Removal of Textile Dye Mixture by Fe3O4/Acrylamide/Triacryloylhexahydro Triazine Composite Hydrogel Polymer
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Synthesis of Fe3O4 Particles
2.3. Synthesis of Fe3O4/Acrylamide/Triazine Composite Polymer
2.4. Hydrolysis of Fe3O4/Acrylamide/Triazine Composite Polymer
2.5. Dye Removal Experiments
2.6. Development of the First Derivative Method
2.7. Characterization
2.8. Antibacterial Activity Assays
3. Results and Discussion
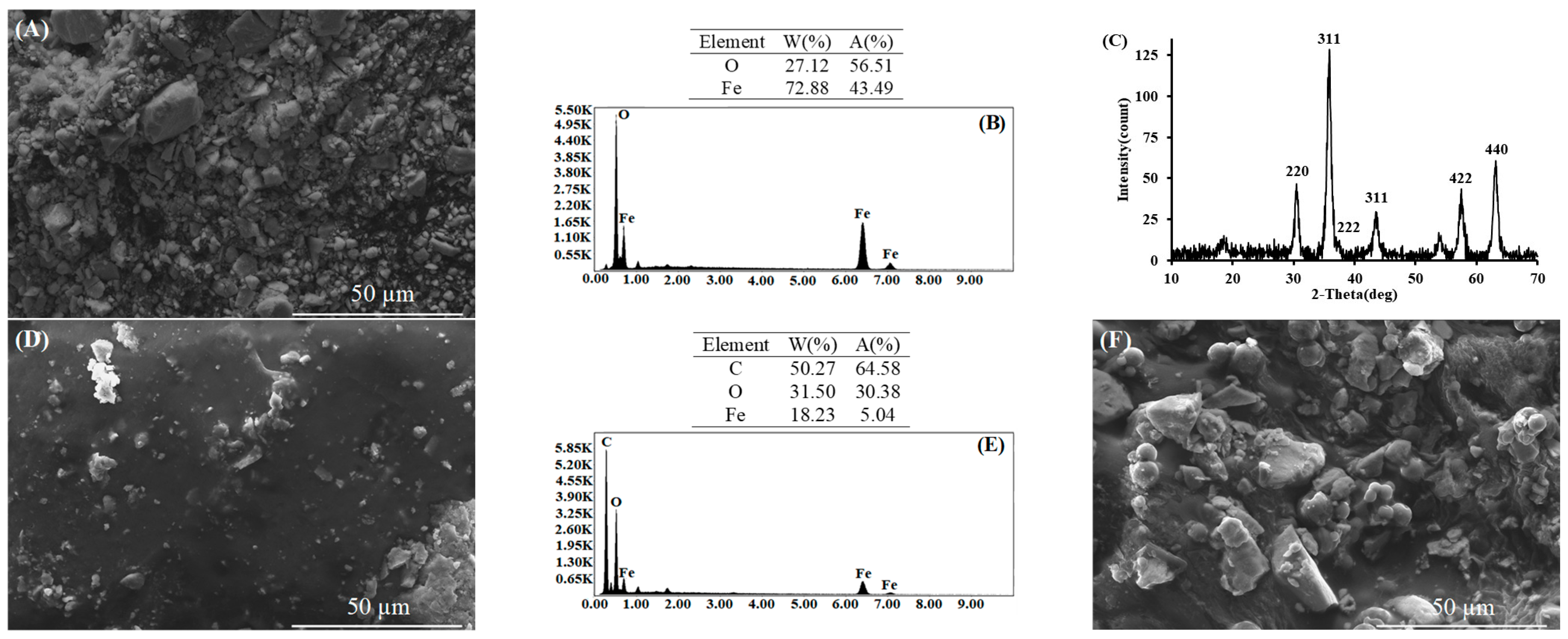
3.1. Characterization of the Materials
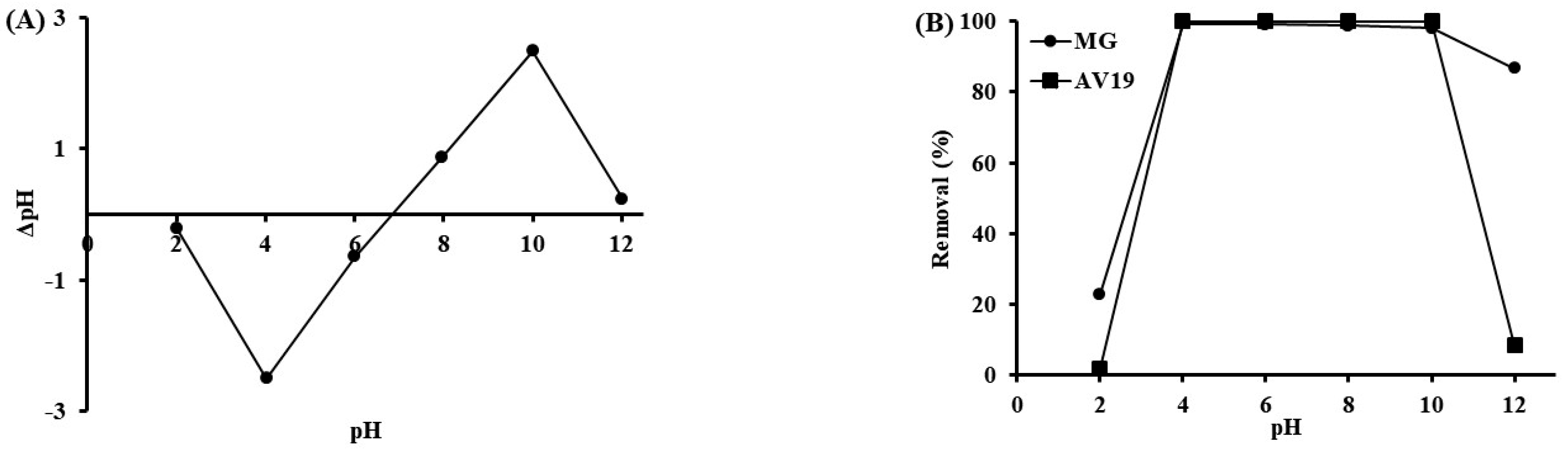
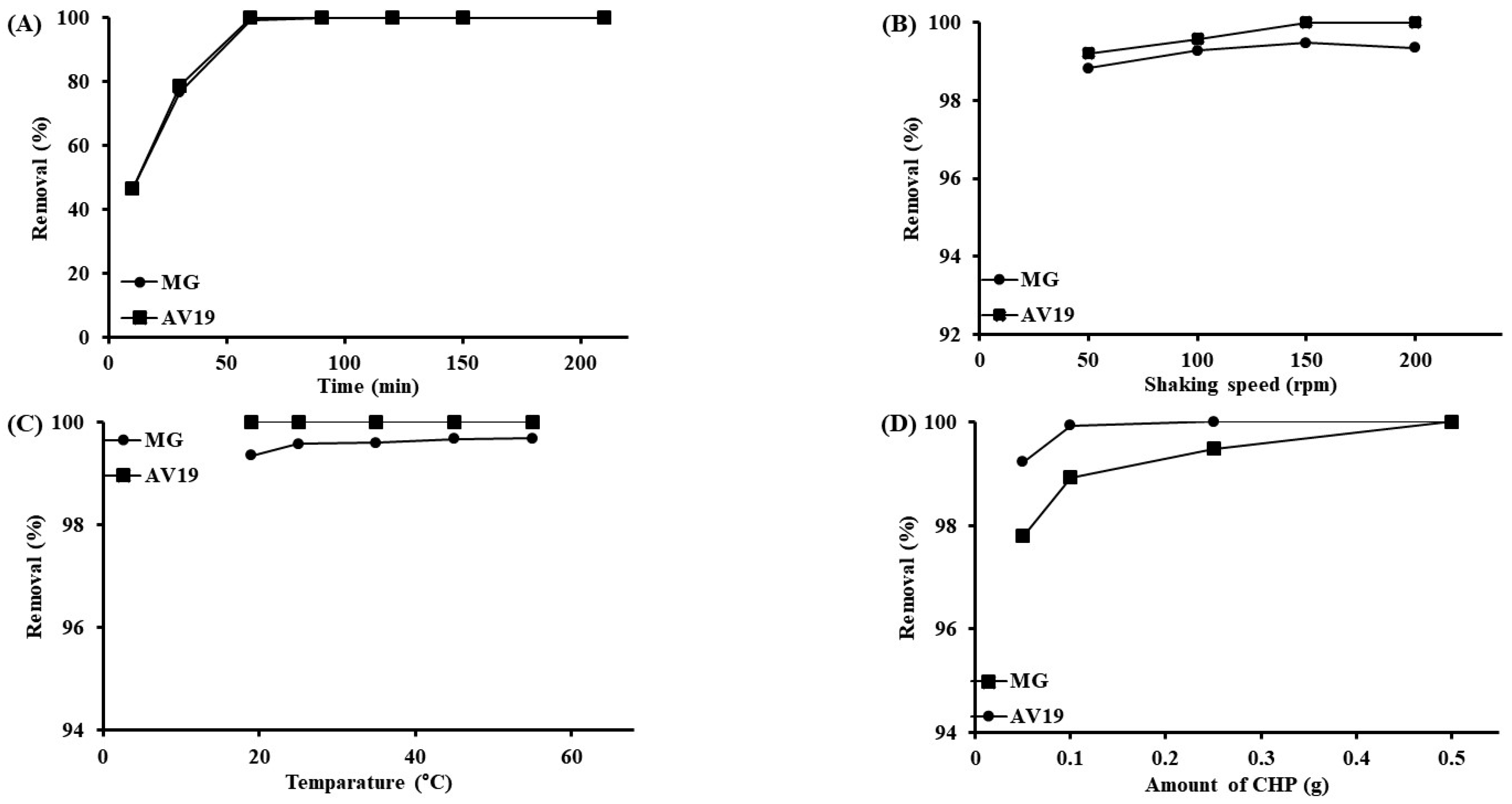
3.2. Effect of Operating Parameters
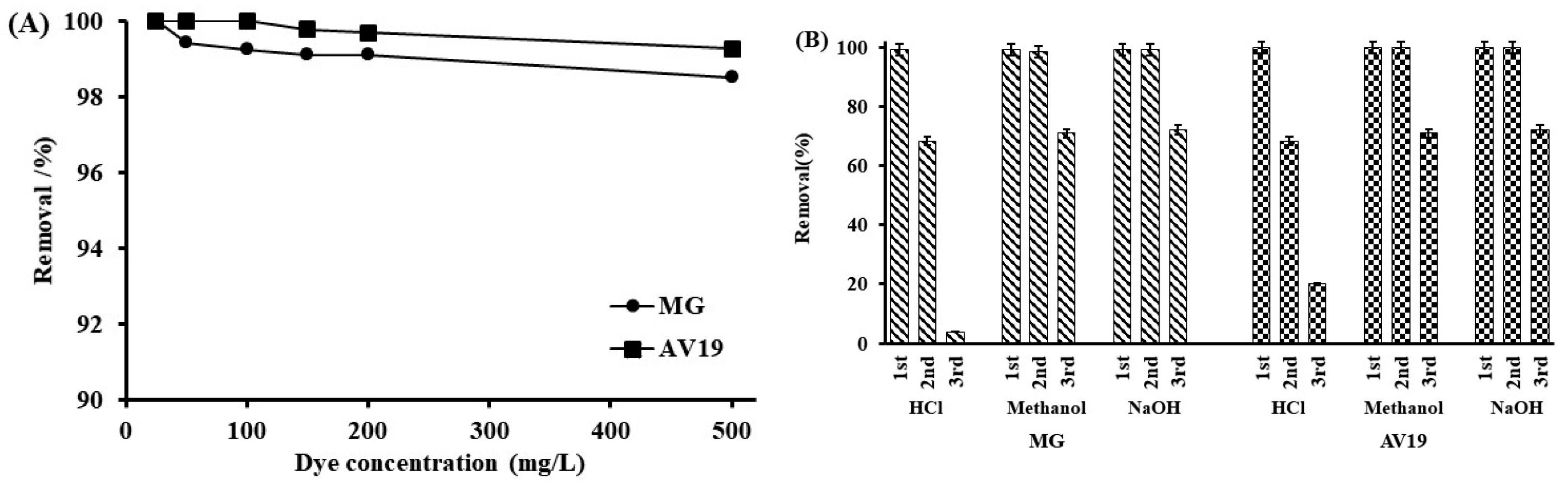
3.3. Kinetic and Isotherm Parameters
3.4. Comparison with Similar Studies
3.5. Antibacterial Activity of CHP
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dehkordi, N.K.; Shojaei, S.; Asefnejad, A.; Hassani, K.; Benisi, S.Z. The effect of three types of cross-linked hydrogels and volume fraction of polyacrylamide on the swelling and thermal behavior using molecular dynamics simulation. J. Mater. Res. Technol. 2023, 24, 4627–4638. [Google Scholar] [CrossRef]
- Lu, K.; Lan, X.; Folkersma, R.; Voet, V.S.D.; Loos, K. Borax Cross-Linked Acrylamide-Grafted Starch Self-Healing Hydrogels. Biomacromolecules 2024, 25, 8026–8037. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Chen, S.; Luo, Y. Adsorption mechanisms of hydrogels for heavy metal and organic dyes removal: A short review. J. Agric. Food Res. 2023, 12, 100552. [Google Scholar] [CrossRef]
- Lellis, B.; Fávaro-Polonio, C.Z.; Pamphile, J.A.; Polonio, J.C. Effects of textile dyes on health and the environment and bioremediation potential of living organisms. Biotechnol. Res. Innov. 2019, 3, 275–290. [Google Scholar] [CrossRef]
- Shahi, Z.; Khajeh Mehrizi, M.; Mirbagheri Firoozabad, M.S. Decolorization of textile dye by isolated bacterial strains from textile waste and their ability to produce polyhydroxyalkanoate. Prog. Color. Colorants Coat. 2025, 18, 99–111. [Google Scholar] [CrossRef]
