Silicone Films Modified with Ethylene Glycol Dicyclopentenyl Ether Acrylate for Antimicrobial Silver Loading
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of SR-g-EGDEA by the Direct Method
2.3. Synthesis of SR-g-EGDEA by the Oxidative Pre-Irradiation Method
2.4. Fourier Transform Infrared Spectroscopy with Attenuated Total Reflectance (FTIR-ATR)
2.5. Thermogravimetric Analysis (TGA)
2.6. Differential Scanning Calorimetry (DSC)-
2.7. Swelling
2.8. Contact Angle
2.9. Mechanical Test
2.10. Synthesis and Loading of Antimicrobial Silver
2.11. Microbiological Trials
3. Results
3.1. Grafting of SR-g-EGDEA by the Direct Method
3.2. Grafting of SR-g-EGDEA by the Oxidative Pre-Irradiation Method
3.3. FTIR-ATR
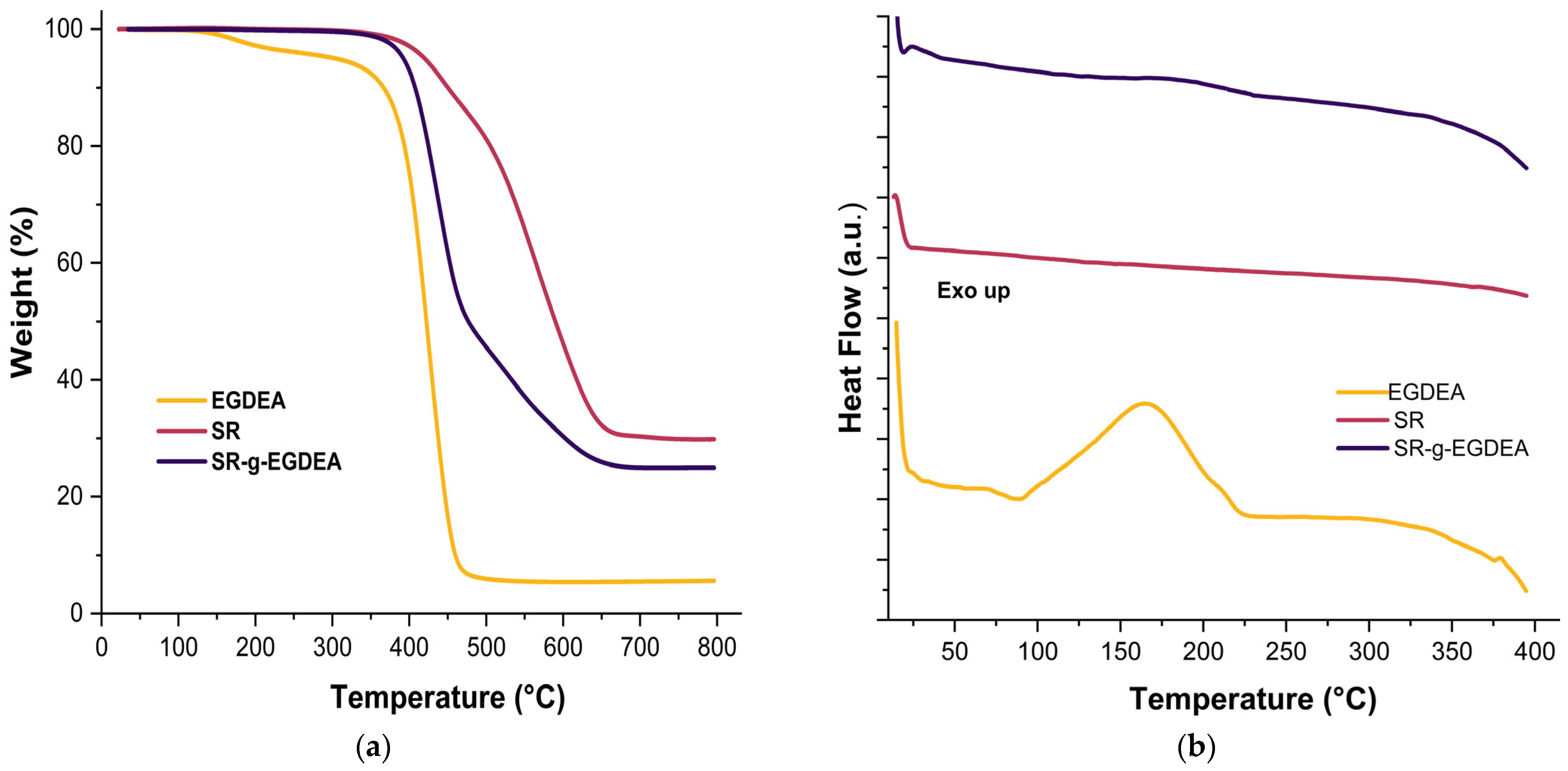
3.4. Thermal Analysis
3.5. Swelling and Contact Angle
3.6. Synthesis of Antimicrobial Silver
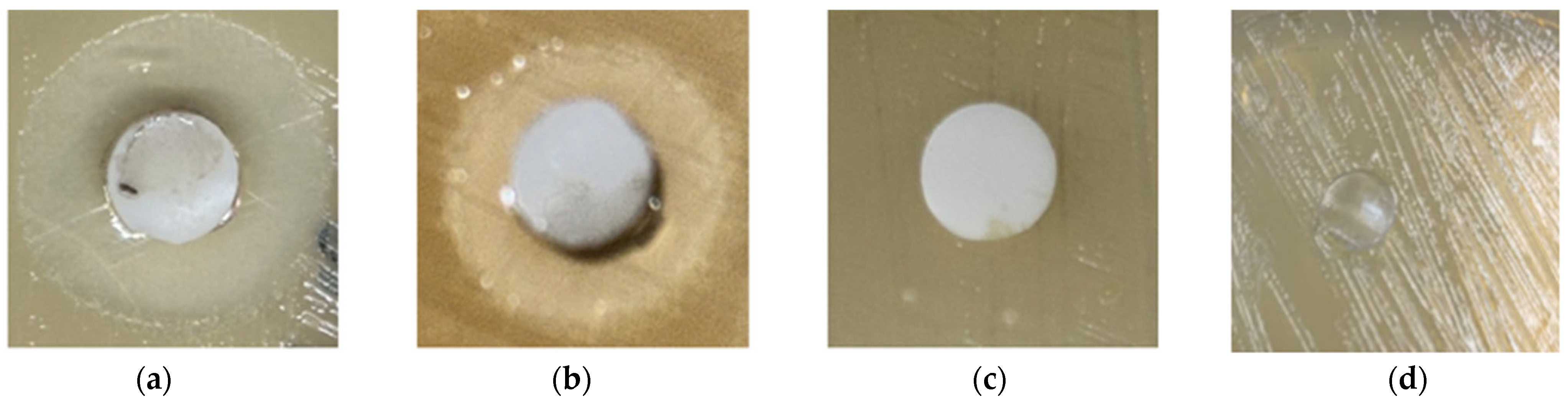
3.7. Antimicrobial Studies
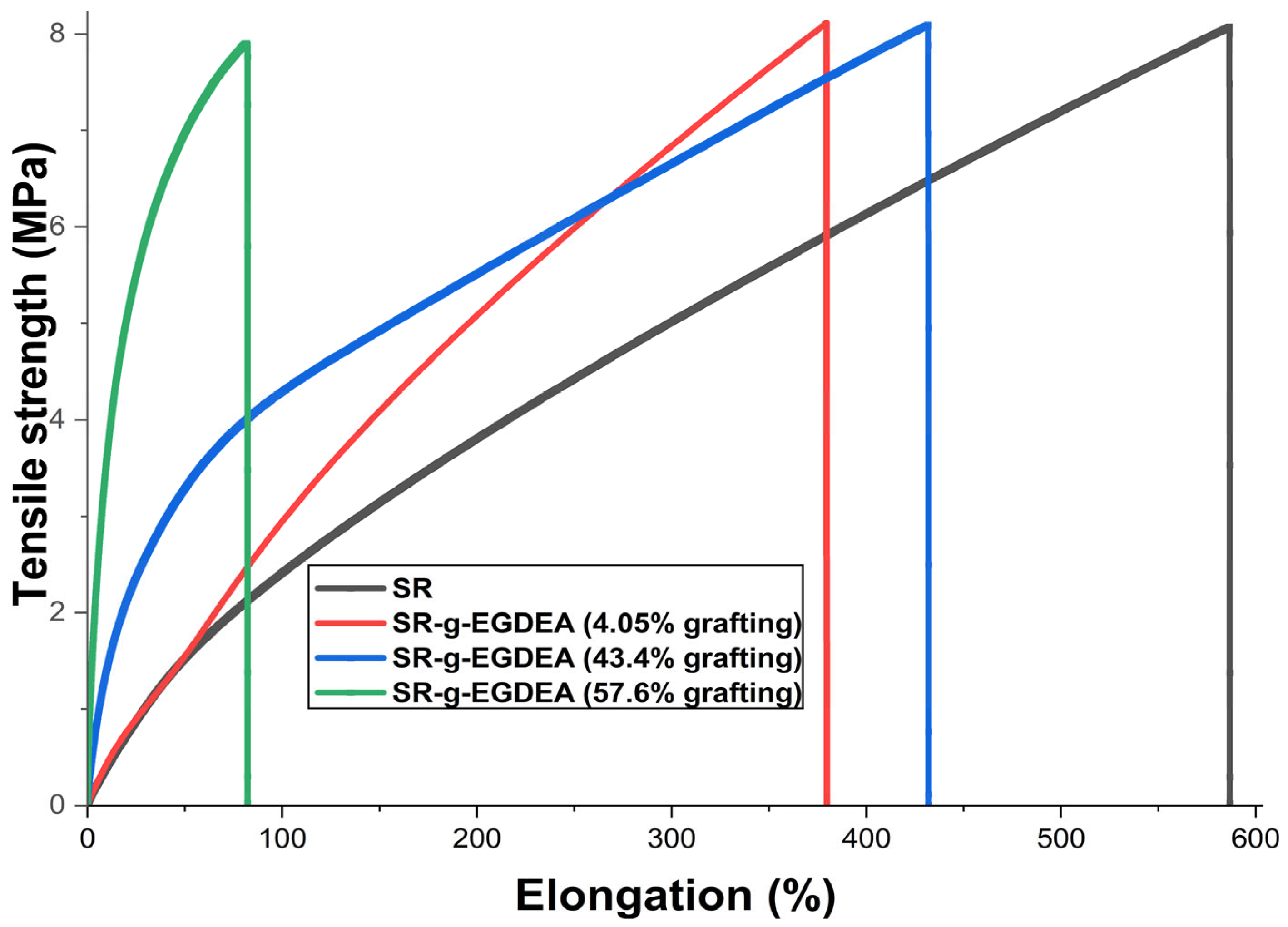
3.8. Results of Mechanical Test
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Divatia, J.V.; Pulinilkunnathil, J.G.; Myatra, S.N. Nosocomial Infections and Ventilator-Associated Pneumonia in Cancer Patients. In Oncologic Critical Care; Springer International Publishing: Berlin/Heidelberg, Germany, 2019; Volume 9, pp. 1419–1439. [Google Scholar] [CrossRef]
- Qiu, H.; Si, Z.; Luo, Y.; Feng, P.; Wu, X.; Hou, W.; Zhu, Y.; Chan-Park, M.B.; Xu, L.; Huang, D. The Mechanisms and the Applications of Antibacterial Polymers in Surface Modification on Medical Devices. Front. Bioeng. Biotechnol. 2020, 8, 910. [Google Scholar] [CrossRef] [PubMed]
- Zare, M.; Ghomi, E.R.; Venkatraman, P.D.; Ramakrishna, S. Silicone-Based Biomaterials for Biomedical Applications: Antimicrobial Strategies and 3D Printing Technologies. J. Appl. Polym. Sci. 2021, 138, 50969. [Google Scholar] [CrossRef]
- Lawrence, E.L.; Turner, I.G. Materials for Urinary Catheters: A Review of Their History and Development in the UK. Med. Eng. Phys. 2005, 27, 443–453. [Google Scholar] [CrossRef]
