Effect of Carboxyl Content on Mechanical Properties of Lignin/Carboxylated Nitrile Rubber Compounds
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Performance Testing and Characterization
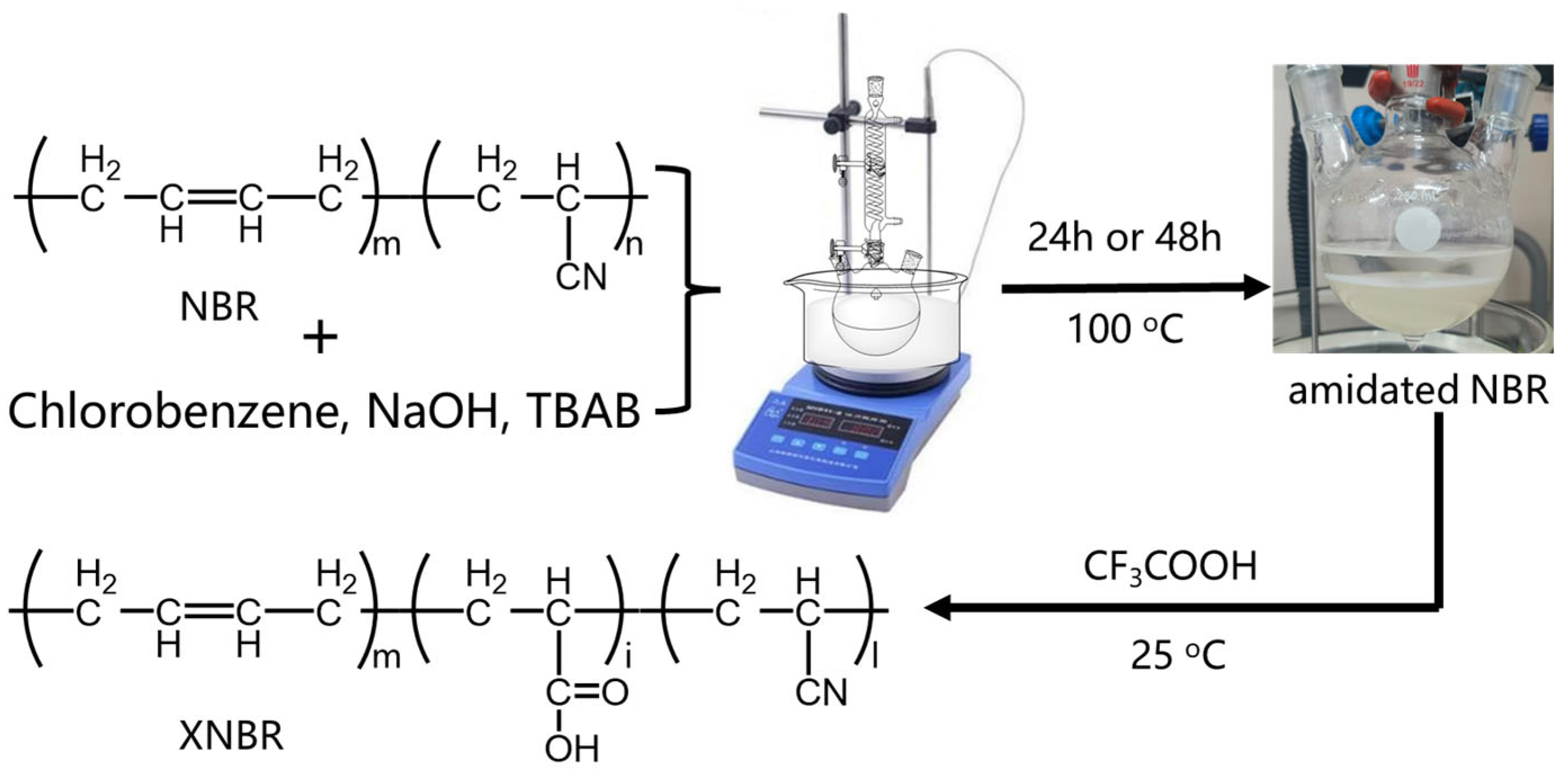
2.3. Synthesis of Carboxylated NBR (XNBR)
2.4. Determination of Carboxyl Content in XNBR
2.5. Mixing and Vulcanization of XNBR/Lignin Compounds
3. Results and Discussion
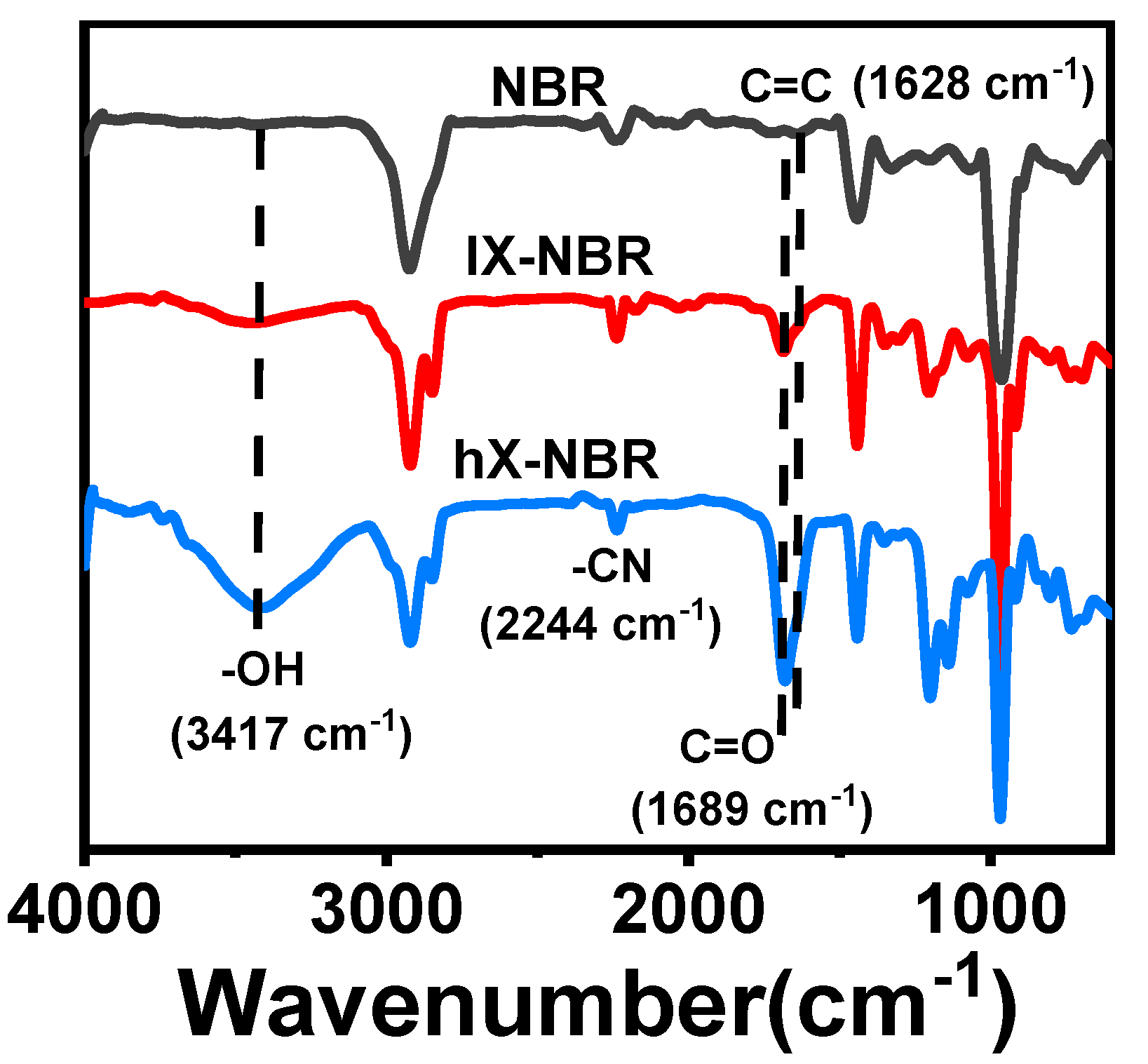
3.1. FT-IR Characterization of Carboxylated NBR
3.2. Characterization of Vulcanization Properties of XNBR/Lignin Compounds
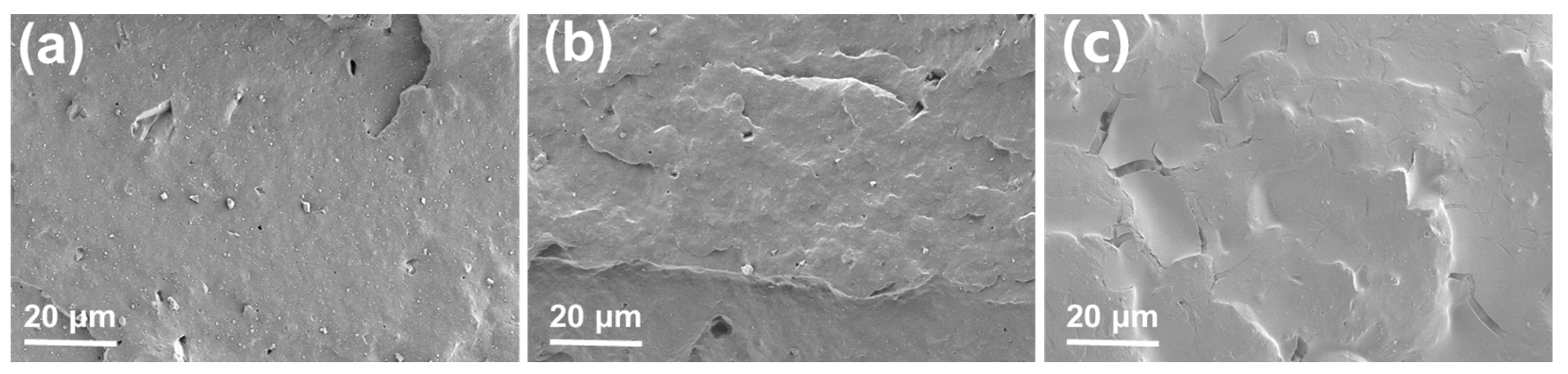
3.3. Morphological Characterization of XNBR/Lignin Compounds
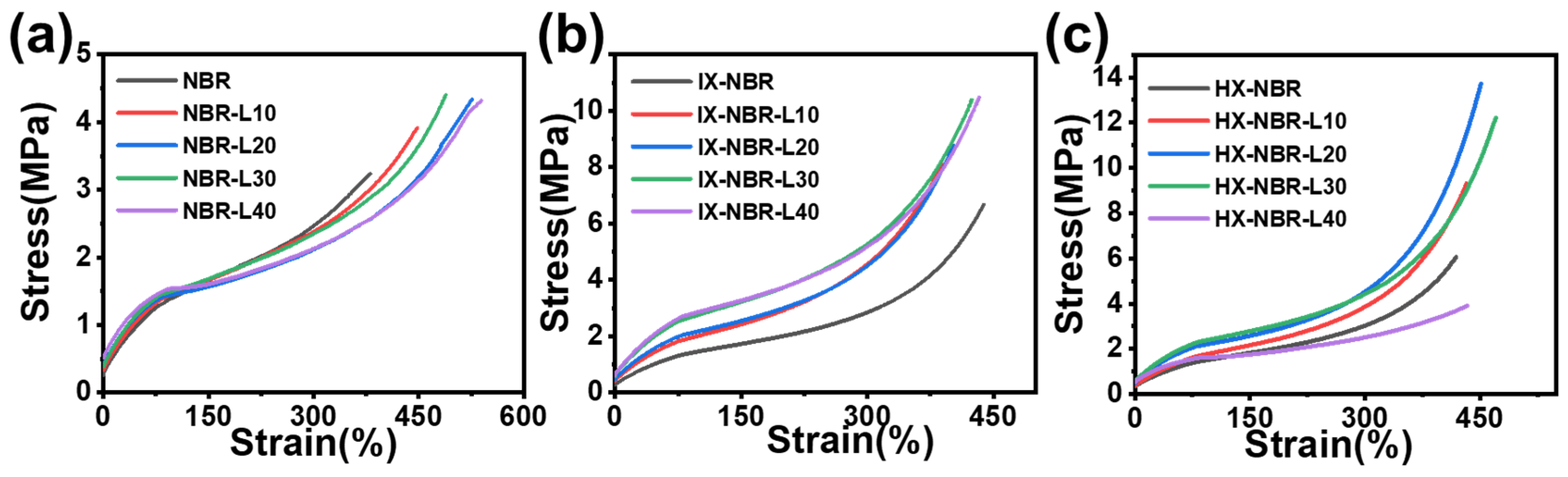
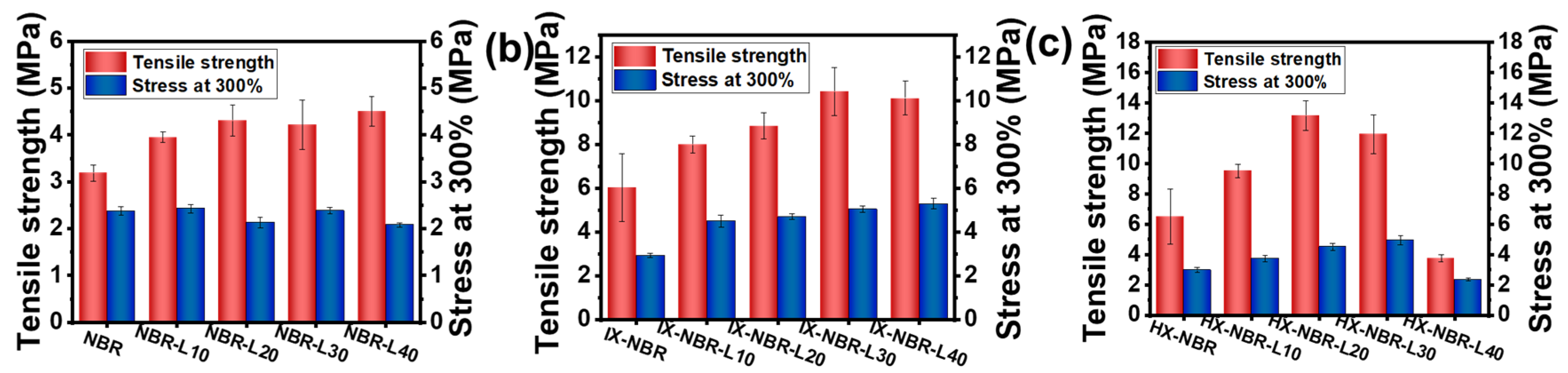
3.4. Mechanical Property Characterization of XNBR/Lignin Compounds
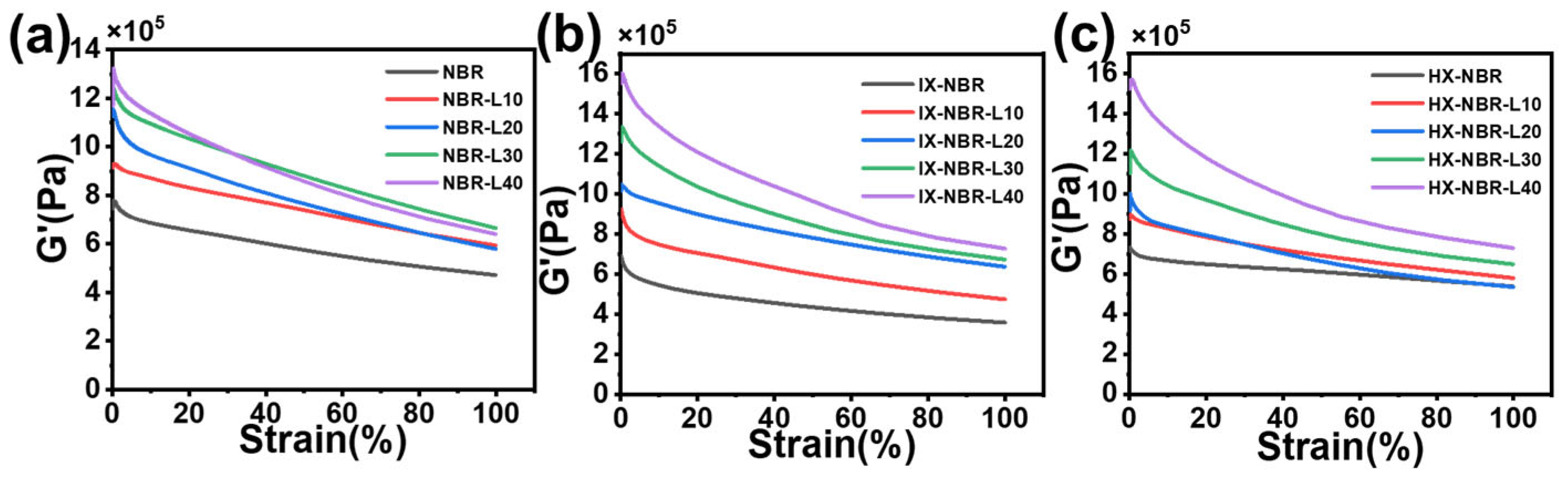
3.5. Strain Scanning Characterization of NBR/Lignin Compounds
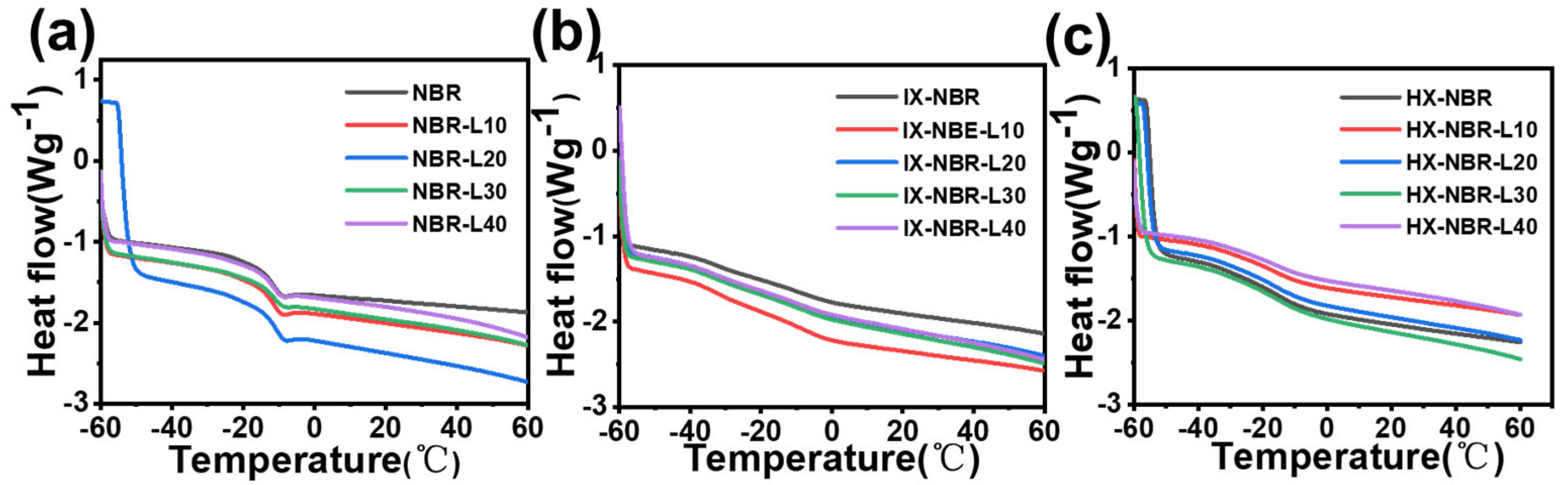
3.6. Dynamic Thermomechanical Characterization of XNBR/Lignin Compounds
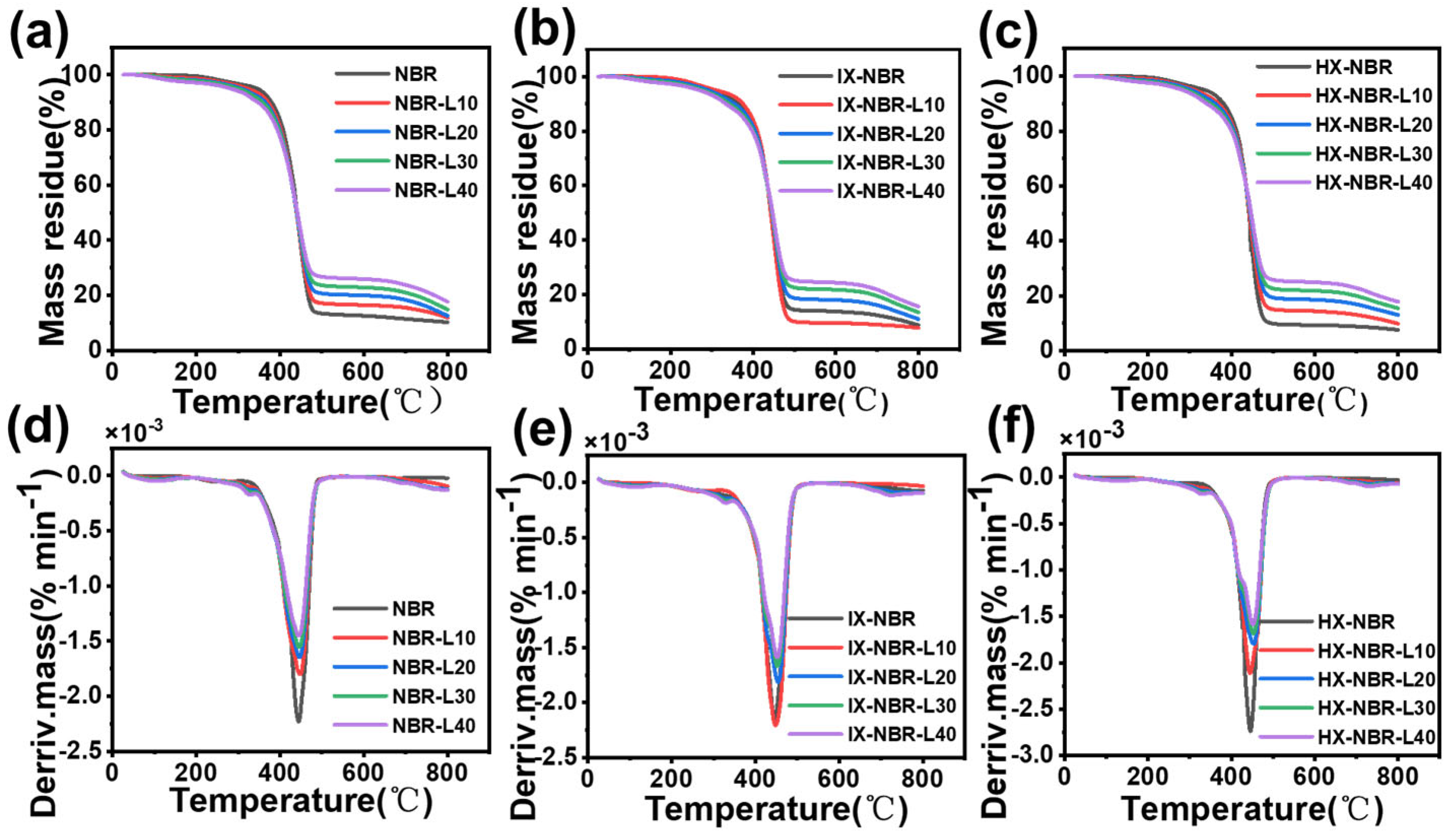
3.7. Characterization of Thermal Stability of XNBR/Lignin Compounds
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ge, Y.; Diao, P.; Li, X.; Zhou, Y.; Xu, Z.; Bian, H.; Xiao, Y.; Wang, C. Application of DPG/KH550 Modified Pyrolysis Carbon Black in Oil and High Temperature-Resistant NBR Composites. J. Appl. Polym. Sci. 2025, 142, e56377. [Google Scholar] [CrossRef]
- Pinedo, B.; Hadfield, M.; Tzanakis, I.; Conte, M.; Anand, M. Thermal Analysis and Tribological Investigation on TPU and NBR Elastomers Applied to Sealing Applications. Tribol. Int. 2018, 127, 24–36. [Google Scholar] [CrossRef]
