectrode arrays. Nanoscale 2024, 16, 16204–16217. [Google Scholar] [CrossRef]
- Weber, C.J.; Strom, N.E.; Vagnoni, E.M.; Simoska, O. Electrochemical deposition of silver nanoparticle assemblies on carbon ultramicroelectrode arrays. Chem. Phys. Chem. 2024, 26, e202400791. [Google Scholar] [CrossRef] [PubMed]
- Bograchev, D.A.; Kabanova, T.B.; Davydov, A.D. Electrodeposition of metals into nano/micropores of templates: A type of electrochemistry under confinement (review). J. Solid State Electrochem. 2024, 29, 1309–1340. [Google Scholar] [CrossRef]
- Gusmão, R.; Browne, M.P.; Sofer, Z.; Pumera, M. The capacitance and electron transfer of 3D-printed graphene electrodes are dramatically influenced by the type of solvent used for pre-treatment. Electrochem. Commun. 2019, 102, 83–88. [Google Scholar] [CrossRef]
- Grujicic, D.; Pesic, B. Electrodeposition of copper: The nucleation mechanisms. Electrochim. Acta 2002, 47, 2901–2912. [Google Scholar] [CrossRef]
- ISO 15705:2002; Water Quality—Determination of the Chemical Oxygen Demand Index (ST-COD)—Small-Scale Sealed-Tube Method. International Organization for Standardization: Geneva, Switzerland, 2002.
- 5220 D; Chemical Oxygen Demand (COD). Sealed-Tube, Miniaturized Colorimetric Method. Federal Register: Washington, DC, USA, 1980.
- Morsi Al-Sarawy, A.; El-Dein, W.S. Electrochemical degradation of some organic dyes by electrochemical oxidation on a Pb/PbO2electrode. Desalination Water Treat. 2011, 26, 301–308. [Google Scholar] [CrossRef]
- Sarno, M.; Spina, D.; Senatore, A. One-step nanohybrid synthesis in waste cooking oil, for direct lower environmental impact and stable lubricant formulation. Tribol. Int. 2019, 135, 355–367. [Google Scholar] [CrossRef]
- Chen, X.; Kalish, J.; Hsu, S.L. Structure evolution of α′-phase poly(lactic acid). J. Polym. Sci. Part B Polym. Phys. 2011, 49, 1446–1454. [Google Scholar] [CrossRef]
- Usman, M.S.; Ibrahim, N.A.; Shameli, K.; Zainuddin, N.; Yunus, W.M.Z.W. Copper nanoparticles mediated by chitosan: Synthesis and characterization via chemical methods. Molecules 2012, 17, 14928–14936. [Google Scholar] [CrossRef]
- Akhter, G.; Khan, A.; Ali, S.G.; Khan, T.A.; Siddiqi, K.S.; Khan, H.M. Antibacterial and nematicidal properties of biosynthesized Cu nanoparticles using extract of holo-parasitic plant. SN Appl. Sci. 2020, 2, 1268. [Google Scholar] [CrossRef]
