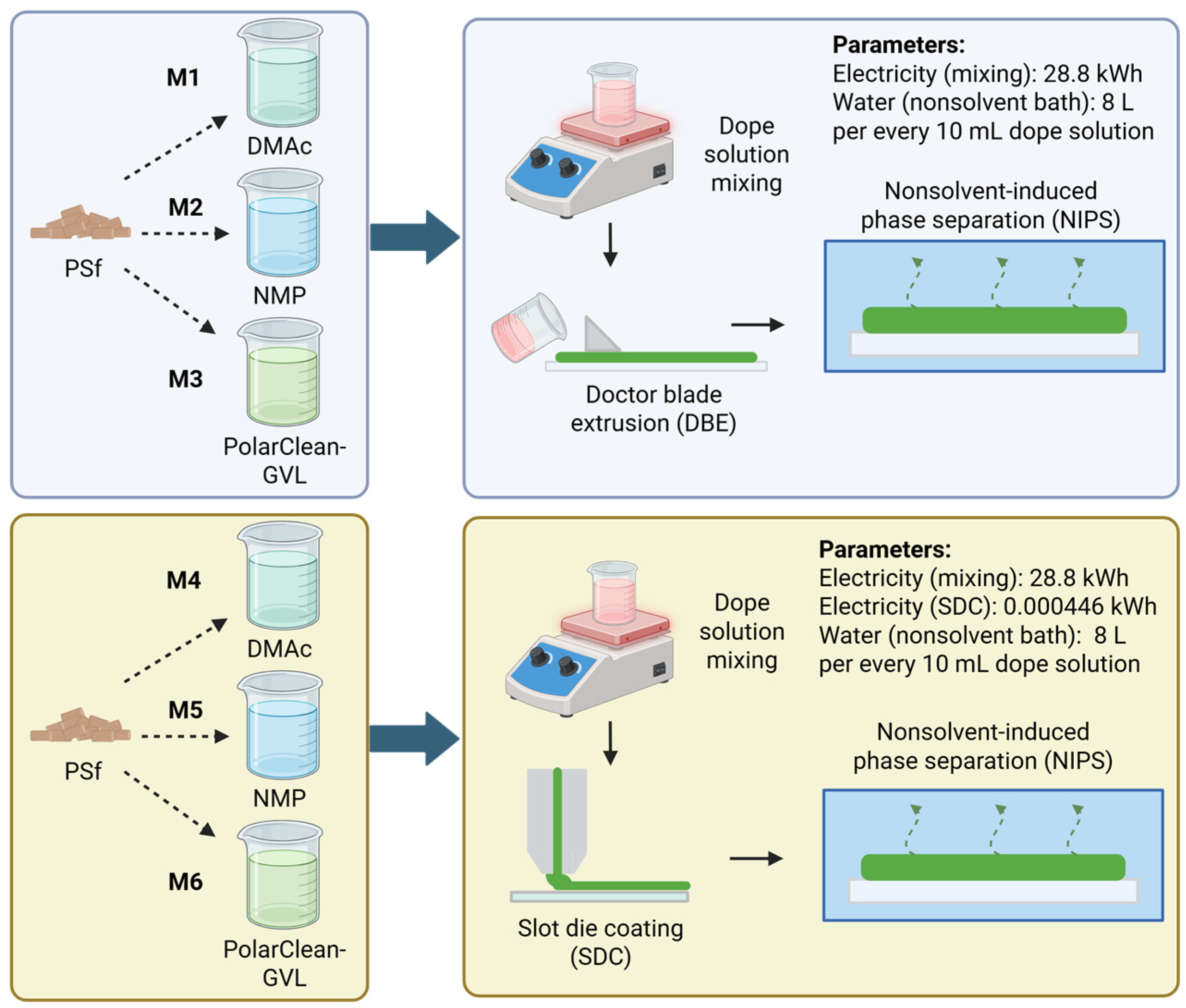
1. Introduction
Organic solvents commonly used in commercial polysulfone membrane fabrication via nonsolvent-induced phase separation are becoming increasingly restricted due to their reported toxic, carcinogenic, irritant, and other operating hazard properties [
1]. These solvents, including dimethylacetamide (DMAc), dimethylformamide (DMF), and N-methyl-2-pyrrolidone (NMP) as commonly used examples, are also derived from fossil fuel sources that limit their sustainability [
1]. In the European Union, DMAc, DMF, and NMP manufacturing and usage are now limited under Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) regulations, including NMP restrictions since 2018, DMF restrictions since 2023, and DMAc restrictions by 2026 [
2,
3,
4]. In 2024, the US Environmental Protection Agency (EPA) announced a proposed rule that would implement similar restrictions on NMP manufacturing, processing, and distribution [
5]. While no timeline for further action has been established, other EPA-led chemical restrictions have occurred within a 5-year timeline [
2].
The growing regulations on commercially available solvents have motivated the emergence of membrane fabrication studies using non-hazardous and eco-friendly solvents. While a myriad of eco-friendly alternatives has been identified, the additional criterion of large-scale production is important to support the economic feasibility of substitution. Among this group of alternatives, methyl 5-(dimethylamino)-2-methyl-5-oxopentanoate (Rhodiasolv
® PolarClean) and γ-valerolactone (GVL), two nontoxic and biodegradable solvents, are considered suitable solvents for producing polysulfone (PSf) membranes. PolarClean is the chemical valorization product of methylglutaronitrile (MGN), a byproduct of nylon-6,6 synthesis [
3]; GVL is produced from levulinic acid sourced from lignocellulosic biomass [
4]. The use of these solvents individually results in PSf membranes hydrolyzing upon formation or experiencing pore collapse during filtration. However, their use in combination has been shown to produce durable PSf membranes with permeability and solute rejection similar to PSf-NMP membranes [
3,
5,
6].
Beyond the lab-scale demonstration of PSf-PolarClean-GVL membrane fabrication, proving the scalability and sustainability of this process is imperative to determining commercial viability. While commonly utilized in lab-scale studies, polymeric membrane fabrication via doctor blade extrusion (DBE) is difficult to scale up and integrate with a roll-to-roll (R2R) system for continuous casting [
7]. Slot die coating (SDC), a manufacturing method commonly used to produce thin films, has been shown to also produce defect-free polymeric membranes and is compatible with an R2R configuration, paving the way for the manufacturing scale-up of PSf membranes with novel materials [
8]. Using SDC, studies by Dong et al. [
7] and Lu et al. [
8] have reported the fabrication of PSf-PolarClean-GVL membranes that possess membrane morphology, surface pore size, total porosity, water permeability, and solute rejection similar to membrane samples prepared via DBE [
7,
8,
9].