- Li, S.; Liu, T.; Wang, L.; Wang, Z. Dynamically Vulcanized Nitrile Butadiene Rubber/Ethylene-Vinyl Acetate Copolymer Blends Compatibilized by Chlorinated Polyethylene. J. Macromol. Sci. B Phys. 2013, 52, 13–21. [Google Scholar] [CrossRef]
- Li, Y.; Wu, J.; Chen, Z.; Zhang, Z.; Su, B.; Wang, Y. The Influence of Oil and Thermal Aging on the Sealing Characteristics of NBR Seals. Polymers 2024, 16, 2501. [Google Scholar] [CrossRef]
- Wei, Q.; Yang, D.; Yu, L.; Ni, Y.; Zhang, L. Fabrication of Carboxyl Nitrile Butadiene Rubber Composites with High Dielectric Constant and Thermal Conductivity Using Al2O3@PCPA@GO Hybrids. Compos. Sci. Technol. 2020, 199, 108344. [Google Scholar] [CrossRef]
- Dunn, J.R.; Vara, R.G. Oil Resistant Elastomers for Hose Applications. Rubber Chem. Technol. 1983, 56, 557–574. [Google Scholar] [CrossRef]
- Hashimoto, K.; Maeda, A.; Hosoya, K.; Todani, Y. Specialty Elastomers for Automotive Applications. Rubber Chem. Technol. 1998, 71, 449–519. [Google Scholar] [CrossRef]
- Pal, K.; Das, T.; Pal, S.K.; Das, C.K. Use of Carboxylated Nitrile Rubber and Natural Rubber Blends as Retreading Compound for OTR Tires. Polym. Eng. Sci. 2008, 48, 2410–2417. [Google Scholar] [CrossRef]
- Sombatsompop, N.; Wimolmala, E.; Sirisinha, C. Fly Ash Particles and Precipitated Silica as Fillers in Rubbers. III. Cure Characteristics and Mechanical and Oil-Resistance Properties of Acrylonitrile-Butadiene Rubber. J. Appl. Polym. Sci. 2008, 110, 2877–2883. [Google Scholar] [CrossRef]
- Zhang, Z.F.; Liu, X.T.; Yang, K.; Zhao, S.G. Design of Coordination-Crosslinked Nitrile Rubber with Self-Healing and Reprocessing Ability. Macromol. Res. 2019, 27, 803–810. [Google Scholar] [CrossRef]
