Potential of Compost-Derived Actinomycetes for Low-Density Polyethylene Degradation
Abstract
1. Introduction
2. Materials and Methods
2.1. Origin of Bacteria
2.2. PCR Amplification and Sequencing of the 16S rRNA Genes
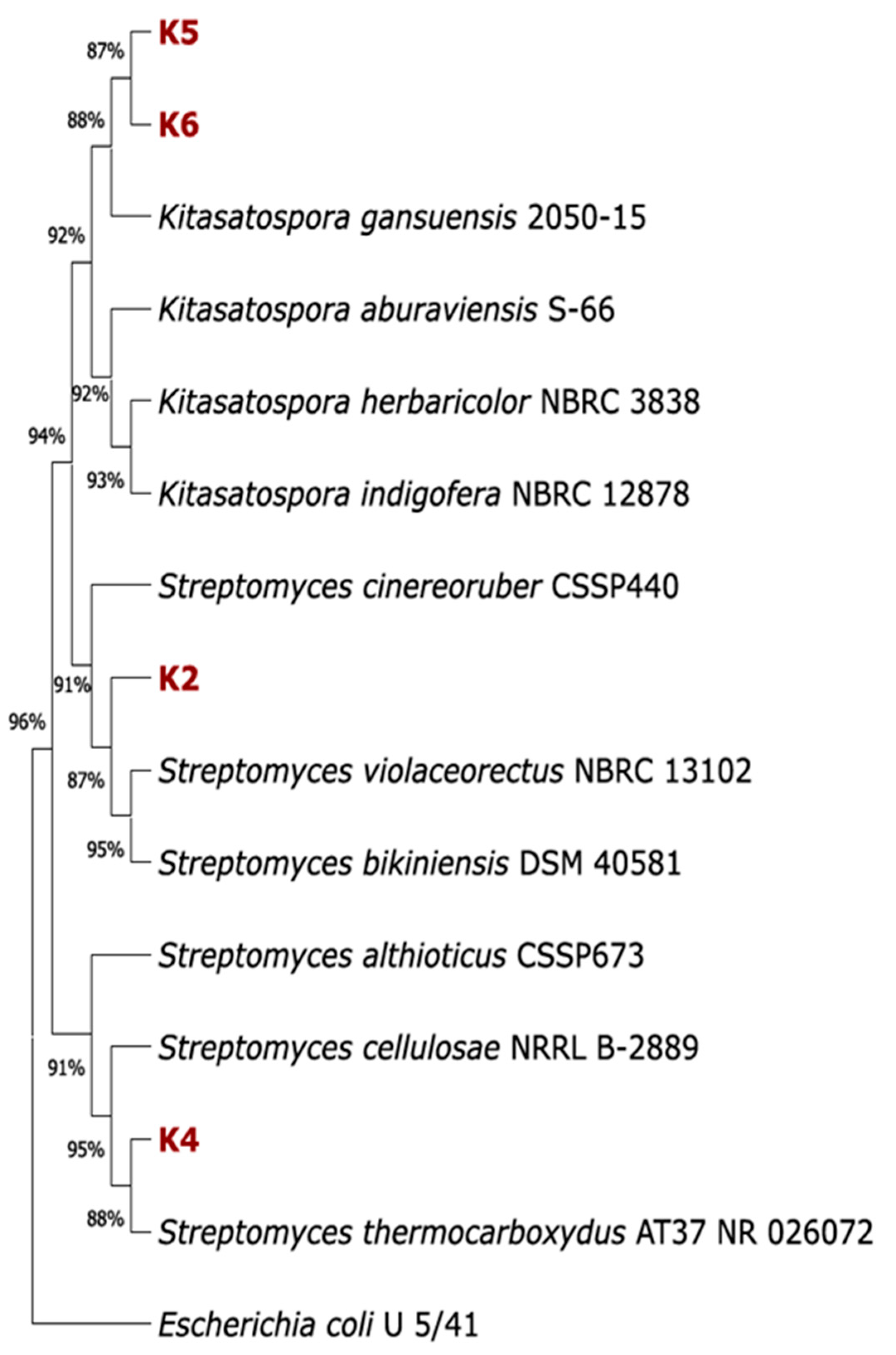
2.3. The Phylogenetic Analysis of Isolated Strains
2.4. Preparation of LDPE
2.5. Biodegradation Tests
2.5.1. Determination of LDPE Degradation
Weight Loss
The Water Contact Angle
pH Change
FTIR Analysis
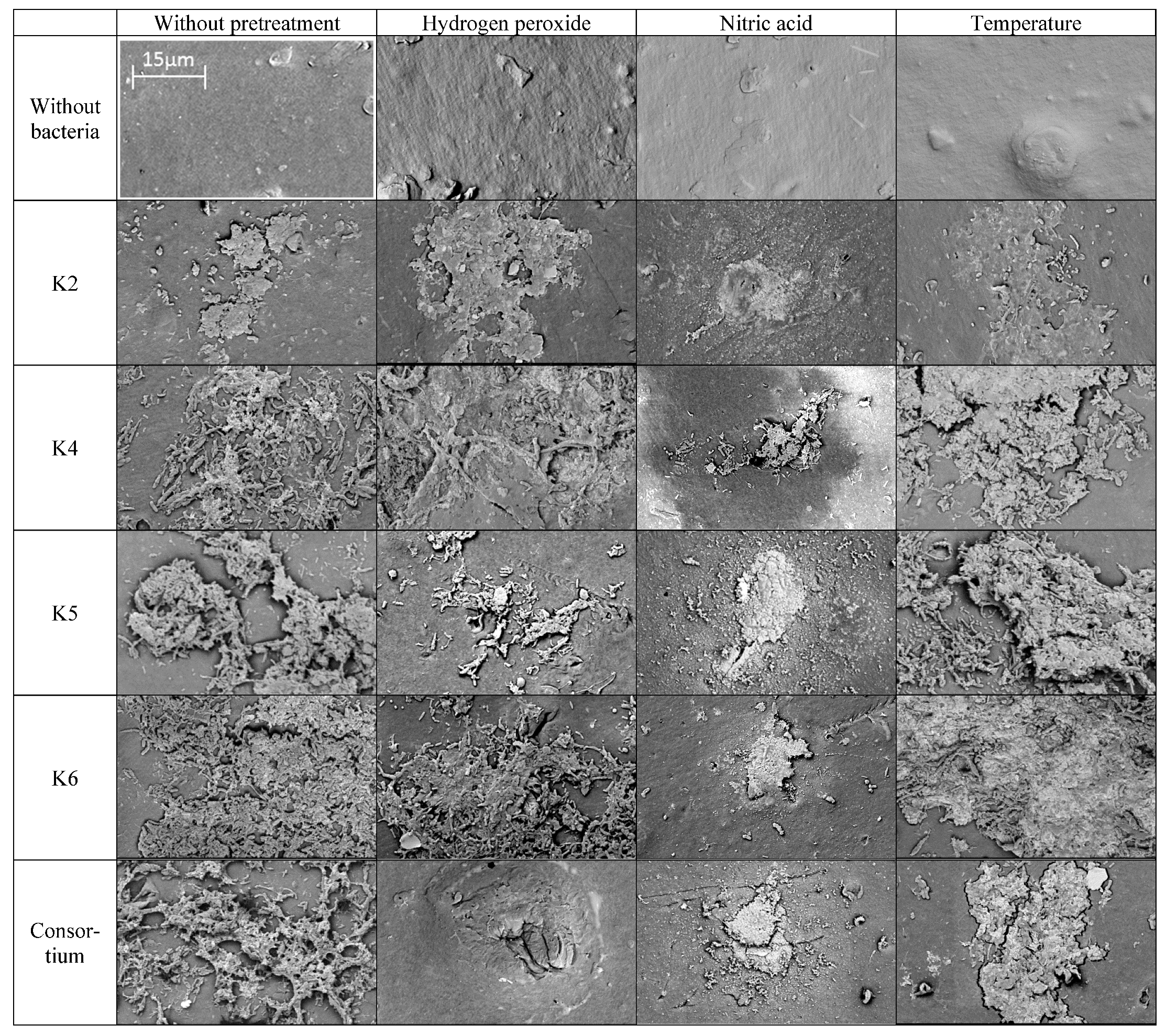
SEM Analysis
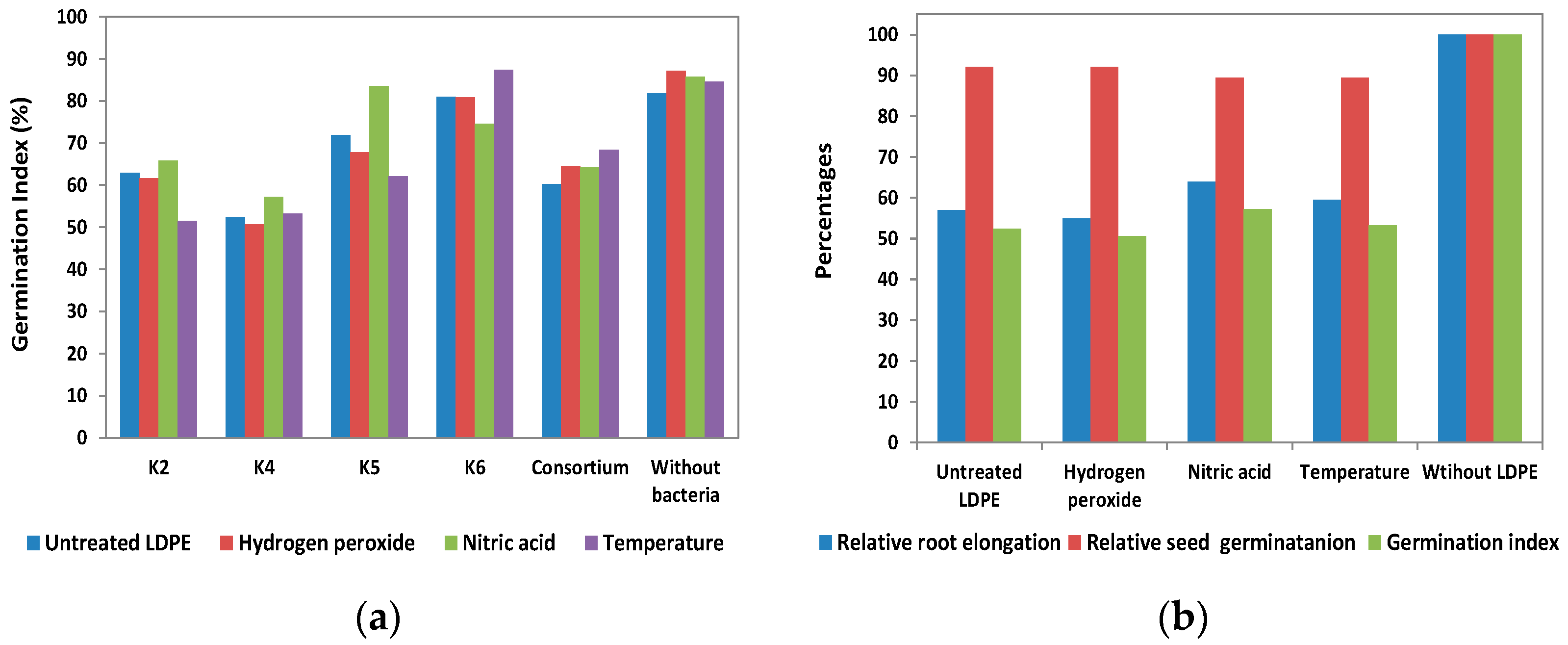
