Early Biological Response to Poly(ε-caprolactone) PCL—Bioactive Glass Composites Obtained by 3D Printing as Bone Substitutes
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Microscopy and EDX Spectroscopy
2.3. Mechanical Properties
2.4. Roughness
2.5. Contact Angle and Surface Free Energy Evaluation
2.6. Ion Release Analysis
2.7. Protein Adsorption
2.8. Cell Culture
2.8.1. Cell Adhesion and Spreading
2.8.2. Cell Viability
2.9. Statistical Analysis
3. Results
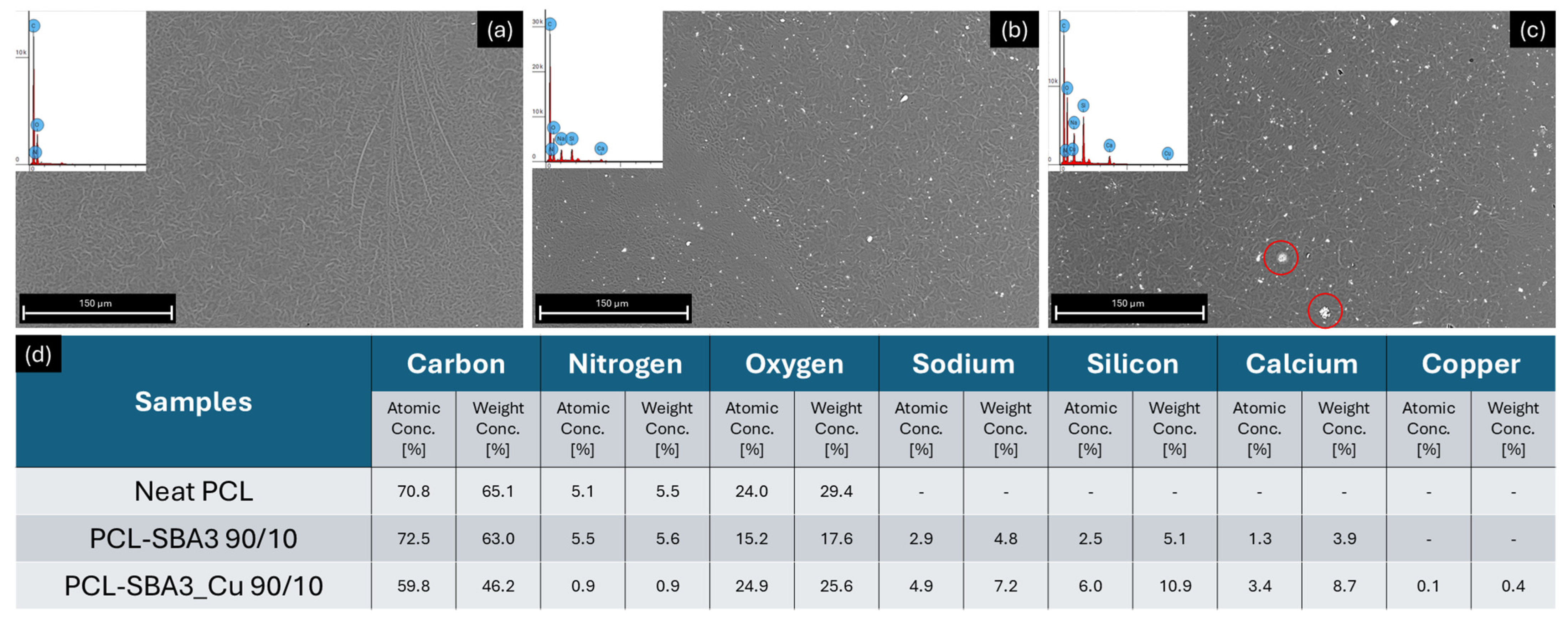
3.1. Scanning Electron Microscopy and Elemental Analyses of the Composites
3.2. Mechanical Characterization of the Composites
