Thioxanthone Skeleton-Based One-Component Macro-Photoinitiator Reduces Oxygen Inhibition and Migration Through Cooperative Effect
Abstract
1. Introduction
2. Materials and Methods
2.1. Material
2.2. General Instruments
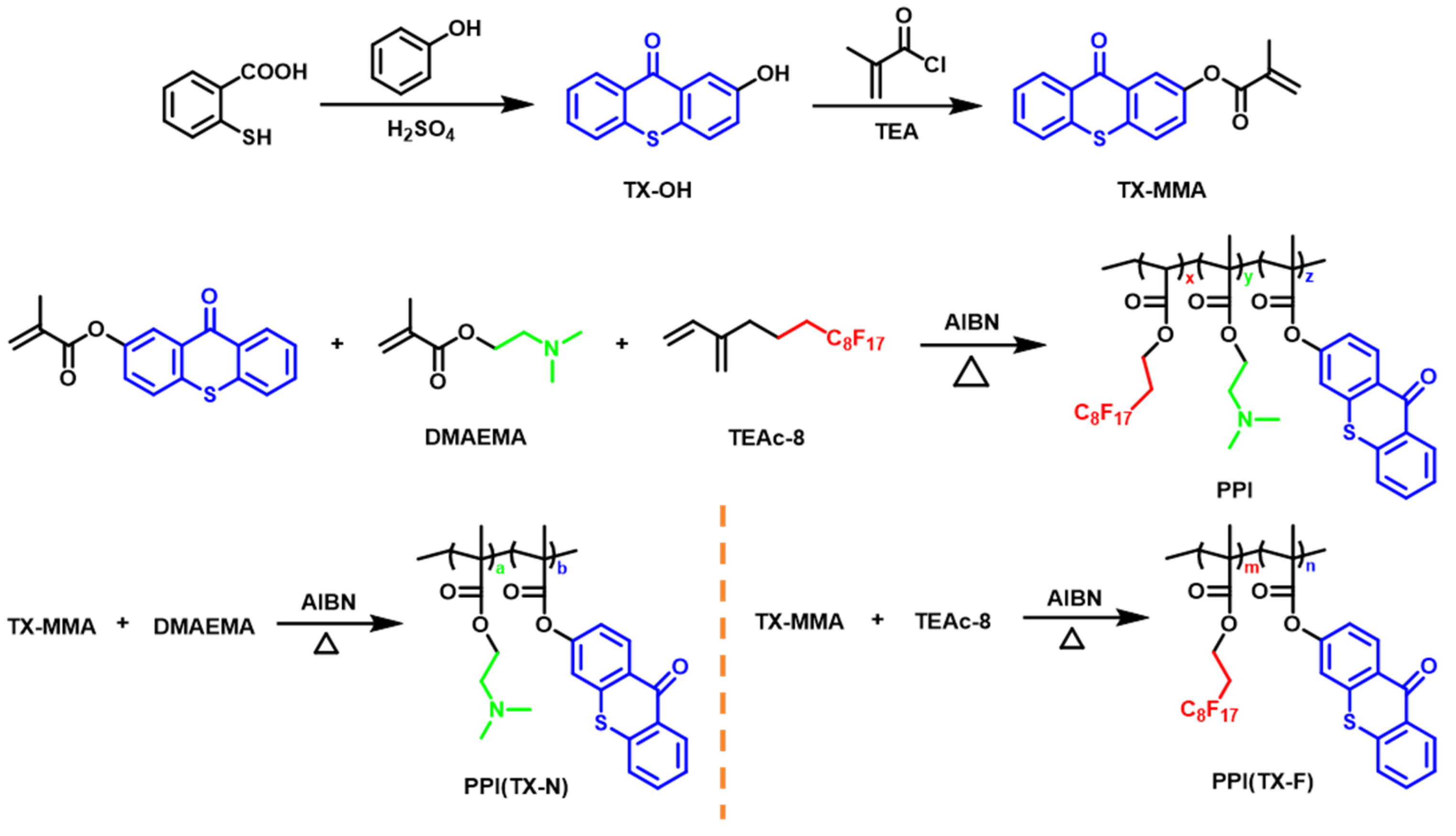
2.3. Synthesis
2.4. Experimental Methods
3. Results
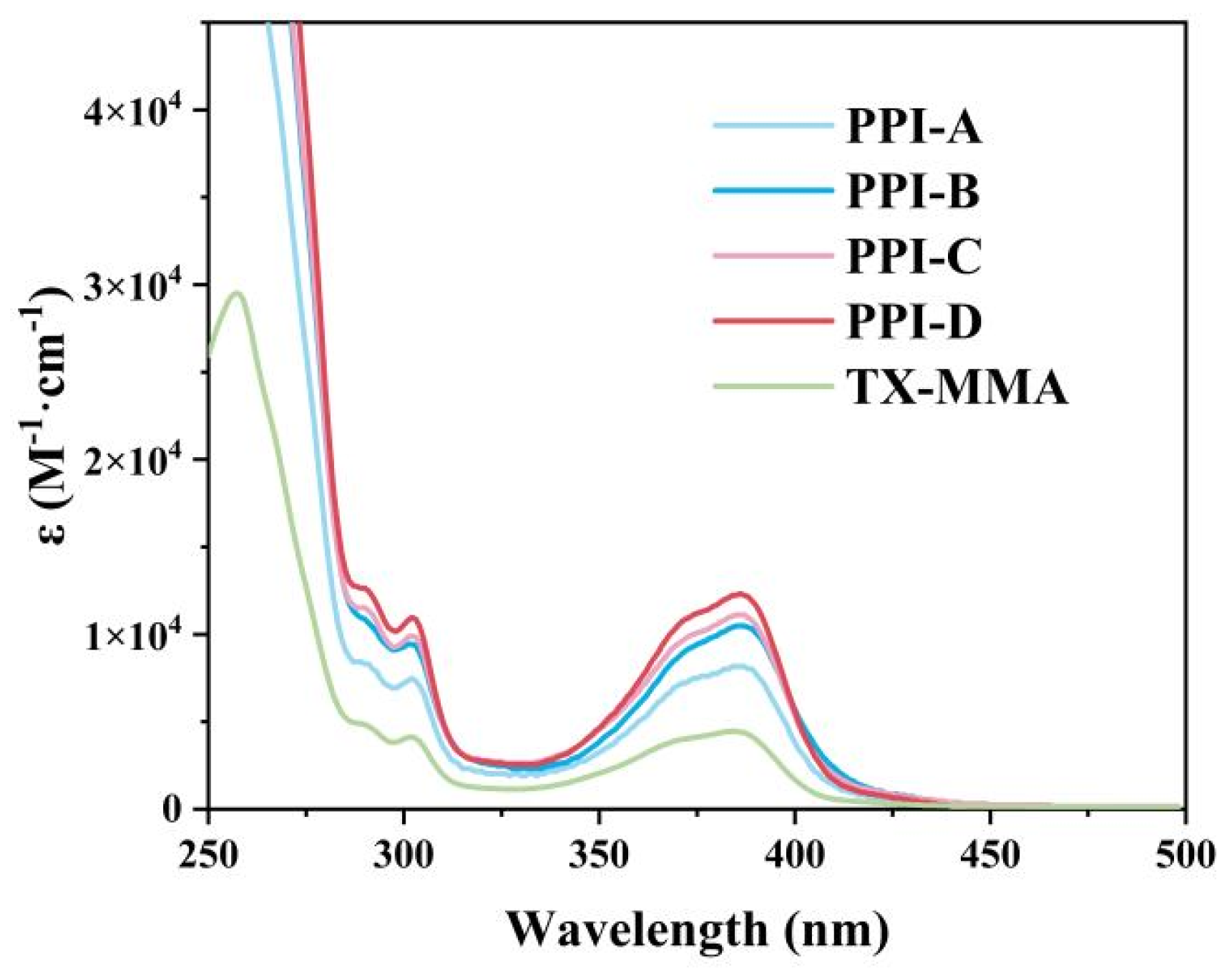
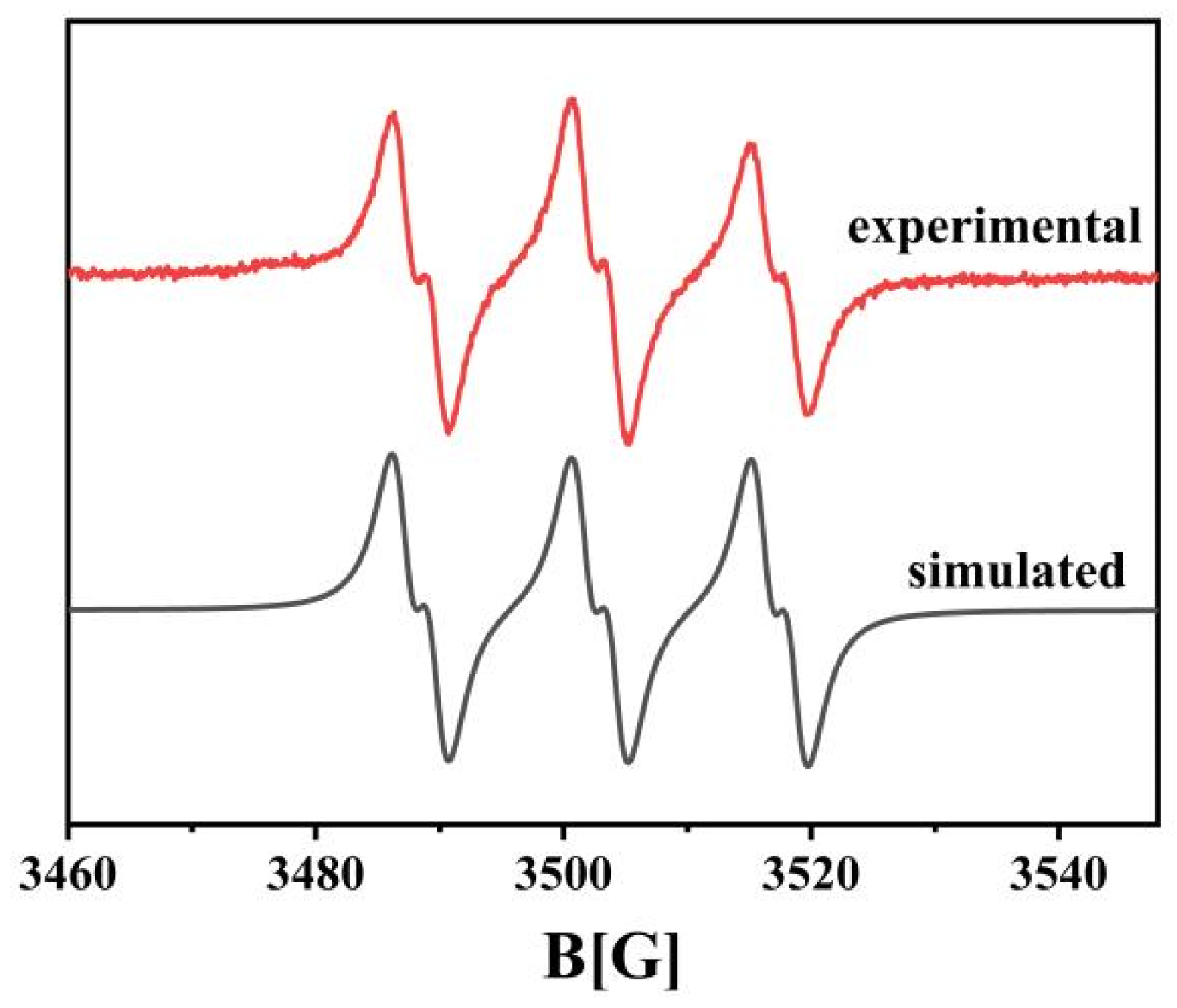
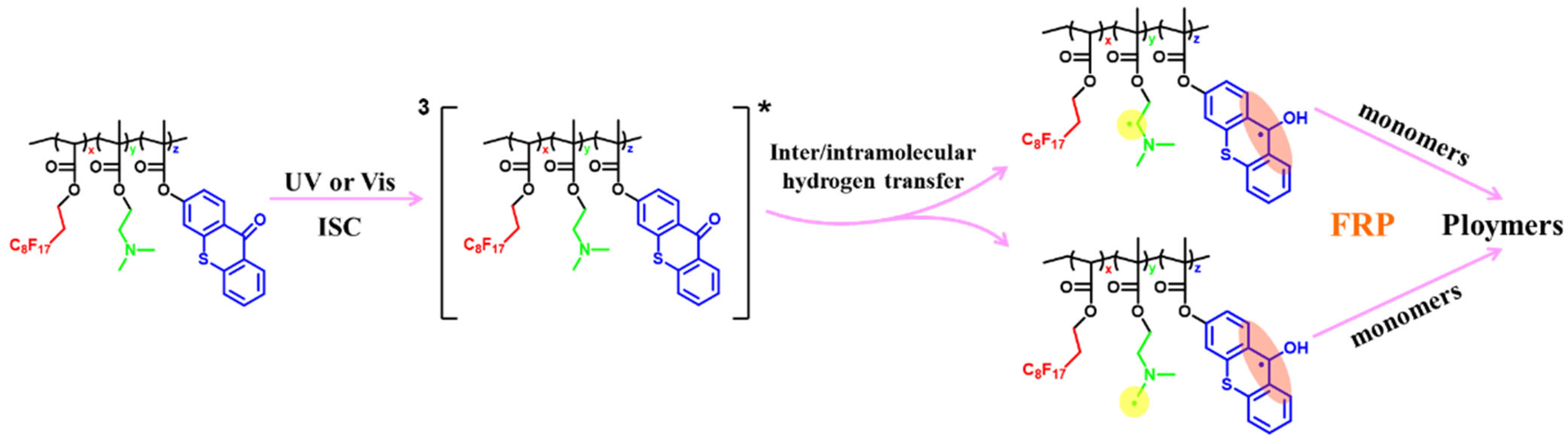
3.1. Photophysical and Photochemical Properties
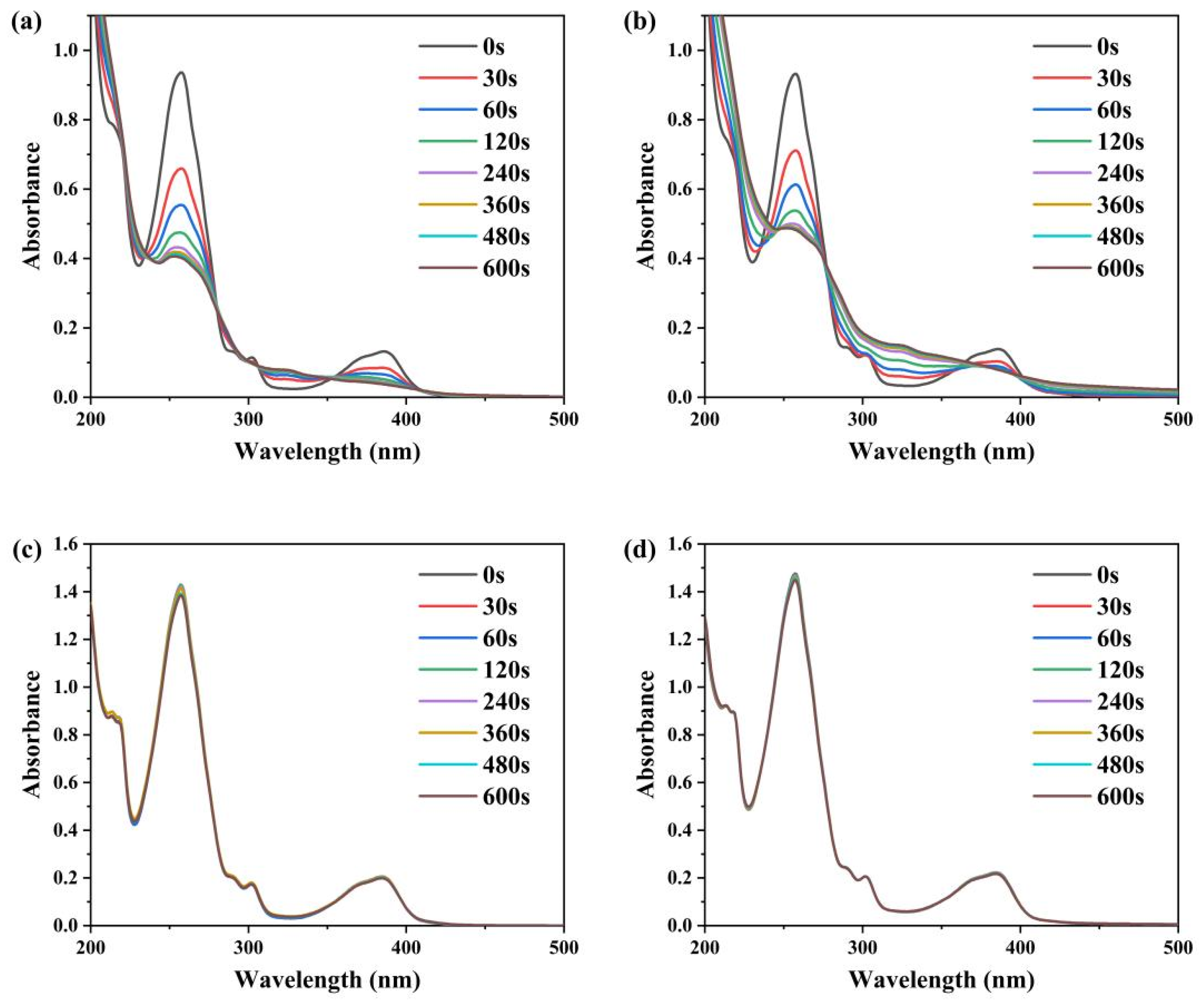
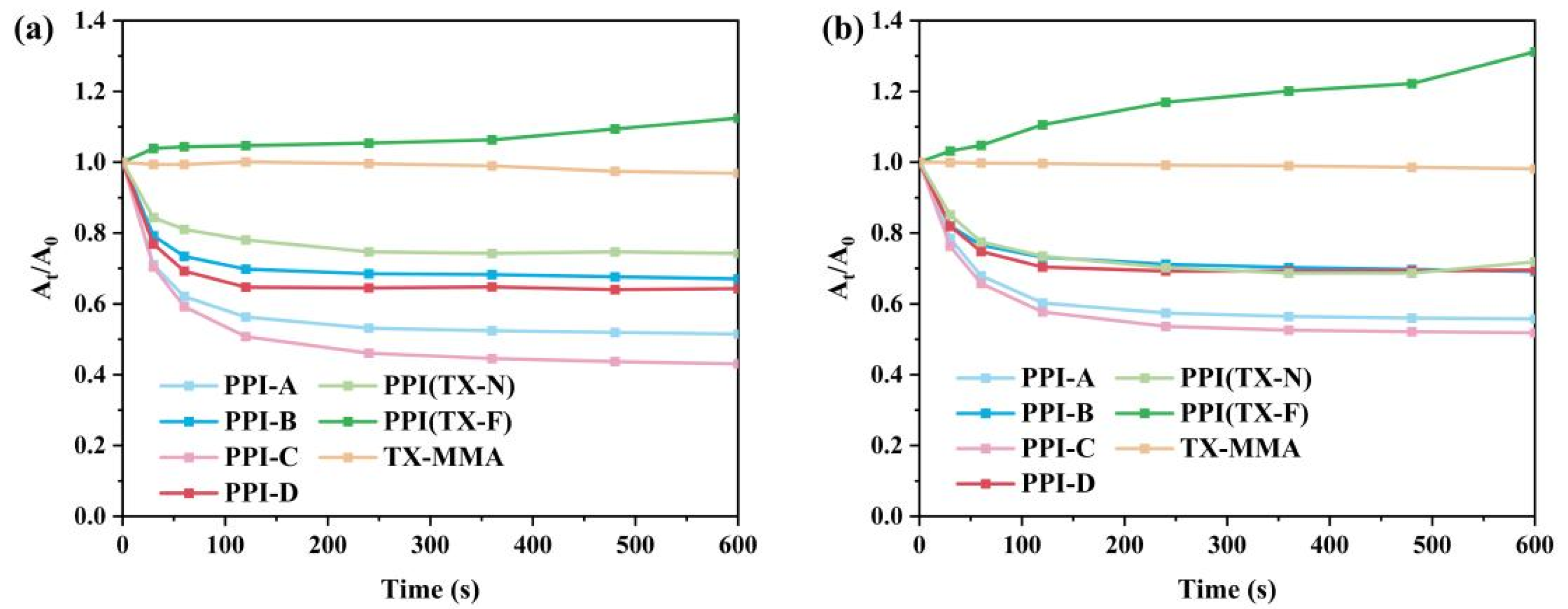
3.2. Photopolymerization Kinetics
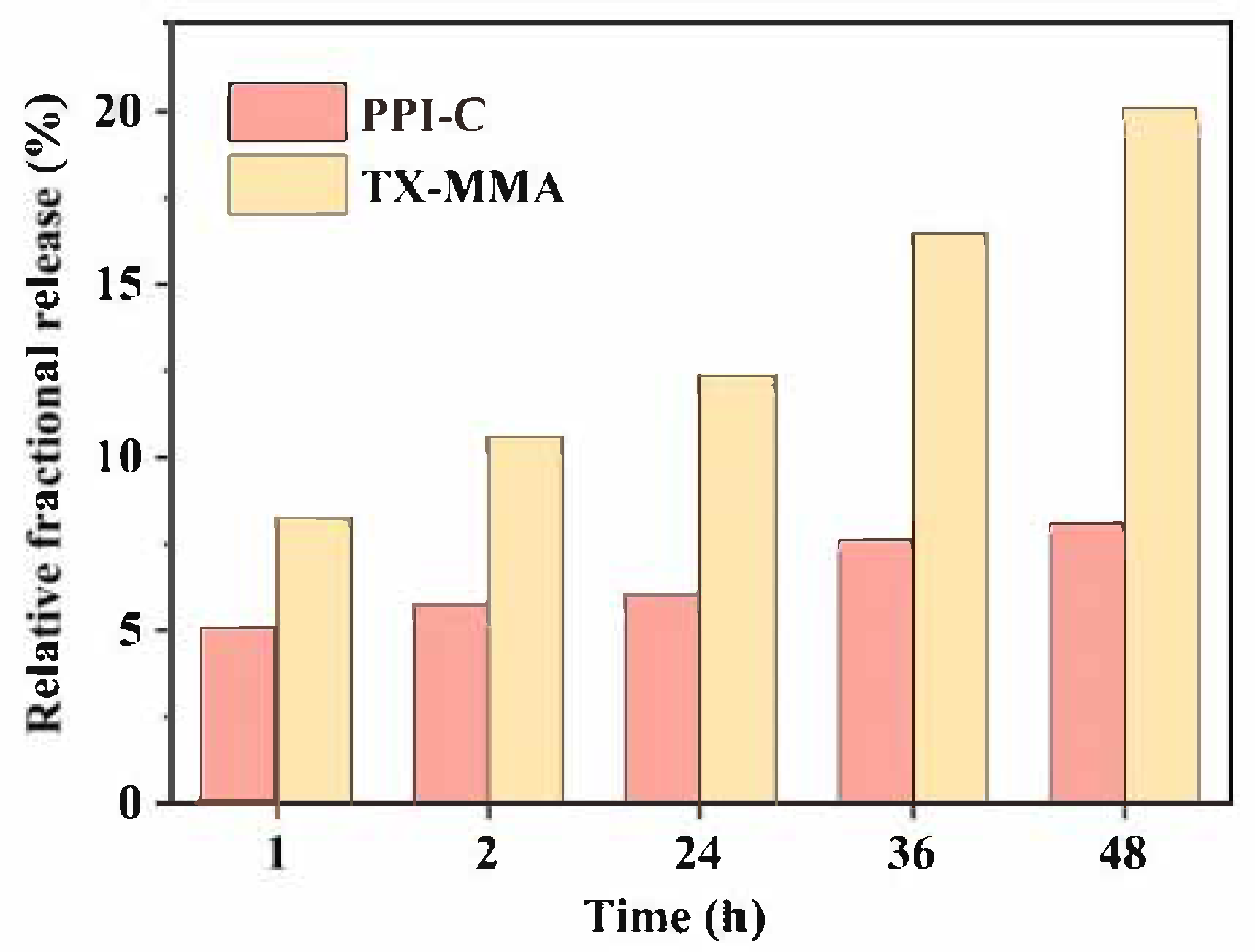
3.3. Migration Stability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Li, C.; Lee, B.; Wang, C.; Bajpayee, A.; Douglas, L.K.; Phillips, B.K.; Yu, G.; Rivera-Gonzalez, N.; Peng, B.-J.; Jiang, Z.; et al. Photopolymerized superhydrophobic hybrid coating enabled by dual-purpose tetrapodal ZnO for liquid/liquid separation. Mater. Horiz. 2022, 9, 452–461. [Google Scholar] [CrossRef]
- Manigrasso, J.; Chillón, I.; Genna, V.; Vidossich, P.; Somarowthu, S.; Pyle, A.M.; Vivo, M.D.; Marcia, M. Visualizing group II intron dynamics between the first and second steps of splicing. Nat. Commun. 2020, 11, 2837. [Google Scholar] [CrossRef] [PubMed]