- Li, M.; Neoh, K.G.; Xu, L.Q.; Wang, R.; Kang, E.T.; Lau, T.; Olszyna, D.P.; Chiong, E. Surface Modification of Silicone for Biomedical Applications Requiring Long-Term Antibacterial, Antifouling, and Hemocompatible Properties. Langmuir 2012, 28, 16408–16422. [Google Scholar] [CrossRef]
- Dizman, B.; Elasri, M.O.; Mathias, L.J. Synthesis, Characterization, and Antibacterial Activities of Novel Methacrylate Polymers Containing Norfloxacin. Biomacromolecules 2004, 6, 514–520. [Google Scholar] [CrossRef]
- Zdyrko, B.; Luzinov, I. Polymer Brushes by the “Grafting to” Method. Macromol. Rapid Commun. 2011, 32, 859–869. [Google Scholar] [CrossRef]
- Sun, Y.; Chmielewski, A.G. Applications of Ionizing Radiation in Materials Processing; Institute of Nuclear Chemistry and Technology: Warsaw, Poland, 2017; Volume 2, pp. 143–167. [Google Scholar]
- Tyler, B.J.; Hook, A.; Pelster, A.; Williams, P.; Alexander, M.; Arlinghaus, H.F. Development and Characterization of a Stable Adhesive Bond between a Poly(Dimethylsiloxane) Catheter Material and a Bacterial Biofilm Resistant Acrylate Polymer Coating. Biointerphases 2017, 12, 02C412. [Google Scholar] [CrossRef]
- Adlington, K.; Nguyen, N.T.; Eaves, E.; Yang, J.; Chang, C.Y.; Li, J.; Gower, A.L.; Stimpson, A.; Anderson, D.G.; Langer, R.; et al. Application of Targeted Molecular and Material Property Optimization to Bacterial Attachment-Resistant (Meth)Acrylate Polymers. Biomacromolecules 2016, 17, 2830–2838. [Google Scholar] [CrossRef]
- Hook, A.L.; Chang, C.Y.; Yang, J.; Atkinson, S.; Langer, R.; Anderson, D.G.; Davies, M.C.; Williams, P.; Alexander, M.R. Discovery of Novel Materials with Broad Resistance to Bacterial Attachment Using Combinatorial Polymer Microarrays. Adv. Mater. 2013, 25, 2542–2547. [Google Scholar] [CrossRef] [PubMed]