- Pal, K.; Pal, S.K.; Das, C.K.; Kim, J.K. Effect of Fillers on Morphological Properties and Wear Characteristics of XNBR/NR Blends. J. Appl. Polym. Sci. 2011, 120, 710–718. [Google Scholar] [CrossRef]
- Severe, G.; White, J.L. Dynamically Vulcanized Blends of Oil-Resistant Elastomers with HNBR. J. Appl. Polym. Sci. 2005, 95, 2–5. [Google Scholar] [CrossRef]
- Chakraborty, S.K.; De, S.K. Epoxy-Resin-Cured Carboxylated Nitrile Rubber. J. Appl. Polym. Sci. 1982, 27, 4561–4576. [Google Scholar] [CrossRef]
- Brown, H.P. Crosslinking Reactions of Carboxylic Elastomers. Rubber Chem. Technol. 1963, 36, 931–962. [Google Scholar] [CrossRef]
- Xu, C.; Wu, W.; Nie, J.; Fu, L.; Lin, B. Preparation of Carboxylic Styrene Butadiene Rubber/Chitosan Composites with Dense Supramolecular Network via Solution Mixing Process. Compos. Part A: Appl. Sci. Manuf. 2019, 117, 116–124. [Google Scholar] [CrossRef]
- Paran, S.M.R.; Naderi, G.; Ghoreishy, M.H.R. XNBR-Grafted Halloysite Nanotube Core-Shell as a Potential Compatibilizer for Immiscible Polymer Systems. Appl. Surf. Sci. 2016, 382, 63–72. [Google Scholar] [CrossRef]
- Cheng, C.; Chen, Z.; Huang, Z.; Zhang, C.; Tusiime, R.; Zhou, J.; Sun, Z.; Liu, Y.; Yu, M.; Zhang, H. Simultaneously Improving Mode I and Mode II Fracture Toughness of the Carbon Fiber/Epoxy Composite Laminates via Interleaved with Uniformly Aligned PES Fiber Webs. Compos. Part A: Appl. Sci. Manuf. 2020, 129, 105696. [Google Scholar] [CrossRef]
- Laskowska, A.; Zaborski, M.; Boiteux, G.; Gain, O.; Marzec, A.; Maniukiewicz, W. Ionic Elastomers Based on Carboxylated Nitrile Rubber (XNBR) and Magnesium Aluminum Layered Double Hydroxide (Hydrotalcite). Express Polym. Lett. 2014, 8, 374–386. [Google Scholar] [CrossRef]