2.6. Phytotoxicity
3. Results
3.1. Phylogenetic Characterisation of the Actinomycetes Strains
3.2. Biodegradation Tests
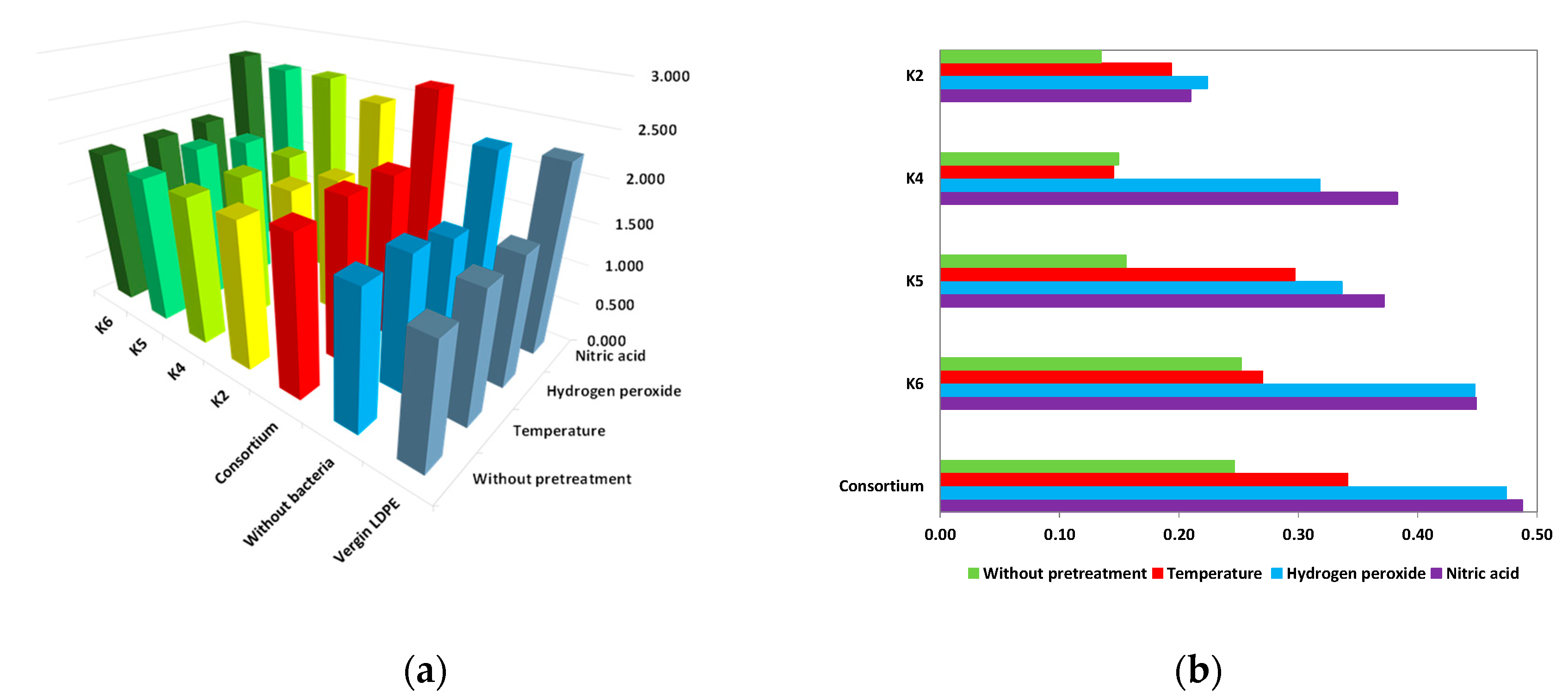
3.2.1. LDPE Film Weight Loss
- A.
- Virgin films
- B.
- Pretreatment
3.2.2. Water Contact Angle
3.2.3. Changes in pH
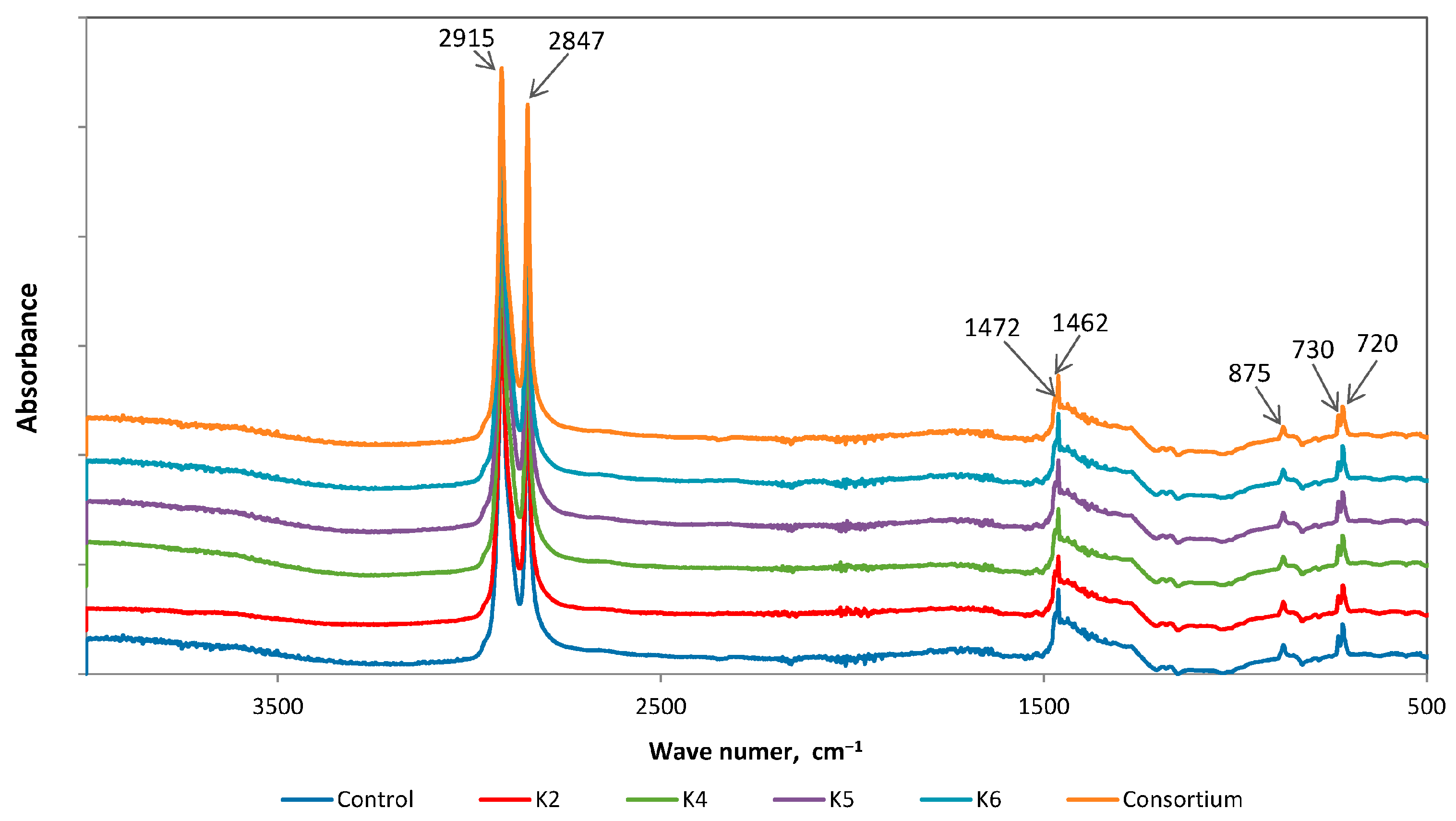
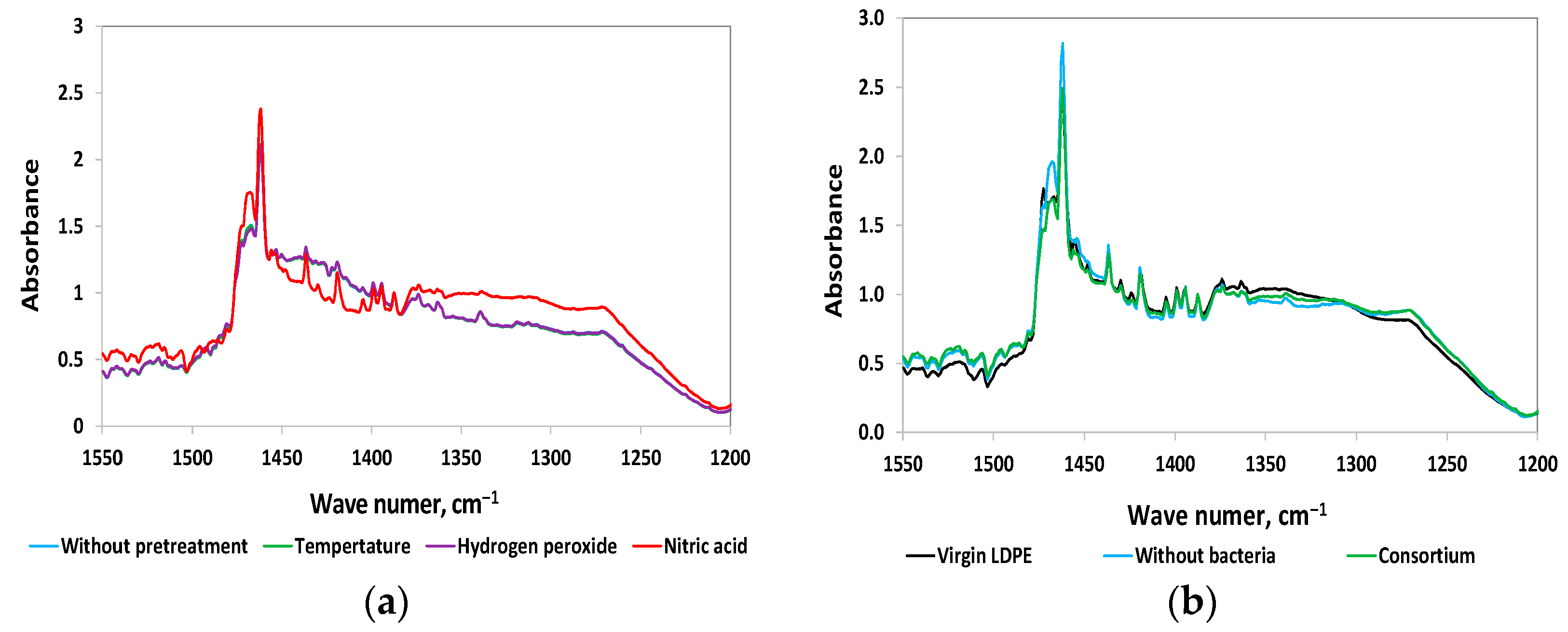
3.2.4. FTIR Analysis
3.2.5. SEM Analysis
3.3. Phytotoxicity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| LDPE | Low-density polyethylene |
| LDPE(WP) | Low-density polyethylene without pretreatment |