- Mijnendonckx, K.; Leys, N.; Mahillon, J.; Silver, S.; Van Houdt, R. Antimicrobial Silver: Uses, Toxicity and Potential for Resistance. BioMetals 2013, 26, 609–621. [Google Scholar] [CrossRef]
- Cano, A.; Cháfer, M.; Chiralt, A.; González-Martínez, C. Development and Characterization of Active Films Based on Starch-PVA, Containing Silver Nanoparticles. Food Packag. Shelf Life 2016, 10, 16–24. [Google Scholar] [CrossRef]
- Melaiye, A.; Youngs, W.J. Silver and Its Application as an Antimicrobial Agent. Expert. Opin. Ther. Pat. 2005, 15, 125–130. [Google Scholar] [CrossRef]
- Furno, F.; Morley, K.S.; Wong, B.; Sharp, B.L.; Arnold, P.L.; Howdle, S.M.; Bayston, R.; Brown, P.D.; Winship, P.D.; Reid, H.J. Silver Nanoparticles and Polymeric Medical Devices: A New Approach to Prevention of Infection? J. Antimicrob. Chemother. 2004, 54, 1019–1024. [Google Scholar] [CrossRef] [PubMed]
- More, P.R.; Pandit, S.; Filippis, A.D.; Franci, G.; Mijakovic, I.; Galdiero, M. Silver Nanoparticles: Bactericidal and Mechanistic Approach Against Drug Resistant Pathogens. Microorganisms 2023, 11, 369. [Google Scholar] [CrossRef]
- Van Phu, D.; Quoc, L.A.; Duy, N.N.; Lan, N.T.K.; Du, B.D.; Luan, L.Q.; Hien, N.Q. Study on Antibacterial Activity of Silver Nanoparticles Synthesized by Gamma Irradiation Method Using Different Stabilizers. Nanoscale Res. Lett. 2014, 9, 162. [Google Scholar] [CrossRef]
- Pérez-Garibay, M.S.; Lara-Rodríguez, G.Á.; Bucio, E. Functionalization of Polyvinylpyrrolidone Films by Grafting Maleic Acid from PVP Gels for Loading Studies of Naringin and Silver Nanoparticles as Potential Wound Dressings. Gels 2025, 11, 147. [Google Scholar] [CrossRef]
- Caballero-Becerra, G.; Esquivel-Lozano, Y.A.; Romero-Fierro, D.; Bucio, E. Two-Step Grafting of Ethylene Glycol Dicyclopentenyl Ether Acrylate and Methacrylic Acid in Silicone Films by Gamma Radiation. Radiat. Phys. Chem. 2025, 237, 113000. [Google Scholar] [CrossRef]
