Polyethylene Terephthalate Glycolysis: Kinetic Modeling and Validation
Abstract
1. Introduction
2. Material and Methods
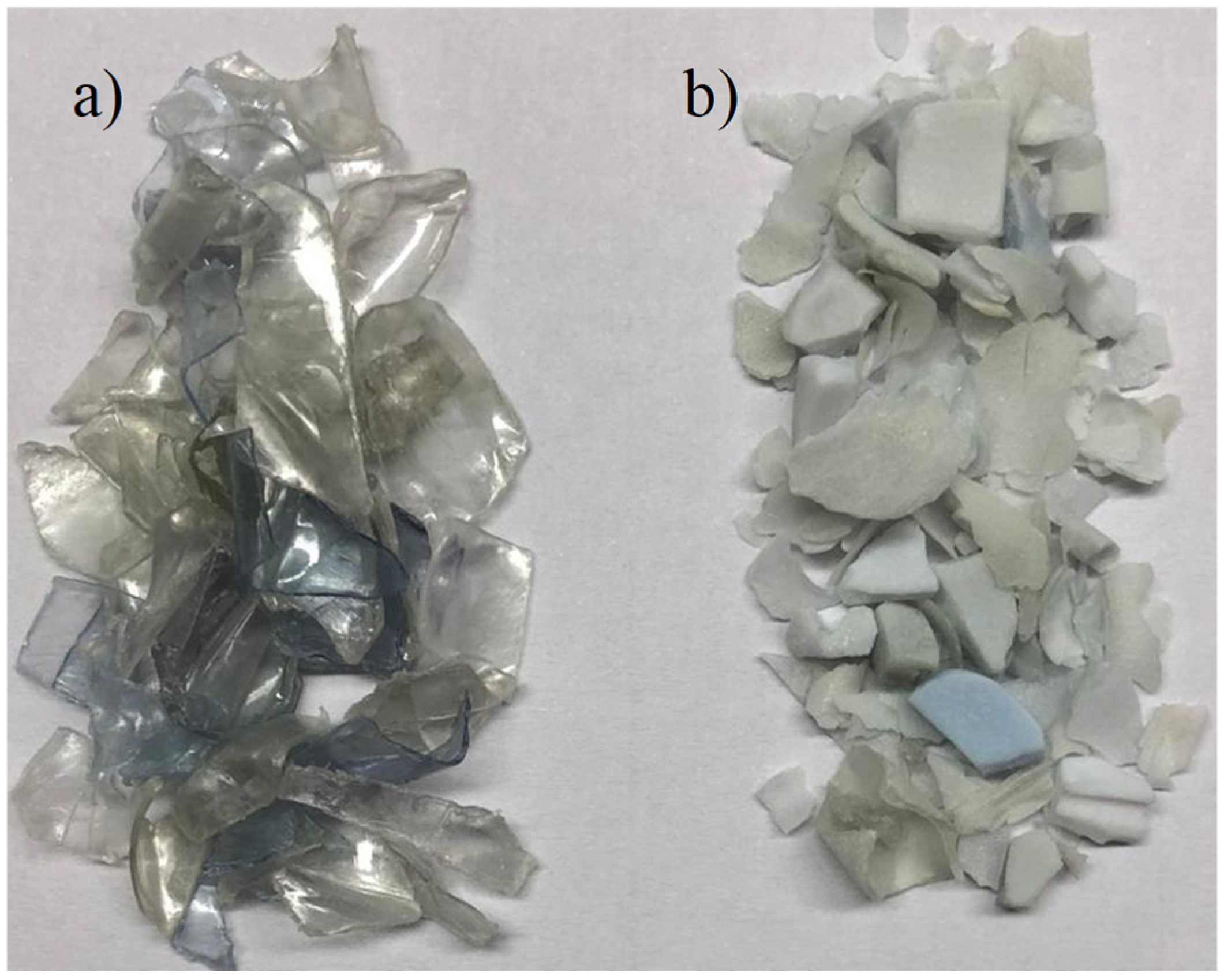
2.1. Reactor Equipment
2.2. Size Exclusion Chromatography
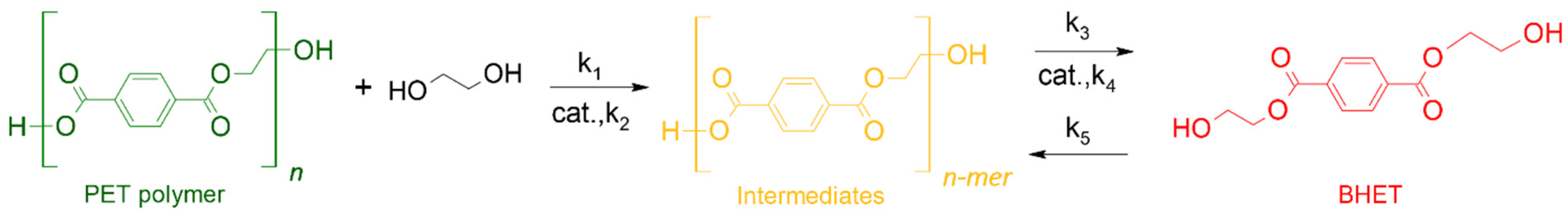
2.3. Kinetic Modeling
3. Results and Discussion
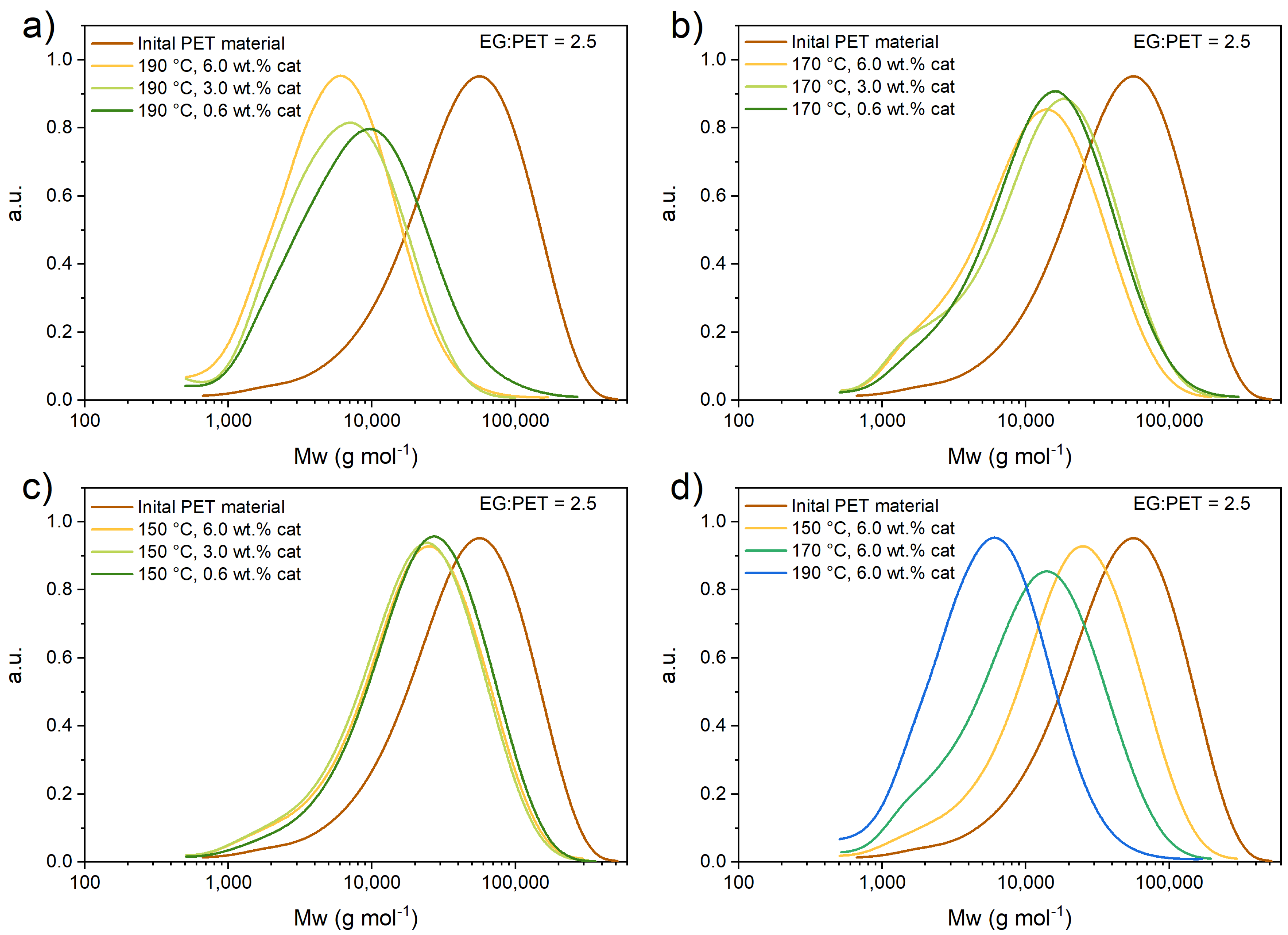
3.1. Size Exclusion Chromatography
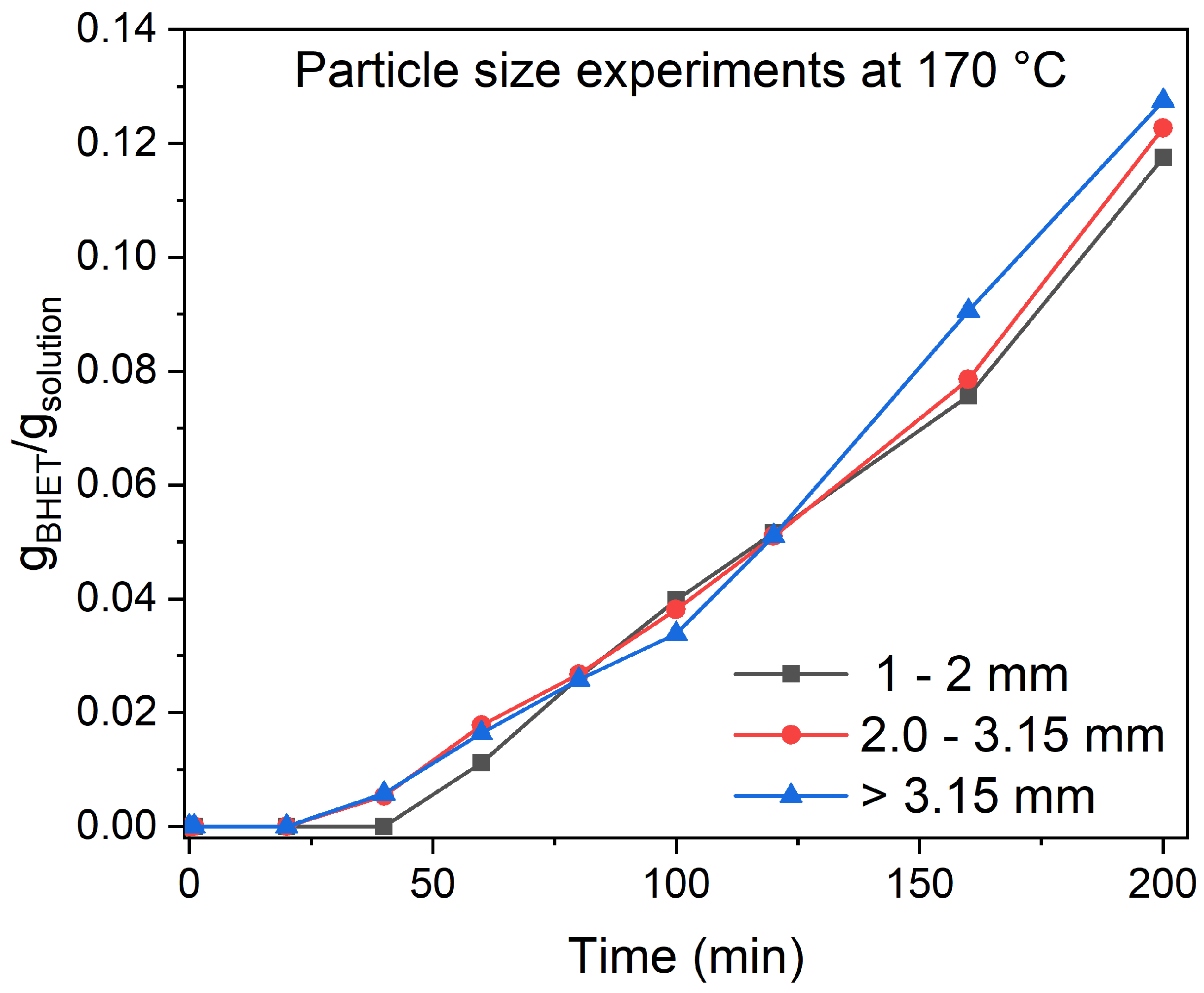
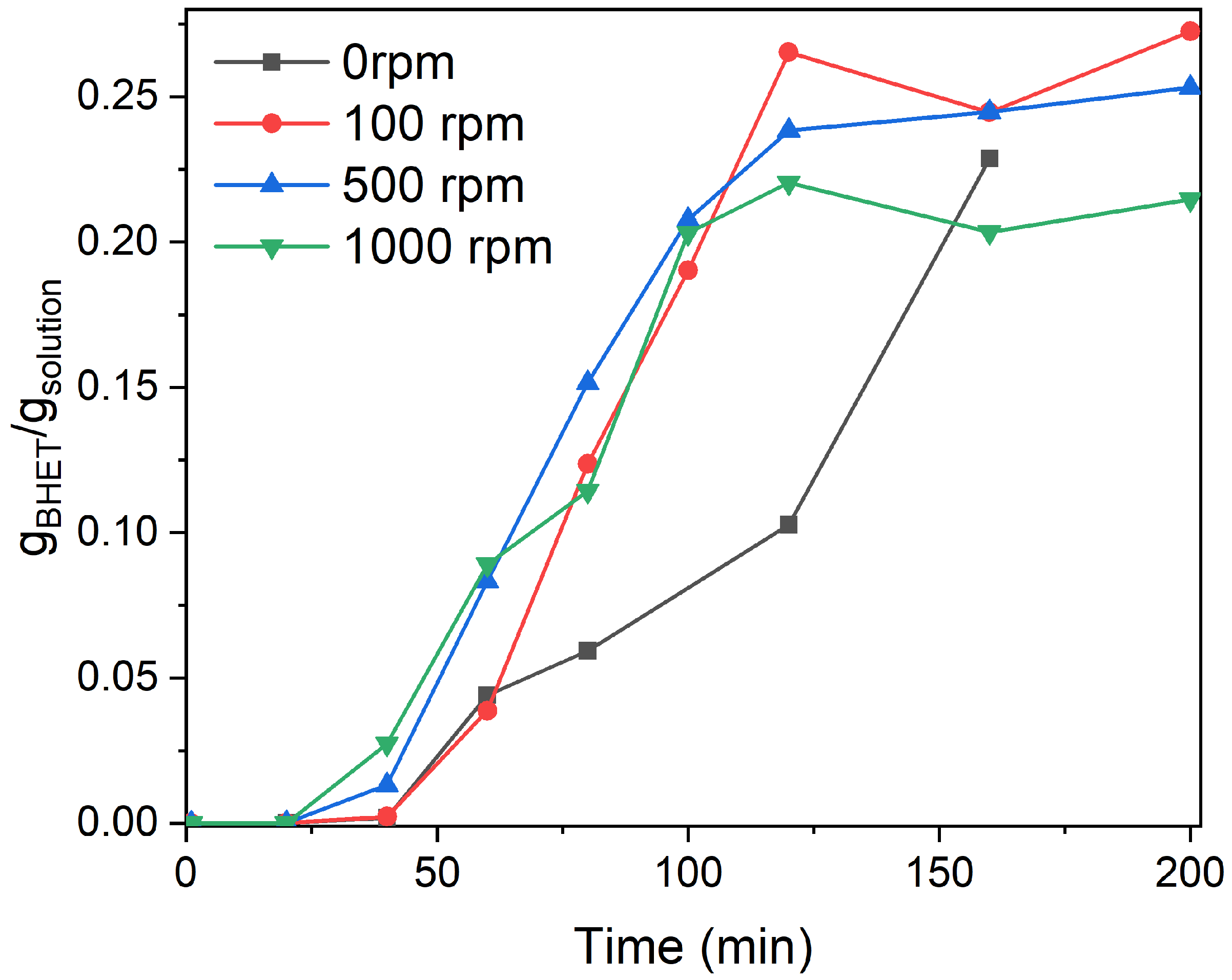
3.2. Particle Size and Stirring Speed Effect
3.3. Glycolysis Kinetics
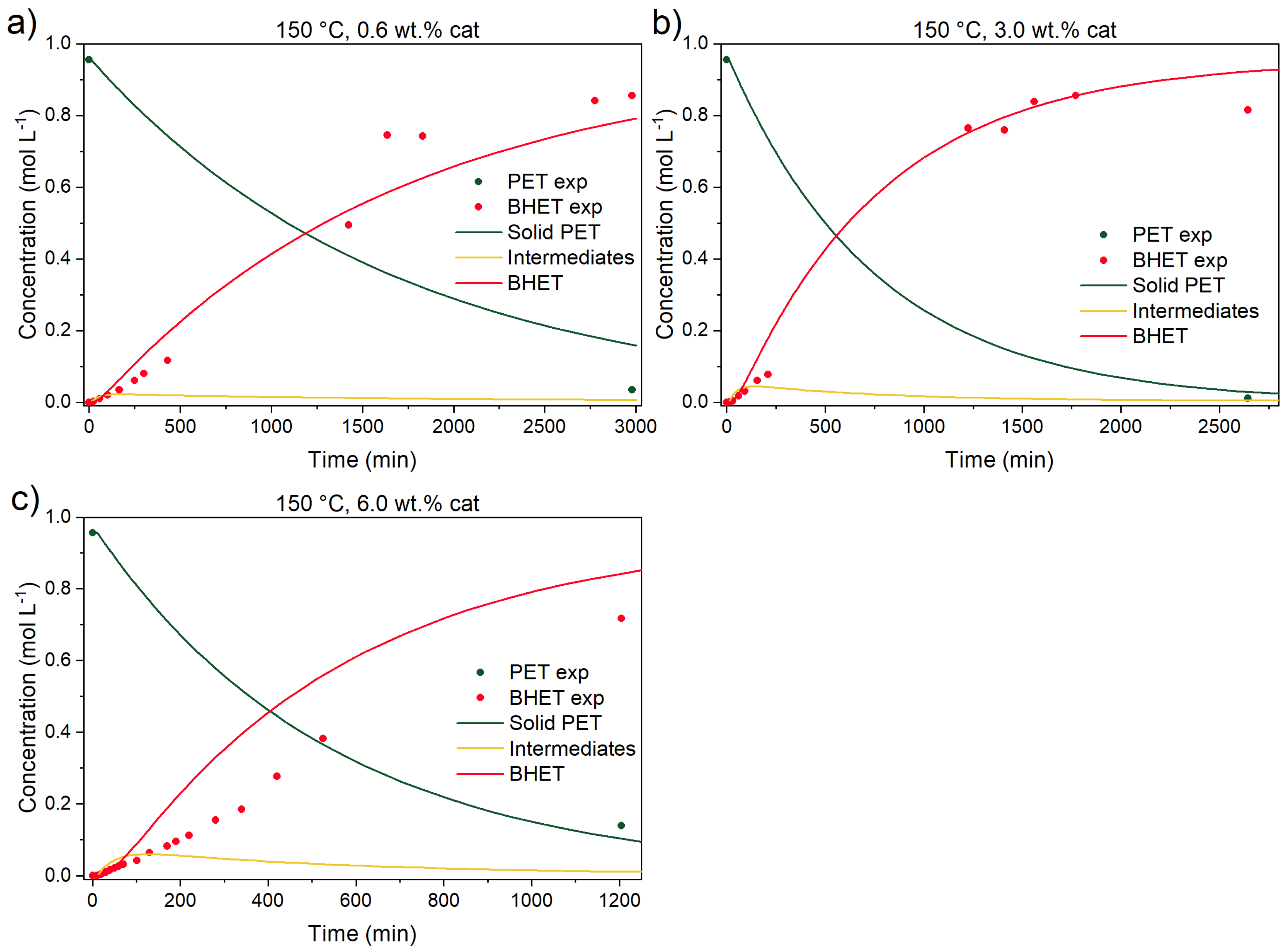
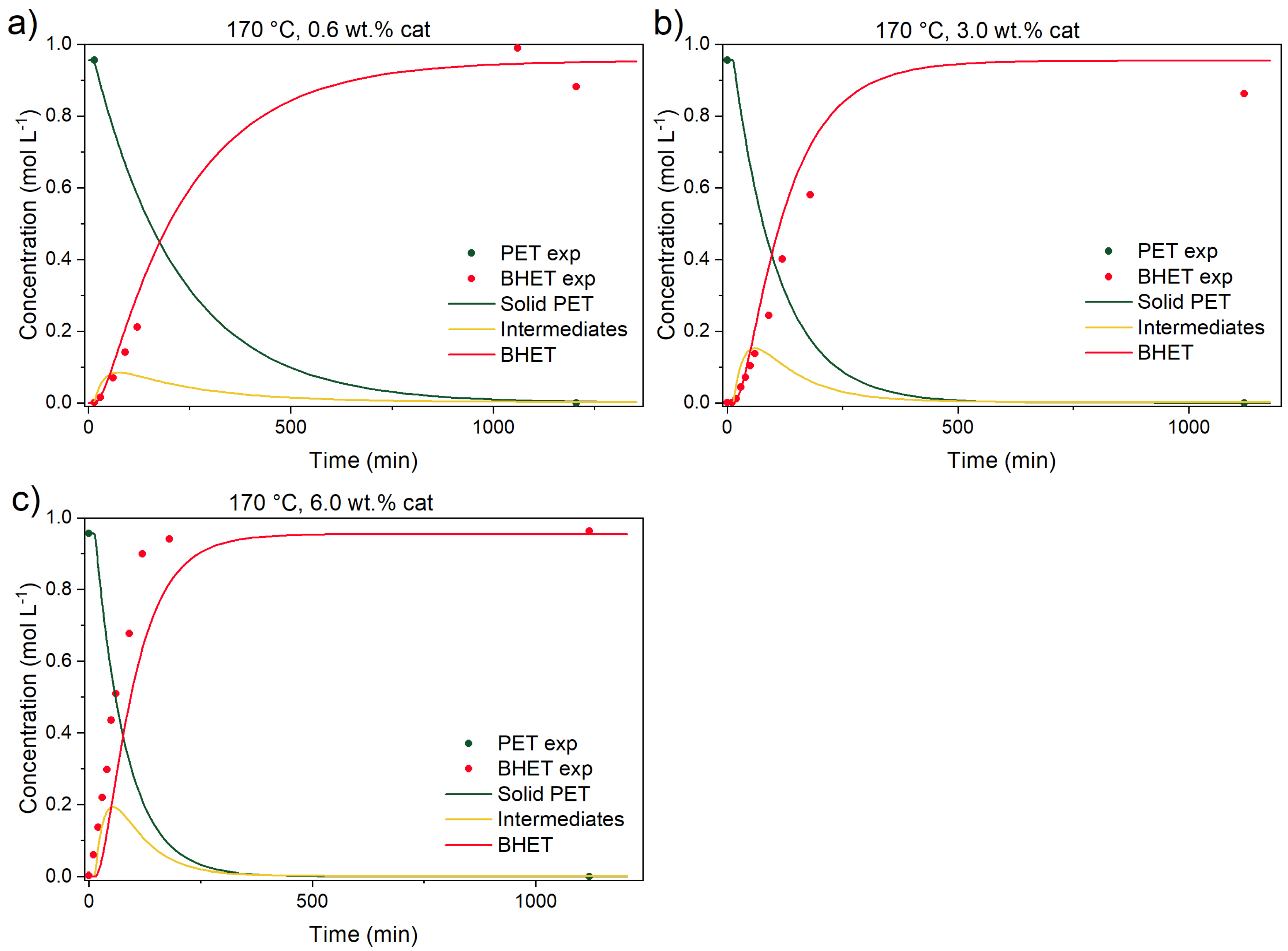
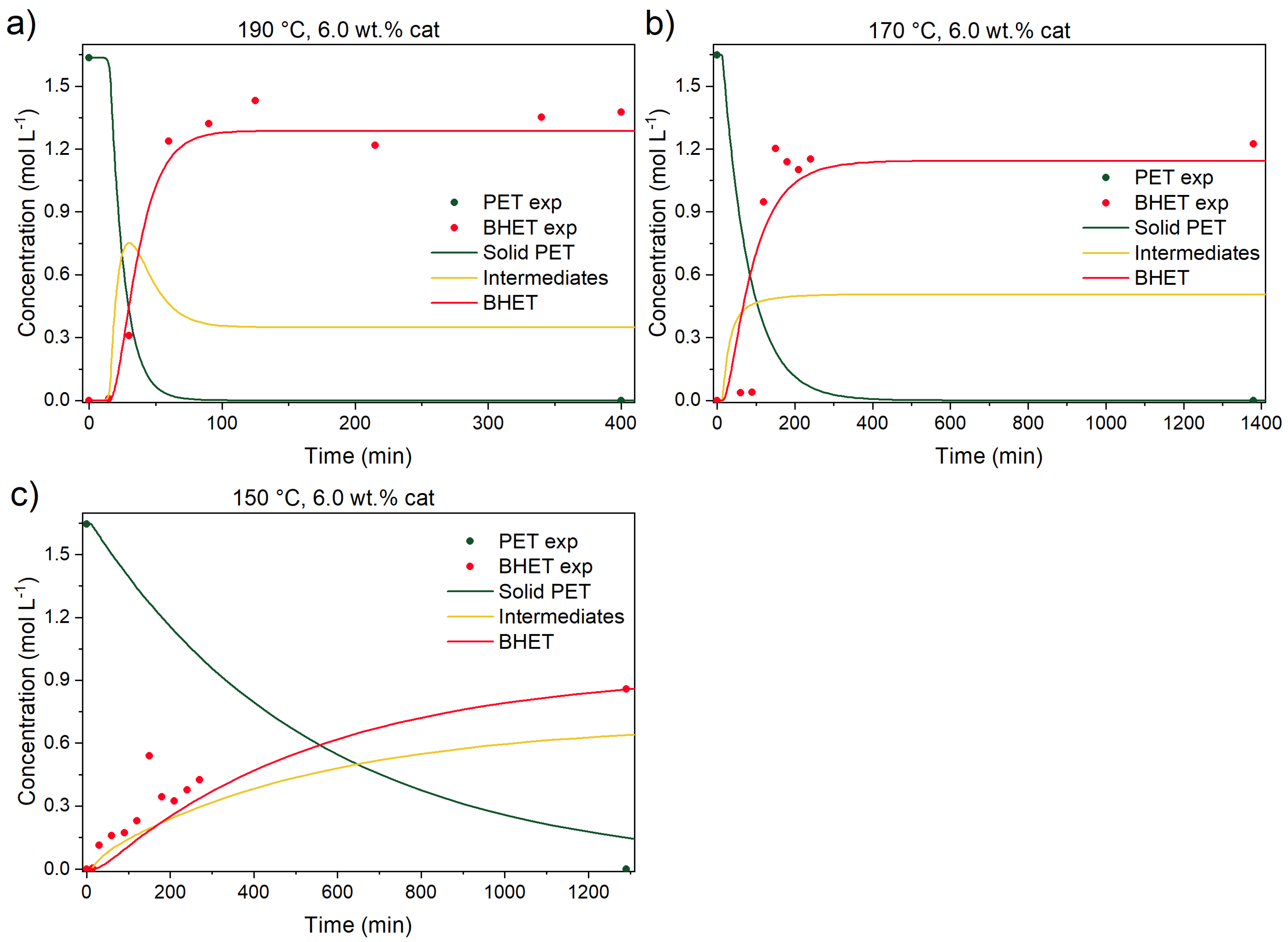
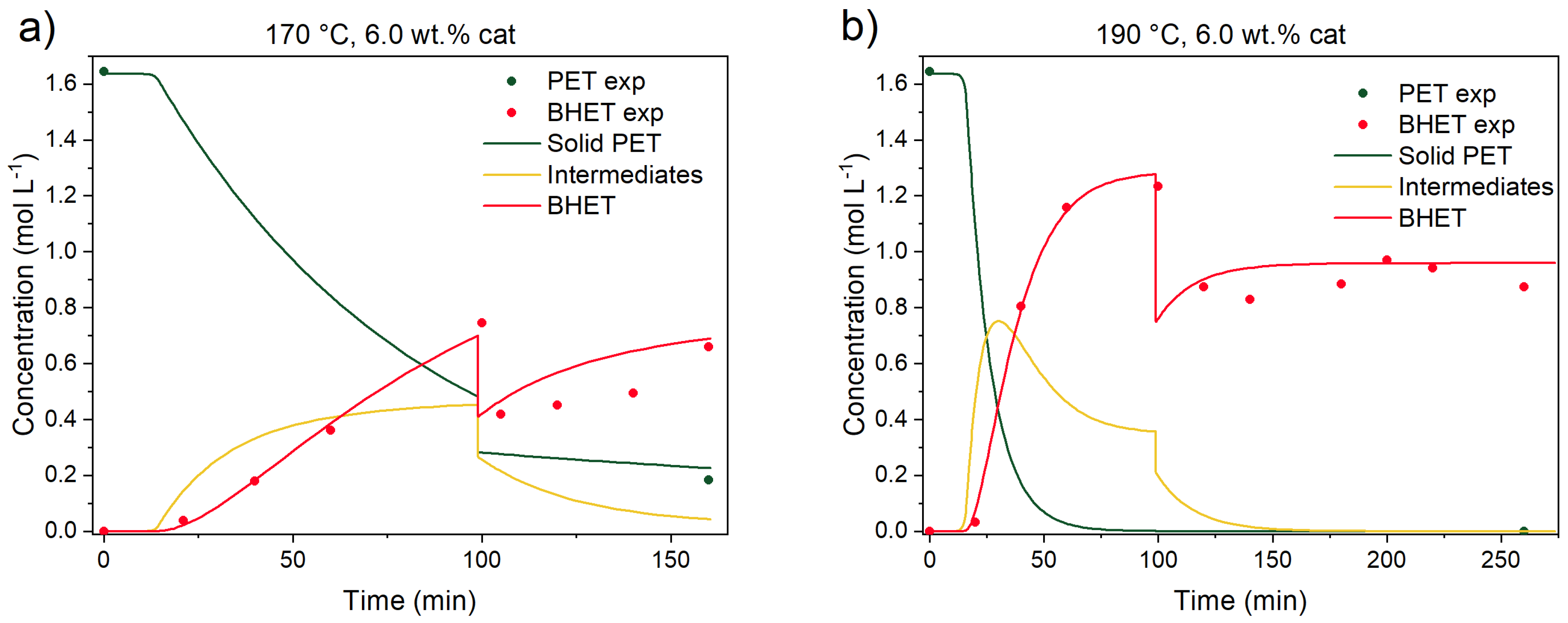
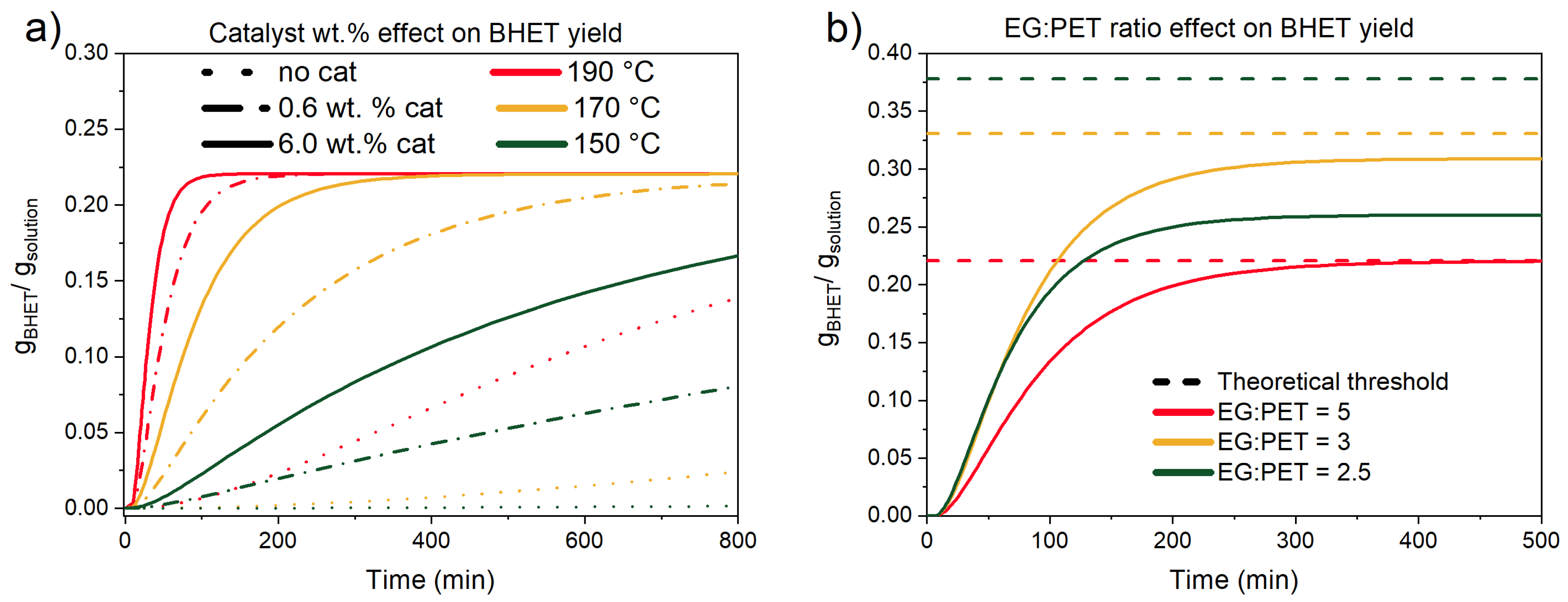
3.3.1. Catalyst Effect on the Reaction
3.3.2. Ethylene Glycol-to-PET Ratio Effect
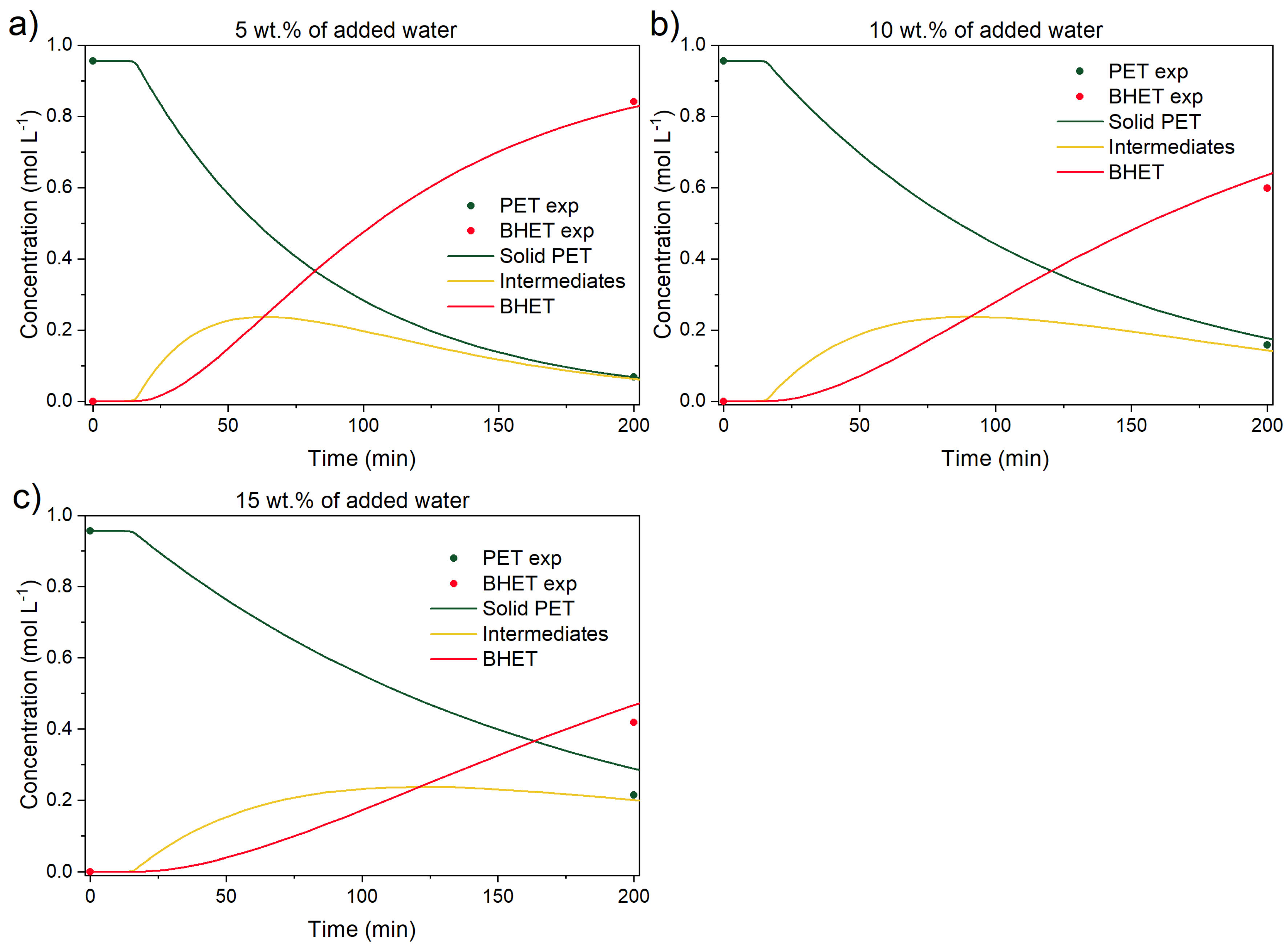
3.3.3. Experiments with Added Water
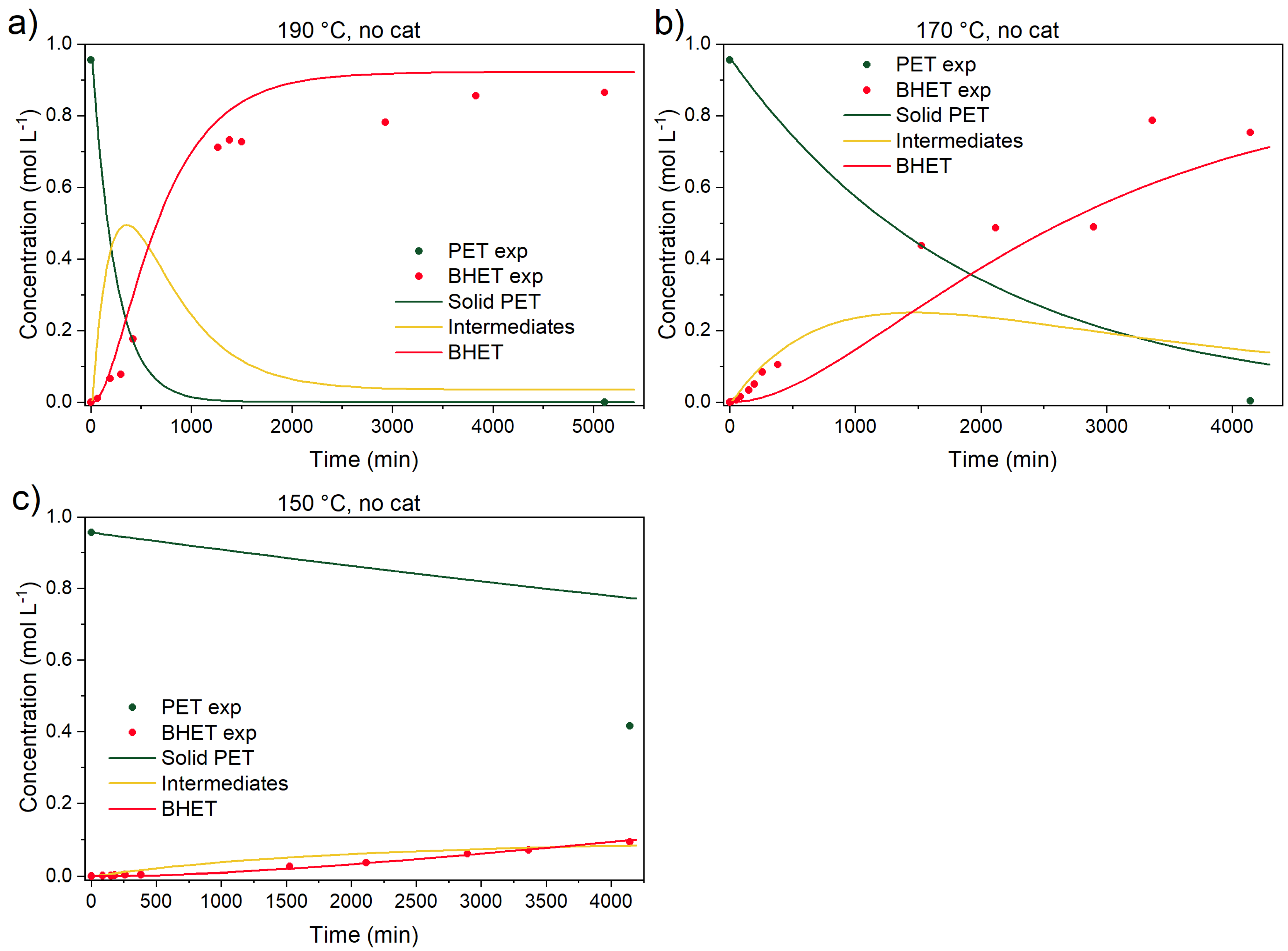
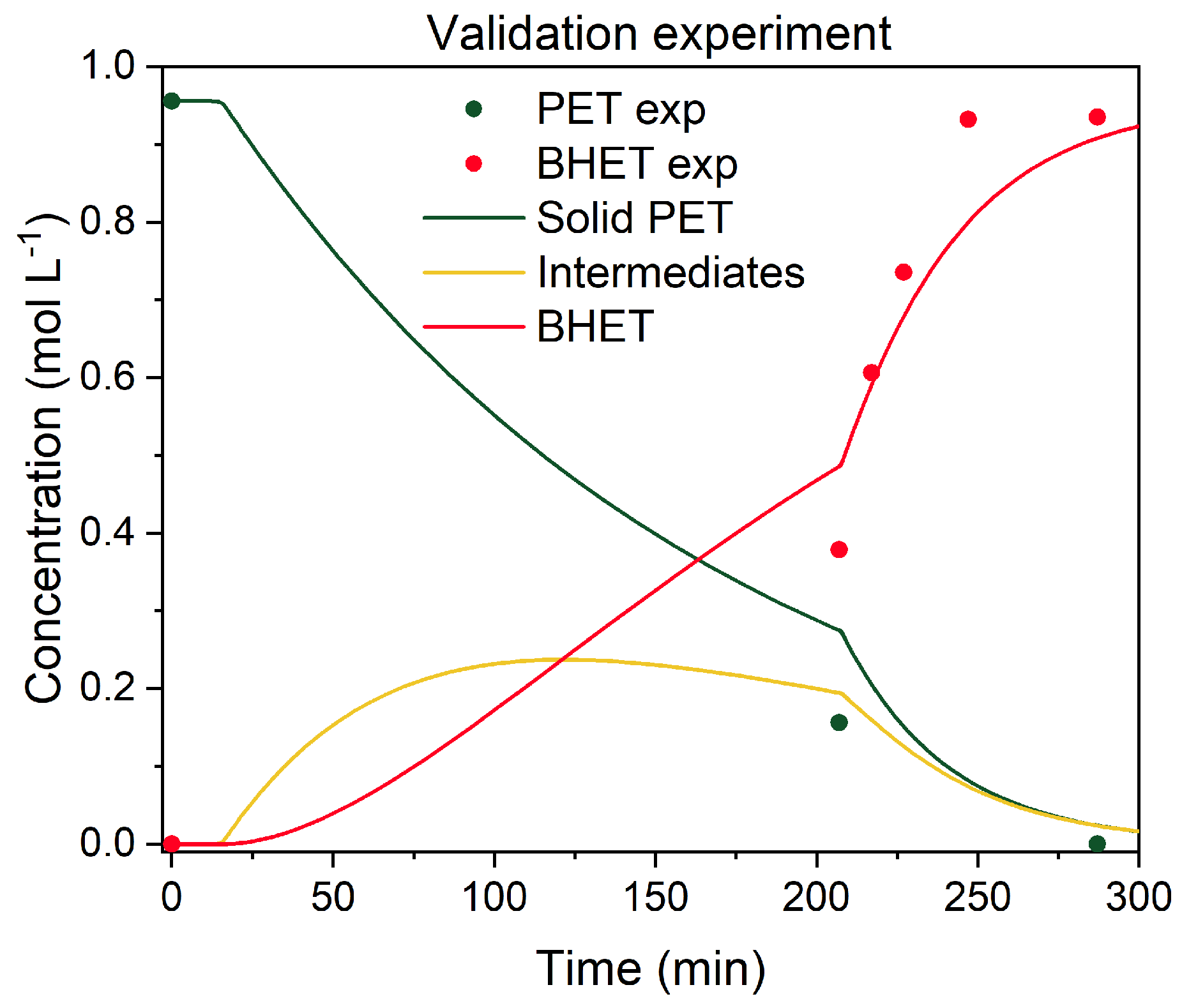
