Green Synthesis of Silver Nanoparticles and Polymeric Nanofiber Composites: Fabrications, Mechanisms, and Applications
Abstract
1. Introduction
2. Nanotechnology
3. Nanoparticles (NPs)
4. Silver Nanoparticles (AgNPs)
4.1. Silver, Background Information
4.2. Characteristics
4.3. Methods of AgNP Synthesis
4.3.1. Top-Down Approach
4.3.2. Bottom-Up Approach
5. Biosynthesis of AgNPs Using Plant Extracts
6. Biosynthesis of AgNPs Using Microorganisms
6.1. Biosynthesis of AgNPs Using Bacteria
6.2. Biosynthesis of AgNPs Using Fungi
6.3. Biosynthesis of AgNPs Using Algae
6.4. Biosynthesis of AgNPs Using Miscellaneous Sources
6.5. Mechanism of Biosynthesis of AgNPs
7. Factors Affecting the Synthesis of AgNPs
8. Characterization of AgNPs
9. Stability, Aggregation, and Shelf Life of AgNPs
10. Applications of AgNPs
11. Mechanism of Antimicrobial Activity of AgNPs
12. Polymeric Nanofiber–Nanosilver Composites
13. Nanofibers
14. Polymer Nanocomposites
15. Electrospinning
16. Application of Polymeric AgNP–Nanofiber Composites
16.1. Antimicrobial Materials
16.2. Wound Dressings
16.3. Food Packaging Materials
16.4. Antimicrobial Nanopaints
16.5. Water Filtration and Treatment
16.6. Catalyst for Hydrolysis/Electrolysis of Polymer Matrix
17. Future Perspectives and Limitations
18. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Verma, A.; Tyagi, S.; Verma, A.; Singh, J.; Joshi, P. Optimization of Different Reaction Conditions for the Bio-Inspired Synthesis of Silver Nanoparticles Using Aqueous Extract of Solanum nigrum Leaves. J. Nanomater. Mol. Nanotechnol. 2017, 6, 2–5. [Google Scholar] [CrossRef]
- Ahmad, S.; Munir, S.; Zeb, N.; Ullah, A.; Khan, B.; Ali, J.; Bilal, M.; Omer, M.; Alamzeb, M.; Salman, S.M.; et al. Green Nanotechnology: A Review on Green Synthesis of Silver Nanoparticles—An Ecofriendly Approach. Int. J. Nanomed. 2019, 14, 5087–5107. [Google Scholar] [CrossRef] [PubMed]
- Mathur, A.; Mathur, A.; Mishra, P.K.; Srivastava, A.K.; Kumar, Y. Multifunctional Materials for Nanotechnology. In Multifunctional Materials; Tripathy, D.B., Gupta, A., Kumar Jain, A., Eds.; Wiley: Hoboken, NJ, USA, 2025; pp. 181–206. [Google Scholar]
- Pallavi, S.S.; Rudayni, H.A.; Bepari, A.; Niazi, S.K.; Nayaka, S. Green Synthesis of Silver Nanoparticles Using Streptomyces hirsutus Strain SNPGA-8 and Their Characterization, Antimicrobial Activity, and Anticancer Activity against Human Lung Carcinoma Cell Line A549. Saudi J. Biol. Sci. 2022, 29, 228–238. [Google Scholar] [CrossRef]
- Birla, S.S.; Gaikwad, S.C.; Gade, A.K.; Rai, M.K. Rapid Synthesis of Silver Nanoparticles from Fusarium oxysporum by Optimizing Physicocultural Conditions. Sci. World J. 2013, 2013, 796018. [Google Scholar] [CrossRef] [PubMed]
- Manisekaran, R.; Chettiar, A.R.; Marasamy, L.; Ibarra, V.C.; Lopez-Ayuso, C.A.; Chavez-Granados, P.A.; Kandasamy, G.; Acosta-Torres, L.S.; Arthikala, M. Silver-Nanoparticles-Based Composites for Antimicrobial Applications: An Update. ChemistrySelect 2024, 9, e202403772. [Google Scholar] [CrossRef]
- Wang, S.; Bai, J.; Li, C.; Zhang, Y.; Zhang, J. Ag Nanoparticle-Embedded One-Dimensional β-CD/PVP Composite Nanofibers Prepared via Electrospinning for Use in Antibacterial Material. Colloid Polym. Sci. 2012, 290, 667–672. [Google Scholar] [CrossRef]
- Govindaraju, K.; Tamilselvan, S.; Kiruthiga, V.; Singaravelu, G. Biogenic Silver Nanoparticles by Solanum torvum and Their Promising Antimicrobial Activity. J. Biopestic. 2010, 3, 394–399. [Google Scholar]
- Alexander, J.W. History of the Medical Use of Silver. Surg. Infect. 2009, 10, 289–292. [Google Scholar] [CrossRef] [PubMed]
- Ebrahiminezhad, A.; Raee, M.J.; Manafi, Z.; Sotoodeh Jahromi, A.; Ghasemi, Y. Ancient and Novel Forms of Silver in Medicine and Biomedicine. J. Adv. Med. Sci. Applied Technol. 2016, 2, 122–128. [Google Scholar] [CrossRef]
- Tian, S.; Saravanan, K.; Mothana, R.A.; Ramachandran, G.; Rajivgandhi, G.; Manoharan, N. Anti-Cancer Activity of Biosynthesized Silver Nanoparticles Using Avicennia marina against A549 Lung Cancer Cells through ROS/Mitochondrial Damages. Saudi J. Biol. Sci. 2020, 27, 3018–3024. [Google Scholar] [CrossRef]
- Dias, M.; Zhang, R.; Lammers, T.; Pallares, R.M. Clinical Translation and Landscape of Silver Nanoparticles. Drug Deliv. Transl. Res. 2025, 15, 789–797. [Google Scholar] [CrossRef]
- Mustapha, T.; Misni, N.; Ithnin, N.R.; Daskum, A.M.; Unyah, N.Z. A Review on Plants and Microorganisms Mediated Synthesis of Silver Nanoparticles, Role of Plants Metabolites and Applications. Int. J. Environ. Res. Public Health 2022, 19, 674. [Google Scholar] [CrossRef]
- Sati, A.; Ranade, T.N.; Mali, S.N.; Ahmad Yasin, H.K.; Pratap, A. Silver Nanoparticles (AgNPs): Comprehensive Insights into Bio/Synthesis, Key Influencing Factors, Multifaceted Applications, and Toxicity—A 2024 Update. ACS Omega 2025, 10, 7549–7582. [Google Scholar] [CrossRef]
- Ahmad, N.; Sharma, S. Green Synthesis of Silver Nanoparticles Using Extracts of Ananas Comosus. Green Sustain. Chem. 2012, 2, 141–147. [Google Scholar] [CrossRef]
- Azmi, S.N.H.; Al-Jassasi, B.M.H.; Al-Sawafi, H.M.S.; Al-Shukaili, S.H.G.; Rahman, N.; Nasir, M. Optimization for Synthesis of Silver Nanoparticles through Response Surface Methodology Using Leaf Extract of Boswellia sacra and Its Application in Antimicrobial Activity. Environ. Monit. Assess. 2021, 193, 497. [Google Scholar] [CrossRef]
- Wang, X.; Lee, S.Y.; Akter, S.; Huq, M.A. Probiotic-Mediated Biosynthesis of Silver Nanoparticles and Their Antibacterial Applications against Pathogenic Strains of Escherichia coli O157:H7. Polymers 2022, 14, 1834. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Chen, J.; Xu, T.; Zhang, Z.; Wu, J. Electrospun Composite Nanofibre Fabrics Containing Green Reduced Ag Nanoparticles as an Innovative Type of Antimicrobial Insole. RSC Adv. 2024, 14, 7891–7904. [Google Scholar] [CrossRef] [PubMed]
- Shahzadi, S.; Fatima, S.; Shafiq, Z.; Janjua, M.R.S.A. A Review on Green Synthesis of Silver Nanoparticles (SNPs) Using Plant Extracts: A Multifaceted Approach in Photocatalysis, Environmental Remediation, and Biomedicine. RSC Adv. 2025, 15, 3858–3903. [Google Scholar] [CrossRef]
- Zehra, S.H.; Ramzan, K.; Viskelis, J.; Viskelis, P.; Balciunaitiene, A. Advancements in Green Synthesis of Silver-Based Nanoparticles: Antimicrobial and Antifungal Properties in Various Films. Nanomaterials 2025, 15, 252. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-López, E.; Gomes, D.; Esteruelas, G.; Bonilla, L.; Lopez-Machado, A.L.; Galindo, R.; Cano, A.; Espina, M.; Ettcheto, M.; Camins, A.; et al. Metal-Based Nanoparticles as Antimicrobial Agents: An Overview. Nanomaterials 2020, 10, 292. [Google Scholar] [CrossRef]
- Kaur, A.; Preet, S.; Kumar, V.; Kumar, R.; Kumar, R. Synergetic Effect of Vancomycin Loaded Silver Nanoparticles for Enhanced Antibacterial Activity. Colloids Surf. B Biointerfaces 2019, 176, 62–69. [Google Scholar] [CrossRef]
- Feizi, S.; Taghipour, E.; Ghadam, P.; Mohammadi, P. Antifungal, Antibacterial, Antibiofilm and Colorimetric Sensing of Toxic Metals Activities of Eco Friendly, Economical Synthesized Ag/AgCl Nanoparticles Using Malva sylvestris Leaf Extracts. Microb. Pathog. 2018, 125, 33–42. [Google Scholar] [CrossRef]
- Rajivgandhi, G.; Maruthupandy, M.; Quero, F.; Li, W.J. Graphene/Nickel Oxide Nanocomposites against Isolated ESBL Producing Bacteria and A549 Cancer Cells. Mater. Sci. Eng. C 2019, 102, 829–843. [Google Scholar] [CrossRef]
- Dixit, D.; Gangadharan, D.; Popat, K.M.; Reddy, C.R.K.; Trivedi, M.; Gadhavi, D.K. Synthesis, Characterization and Application of Green Seaweed Mediated Silver Nanoparticles (AgNPs) as Antibacterial Agents for Water Disinfection. Water Sci. Technol. 2018, 78, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Fahim, M.; Shahzaib, A.; Nishat, N.; Jahan, A.; Bhat, T.A.; Inam, A. Green Synthesis of Silver Nanoparticles: A Comprehensive Review of Methods, Influencing Factors, and Applications. JCIS Open 2024, 16, 100125. [Google Scholar] [CrossRef]
- Rodrigues, A.S.; Batista, J.G.S.; Rodrigues, M.Á.V.; Thipe, V.C.; Minarini, L.A.R.; Lopes, P.S.; Lugão, A.B. Advances in Silver Nanoparticles: A Comprehensive Review on Their Potential as Antimicrobial Agents and Their Mechanisms of Action Elucidated by Proteomics. Front. Microbiol. 2024, 15, 1440065. [Google Scholar] [CrossRef]
- Singh, H.; Du, J.; Singh, P.; Yi, T.H. Extracellular Synthesis of Silver Nanoparticles by Pseudomonas Sp. THG-LS1.4 and Their Antimicrobial Application. J. Pharm. Anal. 2018, 8, 258–264. [Google Scholar] [CrossRef]
- Ranjbar, S.; Bakhtiari, A.; Khosravi, N.; Jafari Ashkavandi, S.; Azamian, F.; Alijaniha, M.; Karbalaee, M. Silver Nanoparticles: Biomedical Applications and Future Perspectives. J. Compos. Compd. 2024, 6, 1–13. [Google Scholar] [CrossRef]
- Nour El-Dein, M.M.; Baka, Z.A.M.; Abou-Dobara, M.I.; El-Sayed, A.K.A.; El-Zahed, M.M. Extracellular Biosynthesis, Optimization, Characterization and Antimicrobial Potential of Escherichia coli D8 Silver Nanoparticles. J. Microbiol. Biotechnol. Food Sci. 2021, 10, 648–656. [Google Scholar] [CrossRef]
- Abdelmoneim, H.M.; Taha, T.H.; Elnouby, M.S.; AbuShady, H.M. Extracellular Biosynthesis, OVAT/Statistical Optimization, and Characterization of Silver Nanoparticles (AgNPs) Using Leclercia adecarboxylata THHM and Its Antimicrobial Activity. Microb. Cell Factories 2022, 21, 277. [Google Scholar] [CrossRef] [PubMed]
- Mangiri, R.; Ramachandran, T.; Kumar, Y.A.; Ghosh, A.; Al-Sehemi, A.G.; Yadav, A.K.; Mani, D. Surface Engineering of M5X4 MXenes for Next-Gen Energy Solutions. Mater. Today Chem. 2025, 48, 102864. [Google Scholar] [CrossRef]
- Velgosova, O.; Mačák, L.; Múdra, E.; Vojtko, M.; Lisnichuk, M. Preparation, Structure, and Properties of PVA–AgNPs Nanocomposites. Polymers 2023, 15, 379. [Google Scholar] [CrossRef] [PubMed]
- Villarreal-Gómez, L.J.; Pérez-González, G.L.; Bogdanchikova, N.; Pestryakov, A.; Nimaev, V.; Soloveva, A.; Cornejo-Bravo, J.M.; Toledaño-Magaña, Y. Antimicrobial Effect of Electrospun Nanofibers Loaded with Silver Nanoparticles: Influence of Ag Incorporation Method. J. Nanomater. 2021, 2021, 9920755. [Google Scholar] [CrossRef]
- Quintero-Quiroz, C.; Botero, L.E.; Zárate-Triviño, D.; Acevedo-Yepes, N.; Escobar, J.S.; Pérez, V.Z.; Cruz Riano, L.J. Synthesis and Characterization of a Silver Nanoparticle-Containing Polymer Composite with Antimicrobial Abilities for Application in Prosthetic and Orthotic Devices. Biomater. Res. 2020, 24, 13. [Google Scholar] [CrossRef]
- Harun-Ur-Rashid, M.; Foyez, T.; Krishna, S.B.N.; Poda, S.; Imran, A. Bin Recent Advances of Silver Nanoparticle-Based Polymer Nanocomposites for Biomedical Applications. RSC Adv. 2025, 15, 8480–8505. [Google Scholar] [CrossRef]
- Martínez-Rodríguez, M.A.; Garza-Navarro, M.A.; Moreno-Cortez, I.E.; Lucio-Porto, R.; González-González, V.A. Silver/Polysaccharide-Based Nanofibrous Materials Synthesized from Green Chemistry Approach. Carbohydr. Polym. 2016, 136, 46–53. [Google Scholar] [CrossRef]
- Maliszewska, I.; Czapka, T. Electrospun Polymer Nanofibers with Antimicrobial Activity. Polymers 2022, 14, 1661. [Google Scholar] [CrossRef]
- Chen, H.; Ni, J.; Chen, J.; Xue, W.; Wang, J.; Na, H.; Zhu, J. Activation of Corn Cellulose with Alcohols to Improve Its Dissolvability in Fabricating Ultrafine Fibers via Electrospinning. Carbohydr. Polym. 2015, 123, 174–179. [Google Scholar] [CrossRef]
- Mostafa, M.; Kandile, N.G.; Mahmoud, M.K.; Ibrahim, H.M. Synthesis and Characterization of Polystyrene with Embedded Silver Nanoparticle Nanofibers to Utilize as Antibacterial and Wound Healing Biomaterial. Heliyon 2022, 8, e08772. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Chen, M.; Dou, Y.; Ding, J.; Yue, H.; Yin, G.; Chen, X.; Cui, Y. Electrospun Silver Nanoparticles-Embedded Feather Keratin/Poly(Vinyl Alcohol)/Poly(Ethylene Oxide) Antibacterial Composite Nanofibers. Polymers 2020, 12, 305. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Yang, H.; Yin, P.; Liu, X.; Zhang, L.; Dou, Y.; Sun, S. Applications of Cyclodextrin-Based Drug Delivery Systems in Inflammation-Related Diseases. Pharmaceutics 2025, 17, 378. [Google Scholar] [CrossRef]
- Costoya, A.; Concheiro, A.; Alvarez-Lorenzo, C. Electrospun Fibers of Cyclodextrins and Poly(Cyclodextrins). Molecules 2017, 22, 230. [Google Scholar] [CrossRef]
- Musuc, A.M. Cyclodextrins: Advances in Chemistry, Toxicology, and Multifaceted Applications. Molecules 2024, 29, 5319. [Google Scholar] [CrossRef]
- Gur’eva, L.L.; Kuzub, L.I.; Anokhin, D.V.; Badamshina, E.R. From Synthesis of Silver Nanoparticles to Polymer Nanocomposites. Russ. Chem. Bull. 2025, 74, 281–304. [Google Scholar] [CrossRef]
- Mikhailova, E.O. Silver Nanoparticles: Mechanism of Action and Probable Bio-Application. J. Funct. Biomater. 2020, 11, 84. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.M.; Morsy, J.M.; Hassanin, H.M.; Othman, E.S.; Mostafa, M.A. New Synthetic Chitosan Schiff Bases Bearing Pyranoquinolinone or Benzonaphthyridine and Their Silver Nanoparticles Derivatives with Potential Activity as Antioxidant and Molecular Docking Study for EGFR Inhibitors. RSC Adv. 2024, 14, 29919–29933. [Google Scholar] [CrossRef] [PubMed]
- Maršík, D.; Thoresen, P.P.; Maťátková, O.; Masák, J.; Sialini, P.; Rova, U.; Tsikourkitoudi, V.; Christakopoulos, P.; Matsakas, L.; Jarošová Kolouchová, I. Synthesis and Characterization of Lignin-Silver Nanoparticles. Molecules 2024, 29, 2360. [Google Scholar] [CrossRef]