- Gauci, S.C.; Vranic, A.; Blasco, E.; Bräse, S.; Wegener, M.; Barner-Kowollik, C. Photochemically Activated 3D Printing Inks: Current Status, Challenges, and Opportunities. Adv. Mater. 2024, 36, 2306468. [Google Scholar] [CrossRef] [PubMed]
- Gerges, T.; Semet, V.; Lombard, P.; Allard, B.; Cabrera, M. Rapid 3D-Plastronics prototyping by selective metallization of 3D printed parts. Addit. Manuf. 2023, 73, 103673. [Google Scholar] [CrossRef]
- Wen, P.; Lu, P.; Shi, X.; Yao, Y.; Shi, H.; Liu, H.; Yu, Y.; Wu, Z.-S. Photopolymerized Gel Electrolyte with Unprecedented Room-Temperature Ionic Conductivity for High-Energy-Density Solid-State Sodium Metal Batteries. Adv. Energy Mater. 2021, 11, 2002930. [Google Scholar] [CrossRef]
- Bao, Y.; Paunović, N.; Leroux, J.-C. Challenges and Opportunities in 3D Printing of Biodegradable Medical Devices by Emerging Photopolymerization Techniques. Adv. Funct. Mater. 2022, 32, 2109864. [Google Scholar] [CrossRef]
- Peng, S.; Thirunavukkarasu, N.; Chen, J.; Zheng, X.; Long, C.; Huang, X.; Weng, Z.; Zheng, L.; Wang, H.; Peng, X.; et al. Vat photopolymerization 3D printing of transparent, mechanically robust, and self-healing polyurethane elastomers for tailored wearable sensors. Chem. Eng. J. 2023, 463, 142312. [Google Scholar] [CrossRef]
- Liu, R.; Mabury, S.A. Identification of Photoinitiators, Including Novel Phosphine Oxides, and Their Transformation Products in Food Packaging Materials and Indoor Dust in Canada. Environ. Sci. Technol. 2019, 53, 4109–4118. [Google Scholar] [CrossRef]