Studies on polymeric membrane fabrication using eco-friendly solvents or scalable fabrication techniques have largely focused on investigating membrane properties and performance, though investigating the environmental and health impacts is another key element to evaluating commercial viability. While it may be difficult to definitively assess material and process sustainability, it is possible to perform a systematic analysis of the produced impacts. To this end, a life cycle assessment (LCA) is an environmental management tool for quantifying the environmental and health impacts over the life cycle of a product, process, or activity to inform decision making [
10]. Depending on the assessment, the life cycle can include raw material extraction, manufacturing, distribution, operation, maintenance, and end-of-life stages [
10]. With the development of a standardized methodological framework and terminology by the International Organization for Standardization (ISO 14040 and ISO 14044), the prominence and merit of LCAs have significantly grown [
11,
12]. As part of the ISO-issued framework, a standard LCA must include a goal and scope definition, inventory analysis, life cycle impact assessment, and interpretation [
10]. Using this common methodology, LCAs have increasingly become a core element in product development and environmental policy worldwide, including applications in waste incineration, building materials, and military systems [
13].
Since their standardization, LCAs have also become a tool for investigating the impacts of polymeric membrane fabrication with novel materials. In particular, recent LCAs on membrane fabrication have compared the impacts of membrane fabrication when substituting conventional solvents with eco-friendly alternatives, including ethylene carbonate (EC), ethyl acetate (EA), γ-butyrolactone (GBL), PolarClean, and GVL [
14,
15,
16]. Yadav et al. [
14] reported that conventional solvents heavily contribute to global warming potential, human toxicity, and fossil resource scarcity impacts during membrane fabrication and that substitution with EC significantly can reduce the solvent’s relative contribution to these impacts. Similarly, Hong et al. [
15] found that substituting NMP with GBL or EA reduces membrane fabrication impacts by at least 10%, particularly during dope solution preparation. However, Fionah et al. [
5,
16] have reported that PSf membranes solely prepared with PolarClean exhibit structural deficiencies during water filtration, while those solely prepared with GVL hydrolyze following casting. Follow-up studies have reported the formation of more durable PSf membranes using a 3:1 ratio of PolarClean and GVL, though the changes in impacts when using both have not been studied [
5,
6,
8].
Another limitation in the existing LCA literature is that LCAs in the field of membrane science have only focused on DBE as the fabrication technique. To date, existing LCAs on polymeric membrane fabrication are limited to bench-scale fabrication techniques. While some have calculated the impacts of large-scale membrane production by increasing the functional unit, assessments continue to use DBE as the fabrication technique despite its difficulty for scaled fabrication; in particular, it is difficult to meter without additional tooling when part of an R2R system [
17,
18]. Conversely, LCAs on SDC are limited to applications in solar cell manufacturing [
19,
20,
21,
22]. Zhao et al. [
19] and Vesce et al. [
22] found impacts to be lower by 1–3 magnitudes of order when substituting spin coating with SDC to fabricate perovskite solar cells, though it remains to be seen if applying SDC for polymeric membrane fabrication yields similar results. With the difference in material and machinery input when using SDC, new LCAs are needed to determine if such a scalable technique can still be used to fabricate PSf membranes without creating significantly higher impacts. Thus, the goal of this study is to build on the previous findings of Fionah et al. [
16] by performing an LCA that estimates the global environmental and health impacts of producing PSf-PolarClean-GVL membranes using SDC and comparing them to conventional solvents and the bench-scale DBE technique. The assessment also pinpoints the parameters that have significant impacts and the need for further analysis. Ultimately, the life cycle impacts identified in this LCA can be used to formulate strategies to reduce impacts from the membrane fabrication scale-up process.
3. Results and Discussion
3.1. Influence of Membrane Fabrication on the Life Cycle Inventory
The dope solution dynamic viscosity measurements were collected using a rheometer (Rheometer DHR3, TA instruments, New Castle, DE, USA) at ambient temperature and over a shear range of 0–1000 s
−1, which was based on procedures from a previous study [
6]. In both fabrication techniques, the dope solution viscosity significantly influenced the size of the membrane sheets produced. At a constant shear rate of 27 s
−1, dynamic viscosities of 17 wt.% PSf-DMAc, PSf-NMP, and PSf-PolarClean-GVL dope solutions were found to measure 0.069 Pa·s, 0.495 Pa·s, and 3.147 Pa·s, respectively. The viscosity results were consistent with those previously reported over shear rates of 1–10 s
−1, indicating enhanced polymer swelling as PSf mixed with eco-friendly solvents across a range of shear rates [
5,
6].
During DBE, a uniform shear force was applied to spread the cast solution across the glass plate to form the membrane. When casting the PSf-DMAc and PSf-NMP dope solutions, the low viscosity allowed the solutions to be spread further across the plate and produce larger membranes. In contrast, the membrane samples produced using the PSf-PolarClean-GVL dope solution were smaller due to the viscosity creating greater resistance during casting. The variation in membrane thickness was evident with samples ranging in thickness between approximately 0.8 and 1.0 mm, in which the solutions with PolarClean and GVL were thinner than those with DMAc and NMP. Thus, a larger material quantity was required to produce the functional unit for the PSf-PolarClean-GVL membranes. For this study, the spreading of the dope solution played a major role in determining the material inventory, since the functional unit was set on a surface area basis; while consistent with other LCAs on membrane fabrication, this basis does not take the membrane thickness into account, which would also influence the material inventory.
Additionally, the solution viscosity influenced the quantity of surface defects that formed on the membranes and residual solution left on the doctor blade. Based on casting experiments pictured in
Figure S1 and summarized in
Table S9, the dope solution viscosity was inversely related to the defective area, total defect dope solution volume, residual solution volume, and the material loss factor: 1.517, 1.133, and 1.0779 for M1, M2, and M3, respectively. This trend was consistent with reported literature on membrane fabrication, in which wrinkles were evidence of membrane shrinkage due to the solvent-nonsolvent de-mixing; as viscous solutions exhibit delayed de-mixing, shrinkage and wrinkle formation were minimized [
33]. Ismail et al. [
34] and Chung et al. [
35] also reported that high dope solution viscosity was due to extensive polymer chain entanglement and essential to producing membranes with minimum defects. Thus, multiplying each material amount with the corresponding factor provided a more realistic material inventory for membrane fabrication by accounting for the formation of both viable and defective regions, with the latter ultimately being removed from the sheet. Despite M1 and M2 exhibiting greater areas of defects, the dope solution for M3 still required the largest solution amount and indicated that the total amount of required solution for M3 was greater than the required amount to compensate for defective areas of M1 and M2. As listed in
Table S10, M1, M2, and M3 required dope solution amounts of 0.131 kg, 0.152 kg, and 0.307 kg, respectively.