- Patterson, A.L. The Scherrer formula for X-ray particle size determination. Phys. Rev. 1939, 56, 978–982. [Google Scholar] [CrossRef]
- Krikorian, V.; Pochan, D.J. Crystallization Behavior of Poly(l-lactic acid) Nanocomposites: Nucleation and Growth Probed by Infrared Spectroscopy. Macromolecules 2005, 38, 6520–6527. [Google Scholar] [CrossRef]
- Jordá-Vilaplana, A.; Fombuena, V.; García-García, D.; Samper, M.; Sánchez-Nácher, L. Surface modification of polylactic acid (PLA) by air atmospheric plasma treatment. Eur. Polym. J. 2014, 58, 23–33. [Google Scholar] [CrossRef]
- Cirillo, C.; Iuliano, M.; Scarpa, D.; Iovane, P.; Borriello, C.; Portofino, S.; Galvagno, S.; Sarno, M. Silver-Doped Reduced Graphene Oxide/PANI-DBSA-PLA Composite 3D-Printed Supercapacitors. Nanomaterials 2024, 14, 1681. [Google Scholar] [CrossRef]
- Morgounova, E.; Shao, Q.; Hackel, B.J.; Thomas, D.D.; Ashkenazi, S. Photoacoustic lifetime contrast between methylene blue monomers and self-quenched dimers as a model for dual-labeled activatable probes. J. Biomed. Opt. 2013, 18, 056004. [Google Scholar] [CrossRef]
- Usacheva, M.N.; Teichert, M.C.; Biel, M.A. The role of the methylene blue and toluidine blue monomers and dimers in the photoinactivation of bacteria. J. Photochem. Photobiol. B Biol. 2003, 71, 87–98. [Google Scholar] [CrossRef]
- Spencer, W.; Sutter, J.R. Kinetic study of the monomer-dimer equilibrium of methylene blue in aqueous solution. J. Phys. Chem. 1979, 83, 1573–1576. [Google Scholar] [CrossRef]
- Yao, Y.; Teng, G.; Yang, Y.; Huang, C.; Liu, B.; Guo, L. Electrochemical oxidation of acetamiprid using Yb-doped PbO2 electrodes: Electrode characterization, influencing factors and degradation pathways. Sep. Purif. Technol. 2019, 211, 456–466. [Google Scholar] [CrossRef]
- Liou, S.; Dodd, M.C. Evaluation of hydroxyl radical and reactive chlorine species generation from the superoxide/hypochlorous acid reaction as the basis for a novel advanced oxidation process. Water Res. 2021, 200, 117142. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Feng, J.; Fan, S.; Zhou, W.; Dai, Q. Fabrication of a multi-layer CNT-PbO2 anode for the degradation of isoniazid: Kinetics and mechanism. Chemosphere 2021, 263, 128069. [Google Scholar] [CrossRef]
- Hasnat, M.; Safwan, J.A.; Islam, M.S.; Rahman, Z.; Karim, M.R.; Pirzada, T.J.; Samed, A.J.; Rahman, M.M. Electrochemical decolorization of Methylene blue at Pt electrode in KCl solution for environmental remediation. J. Ind. Eng. Chem. 2014, 21, 787–791. [Google Scholar] [CrossRef]