- Tumbleston, J.R.; Shirvanyants, D.; Ermoshkin, N.; Janusziewicz, R.; Johnson, A.R.; Kelly, D.; Chen, K.; Pinschmidt, R.; Rolland, J.P.; Ermoshkin, A.; et al. Continuous liquid interface production of 3D objects. Science 2015, 347, 1349–1352. [Google Scholar] [CrossRef]
- Han, L.; Wu, Y.; Fang, K.; Sweeney, S.; Roesner, U.K.; Parrish, M.; Patel, K.; Walter, T.; Piermattei, J.; Trimboli, A.; et al. The splanchnic mesenchyme is the tissue of origin for pancreatic fibroblasts during homeostasis and tumorigenesis. Nat. Commun. 2023, 14, 2381. [Google Scholar] [CrossRef]
- O’Brien, A.K.; Bowman, C.N. Impact of Oxygen on Photopolymerization Kinetics and Polymer Structure. Macromolecules 2006, 39, 2501–2506. [Google Scholar] [CrossRef]
- Aparicio, J.L.; Elizalde, M. Migration of Photoinitiators in Food Packaging: A Review. Packag. Technol. Sci. 2015, 28, 181–203. [Google Scholar] [CrossRef]
- He, M.-H.; Xu, R.-X.; Chen, G.-X.; Zeng, Z.-H.; Yang, J.-W. A thioxanthone-based photocaged superbase for highly effective free radical photopolymerization. Chin. Chem. Lett. 2014, 25, 1445–1448. [Google Scholar] [CrossRef]
- Wessels, M.; Rimkus, J.; Leyhausen, G.; Volk, J.; Geurtsen, W. Genotoxic effects of camphorquinone and DMT on human oral and intestinal cells. Dent. Mater. 2015, 30, 1159–1168. [Google Scholar] [CrossRef]