- Sahoo, S.; Bhowmick, A.K. Influence of ZnO Nanoparticles on the Cure Characteristics and Mechanical Properties of Carboxylated Nitrile Rubber. J. Appl. Polym. Sci. 2007, 106, 3077–3083. [Google Scholar] [CrossRef]
- Zainal Abidin, Z.; Mamauod, S.N.L.; Romli, A.Z.; Sarkawi, S.S.; Zainal, N.H. Synergistic Effect of Partial Replacement of Carbon Black by Palm Kernel Shell Biochar in Carboxylated Nitrile Butadiene Rubber Composites. Polymers 2023, 15, 943. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Liang, P.; Hua, K.; Peng, X.; Zhou, Y.; Cai, Z. Preparation of Carboxylated Nitrile Butadiene Rubber/Fly Ash Composites by in-Situ Carboxylate Reaction. Compos. Sci. Technol. 2018, 167, 294–300. [Google Scholar] [CrossRef]
- Szadkowski, B.; Marzec, A.; Zaborski, M. Effect of in Situ Silanization of Multiwalled Carbon Nanotubes on the Properties of NBR/MWCNT-OH Composites. Polym. Plast. Technol. Eng. 2019, 58, 1327–1341. [Google Scholar] [CrossRef]
- Pingot, M.; Szadkowski, B.; Zaborski, M. Effect of Carbon Nanofibers on Mechanical and Electrical Behaviors of Acrylonitrile-butadiene Rubber Composites. Polym. Adv. Technol. 2018, 29, 1661–1669. [Google Scholar] [CrossRef]
- Ha, C.S. Carboxylated Nitrile Elastomer/Filler Nanocomposite: Effect of Silica Nanofiller in Thermal, Dynamic Mechanical Behavior, and Interfacial Adhesion. Macromol. Res. 2005, 13, 306–313. [Google Scholar] [CrossRef]