- Xu, T.; Fu, L.; Lu, H.; Zhang, M.; Wang, W.; Hu, B.; Zhou, Y.; Yu, G. Electrochemical oxidation degradation of Rhodamine B dye on boron-doped diamond electrode: Input mode of power attenuation. J. Clean. Prod. 2023, 401, 136794. [Google Scholar] [CrossRef]
- Nwanebu, E.O.; Liu, X.; Pajootan, E.; Yargeau, V.; Omanovic, S. Electrochemical degradation of methylene blue using a Ni-Co-Oxide anode. Catalysts 2021, 11, 793. [Google Scholar] [CrossRef]
- Panizza, M.; Barbucci, A.; Ricotti, R.; Cerisola, G. Electrochemical degradation of methylene blue. Sep. Purif. Technol. 2006, 54, 382–387. [Google Scholar] [CrossRef]
- Panizza, M.; Michaud, P.; Cerisola, G.; Comninellis, C. Anodic oxidation of 2-naphthol at boron-doped diamond electrodes. J. Electroanal. Chem. 2001, 507, 206–214. [Google Scholar] [CrossRef]
- Aravind, P.; Selvaraj, H.; Ferro, S.; Sundaram, M. An integrated (electro- and bio-oxidation) approach for remediation of industrial wastewater containing azo-dyes: Understanding the degradation mechanism and toxicity assessment. J. Hazard. Mater. 2016, 318, 203–215. [Google Scholar] [CrossRef] [PubMed]
- Haider, M.R.; Jiang, W.-L.; Han, J.-L.; Sharif, H.M.A.; Ding, Y.-C.; Cheng, H.-Y.; Wang, A.-J. In-situ electrode fabrication from polyaniline derived N-doped carbon nanofibers for metal-free electro-Fenton degradation of organic contaminants. Appl. Catal. B Environ. 2019, 256, 117774. [Google Scholar] [CrossRef]
- Zhou, Q.; Liu, D.; Yuan, G.; Tang, Y.; Cui, K.; Jiang, S.; Xia, Y.; Xiong, W. Efficient degradation of phenolic wastewaters by a novel Ti/PbO2-Cr-PEDOT electrode with enhanced electrocatalytic activity and chemical stability. Sep. Purif. Technol. 2022, 281, 119735. [Google Scholar] [CrossRef]



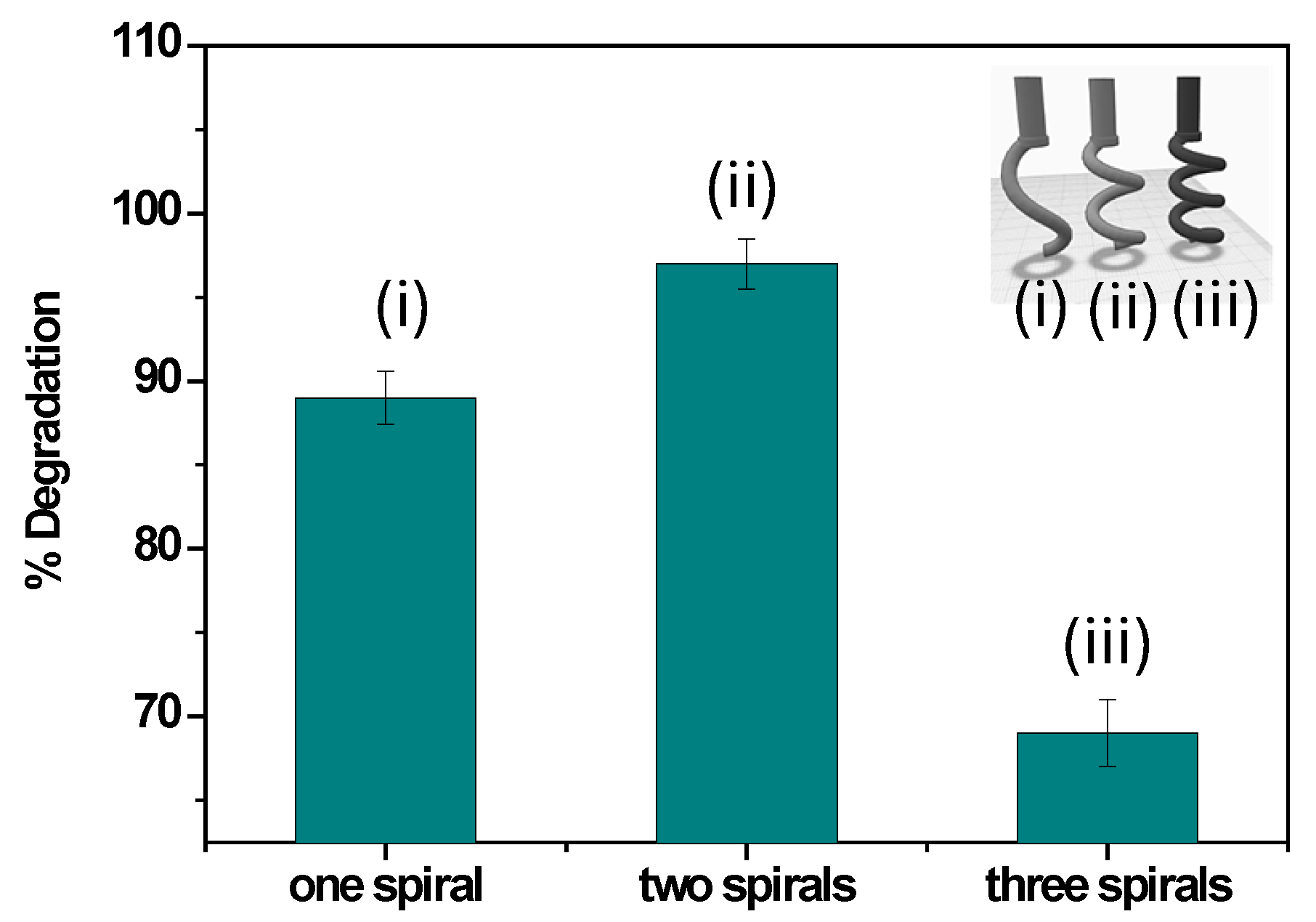
| 3D-Printed Electrode | Metal Coating | Application | Ref. |
|---|---|---|---|
| PLA/Carbon composite | Au electroplating | Heavy metal sensing (Hg2+) | [28] |
| Graphene/PLA | Ni microparticles (activated) | Glucose sensing | [29] |
| Graphene/PLA | Cu/Ni coating | Glucose and sucrose sensing | [30] |
| Graphene/PLA | Cu electroplating | CO2 reduction | [31] |
| Graphene/PLA | Ni-Pt coating | Hydrogen Evolution Reaction (HER) | [32] |
| Graphene/PLA | Ni-Co coating | [33] | |