- Ashfaq, A.; Clochard, M.C.; Coqueret, X.; Dispenza, C.; Driscoll, M.S.; Ulański, P.; Al-Sheikhly, M. Polymerization Reactions and Modifications of Polymers by Ionizing Radiation. Polymers 2020, 12, 2877. [Google Scholar] [CrossRef] [PubMed]
- Cruz, E. Rentabilidad de un Irradiador de Co 60 Semi-Industrial Tipo Gamma Beam 651 Pt; Instituto de Ciencias Nucleares UNAM: Mexico City, Mexico, 1997. [Google Scholar]
- Romero-Fierro, D.; Esquivel-Lozano, Y.A.; Camacho-Cruz, A.; Bucio, E. Thermo- and PH-Responsive Cotton Gauzes as Drug Delivery System Obtained by Gamma Radiation and Chemical Initiator. Cellulose 2023, 30, 11273–11294. [Google Scholar] [CrossRef]
- Salih, S.I.; Oleiwi, J.K.; Ali, H.M. Study the Mechanical Properties of Polymeric Blends (SR/PMMA) Using for Maxillofacial Prosthesis Application. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Melbourne, Australia, 15–16 September 2018; Institute of Physics Publishing: Bristol, UK, 2018; p. 454. [Google Scholar] [CrossRef]
- Maupu, A.; Kanawati, Y.; Métafiot, A.; Maric, M. Ethylene Glycol Dicyclopentenyl (Meth)Acrylate Homo and Block Copolymers via Nitroxide Mediated Polymerization. Materials 2019, 12, 1547. [Google Scholar] [CrossRef]
- Li, X.; Zhou, X.; Chen, Y.; Yu, S.; Chen, X.; Xia, X.; Shi, X.; Zhang, Y.; Fan, D. Surface Changes of Nanotopography by Carbon Ion Implantation to Enhance the Biocompatibility of Silicone Rubber: An in Vitro Study of the Optimum Ion Fluence and Adsorbed Protein. J. Mater. Sci. Mater. Med. 2017, 28, 167. [Google Scholar] [CrossRef]
- Murphy, M.; Ting, K.; Zhang, X.; Soo, C.; Zheng, Z. Current Development of Silver Nanoparticle Preparation, Investigation, and Application in the Field of Medicine. J. Nanomater. 2015, 2015, 696918. [Google Scholar] [CrossRef]
- Malina, D.; Sobczak-Kupiec, A.; Wzorek, Z.; Kowalski, Z. Silver Nanoparticles Synthesis with Different Concentrations of Polyvinylpyrrolidone. Dig. J. Nanomater. Biostruct 2012, 7, 1527–1534. [Google Scholar]