- Neto, F.N.S.; Morais, L.A.; Gorup, L.F.; Ribeiro, L.S.; Martins, T.J.; Hosida, T.Y.; Francatto, P.; Barbosa, D.B.; Camargo, E.R.; Delbem, A.C.B. Facile Synthesis of PVP-Coated Silver Nanoparticles and Evaluation of Their Physicochemical, Antimicrobial and Toxic Activity. Colloids Interfaces 2023, 7, 66. [Google Scholar] [CrossRef]
- Malik, M.A.; Batterjee, M.G.; Kamli, M.R.; Alzahrani, K.A.; Danish, E.Y.; Nabi, A. Polyphenol-Capped Biogenic Synthesis of Noble Metallic Silver Nanoparticles for Antifungal Activity against Candida auris. J. Fungi 2022, 8, 639. [Google Scholar] [CrossRef]
- Butola, B.S.; Kumar, A. Green Chemistry Based In-Situ Synthesis of Silver Nanoparticles for Multifunctional Finishing of Chitosan Polysaccharide Modified Cellulosic Textile Substrate. Int. J. Biol. Macromol. 2020, 152, 1135–1145. [Google Scholar] [CrossRef] [PubMed]
- Rónavári, A.; Bélteky, P.; Boka, E.; Zakupszky, D.; Igaz, N.; Szerencsés, B.; Pfeiffer, I.; Kónya, Z.; Kiricsi, M. Polyvinyl-Pyrrolidone-Coated Silver Nanoparticles—The Colloidal, Chemical and Biological Consequences of Steric Stabilization under Biorelevant Conditions. Int. J. Mol. Sci. 2021, 22, 8673. [Google Scholar] [CrossRef] [PubMed]
- Novikov, I.V.; Pigaleva, M.A.; Levin, E.E.; Abramchuk, S.S.; Naumkin, A.V.; Li, H.; Pich, A.; Gallyamov, M.O. The Mechanism of Stabilization of Silver Nanoparticles by Chitosan in Carbonic Acid Solutions. Colloid Polym. Sci. 2020, 298, 1135–1148. [Google Scholar] [CrossRef]
- Hassan, T.; Salam, A.; Khan, A.; Khan, S.U.; Khanzada, H.; Wasim, M.; Khan, M.Q.; Kim, I.S. Functional Nanocomposites and Their Potential Applications: A Review. J. Polym. Res. 2021, 28, 36. [Google Scholar] [CrossRef]
- Singh, N.B.; Kumar, B.; Usman, U.L.; Susan, M.A.B.H. Nano Revolution: Exploring the Frontiers of Nanomaterials in Science, Technology, and Society. Nano-Struct. Nano-Objects 2024, 39, 101299. [Google Scholar] [CrossRef]
- Priya, S.; Murali, A.; Preeth, D.R.; Dharanibalaji, K.C.; Jeyajothi, G. Green Synthesis of Silver Nanoparticle-Embedded Poly(Methyl Methacrylate-Co-Methacrylic Acid) Copolymer for Fungal-Free Leathers. Polym. Bull. 2022, 79, 4607–4626. [Google Scholar] [CrossRef]
- Paul, D.R.; Robeson, L.M. Polymer Nanotechnology: Nanocomposites. Polymer 2008, 49, 3187–3204. [Google Scholar] [CrossRef]
- Taghavizadeh Yazdi, M.E.; Darroudi, M.; Amiri, M.S.; Zarrinfar, H.; Hosseini, H.A.; Mashreghi, M.; Mozafarri, H.; Ghorbani, A.; Mousavi, S.H. Antimycobacterial, Anticancer, Antioxidant and Photocatalytic Activity of Biosynthesized Silver Nanoparticles Using Berberis Integerrima. Iran. J. Sci. Technol. Trans. A Sci. 2022, 46, 1–11. [Google Scholar] [CrossRef]
- Jo, J.H.; Singh, P.; Kim, Y.J.; Wang, C.; Mathiyalagan, R.; Jin, C.G.; Yang, D.C. Pseudomonas deceptionensis DC5-Mediated Synthesis of Extracellular Silver Nanoparticles. Artif. Cells Nanomed. Biotechnol. 2016, 44, 1576–1581. [Google Scholar] [CrossRef]
- Bastos Araruna, F.; Oliveira Sousa Araruna, F.; Lima Alves Pereira, L.P.; Aranha Brito, M.C.; Veras Quelemes, P.; de Araújo-Nobre, A.R.; de Oliveira, T.M.; da Silva, D.A.; de Souza de Almeida Leite, J.R.; Fernandes Coutinho, D.; et al. Green Syntheses of Silver Nanoparticles Using Babassu Mesocarp Starch (Attalea speciosa Mart. Ex Spreng.) and Their Antimicrobial Applications. Environ. Nanotechnol. Monit. Manag. 2020, 13, 100281. [Google Scholar] [CrossRef]
- Ibrahim, H.M.; Reda, M.M.; Klingner, A. Preparation and Characterization of Green Carboxymethylchitosan (CMCS)—Polyvinyl Alcohol (PVA) Electrospun Nanofibers Containing Gold Nanoparticles (AuNPs) and Its Potential Use as Biomaterials. Int. J. Biol. Macromol. 2020, 151, 821–829. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.; Soumya, K.R.; Mathew, J.; Radhakrishnan, E.K. Electrospun Polycaprolactone Membrane Incorporated with Biosynthesized Silver Nanoparticles as Effective Wound Dressing Material. Appl. Biochem. Biotechnol. 2015, 176, 2213–2224. [Google Scholar] [CrossRef]
- Kowsalya, E.; MosaChristas, K.; Balashanmugam, P.; Tamil Selvi, A.; Jaquline Chinna Rani, I. Biocompatible Silver Nanoparticles/Poly(Vinyl Alcohol) Electrospun Nanofibers for Potential Antimicrobial Food Packaging Applications. Food Packag. Shelf Life 2019, 21, 100379. [Google Scholar] [CrossRef]
- Premkumar, J.; Sudhakar, T.; Dhakal, A.; Shrestha, J.B.; Krishnakumar, S.; Balashanmugam, P. Synthesis of Silver Nanoparticles (AgNPs) from Cinnamon against Bacterial Pathogens. Biocatal. Agric. Biotechnol. 2018, 15, 311–316. [Google Scholar] [CrossRef]
- Burdușel, A.C.; Gherasim, O.; Grumezescu, A.M.; Mogoantă, L.; Ficai, A.; Andronescu, E. Biomedical Applications of Silver Nanoparticles: An up-to-Date Overview. Nanomaterials 2018, 8, 681. [Google Scholar] [CrossRef] [PubMed]
- Baktash, E.B.; Kazemi, N.M.; Hamedi, S. Preparation of Silver-Containing Starch Nanocomposite Prepared from Green Synthesis with Green Tea Plant Extract and Investigation of Dye Degradation and Antibacterial Activity. Nanomed. Res. J. 2024, 9, 61–70. [Google Scholar] [CrossRef]
- Agarwal, H.; Venkat Kumar, S.; Rajeshkumar, S. A Review on Green Synthesis of Zinc Oxide Nanoparticles—An Eco-Friendly Approach. Resour.-Effic. Technol. 2017, 3, 406–413. [Google Scholar] [CrossRef]
- Duman, H.; Eker, F.; Akdaşçi, E.; Witkowska, A.M.; Bechelany, M.; Karav, S. Silver Nanoparticles: A Comprehensive Review of Synthesis Methods and Chemical and Physical Properties. Nanomaterials 2024, 14, 1527. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, Applications and Toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Kirubakaran, D.; Wahid, J.B.A.; Karmegam, N.; Jeevika, R.; Sellapillai, L.; Rajkumar, M.; SenthilKumar, K.J. A Comprehensive Review on the Green Synthesis of Nanoparticles: Advancements in Biomedical and Environmental Applications. Biomed. Mater. Devices 2025. [Google Scholar] [CrossRef]
- Altammar, K.A. A Review on Nanoparticles: Characteristics, Synthesis, Applications, and Challenges. Front. Microbiol. 2023, 14, 1155622. [Google Scholar] [CrossRef]
- Esmail, R.; Afshar, A.; Morteza, M.; Abolfazl, A.; Akhondi, E. Synthesis of Silver Nanoparticles with High Efficiency and Stability by Culture Supernatant of Bacillus ROM6 Isolated from Zarshouran Gold Mine and Evaluating Its Antibacterial Effects. BMC Microbiol. 2022, 22, 97. [Google Scholar] [CrossRef]
- Ghosh Chaudhuri, R.; Paria, S. Core/Shell Nanoparticles: Classes, Properties, Synthesis Mechanisms, Characterization, and Applications. Chem. Rev. 2012, 112, 2373–2433. [Google Scholar] [CrossRef]
- Nikzamir, M.; Akbarzadeh, A.; Panahi, Y. An Overview on Nanoparticles Used in Biomedicine and Their Cytotoxicity. J. Drug Deliv. Sci. Technol. 2021, 61, 102316. [Google Scholar] [CrossRef]
- Khalil, M.A.; El-Shanshoury, A.E.-R.R.; Alghamdi, M.A.; Alsalmi, F.A.; Mohamed, S.F.; Sun, J.; Ali, S.S. Biosynthesis of Silver Nanoparticles by Marine Actinobacterium Nocardiopsis dassonvillei and Exploring Their Therapeutic Potentials. Front. Microbiol. 2022, 12, 705673. [Google Scholar] [CrossRef]
- Singh, P.; Pandit, S.; Balusamy, S.R.; Madhusudanan, M.; Singh, H.; Amsath Haseef, H.M.; Mijakovic, I. Advanced Nanomaterials for Cancer Therapy: Gold, Silver, and Iron Oxide Nanoparticles in Oncological Applications. Adv. Healthc. Mater. 2025, 14, 2403059. [Google Scholar] [CrossRef]
- Zhang, M.; Peltier, R.; Zhang, M.; Lu, H.; Bian, H.; Li, Y.; Xu, Z.; Shen, Y.; Sun, H.; Wang, Z. In Situ Reduction of Silver Nanoparticles on Hybrid Polydopamine–Copper Phosphate Nanoflowers with Enhanced Antimicrobial Activity. J. Mater. Chem. B 2017, 5, 5311–5317. [Google Scholar] [CrossRef]
- Obliosca, J.M.; Liu, C.; Yeh, H.-C. Fluorescent Silver Nanoclusters as DNA Probes. Nanoscale 2013, 5, 8443–8461. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Zhang, Z.; Liu, K.; Dong, B. Direct Evidence of Plasmonic Enhancement on Catalytic Reduction of 4-Nitrophenol over Silver Nanoparticles Supported on Flexible Fibrous Networks. Appl. Catal. B 2016, 188, 245–252. [Google Scholar] [CrossRef]
- Schoen, D.T.; Schoen, A.P.; Hu, L.; Kim, H.S.; Heilshorn, S.C.; Cui, Y. High Speed Water Sterilization Using One-Dimensional Nanostructures. Nano Lett. 2010, 10, 3628–3632. [Google Scholar] [CrossRef]
- Wei, X.; Cai, J.; Lin, S.; Li, F.; Tian, F. Controlled Release of Monodisperse Silver Nanoparticles via in Situ Cross-Linked Polyvinyl Alcohol as Benign and Antibacterial Electrospun Nanofibers. Colloids Surf. B Biointerfaces 2021, 197, 111370. [Google Scholar] [CrossRef]
- Chen, X.; Schluesener, H.J. Nanosilver: A Nanoproduct in Medical Application. Toxicol. Lett. 2008, 176, 1–12. [Google Scholar] [CrossRef]
- Wijnhoven, S.W.P.; Peijnenburg, W.J.G.M.; Herberts, C.A.; Hagens, W.I.; Oomen, A.G.; Heugens, E.H.W.; Roszek, B.; Bisschops, J.; Gosens, I.; Van De Meent, D.; et al. Nano-Silver—A Review of Available Data and Knowledge Gaps in Human and Environmental Risk Assessment. Nanotoxicology 2009, 3, 109–138. [Google Scholar] [CrossRef]
- Pandey, R.; Wang, N.; Sahu, B.B.; Moharana, S.; Tiwari, S.K. Metal and Materials Engineering: Historical Prospect. In Nanoparticles Reinforced Metal Nanocomposites; Springer Nature Singapore: Singapore, 2023; pp. 1–21. [Google Scholar]
- Nordberg, G.; Gerhardsson, L. Silver. In Handbook on Toxicity of Inorganic Compounds; Seiler, H.G., Sigel, H., Sigel, A., Eds.; Marcel Dekker: New York, NY, USA, 1988; pp. 619–624. [Google Scholar]
- Galatage, S.T.; Hebalkar, A.S.; Gote, R.V.; Mali, O.R.; Killedar, S.G. Silver Nano Particles by Green Synthesis: An Overview. Res. J. Pharm. Technol. 2020, 13, 1503. [Google Scholar] [CrossRef]
- Medici, S.; Peana, M.; Nurchi, V.M.; Zoroddu, M.A. Medical Uses of Silver: History, Myths, and Scientific Evidence. J. Med. Chem. 2019, 62, 5923–5943. [Google Scholar] [CrossRef] [PubMed]
- Sabarees, G.; Velmurugan, V.; Tamilarasi, G.P.; Alagarsamy, V.; Raja Solomon, V. Recent Advances in Silver Nanoparticles Containing Nanofibers for Chronic Wound Management. Polymers 2022, 14, 3994. [Google Scholar] [CrossRef] [PubMed]
- Warheit, D.B.; Borm, P.J.A.; Hennes, C.; Lademann, J. Testing Strategies to Establish the Safety of Nanomaterials: Conclusions of an ECETOC Workshop. Inhal. Toxicol. 2007, 19, 631–643. [Google Scholar] [CrossRef]
- Satyavani, K.; Gurudeeban, S.; Ramanathan, T.; Balasubramanian, T. Biomedical Potential of Silver Nanoparticles Synthesized from Calli Cells of Citrullus colocynthis (L.) Schrad. J. Nanobiotechnol. 2011, 9, 43. [Google Scholar] [CrossRef]
- Satyavani, K.; Gurudeeban, S.; Deepak, V.; Ramanathan, T. Heliotropium Curassavicum Mediated Silver Nanoparticles for Environmental Application. Res. J. Chem. Environ. 2013, 17, 27–33. [Google Scholar]
- Kaliamurthi, S.; Selvaraj, G.; Çakmak, Z.E.; Çakmak, T. Production and Characterization of Spherical Thermostable Silver Nanoparticles from Spirulina platensis (Cyanophyceae). Phycologia 2016, 55, 568–576. [Google Scholar] [CrossRef]
- Chaloupka, K.; Malam, Y.; Seifalian, A.M. Nanosilver as a New Generation of Nanoproduct in Biomedical Applications. Trends Biotechnol. 2010, 28, 580–588. [Google Scholar] [CrossRef]
- Zhang, X.F.; Liu, Z.G.; Shen, W.; Gurunathan, S. Silver Nanoparticles: Synthesis, Characterization, Properties, Applications, and Therapeutic Approaches. Int. J. Mol. Sci. 2016, 17, 1534. [Google Scholar] [CrossRef]
- Abbas, R.; Luo, J.; Qi, X.; Naz, A.; Khan, I.A.; Liu, H.; Yu, S.; Wei, J. Silver Nanoparticles: Synthesis, Structure, Properties and Applications. Nanomaterials 2024, 14, 1425. [Google Scholar] [CrossRef] [PubMed]
- Morones, J.R.; Elechiguerra, J.L.; Camacho, A.; Holt, K.; Kouri, J.B.; Ramírez, J.T.; Yacaman, M.J. The Bactericidal Effect of Silver Nanoparticles. Nanotechnology 2005, 16, 2346–2353. [Google Scholar] [CrossRef] [PubMed]
- Benelli, G. Plant-Mediated Biosynthesis of Nanoparticles as an Emerging Tool against Mosquitoes of Medical and Veterinary Importance: A Review. Parasitol. Res. 2016, 115, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Shanmuganathan, R.; Karuppusamy, I.; Saravanan, M.; Muthukumar, H.; Ponnuchamy, K.; Ramkumar, V.S.; Pugazhendhi, A. Synthesis of Silver Nanoparticles and Their Biomedical Applications—A Comprehensive Review. Curr. Pharm. Des. 2019, 25, 2650–2660. [Google Scholar] [CrossRef]
- Wei, L.; Lu, J.; Xu, H.; Patel, A.; Chen, Z.S.; Chen, G. Silver Nanoparticles: Synthesis, Properties, and Therapeutic Applications. Drug Discov. Today 2015, 20, 595–601. [Google Scholar] [CrossRef]
- Sondi, I.; Salopek-Sondi, B. Silver Nanoparticles as Antimicrobial Agent: A Case Study on E. coli as a Model for Gram-Negative Bacteria. J. Colloid. Interface Sci. 2004, 275, 177–182. [Google Scholar] [CrossRef]
- Martínez-Castañón, G.A.; Niño-Martínez, N.; Martínez-Gutierrez, F.; Martínez-Mendoza, J.R.; Ruiz, F. Synthesis and Antibacterial Activity of Silver Nanoparticles with Different Sizes. J. Nanoparticle Res. 2008, 10, 1343–1348. [Google Scholar] [CrossRef]
- Guzman, M.; Dille, J.; Godet, S. Synthesis and Antibacterial Activity of Silver Nanoparticles against Gram-Positive and Gram-Negative Bacteria. Nanomedicine 2012, 8, 37–45. [Google Scholar] [CrossRef]
- Kim, K.J.; Sung, W.S.; Suh, B.K.; Moon, S.K.; Choi, J.S.; Kim, J.G.; Lee, D.G. Antifungal Activity and Mode of Action of Silver Nano-Particles on Candida albicans. BioMetals 2009, 22, 235–242. [Google Scholar] [CrossRef]