A similar trend was found when developing the material inventories for membranes produced via SDC. As the coating bead must first reach a steady state to form a viable membrane, an initial portion of the solution was allocated for tool fluid loss, which was considered analogous to the solution volume that formed defective membrane areas and the residual dope solution volume during DBE. From lab-scale coating experiments, tool fluid loss amounts for M4, M5, and M6 were 9.4 g, 11.89 g, and 21.01 g, respectively. The relatively high viscosity of the PSf-PolarClean-GVL dope solution required increased pressure and solution to fill the SDC reservoir before exiting the slot, thereby increasing its tool fluid loss. When combined with the dope solution amounts for producing the membrane sheet and scaled to the functional unit, 0.913 kg, 0.979 kg, and 1.273 kg were required for M4, M5, and M6, respectively (
Table S10). With both fabrication techniques, the larger dope solution amount due to viscosity resulted in larger material inventory amounts, potentially increasing the impacts.
3.2. Parameter Relative Contributions to Membrane Fabrication Impacts
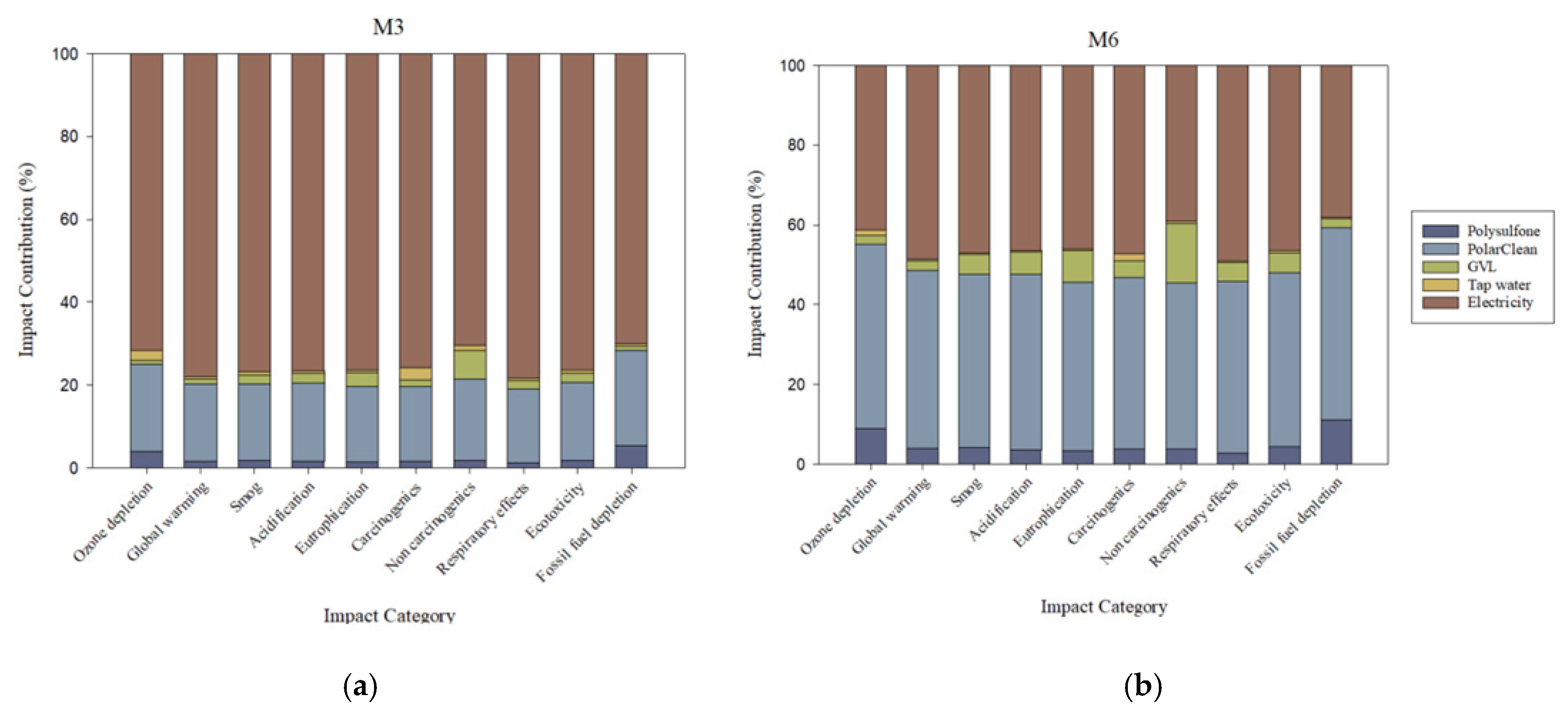
The relative contributions of each material to impacts from fabricating PSf-PolarClean-GVL membranes are presented in
Figure 2. For comparison, relative material impact contributions from fabricating PSf-DMAc and PSf-NMP membranes are presented in
Figure S2. Across all membranes, electricity followed by the solvent(s) and PSf were the main contributing parameters in all 10 categories. Among the three parameters, electricity was the most dominant in contributions, accounting for approximately 70% of impacts in M3 and at least 90% in M1 and M2 with respect to all 10 categories. Of the solvents used in DBE membranes, PolarClean exhibited the highest contributions and accounted for approximately 20% of all impacts, whereas DMAc and NMP only contributed up to 10% in most impact categories (shown in
Figure S2). This disparity between the eco-friendly and conventional solvents was partially attributed to the larger quantity of PolarClean required to produce the functional unit of the membrane, though the impacts of PolarClean synthesis also played a role, as discussed later. Across M1, M2, and M3, contributions due to PSf were approximately 5% at most for each category.
When SDC was used, the additional electricity input for the equipment (0.000446 kWh) did not result in a notable increase in impacts in each category. In fact, the increased quantity of solvents caused the solvent relative contribution to rise and the electricity relative contribution to decline across all categories. Most electricity input from the fabrication process was attributed to solution mixing (28.8 kWh), indicating that the energy input was most significant for dope solution preparation and negligible for the coating process. Impacts from electricity in all categories were attributed to the global energy mix being primarily comprised of fossil fuel sources (e.g., coal, oil, and natural gas) that produce environmental emissions from extraction and combustion, as well as additional health impacts from toxic materials released during extraction [
36].