- Hola, E.; Fiedor, P.; Dzienia, A.; Ortyl, J. Visible-Light Amine Thioxanthone Derivatives as Photoredox Catalysts for Photopolymerization Processes. ACS Appl. Polym. Mater. 2021, 3, 5547–5558. [Google Scholar] [CrossRef]
- Ogasahara, R.; Mae, M.; Itabashi, Y.; Ohkubo, K.; Matsuura, K.; Shimizu, H.; Ban, K.; Togami, M.; Udagawa, T.; Fujioka, H.; et al. Photocatalytic and Chemoselective H/D Exchange at α-Thio C(sp3)-H Bonds. J. Am. Chem. Soc. 2025, 147, 15499–15509. [Google Scholar] [CrossRef]
- Dogruyol, S.K.; Dogruyol, Z.; Kazancioglu, E.O.; Gokcek, H.; Arsu, N. Thioxanthonation of 2-naphthalene-thioacetic acid as a visible-light photoinitiator: The investigation of photophysical and photochemical properties. Eur. Polym. J. 2023, 198, 112440. [Google Scholar] [CrossRef]
- Kreutzer, J.; Kaya, K.; Yagci, Y. Poly(propylene oxide)-thioxanthone as one-component Type II polymeric photoinitiator for free radical polymerization with low migration behavior. Eur. Polym. J. 2017, 95, 71–81. [Google Scholar] [CrossRef]
- Yang, J.; Shi, S.; Nie, J. Reasons for the yellowness of photocured samples by the benzophenone/1,3-benzodioxole photoinitiating system. New J. Chem. 2015, 39, 5453–5458. [Google Scholar] [CrossRef]
- Corrales, T.; Catalina, F.; Peinado, C.; Allen, N.S. Free radical macrophotoinitiators: An overview on recent advances. J. Photochem. Photobiol. A Chem. 2003, 159, 103–114. [Google Scholar] [CrossRef]