- Jo, Y.K.; Kim, B.H.; Jung, G. Antifungal Activity of Silver Ions and Nanoparticles on Phytopathogenic Fungi. Plant Dis. 2009, 93, 1037–1043. [Google Scholar] [CrossRef]
- Lara, H.H.; Garza-Treviño, E.N.; Ixtepan-Turrent, L.; Singh, D.K. Silver Nanoparticles Are Broad-Spectrum Bactericidal and Virucidal Compounds. J. Nanobiotechnol. 2011, 9, 30. [Google Scholar] [CrossRef]
- Manea, F.; Motoc, S.; Pop, A.; Remes, A.; Schoonman, J. Silver-Functionalized Carbon Nanofiber Composite Electrodes for Ibuprofen Detection. Nanoscale Res. Lett. 2012, 7, 331. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-León, E.; Iñiguez-Palomares, R.; Navarro, R.E.; Herrera-Urbina, R.; Tánori, J.; Iñiguez-Palomares, C.; Maldonado, A. Synthesis of Silver Nanoparticles Using Reducing Agents Obtained from Natural Sources (Rumex hymenosepalus Extracts). Nanoscale Res. Lett. 2013, 8, 318–327. [Google Scholar] [CrossRef]
- Jamkhande, P.G.; Ghule, N.W.; Bamer, A.H.; Kalaskar, M.G. Metal Nanoparticles Synthesis: An Overview on Methods of Preparation, Advantages and Disadvantages, and Applications. J. Drug Deliv. Sci. Technol. 2019, 53, 101174. [Google Scholar] [CrossRef]
- Begum, S.J.P.; Pratibha, S.; Rawat, J.M.; Venugopal, D.; Sahu, P.; Gowda, A.; Qureshi, K.A.; Jaremko, M. Recent Advances in Green Synthesis, Characterization, and Applications of Bioactive Metallic Nanoparticles. Pharmaceuticals 2022, 15, 455. [Google Scholar] [CrossRef]
- Zahin, N.; Anwar, R.; Tewari, D.; Kabir, M.T.; Sajid, A.; Mathew, B.; Uddin, M.S.; Aleya, L.; Abdel-Daim, M.M. Nanoparticles and Its Biomedical Applications in Health and Diseases: Special Focus on Drug Delivery. Environ. Sci. Pollut. Res. 2020, 27, 19151–19168. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xia, Y. Bottom-Up and Top-Down Approaches to the Synthesis of Monodispersed Spherical Colloids of Low Melting-Point Metals. Nano Lett. 2004, 4, 2047–2050. [Google Scholar] [CrossRef]
- Nyabadza, A.; McCarthy, É.; Makhesana, M.; Heidarinassab, S.; Plouze, A.; Vazquez, M.; Brabazon, D. A Review of Physical, Chemical and Biological Synthesis Methods of Bimetallic Nanoparticles and Applications in Sensing, Water Treatment, Biomedicine, Catalysis and Hydrogen Storage. Adv. Colloid. Interface Sci. 2023, 321, 103010. [Google Scholar] [CrossRef]
- Iravani, S. Green Synthesis of Metal Nanoparticles Using Plants. Green Chem. 2011, 13, 2638. [Google Scholar] [CrossRef]
- Daniel, M.C.; Astruc, D. Gold Nanoparticles: Assembly, Supramolecular Chemistry, Quantum-Size-Related Properties, and Applications toward Biology, Catalysis, and Nanotechnology. Chem. Rev. 2004, 104, 293–346. [Google Scholar] [CrossRef]
- Ahmed, S.; Ahmad, M.; Swami, B.L.; Ikram, S. A Review on Plants Extract Mediated Synthesis of Silver Nanoparticles for Antimicrobial Applications: A Green Expertise. J. Adv. Res. 2016, 7, 17–28. [Google Scholar] [CrossRef]
- Kapoor, R.T.; Salvadori, M.R.; Rafatullah, M.; Siddiqui, M.R.; Khan, M.A.; Alshareef, S.A. Exploration of Microbial Factories for Synthesis of Nanoparticles—A Sustainable Approach for Bioremediation of Environmental Contaminants. Front. Microbiol. 2021, 12, 658294. [Google Scholar] [CrossRef]
- Siddiqi, K.S.; Husen, A.; Rao, R.A.K. A Review on Biosynthesis of Silver Nanoparticles and Their Biocidal Properties. J. Nanobiotechnol. 2018, 16, 14. [Google Scholar] [CrossRef]
- Mohammadlou, M.; Maghsoudi, H.; Jafarizadeh-Malmiri, H. A Review on Green Silver Nanoparticles Based on Plants: Synthesis, Potential Applications and Eco-Friendly Approach. Int. Food Res. J. 2016, 23, 446–463. [Google Scholar]
- Gardea-Torresdey, J.L.; Gomez, E.; Peralta-Videa, J.R.; Parsons, J.G.; Troiani, H.; Jose-Yacaman, M. Alfalfa Sprouts: A Natural Source for the Synthesis of Silver Nanoparticles. Langmuir 2003, 19, 1357–1361. [Google Scholar] [CrossRef]
- Velmurugan, P.; Sivakumar, S.; Young-Chae, S.; Seong-Ho, J.; Pyoung-In, Y.; Jeong-Min, S.; Sung-Chul, H. Synthesis and Characterization Comparison of Peanut Shell Extract Silver Nanoparticles with Commercial Silver Nanoparticles and Their Antifungal Activity. J. Ind. Eng. Chem. 2015, 31, 51–54. [Google Scholar] [CrossRef]
- Banerjee, P.; Nath, D. A Phytochemical Approach to Synthesize Silver Nanoparticles for Non-Toxic Biomedical Application and Study on Their Antibacterial Efficacy. Nanosci. Technol. 2015, 2, 1–14. [Google Scholar] [CrossRef]
- Ibrahim, H.M.M. Green Synthesis and Characterization of Silver Nanoparticles Using Banana Peel Extract and Their Antimicrobial Activity against Representative Microorganisms. J. Radiat. Res. Appl. Sci. 2015, 8, 265–275. [Google Scholar] [CrossRef]
- Roy, K.; Biswas, S.; Banerjee, P.C. “Green” Synthesis of Silver Nanoparticles by Using Grape (Vitis vinifera) Fruit Extract: Characterization of the Particles and Study of Antibacterial Activity. Res. J. Pharm. Biol. Chem. Sci. 2013, 4, 1271–1278. [Google Scholar]
- Kumar, B.; Smita, K.; Cumbal, L.; Debut, A. Green Synthesis of Silver Nanoparticles Using Andean Blackberry Fruit Extract. Saudi J. Biol. Sci. 2017, 24, 45–50. [Google Scholar] [CrossRef]
- Kumar, C.; Yugandhar, P.; Savithramma, N. Biological Synthesis of Silver Nanoparticles from Adansonia digitata L. Fruit Pulp Extract, Characterization, and Its Antimicrobial Properties. J. Intercult. Ethnopharmacol. 2016, 5, 79. [Google Scholar] [CrossRef]
- Rad, M.S.; Sharifi, J.; Heshmati, G.A.; Miri, A.; Jyoti Sen, D. Biological Synthesis of Gold and Silver Nanoparticles by Nitraria schoberi Fruits. Am. J. Adv. Drug Deliv. 2013, 1, 174–179. [Google Scholar]
- Naganathan, K.; Thirunavukkarasu, S. Green Way Genesis of Silver Nanoparticles Using Multiple Fruit Peels Waste and Its Antimicrobial, Anti-Oxidant and Anti-Tumor Cell Line Studies. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2017; Volume 191, p. 012009. [Google Scholar] [CrossRef]
- Song, J.Y.; Kim, B.S. Rapid Biological Synthesis of Silver Nanoparticles Using Plant Leaf Extracts. Bioprocess Biosyst. Eng. 2009, 32, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.A.; Ahmed, T.; Wu, W.; Hossain, A.; Hafeez, R.; Islam Masum, M.M.; Wang, Y.; An, Q.; Sun, G.; Li, B. Advancements in Plant and Microbe-Based Synthesis of Metallic Nanoparticles and Their Antimicrobial Activity against Plant Pathogens. Nanomaterials 2020, 10, 1146. [Google Scholar] [CrossRef] [PubMed]
- Rafique, M.; Sadaf, I.; Rafique, M.S.; Tahir, M.B. A Review on Green Synthesis of Silver Nanoparticles and Their Applications. Artif. Cells Nanomed. Biotechnol. 2017, 45, 1272–1291. [Google Scholar] [CrossRef]
- Singh, P.; Kim, Y.J.; Zhang, D.; Yang, D.C. Biological Synthesis of Nanoparticles from Plants and Microorganisms. Trends Biotechnol. 2016, 34, 588–599. [Google Scholar] [CrossRef] [PubMed]
- Bahrulolum, H.; Nooraei, S.; Javanshir, N.; Tarrahimofrad, H.; Mirbagheri, V.S.; Easton, A.J.; Ahmadian, G. Green Synthesis of Metal Nanoparticles Using Microorganisms and Their Application in the Agrifood Sector. J. Nanobiotechnol. 2021, 19, 1–26. [Google Scholar] [CrossRef]
- Haefeli, C.; Franklin, C.; Hardy, K. Plasmid-Determined Silver Resistance in Pseudomonas stutzeri Isolated from a Silver Mine. J. Bacteriol. 1984, 158, 389–392. [Google Scholar] [CrossRef]
- Iravani, S. Bacteria in Nanoparticle Synthesis: Current Status and Future Prospects. Int. Sch. Res. Not. 2014, 2014, 1–18. [Google Scholar] [CrossRef]
- Klaus, T.; Joerger, R.; Olsson, E.; Granqvist, C.G. Silver-Based Crystalline Nanoparticles, Microbially Fabricated. Proc. Natl. Acad. Sci. USA 1999, 96, 13611–13614. [Google Scholar] [CrossRef]
- Kalimuthu, K.; Suresh Babu, R.; Venkataraman, D.; Bilal, M.; Gurunathan, S. Biosynthesis of Silver Nanocrystals by Bacillus licheniformis. Colloids Surf. B Biointerfaces 2008, 65, 150–153. [Google Scholar] [CrossRef] [PubMed]
- Annamalai, J.; Nallamuthu, T. Green Synthesis of Silver Nanoparticles: Characterization and Determination of Antibacterial Potency. Appl. Nanosci. 2016, 6, 259–265. [Google Scholar] [CrossRef]
- Saravanan, M.; Vemu, A.K.; Barik, S.K. Rapid Biosynthesis of Silver Nanoparticles from Bacillus Megaterium (NCIM 2326) and Their Antibacterial Activity on Multi Drug Resistant Clinical Pathogens. Colloids Surf. B Biointerfaces 2011, 88, 325–331. [Google Scholar] [CrossRef]
- Arif, R.; Uddin, R. A Review on Recent Developments in the Biosynthesis of Silver Nanoparticles and Its Biomedical Applications. Med. Devices Sens. 2021, 4, e10158. [Google Scholar] [CrossRef]
- Shahverdi, A.R.; Minaeian, S.; Shahverdi, H.R.; Jamalifar, H.; Nohi, A.A. Rapid Synthesis of Silver Nanoparticles Using Culture Supernatants of Enterobacteria: A Novel Biological Approach. Process Biochem. 2007, 42, 919–923. [Google Scholar] [CrossRef]
- Lagopati, N.; Gatou, M.A.; Tsoukleris, D.S.; Pavlatou, E.A. Biogenic Synthesis of Silver Nanoparticles with Antimicrobial Properties. Nanomed. Nanotechnol. 2020, 5, 000185. [Google Scholar] [CrossRef]
- Gade, A.K.; Bonde, P.; Ingle, A.P.; Marcato, P.D.; Durán, N.; Rai, M.K. Exploitation of Aspergillus niger for Synthesis of Silver Nanoparticles. J. Biobased Mater. Bioenergy 2008, 2, 243–247. [Google Scholar] [CrossRef]
- Ingle, A.; Rai, M.; Gade, A.; Bawaskar, M. Fusarium Solani: A Novel Biological Agent for the Extracellular Synthesis of Silver Nanoparticles. J. Nanoparticle Res. 2009, 11, 2079–2085. [Google Scholar] [CrossRef]
- Kathiresan, K.; Manivannan, S.; Nabeel, M.A.; Dhivya, B. Studies on Silver Nanoparticles Synthesized by a Marine Fungus, Penicillium fellutanum Isolated from Coastal Mangrove Sediment. Colloids Surf. B Biointerfaces 2009, 71, 133–137. [Google Scholar] [CrossRef]
- Jain, N.; Bhargava, A.; Majumdar, S.; Tarafdar, J.C.; Panwar, J. Extracellular Biosynthesis and Characterization of Silver Nanoparticles Using Aspergillus flavus NJP08: A Mechanism Perspective. Nanoscale 2011, 3, 635–641. [Google Scholar] [CrossRef]
- Ma, L.; Su, W.; Liu, J.X.; Zeng, X.X.; Huang, Z.; Li, W.; Liu, Z.C.; Tang, J.X. Optimization for Extracellular Biosynthesis of Silver Nanoparticles by Penicillium aculeatum Su1 and Their Antimicrobial Activity and Cytotoxic Effect Compared with Silver Ions. Mater. Sci. Eng. C 2017, 77, 963–971. [Google Scholar] [CrossRef]
- Lotfy, W.A.; Alkersh, B.M.; Sabry, S.A.; Ghozlan, H.A. Biosynthesis of Silver Nanoparticles by Aspergillus terreus: Characterization, Optimization, and Biological Activities. Front. Bioeng. Biotechnol. 2021, 9, 633468. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, P.; Gupta, R.; Agarwal, N. Advances in Synthesis and Applications of Microalgal Nanoparticles for Wastewater Treatment. J. Nanotechnol. 2019, 2019, 7392713. [Google Scholar] [CrossRef]
- Teixeira, L.M.; Reis, C.P.; Pacheco, R. Marine-Derived Compounds Combined with Nanoparticles: A Focus on the Biomedical and Pharmaceutical Sector. Mar. Drugs 2025, 23, 207. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Lee, J.Y.; Wang, D.I.C.; Ting, Y.P. Silver Nanoplates: From Biological to Biomimetic Synthesis. ACS Nano 2007, 1, 429–439. [Google Scholar] [CrossRef]
- Merin, D.D.; Prakash, S.; Bhimba, B.V. Antibacterial Screening of Silver Nanoparticles Synthesized by Marine Micro Algae. Asian Pac. J. Trop. Med. 2010, 3, 797–799. [Google Scholar] [CrossRef]
- Prasad, T.N.; Kambala, V.S.R.; Naidu, R. Phyconanotechnology: Synthesis of Silver Nanoparticles Using Brown Marine Algae Cystophora moniliformis and Their Characterisation. J. Appl. Phycol. 2013, 25, 177–182. [Google Scholar] [CrossRef]
- El-Sheekh, M.M.; El-Kassas, H.Y. Algal Production of Nano-Silver and Gold: Their Antimicrobial and Cytotoxic Activities: A Review. J. Genet. Eng. Biotechnol. 2016, 14, 299–310. [Google Scholar] [CrossRef]
- Wei, G.; Zhou, H.; Liu, Z.; Song, Y.; Wang, L.; Sun, L.; Li, Z. One-Step Synthesis of Silver Nanoparticles, Nanorods, and Nanowires on the Surface of DNA Network. J. Phys. Chem. B 2005, 109, 8738–8743. [Google Scholar] [CrossRef]
- Nithyaja, B.; Misha, H.; Nampoori, V.P.N. Synthesis of Silver Nanoparticles in DNA Template and Its Influence on Nonlinear Optical Properties. Nanosci. Nanotechnol. 2012, 2, 99–103. [Google Scholar] [CrossRef]
- Almatroudi, A. Silver Nanoparticles: Synthesis, Characterisation and Biomedical Applications. Open Life Sci. 2020, 15, 819–839. [Google Scholar] [CrossRef]
- Kasyanenko, N.; Varshavskii, M.; Ikonnikov, E.; Tolstyko, E.; Belykh, R.; Sokolov, P.; Bakulev, V.; Rolich, V.; Lopatko, K. DNA Modified with Metal Nanoparticles: Preparation and Characterization of Ordered Metal-DNA Nanostructures in a Solution and on a Substrate. J. Nanomater. 2016, 2016, 1–12. [Google Scholar] [CrossRef]
- Dai, S.; Zhang, X.; Li, T.; Du, Z.; Dang, H. Preparation of Silver Nanopatterns on DNA Templates. Appl. Surf. Sci. 2005, 249, 346–353. [Google Scholar] [CrossRef][Green Version]
- Kulkarni, D.; Sherkar, R.; Shirsathe, C.; Sonwane, R.; Varpe, N.; Shelke, S.; More, M.P.; Pardeshi, S.R.; Dhaneshwar, G.; Junnuthula, V.; et al. Biofabrication of Nanoparticles: Sources, Synthesis, and Biomedical Applications. Front. Bioeng. Biotechnol. 2023, 11, 1159193. [Google Scholar] [CrossRef]
- Huang, W.; Yan, M.; Duan, H.; Bi, Y.; Cheng, X.; Yu, H. Synergistic Antifungal Activity of Green Synthesized Silver Nanoparticles and Epoxiconazole against Setosphaeria turcica. J. Nanomater. 2020, 2020, 1–7. [Google Scholar] [CrossRef]