- Ragauskas, A.J.; Beckham, G.T.; Biddy, M.J.; Chandra, R.; Chen, F.; Davis, M.F.; Davison, B.H.; Dixon, R.A.; Gilna, P.; Keller, M.; et al. Lignin Valorization: Improving Lignin Processing in the Biorefinery. Science 2014, 344, 1246843. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, X.; Yang, C.; Haung, J.; Wang, P. Effect of interfacial interaction between Nano-SiO2 and NBR on tribological properties of NBR water-lubricated bearings. Wear 2022, 490, 204191. [Google Scholar] [CrossRef]
- Collins, M.N.; Nechifor, M.; Tanasă, F.; Zănoagă, M.; McLoughlin, A.; Stróżyk, M.A.; Culebras, M.; Teacă, C.-A. Valorization of Lignin in Polymer and Composite Systems for Advanced Engineering Applications—A Review. Int. J. Biol. Macromol. 2019, 131, 828–849. [Google Scholar] [CrossRef] [PubMed]
- Xia, Z.; Li, J.; Zhang, J.; Zhang, X.; Zheng, X.; Zhang, J. Processing and Valorization of Cellulose, Lignin and Lignocellulose Using Ionic Liquids. J. Bioresour. Bioprod. 2020, 5, 79–95. [Google Scholar] [CrossRef]
- Lizundia, E.; Sipponen, M.H.; Greca, L.G.; Balakshin, M.; Tardy, B.L.; Rojas, O.J.; Puglia, D. Multifunctional Lignin-Based Nanocomposites and Nanohybrids. Green Chem. 2021, 23, 6698–6760. [Google Scholar] [CrossRef]
- Barana, D.; Ali, S.D.; Salanti, A.; Orlandi, M.; Castellani, L.; Hanel, T.; Zoia, L. Influence of Lignin Features on Thermal Stability and Mechanical Properties of Natural Rubber Compounds. ACS Sustain. Chem. Eng. 2016, 4, 5258–5267. [Google Scholar] [CrossRef]
- Parvathy, G.; Sethulekshmi, A.S.; Jayan, J.S.; Raman, A.; Saritha, A. Lignin Based Nano-Composites: Synthesis and Applications. Process Saf. Environ. Prot. 2021, 145, 395–410. [Google Scholar]
- Liu, Z.-H.; Li, B.-Z.; Yuan, J.S.; Yuan, Y.-J. Creative Biological Lignin Conversion Routes toward Lignin Valorization. Trends Biotechnol. 2022, 40, 1550–1566. [Google Scholar] [CrossRef]