- Akat, H.; Ozkan, M. Synthesis and characterization of poly(vinylchloride) type macrophotoinitiator comprising side-chain thioxanthone via click chemistry. Express Polym. Lett. 2011, 5, 318–326. [Google Scholar] [CrossRef]
- Yilmaz, G.; Acik, G.; Yagci, Y. Counteranion Sensitization Approach to Photoinitiated Free Radical Polymerization. Macromolecules 2012, 45, 2219–2224. [Google Scholar] [CrossRef]
- Akat, H.; Gacal, B.; Balta, D.K.; Arsu, N.; Yagci, Y. Poly(ethylene glycol)-thioxanthone prepared by Diels–Alder click chemistry as one-component polymeric photoinitiator for aqueous free-radical polymerization. J. Polym. Sci. A Polym. Chem. 2010, 48, 2109–2114. [Google Scholar] [CrossRef]
- Jin, J.; Lu, G.; Nie, J.; Zhu, X. Low migration and high performance thioxanthone based photoinitiators. Eur. Polym. J. 2024, 202, 112625. [Google Scholar] [CrossRef]
- Eren, T.N.; Okte, N.; Morlet-Savary, F.; Fouassier, J.P.; Lalevee, J.; Avci, D. One-component thioxanthone-based polymeric photoinitiators. J. Polym. Sci. A Polym. Chem. 2016, 54, 3370–3378. [Google Scholar] [CrossRef]
- Jiang, X.; Yin, J. Polymeric Photoinitiator Containing In-Chain Thioxanthone and Coinitiator Amines. Macromol. Rapid Commun. 2004, 25, 748–752. [Google Scholar] [CrossRef]
- Jiang, X.; Yin, J. Study of macrophotoinitiator containing in-chain thioxanthone and coinitiator amines. Polymer 2004, 45, 5057–5063. [Google Scholar] [CrossRef]