3.3. Membrane Fabrication Impacts Comparison
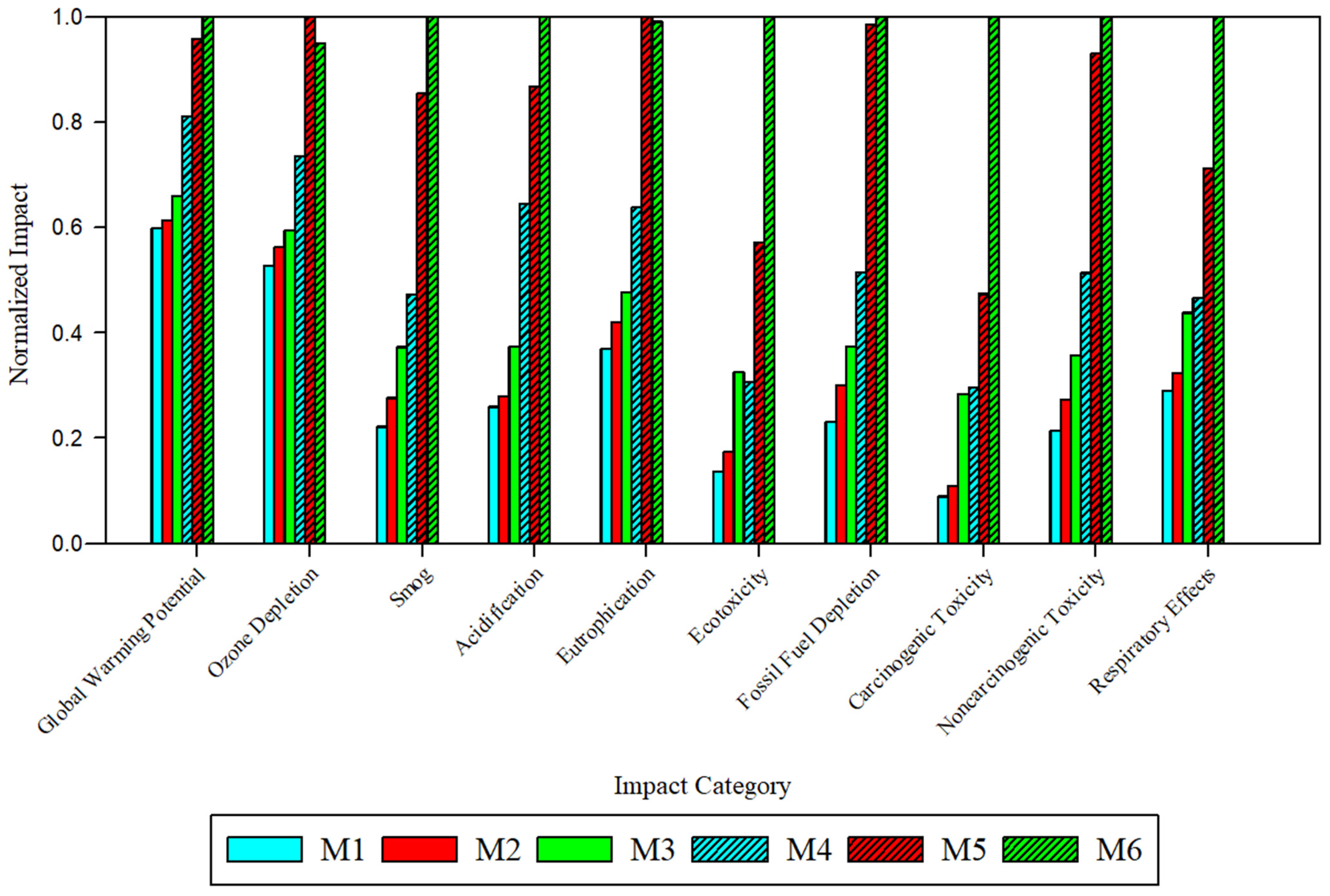
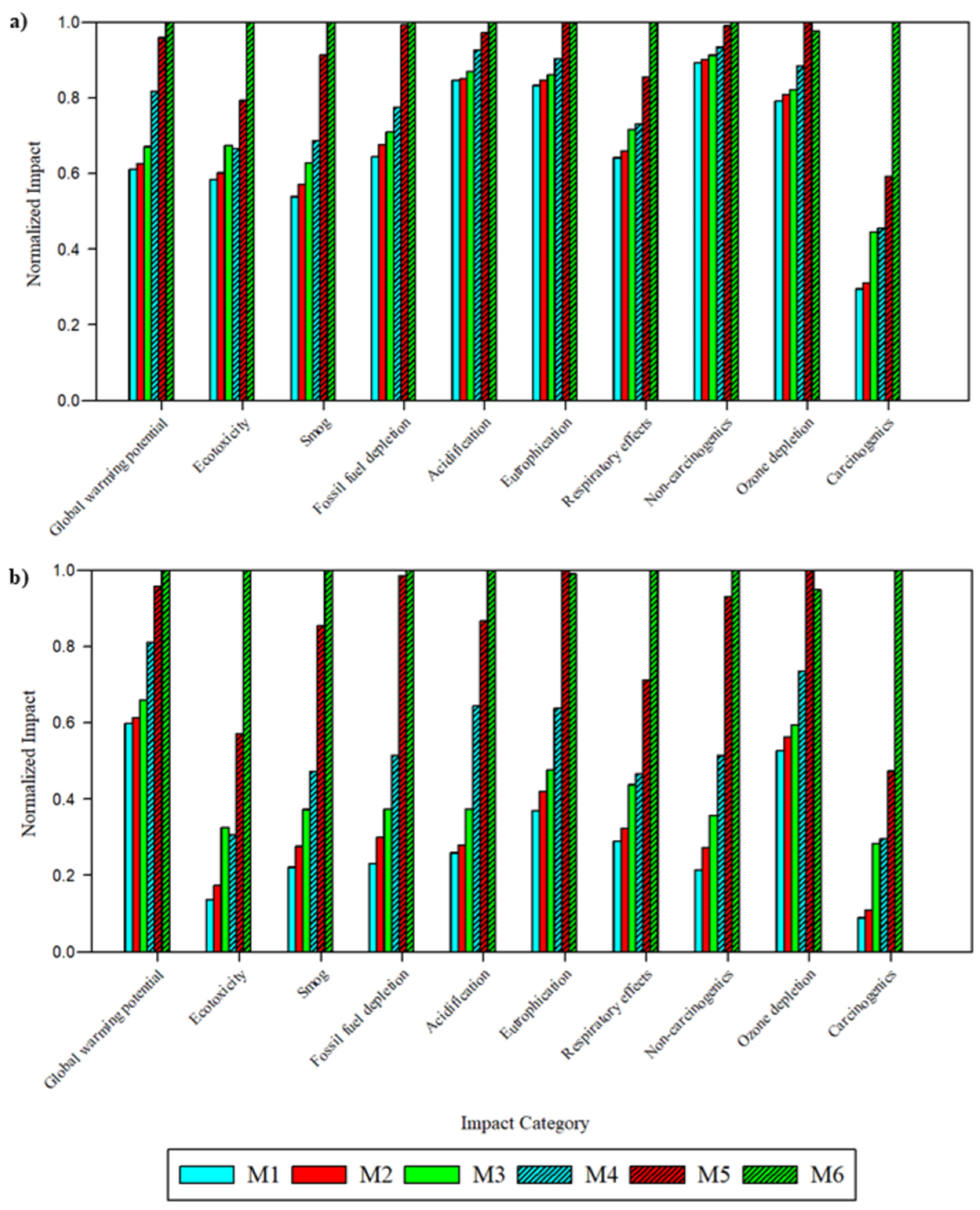
To compare the impacts of membrane fabrication, the global environmental and health impacts were normalized to the fabrication of 1 m
2 of viable flat sheet membrane (
Figure 3,
Table S12). Across all ten impact categories, the solvent and fabrication technique type produced notable changes to the magnitude of impacts. Across all ten categories, M5 and M6 exhibited the highest impacts. M6, derived from PolarClean and GVL and fabricated via SDC, exhibited the highest impacts across 8 categories: global warming potential (2.6 × 10
−6 kg CO
2 eq), smog (1.7 kg O
3 eq), acidification (0.12 kg SO
2 eq), ecotoxicity (36.0 CTUe), fossil fuel depletion (0.13 MJ surplus), carcinogenic toxicity (56.1 CTUh), noncarcinogenic toxicity (0.04 CTUh), and respiratory effects (8.19 × 10
−6 kg PM
2.5 eq). M5, derived from NMP and fabricated via SDC, exhibited the highest impacts in eutrophication (1.64 × 10
−6 kg N eq) and ozone depletion (203.1 kg CFC-
11 eq). While M5 and M6 impacts with respect to most categories were relatively similar, M6 impacts were significantly higher than all other membranes with respect to ecotoxicity and carcinogenic toxicity.
Regarding the absolute values for impacts, the relative significance varied depending on the category and comparison with related LCA studies.
Table 2 lists compiled LCA impact data between M6 and other polymeric membrane fabrication processes that were also assessed on a cradle-to-gate system. Categories such as global warming potential and respiratory effects saw relatively insignificant impact values and were several orders of magnitude lower than those reported in other studies [
23,
24]. Other categories, including ozone depletion and carcinogenic toxicity, exhibited relatively significant impact values and were several orders of magnitude higher than those in literature [
23,
24]. When analyzing the LCIA, parameters that heavily contribute to carcinogenic and ozone depletion impacts (
Figure 2), including the solvent and electricity, were used in quantities ranging between approximately 0.02 and 0.81 kg and 28.8 kWh, respectively. In contrast, related studies that used the same functional unit reported electricity amounts between 117–310 kWh, thereby requiring larger amounts of extracted fossil fuels that release more carcinogenic toxins [
37]. However, comparison with literature values is also not a completely straightforward measure due to the relatively small number of studies with the same functional unit and each using different membrane materials and configurations. In this case, other studies used other or additional fabrication techniques, including electrospinning and drying steps that could consume additional electricity and require longer operating times [
15,
24]. Other materials, including LiCl and aluminum foil (used in the electrospinning process), were included in these studies that were additional contributors to these impacts and complicate the comparison process [
24].
3.3.1. Impacts Comparison for Fabrication Scale-Up