- Njuguna, D.G.; Schönherr, H. Xanthan gum hydrogels as high-capacity adsorbents for dye removal. ACS Appl. Polym. Mater. 2021, 3, 3142–3152. [Google Scholar] [CrossRef]
- Jain, P.; Sahoo, K.; Mahiya, L.; Ojha, H.; Trivedi, H.; Parmar, A.S.; Kumar, M. Color removal from model dye effluent using PVA-GA hydrogel beads. J. Environ. Manag. 2021, 281, 111797. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Li, R.; Wang, X.; Li, M.; Yang, W. A superabsorbent hydrogel for removal of dyes from aqueous solution. J. Polym. Environ. 2022, 30, 3327–3339. [Google Scholar] [CrossRef]
- Lin, X.; Liu, Z.; Chen, R.; Hou, Y.; Lu, R.; Li, S.; Ren, S.; Gao, Z. A multifunctional polyacrylamide/chitosan hydrogel for dyes adsorption and metal ions detection in water. Int. J. Biol. Macromol. 2023, 246, 125613. [Google Scholar] [CrossRef]
- Halyal, U.A.; Pal, S.; Sharma, V.K.; Tyagi, R.; Yusuf, M. Adsorption and Kinetic Studies of Polyacrylamide (PAA) Hydrogels for Efficient Removal of Methylene Blue (MB) in Aqueous Media. Biointerface Res. Appl. Chem. 2023, 13, 570. [Google Scholar] [CrossRef]
- Faizan, S.; Bakhtawara, M.; Fatima, M.; Ayub, R.; Ul-Ain, Q.; Shafique, S. Optimizing the % swelling and removal of carcinogenic dye from wastewater using polyacrylamide-based hydrogels. J. Dispers. Sci. Technol. 2024, 1–14. [Google Scholar] [CrossRef]
- Shaki, H. Evaluation of removal of acid dyes using PAM-FeSO4 hybrid polymer as flocculant. Pigment. Resin Technol. 2023, 52, 302–309. [Google Scholar] [CrossRef]
- Salama, A. New sustainable hybrid material as adsorbent for dye removal from aqueous solutions. J. Colloid Interface Sci. 2017, 487, 348–353. [Google Scholar] [CrossRef]
- Deb, A.; Debnath, A.; Bhattacharjee, N.; Saha, B. Ultrasonically enhanced dye removal using conducting polymer functionalised ZnO nanocomposite at near neutral pH: Kinetic study, isotherm modelling and adsorbent cost analysis. Int. J. Environ. Anal. Chem. 2022, 102, 8055–8074. [Google Scholar] [CrossRef]