3.3.4. Kinetic Parameters, Validation Experiments, and Model Predictions
4. Conclusions and Outlook
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Abbreviation | Definition |
| BHET | Bis(2-hydroxyethyl) terephthalate |
| DMT | Dimethyl terephthalate |
| EG | Ethylene glycol |
| HFIP | 1,1,1,3,3,3-Hexafluoro-2-propanol |
| HPLC | High-performance liquid chromatography |
| MHET | Monohydroxyethyl terephthalate |
| NaTFA | Sodium trifluoroacetate |
| PET | Polyethylene terephthalate |
| PDI | Polydispersity index |
| PMMA | Polymethyl methacrylate |
| RI | Refractive index |
| SEC | Size exclusion chromatography |
| TPA | Terephthalic acid |
References
- Sarda, P.; Hanan, J.C.; Lawrence, J.G.; Allahkarami, M. Sustainability performance of polyethylene terephthalate, clarifying challenges and opportunities. J. Polym. Sci. 2022, 60, 7–31. [Google Scholar] [CrossRef]
- Schyns, Z.O.G.; Shaver, M.P. Mechanical Recycling of Packaging Plastics: A Review. Macromol. Rapid Commun. 2021, 42, 2000415. [Google Scholar] [CrossRef] [PubMed]
- Kurokawa, H.; Ohshima, M.; Sugiyama, K.; Miura, H. Methanolysis of polyethylene terephthalate (PET) in the presence of aluminium tiisopropoxide catalyst to form dimethyl terephthalate and ethylene glycol. Polym. Degrad. Stab. 2003, 79, 529–533. [Google Scholar] [CrossRef]
- Genta, M.; Iwaya, T.; Sasaki, M.; Goto, M.; Hirose, T. Depolymerization Mechanism of Poly(ethylene terephthalate) in Supercritical Methanol. Ind. Eng. Chem. Res. 2005, 44, 3894–3900. [Google Scholar] [CrossRef]
- Zhu, M.; Li, S.; Li, Z.; Lu, X.; Zhang, S. Investigation of solid catalysts for glycolysis of polyethylene terephthalate. Chem. Eng. J. 2012, 185–186, 168–177. [Google Scholar] [CrossRef]
- Zhu, M.; Li, Z.; Wang, Q.; Zhou, X.; Lu, X. Characterization of Solid Acid Catalysts and Their Reactivity in the Glycolysis of Poly(ethylene terephthalate). Ind. Eng. Chem. Res. 2012, 51, 11659–11666. [Google Scholar] [CrossRef]