- Liang, S.; Yang, Y.D.; Zhou, H.Y.; Li, Y.Q.; Wang, J.X. Fluorinated photoinitiators: Synthesis and photochemical behaviors. Prog. Org. Coat. 2018, 114, 102–108. [Google Scholar] [CrossRef]
- Wu, Q.; Liao, W.; Wang, X.; Xiong, Y.; Tang, H. Sole-Component Visible Macrophotoinitiators with Si-H: Decreased Oxygen Inhibition and Modified Cured Polymer Materials. ChemistrySelect 2020, 5, 10243–10249. [Google Scholar] [CrossRef]
- Hou, H.; Gan, Y.; Yin, J.; Jiang, X. Multifunctional POSS-Based Nano-Photo-Initiator for Overcoming the Oxygen Inhibition of Photo-Polymerization and for Creating Self-Wrinkled Patterns. Adv. Mater. Interfaces 2014, 1, 1400385. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, L.; Zhang, Y.; Ou, Y.; Zhang, J.; Yagic, Y.; Liu, R. Broad wavelength sensitive coumarin sulfonium salts as photoinitiators for cationic, free radical and hybrid photopolymerizations. Prog. Org. Coat. 2023, 174, 107272. [Google Scholar] [CrossRef]
- Tehfe, M.-A.; Dumur, F.; Graff, B.; Morlet-Savary, F.; Fouassier, J.-P.; Gigmes, D.; Lalevée, J. New Push–Pull Dyes Derived from Michler’s Ketone for Polymerization Reactions upon Visible Lights. Macromolecules 2013, 46, 3761–3770. [Google Scholar] [CrossRef]


| Name of Material | Manufacturer |
|---|---|
| Thiosalicylic acid | Adamas Reagents (Shanghai, China) |