Impact changes from transitioning membrane fabrication from DBE to SDC were notable in most categories. Across all membrane types, the largest increase in impacts was observed in global warming potential and carcinogenic toxicity. For PSf-DMAc and PSf-NMP membranes, carcinogenic toxicity increased by 51.2% and 85.4% when transitioning to SDC, respectively. The disparity was found to be larger for PSf-PolarClean-GVL membranes; comparing M3 and M6, impacts increased by 58.5% and 119.3% in global warming potential and carcinogenic toxicity, respectively. In other categories, the change from DBE to SDC resulted in impact increases generally between 10–30%. Together, the increases in impacts correspond to the increased material quantity, particularly increases in electricity usage from solvent production and other materials in solvent production that required fossil fuels and hazardous chemicals in upstream processes. Thus, the increased impacts for this membrane were attributed to upstream synthesis processes and not end-use properties of the membrane.
3.3.2. Impacts Comparison for Solvent Type
In sharp contrast to the reported benefits of using eco-friendly solvents, the impact assessment indicated that membranes produced with PolarClean and GVL produced higher impacts across most categories (excluding eutrophication and ozone depletion) compared to those derived from DMAc and NMP. Differences in impacts were smaller between DBE-produced membranes. On average, impacts of M3 were 9.8% and 7.5% higher than those of M1 and M2, respectively. A larger disparity was found among pristine SDC membranes: impacts of M9 were 21.7% and 8.9% higher than those of M7 and M8, respectively. Thus, the higher amounts of PolarClean and GVL when using SDC enhanced the difference in impacts from conventional solvent use. In the larger context of all membrane types, however, the results pose a concern about using the PSf-PolarClean-GVL system as an alternative to conventional PSf membranes. With small to negligible GVL impact contributions, PolarClean becomes the eco-friendly solvent under scrutiny. Despite the non-hazardous properties of the final product, impacts generated by the synthesis route (PolarClean Commercial A1) produce notable relative contributions to the membrane fabrication impacts. A breakdown of material impact contributions in the PolarClean Commercial A1 synthesis route in
Figure S3 shows that electricity, sulfuric acid, methanol, and acetic anhydride are major contributors. The production of these materials largely involves petrochemicals and energy-intensive operations, though the integration of cleaner energy technologies may reduce the energy footprint [
40,
41,
42]. The conclusion of these findings is neatly summarized by Yadav et al. [
14], positing that “it is important to also consider the impact of producing the green solvent; a green solvent produced via a toxic procedure cannot be expected to greatly increase the sustainability of membrane production.” Further research on the upstream solvent synthesis processes and LCA data on solvent synthesis is needed to reliably determine if eco-friendly solvents such as PolarClean and GVL can lower membrane fabrication impacts.