| LDPE(H2O2) | Low-density polyethylene after hydrogen peroxide pretreatment |
| LDPE(HNO3) | Low-density polyethylene after nitric acid pretreatment |
| LDPE(T) | Low-density polyethylene after thermal pretreatment |
| ATR-FTIR | Attenuated total reflection–Fourier-transform infrared spectroscopy |
| OD | Optical density |
| CI | Carbonyl index |
| SEM | Scanning electron microscopy |
| WCA | Water contact angle |
| GI | Germination index |
References
- Plastics Europe. Plastics—The Fast Facts 2023. Available online: https://plasticseurope.org/knowledge-hub/plastics-the-fast-facts-2023/ (accessed on 15 April 2025).
- Mohanan, N.; Montazer, Z.; Sharma, P.K.; Levin, D.B. Microbial and Enzymatic Degradation of Synthetic Plastics. Front. Microbiol. 2020, 11, 580709. [Google Scholar] [CrossRef]
- Yao, Z.; Seong, H.J.; Jang, Y.S. Environmental toxicity and decomposition of polyethylene. Ecotoxicol. Environ. Saf. 2022, 242, 113933. [Google Scholar] [CrossRef]
- EEA. European Environment Agency. 2024. Available online: https://www.eea.europa.eu/en/topics/in-depth/plastics (accessed on 15 April 2025).