| Dioxane | Adamas Reagents (Shanghai, China) |
| Methacryloyl chloride | Adamas Reagents (Shanghai, China) |
| Perfluorooctyl ethyl acrylates (TEAc-8) | Adamas Reagents (Shanghai, China) |
| N-tert-butyl-α-phenylnitrone (PBN) | Adamas Reagents (Shanghai, China) |
| Triethylamine | Sinopharm Chemical Reagent (Shanghai, China) |
| Acetonitrile (CH3CN) | Sinopharm Chemical Reagent (Shanghai, China) |
| Petroleum ether | Sinopharm Chemical Reagent (Shanghai, China) |
| Sodium chloride | Sinopharm Chemical Reagent (Shanghai, China) |
| Sodium bicarbonate | Sinopharm Chemical Reagent (Shanghai, China) |
| Anhydrous sodium sulfate | Sinopharm Chemical Reagent (Shanghai, China) |
| Toluene | Sinopharm Chemical Reagent (Shanghai, China) |
| Concentrated sulfuric acid | Sinopharm Chemical Reagent (Shanghai, China) |
| Phenol | Sinopharm Chemical Reagent (Shanghai, China) |
| Dimethylaminoethyl methacrylate (DMAEMA) | Sinopharm Chemical Reagent (Shanghai, China) |
| Azobisisobutyronitrile (AIBN) | Sinopharm Chemical Reagent (Shanghai, China) |
| Tetrahydrofuran (THF) | Sinopharm Chemical Reagent (Shanghai, China) |
| Dichloromethane (DCM) | Sinopharm Chemical Reagent (Shanghai, China) |
| Trimethylolpropane triacrylate (TMPTA) | Kailin Ruiyang Chemical (Changzhou, China) |
| PIs. | TX-MMA/g | DMAEMA/g | TEAc-8/g | AIBN/g | Time/h | Mn/g/mol | Mw/Mn |