- Scaria, J.; Gopinath, A.; Nidheesh, P.V. A versatile strategy to eliminate emerging contaminants from the aqueous environment: Heterogeneous Fenton process. J. Clean. Prod. 2021, 278, 124014. [Google Scholar] [CrossRef]
- Gao, L.; Lu, Y.; Chen, S.; Ma, X.; Zhao, W. Fe3O4 Nanoparticle/Hyper-Cross-Linked Polymer Composites for Dye Removal. ACS Appl. Nano Mater. 2024, 7, 9960–9967. [Google Scholar] [CrossRef]
- Mu, B.; Tang, J.; Zhang, L.; Wang, A. Facile fabrication of superparamagnetic graphene/polyaniline/Fe3O4 nanocomposites for fast magnetic separation and efficient removal of dye. Sci. Rep. 2017, 7, 5347. [Google Scholar] [CrossRef]
- Ali, H.; Ismail, A.M. Fabrication of Magnetic Fe3O4/Polypyrrole/Carbon Black Nanocomposite for Effective Uptake of Congo Red and Methylene Blue Dye: Adsorption Investigation and Mechanism. J. Polym. Environ. 2023, 31, 976–998. [Google Scholar] [CrossRef]
- Huaman, M.A.L.; Manco, A.E.Q.; López, F.d.L.M.; Carrasco, R.L.A.; Chacón, A.M.L.; Khan, S. Removal of methylene blue dye from water with Fe3O4/poly(HEMA-co-AMPS) magnetic hydrogels. Results Chem. 2024, 7, 101454. [Google Scholar] [CrossRef]
- Jiang, R.; Shen, T.-T.; Zhu, H.-Y.; Fu, Y.-Q.; Jiang, S.-T.; Li, J.-B.; Wang, J.-L. Magnetic Fe3O4 embedded chitosan–crosslinked-polyacrylamide composites with enhanced removal of food dye: Characterization, adsorption and mechanism. Int. J. Biol. Macromol. 2023, 227, 1234–1244. [Google Scholar] [CrossRef]
- Keskin, C.S. Removal of disperse blue 56 dye by Fe3O4 embedded acrylamide based polymer. SAÜ Fen. Bilim. Enstitüsü Derg. 2017, 21, 363–371. [Google Scholar] [CrossRef][Green Version]
- Keskin, C.S. Arsenic and molybdenum ions removal by Fe3O4 embedded acrylamide—Triazine hybrid—Polymer. Rev. Roum. Chim. 2017, 62, 139–148. [Google Scholar][Green Version]
- Keskin, C.S. Rapid Removal of Cd2+, Pb2+ and Cr3+ by Polymer/Fe3O4 Composite. J. Water Chem. Technol. 2019, 41, 299–306. [Google Scholar] [CrossRef]