- Mendoza, J.E.; Tineo, D.; Chuquibala-Checan, B.; Atalaya-Marin, N.; Taboada-Mitma, V.H.; Tafur-Culqui, J.; Tarrillo, E.; Gómez-Fernández, D.; Goñas, M.; Reyes-Reyes, M.A. Global perspectives on the biodegradation of LDPE in agricultural systems. Front. Microbiol. 2025, 15, 1510817. [Google Scholar] [CrossRef]
- Tiago, G.A.O.; Mariquito, A.; Martins-Dias, S.; Marques, A.C. The problem of polyethylene waste—Recent attempts for its mitigation. Sci. Total Environ. 2023, 892, 164629. [Google Scholar] [CrossRef]
- Zhang, N.; Ding, M.; Yuan, Y. Current Advances in Biodegradation of Polyolefins. Microorganisms 2022, 10, 1537. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Wang, R.; Zhou, Q.; Li, J.; Fan, Y.; Chen, Q. Recent progresses and perspectives of polyethylene biodegradation by bacteria and fungi: A review. J. Contam. Hydrol. 2025, 269, 104499. [Google Scholar] [CrossRef]
- Montazer, Z.; Habibi Najafi, M.B.; Levin, D.B. Challenges with Verifying Microbial Degradation of Polyethylene. Polymers 2020, 12, 123. [Google Scholar] [CrossRef]
- Zheng, S.; Bawazir, M.; Dhall, A.; Kim, H.-E.; He, L.; Heo, J.; Hwang, G. Implication of Surface Properties, Bacterial Motility, and Hydrodynamic Conditions on Bacterial Surface Sensing and Their Initial Adhesion. Front. Bioeng. Biotechnol. 2021, 9, 643722. [Google Scholar] [CrossRef]
- Flores-Díaz, A.; Arang, J.A.; Calvo, D.C.; Rangel-Mendez, R.; Ontiveros-Valencia, A. Physicochemical and microbial treatments for plastics, microplastics, and nanoplastics. Environ. Dev. 2025, 54, 101181. [Google Scholar] [CrossRef]
- Wei, R.; Zimmermann, W. Microbial enzymes for the recycling of recalcitrant petroleum-based plastics: How far are we? Microb. Biotechnol. 2017, 10, 1308–1322. [Google Scholar] [CrossRef] [PubMed]
- Lv, S.; Li, Y.; Zhao, S.; Shao, Z. Biodegradation of Typical Plastics: From Microbial Diversity to Metabolic Mechanisms. Int. J. Mol. Sci. 2024, 25, 593. [Google Scholar] [CrossRef] [PubMed]
- Rong, Z.; Ding, Z.H.; Wu, Y.H.; Xu, X.W. Degradation of low-density polyethylene by the bacterium Rhodococcus sp. C-2 isolated from seawater. Sci. Total Environ. 2024, 907, 167993. [Google Scholar] [CrossRef] [PubMed]
- Zaini, N.; Kasmuri, N.; Mojiri, A.; Kindaichi, T.; Nayono, S.E. Plastic pollution and degradation pathways: A review on the treatment technologies. Heliyon 2024, 10, e28849. [Google Scholar] [CrossRef]
- Huang, Y.; Zhao, Y.; Wang, J.; Zhang, M.; Jia, W.; Qin, X. LDPE microplastic films alter microbial community composition and enzymatic activities in soil. Environ. Pollut. 2019, 254, 112983. [Google Scholar] [CrossRef]
- Wang, J.; Huang, M.; Wang, Q.; Sun, Y.; Zhao, Y.; Huang, Y. LDPE microplastics significantly alter the temporal turnover of soil microbial communities. Sci. Total. Environ. 2020, 726, 138682. [Google Scholar] [CrossRef] [PubMed]
- Biki, S.P.; Mahmud, S.; Akhter, S.; Rahman, J.; Rix, J.J.; Al Bachchu, A.; Ahmed, M. Polyethylene degradation by Ralstonia sp. strain SKM2 and Bacillus sp. strain SM1 isolated from land fill soil site. Environ. Technol. Innov. 2021, 22, 101495. [Google Scholar] [CrossRef]
- El-Sherif, D.M.; Eloffy, M.G.; Elmesery, A.; Abouzid, M.; Gad, M.; El-Seedi, H.R.; Brinkmann, M.; Wang, K.; Al Naggar, Y. Environmental risk, toxicity, and biodegradation of polyethylene: A review. Environ. Sci. Pollut. Res. 2022, 29, 81166–81182. [Google Scholar] [CrossRef]
- Joshi, G.; Goswami, P.; Verma, P.; Prakash, G.; Simon, P.; Vinithkumar, N.V.; Dharani, G. Unraveling the plastic degradation potentials of the plastisphere-associated marine bacterial consortium as a key player for the low-density polyethylene degradation. J. Hazard. Mater. 2022, 425, 128005. [Google Scholar] [CrossRef]
- Pondala, S.; Botsa, S.M. Physical, thermal, chemical and biological approaches for plastics degradation–A review. Clean. Chem. Eng. 2025, 11, 100162. [Google Scholar] [CrossRef]
- Kumari, A.; Chaudhary, D.R.; Jha, B. Destabilization of polyethylene and polyvinylchloride structure by marine bacterial strain. Environ Sci. Pollut. Res. 2019, 26, 1507–1516. [Google Scholar] [CrossRef]
- Kyaw, B.M.; Champakalakshmi, R.; Sakharkar, M.K.; Lim, C.S.; Sakharkar, K.R. Biodegradation of Low Density Polythene (LDPE) by Pseudomonas Species. Indian J. Microbiol. 2012, 52, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Nadeem, H.; Alia, K.B.; Muneer, F.; Rasul, I.; Siddique, M.H.; Azeem, F.; Zubair, M. Isolation and identification of low-density polyethylene degrading novel bacterial strains. Arch. Microbiol. 2021, 203, 5417–5423. [Google Scholar] [CrossRef]
- Zhang, H.; Lu, Y.; Wu, H.; Liu, Q.; Sun, W. Effect of an Acinetobacter pittobacter on low-density polyethylene. Environ. Sci. Pollut. Res. 2022, 30, 10495–10504. [Google Scholar] [CrossRef]
- Ciuffi, B.; Fratini, E.; Rosi, L. Plastic pretreatment: The key for efficient enzymatic and biodegradation processes. Polym. Degrad. Stab. 2024, 222, 110698. [Google Scholar] [CrossRef]
- Ormsby, M.J.; Woodford, L.; Fellows, R.; White, H.L.; Quilliam, R.S. Rapid colonisation of environmental plastic waste by pathogenic bacteria drives adaptive phenotypic changes. J. Hazard. Mater. 2024, 480, 136359. [Google Scholar] [CrossRef]