- Taleb Safa, M.A.; Koohestani, H. Green Synthesis of Silver Nanoparticles with Green Tea Extract from Silver Recycling of Radiographic Films. Results Eng. 2024, 21, 101808. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Elshafie, H.S.; Pohl, P. Green Synthesis of Silver Nanoparticles (AgNPs) by Lallemantia royleana Leaf Extract: Their Bio-Pharmaceutical and Catalytic Properties. J. Photochem. Photobiol. A Chem. 2024, 448, 115318. [Google Scholar] [CrossRef]
- Baran, M.F.; Keskin, C.; Baran, A.; Hatipoğlu, A.; Yildiztekin, M.; Küçükaydin, S.; Kurt, K.; Hoşgören, H.; Sarker, M.M.R.; Sufianov, A.; et al. Green Synthesis of Silver Nanoparticles from Allium cepa L. Peel Extract, Their Antioxidant, Antipathogenic, and Anticholinesterase Activity. Molecules 2023, 28, 2310. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Kotteeswaran, V. Green Synthesis of Silver Nanoparticles from Aqueous Leaf Extract of Pomegranate (Punica granatum) and Their Anticancer Activity on Human Cervical Cancer Cells. Adv. Nat. Sci. Nanosci. Nanotechnol. 2018, 9, 025014. [Google Scholar] [CrossRef]
- Tongwanichniyom, S.; Phewrat, N.; Rangsarikorn, N.; Leasen, S.; Luangkamin, S.; Chumnanvej, N. Green Synthesis of Silver Nanoparticles Using Mature-Pseudostem Extracts of Alpinia nigra and Their Bioactivities. Green Process. Synth. 2024, 13, 20230226. [Google Scholar] [CrossRef]
- Behravan, M.; Hossein Panahi, A.; Naghizadeh, A.; Ziaee, M.; Mahdavi, R.; Mirzapour, A. Facile Green Synthesis of Silver Nanoparticles Using Berberis vulgaris Leaf and Root Aqueous Extract and Its Antibacterial Activity. Int. J. Biol. Macromol. 2019, 124, 148–154. [Google Scholar] [CrossRef]
- Chakraborty, A.; Haque, S.M.; Ghosh, D.; Dey, D.; Mukherjee, S.; Maity, D.K.; Ghosh, B. Silver Nanoparticle Synthesis and Their Potency against Multidrug-Resistant Bacteria: A Green Approach from Tissue-Cultured Coleus forskohlii. 3 Biotech 2022, 12, 228. [Google Scholar] [CrossRef]
- Saravanan, M.; Barik, S.K.; MubarakAli, D.; Prakash, P.; Pugazhendhi, A. Synthesis of Silver Nanoparticles from Bacillus brsevis (NCIM 2533) and Their Antibacterial Activity against Pathogenic Bacteria. Microb. Pathog. 2018, 116, 221–226. [Google Scholar] [CrossRef]
- Ajaz, S.; Ahmed, T.; Shahid, M.; Noman, M.; Shah, A.A.; Mehmood, M.A.; Abbas, A.; Cheema, A.I.; Iqbal, M.Z.; Li, B. Bioinspired Green Synthesis of Silver Nanoparticles by Using a Native Bacillus Sp. Strain AW1-2: Characterization and Antifungal Activity against Colletotrichum falcatum Went. Enzym. Microb. Technol. 2021, 144, 109745. [Google Scholar] [CrossRef] [PubMed]
- Huq, M.A. Green Synthesis of Silver Nanoparticles Using Pseudoduganella eburnea MAHUQ-39 and Their Antimicrobial Mechanisms Investigation against Drug Resistant Human Pathogens. Int. J. Mol. Sci. 2020, 21, 1510. [Google Scholar] [CrossRef] [PubMed]
- Akther, T.; Khan, M.S. Biosynthesis of Silver Nanoparticles via Fungal Cell Filtrate and Their Anti-Quorum Sensing against Pseudomonas aeruginosa. J. Environ. Chem. Eng. 2020, 8, 104365. [Google Scholar] [CrossRef]
- Feroze, N.; Arshad, B.; Younas, M.; Afridi, M.I.; Saqib, S.; Ayaz, A. Fungal Mediated Synthesis of Silver Nanoparticles and Evaluation of Antibacterial Activity. Microsc. Res. Tech. 2020, 83, 72–80. [Google Scholar] [CrossRef]
- Mukherjee, P.; Roy, M.; Mandal, B.P.; Dey, G.K.; Mukherjee, P.K.; Ghatak, J.; Tyagi, A.K.; Kale, S.P. Green Synthesis of Highly Stabilized Nanocrystalline Silver Particles by a Non-Pathogenic and Agriculturally Important Fungus T. asperellum. Nanotechnology 2008, 19, 075103. [Google Scholar] [CrossRef]
- Xue, B.; He, D.; Gao, S.; Wang, D.; Yokoyama, K.; Wang, L. Biosynthesis of Silver Nanoparticles by the Fungus Arthroderma Fulvum and Its Antifungal Activity against Genera of Candida, Aspergillus and Fusarium. Int. J. Nanomed. 2016, 11, 1899–1906. [Google Scholar] [CrossRef]
- Sharma, A.; Sagar, A.; Rana, J.; Rani, R. Green Synthesis of Silver Nanoparticles and Its Antibacterial Activity Using Fungus Talaromyces purpureogenus Isolated from Taxus baccata Linn. Micro Nano Syst. Lett. 2022, 10, 2. [Google Scholar] [CrossRef]
- Fatima, R.; Priya, M.; Indurthi, L.; Radhakrishnan, V.; Sudhakaran, R. Biosynthesis of Silver Nanoparticles Using Red Algae Portieria hornemannii and Its Antibacterial Activity against Fish Pathogens. Microb. Pathog. 2020, 138, 103780. [Google Scholar] [CrossRef]
- Do, J.-M.; Hong, J.W.; Yoon, H.-S. Microalgae-Mediated Green Synthesis of Silver Nanoparticles: A Sustainable Approach Using Extracellular Polymeric Substances from Graesiella emersonii KNUA204. Front. Microbiol. 2025, 16, 1589285. [Google Scholar] [CrossRef]
- Ramkumar, V.S.; Pugazhendhi, A.; Gopalakrishnan, K.; Sivagurunathan, P.; Saratale, G.D.; Dung, T.N.B.; Kannapiran, E. Biofabrication and Characterization of Silver Nanoparticles Using Aqueous Extract of Seaweed Enteromorpha compressa and Its Biomedical Properties. Biotechnol. Rep. 2017, 14, 1–7. [Google Scholar] [CrossRef]
- Jha, A.K.; Prasad, K.; Prasad, K.; Kulkarni, A.R. Plant System: Nature’s Nanofactory. Colloids Surf. B Biointerfaces 2009, 73, 219–223. [Google Scholar] [CrossRef]
- Nguyen, N.P.U.; Dang, N.T.; Doan, L.; Nguyen, T.T.H. Synthesis of Silver Nanoparticles: From Conventional to ‘Modern’ Methods—A Review. Processes 2023, 11, 2617. [Google Scholar] [CrossRef]
- Srikar, S.K.; Giri, D.D.; Pal, D.B.; Mishra, P.K.; Upadhyay, S.N. Green Synthesis of Silver Nanoparticles: A Review. Green Sustain. Chem. 2016, 6, 34–56. [Google Scholar] [CrossRef]
- Kora, A.J.; Sashidhar, R.B.; Arunachalam, J. Gum Kondagogu (Cochlospermum gossypium): A Template for the Green Synthesis and Stabilization of Silver Nanoparticles with Antibacterial Application. Carbohydr. Polym. 2010, 82, 670–679. [Google Scholar] [CrossRef]
- Salem, S.S.; Fouda, A. Green Synthesis of Metallic Nanoparticles and Their Prospective Biotechnological Applications: An Overview. Biol. Trace Elem. Res. 2021, 199, 344–370. [Google Scholar] [CrossRef] [PubMed]
- Seifipour, R.; Nozari, M.; Pishkar, L. Green Synthesis of Silver Nanoparticles Using Tragopogon collinus Leaf Extract and Study of Their Antibacterial Effects. J. Inorg. Organomet. Polym. Mater. 2020, 30, 2926–2936. [Google Scholar] [CrossRef]
- Theivasanthi, T.; Alagar, M. Anti-Bacterial Studies of Silver Nanoparticles. arXiv 2011, arXiv:1101.0348. [Google Scholar] [CrossRef]
- Mikhailova, E.O. Green Silver Nanoparticles: An Antibacterial Mechanism. Antibiotics 2024, 14, 5. [Google Scholar] [CrossRef]
- Roopan, S.M.; Rohit; Madhumitha, G.; Rahuman, A.A.; Kamaraj, C.; Bharathi, A.; Surendra, T.V. Low-Cost and Eco-Friendly Phyto-Synthesis of Silver Nanoparticles Using Cocos Nucifera Coir Extract and Its Larvicidal Activity. Ind. Crops Prod. 2013, 43, 631–635. [Google Scholar] [CrossRef]
- Sadeghi, B.; Gholamhoseinpoor, F. A Study on the Stability and Green Synthesis of Silver Nanoparticles Using Ziziphora tenuior (Zt) Extract at Room Temperature. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 134, 310–315. [Google Scholar] [CrossRef]
- Rahimi-Nasrabadi, M.; Pourmortazavi, S.M.; Shandiz, S.A.S.; Ahmadi, F.; Batooli, H. Green Synthesis of Silver Nanoparticles Using Eucalyptus leucoxylon Leaves Extract and Evaluating the Antioxidant Activities of Extract. Nat. Prod. Res. 2014, 28, 1964–1969. [Google Scholar] [CrossRef]
- Sana, S.S.; Dogiparthi, L.K. Green Synthesis of Silver Nanoparticles Using Givotia moluccana Leaf Extract and Evaluation of Their Antimicrobial Activity. Mater. Lett. 2018, 226, 47–51. [Google Scholar] [CrossRef]
- Edison, T.J.I.; Sethuraman, M.G. Instant Green Synthesis of Silver Nanoparticles Using Terminalia chebula Fruit Extract and Evaluation of Their Catalytic Activity on Reduction of Methylene Blue. Process Biochem. 2012, 47, 1351–1357. [Google Scholar] [CrossRef]
- Khalil, M.M.H.; Ismail, E.H.; El-Baghdady, K.Z.; Mohamed, D. Green Synthesis of Silver Nanoparticles Using Olive Leaf Extract and Its Antibacterial Activity. Arab. J. Chem. 2014, 7, 1131–1139. [Google Scholar] [CrossRef]
- Das, S.K.; Khan, M.M.R.; Guha, A.K.; Das, A.R.; Mandal, A.B. Silver-Nano Biohybride Material: Synthesis, Characterization and Application in Water Purification. Bioresour. Technol. 2012, 124, 495–499. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, S.; Abbasi, A.Z.; Pfeiffer, C.; Hussain, S.Z.; Khalid, Z.M.; Gil, P.R.; Parak, W.J.; Hussain, I. Protein-Mediated Synthesis, PH-Induced Reversible Agglomeration, Toxicity and Cellular Interaction of Silver Nanoparticles. Colloids Surf. B Biointerfaces 2013, 102, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Moldovan, B.; Sincari, V.; Perde-Schrepler, M.; David, L. Biosynthesis of Silver Nanoparticles Using Ligustrum ovalifolium Fruits and Their Cytotoxic Effects. Nanomaterials 2018, 8, 627. [Google Scholar] [CrossRef]
- Ortega-Arroyo, L.; Martin-Martinez, E.S.; Aguilar-Mendez, M.A.; Cruz-Orea, A.; Hernandez-Pérez, I.; Glorieux, C. Green Synthesis Method of Silver Nanoparticles Using Starch as Capping Agent Applied the Methodology of Surface Response. Starch-Stärke 2013, 65, 814–821. [Google Scholar] [CrossRef]
- Sathishkumar, G.; Gobinath, C.; Karpagam, K.; Hemamalini, V.; Premkumar, K.; Sivaramakrishnan, S. Phyto-Synthesis of Silver Nanoscale Particles Using Morinda citrifolia L. and Its Inhibitory Activity against Human Pathogens. Colloids Surf. B Biointerfaces 2012, 95, 235–240. [Google Scholar] [CrossRef]
- Bala, A.; Rani, G. A Review on Phytosynthesis, Affecting Factors and Characterization Techniques of Silver Nanoparticles Designed by Green Approach. Int. Nano Lett. 2020, 10, 159–176. [Google Scholar] [CrossRef]
- Verma, A.; Mehata, M.S. Controllable Synthesis of Silver Nanoparticles Using Neem Leaves and Their Antimicrobial Activity. J. Radiat. Res. Appl. Sci. 2016, 9, 109–115. [Google Scholar] [CrossRef]
- Ahmed, R.H.; Mustafa, D.E. Green Synthesis of Silver Nanoparticles Mediated by Traditionally Used Medicinal Plants in Sudan. Int. Nano Lett. 2020, 10, 1–14. [Google Scholar] [CrossRef]
- El-Rafie, M.H.; El-Naggar, M.E.; Ramadan, M.A.; Fouda, M.M.G.; Al-Deyab, S.S.; Hebeish, A. Environmental Synthesis of Silver Nanoparticles Using Hydroxypropyl Starch and Their Characterization. Carbohydr. Polym. 2011, 86, 630–635. [Google Scholar] [CrossRef]
- Mohammed Fayaz, A.; Balaji, K.; Kalaichelvan, P.T.; Venkatesan, R. Fungal Based Synthesis of Silver Nanoparticles-An Effect of Temperature on the Size of Particles. Colloids Surf. B Biointerfaces 2009, 74, 123–126. [Google Scholar] [CrossRef] [PubMed]
- Fernando, K.M.; Gunathilake, C.A.; Yalegama, C.; Samarakoon, U.K.; Fernando, C.A.N.; Weerasinghe, G.; Pamunuwa, G.K.; Soliman, I.; Ghulamullah, N.; Rajapaksha, S.M.; et al. Synthesis of Silver Nanoparticles Using Green Reducing Agent: Ceylon Olive (Elaeocarpus serratus): Characterization and Investigating Their Antimicrobial Properties. J. Compos. Sci. 2024, 8, 43. [Google Scholar] [CrossRef]
- Senthil, B.; Devasena, T.; Prakash, B.; Rajasekar, A. Non-Cytotoxic Effect of Green Synthesized Silver Nanoparticles and Its Antibacterial Activity. J. Photochem. Photobiol. B Biol. 2017, 177, 1–7. [Google Scholar] [CrossRef]
- Arya, G.; Kumari, R.M.; Gupta, N.; Kumar, A.; Chandra, R.; Nimesh, S. Green Synthesis of Silver Nanoparticles Using Prosopis juliflora Bark Extract: Reaction Optimization, Antimicrobial and Catalytic Activities. Artif. Cells Nanomed. Biotechnol. 2018, 46, 985–993. [Google Scholar] [CrossRef]
- Jasrotia, T.; Chaudhary, S.; Kaushik, A.; Kumar, R.; Chaudhary, G.R. Green Chemistry-Assisted Synthesis of Biocompatible Ag, Cu, and Fe2O3 Nanoparticles. Mater. Today Chem. 2020, 15, 100214. [Google Scholar] [CrossRef]
- Verma, D.K.; Hasan, S.H.; Banik, R.M. Photo-Catalyzed and Phyto-Mediated Rapid Green Synthesis of Silver Nanoparticles Using Herbal Extract of Salvinia molesta and Its Antimicrobial Efficacy. J. Photochem. Photobiol. B 2016, 155, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Katta, V.K.M.; Dubey, R.S. Green Synthesis of Silver Nanoparticles Using Tagetes Erecta Plant and Investigation of Their Structural, Optical, Chemical and Morphological Properties. Mater. Today Proc. 2020, 45, 794–798. [Google Scholar] [CrossRef]
- Roy, K.; Sarkar, C.K.; Ghosh, C.K. Plant-Mediated Synthesis of Silver Nanoparticles Using Parsley (Petroselinum crispum) Leaf Extract: Spectral Analysis of the Particles and Antibacterial Study. Appl. Nanosci. 2015, 5, 945–951. [Google Scholar] [CrossRef]
- Naganthran, A.; Verasoundarapandian, G.; Khalid, F.E.; Masarudin, M.J.; Zulkharnain, A.; Nawawi, N.M.; Karim, M.; Che Abdullah, C.A.; Ahmad, S.A. Synthesis, Characterization and Biomedical Application of Silver Nanoparticles. Materials 2022, 15, 427. [Google Scholar] [CrossRef]
- Ibrahim, N.H.; Taha, G.M.; Hagaggi, N.S.A.; Moghazy, M.A. Green Synthesis of Silver Nanoparticles and Its Environmental Sensor Ability to Some Heavy Metals. BMC Chem. 2024, 18, 7. [Google Scholar] [CrossRef] [PubMed]
- Fares, A.; Mahdy, A.; Ahmed, G. Unraveling the Mysteries of Silver Nanoparticles: Synthesis, Characterization, Antimicrobial Effects and Uptake Translocation in Plant—A Review. Planta 2024, 260, 7. [Google Scholar] [CrossRef]
- Sastry, M.; Mayya, K.S.; Bandyopadhyay, K. PH Dependent Changes in the Optical Properties of Carboxylic Acid Derivatized Silver Colloidal Particles. Colloids Surf. A Physicochem. Eng. Asp. 1997, 127, 221–228. [Google Scholar] [CrossRef]