- Batra, B.; Lata, S.; Pundir, C.S. Construction of an improved amperometric acrylamide biosensor based on hemoglobin immobilized onto carboxylated multi-walled carbon nanotubes/iron oxide nanoparticles/chitosan composite film. Bioprocess Biosyst. Eng. 2013, 36, 1591–1599. [Google Scholar] [CrossRef]
- Özdemir, A.; Keskin, C.S. Development of a new sensor material based on crystalline colloidal arrays embedded into polymer network. J. Anal. Chem. 2011, 66, 763–769. [Google Scholar] [CrossRef]
- Asher, S.A.; Sharma, A.C.; Goponenko, A.V.; Ward, M.M. Photonic crystal aqueous metal cation sensing materials. Anal. Chem. 2003, 75, 1676–1683. [Google Scholar] [CrossRef] [PubMed]
- Al-Gaashani, R.; Pasha, M.; Jabbar, K.A.; Shetty, A.R.; Baqiah, H.; Mansour, S.; Kochkodan, V.; Lawler, J. Antimicrobial activity of ZnO-Ag-MWCNTs nanocomposites prepared by a simple impregnation–calcination method. Sci. Rep. 2023, 13, 21418. [Google Scholar] [CrossRef]
- Yılmazer Keskin, S.; Avcı, A.; Arif Ali Ali, L.; Keskin, C.S. Analysis of Polyphenols from Polygala major Jacq. Antioxidants 2025, 14, 153. [Google Scholar] [CrossRef]
- Prabakaran, R.; Arivoli, S. Thermodynamic and isotherm analysis on the removal of malachite green dye using thespesia populnea bark. J. Chem. 2012, 9, 2575–2588. [Google Scholar] [CrossRef]
- Alshamrani, A.A.; Raju, R.; Ellakwa, A. Effect of printing layer thickness and postprinting conditions on the flexural strength and hardness of a 3D-printed resin. Biomed Res. Int. 2022, 2022, 8353137. [Google Scholar] [CrossRef]
- Fu, J.; Liu, W.; Hao, Z.; Wu, X.; Yin, J.; Panjiyar, A.; Liu, X.; Shen, J.; Wang, H. Characterization of a low shrinkage dental composite containing bismethylene spiroorthocarbonate expanding monomer. Int. J. Mol. Sci. 2014, 15, 2400–2412. [Google Scholar] [CrossRef] [PubMed]
- Narkbuakaew, T.; Sujaridworakun, P. Synthesis of tri-s-triazine based g-C3N4 photocatalyst for cationic rhodamine b degradation under visible light. Top. Catal. 2020, 63, 1086–1096. [Google Scholar] [CrossRef]
- Patil, M.R.; Shrivastava, V.S. Adsorption removal of carcinogenic acid violet19 dye from aqueous solution by polyaniline-Fe2O3 magnetic nanocomposite. J. Mater. Environ. Sci. 2015, 6, 11–21. [Google Scholar]