|---|---|---|---|---|---|---|---|
| PPI-A | 1 | 3 | 1.5 | 0.12 | 6 | 1834 | 44.696 |
| PPI-B | 2 | 3 | 1.5 | 0.12 | 6 | 1173 | 33.066 |
| PPI-C | 1 | 3 | 1.5 | 0.12 | 24 | 1483 | 48.132 |
| PPI-D | 1 | 3 | 1.5 | 0.06 | 6 | 1767 | 49.204 |
| PPI (TX-N) | 1 | 3 | 0 | 0.12 | 24 | 1001 | 34.583 |
| PPI (TX-F) | 1 | 0 | 1.5 | 0.12 | 24 | 4994 | 2.935 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, Y.; Zhang, J.; Han, T.; Zhu, Y. Thioxanthone Skeleton-Based One-Component Macro-Photoinitiator Reduces Oxygen Inhibition and Migration Through Cooperative Effect. Polymers 2025, 17, 2252. https://doi.org/10.3390/polym17162252
Du Y, Zhang J, Han T, Zhu Y. Thioxanthone Skeleton-Based One-Component Macro-Photoinitiator Reduces Oxygen Inhibition and Migration Through Cooperative Effect. Polymers. 2025; 17(16):2252. https://doi.org/10.3390/polym17162252
Chicago/Turabian StyleDu, Yiyun, Jingyan Zhang, Tianyi Han, and Yi Zhu. 2025. "Thioxanthone Skeleton-Based One-Component Macro-Photoinitiator Reduces Oxygen Inhibition and Migration Through Cooperative Effect" Polymers 17, no. 16: 2252. https://doi.org/10.3390/polym17162252
APA StyleDu, Y., Zhang, J., Han, T., & Zhu, Y. (2025). Thioxanthone Skeleton-Based One-Component Macro-Photoinitiator Reduces Oxygen Inhibition and Migration Through Cooperative Effect. Polymers, 17(16), 2252. https://doi.org/10.3390/polym17162252