- Matjašič, T.; Simčič, T.; Medvešček, N.; Bajt, O.; Dreo, T.; Mori, N. Critical evaluation of biodegradation studies on synthetic plastics through a systematic literature review. Sci. Total Environ. 2021, 752, 141959. [Google Scholar] [CrossRef]
- Szczyrba, E.; Pokynbroda, T.; Koretska, N.; Gąszczak, A. Isolation and characterization of new bacterial strains degrading low-density polyethylene. Chem. Process. Eng. New Front. 2024, 45, e60. [Google Scholar] [CrossRef]
- Kieser, B.; Buttner, M.; Chater, K.; Hopwood, B. Practical Streptomyces Genetics; John Innes Foundation: Norwich, UK, 2000. [Google Scholar]
- Kearse, M.; Moir, R.; Wilson, A.; Stones-Havas, S.; Cheung, M.; Sturrock, S.; Buxton, S.; Cooper, A.; Markowitz, S.; Duran, C.; et al. Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 2012, 28, 1647–1649. [Google Scholar] [CrossRef]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef] [PubMed]
- Kimura, M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 1980, 16, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA 11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Celik, M.; Yang, Z.; Xu, H.; Nakano, H.; Isobe, A.; Arakawa, H. Carbonyl index of miniaturized microplastics at the sea surface. Mar. Pollut. Bull. 2025, 211, 117376. [Google Scholar] [CrossRef] [PubMed]
- Almond, J.; Sugumaar, P.; Wenzel, M.; Hill, G.; Wallis, C. Determination of the carbonyl index of polyethylene and polypropylene using specified area under band methodology with ATR-FTIR spectroscopy. e-Polymers 2020, 20, 369–381. [Google Scholar] [CrossRef]
- Harrat, R.; Bourzama, G.; Sadrati, N.; Zerroug, A.; Burgaud, G.; Ouled-Haddar, H.; Soumati, B. A comparative study on biodegradation of low density polyethylene bags by a Rhizopus arrhizus SLNEA1 strain in batch and continuous cultures. Braz. J. Microbiol. 2024, 55, 3449–3463. [Google Scholar] [CrossRef] [PubMed]
- Celina, M.C.; Linde, E.; Martinez, E. Carbonyl Identification and Quantification Uncertainties for Oxidative Polymer Degradation. Polym. Degrad. Stab. 2021, 188, 109550. [Google Scholar] [CrossRef]
- Buron-Moles, G.; Vandenbossche, V.; Gorret, N.; Santonja-Blasco, L.; González-Aranda, T.; Cameleyre, X.; Guillouet, S. Biodegradation of pre-treated low-density polyethylene (LDPE) by Yarrowia lipolytica determined by oxidation and molecular weight reduction. Polym. Degrad. Stab. 2025, 236, 111292. [Google Scholar] [CrossRef]
- Celik, M.; Nakano, H.; Uchida, K.; Isobe, A.; Arakawa, H. Comparative evaluation of the carbonyl index of microplastics around the Japan coast. Mar. Pollut. Bull. 2023, 190, 114818. [Google Scholar] [CrossRef]
- Yang, Z.; Çelik, M.; Nakano, H.; Arakawa, H. Accessing the intrinsic factors of carbonyl index of microplastics: Physical and spectral properties, baseline correction, calculation methods, and their interdependence. Mar. Pollut. Bull. 2023, 197, 115700. [Google Scholar] [CrossRef]
- Angelini, V.A.; Orejas, J.; Medina, M.I.; Agostini, E. Scale up of 2,4-dichlorophenol removal from aqueous solutions using Brassica napus hairy roots. J. Hazard. Mater. 2011, 185, 269–274. [Google Scholar] [CrossRef]
- Kebrom, T.H.; Woldesenbet, S.; Bayabil, H.K.; Garcia, M.; Gao, M.; Ampim, P.; Awal, R.; Fares, A. Evaluation of phytotoxicity of three organic amendments to collard greens using the seed germination bioassay. Environ. Sci. Pollut. Res. 2019, 26, 5454–5462. [Google Scholar] [CrossRef]
- Burton, G.A., Jr.; Baudo, R.; Beltrami, M.; Rowlnad, C. Assessing sediment contamination using six toxicity assays. J. Limnol. 2001, 60, 263–267. [Google Scholar] [CrossRef]
- Rodríguez-Fonseca, M.F.; Sánchez-Suárez, J.; Valero, M.F.; Ruiz-Balaguera, S.; Díaz, L.E. Streptomyces as potential synthetic polymer degraders: A systematic review. Bioengineering 2021, 8, 154. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, H.; Zhu, B.; Yao, Z.; Jiang, L. Purification and biochemical characterization of a novel chitosanase cloned from the gene of Kitasatospora setae KM-6054 and its application in the production of chitooligosaccharides. World J. Microbiol. Biotechnol. 2023, 39, 111. [Google Scholar] [CrossRef] [PubMed]
- Yun, B.R.; Malik, A.; Kim, S.B. Genome based characterization of Kitasatospora sp. MMS16-BH015, a multiple heavy metal resistant soil actinobacterium with high antimicrobial potential. Gene 2020, 733, 144379. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.N.; Wei, M.; Han, F.; Fang, C.; Wang, D.; Zhong, Y.J.; Guo, C.L.; Shi, X.Y.; Xie, Z.K.; Li, F.M. Greater Biofilm Formation and Increased Biodegradation of Polyethylene Film by a Microbial Consortium of Arthrobacter sp. and Streptomyces sp. Microorganisms 2020, 8, 1979. [Google Scholar] [CrossRef]
- Duddu, M.K.; Tripura, K.L.; Guntuku, G.; Divya, D.S. Biodegradation of low density polyethylene (LDPE) by a new biosurfactant-producing thermophilic Streptomyces coelicoflavus NBRC 15399T. Afr. J. Biotechnol. 2015, 14, 327–340. [Google Scholar] [CrossRef]
- Priyadarshini, P.; Rafiq, S.; Shahina, S.K.J.; Ramesh, K.V. Biodegradation of Low Density Polyethylene(LDPE) by Nocardiopsis alba from municipal landfill in Chennai. Int. J. Adv. Sci. Res. Manag. 2018, 3, 8. [Google Scholar]