3.4. Uncertainty and Sensitivity Analyses Results
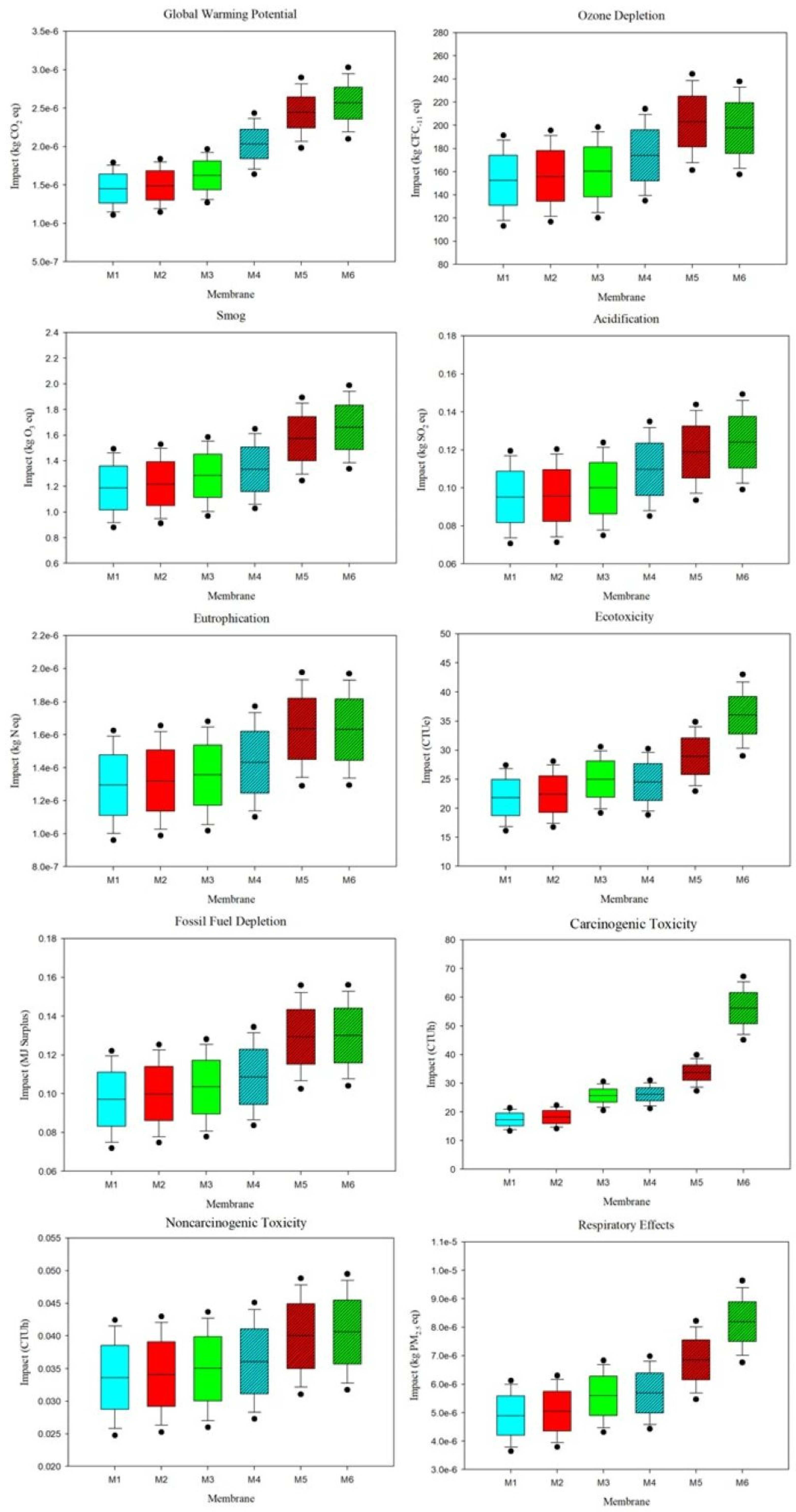
By varying the material quantities, uncertainty in the impacts was calculated and shown in
Figure 4. Uncertainty ranges in the form of minimum, median, and maximum values for each impact category are listed in
Table S13. Echoing the impact assessment results, the uncertainty analysis found M5 and M6 to consistently produce significantly higher impacts than other membranes. In categories such as global warming potential, ecotoxicity, carcinogenic toxicity, and respiratory effects, the median values of M6 impacts were nearly twice as high as M1, M2, and M3. This significant disparity highlights the significant influence of PolarClean and electricity from larger parameter amounts in these categories. Across all impact categories except carcinogenic toxicity, impact variability was relatively uniform for each membrane. For carcinogenic toxicity, the interquartile range and extremes of M6 were approximately an order of magnitude larger than the ranges of the other membranes, including M4 and M5. Based on the Monte Carlo simulation approach, impact results for membranes M1-M5 with respect to carcinogenic toxicity had a high confidence interval, whereas impacts for M6 in this category and all membranes with respect to the other 9 categories were relatively lower, indicating the need for further parameter assessment to raise the confidence interval.
Sensitivity analysis of the inventory materials highlighted the role of electricity and solvent amounts in affecting the environmental and health impacts.