- Kim, J.; Kang, S.W.; Kang, Y.S. Partially Positively Charged Silver Nanoparticles Prepared by P-Benzoquinone. Colloids Surf. A Physicochem. Eng. Asp. 2008, 320, 189–192. [Google Scholar] [CrossRef]
- Sambalova, O.; Thorwarth, K.; Heeb, N.V.; Bleiner, D.; Zhang, Y.; Borgschulte, A.; Kroll, A. Carboxylate Functional Groups Mediate Interaction with Silver Nanoparticles in Biofilm Matrix. ACS Omega 2017, 3, 724–733. [Google Scholar] [CrossRef]
- Tang, S.; Zheng, J. Antibacterial Activity of Silver Nanoparticles: Structural Effects. Adv. Healthc. Mater. 2018, 7, 1701503. [Google Scholar] [CrossRef]
- Lee, H.-S.; Ryu, D.-S.; Choi, S.-J.; Lee, D.-S. Antibacterial Activity of Silver-Nanoparticles Against Staphylococcus Aureus and Escherichia Coli. Korean J. Microbiol. Biotechnol. 2011, 39, 77–85. [Google Scholar]
- Guzmán, M.G.; Dille, J.; Godet, S. Synthesis of silver nanoparticles by chemical reduction method and their antibacterial activity. Int. J. Chem. Biomol. Eng. 2009, 2, 104–111. [Google Scholar]
- Rodrigues, A.S.; Batista, J.G.S.; Rodrigues, M.Á.V.; Thipe, V.C.; Minarini, L.A.R.; Lopes, P.S.; Lugão, A.B. Advances in Silver Nanoparticles: A Comprehensive Review on Their Potential as Antimicrobial Agents and Their Mechanisms of Action Elucidated by Proteomics. Front. Microbiol. 2024, 15, 1440065. [Google Scholar] [CrossRef] [PubMed]
- McKee, C.T.; Last, J.A.; Russell, P.; Murphy, C.J. Indentation Versus Tensile Measurements of Young’s Modulus for Soft Biological Tissues. Tissue Eng. Part B Rev. 2011, 17, 155–164. [Google Scholar] [CrossRef]
- Vargas-Machado, P.J.; López-Saucedo, F.; Bucio, E. Poly(Vinylpyrrolidone) Graft in Poly(Vinyl Chloride) Catheters Using Gamma Radiation for Ciprofloxacin Loading and Release. Polymers 2025, 17, 612. [Google Scholar] [CrossRef] [PubMed]




| Dose (kGy) | EGDEA 25%(v/v) | Grafting (%) (a) |
|---|---|---|
| 10 | Hexanes | 43.6 ± 3.6 |
| 10 | Petroleum ether | 57.2 ± 4.5 |
| 30 | Hexanes | 60.1 ± 6.0 |
| 30 | Petroleum ether | 59.6 ± 4.5 |
| 50 | Hexanes | 61.6 ± 2.6 |