- Sha, X.; Sheng, X.; Zhou, Y.; Wang, B.; Liu, Y.; Bao, J. High Catalytic Performance of Mesoporous Dual Brønsted Acidic Ternary Poly (Ionic Liquids) for Friedel-Crafts Alkylation. Appl. Organomet. Chem. 2019, 33, e5180. [Google Scholar] [CrossRef]
- Yang, K.; Peng, H.; Wen, Y.; Li, N. Re-examination of characteristic FTIR spectrum of secondary layer in bilayer oleic acid-coated Fe3O4 nanoparticles. Appl. Surf. Sci. 2010, 256, 3093–3097. [Google Scholar] [CrossRef]
- Xiong, Y.; Qin, Y.; Su, L.; Ye, F. Bioinspired Synthesis of Cu2+-Modified Covalent Triazine Framework: A New Highly Efficient and Promising Peroxidase Mimic. Chem.—A Eur. J. 2017, 23, 11037–11045. [Google Scholar] [CrossRef]
- Zhuang, L.; Zhang, W.; Zhao, Y.; Shen, H.; Lin, H.; Liang, J. Preparation and characterization of Fe3O4 particles with novel nanosheets morphology and magnetochromatic property by a modified solvothermal method. Sci. Rep. 2015, 5, 9320. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.S.; McKay, G. The kinetics of sorption of basic dyes from aqueous solution by sphagnum moss peat. Can. J. Chem. Eng. 1998, 76, 822–827. [Google Scholar] [CrossRef]
- Popoola, L.T. Characterization and adsorptive behaviour of snail shell-rice husk (SS-RH) calcined particles (CPs) towards cationic dye. Heliyon 2019, 5, e01153. [Google Scholar] [CrossRef] [PubMed]
- Zahuri, A.A.; Abdul Patah, M.F.; Kamarulzaman, Y.; Hashim, N.H.; Thirumoorthi, T.; Wan Mohtar, H.M.W.; Mohd Hanafiah, Z.; Amir, Z.; Wan-Mohtar, W.A.A.Q.I. Decolourisation of Real Industrial and Synthetic Textile Dye Wastewater Using Activated Dolomite. Water 2023, 15, 1172. [Google Scholar] [CrossRef]
- El-Khawaga, A.M.; Orlandini, M.; Raucci, L.; Elmaghraby, K. Magnetic nanoparticles as a promising antimicrobial agent for combating multidrug resistant bacteria: A review. Discov. Appl. Sci. 2025, 7, 652. [Google Scholar] [CrossRef]