- Čolnik, M.; Pečar, D.; Knez, Ž.; Goršek, A.; Škerget, M. Kinetics Study of Hydrothermal Degradation of PET Waste into Useful Products. Processes 2021, 10, 24. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, M.; Pan, Z. Catalytic depolymerization of polyethylene terephthalate in hot compressed water. J. Supercrit. Fluids 2012, 62, 226–231. [Google Scholar] [CrossRef]
- Zhang, S.; Xu, W.; Du, R.; An, W.; Liu, X.; Xu, S.; Wang, Y.-Z. Selective depolymerization of PET to monomers from its waste blends and composites at ambient temperature. Chem. Eng. J. 2023, 470, 144032. [Google Scholar] [CrossRef]
- Kang, M.J.; Yu, H.J.; Jegal, J.; Kim, H.S.; Cha, H.G. Depolymerization of PET into terephthalic acid in neutral media catalyzed by the ZSM-5 acidic catalyst. Chem. Eng. J. 2020, 398, 125655. [Google Scholar] [CrossRef]
- Rollo, M.; Raffi, F.; Rossi, E.; Tiecco, M.; Martinelli, E.; Ciancaleoni, G. Depolymerization of polyethylene terephthalate (PET) under mild conditions by Lewis/Brønsted acidic deep eutectic solvents. Chem. Eng. J. 2023, 456, 141092. [Google Scholar] [CrossRef]