- Zhou, Y.; Fan, M.; Chen, L.; Zhuang, J. Lignocellulosic Fibre Mediated Rubber Composites: An Overview. Compos. Part B Eng. 2015, 76, 180–191. [Google Scholar] [CrossRef]
- Chang, B.P.; Gupta, A.; Muthuraj, R.; Mekonnen, T.H. Bioresourced Fillers for Rubber Composite Sustainability: Current Development and Future Opportunities. Green Chem. 2021, 23, 5337–5378. [Google Scholar] [CrossRef]
- Burfield, D.R.; Lim, K.; Law, K. Epoxidation of Natural Rubber Latices: Methods of Preparation and Properties of Modified Rubbers. J. Appl. Polym. Sci. 1984, 29, 1661–1673. [Google Scholar] [CrossRef]
- Qiu, J.; Yuan, S.; Xiao, H.; Liu, J.; Shen, T.; Tan, Z.; Zhuang, W.; Ying, H.; Li, M.; Zhu, C. Study on Lignin Amination for Lignin/SiO2 Nano-Hybrids towards Sustainable Natural Rubber Composites. Int. J. Biol. Macromol. 2023, 233, 123547. [Google Scholar] [CrossRef] [PubMed]
- Mei, J.; Liu, W.; Huang, J.; Qiu, X. Lignin-Reinforced Ethylene-Propylene-Diene Copolymer Elastomer via Hydrogen Bonding Interactions. Macromol. Mater. Eng. 2019, 304, 1800689. [Google Scholar] [CrossRef]
- Roy, K.; Debnath, S.C.; Pongwisuthiruchte, A.; Potiyaraj, P. Recent Advances of Natural Fibers Based Green Rubber Composites: Properties, Current Status, and Future Perspectives. J. Appl. Polym. Sci. 2021, 138, 50866. [Google Scholar] [CrossRef]
- Campos, G.N.; Rocha, E.B.D.; Furtado, C.R.G.; Figueiredo, M.A.G.; Sousa, A.M.F. Using carboxyl groups to improve the compatibility of XNBR/lignin composites. Polym. Compos. 2024, 45, 4124–4137. [Google Scholar] [CrossRef]
- GB/T 528-2009; Vulcanized rubbers or thermoplastics—Determination of tensile stress-strain proper-ties. Standardization Administration of China (SAC): Beijing, China, 2009.