- Yang, R.; Liang, B.; Han, D.; Guo, Z.; Yang, C.; Yang, J.; Qiu, Y.; Li, Q.; Guo, S.; Shi, J.; et al. Synthesis and antibacterial activity of magnetic Fe3O4-loaded silver nanocomposites. J. Alloys Compd. 2024, 973, 172849. [Google Scholar] [CrossRef]
- Allafchian, A.; Hosseini, S.S. Antibacterial magnetic nanoparticles for therapeutics: A review. IET Nanobiotechnol. 2019, 13, 786–799. [Google Scholar] [CrossRef] [PubMed]
- Prameela, P.; Ramana Ramya, J.; Gajendiran, J.; Gnanam, S.; Ramachandran, K.; Gokul Raj, S.; Ramesh Kumar, G. Structural, magnetic, and antibacterial activity of the pure, Zn-doped, and Zn-doped/sugar-assisted co-precipitation synthesized semicrystalline Co3O4 compound. J. Mol. Struct. 2023, 1292, 136154. [Google Scholar] [CrossRef]
- Sharaf, E.M.; Hassan, A.; AL-Salmi, F.A.; Albalwe, F.M.; Albalawi, H.M.R.; Darwish, D.B.; Fayad, E. Synergistic antibacterial activity of compact silver/magnetite core-shell nanoparticles core shell against Gram-negative foodborne pathogens. Front. Microbiol. 2022, 13, 929491. [Google Scholar] [CrossRef] [PubMed]
- Abdulsada, F.M.; Hussein, N.N.; Sulaiman, G.M.; Al Ali, A.; Alhujaily, M. Evaluation of the Antibacterial Properties of Iron Oxide, Polyethylene Glycol, and Gentamicin Conjugated Nanoparticles against Some Multidrug-Resistant Bacteria. J. Funct. Biomater. 2022, 13, 138. [Google Scholar] [CrossRef]
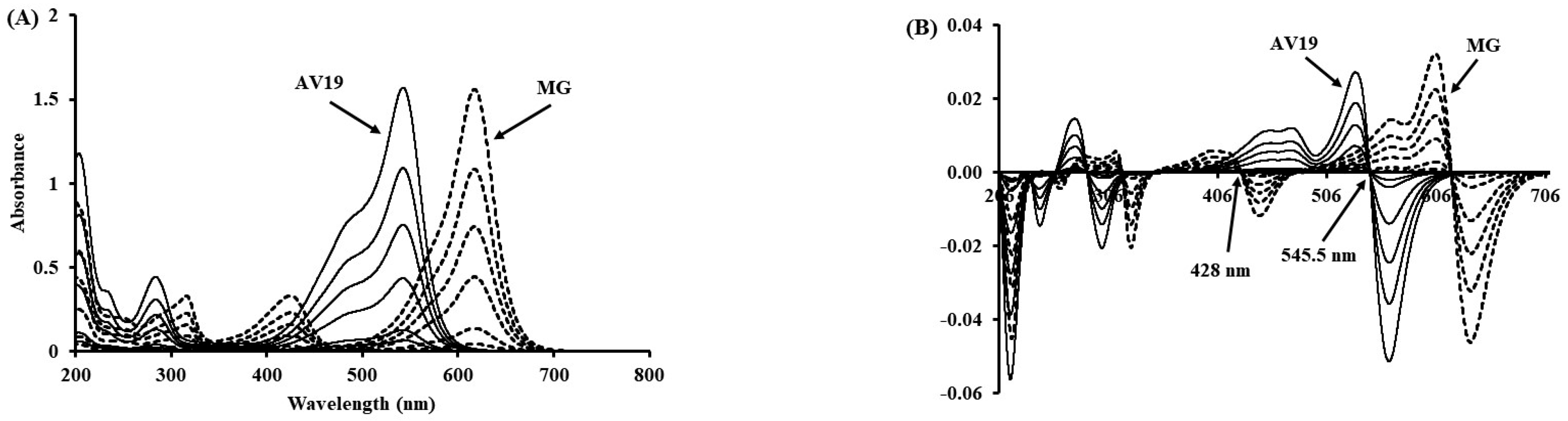
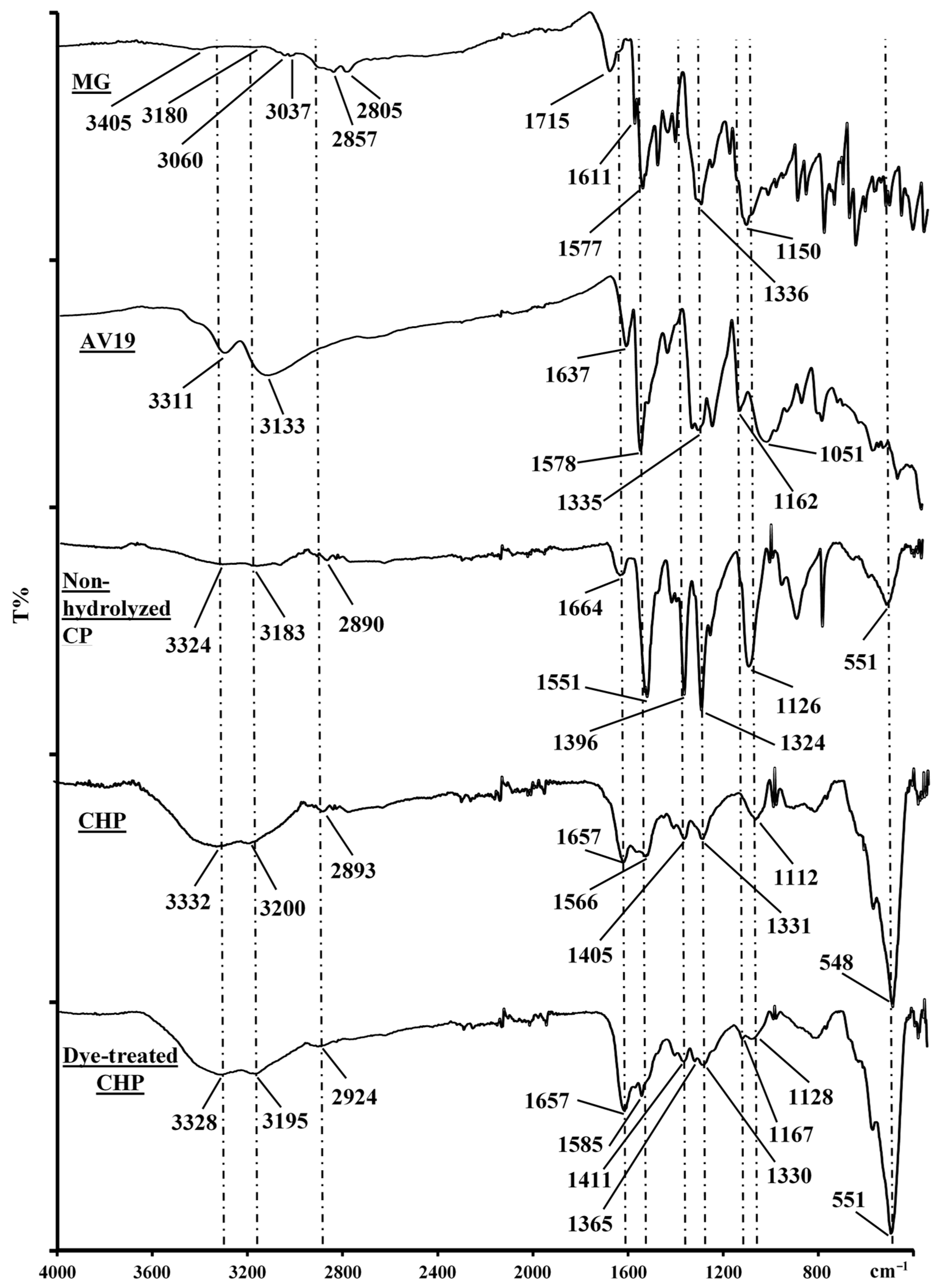
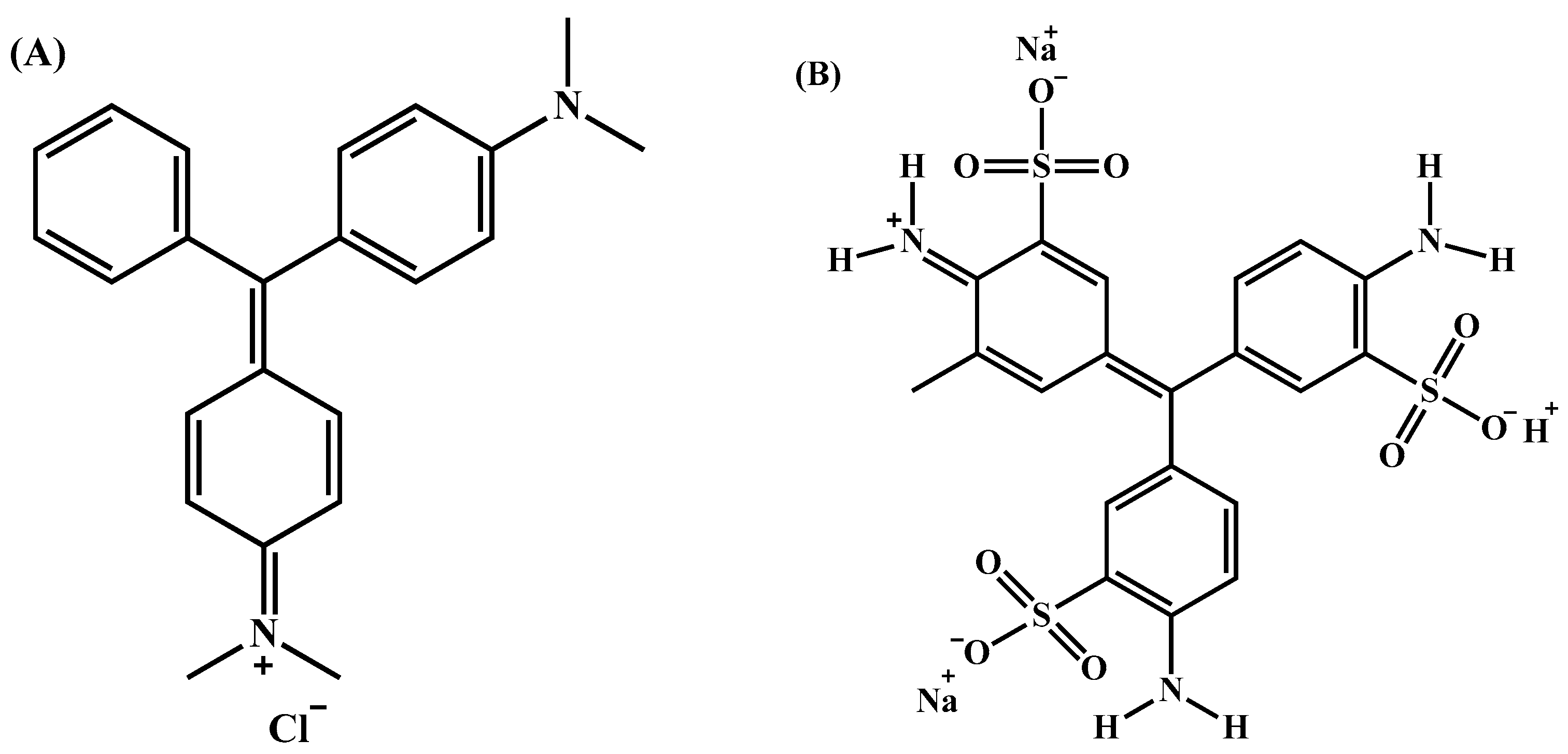
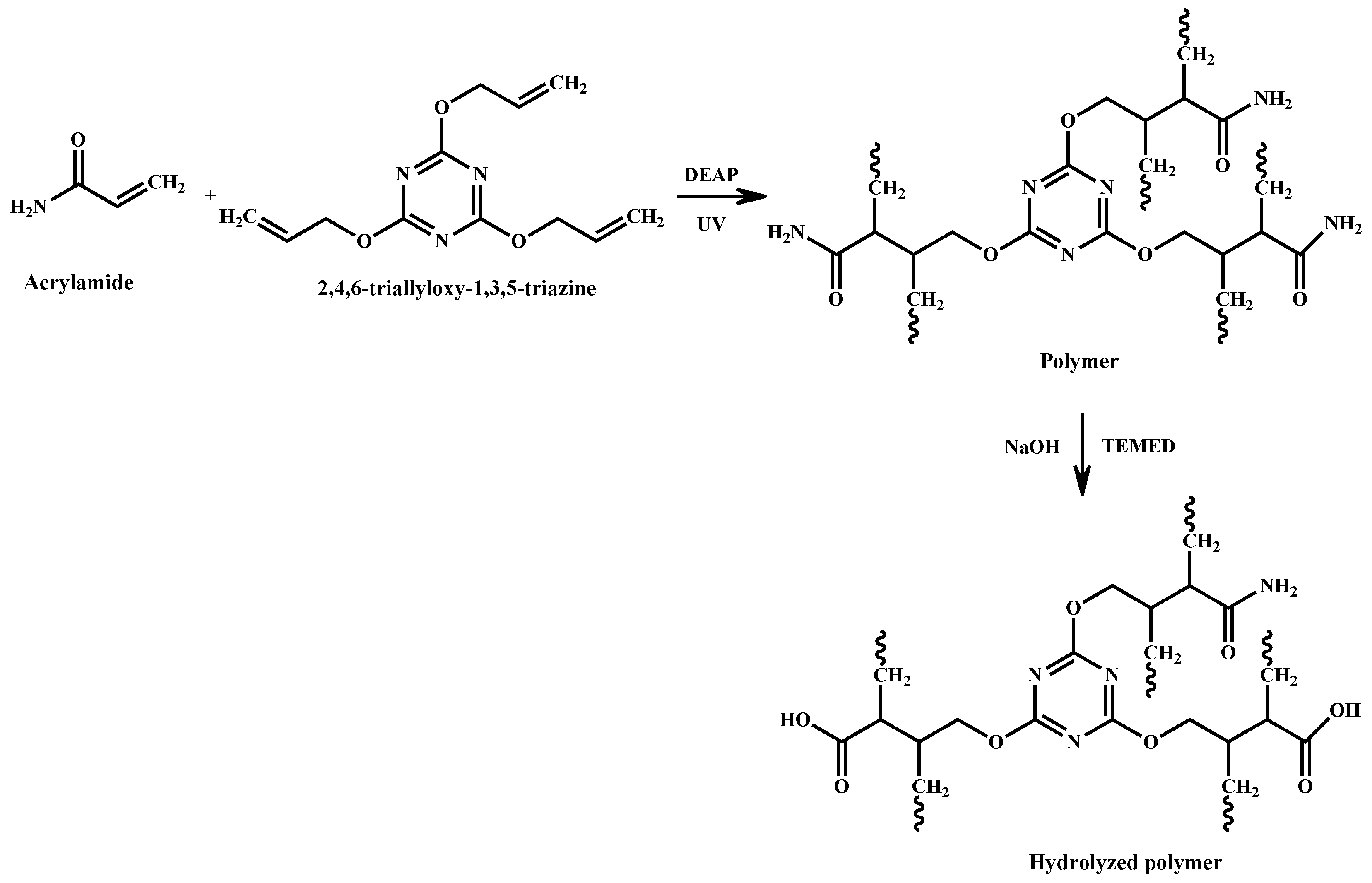


| Dye | λ (nm) | Calibration Equations | R2 | LOD (mg/L) | LOQ (mg/L) |
|---|---|---|---|---|---|
| AV19 | 428.0 | A = 0.0007CAV19 − 0.0002 | 0.9996 | 0.0754 | 0.2283 |
| MG | 545.5 | A = 0.0009CMG − 0.0001 | 1.0000 | 0.0305 | 0.0924 |
| Mixture | Added (mg/L) | Found (mg/L) | Recovery (%) | |||
|---|---|---|---|---|---|---|
| AV19 | MG | AV19 | MG | AV19 | MG | |
| 1 | 3.00 | 1.00 | 3.13 | 0.98 | 104.33 | 98.00 |
| 2 | 5.00 | 1.00 | 5.11 | 0.96 | 102.20 | 96.00 |
| 3 | 7.00 | 1.00 | 7.14 | 0.98 | 102.00 | 98.00 |
| 4 | 10.00 | 1.00 | 10.28 | 1.03 | 102.80 | 103.00 |
| 5 | 1.00 | 3.00 | 1.03 | 2.98 | 103.00 | 99.33 |