- Westover, C.C.; Long, T.E. Envisioning a BHET Economy: Adding Value to PET Waste. Sustain. Chem. 2023, 4, 363–393. [Google Scholar] [CrossRef]
- Enache, A.-C.; Grecu, I.; Samoila, P. Polyethylene Terephthalate (PET) Recycled by Catalytic Glycolysis: A Bridge toward Circular Economy Principles. Materials 2024, 17, 2991. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Zhou, Q.; Bu, R.; Yang, F.; Li, W. Kinetics of poly(ethylene terephthalate) fiber glycolysis in ethylene glycol. Fibers Polym. 2015, 16, 1213–1219. [Google Scholar] [CrossRef]
- Chen, J.-W.; Chen, L.-W.; Cheng, W.-H. Kinetics of glycolysis of polyethylene terephthalate with zinc catalyst. Polym. Int. 1999, 48, 885–888. [Google Scholar] [CrossRef]
- Viana, M.E.; Riul, A.; Carvalho, G.M.; Rubira, A.F.; Muniz, E.C. Chemical recycling of PET by catalyzed glycolysis: Kinetics of the heterogeneous reaction. Chem. Eng. J. 2011, 173, 210–219. [Google Scholar] [CrossRef]
- Capeletti, M.R.; Passamonti, F.J. Optimization of reaction parameters in the conversion of PET to produce BHET. Polym. Eng. Sci. 2018, 58, 1500–1507. [Google Scholar] [CrossRef]
- López-Fonseca, R.; Duque-Ingunza, I.; de Rivas, B.; Flores-Giraldo, L.; Gutiérrez-Ortiz, J.I. Kinetics of catalytic glycolysis of PET wastes with sodium carbonate. Chem. Eng. J. 2011, 168, 312–320. [Google Scholar] [CrossRef]
- Meira, G.; Netopilík, M.; Potschka, M.; Schnöll-Bitai, I.; Vega, J. Band Broadening Function in Size Exclusion Chromatography of Polymers: Review of Some Recent Developments. Macromol. Symp. 2007, 258, 186–197. [Google Scholar] [CrossRef]
- Preis, H.B.J. Characterization of Polyethylene Terephthalate with GPC/SEC. Available online: https://www.agilent.com/cs/library/applications/ab-pfg-polyethylene-terephthalate-pet-gpc-sec-5994-5724en-agilent.pdf (accessed on 19 November 2024).