- Sastry, M.; Patil, V.; Sainkar, S.R. Electrostatically Controlled Diffusion of Carboxylic Acid Derivatized Silver Colloidal Particles in Thermally Evaporated Fatty Amine Films. J. Phys. Chem. B 1998, 102, 1404–1410. [Google Scholar] [CrossRef]
- Mittal, A.K.; Chisti, Y.; Banerjee, U.C. Synthesis of Metallic Nanoparticles Using Plant Extracts. Biotechnol. Adv. 2013, 31, 346–356. [Google Scholar] [CrossRef]
- Lin, P.-C.; Lin, S.; Wang, P.C.; Sridhar, R. Techniques for Physicochemical Characterization of Nanomaterials. Biotechnol. Adv. 2014, 32, 711–726. [Google Scholar] [CrossRef]
- Rauwel, P.; Küünal, S.; Ferdov, S.; Rauwel, E. A Review on the Green Synthesis of Silver Nanoparticles and Their Morphologies Studied via TEM. Adv. Mater. Sci. Eng. 2015, 2015, 682749. [Google Scholar] [CrossRef]
- Hall, J.B.; Dobrovolskaia, M.A.; Patri, A.K.; McNeil, S.E. Characterization of Nanoparticles for Therapeutics. Nanomedicine 2007, 2, 789–803. [Google Scholar] [CrossRef]
- Fissan, H.; Ristig, S.; Kaminski, H.; Asbach, C.; Epple, M. Comparison of Different Characterization Methods for Nanoparticle Dispersions before and after Aerosolization. Anal. Methods 2014, 6, 7324–7334. [Google Scholar] [CrossRef]
- Akintelu, S.A.; Bo, Y.; Folorunso, A.S. A Review on Synthesis, Optimization, Mechanism, Characterization, and Antibacterial Application of Silver Nanoparticles Synthesized from Plants. J. Chem. 2020, 2020, 3189043. [Google Scholar] [CrossRef]
- Mourdikoudis, S.; Pallares, R.M.; Thanh, N.T.K. Characterization Techniques for Nanoparticles: Comparison and Complementarity upon Studying Nanoparticle Properties. Nanoscale 2018, 10, 12871–12934. [Google Scholar] [CrossRef]
- Pasieczna-Patkowska, S.; Cichy, M.; Flieger, J. Application of Fourier Transform Infrared (FTIR) Spectroscopy in Characterization of Green Synthesized Nanoparticles. Molecules 2025, 30, 684. [Google Scholar] [CrossRef] [PubMed]
- Shameli, K.; Ahmad, M.; Shabanzadeh, P.; Zamanian, A.; Sangpour, P.; Abdollahi, Y.; Mohsen, Z. Green Biosynthesis of Silver Nanoparticles Using Curcuma longa Tuber Powder. Int. J. Nanomed. 2012, 7, 5603–5610. [Google Scholar] [CrossRef]
- Okka, E.Z.; Tongur, T.; Aytas, T.T.; Yılmaz, M.; Topel, Ö.; Sahin, R. Green Synthesis and the Formation Kinetics of Silver Nanoparticles in Aqueous Inula viscosa Extract. Optik 2023, 294, 171487. [Google Scholar] [CrossRef]
- Serrano-Díaz, P.; Williams, D.W.; Vega-Arreguin, J.; Manisekaran, R.; Twigg, J.; Morse, D.; García-Contreras, R.; Arenas-Arrocena, M.C.; Acosta-Torres, L.S. Geranium Leaf-Mediated Synthesis of Silver Nanoparticles and Their Transcriptomic Effects on Candida albicans. Green Process. Synth. 2023, 12, 20228105. [Google Scholar] [CrossRef]
- Gharari, Z.; Hanachi, P.; Sadeghinia, H.; Walker, T.R. Eco-Friendly Green Synthesis and Characterization of Silver Nanoparticles by Scutellaria Multicaulis Leaf Extract and Its Biological Activities. Pharmaceuticals 2023, 16, 992. [Google Scholar] [CrossRef] [PubMed]
- Abd-Elhady, H.M.; Ashor, M.A.; Hazem, A.; Saleh, F.M.; Selim, S.; El Nahhas, N.; Abdel-Hafez, S.H.; Sayed, S.; Hassan, E.A. Biosynthesis and Characterization of Extracellular Silver Nanoparticles from Streptomyces aizuneusis: Antimicrobial, Anti Larval, and Anticancer Activities. Molecules 2021, 27, 212. [Google Scholar] [CrossRef]
- Răut, I.; Constantin, M.; Șuică-Bunghez, R.; Firincă, C.; Alexandrescu, E.; Gîfu, I.C.; Doni, M.; Zamfir, L.-G.; Gurban, A.-M.; Jecu, L. Extracellular Biosynthesis, Characterization and Antimicrobial Activity of Silver Nanoparticles Synthesized by Filamentous fungi. J. Fungi 2024, 10, 798. [Google Scholar] [CrossRef]
- Bhatnagar, S.; Kobori, T.; Ganesh, D.; Ogawa, K.; Aoyagi, H. Biosynthesis of Silver Nanoparticles Mediated by Extracellular Pigment from Talaromyces purpurogenus and Their Biomedical Applications. Nanomaterials 2019, 9, 1042. [Google Scholar] [CrossRef]
- Restrepo, C.V.; Villa, C.C. Synthesis of Silver Nanoparticles, Influence of Capping Agents, and Dependence on Size and Shape: A Review. Environ. Nanotechnol. Monit. Manag. 2021, 15, 100428. [Google Scholar] [CrossRef]
- Clogston, J.D.; Patri, A.K. Zeta Potential Measurement. In Characterization of Nanoparticles Intended for Drug Delivery; Humana Press: Totowa, NJ, USA, 2011; pp. 63–70. [Google Scholar] [CrossRef]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef] [PubMed]
- Velgosova, O.; Mačák, L.; Mára, V.; Múdra, E.; Vojtko, M.; Lisnichuk, M.; Čižmárová, E. The Influence of Reagents on the Shape, Stability, and Toxicity of AgNPs and Their Use to Produce Polymer-AgNPs Composites. Metals 2023, 13, 1996. [Google Scholar] [CrossRef]
- Mulfinger, L.; Solomon, S.D.; Bahadory, M.; Jeyarajasingam, A.V.; Rutkowsky, S.A.; Boritz, C. Synthesis and Study of Silver Nanoparticles. J. Chem. Educ. 2007, 84, 322. [Google Scholar] [CrossRef]
- Shehzad, A.; Tariq, F.; Al Saidi, A.K.; Khan, K.A.; Khan, S.A.; Alshammari, F.H.; Ul-Islam, M. Green-Synthesized Silver Nanoparticle-Infused PVA Hydrogels: A Sustainable Solution for Skin Repair. Results Chem. 2025, 17, 102584. [Google Scholar] [CrossRef]
- Choi, O.; Deng, K.K.; Kim, N.-J.; Ross, L.; Surampalli, R.Y.; Hu, Z. The Inhibitory Effects of Silver Nanoparticles, Silver Ions, and Silver Chloride Colloids on Microbial Growth. Water Res. 2008, 42, 3066–3074. [Google Scholar] [CrossRef]
- Natsuki, J.; Natsuki, T.; Hashimoto, Y. A Review of Silver Nanoparticles: Synthesis Methods, Properties and Applications. Int. J. Mater. Sci. Appl. 2015, 4, 325. [Google Scholar] [CrossRef]
- Rupanshi; Kumar, V.; Yadav, N.; Singh, D.; Beniwal, V.; Chhabra, J.; Singh, B. Biogenic Silver Nanoparticles as Next-Generation Green Catalysts for Multifaceted Applications. Trans. Tianjin Univ. 2025, 31, 145–178. [Google Scholar] [CrossRef]
- Gerardo, C.; Cretu, E.; Rohling, R. Fabrication of Circuits on Flexible Substrates Using Conductive SU-8 for Sensing Applications. Sensors 2017, 17, 1420. [Google Scholar] [CrossRef]
- Yeo, C.I.; Choi, J.H.; Kim, J.B.; Lee, J.C.; Lee, Y.T. Spin-Coated Ag Nanoparticles for Enhancing Light Absorption of Thin Film a-Si:H Solar Cells. Opt. Mater. Express 2014, 4, 346–351. [Google Scholar] [CrossRef]
- Bindumadhavan, K.; Chang, P.-Y.; Doong, R. Silver Nanoparticles Embedded Boron-Doped Reduced Graphene Oxide as Anode Material for High Performance Lithium Ion Battery. Electrochim. Acta 2017, 243, 282–290. [Google Scholar] [CrossRef]
- Fei Guo, C.; Sun, T.; Cao, F.; Liu, Q.; Ren, Z. Metallic Nanostructures for Light Trapping in Energy-Harvesting Devices. Light Sci. Appl. 2014, 3, e161. [Google Scholar] [CrossRef]
- Jo, Y.K.; Seo, J.H.; Choi, B.H.; Kim, B.J.; Shin, H.H.; Hwang, B.H.; Cha, H.J. Surface-Independent Antibacterial Coating Using Silver Nanoparticle-Generating Engineered Mussel Glue. ACS Appl. Mater. Interfaces 2014, 6, 20242–20253. [Google Scholar] [CrossRef] [PubMed]
- Ghaedi, M.; Yousefinejad, M.; Safarpoor, M.; Khafri, H.Z.; Purkait, M.K. Rosmarinus Officinalis Leaf Extract Mediated Green Synthesis of Silver Nanoparticles and Investigation of Its Antimicrobial Properties. J. Ind. Eng. Chem. 2015, 31, 167–172. [Google Scholar] [CrossRef]
- Ahmadi, F.; Lackner, M. Green Synthesis of Silver Nanoparticles from Cannabis Sativa: Properties, Synthesis, Mechanistic Aspects, and Applications. ChemEngineering 2024, 8, 64. [Google Scholar] [CrossRef]
- Ershov, V.A.; Ershov, B.G. Effect of Silver Nanoparticle Size on Antibacterial Activity. Toxics 2024, 12, 801. [Google Scholar] [CrossRef]
- Yin, I.X.; Zhang, J.; Zhao, I.S.; Mei, M.L.; Li, Q.; Chu, C.H. The Antibacterial Mechanism of Silver Nanoparticles and Its Application in Dentistry. Int. J. Nanomed. 2020, 15, 2555–2562. [Google Scholar] [CrossRef]
- Gahlawat, G.; Choudhury, A.R. A Review on the Biosynthesis of Metal and Metal Salt Nanoparticles by Microbes. RSC Adv. 2019, 9, 12944–12967. [Google Scholar] [CrossRef]
- Qing, Y.; Cheng, L.; Li, R.; Liu, G.; Zhang, Y.; Tang, X.; Wang, J.; Liu, H.; Qin, Y. Potential Antibacterial Mechanism of Silver Nanoparticles and the Optimization of Orthopedic Implants by Advanced Modification Technologies. Int. J. Nanomed. 2018, 13, 3311–3327. [Google Scholar] [CrossRef] [PubMed]
- Nqoro, X.; Taziwa, R. Polymer-Based Functional Materials Loaded with Metal-Based Nanoparticles as Potential Scaffolds for the Management of Infected Wounds. Pharmaceutics 2024, 16, 155. [Google Scholar] [CrossRef]
- Gomaa, E.Z. Silver Nanoparticles as an Antimicrobial Agent: A Case Study on Staphylococcus aureus and Escherichia coli as Models for Gram-Positive and Gram-Negative Bacteria. J. Gen. Appl. Microbiol. 2017, 63, 36–43. [Google Scholar] [CrossRef]
- Ninganagouda, S.; Rathod, V.; Singh, D.; Hiremath, J.; Singh, A.K.; Mathew, J.; Ul-Haq, M. Growth Kinetics and Mechanistic Action of Reactive Oxygen Species Released by Silver Nanoparticles from Aspergillus niger on Escherichia coli. BioMed Res. Int. 2014, 2014, 753419. [Google Scholar] [CrossRef]
- Buszewski, B.; Railean-Plugaru, V.; Pomastowski, P.; Rafińska, K.; Szultka-Mlynska, M.; Golinska, P.; Wypij, M.; Laskowski, D.; Dahm, H. Antimicrobial Activity of Biosilver Nanoparticles Produced by a Novel Streptacidiphilus durhamensis Strain. J. Microbiol. Immunol. Infect. 2018, 51, 45–54. [Google Scholar] [CrossRef]
- Gahlawat, G.; Shikha, S.; Chaddha, B.S.; Chaudhuri, S.R.; Mayilraj, S.; Choudhury, A.R. Microbial Glycolipoprotein-Capped Silver Nanoparticles as Emerging Antibacterial Agents against Cholera. Microb. Cell Factories 2016, 15, 25. [Google Scholar] [CrossRef] [PubMed]
- Soenen, S.J.; Rivera-Gil, P.; Montenegro, J.M.; Parak, W.J.; De Smedt, S.C.; Braeckmans, K. Cellular Toxicity of Inorganic Nanoparticles: Common Aspects and Guidelines for Improved Nanotoxicity Evaluation. Nano Today 2011, 6, 446–465. [Google Scholar] [CrossRef]
- Chandimali, N.; Bak, S.G.; Park, E.H.; Lim, H.-J.; Won, Y.-S.; Kim, E.-K.; Park, S.-I.; Lee, S.J. Free Radicals and Their Impact on Health and Antioxidant Defenses: A Review. Cell Death Discov. 2025, 11, 19. [Google Scholar] [CrossRef]
- Hassabo, A.G.; Nada, A.A.; Ibrahim, H.M.; Abou-Zeid, N.Y. Impregnation of Silver Nanoparticles into Polysaccharide Substrates and Their Properties. Carbohydr. Polym. 2015, 122, 343–350. [Google Scholar] [CrossRef]
- Dakal, T.C.; Kumar, A.; Majumdar, R.S.; Yadav, V. Mechanistic Basis of Antimicrobial Actions of Silver Nanoparticles. Front. Microbiol. 2016, 7, 1831. [Google Scholar] [CrossRef]
- González-Fernández, S.; Blanco-Agudín, N.; Rodríguez, D.; Fernández-Vega, I.; Merayo-Lloves, J.; Quirós, L.M. Silver Nanoparticles: A Versatile Tool Against Infectious and Non-Infectious Diseases. Antibiotics 2025, 14, 289. [Google Scholar] [CrossRef] [PubMed]
- Rahman, S.; Rahman, L.; Khalil, A.T.; Ali, N.; Zia, D.; Ali, M.; Shinwari, Z.K. Endophyte-Mediated Synthesis of Silver Nanoparticles and Their Biological Applications. Appl. Microbiol. Biotechnol. 2019, 103, 2551–2569. [Google Scholar] [CrossRef] [PubMed]
- Nurani, S.J.; Saha, C.K.; Rahman Khan, M.A.; Sunny, S.M.H. Silver Nanoparticles Synthesis, Properties, Applications and Future Perspectives: A Short Review. IOSR J. Electr. Electron. Eng. 2015, 10, 117–126. [Google Scholar] [CrossRef]
- Yousef, N.; Temerk, H. Enhancement the Antibacterial Potential of the Biosynthesized Silver Nanoparticles Using Hydrophilic Polymers. Microbiol. Res. J. Int. 2017, 19, 1–9. [Google Scholar] [CrossRef]
- Zook, J.M.; Halter, M.D.; Cleveland, D.; Long, S.E. Disentangling the Effects of Polymer Coatings on Silver Nanoparticle Agglomeration, Dissolution, and Toxicity to Determine Mechanisms of Nanotoxicity. J. Nanoparticle Res. 2012, 14, 1165. [Google Scholar] [CrossRef]
- Pereira, D.; Ferreira, S.; Ramírez-Rodríguez, G.B.; Alves, N.; Sousa, Â.; Valente, J.F.A. Silver and Antimicrobial Polymer Nanocomplexes to Enhance Biocidal Effects. Int. J. Mol. Sci. 2024, 25, 1256. [Google Scholar] [CrossRef] [PubMed]
- Bhong, M.; Khan, T.K.H.; Devade, K.; Vijay Krishna, B.; Sura, S.; Eftikhaar, H.K.; Pal Thethi, H.; Gupta, N. Review of Composite Materials and Applications. Mater. Today Proc. 2023, 93, 26–30. [Google Scholar] [CrossRef]
- Sharma, A.K.; Priya; Kaith, B.S. Polymer Nanocomposite Matrices: Classification, Synthesis Methods, and Applications; Hussain, C.M., Thomas, S., Eds.; Springer International Publishing: Cham, Switzerland, 2021; ISBN 978-3-030-40512-0. [Google Scholar]
- Vedarethinam, V.; Arun, C. Nanocomposites and Nanofillers. In Handbook of Nanofillers; Mallakpour, S., Hussain, C.M., Eds.; Springer Nature Singapore: Singapore, 2024; pp. 1–18. [Google Scholar]
- Schadler, L.S. Polymer-Based and Polymer-Filled Nanocomposites. In Nanocomposite Science and Technology; Ajayan, P.M., Schadler, L.S., Braun, P.V., Eds.; Wiley-VCH: Weinheim, Germany, 2003; pp. 77–154. ISBN 3527303596. [Google Scholar]
- Musa, A.A.; Bello, A.; Adams, S.M.; Onwualu, A.P.; Anye, V.C.; Bello, K.A.; Obianyo, I.I. Nano-Enhanced Polymer Composite Materials: A Review of Current Advancements and Challenges. Polymers 2025, 17, 893. [Google Scholar] [CrossRef]
- Saha, R.; Munshi, M.H.; Akter, M.; Shikder, A.A.R.; Islam, T. Synthesis Techniques, Fundamental Properties, and Emerging Applications of Nanocomposites: A Comprehensive Review. SPE Polym. 2025, 6, e70004. [Google Scholar] [CrossRef]