| PLA | Ni/Cu electrodeposition | [34] | |
| Graphene/PLA | MoSₓ deposition (metal sulfide) | [35] | |
| Ti-based 3DPE | MoS2 via ALD | [36] | |
| Nano-carbon (3DPE) | MXene + dichalcogenides (MoS2, WS2, WSe2) | [37] |
| Property | Value/Description |
|---|---|
| Base material | PLA |
| Characteristics | Low odor, non-toxic, renewably sourced |
| Molecular structure | Amorphous |
| Additives | Carbon black/Polymer blend |
| Density | ~1.24 g/cm3 |
| Minimum bend diameter | 25 mm (Ø 1.75 mm) |
| Melting point (Tm) onset | ~155 °C (310 °F) |
| Materials | Conditions | Pollutants | Removal Efficiency@Time | Ref. |
|---|---|---|---|---|
| Cu@AC-3DPE | pH: 6.0, V: 2 V, C(MB): 50 mg/L, C(NaCl): 0.1 M | MB | ≈97%@60 min | This Work |
| Carbon–PTFE diffusion | pH = 3.0, Applied current = 33.3 mA/cm2, C(Ponceau 4R) = 254.0 mg/dm3, V = 130.0 cm3, C(Na2SO4): = 0.05 M | Ponceau 4R | 100%@6 h | [69] |
| PANI/Gr | pH = 3.0, V: −0.6V, C(MO) = 50 mg L−1, C(Na2SO4): = 0.05 M | MO | 100%@90 min | [70] |
| Ti/PbO2-Cr-PEDOT | pH: n.d. Applied current = 15 mA/cm2, C(Phenol) = 1000 mg/L, C(Na2SO4): = 0.028 M | Phenol | 100%@120 min | [71] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cirillo, C.; Iuliano, M.; Shahzad, M.; Di Martino, E.G.; Gallucci, L.; Funicello, N.; Iannone, G.; De Pasquale, S.; Sarno, M. Electrochemical Oxidation Degradation of Methylene Blue Dye on 3D-Printed Anode Electrodes. Polymers 2025, 17, 2499. https://doi.org/10.3390/polym17182499
Cirillo C, Iuliano M, Shahzad M, Di Martino EG, Gallucci L, Funicello N, Iannone G, De Pasquale S, Sarno M. Electrochemical Oxidation Degradation of Methylene Blue Dye on 3D-Printed Anode Electrodes. Polymers. 2025; 17(18):2499. https://doi.org/10.3390/polym17182499
Chicago/Turabian StyleCirillo, Claudia, Mariagrazia Iuliano, Muhammad Shahzad, Emanuela Grazia Di Martino, Luca Gallucci, Nicola Funicello, Gerardo Iannone, Salvatore De Pasquale, and Maria Sarno. 2025. "Electrochemical Oxidation Degradation of Methylene Blue Dye on 3D-Printed Anode Electrodes" Polymers 17, no. 18: 2499. https://doi.org/10.3390/polym17182499
APA StyleCirillo, C., Iuliano, M., Shahzad, M., Di Martino, E. G., Gallucci, L., Funicello, N., Iannone, G., De Pasquale, S., & Sarno, M. (2025). Electrochemical Oxidation Degradation of Methylene Blue Dye on 3D-Printed Anode Electrodes. Polymers, 17(18), 2499. https://doi.org/10.3390/polym17182499