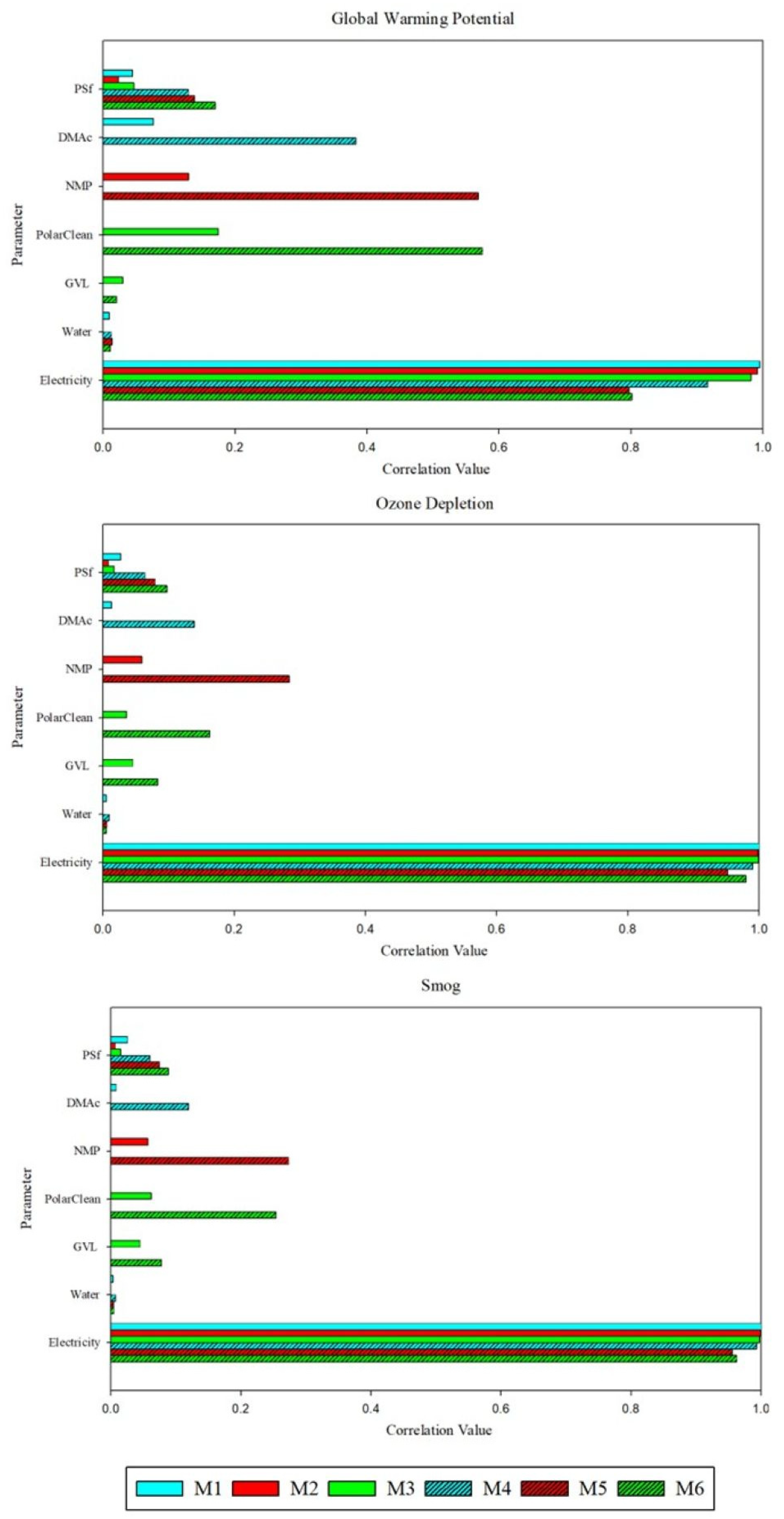
Figure 5 shows correlation values (ρ) for the polymer, solvents, and electricity amounts calculated using Spearman’s rank coefficient for select impact categories. Membranes fabricated using both techniques were particularly sensitive to electricity. For M3, the electricity amount ρ was highest with respect to ozone depletion (0.998), acidification (0.998), eutrophication (0.998), fossil fuel depletion (0.998), noncarcinogenic toxicity (0.999), and respiratory effects (0.992); similarly, ρ for M1 and M2 was at least 0.990 in the same categories. Additionally, a distinct trend was found in impact sensitivity with respect to solvent type. For SDC membranes, the impact sensitivity of PolarClean was highest in all categories, followed by NMP, DMAc, and GVL. Among all membranes, M6 had the highest ρ, with values between 0.6 and 0.8 in each category. Thus, all impact categories were at least moderately sensitive to PolarClean for M6. Moreover, accurate assessments of PolarClean and electricity are necessary to ensure reliable LCA results. It is important to note that sensitivity analysis results are connected to the Monte Carlo uncertainty analysis. As a conservative estimate, material quantities were varied by +/−30% to account for uncertainty; however, as these technologies become more developed, more specific probability distributions would improve the uncertainty and sensitivity analyses.
3.5. Scenario Analysis Results
Following findings on the significant impact contribution and sensitivity of electricity, a scenario analysis was performed that substituted the regional electricity market to determine the relationship between energy grid composition and life cycle impacts. In this analysis, the global electricity market was first substituted with the US electricity market and then the Swedish (SE) electricity market. The majority of power generation in the US grid is sourced from fossil fuels (60%), while nuclear and other renewable technologies account for 19% and 21%, respectively [
43]. In contrast, the Swedish grid is mainly comprised of non-fossil fuel sources, with hydro and nuclear accounting for 42% and 41% of power generation, respectively [
32].
Figure 6a shows the normalized impacts of membrane fabrication using the US electricity market. Impact assessment results and percent changes relative to the global grid are presented in
Table S14 and Table S15, respectively. While M6 maintained its position as the membrane type with the highest impacts in most categories, changes in impact magnitudes were observed. In comparison with global impacts, substitution with the US electricity market decreased impacts in ecotoxicity, smog, fossil fuel depletion, and carcinogenic toxicity. Pristine membranes, which had higher impact contributions from electricity, exhibited the largest declines in impacts. Smog impacts were the largest, with a −49.3% change for M3 and a −37.8% change for M6. In contrast, increased impacts were found with respect to acidification, eutrophication, and noncarcinogenic toxicity. Noncarcinogenic toxicity impact increases were the largest, ranging from 28.5% for M12 to 75.7% for M1, followed by acidification ranging between 6.4% and 68.6%. The changes in this scenario reflect differences between the fossil fuel energy mix in the US and globally. A larger reliance on natural gas than coal in the US reduces the release of heavy metals and the emission of sulfurous components that exacerbate smog formation, though higher methane emissions harm the environment in other categories [
44,
45].
Figure 6b shows the normalized impacts of membrane fabrication using the Swedish electricity market. Impact assessment results and percent changes relative to the global grid are presented in
Table S16 and Table S17, respectively. Here, the difference in the power generation mix resulted in significant impact declines across all categories except global warming potential, which had marginal increases. For M1 and M2, impacts declined by greater than 70% in 8 categories when switching to the Swedish electricity mix. Even with a smaller impact contribution from electricity in membranes derived from PolarClean and GVL, significant impact declines were observed across most categories, including M3 and M6 decreasing in noncarcinogenic toxicity by 90.7% and 77.9%, respectively. With more electricity sourced from nuclear and hydro technologies, emissions to the environment were minimized with the extraction and combustion of fossil fuels avoided. Together with results from the US grid, the Swedish grid scenario found that the source of electricity played an important role in the impacts, and the integration of clean energy technologies can, in certain cases, significantly reduce life cycle impacts.
3.6. Discussion of LCA Limitations
In the review of the LCA results, it is important to discuss limitations in accuracy and potential impact reduction strategies. Of the processes identified to significantly contribute to impacts, electricity may be the easiest to modify. As shown in the scenario analysis, selecting a region with more renewable energy sources produced significant declines in most impact categories. Moreover, since all or most electricity was used for dope solution preparation, future investigations are into accelerating the mixing time to reduce electricity consumption.