| 6 | 5.00 | 3.00 | 5.21 | 2.92 | 104.20 | 97.33 |
| 7 | 7.00 | 3.00 | 7.24 | 3.09 | 103.43 | 103.00 |
| 8 | 10.00 | 3.00 | 10.26 | 3.02 | 102.60 | 100.67 |
| 9 | 1.00 | 5.00 | 1.00 | 5.02 | 100.00 | 100.40 |
| 10 | 3.00 | 5.00 | 2.88 | 4.94 | 96.00 | 98.80 |
| 11 | 7.00 | 5.00 | 7.19 | 5.20 | 102.71 | 104.00 |
| 12 | 10.00 | 5.00 | 10.34 | 5.18 | 103.40 | 103.60 |
| 13 | 1.00 | 7.00 | 0.98 | 6.90 | 98.00 | 98.57 |
| 14 | 3.00 | 7.00 | 2.90 | 6.92 | 96.67 | 98.86 |
| 15 | 5.00 | 7.00 | 5.03 | 7.11 | 100.60 | 101.57 |
| 16 | 10.00 | 7.00 | 10.16 | 7.37 | 101.60 | 105.29 |
| 17 | 1.00 | 10.00 | 0.98 | 10.03 | 98.00 | 100.30 |
| 18 | 3.00 | 10.00 | 2.88 | 10.12 | 96.00 | 101.20 |
| 19 | 5.00 | 10.00 | 4.91 | 10.18 | 98.20 | 101.80 |
| 20 | 7.00 | 10.00 | 6.91 | 10.22 | 98.71 | 102.20 |
| 21 | 1.00 | 1.00 | 1.02 | 0.98 | 102.00 | 98.00 |
| 22 | 3.00 | 3.00 | 3.03 | 2.95 | 101.00 | 98.33 |
| 23 | 5.00 | 5.00 | 5.08 | 5.02 | 101.60 | 100.40 |
| 24 | 7.00 | 7.00 | 7.09 | 7.20 | 101.29 | 102.86 |
| 25 | 10.00 | 10.00 | 10.06 | 10.41 | 100.60 | 104.10 |
| Mean | 100.84 | 100.62 | ||||
| SD | 2.45 | 2.42 | ||||
| RSD% | 2.43 | 2.40 | ||||
| Parameters | MG | AV19 | |
|---|---|---|---|
| Pseudo-first-order kinetic | qe (mg/g) | 94.80 | 156.85 |
| k1 (1/min) | 0.0467 | 0.0526 | |
| R2 | 0.9876 | 0.9616 | |
| Pseudo-second-order kinetic | qe (mg/g) | 90.17 | 118.36 |
| k2 (1/min) | 0.0007 | 0.0006 | |
| R2 | 0.9985 | 0.9986 | |
| Langmuir isotherm | Qm (mg/g) | 105.33 | 112.30 |
| KL (L/mg) | 0.37 | 2.66 | |
| R2 | 0.9983 | 0.9974 | |
| Freundlich isotherm | Kf (mg/g) | 23.30 | 52.41 |
| n | 1.81 | 2.92 | |
| R2 | 0.8811 | 0.8448 | |
| Material | Adsorbate pH | Removal Efficiency | Calculated Adsorption Capacity | Ref. |
|---|---|---|---|---|
| Fe3O4/hyper-cross-linked polymer composites | Crystal Violet/pH 7 | 94.1% | Qm = 104.3 mg/g KF = 58.431 (mg/g)·(L/mg)1/n | [16] |
| Graphene/polyaniline/ Fe3O4 | Congo Red pH 6.3 | 92.4% | Qm = 248.76 mg/g KF = 82.41 | [17] |
| Fe3O4/ polypyrrole/carbon black | Congo Red/pH 7 Methylene Blue/pH 8 | 96.9% 95.9% | Qm = 500 mg/g KF = 120 L/mg Qm = 90.9 mg/g KF = 89.2 L/mg | [18] |