- Nichols, S.P.; Koh, A.; Storm, W.L.; Shin, J.H.; Schoenfisch, M.H. Biocompatible Materials for Continuous Glucose Monitoring Devices. Chem. Rev. 2013, 113, 2528–2549. [Google Scholar] [CrossRef]
- Khdary, N.H.; Almuarqab, B.T.; El Enany, G. Nanoparticle-Embedded Polymers and Their Applications: A Review. Membranes 2023, 13, 537. [Google Scholar] [CrossRef] [PubMed]
- Kharazmi, A.; Saion, E.; Faraji, N.; Soltani, N.; Dehzangi, A. Optical Properties of CdS/PVA Nanocomposite Films Synthesized Using the Gamma-Irradiation-Induced Method. Chin. Phys. Lett. 2013, 30, 057803. [Google Scholar] [CrossRef]
- Yang, Q.; Li, D.; Hong, Y.; Li, Z.; Wang, C.; Qiu, S.; Wei, Y. Preparation and Characterization of a PAN Nanofibre Containing Ag Nanoparticles via Electrospinning. In Proceedings of the Synthetic Metals, Shanghai, China, 4 April 2003; Volume 137, pp. 973–974. [Google Scholar] [CrossRef]
- Shalumon, K.T.; Anulekha, K.H.; Nair, S.V.; Nair, S.V.; Chennazhi, K.P.; Jayakumar, R. Sodium Alginate/Poly(Vinyl Alcohol)/Nano ZnO Composite Nanofibers for Antibacterial Wound Dressings. Int. J. Biol. Macromol. 2011, 49, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Bruening, M.L. Catalytic Nanoparticles Formed by Reduction of Metal Ions in Multilayered Polyelectrolyte Films. Nano Lett. 2002, 2, 497–501. [Google Scholar] [CrossRef]
- Li, Z.; Huang, H.; Shang, T.; Yang, F.; Zheng, W.; Wang, C.; Manohar, S.K. Facile Synthesis of Single-Crystal and Controllable Sized Silver Nanoparticles on the Surfaces of Polyacrylonitrile Nanofibres. Nanotechnology 2006, 17, 917–920. [Google Scholar] [CrossRef]
- Bottino, A.; Capannelli, G.; Comite, A. Preparation and Characterization of Novel Porous PVDF-ZrO2 Composite Membranes. Desalination 2002, 146, 35–40. [Google Scholar] [CrossRef]
- Bottino, A.; Capannelli, G.; D’Asti, V.; Piaggio, P. Preparation and Properties of Novel Organic-Inorganic Porous Membranes. Sep. Purif. Technol. 2001, 22–23, 269–275. [Google Scholar] [CrossRef]
- Haider, A.; Kang, I.K. Preparation of Silver Nanoparticles and Their Industrial and Biomedical Applications: A Comprehensive Review. Adv. Mater. Sci. Eng. 2015, 2015, 165257. [Google Scholar] [CrossRef]
- Radha, K.V.; Selvi, V.S.; Aarcha, J. Silver Nanofiber Membranes for Indoor Air Pollution Treatment. Sustain. Chem. Clim. Action 2025, 6, 100056. [Google Scholar] [CrossRef]
- Dallas, P.; Tucek, J.; Jancik, D.; Kolar, M.; Panacek, A.; Zboril, R. Magnetically Controllable Silver Nanocomposite with Multifunctional Phosphotriazine Matrix and High Antimicrobial Activity. Adv. Funct. Mater. 2010, 20, 2347–2354. [Google Scholar] [CrossRef]
- Tunç, T. Synthesis and Characterization of Silver Nanoparticles Loaded with Carboplatin as a Potential Antimicrobial and Cancer Therapy. Cancer Nanotechnol. 2024, 15, 2. [Google Scholar] [CrossRef]
- Gan, Y.X. Nanofibers and Their Composites: Fabrication, Characterization, and Structure with Particular Emphasis on Their Advantages and Disadvantages. In Polymeric Nanofibers and Their Composites: Recent Advances and Applications; Elsevier Science Ltd.: Amsterdam, The Netherlands, 2024; pp. 1–30. ISBN 9780443141287. [Google Scholar]
- Frenot, A.; Chronakis, I.S. Polymer Nanofibers Assembled by Electrospinning. Curr. Opin. Colloid Interface Sci. 2003, 8, 64–75. [Google Scholar] [CrossRef]
- Wang, H.; Tian, Z.; Wang, L.; Wang, H.; Zhang, Y.; Shi, Z. Advancements, Functionalization Techniques, and Multifunctional Applications in Biomedical and Industrial Fields of Electrospun Pectin Nanofibers: A Review. Int. J. Biol. Macromol. 2025, 307, 141964. [Google Scholar] [CrossRef]
- Phan, D.-N.; Dorjjugder, N.; Saito, Y.; Taguchi, G.; Lee, H.; Lee, J.S.; Kim, I.-S. The Mechanistic Actions of Different Silver Species at the Surfaces of Polyacrylonitrile Nanofibers Regarding Antibacterial Activities. Mater. Today Commun. 2019, 21, 100622. [Google Scholar] [CrossRef]
- Xu, X.; Yang, Q.; Wang, Y.; Yu, H.; Chen, X.; Jing, X. Biodegradable Electrospun Poly(l-Lactide) Fibers Containing Antibacterial Silver Nanoparticles. Eur. Polym. J. 2006, 42, 2081–2087. [Google Scholar] [CrossRef]
- Fan, L.; Dong, Y.; Ismail, B.B.; Zhang, L.; Shi, Y.; Wu, D.; Wu, Y.; Li, G. The Antimicrobial Activity and Resistance Evolution of Nanomaterials: A Review. ACS Mater. Lett. 2025, 7, 1085–1111. [Google Scholar] [CrossRef]
- Burger, C.; Hsiao, B.S.; Chu, B. Nanofibrous Materials and Their Applications. Annu. Rev. Mater. Res. 2006, 36, 333–368. [Google Scholar] [CrossRef]
- Huang, Z.-M.; Zhang, Y.-Z.; Kotaki, M.; Ramakrishna, S. A Review on Polymer Nanofibers by Electrospinning and Their Applications in Nanocomposites. Compos. Sci. Technol. 2003, 63, 2223–2253. [Google Scholar] [CrossRef]
- Kriegel, C.; Arrechi, A.; Kit, K.; McClements, D.J.; Weiss, J. Fabrication, Functionalization, and Application of Electrospun Biopolymer Nanofibers. Crit. Rev. Food Sci. Nutr. 2008, 48, 775–797. [Google Scholar] [CrossRef]
- Gavande, V.; Nagappan, S.; Seo, B.; Lee, W.-K. A Systematic Review on Green and Natural Polymeric Nanofibers for Biomedical Applications. Int. J. Biol. Macromol. 2024, 262, 130135. [Google Scholar] [CrossRef]
- Farboudi, A.; Mahboobnia, K.; Chogan, F.; Karimi, M.; Askari, A.; Banihashem, S.; Davaran, S.; Irani, M. UiO-66 Metal Organic Framework Nanoparticles Loaded Carboxymethyl Chitosan/Poly Ethylene Oxide/Polyurethane Core-Shell Nanofibers for Controlled Release of Doxorubicin and Folic Acid. Int. J. Biol. Macromol. 2020, 150, 178–188. [Google Scholar] [CrossRef]
- Cordoba, A.; Saldias, C.; Urzúa, M.; Montalti, M.; Guernelli, M.; Focarete, M.L.; Leiva, A. On the Versatile Role of Electrospun Polymer Nanofibers as Photocatalytic Hybrid Materials Applied to Contaminated Water Remediation: A Brief Review. Nanomaterials 2022, 12, 756. [Google Scholar] [CrossRef]
- Elsayed, R.; Teow, Y.H. Advanced Functional Polymer Materials for Biomedical Applications. J. Appl. Polym. Sci. 2025, 142, 1–34. [Google Scholar] [CrossRef]
- Aktürk, A.; Cenik, B.; Aydoğdu, Z.; Taygun, M.E.; Güler, F.K.; Küçükbayrak, S. Fabrication and Characterization of Polyvinyl Alcohol/Gelatin/ Silver Nanoparticle Nanocomposite Materials. Eurasian J. Biol. Chem. Sci. 2019, 2, 1–6. [Google Scholar]
- Raei, M.; Shabani, M.; Parivar, K.; Najafi, M.; Adabi, M. Development, Characterization and Bioactivity of PVA/VPA/Aloin Nanofibrous Scaffolds for Biomedical Applications. Nanomed. Res. J. 2024, 9, 356–372. [Google Scholar] [CrossRef]
- Fu, S.; Sun, Z.; Huang, P.; Li, Y.; Hu, N. Some Basic Aspects of Polymer Nanocomposites: A Critical Review. Nano Mater. Sci. 2019, 1, 2–30. [Google Scholar] [CrossRef]
- Kaur, S.; Sundarrajan, S.; Rana, D.; Sridhar, R.; Gopal, R.; Matsuura, T.; Ramakrishna, S. Review: The Characterization of Electrospun Nanofibrous Liquid Filtration Membranes. J. Mater. Sci. 2014, 49, 6143–6159. [Google Scholar] [CrossRef]
- Opriș, O.; Mormile, C.; Lung, I.; Stegarescu, A.; Soran, M.-L.; Soran, A. An Overview of Biopolymers for Drug Delivery Applications. Appl. Sci. 2024, 14, 1383. [Google Scholar] [CrossRef]
- Hong, K.H.; Park, J.L.; Hwan Sul, I.N.; Youk, J.H.; Kang, T.J. Preparation of Antimicrobial Poly(Vinyl Alcohol) Nanofibers Containing Silver Nanoparticles. J. Polym. Sci. B Polym. Phys. 2006, 44, 2468–2474. [Google Scholar] [CrossRef]
- Mansur, H.S.; Mansur, A.A.P. CdSe Quantum Dots Stabilized by Carboxylic-Functionalized PVA: Synthesis and UV–Vis Spectroscopy Characterization. Mater. Chem. Phys. 2011, 125, 709–717. [Google Scholar] [CrossRef]
- Fathollahipour, S.; Abouei Mehrizi, A.; Ghaee, A.; Koosha, M. Electrospinning of PVA/Chitosan Nanocomposite Nanofibers Containing Gelatin Nanoparticles as a Dual Drug Delivery System. J. Biomed. Mater. Res. A 2015, 103, 3852–3862. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Wu, X.; Zhao, Y.; Xu, L.; Wei, S. Nanofibrous Scaffold Prepared by Electrospinning of Poly(Vinyl Alcohol)/Gelatin Aqueous Solutions. J. Appl. Polym. Sci. 2011, 121, 3047–3055. [Google Scholar] [CrossRef]
- Lin, W.-C.; Yu, D.-G.; Yang, M.-C. Blood Compatibility of Novel Poly(γ-Glutamic Acid)/Polyvinyl Alcohol Hydrogels. Colloids Surf. B Biointerfaces 2006, 47, 43–49. [Google Scholar] [CrossRef]
- Krevelen, D.W. Some Basic Aspects of Flame Resistance of Polymeric Materials. Polymer 1975, 16, 615–620. [Google Scholar] [CrossRef]
- Türkoğlu, G.C.; Khomarloo, N.; Mohsenzadeh, E.; Gospodinova, D.N.; Neznakomova, M.; Salaün, F. PVA-Based Electrospun Materials—A Promising Route to Designing Nanofiber Mats with Desired Morphological Shape—A Review. Int. J. Mol. Sci. 2024, 25, 1668. [Google Scholar] [CrossRef]
- Khanna, P.K.; Singh, N.; Charan, S.; Subbarao, V.V.V.S.; Gokhale, R.; Mulik, U.P. Synthesis and Characterization of Ag/PVA Nanocomposite by Chemical Reduction Method. Mater. Chem. Phys. 2005, 93, 117–121. [Google Scholar] [CrossRef]
- Kharazmi, A.; Faraji, N.; Mat Hussin, R.; Saion, E.; Yunus, W.M.M.; Behzad, K. Structural, Optical, Opto-Thermal and Thermal Properties of ZnS–PVA Nanofluids Synthesized through a Radiolytic Approach. Beilstein J. Nanotechnol. 2015, 6, 529–536. [Google Scholar] [CrossRef]
- Balakrishnan, S.B.; Thambusamy, S. Preparation of Silver Nanoparticles and Riboflavin Embedded Electrospun Polymer Nanofibrous Scaffolds for in Vivo Wound Dressing Application. Process Biochem. 2020, 88, 148–158. [Google Scholar] [CrossRef]
- Zhang, C.Q.; Wang, Y.M.; Li, S.Z.; Feng, X.D.; Liu, L.H.; Wang, Y.; Zhao, L.P. Electrospinning and Catalytic Properties of Cyclodextrin Functionalized Polyoxymethylene (POM) Nanofibers Supported by Silver Nanoparticles. Adv. Polym. Technol. 2021, 2021, 8272626. [Google Scholar] [CrossRef]
- Andrade, P.F.; de Faria, A.F.; da Silva, D.S.; Bonacin, J.A.; do Carmo Gonçalves, M. Structural and Morphological Investigations of β-Cyclodextrin-Coated Silver Nanoparticles. Colloids Surf. B Biointerfaces 2014, 118, 289–297. [Google Scholar] [CrossRef]
- Teaima, M.H.; Abdelnaby, F.A.; Fadel, M.; El-Nabarawi, M.A.; Shoueir, K.R. Synthesis of Biocompatible and Environmentally Nanofibrous Mats Loaded with Moxifloxacin as a Model Drug for Biomedical Applications. Pharmaceutics 2020, 12, 1029. [Google Scholar] [CrossRef] [PubMed]
- Bouzitoun, M.; Mlika, R.; Gam, H.; Ouada, H.B.; Majdoub, M.; Sfihi, H. A Non-Water-Soluble Modified β-Cyclodextrin for Sensitive Electrode. Mater. Sci. Eng. C 2006, 26, 481–485. [Google Scholar] [CrossRef]
- Tingry, S.; Innocent, C.; Touil, S.; Deratani, A.; Seta, P. Carbon Paste Biosensor for Phenol Detection of Impregnated Tissue: Modification of Selectivity by Using β-Cyclodextrin-Containing PVA Membrane. Mater. Sci. Eng. C 2006, 26, 222–226. [Google Scholar] [CrossRef]
- Li, R.; Dou, J.; Jiang, Q.; Li, J.; Xie, Z.; Liang, J.; Ren, X. Preparation and Antimicrobial Activity of β-Cyclodextrin Derivative Copolymers/Cellulose Acetate Nanofibers. Chem. Eng. J. 2014, 248, 264–272. [Google Scholar] [CrossRef]
- Anceschi, A.; Caldera, F.; Bertasa, M.; Cecone, C.; Trotta, F.; Bracco, P.; Zanetti, M.; Malandrino, M.; Mallon, P.E.; Scalarone, D. New Poly(β-Cyclodextrin)/Poly(Vinyl Alcohol) Electrospun Sub-Micrometric Fibers and Their Potential Application for Wastewater Treatments. Nanomaterials 2020, 10, 482. [Google Scholar] [CrossRef]
- Morin-Crini, N.; Crini, G. Environmental Applications of Water-Insoluble β-Cyclodextrin–Epichlorohydrin Polymers. Prog. Polym. Sci. 2013, 38, 344–368. [Google Scholar] [CrossRef]
- Dos Santos, J.P.; da Rosa Zavareze, E.; Dias, A.R.G.; Vanier, N.L. Immobilization of Xylanase and Xylanase–β-Cyclodextrin Complex in Polyvinyl Alcohol via Electrospinning Improves Enzyme Activity at a Wide PH and Temperature Range. Int. J. Biol. Macromol. 2018, 118, 1676–1684. [Google Scholar] [CrossRef]
- Celebioglu, A.; Aytac, Z.; Umu, O.C.O.; Dana, A.; Tekinay, T.; Uyar, T. One-Step Synthesis of Size-Tunable Ag Nanoparticles Incorporated in Electrospun PVA/Cyclodextrin Nanofibers. Carbohydr. Polym. 2014, 99, 808–816. [Google Scholar] [CrossRef]
- Norouzi, Z.; Abdouss, M. Preparation and Characterization of β-Cyclodextrin Grafted Chitosan Nanofibers via Electrospinning for Dye Removal. Polym. Bull. 2024, 81, 3641–3677. [Google Scholar] [CrossRef]
- Kianfar, P.; Vitale, A.; Dalle Vacche, S.; Bongiovanni, R. Enhancing Properties and Water Resistance of PEO-Based Electrospun Nanofibrous Membranes by Photo-Crosslinking. J. Mater. Sci. 2021, 56, 1879–1896. [Google Scholar] [CrossRef]
- Subramanian, A.; Vu, D.; Larsen, G.F.; Lin, H.-Y. Preparation and Evaluation of the Electrospun Chitosan/PEO Fibers for Potential Applications in Cartilage Tissue Engineering. J. Biomater. Sci. Polym. Ed. 2005, 16, 861–873. [Google Scholar] [CrossRef] [PubMed]
- Schneider, H.E.; Steuber, J.; Du, W.; Mortazavi, M.; Bullock, D. Polyethylene Oxide Nanofiber Production by Electrospinning. J. Ark. Acad. Sci. 2016, 70, 35. [Google Scholar] [CrossRef]
- Xu, F.; Gough, I.; Dorogin, J.; Sheardown, H.; Hoare, T. Nanostructured Degradable Macroporous Hydrogel Scaffolds with Controllable Internal Morphologies via Reactive Electrospinning. Acta Biomater. 2020, 104, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Rajam, S.; Ho, C.C. Graft Coupling of PEO to Mixed Cellulose Esters Microfiltration Membranes by UV Irradiation. J. Membr. Sci. 2006, 281, 211–218. [Google Scholar] [CrossRef]
- Aqil, A.; Vasseur, S.; Duguet, E.; Passirani, C.; Benoît, J.P.; Roch, A.; Müller, R.; Jérôme, R.; Jérôme, C. PEO Coated Magnetic Nanoparticles for Biomedical Application. Eur. Polym. J. 2008, 44, 3191–3199. [Google Scholar] [CrossRef]