| 50 | Petroleum ether | 67.9 ± 5.5 |
| Dose (kGy) | EGDEA 25% (v/v) | Grafting (%) (a) | Temperature (°C) | Time (hr) |
|---|---|---|---|---|
| 10 | Hexanes | 4.58 ± 1.1 | 80 | 72 |
| 30 | Hexanes | 4.98 ± 2.1 | 80 | 72 |
| 50 | Hexanes | 4.51 ± 1.3 | 80 | 72 |
| Film | Time (30 min) Swelling (%) | Time (60 min) Swelling (%) |
|---|---|---|
| Pristine SR | - | - |
| SR-g-EGDEA (46.7%) | 5.32 | 8.66 |
| SR-g-EGDEA (62.2%) | 5.77 | 8.44 |
| E. coli | S. aureus |
|---|---|
| Clear zone (mm) | Clear zone (mm) |
| 10.06 ± 0.04 | 0.9 ± 0.081 |
| 11.50 ± 0.06 | 11.3 ± 0.28 |
| Film | Tensile Strength (MPa) | Elongation at Break (%) | Young Modulus (MPa) |
|---|---|---|---|
| SR | 7.6 ± 0.47 | 566 ± 19.5 | 1.3 ± 0.07 |
| SR-g-EGDEA (4%) | 11.9 ± 0.81 | 324 ± 55 | 1.84 ± 0.06 |
| SR-g-EGDEA (43.4%) | 6.7 ± 0.89 | 417.5 ± 13.5 | 1.6 ± 0.16 |
| SR-g-EGDEA (57.6%) | 4.6% ± 0.16 | 72 ± 9 | 12.9 ± 0.26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Padilla, O.; Pérez-Garibay, M.S.; Camacho-Cruz, A.; Bucio, E. Silicone Films Modified with Ethylene Glycol Dicyclopentenyl Ether Acrylate for Antimicrobial Silver Loading. Polymers 2025, 17, 2482. https://doi.org/10.3390/polym17182482
Padilla O, Pérez-Garibay MS, Camacho-Cruz A, Bucio E. Silicone Films Modified with Ethylene Glycol Dicyclopentenyl Ether Acrylate for Antimicrobial Silver Loading. Polymers. 2025; 17(18):2482. https://doi.org/10.3390/polym17182482
Chicago/Turabian StylePadilla, Orlando, Miguel S. Pérez-Garibay, Alejandro Camacho-Cruz, and Emilio Bucio. 2025. "Silicone Films Modified with Ethylene Glycol Dicyclopentenyl Ether Acrylate for Antimicrobial Silver Loading" Polymers 17, no. 18: 2482. https://doi.org/10.3390/polym17182482
APA StylePadilla, O., Pérez-Garibay, M. S., Camacho-Cruz, A., & Bucio, E. (2025). Silicone Films Modified with Ethylene Glycol Dicyclopentenyl Ether Acrylate for Antimicrobial Silver Loading. Polymers, 17(18), 2482. https://doi.org/10.3390/polym17182482