- Llopis, S.F.; Verdejo, E.; Gil-Castell, O.; Ribes-Greus, A. Partial glycolytic depolymerisation of poly(ethylene terephthalate) (PET) in the solid state: Modelling the contribution of time and temperature. Polym. Degrad. Stab. 2024, 221, 110695. [Google Scholar] [CrossRef]


| Uncatalyzed Reaction Rate Constants and Corresponding Activation Energies | |||||
|---|---|---|---|---|---|
| k1 [min−1] | k3 [min−1] | k5 [min−1] | Ea1 [kJ mol−1] | Ea3 [kJ mol−1] | Ea5 [kJ mol−1] |
| (5.17 ± 0.5) × 10−4 | 0.0010 ± 0.00034 | (2.88 ± 0.4) × 10−7 | 180 ± 23 | 57 ± 12 | <5 |
| Catalyzed Reaction Rate Constants and Corresponding Activation Energies | |||
|---|---|---|---|
| k2 [Lα mol−α min−1] | k4 [Lβ mol−β min−1] | Ea2 [kJ mol−1] | Ea4 [kJ mol−1] |
| 0.06 ± 0.005 | 0.039 ± 0.004 | 158 ± 6 | 37 ± 6 |
| Catalyst Concentration Order | Ratio Parameter | Water Inhibition | |
|---|---|---|---|
| α | β | S1 | Inhib |