As shown in the impact assessment results, the impacts of membrane fabrication with PolarClean produced were higher than those derived from DMAc and NMP. In contrast, impact assessment results from Fionah et al. [
16] found differing results in which PSf membranes produced with PolarClean showed the lowest global environmental impacts in the respective comparison study. Key differences were found in the PolarClean LCI between the two studies, notably the simpler inventory in Fionah et al. [
16] that consisted of isobutanol, dimethylamine, acetic anhydride, and electricity. In contrast, the inventory in this study was expanded with the inclusion of butadiene, hydrogen cyanide, sodium hydroxide, sulfuric acid, methanol, thionyl chloride, triethylamine, and toluene (
Table S6), which created a disparity in impacts from producing 1 kg of PolarClean. Similar parameters between the two studies also had larger quantities in this study, including 0.174 kg of DMAc and 28.8 kWh of electricity compared to 0.0712 kg of dimethylamine and 0.1 kWh of electricity, respectively. The electricity amount of 28.8 kWh to produce 1 kg of PolarClean was an assumption in order to be uniform with the electricity amount needed to produce 1 m
2 of PSf membrane, which was a limitation to the LCIA accuracy and highlighted the lack of available data on commercial PolarClean synthesis. As discussed in 3.3, the production of numerous hazardous, petrochemical-derived, and energy-intensive chemical feedstocks for PolarClean production raised impacts to exceed those of conventional solvents in multiple impact categories; the larger amount of electricity used to produce PolarClean in this study was another contributing factor to the disparity in results.
Together, the results of these studies highlight the importance of identifying underlying differences in LCIs and LCA methodologies when making comparisons and drawing conclusions. As different assumptions regarding synthesis routes could be used within the LCI (the synthesis route used in Fionah et al. [
16] having an inventory assumed from mass balance based on Solvay’s Sustainable Portfolio Management Guide [
46] and the Commercial Route A1 reported by Cseri and Szekely [
26] being used for general large-scale synthesis), the studies indicate the difference in impacts is not only due to membrane fabrication scale-up but also due to nuances in solvent synthesis. As such, the results do not necessarily expand the scope of LCAs on membrane fabrication when using PolarClean as a solvent.
To leverage its eco-friendly properties while reducing impacts from its usage, alternative synthesis routes for PolarClean should be considered. Notably, Cseri and Szekely [
26] developed a one-step synthesis route for PolarClean by using Michael addition reactions to build the carbon backbone and catalyzed using KO
tBu and phosphazene bases at 0–5 °C. This alternative route aligned with green chemistry principles with reductions of E factor and carbon intensity by 78% and 87%, respectively, compared to the patented process [
26]. Moreover, an alternative route avoids the use of reagents, including methanol and DMAc, that produced notable impact contributions in this assessment.
Other limitations in the methodology were tied to the lab casting experiments for developing the material inventory and the LCA assumptions. As previously mentioned, membrane sheet thickness was not accounted for in the functional unit and the membrane samples prepared in the lab. Due to the dope solution viscosity, the thickness may have varied to an extent as each solution spread into a different surface area. As previously mentioned, the spreading of the dope solution resulted in a range of thicknesses between approximately 0.8 and 1.0 mm that influenced the LCI. In review of this trend, creating a PSf-PolarClean-GVL membrane with the same thickness as those prepared with DMAc and NMP would have lowered the inventory quantities and, subsequently, the magnitude of impacts. A sensitivity analysis of the parameter amounts due to membrane thickness could add quantified insight into the importance of controlling this dimension during membrane fabrication. Moreover, the inclusion of the ± 30% input variation in the uncertainty analysis would have also potentially covered variations in the membrane parameter inventories due to thickness differences.
Additionally, measurements of the surface defect areas on each DBE sample were done with defect areas approximated to polygonal areas. More accurate measurements of these areas would change the ratio of material lost during casting processes. The exclusion of impacts due to material transportation to the lab discounted a portion of impacts from the LCA impact assessment results. Specifying the manufacturing location of each material and calculating the distance and the transportation modes would provide further clarity on this information for impact assessment.
Furthermore, the focus of producing lab-scale membranes using SDC also created limitations on the practicality of the results when considering more realistic pilot or commercial-scale operations. While more akin to the equipment needed in commercial membrane fabrication, the SDC machinery used in this study was relatively simplistic, producing lower impacts than those expected in industry. When considering pilot or commercial scale, increased material throughput would require additional electricity for pumping solution through the slot die and additional material for tool priming, both of which would increase impacts. Additionally, the integration of a functioning R2R system to create a roll of membranes during continuous casting may require additional electricity input or potentially offer efficiency gains over DBE machinery. Nonsolvent water, which would inevitably be periodically replaced since the solvent concentration in the bath influences NIPS, would also create additional water amounts not reflected in this study [
1]. When considering the materials spent as tool fluid loss during SDC, changes in tooling equipment may result in a non-linear increase in fluid tooling loss. Additionally, the ratio of material used for tooling to material that becomes a viable membrane decreases as the functional unit increases to match a more realistic commercial scale. Thus, the materials consumption and impacts during the tooling process become more negligible as the size of the membrane sheet produced increases. Further consideration is needed when translating the system design and outputs in this LCA to operations needed for more realistic fabrication scale-up. A review of scale-up factors and predefined ratios used in this study, including the dope solution-nonsolvent ratio from lab-scale experiments and scaling calculations to translate material amounts from lab-scale samples to the functional unit, would be beneficial to determine uncertainty when considering a more realistic industrial scenario.
The boundary decisions in this study also played a major role in defining impact sources and the impact types. As previously stated, a cradle-to-gate system was created for this LCA for two major reasons. First, the multitude of variables in the membrane life cycle beyond fabrication, entailing filtration application and configuration, membrane cleaning, and membrane end-of-life, would require context-specific assumptions and limit the ability to compare to existing studies [
24]. This uncertainty has led LCAs on polymeric membrane fabrication to largely be bound between materials extraction and membrane production [
15,
23,
38]. Given the common trend of a cradle-to-gate system for fabrication LCAs within the relatively sparsely reported field of polymeric membrane LCAs, following the same boundaries allowed for a fairer comparison between studies. Focusing on the impacts from the fabrication processes also fits the scope of determining strategies to modify input parameter synthesis and product fabrication to reduce impacts. Limiting the boundaries to a cradle-to-gate system created several limitations in the assessment. Without accounting for the impacts that would be produced from materials to produce a module, electricity input for pumping feed solution through the membrane, cleaning chemicals to counter fouling, and the membrane disposal process, a systematic assessment of the entire polymeric membrane lifecycle remains absent. Future studies that delve beyond the gate boundary and into the operation and end-of-life phases could identify parameters and operating steps that produce significant impacts or carry higher uncertainty that would prove useful in optimizing the rest of the life cycle.