| Fe3O4/poly(2-hydroxyethylmethacrylateco- 2-acrylamido-2-methylpropanesulfonic acid) | Methylene Blue/pH 6.5 | 99.01% | Qm = 445.35 mg/g KF = 95.59 mg/g | [19] |
| Fe3O4 embedded chitosan–crosslinked-polyacrylamide | Sunset yellow pH 2–10 | >99% | Qm = 359.71 mg/g Kf = 33.21 mg1- (1/n) L1/n g−1 | [20] |
| Fe3O4 embedded acrylamide- 1,3,5-Triacryloylhexahydro-1,3,5-triazine polymer | Disperse Blue 56 pH 2 | 96.6% | Qm = 0.9063 mg/g KF = 1.2735 mg/g | [21] |
| Fe3O4/Acrylamide/2,4,6-triallyloxy-1,3,5-triazine composite hydrogel polymer | Malachite Green/pH 4 Acid Violet 19/pH 4 | ~99% ~100% | Qm = 105.33 mg/g KF = 23.30 mg/g Qm = 112.30 mg/g KF = 52.41 mg/g | This study |
| Bacteria | Zone of Inhibition (mm) |
|---|---|
| Pseudomonas aeruginosa | 22.58 ± 0.68 |
| Escherichia coli O157:H7 | 16.63 ± 0.40 |
| Escherichia coli type 1 | 22.48 ± 0.63 |
| Staphylococcus aureus | 23.53 ± 0.55 |
| Listeria monocytogenes | 29.55 ± 0.89 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Erdağı, S.S.; Keskin, C.S.; Yılmazer Keskin, S.; Avcı, A. Removal of Textile Dye Mixture by Fe3O4/Acrylamide/Triacryloylhexahydro Triazine Composite Hydrogel Polymer. Polymers 2025, 17, 2469. https://doi.org/10.3390/polym17182469
Erdağı SS, Keskin CS, Yılmazer Keskin S, Avcı A. Removal of Textile Dye Mixture by Fe3O4/Acrylamide/Triacryloylhexahydro Triazine Composite Hydrogel Polymer. Polymers. 2025; 17(18):2469. https://doi.org/10.3390/polym17182469
Chicago/Turabian StyleErdağı, Sude Sena, Can Serkan Keskin, Semra Yılmazer Keskin, and Ayşe Avcı. 2025. "Removal of Textile Dye Mixture by Fe3O4/Acrylamide/Triacryloylhexahydro Triazine Composite Hydrogel Polymer" Polymers 17, no. 18: 2469. https://doi.org/10.3390/polym17182469
APA StyleErdağı, S. S., Keskin, C. S., Yılmazer Keskin, S., & Avcı, A. (2025). Removal of Textile Dye Mixture by Fe3O4/Acrylamide/Triacryloylhexahydro Triazine Composite Hydrogel Polymer. Polymers, 17(18), 2469. https://doi.org/10.3390/polym17182469