- Harshvardhan, K.; Jha, B. Biodegradation of low-density polyethylene by marine bacteria from pelagic waters, Arabian Sea, India. Mar. Pollut. Bull. 2013, 77, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Khandare, D.; Chaudhary, D.R.; Jha, B. Marine bacterial biodegradation of low-density polyethylene (LDPE) plastic. Biodegradation 2021, 32, 127–143. [Google Scholar] [CrossRef]
- He, Y.; Li, H.; Xiao, X.; Zhao, X. Polymer Degradation: Category, Mechanism and Development Prospect. In Proceedings of the 2021 3rd International Conference on Geoscience and Environmental Chemistry, Xining, China, 18–20 June 2021; Volume 290, p. 01012. [Google Scholar] [CrossRef]
- Balasubramanian, V.; Natarajan, K.; Rajeshkannan, V.; Perumal, P. Enhancement of in vitro high-density polyethylene (HDPE) degradation by physical, chemical, and biological treatments. Environ. Sci. Pollut. Res. Int. 2014, 21, 12549–12562. [Google Scholar] [CrossRef]
- Arkatkar, A.; Juwarkar, A.A.; Bhaduri, S.; Uppara, P.V.; Doble, M. Growth of Pseudomonas and Bacillus biofilms on pretreated polypropylene surface. Int. Biodeterior. Biodegrad. 2010, 64, 530–536. [Google Scholar] [CrossRef]
- Rajandas, H.; Parimannan, S.; Sathasivam, K.; Ravichandran, M.; Yin, L.S. A novel FTIR-ATR spectroscopy based technique for the estimation of low-density polyethylene biodegradation. Polym. Test. 2012, 31, 1094–1099. [Google Scholar] [CrossRef]
- Hirsch, S.G.; Barel, B.; Segal, E. Characterization of surface phenomena: Probing early stage degradation of low-density polyethylene films. Polym. Eng. Sci. 2019, 59, E129–E137. [Google Scholar] [CrossRef]
- Seong, H.J.; Kim, H.; Ko, Y.J.; Yao, Z.; Baek, S.-B.; Kim, N.-J.; Janget, Y.-S. Enhancing polyethylene degradation: A novel bioprocess approach using Acinetobacter nosocomialis pseudo-resting cells. Appl. Microbiol. Biotechnol. 2024, 108, 86. [Google Scholar] [CrossRef]
- Samanta, S.; Datta, D.; Halder, G. Biodegradation efficacy of soil inherent novel sp. Bacillus tropicus (MK318648) onto low density polyethylene matrix. J. Polym. Res. 2020, 27, 324. [Google Scholar] [CrossRef]
- Awasthi, S.; Srivastava, P.; Singh, P.; Tiwary, D.; Mishra, P.K. Biodegradation of thermally treated high-density polyethylene (HDPE) by Klebsiella pneumoniae CH001. 3 Biotech 2017, 7, 332. [Google Scholar] [CrossRef] [PubMed]
- Gowthami, A.; Marjuk, M.S.; Raju, P.; Devi, K.N.; Santhanam, P.; Kumar, S.D.; Perumal, P. Biodegradation efficacy of selected marine microalgae against Low-Density Polyethylene (LDPE): An environment friendly green approach. Mar. Pollut. Bull. 2023, 190, 114889. [Google Scholar] [CrossRef]
- Devi, D.; Gupta, K.K.; Chandra, H.; Sharma, K.K.; Sagar, K.; Mori, E.; de Farias, P.A.M.; Coutinho, H.D.M.; Mishra, A.P. Biodegradation of low-density polyethylene (LDPE) through application of indigenous strain Alcaligenes faecalis ISJ128. Environ. Geochem. Health 2023, 45, 9391–9409. [Google Scholar] [CrossRef]
- Shilpa; Basak, N.; Meena, S.S. Biodegradation of low-density polythene (LDPE) by a novel strain of Pseudomonas aeruginosa WD4 isolated from plastic dumpsite. Biodegradation 2024, 35, 641–655. [Google Scholar] [CrossRef] [PubMed]
- Bajo, K.; Romano, R.; Kolvenbach, B.; Nazemi, S.A.; Shahgaldian, P.; Corvini, P.F.-X.; Fava, F.; Raddadi, N. Biodegradation of untreated plasticizers-free linear low-density polyethylene films by marine bacteria. Mar. Pollut. Bull. 2024, 209 Pt A, 117115. [Google Scholar] [CrossRef]
- Kim, Y.J.; Kim, Y.H.; Shin, Y.R.; Choi, S.Y.; Park, J.A.; Kim, H.O.; Lim, K.S.; Ha, S.J. Efficient biodegradation of low-density polyethylene by Pseudomonas plecoglossicida SYp2123 was observed through FT-IR and FE-SEM analysis. Biotechnol. Bioproc. E 2024, 29, 743–750. [Google Scholar] [CrossRef]
- Alamer, N.J.; Aldayel, M.F.; Khalifa, A. Isolation and Characterization of Brucella spp., Low-Density Polyethylene (LDPE) Plastic Degrading Bacteria in Al-Ahsa Region, Saudi Arabia. Appl. Sci. 2023, 13, 4629. [Google Scholar] [CrossRef]
- Andrady, A.L. Assessment of Environmental Biodegradation of Synthetic Polymers. J. Macromol. Sci. C 1994, 34, 25–76. [Google Scholar] [CrossRef]
- Gomez, N.C.F.; Onda, D.F.L. Potential of sediment bacterial communities from Manila Bay (Philippines) to degrade low-density polyethylene (LDPE). Arch. Microbiol. 2023, 205, 38. [Google Scholar] [CrossRef]
- Miranda, M.N.; Sampaio, M.J.; Tavares, P.B.; Silva, A.M.T.; Pereira, F.M.R. Aging assessment of microplastics (LDPE, PET and uPVC) under urban environment stressors. Sci. Total Environ. 2021, 796, 148914. [Google Scholar] [CrossRef]
- Sudhakar, M.; Doble, M.; Murthy, P.S.; Venkatesan, R. Marine microbe-mediated biodegradation of low-and high-density polyethylenes. Int. Biodeterior. Biodegrad. 2008, 61, 203–213. [Google Scholar] [CrossRef]
- Fajdek-Bieda, A.; Wróblewska, A. The Use of Natural Minerals as Reinforcements in Mineral-Reinforced Polymers: A Review of Current Developments and Prospects. Polymers 2024, 16, 2505. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.D.C.; Brites, P.; Martins, C.; Nunes, C.; Coimbra, M.A.; Ferreira, P.; Gonçalves, I. Starch consolidation of calcium carbonate as a tool to develop lightweight fillers for LDPE-based plastics. Int. J. Biol. Macromol. 2023, 226, 1021–1030. [Google Scholar] [CrossRef]