- Liu, S.L.; Shao, L.; Chua, M.L.; Lau, C.H.; Wang, H.; Quan, S. Recent Progress in the Design of Advanced PEO-Containing Membranes for CO2 Removal. Prog. Polym. Sci. 2013, 38, 1089–1120. [Google Scholar] [CrossRef]
- Deng, F.; Wang, X.; He, D.; Hu, J.; Gong, C.; Ye, Y.S.; Xie, X.; Xue, Z. Microporous Polymer Electrolyte Based on PVDF/PEO Star Polymer Blends for Lithium Ion Batteries. J. Membr. Sci. 2015, 491, 82–89. [Google Scholar] [CrossRef]
- Li, D.; Xia, Y. Direct Fabrication of Composite and Ceramic Hollow Nanofibers by Electrospinning. Nano Lett. 2004, 4, 933–938. [Google Scholar] [CrossRef]
- Filip, P.; Peer, P. Characterization of Poly(Ethylene Oxide) Nanofibers—Mutual Relations between Mean Diameter of Electrospun Nanofibers and Solution Characteristics. Processes 2019, 7, 948. [Google Scholar] [CrossRef]
- Zhang, M.; Song, W.; Tang, Y.; Xu, X.; Huang, Y.; Yu, D. Polymer-Based Nanofiber–Nanoparticle Hybrids and Their Medical Applications. Polymers 2022, 14, 351. [Google Scholar] [CrossRef] [PubMed]
- Liang, D.; Hsiao, B.S.; Chu, B. Functional Electrospun Nanofibrous Scaffolds for Biomedical Applications. Adv. Drug Deliv. Rev. 2007, 59, 1392–1412. [Google Scholar] [CrossRef]
- Matsumura, S.; Shimura, Y.; Terayama, K.; Kiyohara, T. Effects of Molecular Weight and Stereoregularity on Biodegradation of Poly(Vinyl Alcohol) by Alcaligenes Faecalis. Biotechnol. Lett. 1994, 16, 1205–1210. [Google Scholar] [CrossRef]
- Mori, T.; Sakimoto, M.; Kagi, T.; Sakai, T. Isolation and Characterization of a Strain of Bacillus Megaterium That Degrades Poly(Vinyl Alcohol). Biosci. Biotechnol. Biochem. 1996, 60, 330–332. [Google Scholar] [CrossRef]
- Zgoła-Grześkowiak, A.; Grześkowiak, T.; Zembrzuska, J.; Łukaszewski, Z. Comparison of Biodegradation of Poly(Ethylene Glycol)s and Poly(Propylene Glycol)s. Chemosphere 2006, 64, 803–809. [Google Scholar] [CrossRef]
- Hassounah, I.; Shehata, N.; Hudson, A.; Orler, B.; Meehan, K. Characteristics and 3D Formation of PVA and PEO Electrospun Nanofibers with Embedded Urea. J. Appl. Polym. Sci. 2014, 131, 39840. [Google Scholar] [CrossRef]
- Rickel, A.P.; Deng, X.; Engebretson, D.; Hong, Z. Electrospun Nanofiber Scaffold for Vascular Tissue Engineering. Mater. Sci. Eng. C 2021, 129, 112373. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Du, M.; Zou, M.; Xu, C.; Li, N.; Fu, Y. Facile and Green Synthesis of Well-Dispersed Au Nanoparticles in PAN Nanofibers by Tea Polyphenols. J. Mater. Chem. 2012, 22, 9301. [Google Scholar] [CrossRef]
- Fernandes, M.; Padrão, J.; Ribeiro, A.I.; Fernandes, R.D.V.; Melro, L.; Nicolau, T.; Mehravani, B.; Alves, C.; Rodrigues, R.; Zille, A. Polysaccharides and Metal Nanoparticles for Functional Textiles: A Review. Nanomaterials 2022, 12, 1006. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Xie, Y.; Ma, K.; Wei, Z.; Ran, X.; Fu, X.; Zhang, C.; Zhao, C. Electrospun Nanofibrous Membranes Meet Antibacterial Nanomaterials: From Preparation Strategies to Biomedical Applications. Bioact. Mater. 2024, 42, 478–518. [Google Scholar] [CrossRef]
- Sunaryono, S.; Rachmawati, A.; Yogihati, C.I.; Susanto, H.; Taufiq, A.; Mufti, N. The Effect of Ag Nanoparticles in Ag/Polyvinyl Alcohol Nanofiber Composites. Polym. Bull. 2022, 79, 555–568. [Google Scholar] [CrossRef]
- Fouda, M.M.G.; El-Aassar, M.R.; Al-Deyab, S.S. Antimicrobial Activity of Carboxymethyl Chitosan/Polyethylene Oxide Nanofibers Embedded Silver Nanoparticles. Carbohydr. Polym. 2013, 92, 1012–1017. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.-H.; Lee, K.-H.; Lee, B.-T. Fabrication of Ag Nanoparticles Dispersed in PVA Nanowire Mats by Microwave Irradiation and Electro-Spinning. Mater. Sci. Eng. C 2010, 30, 944–950. [Google Scholar] [CrossRef]
- Xiao, S.; Xu, W.; Ma, H.; Fang, X. Size-Tunable Ag Nanoparticles Immobilized in Electrospun Nanofibers: Synthesis, Characterization, and Application for Catalytic Reduction of 4-Nitrophenol. RSC Adv. 2012, 2, 319–327. [Google Scholar] [CrossRef]
- George, N.; Chakraborty, S.; Mary, N.L.; Suguna, L. Incorporating Silver Nanoparticles into Electrospun Nanofibers of Casein/Polyvinyl Alcohol to Develop Scaffolds for Tissue Engineering. Int. J. Biol. Macromol. 2024, 267, 131501. [Google Scholar] [CrossRef]
- Celebioglu, A.; Topuz, F.; Yildiz, Z.I.; Uyar, T. One-Step Green Synthesis of Antibacterial Silver Nanoparticles Embedded in Electrospun Cyclodextrin Nanofibers. Carbohydr. Polym. 2019, 207, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y. A Sustainable Approach to Fabricating Ag Nanoparticles/PVA Hybrid Nanofiber and Its Catalytic Activity. Nanomaterials 2015, 5, 1124–1135. [Google Scholar] [CrossRef]
- Hong, K.H. Preparation and Properties of Electrospun Poly(Vinyl Alcohol)/Silver Fiber Web as Wound Dressings. Polym. Eng. Sci. 2007, 47, 43–49. [Google Scholar] [CrossRef]
- Li, C.; Fu, R.; Yu, C.; Li, Z.; Guan, H.; Hu, D.; Zhao, D.; Lu, L. Silver Nanoparticle/Chitosan Oligosaccharide/Poly(Vinyl Alcohol) Nanofibers as Wound Dressings: A Preclinical Study. Int. J. Nanomed. 2013, 8, 4131–4145. [Google Scholar] [CrossRef]
- Hamza, A.M.; Alhtheal, E.D.; Shakir, A.K. Enhancement the Efficiency of ZnOnanofiber Mats Antibacterial Using Novel PVA/Ag Nanoparticles. Energy Procedia 2017, 119, 615–621. [Google Scholar] [CrossRef]
- Abdulhussain, R.; Adebisi, A.; Conway, B.R.; Asare-Addo, K. Electrospun Nanofibers: Exploring Process Parameters, Polymer Selection, and Recent Applications in Pharmaceuticals and Drug Delivery. J. Drug Deliv. Sci. Technol. 2023, 90, 105156. [Google Scholar] [CrossRef]
- Lee, S.J.; Heo, D.N.; Moon, J.-H.; Ko, W.-K.; Lee, J.B.; Bae, M.S.; Park, S.W.; Kim, J.E.; Lee, D.H.; Kim, E.-C.; et al. Electrospun Chitosan Nanofibers with Controlled Levels of Silver Nanoparticles. Preparation, Characterization and Antibacterial Activity. Carbohydr. Polym. 2014, 111, 530–537. [Google Scholar] [CrossRef] [PubMed]
- Eatemadi, A.; Daraee, H.; Zarghami, N.; Melat Yar, H.; Akbarzadeh, A. Nanofiber: Synthesis and Biomedical Applications. Artif. Cells Nanomed. Biotechnol. 2016, 44, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Nemati, S.; Kim, S.; Shin, Y.M.; Shin, H. Current Progress in Application of Polymeric Nanofibers to Tissue Engineering. Nano Converg. 2019, 6, 36. [Google Scholar] [CrossRef]
- Cho, Y.; Beak, J.W.; Sagong, M.; Ahn, S.; Nam, J.S.; Kim, I. Electrospinning and Nanofiber Technology: Fundamentals, Innovations, and Applications. Adv. Mater. 2025, 37, 2500162. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, H.M.; Klingner, A. A Review on Electrospun Polymeric Nanofibers: Production Parameters and Potential Applications. Polym. Test. 2020, 90, 106647. [Google Scholar] [CrossRef]
- Tao, G.; Cai, R.; Wang, Y.; Zuo, H.; He, H. Fabrication of Antibacterial Sericin Based Hydrogel as an Injectable and Mouldable Wound Dressing. Mater. Sci. Eng. C 2021, 119, 111597. [Google Scholar] [CrossRef]
- Li, H.M.; Zhang, Q.G.; Guo, N.N.; Zhu, A.M.; Liu, Q.L. Ultrafine Polystyrene Nanofibers and Its Application in Nanofibrous Membranes. Chem. Eng. J. 2015, 264, 329–335. [Google Scholar] [CrossRef]
- Uyar, T.; Besenbacher, F. Electrospinning of Uniform Polystyrene Fibers: The Effect of Solvent Conductivity. Polymer 2008, 49, 5336–5343. [Google Scholar] [CrossRef]
- Huan, S.; Liu, G.; Han, G.; Cheng, W.; Fu, Z.; Wu, Q.; Wang, Q. Effect of Experimental Parameters on Morphological, Mechanical and Hydrophobic Properties of Electrospun Polystyrene Fibers. Materials 2015, 8, 2718–2734. [Google Scholar] [CrossRef]
- Abrigo, M.; Kingshott, P.; McArthur, S.L. Electrospun Polystyrene Fiber Diameter Influencing Bacterial Attachment, Proliferation, and Growth. ACS Appl. Mater. Interfaces 2015, 7, 7644–7652. [Google Scholar] [CrossRef] [PubMed]
- Eda, G.; Shivkumar, S. Bead Structure Variations during Electrospinning of Polystyrene. J. Mater. Sci. 2006, 41, 5704–5708. [Google Scholar] [CrossRef]
- Wannatong, L.; Sirivat, A.; Supaphol, P. Effects of Solvents on Electrospun Polymeric Fibers: Preliminary Study on Polystyrene. Polym. Int. 2004, 53, 1851–1859. [Google Scholar] [CrossRef]
- Ibrahim, H.M.; Zairy, E. Carboxymethylchitosan Nanofibers Containing Silver Nanoparticles: Preparation, Characterization and Antibacterial Activity. J. Appl. Pharm. Sci. 2016, 6, 043–048. [Google Scholar] [CrossRef][Green Version]
- Chowdhury, M.F.M.; Khan, M.N.; Rahman, M.M. Metal Nanoparticles Incorporated Chitosan-Based Electrospun Nanofibre Mats for Wound Dressing Applications: A Review. Int. J. Biol. Macromol. 2024, 282, 137352. [Google Scholar] [CrossRef]
- Stevenson, C.L.; Santini, J.T.; Langer, R. Reservoir-Based Drug Delivery Systems Utilizing Microtechnology. Adv. Drug Deliv. Rev. 2012, 64, 1590–1602. [Google Scholar] [CrossRef]
- Prasher, P.; Singh, M.; Mudila, H. Silver Nanoparticles as Antimicrobial Therapeutics: Current Perspectives and Future Challenges. Biotech 2018, 8, 411. [Google Scholar] [CrossRef]
- Rai, M.K.; Deshmukh, S.D.; Ingle, A.P.; Gade, A.K. Silver Nanoparticles: The Powerful Nanoweapon against Multidrug-Resistant Bacteria. J. Appl. Microbiol. 2012, 112, 841–852. [Google Scholar] [CrossRef]
- Wang, L.S.; Wang, C.Y.; Yang, C.H.; Hsieh, C.L.; Chen, S.Y.; Shen, C.Y.; Wang, J.J.; Huang, K.S. Synthesis and Anti-Fungal Effect of Silver Nanoparticles–Chitosan Composite Particles. Int. J. Nanomed. 2015, 10, 2685–2696. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Ahmad, H.; Parveen, T.; Ahmad, A.; Oves, M.; Ismail, I.M.; Qari, H.A.; Umar, K.; Mohamad Ibrahim, M.N. Recent Advances in Metal Decorated Nanomaterials and Their Various Biological Applications: A Review. Front. Chem. 2020, 8, 341. [Google Scholar] [CrossRef]
- Mavani, K.; Shah, M. Synthesis of Silver Nanoparticles by Using Sodium Borohydride as a Reducing Agent. Int. J. Eng. Res. Technol. 2013, 2, 1–5. [Google Scholar] [CrossRef]
- Oliveira, M.M.; Ugarte, D.; Zanchet, D.; Zarbin, A.J.G. Influence of Synthetic Parameters on the Size, Structure, and Stability of Dodecanethiol-Stabilized Silver Nanoparticles. J. Colloid Interface Sci. 2005, 292, 429–435. [Google Scholar] [CrossRef]
- Durán, N.; Durán, M.; De Jesus, M.B.; Seabra, A.B.; Fávaro, W.J.; Nakazato, G. Silver Nanoparticles: A New View on Mechanistic Aspects on Antimicrobial Activity. Nanomedicine 2016, 12, 789–799. [Google Scholar] [CrossRef]
- Zawadzka, K.; Kądzioła, K.; Felczak, A.; Wrońska, N.; Piwoński, I.; Kisielewska, A.; Lisowska, K. Surface Area or Diameter—Which Factor Really Determines the Antibacterial Activity of Silver Nanoparticles Grown on TiO2 Coatings? New J. Chem. 2014, 38, 3275–3281. [Google Scholar] [CrossRef]
- Shi, Q.; Vitchuli, N.; Nowak, J.; Noar, J.; Caldwell, J.M.; Breidt, F.; Bourham, M.; McCord, M.; Zhang, X. One-Step Synthesis of Silver Nanoparticle-Filled Nylon 6 Nanofibers and Their Antibacterial Properties. J. Mater. Chem. 2011, 21, 10330. [Google Scholar] [CrossRef]
- Rzayev, Z.M.O.; Erdönmez, D.; Erkan, K.; Şimşek, M.; Bunyatova, U. Functional Copolymer/Organo-MMT Nanoarchitectures. XXII. Fabrication and Characterization of Antifungal and Antibacterial Poly (Vinyl Alcohol-Co-Vinyl Acetate/ODA-MMT/AgNPs Nanofibers and Nanocoatings by e-Spinning and c-Spinning Methods. Int. J. Polym. Mater. Polym. Biomater. 2015, 64, 267–278. [Google Scholar] [CrossRef]
- Sotiriou, G.A.; Pratsinis, S.E. Antibacterial Activity of Nanosilver Ions and Particles. Environ. Sci. Technol. 2010, 44, 5649–5654. [Google Scholar] [CrossRef]
- Bondarenko, O.; Ivask, A.; Käkinen, A.; Kurvet, I.; Kahru, A. Particle-Cell Contact Enhances Antibacterial Activity of Silver Nanoparticles. PLoS ONE 2013, 8, e64060. [Google Scholar] [CrossRef]
- Godakhindi, V.; Kravitz, E.; Vivero-Escoto, J.L. Light-Activable Silver Nanoparticles for Combatting Antibiotic-Resistant Bacteria and Biofilms. Molecules 2025, 30, 626. [Google Scholar] [CrossRef]
- More, P.R.; Pandit, S.; De Filippis, A.; Franci, G.; Mijakovic, I.; Galdiero, M. Silver Nanoparticles: Bactericidal and Mechanistic Approach against Drug Resistant Pathogens. Microorganisms 2023, 11, 369. [Google Scholar] [CrossRef]
- Alcolea-Rodriguez, V.; Dumit, V.I.; Ledwith, R.; Portela, R.; Bañares, M.A.; Haase, A. Differentially Induced Autophagy by Engineered Nanomaterial Treatment Has an Impact on Cellular Homeostasis and Cytotoxicity. Nano Lett. 2024, 24, 11793–11799. [Google Scholar] [CrossRef]
- Zhao, L.; Ling, L.; Lu, J.; Jiang, F.; Sun, J.; Zhang, Z.; Huang, Y.; Liu, X.; Zhu, Y.; Fu, X.; et al. Reactive oxygen species-responsive mitochondria-targeted liposomal quercetin attenuates retinal ischemia–reperfusion injury via regulating SIRT1/FOXO3A and p38 MAPK signaling pathways. Bioeng. Transl. Med. 2023, 8, e10460. [Google Scholar] [CrossRef]
- Yang, T.; Paulose, T.; Redan, B.W.; Mabon, J.C.; Duncan, T.V. Food and Beverage Ingredients Induce the Formation of Silver Nanoparticles in Products Stored within Nanotechnology-Enabled Packaging. ACS Appl. Mater. Interfaces 2021, 13, 1398–1412. [Google Scholar] [CrossRef]
- Han, J.; Kiss, L.; Mei, H.; Remete, A.M.; Ponikvar-Svet, M.; Sedgwick, D.M.; Roman, R.; Fustero, S.; Moriwaki, H.; Soloshonok, V.A. Chemical Aspects of Human and Environmental Overload with Fluorine. Chem. Rev. 2021, 121, 4678–4742. [Google Scholar] [CrossRef]