| 0.51 ± 0.0 | 5.43 ± 0.16 × 10−6 | 27.6 ± 0.1 | 0.34 ± 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gabrič, M.; Lavrič, Ž.; Schwiderski, M.; Marc, L.; Temmel, E.; Grilc, M.; Likozar, B. Polyethylene Terephthalate Glycolysis: Kinetic Modeling and Validation. Polymers 2025, 17, 2246. https://doi.org/10.3390/polym17162246
Gabrič M, Lavrič Ž, Schwiderski M, Marc L, Temmel E, Grilc M, Likozar B. Polyethylene Terephthalate Glycolysis: Kinetic Modeling and Validation. Polymers. 2025; 17(16):2246. https://doi.org/10.3390/polym17162246
Chicago/Turabian StyleGabrič, Maja, Žan Lavrič, Martin Schwiderski, Laureline Marc, Erik Temmel, Miha Grilc, and Blaž Likozar. 2025. "Polyethylene Terephthalate Glycolysis: Kinetic Modeling and Validation" Polymers 17, no. 16: 2246. https://doi.org/10.3390/polym17162246
APA StyleGabrič, M., Lavrič, Ž., Schwiderski, M., Marc, L., Temmel, E., Grilc, M., & Likozar, B. (2025). Polyethylene Terephthalate Glycolysis: Kinetic Modeling and Validation. Polymers, 17(16), 2246. https://doi.org/10.3390/polym17162246