- Syranidou, E.; Karkanorachaki, K.; Barouta, D.; Papadaki, E.; Moschovas, D.; Avgeropoulos, A.; Kalogerakis, N. Relationship between the Carbonyl Index (CI) and Fragmentation of Polyolefin Plastics during Aging. Environ. Sci. Technol. 2023, 57, 8130–8138. [Google Scholar] [CrossRef] [PubMed]
- Arutchelvi, J.; Sudhakar, M.; Arkatkar, A.; Doble, M.; Bhaduri, S.; Uppara, P. Biodegradation of polyethylene and polypropylene. Indian. J. Biotechnol. 2008, 7, 9–22. [Google Scholar]
- Gilan, I.; Hadar, Y.; Sivan, A. Colonization, biofilm formation and biodegradation of polyethylene by a strain of Rhodococcus ruber. Appl. Microbiol. Biotechnol. 2004, 65, 97–104. [Google Scholar] [CrossRef]
- Howard, S.A.; McCarthy, R.R. Modulating biofilm can potentiate activity of novel plastic-degrading enzymes. npj Biofilms Microbiomes 2023, 9, 72. [Google Scholar] [CrossRef]
- Mazaheri, H.; Nazeri, S. Biodegradation and Detoxification of Low-Density Polyethylene (LDPE) by Stenotrophomonas sp. and Alcaligenaceae bacterium. Bull. Environ. Contam. Toxicol. 2024, 112, 19. [Google Scholar] [CrossRef] [PubMed]
- Rajapandi, J.D.; Rajamanickam, U. Low–density polyethylene management by using selective bacterial strains from garbage soil. Biologia 2024, 79, 985–1001. [Google Scholar] [CrossRef]
- Rani, R.; Rathee, J.; Kumari, P.; Singh, N.P.; Santal, A.R. Biodegradation and detoxification of low-density polyethylene by an indigenous strain Bacillus licheniformis. J. Appl. Biol. Biotech. 2022, 10, 9–21. [Google Scholar] [CrossRef]
- Koriem, A.; Abdalla, M.; Ollick, A.M.; Elhadary, M. The effect of artificial weathering and hardening on mechanical properties of HDPE with and without UV stabilizers. Alex. Eng. J. 2021, 60, 4167–4175. [Google Scholar] [CrossRef]
- Yao, Z.; Seong, H.J.; Jang, Y.S. Degradation of low density polyethylene by Bacillus species. Appl. Biol. Chem. 2022, 65, 84. [Google Scholar] [CrossRef]
- Shahnawaz, M.; Sangale, M.K.; Ade, A.B. Analysis of the Plastic Degradation Products. In Bioremediation Technology for Plastic Waste; Springer: Singapore, 2019; pp. 93–101. [Google Scholar] [CrossRef]
- Mahalakshmi, V.; Siddiq, A.; Niren, A. Analysis of polyethylene degrading potentials of microorganisms isolated from compost soil. Int. J. Pharm. Biol. Arch. 2012, 3, 1230–1236. [Google Scholar]
- Abo El-Souad, S.M.S. Biodegradation of Low-density Polyethylene (LDPE) Strips from Waste Plastic Bags Using Marine-derived Fungi. J. Appl. Biotechnol. Rep. 2025, 12, 1586–1593. [Google Scholar] [CrossRef]
- Sangale, M.K.; Shahnawaz, M.; Ade, A.B. Gas chromatography-Mass Spectra analysis and deleterious potential of fungal based polythene-degradation products. Sci. Rep. 2019, 9, 1599. [Google Scholar] [CrossRef]

| K2 | Untreated LDPE | LDPE(H2O2) | LDPE(HNO3) | LDPE(T) |
|---|---|---|---|---|
| Biodegradation [%] | 1.9 | 0.8 | 2.7 | 2.4 |
| Water contact angle change [%] | 1.1 | 0.0 | 16.7 | 1.1 |
| pH change [%] | 3.8 | 3.3 | 2.4 | 3.6 |
| K4 | Untreated LDPE | LDPE(H2O2) | LDPE(HNO3) | LDPE(T) |
| Biodegradation [%] | 3.2 | 1.8 | 4.6 | 4.1 |
| Water contact angle change [%] | 1.1 | 8.9 | 12.2 | 0.0 |
| pH change [%] | 5.9 | 5.2 | 5.3 | 5.2 |
| K5 | Untreated LDPE | LDPE(H2O2) | LDPE(HNO3) | LDPE(T) |
| Biodegradation [%] | 0.9 | 3.0 | 2.3 | 2.5 |
| Water contact angle change [%] | 10.0 | 6.7 | 8.9 | 4.4 |
| pH change [%] | 3.5 | 4.5 | 4.5 | 4.5 |
| K6 | Untreated LDPE | LDPE(H2O2) | LDPE(HNO3) | LDPE(T) |
| Biodegradation [%] | 2.0 | 1.7 | 2.2 | 2.5 |
| Water contact angle change [%] | 0.0 | 10.0 | 21.1 | 10.0 |
| pH change [%] | 6.2 | 3.8 | 3.3 | 3.6 |
| Consortium | Untreated LDPE | LDPE(H2O2) | LDPE(HNO3) | LDPE(T) |
| Biodegradation [%] | 2.1 | 1.6 | 4.3 | 2.9 |
| Water contact angle change [%] | 8.9 | 10.0 | 11.1 | 5.6 |
| pH change [%] | 3.3 | 1.8 | 2.7 | 2.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szczyrba, E.; Pokynbroda, T.; Gąszczak, A.; Koretska, N.; Tistechok, S.; Roman, I.; Gromyko, O. Potential of Compost-Derived Actinomycetes for Low-Density Polyethylene Degradation. Polymers 2025, 17, 2318. https://doi.org/10.3390/polym17172318
Szczyrba E, Pokynbroda T, Gąszczak A, Koretska N, Tistechok S, Roman I, Gromyko O. Potential of Compost-Derived Actinomycetes for Low-Density Polyethylene Degradation. Polymers. 2025; 17(17):2318. https://doi.org/10.3390/polym17172318
Chicago/Turabian StyleSzczyrba, Elżbieta, Tetiana Pokynbroda, Agnieszka Gąszczak, Nataliia Koretska, Stepan Tistechok, Ivan Roman, and Oleksandr Gromyko. 2025. "Potential of Compost-Derived Actinomycetes for Low-Density Polyethylene Degradation" Polymers 17, no. 17: 2318. https://doi.org/10.3390/polym17172318
APA StyleSzczyrba, E., Pokynbroda, T., Gąszczak, A., Koretska, N., Tistechok, S., Roman, I., & Gromyko, O. (2025). Potential of Compost-Derived Actinomycetes for Low-Density Polyethylene Degradation. Polymers, 17(17), 2318. https://doi.org/10.3390/polym17172318