- Jayakumar, R.; Prabaharan, M.; Sudheesh Kumar, P.T.; Nair, S.V.; Tamura, H. Biomaterials Based on Chitin and Chitosan in Wound Dressing Applications. Biotechnol. Adv. 2011, 29, 322–337. [Google Scholar] [CrossRef]
- Le Ouay, B.; Stellacci, F. Antibacterial Activity of Silver Nanoparticles: A Surface Science Insight. Nano Today 2015, 10, 339–354. [Google Scholar] [CrossRef]
- Liang, D.; Lu, Z.; Yang, H.; Gao, J.; Chen, R. Novel Asymmetric Wettable AgNPs/Chitosan Wound Dressing: In Vitro and In Vivo Evaluation. ACS Appl. Mater. Interfaces 2016, 8, 3958–3968. [Google Scholar] [CrossRef] [PubMed]
- Kharaghani, D.; Kee Jo, Y.; Khan, M.Q.; Jeong, Y.; Cha, H.J.; Kim, I.S. Electrospun Antibacterial Polyacrylonitrile Nanofiber Membranes Functionalized with Silver Nanoparticles by a Facile Wetting Method. Eur. Polym. J. 2018, 108, 69–75. [Google Scholar] [CrossRef]
- Lv, H.; Cui, S.; Yang, Q.; Song, X.; Wang, D.; Hu, J.; Zhou, Y.; Liu, Y. AgNPs-Incorporated Nanofiber Mats: Relationship between AgNPs Size/Content, Silver Release, Cytotoxicity, and Antibacterial Activity. Mater. Sci. Eng. C 2021, 118, 111331. [Google Scholar] [CrossRef] [PubMed]
- Abdelgawad, A.M.; Hudson, S.M.; Rojas, O.J. Antimicrobial Wound Dressing Nanofiber Mats from Multicomponent (Chitosan/Silver-NPs/Polyvinyl Alcohol) Systems. Carbohydr. Polym. 2014, 100, 166–178. [Google Scholar] [CrossRef]
- Anisha, B.S.; Biswas, R.; Chennazhi, K.P.; Jayakumar, R. Chitosan–Hyaluronic Acid/Nano Silver Composite Sponges for Drug Resistant Bacteria Infected Diabetic Wounds. Int. J. Biol. Macromol. 2013, 62, 310–320. [Google Scholar] [CrossRef]
- Huang, S.; Zhou, L.; Li, M.-C.; Wu, Q.; Kojima, Y.; Zhou, D. Preparation and Properties of Electrospun Poly (Vinyl Pyrrolidone)/Cellulose Nanocrystal/Silver Nanoparticle Composite Fibers. Materials 2016, 9, 523. [Google Scholar] [CrossRef]
- Chaudhary, A.; Gupta, A.; Mathur, R.B.; Dhakate, S.R. Effective Antimicrobial Filter from Electrospun Polyacrylonitrile-Silver Composite Nanofibers Membrane for Conductive Environments. Adv. Mater. Lett. 2014, 5, 562–568. [Google Scholar] [CrossRef]
- Castro-Mayorga, J.; Fabra, M.; Cabedo, L.; Lagaron, J. On the Use of the Electrospinning Coating Technique to Produce Antimicrobial Polyhydroxyalkanoate Materials Containing In Situ-Stabilized Silver Nanoparticles. Nanomaterials 2016, 7, 4. [Google Scholar] [CrossRef] [PubMed]
- Kumarasinghe, K.G.U.R.; Silva, W.C.H.; Fernando, M.D.A.; Palliyaguru, L.; Jayawardena, P.S.; Shimomura, M.; Fernando, S.S.N.; Gunasekara, T.D.C.P.; Jayaweera, P.M. One-Pot Reducing Agent-Free Synthesis of Silver Nanoparticles/Nitrocellulose Composite Surface Coating with Antimicrobial and Antibiofilm Activities. BioMed Res. Int. 2021, 2021, 6666642. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Yuan, W.; Lu, Z.; Li, C.M. Polymer/Nanosilver Composite Coatings for Antibacterial Applications. Colloids Surf. A Physicochem. Eng. Asp. 2013, 439, 69–83. [Google Scholar] [CrossRef]
- Kumar, A.; Vemula, P.K.; Ajayan, P.M.; John, G. Silver-Nanoparticle-Embedded Antimicrobial Paints Based on Vegetable Oil. Nat. Mater. 2008, 7, 236–241. [Google Scholar] [CrossRef]
- Garipov, I.T.; Khaydarov, R.R.; Gapurova, O.U.; Khaydarov, R.A.; Lutfi, F.M.; Efimova, I.L.; Evgrafova, S.Y. Silver Nanoparticles as a New Generation of Antimicrobial Prophylaxis. J. Sib. Fed. Univ. Biol. 2019, 12, 266–276. [Google Scholar] [CrossRef]
- Zhang, L.; Jiang, Y.; Ding, Y.; Povey, M.; York, D. Investigation into the Antibacterial Behaviour of Suspensions of ZnO Nanoparticles (ZnO Nanofluids). J. Nanoparticle Res. 2007, 9, 479–489. [Google Scholar] [CrossRef]
- Moustafa, M.; Alamri, S.; Elnouby, M.; Taha, T.; Abu-Saied, M.A.; Shati, A.; Al-Kahtani, M.; Alrumman, S. Hydrothermal Preparation of TiO2-Ag Nanoparticles and Its Antimicrobial Performance against Human Pathogenic Microbial Cells in Water. Biocell 2018, 42, 93–97. [Google Scholar] [CrossRef]
- Lee, D.; Cohen, R.E.; Rubner, M.F. Antibacterial Properties of Ag Nanoparticle Loaded Multilayers and Formation of Magnetically Directed Antibacterial Microparticles. Langmuir 2005, 21, 9651–9659. [Google Scholar] [CrossRef] [PubMed]
- Gong, P.; Li, H.; He, X.; Wang, K.; Hu, J.; Tan, W.; Zhang, S.; Yang, X. Preparation and Antibacterial Activity of Fe3O4 @Ag Nanoparticles. Nanotechnology 2007, 18, 285604. [Google Scholar] [CrossRef]
- Jain, P.; Pradeep, T. Potential of Silver Nanoparticle-Coated Polyurethane Foam as an Antibacterial Water Filter. Biotechnol. Bioeng. 2005, 90, 59–63. [Google Scholar] [CrossRef]
- Bu, Y.; Chen, Z.; Li, W. Dramatically Enhanced Photocatalytic Properties of Ag-Modified Graphene–ZnO Quasi-Shell–Core Heterojunction Composite Material. RSC Adv. 2013, 3, 24118. [Google Scholar] [CrossRef]
- Salehi-Khojin, A.; Jhong, H.M.; Rosen, B.A.; Zhu, W.; Ma, S.; Kenis, P.J.A.; Masel, R.I. Nanoparticle Silver Catalysts That Show Enhanced Activity for Carbon Dioxide Electrolysis. J. Phys. Chem. C 2013, 117, 1627–1632. [Google Scholar] [CrossRef]
- Zhai, H.J.; Sun, D.W.; Wang, H.S. Catalytic Properties of Silica/Silver Nanocomposites. J. Nanosci. Nanotechnol. 2006, 6, 1968–1972. [Google Scholar] [CrossRef]
- Huang, C.-J.; Shieu, F.-S.; Hsieh, W.-P.; Chang, T.-C. Acidic Hydrolysis of a Poly(Vinyl Acetate) Matrix by the Catalytic Effect of Ag Nanoparticles and the Micellization of Ag-Metal-Containing Polymer. J. Appl. Polym. Sci. 2006, 100, 1457–1464. [Google Scholar] [CrossRef]

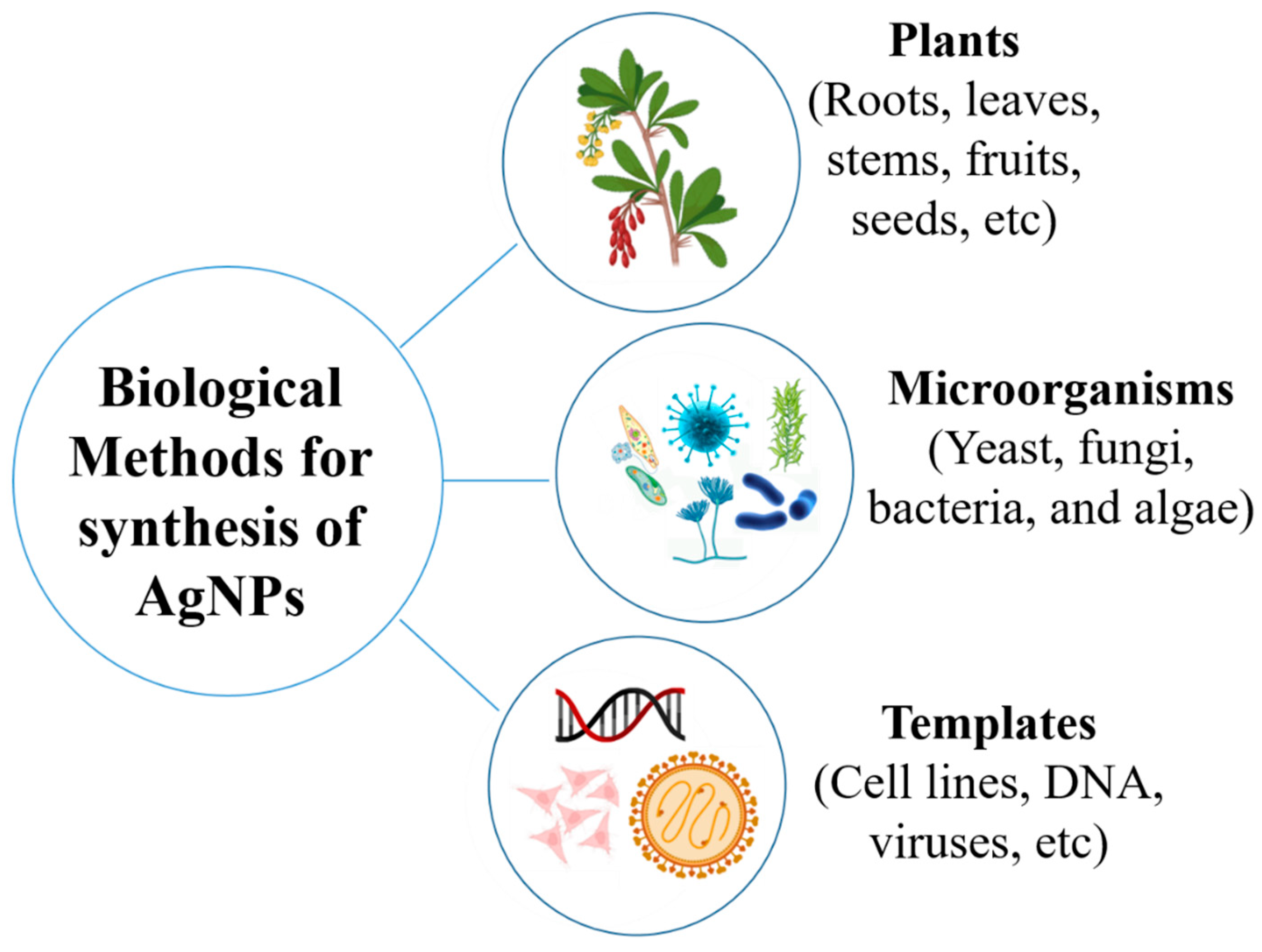
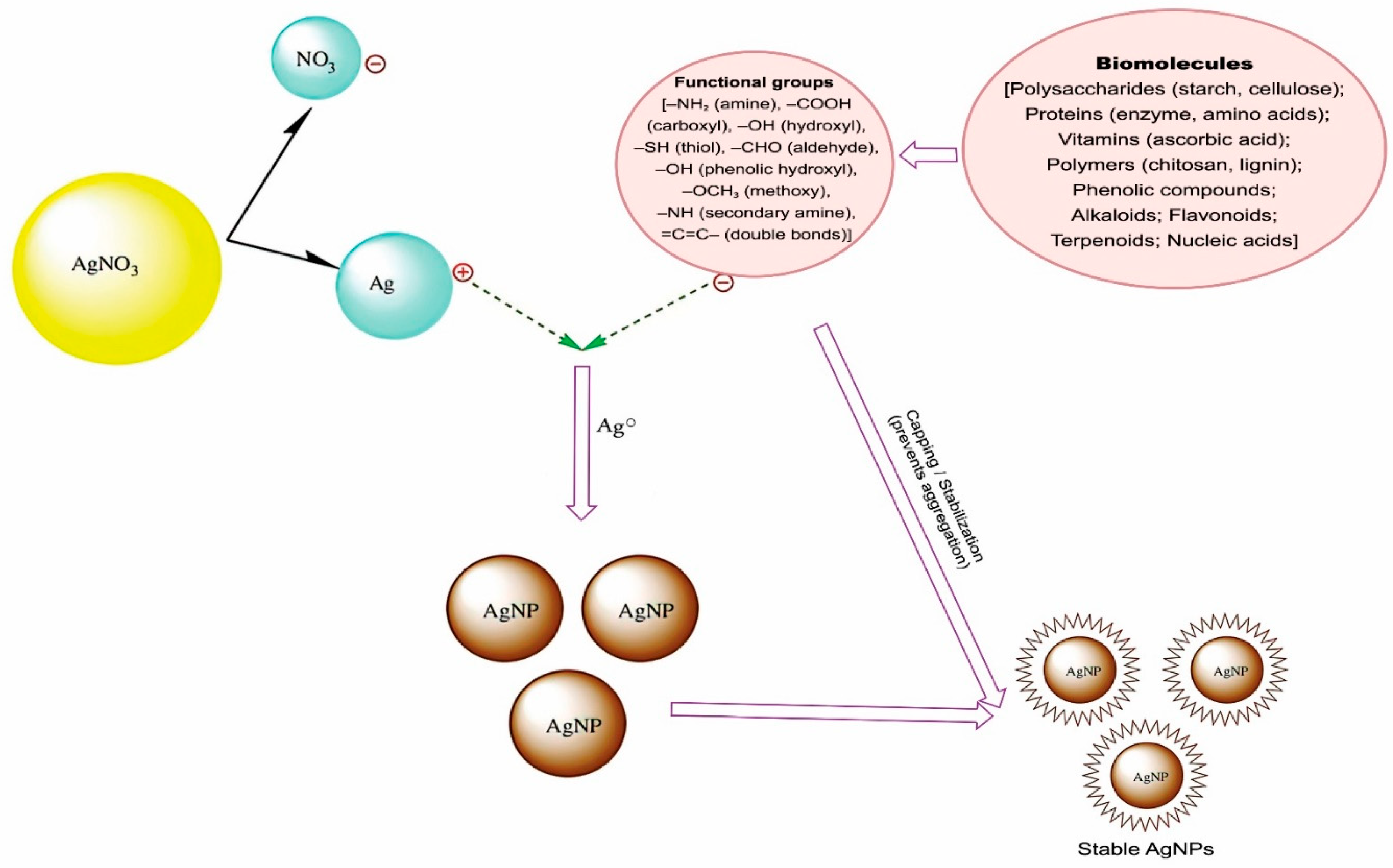
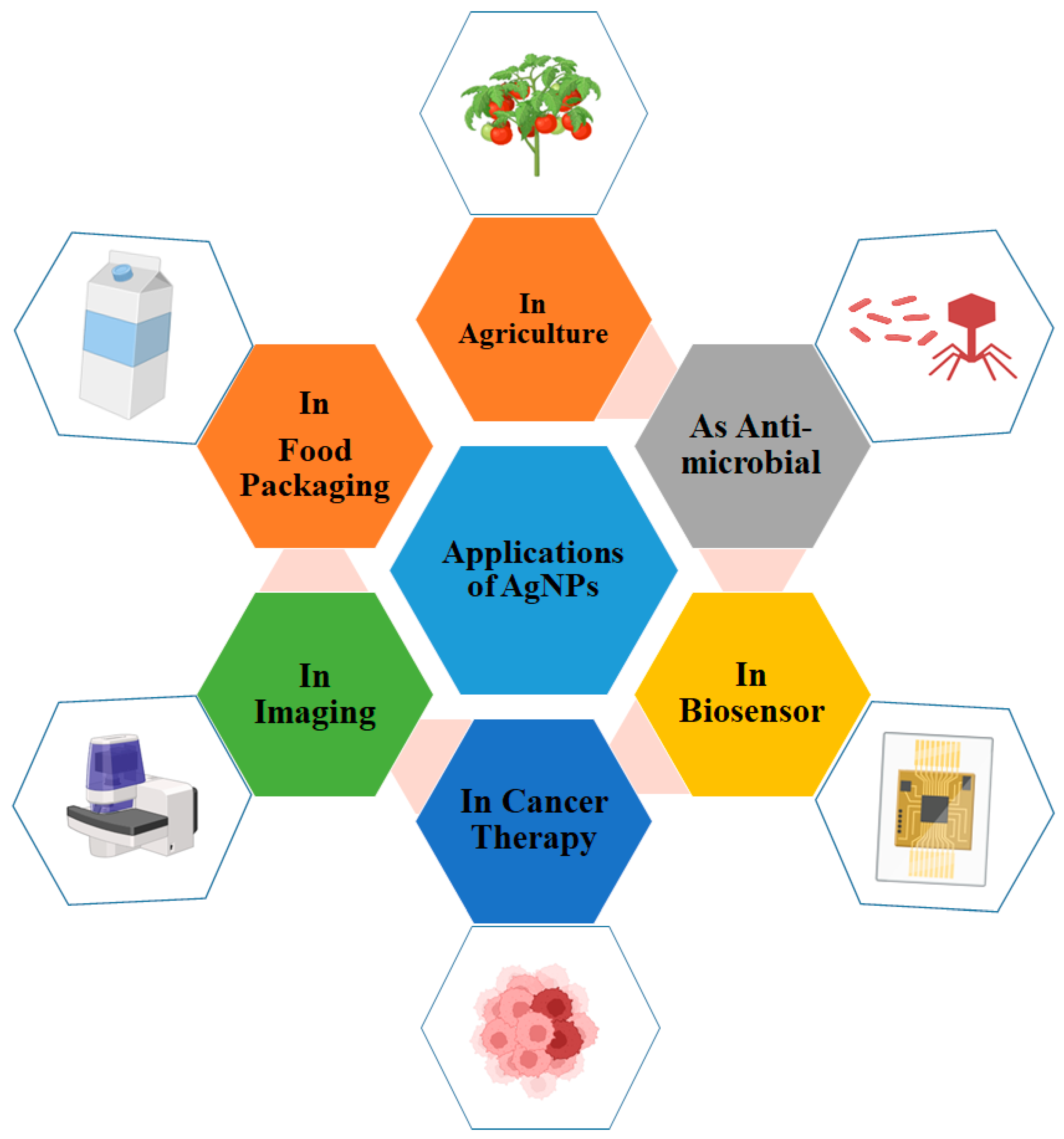

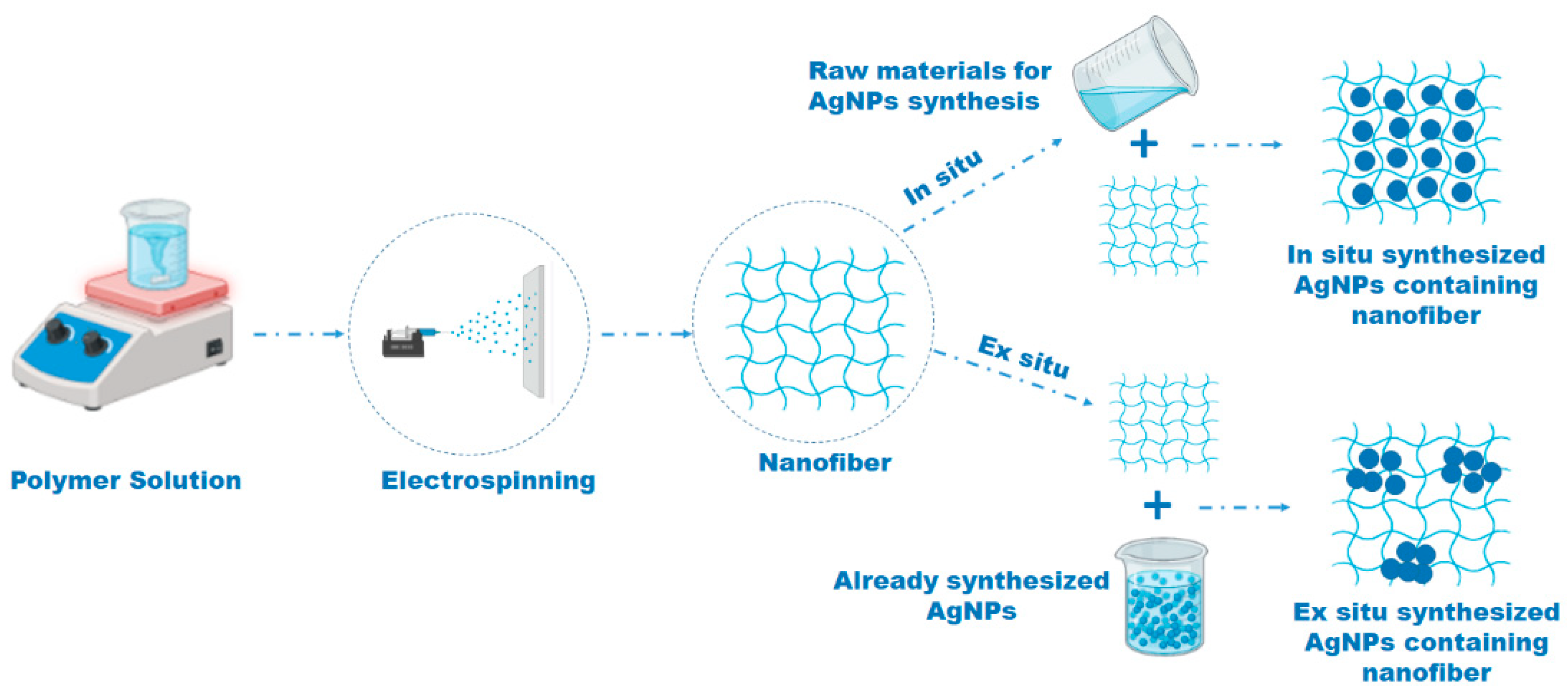
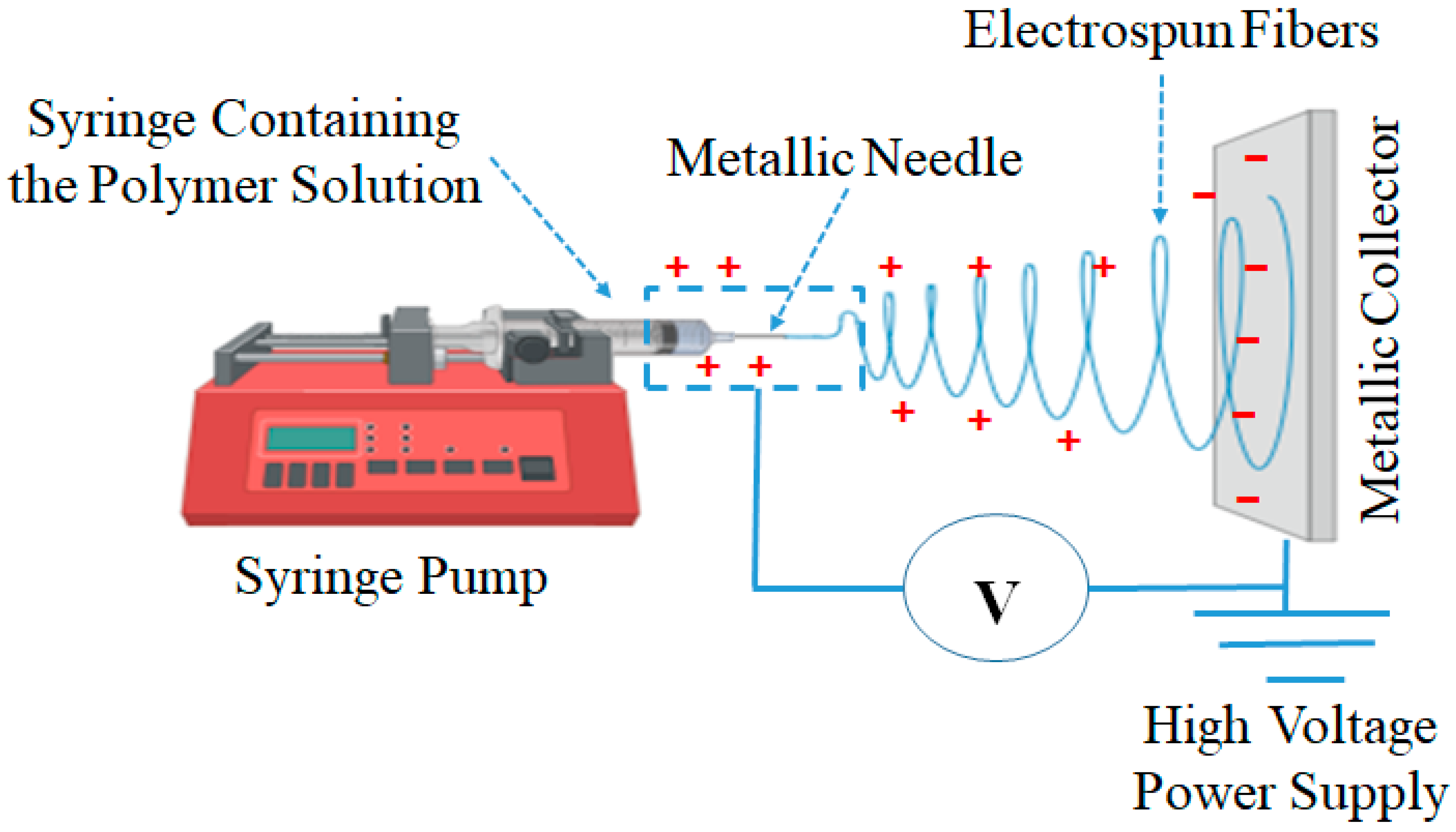
| Green Source | Scientific Names | Size (nm) | Shape | Wavelength (nm) | Zeta Potential (mV) | Applications | Reference |
|---|---|---|---|---|---|---|---|
| plant | Ligustrum lucidum | 13 | Spherical, triangular, polygonal, irregular | 438 | - | A novel fungistat not only for comprehensive control of plant fungi | [160] |
| plant | tea | 20–50 | spherical | 405–415 | −24.6 | Antibacterial activity | [161] |
| plant | Lallemantia royleana (Benth. in Wall.) | 34.47 ± 1.6 | spherical | 425 | −24.1 | Biopharmaceuticals and catalytic applications | [162] |
| plant | Allium cepa | 19.47 ± 1.12 | spherical | 439 | −13.1 | Biomedical activities and also has other possible industrial applications | [163] |
| plant | Juglans regia | 80-90 | spherical | 420 | −67.2 | Photocatalytic degradation of effluent dye | [164] |
| plant | Alpinia nigra | 49.36 | Spherical | 400–500 | - | Antimicrobial activity | [165] |
| plant | Berberis vulgaris | 30–70 | Spherical | 450 | 84.85 | Antibacterial activity | [166] |
| plant | Coleus forskohlii | 10–50 | Trigonal, hexagonal, spherical, rod | 420 | - | Antimicrobial activity | [167] |
| Bacteria | Leclercia acarboxylata | 17.43 | spherical | 423 | - | Antimicrobial activity | [31] |
| Bacteria | Bacillus brevis | 41–68 | Spherical | 420 | - | Antibacterial activity | [168] |
| Bacteria | Bacillus sp. | 22–41 | Spherical | 447 | - | Antifungal activity | [169] |
| Bacteria | Pseudoduganella eburnean | 8–24 | Spherical | 448 | - | Antimicrobial activity | [170] |
| Fungus | Setosphaeria rostrata | 2–20 | Spherical | 400 | - | Antibacterial activity | [171] |
| Fungus | Penicillium oxalicum | 60–80 | Spherical | 600 | - | Antibacterial activity | [172] |
| Fungus | Trichoderma asperellum | 15.5 ± 2.5 | Spherical | 410 | - | Antifungal activity | [173] |
| Fungus | Arthroderma fulvum | 15.5 ± 2.5 | Spherical | 420 | - | Antifungal activity | [174] |
| Fungus | Talaromyces purpureogenus | 30–60 | Spherical | 380–470 | −19.6 | Antibacterial and antioxidant activities | [175] |
| Algae | Portieria hornemannii | 35–50 | Spherical | 418 | −44.5 | Skyscraping activity against fish pathogens | [176] |
| Algae | Graesiella emersonii | 4–35.02 | Spherical | 400–415 | ±20–30 | Antibacterial activity | [177] |
| Algae | Enteromorpha compressa | 4–24 | Spherical | 421 | - | Biomedical and pharmaceutical applications. | [178] |
| Technique | Acronym | Information Provided | Principle |
|---|---|---|---|
| UV–Visible Spectroscopy | UV-Vis | Confirms the formation of AgNPs and provides preliminary data on size and stability. | Measures the absorption of light. AgNPs exhibit a unique SPR peak, typically between 400–450 nm. |
| Transmission Electron Microscopy | TEM | Determines particle size, size distribution, and morphology (shape). | An electron beam is transmitted through an ultra-thin sample, creating a high-resolution 2D projection image of the nanoparticles. |
| Scanning Electron Microscopy | SEM | Visualizes the surface morphology of AgNPs, especially when deposited on a substrate or embedded in nanofibers. | Scans the sample surface with a focused electron beam to produce images of the surface topography and composition. |
| Dynamic Light Scattering | DLS | Measures the hydrodynamic diameter (size in solution) and size distribution. | Analyzes the fluctuations in scattered light intensity caused by the Brownian motion of particles in a suspension. |
| Zeta Potential Analysis | Determines the surface charge and predicts the colloidal stability of the AgNPs suspension. | Measures the electrophoretic mobility of particles in an electric field. High absolute values (>±30 mV) indicate good stability. | |
| X-ray Diffraction | XRD | Identifies the crystalline structure and phase purity of the AgNPs. | Measures the scattering of X-rays as they pass through a sample, producing a diffraction pattern characteristic of the material’s crystal lattice. |
| Fourier-Transform Infrared Spectroscopy | FTIR | Identifies the functional groups of capping agents on the nanoparticle surface. | Measures the absorption of infrared radiation by the sample, revealing the vibrational modes of chemical bonds present. |
| Energy-Dispersive X-ray Spectroscopy | EDS/EDX | Confirms the elemental composition and purity of the sample. | Analyzes the X-rays emitted from a sample bombarded by an electron beam to identify the elements present. Often coupled with SEM or TEM. |
| Synthesis Method | Particle Size | Polydispersity/PDI | Zeta Potential (mV) | Scalability Challenges | Reference |
|---|---|---|---|---|---|
| Aqueous rhizome extract | TEM: ~5–40 nm (average < 20 nm) | NR | NR | Reproducibility and standardization of extract concentration; lack of absolute yield metrics—both limit direct scale-up planning | [223] |
| Aqueous leaf extract | TEM/XRD: ~15 ± 5 nm | Described as monodispersed | NR | Good size control in lab but process depends strongly on extract composition and kinetics (batch-to-batch variability); scale-up needs extract standardization and control of mixing/heat transfer. | [224] |
| Aqueous Geranium (Pelargonium) leaf extract | TEM/SEM: ~30–44 nm | narrow size distribution | −20 to −30 mV | Controlling extract phytochemical content at large scale is a major challenge. | [225] |
| Aqueous leaf extract | DLS/FESEM: ~28–32 nm | NR | −41.4 mV | Scaling requires consistent extract composition and filtration | [226] |
| Culture filtrate | DLS: 11–42 nm | Described as high dispersity | −26 ± 0.2 mV | Variability in microbial metabolite composition; absence of quantitative yield measurement; controlling consistency at scale | [227] |
| Extracellular secretions from fungi (Cladosporium, Penicillium, Purpureocillium) | TEM: mostly < 20 nm; DLS: Cladosporium/Purpureocillium ~166 nm; Penicillium ~124 nm | PDI: Cladosporium 0.074; Purpureocillium 0.279 | Cladosporium −15.7 mV; Penicillium −17.8 mV; Purpureocillium −13.0 mV | Scaling extracellular fungal systems requires consistent enzyme/metabolite production; larger hydrodynamic sizes might affect functionality and processing | [228] |
| Fungal extract | DLS Z-average: 240.2 nm | PDI: 0.720 (high; polydisperse) | −19.5 mV | Very large, polydisperse particles with borderline zeta (moderate stability); significant challenges for reproducibility and downstream uniformity | [175] |
| Extracellular pigment from fungus | NR | Not detailed | −24.8 ± 7.2 mV | Downstream drying adds step; pigment variability; moderate zeta suggests reasonable stability but consistency still a hurdle | [229] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdelmoneim, H.M.; Taha, T.H.; Alhudhaibi, A.M.; Afifi, F.M.; Faqihi, A.A.; Alsalamah, S.A.; Bendif, H. Green Synthesis of Silver Nanoparticles and Polymeric Nanofiber Composites: Fabrications, Mechanisms, and Applications. Polymers 2025, 17, 2327. https://doi.org/10.3390/polym17172327
Abdelmoneim HM, Taha TH, Alhudhaibi AM, Afifi FM, Faqihi AA, Alsalamah SA, Bendif H. Green Synthesis of Silver Nanoparticles and Polymeric Nanofiber Composites: Fabrications, Mechanisms, and Applications. Polymers. 2025; 17(17):2327. https://doi.org/10.3390/polym17172327
Chicago/Turabian StyleAbdelmoneim, Hany M., Tarek H. Taha, Abdulrahman Mohammed Alhudhaibi, Feras M. Afifi, Abdullah A. Faqihi, Sulaiman A. Alsalamah, and Hamdi Bendif. 2025. "Green Synthesis of Silver Nanoparticles and Polymeric Nanofiber Composites: Fabrications, Mechanisms, and Applications" Polymers 17, no. 17: 2327. https://doi.org/10.3390/polym17172327
APA StyleAbdelmoneim, H. M., Taha, T. H., Alhudhaibi, A. M., Afifi, F. M., Faqihi, A. A., Alsalamah, S. A., & Bendif, H. (2025). Green Synthesis of Silver Nanoparticles and Polymeric Nanofiber Composites: Fabrications, Mechanisms, and Applications. Polymers, 17(17), 2327. https://doi.org/10.3390/polym17172327