- Kantala, C.; Wimolmala, E.; Sirisinha, C.; Sombatsompop, N. Reinforcement of Compatibilized NR/NBR Blends by Fly Ash Particles and Precipitated Silica. Polym. Adv. Technol. 2009, 20, 448–458. [Google Scholar] [CrossRef]
- Gregorová, A.; Košíková, B.; Moravčík, R. Stabilization Effect of Lignin in Natural Rubber. Polym. Degrad. Stabil. 2006, 91, 229–233. [Google Scholar] [CrossRef]
- Paran, S.M.R.; Naderi, G.; Mosallanezhad, H.; Movahedifar, E.; Formela, K.; Saeb, M.R. Microstructure and Mechanical Properties of Carboxylated Nitrile Butadiene Rubber/Epoxy/XNBR-Grafted Halloysite Nanotubes Nanocomposites. Polymers 2020, 12, 1192. [Google Scholar] [CrossRef]
- Mousa, A.; Heinrich, G.; Wagenknecht, U. Cure Characteristics and Mechanical Properties of Carboxylated Nitrile Butadiene Rubber (XNBR) Vulcanizate Reinforced by Organic Filler. Polym. Plast. Technol. Mater. 2011, 50, 1388–1392. [Google Scholar] [CrossRef]
- Mohamad Aini, N.A.; Othman, N.; Hussin, M.H.; Sahakaro, K.; Hayeemasae, N. Lignin as Alternative Reinforcing Filler in the Rubber Industry: A Review. Front. Mater. 2020, 6, 329. [Google Scholar] [CrossRef]
- Ferruti, F.; Carnevale, M.; Giannini, L.; Guerra, S.; Tadiello, L.; Orlandi, M.; Zoia, L. Mechanochemical Methacrylation of Lignin for Biobased Reinforcing Filler in Rubber Compounds. ACS Sustain. Chem. Eng. 2024, 12, 14028–14037. [Google Scholar] [CrossRef]
- Thakur, V.K.; Thakur, M.K.; Raghavan, P.; Kessler, M.R. Progress in Green Polymer Composites from Lignin for Multifunctional Applications: A Review. ACS Sustainable Chem. Eng. 2014, 2, 1072–1092. [Google Scholar] [CrossRef]
- Barana, D.; Orlandi, M.; Zoia, L.; Castellani, L.; Hanel, T.; Bolck, C.; Gosselink, R. Lignin Based Functional Additives for Natural Rubber. ACS Sustain. Chem. Eng. 2018, 6, 11843–11852. [Google Scholar] [CrossRef]
- Shi, X.; Sun, S.; Zhao, A.; Zhang, H.; Zuo, M.; Song, Y.; Zheng, Q. Influence of Carbon Black on the Payne Effect of Filled Natural Rubber Compounds. Compos. Sci. Technol. 2021, 203, 108586. [Google Scholar] [CrossRef]
- Wu, Z.; Lin, X.; Teng, J.; Li, M.; Song, J.; Huang, C.; Wang, R.; Ying, H.; Zhang, L.; Zhu, C. Recent Advances of Lignin Functionalization for High-Performance and Advanced Functional Rubber Composites. Biomacromolecules 2023, 24, 4553–4567. [Google Scholar] [CrossRef]
- Kruželák, J.; Džuganová, M.; Hložeková, K.; Kvasničáková, A.; Ház, A.; Nadányi, R.; Krump, H.; Hudec, I. Sulfur and Peroxide Curing of NBR Based Rubber Compounds Filled with Kraft Lignin and Calcium Lignosulfonate. J. Appl. Polym. Sci. 2024, 141, e55593. [Google Scholar] [CrossRef]
- Jang, G.G.; Nguyen, N.A.; Bowland, C.C.; Ho, H.C.; Keum, J.K.; Naskar, A.K. Effects of Graphene Surface Functionalities towards Controlled Reinforcement of a Lignin Based Renewable Thermoplastic Rubber. Compos. Sci. Technol. 2020, 199, 108352. [Google Scholar] [CrossRef]
- Košíková, B.; Gregorová, A.; Osvald, A.; Krajčovičová, J. Role of Lignin Filler in Stabilization of Natural Rubber–Based Composites. J. Appl. Polym. Sci. 2007, 103, 1226–1231. [Google Scholar] [CrossRef]
- Datta, J.; Parcheta, P.; Surówka, J. Softwood-Lignin/Natural Rubber Composites Containing Novel Plasticizing Agent: Preparation and Characterization. Ind. Crops Prod. 2017, 95, 675–685. [Google Scholar] [CrossRef]


| Compound Code | NBR (phr) | IX-NBR (phr) | HX-NBR (phr) | Lignin (phr) |
|---|---|---|---|---|
| NBR | 100 | - | - | 0 |
| NBR-L10 | 100 | - | - | 10 |
| NBR-L20 | 100 | - | - | 20 |
| NBR-L30 | 100 | - | - | 30 |
| NBR-L40 | 100 | - | - | 40 |
| IX-NBR | - | 100 | - | 0 |
| IX-NBR-L10 | - | 100 | - | 20 |
| IX-NBR-L20 | - | 100 | - | 30 |
| IX-NBR-L30 | - | 100 | - | 40 |
| IX-NBR-L40 | - | 100 | - | 50 |
| HX-NBR | - | - | 100 | 0 |
| HX-NBR-L10 | - | - | 100 | 10 |
| HX-NBR-L20 | - | - | 100 | 20 |
| HX-NBR-L30 | - | - | 100 | 30 |
| HX-NBR-L40 | - | - | 100 | 40 |
| Samples | t10 (min) | t90 (min) | ML (dN·m) | MH (dN·m) | ΔM (MH-ML) (dN·m) |
|---|---|---|---|---|---|
| NBR | 2.4 | 8.6 | 0.04 | 5.21 | 5.17 |
| NBR-L10 | 1.2 | 6.5 | 0.04 | 6.05 | 6.01 |
| NBR-L20 | 1.1 | 4.5 | 0.06 | 6.69 | 6.63 |
| NBR-L30 | 0.9 | 4.4 | 0.04 | 7.54 | 7.50 |
| NBR-L40 | 0.8 | 5.2 | 0.09 | 8.1 | 8.01 |
| IX-NBR | 4.6 | 13.1 | 0.04 | 4.03 | 3.99 |
| IX-NBR-L10 | 2.7 | 9.9 | 0.05 | 5.27 | 5.22 |
| IX-NBR-L20 | 2.2 | 8.1 | 0.09 | 5.29 | 5.20 |
| IX-NBR-L30 | 1.3 | 5.8 | 0.11 | 6.79 | 6.68 |
| IX-NBR-L40 | 1.2 | 5.7 | 0.13 | 7.78 | 7.65 |
| HX-NBR | 3.8 | 11.5 | 0.03 | 4.45 | 4.42 |
| HX-NBR-L10 | 2.1 | 9.3 | 0.07 | 4.93 | 4.86 |
| HX-NBR-L20 | 1.3 | 6.8 | 0.13 | 5.73 | 5.60 |
| HX-NBR-L30 | 1.1 | 5.8 | 0.14 | 6.55 | 6.41 |
| HX-NBR-L40 | 1.0 | 5.6 | 0.20 | 6.98 | 6.78 |
| Samples | Tensile Strength (MPa) | Elongation at Break (%) | Stress at 100% (MPa) | Stress at 300% (MPa) |
|---|---|---|---|---|
| NBR | 3.3 ± 0.2 | 392.1 ± 43.6 | 1.4 ± 0.1 | 2.3 ± 0.1 |
| NBR-L10 | 3.9 ± 0.1 | 498.9 ± 31.0 | 1.4 ± 0.1 | 2.4 ± 0.1 |
| NBR-L20 | 4.5 ± 0.3 | 524.6 ± 46.8 | 1.5 ± 0.1 | 2.2 ± 0.1 |
| NBR-L30 | 4.4 ± 0.5 | 488.4 ± 26.9 | 1.4 ± 0.1 | 2.6 ± 0.1 |
| NBR-L40 | 4.3 ± 0.3 | 541.0 ± 9.8 | 1.4 ± 0.1 | 2.1 ± 0.1 |
| IX-NBR | 6.7 ± 1.5 | 439.3 ± 43 | 1.4 ± 0.1 | 2.8 ± 0.1 |
| IX-NBR-L10 | 8.1 ± 0.4 | 389.0 ± 11 | 2.0 ± 0.1 | 4.6 ± 0.3 |
| IX-NBR-L20 | 8.8 ± 0.6 | 402.7 ± 13.0 | 2.1 ± 0.1 | 4.8 ± 0.1 |
| IX-NBR-L30 | 10.4 ± 1.1 | 427.2 ± 17.0 | 2.8 ± 0.1 | 5.2 ± 0.1 |
| IX-NBR-L40 | 10.5 ± 0.8 | 432.9 ± 11.7 | 2.9 ± 0.1 | 5.7 ± 0.2 |
| HX-NBR | 6.1 ± 1.81 | 418.8 ± 28.0 | 1.6 ± 0.1 | 3.0 ± 0.2 |
| HX-NBR-L10 | 9.3 ± 0.45 | 432.4 ± 24.0 | 1.8 ± 0.1 | 3.9 ± 0.2 |
| HX-NBR-L20 | 13.8 ± 1 | 451.3 ± 10.4 | 2.2 ± 0.1 | 4.5 ± 0.2 |
| HX-NBR-L30 | 12.2 ± 1.3 | 470.7 ± 22.7 | 2.6 ± 0.1 | 5.1 ± 0.3 |
| HX-NBR-L40 | 3.9 ± 0.2 | 433.6 ± 74 | 1.6 ± 0.1 | 2.3 ± 0.1 |
| Samples | T05% (℃) | T50% (℃) | Char Residue Values (%) |
|---|---|---|---|
| NBR | 345.3 | 440.3 | 10.3 |
| NBR-L10 | 322.3 | 439.2 | 11.9 |
| NBR-L20 | 308.2 | 439.7 | 12.6 |
| NBR-L30 | 299.5 | 440.7 | 14.8 |
| NBR-L40 | 282.2 | 441.2 | 17.6 |
| IX-NBR | 302.5 | 443.2 | 8.7 |
| IX-NBR-L10 | 311.1 | 442.8 | 7.69 |
| IX-NBR-L20 | 291.6 | 445.8 | 10.9 |
| IX-NBR-L30 | 279.5 | 445.6 | 13.4 |
| IX-NBR-L40 | 269.8 | 447.3 | 15.5 |
| HX-NBR | 335.1 | 442.3 | 7.5 |
| HX-NBR-L10 | 310.5 | 443.5 | 9.9 |
| HX-NBR-L20 | 303.1 | 445.5 | 13.0 |
| HX-NBR-L30 | 291.2 | 447.2 | 15.4 |
| HX-NBR-L40 | 281.6 | 447.7 | 17.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, H.; Yue, D. Effect of Carboxyl Content on Mechanical Properties of Lignin/Carboxylated Nitrile Rubber Compounds. Polymers 2025, 17, 2332. https://doi.org/10.3390/polym17172332
Zheng H, Yue D. Effect of Carboxyl Content on Mechanical Properties of Lignin/Carboxylated Nitrile Rubber Compounds. Polymers. 2025; 17(17):2332. https://doi.org/10.3390/polym17172332
Chicago/Turabian StyleZheng, Hongbing, and Dongmei Yue. 2025. "Effect of Carboxyl Content on Mechanical Properties of Lignin/Carboxylated Nitrile Rubber Compounds" Polymers 17, no. 17: 2332. https://doi.org/10.3390/polym17172332
APA StyleZheng, H., & Yue, D. (2025). Effect of Carboxyl Content on Mechanical Properties of Lignin/Carboxylated Nitrile Rubber Compounds. Polymers, 17(17), 2332. https://doi.org/10.3390/polym17172332