3.3. Roughness
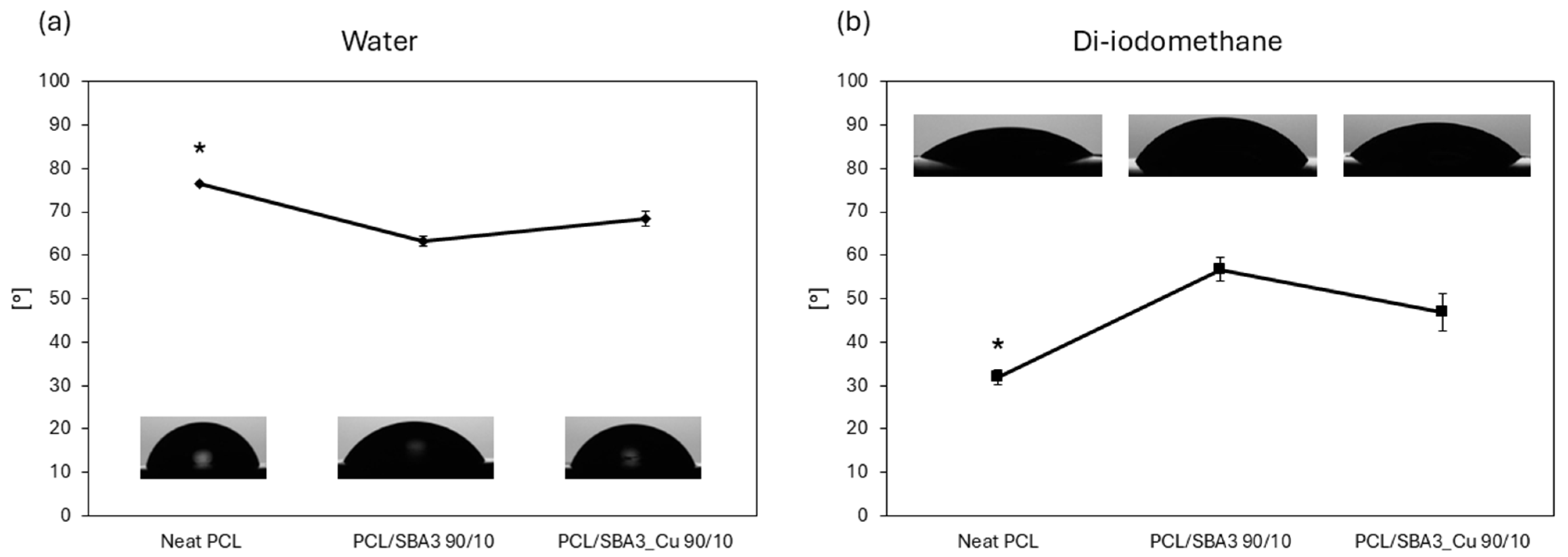
3.4. Contact Angle and Surface Free Energy Evaluation
3.5. Analysis of the Ions in Culture Medium
3.6. Protein Adsorption
3.7. Cell Experiments
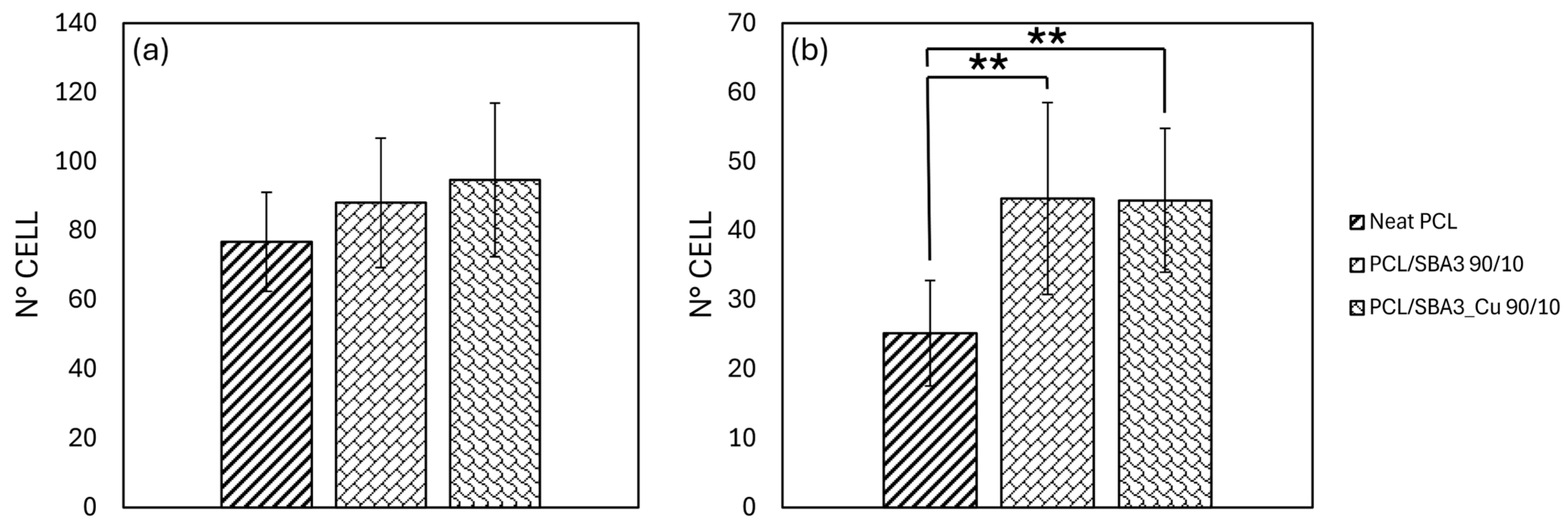
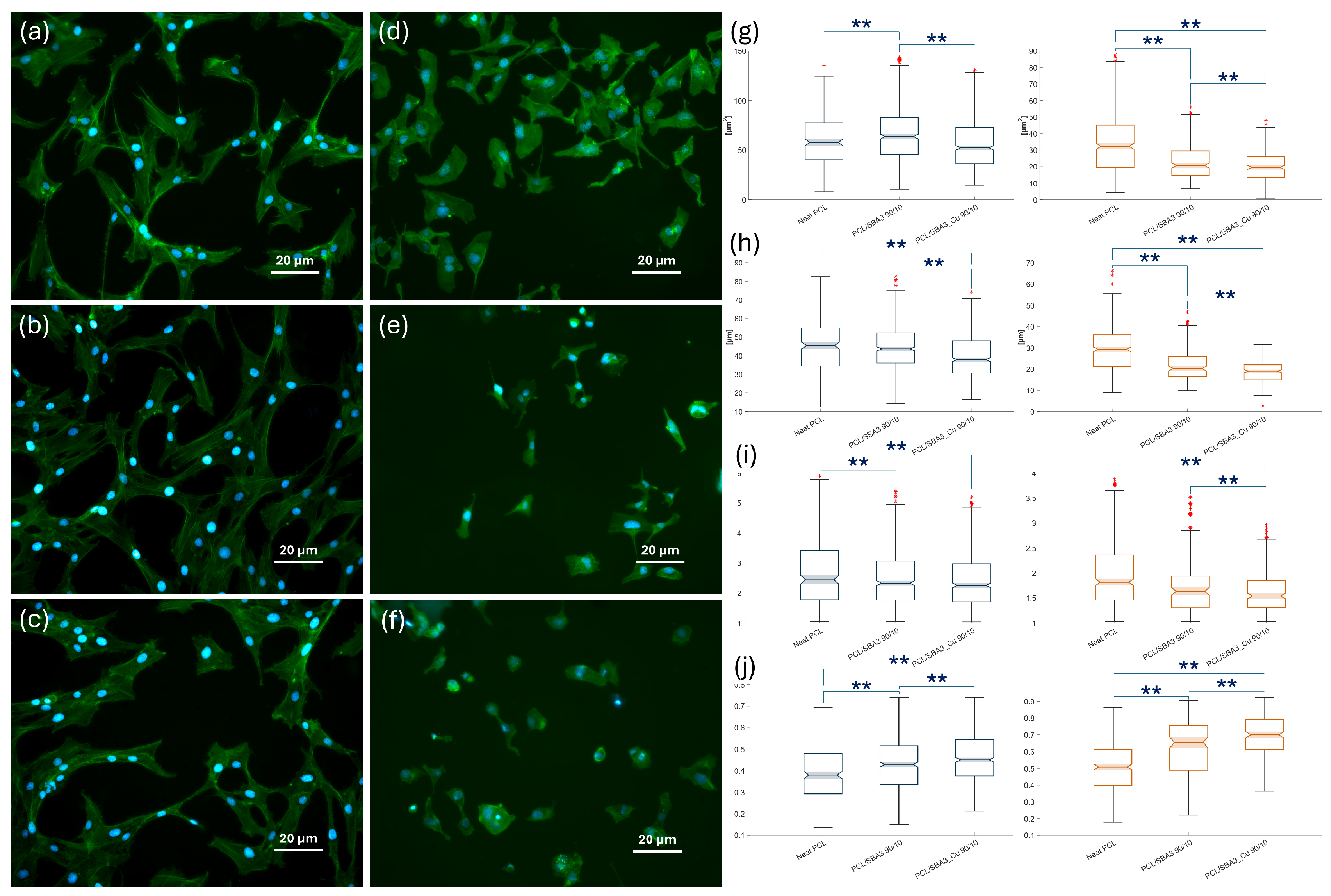
3.7.1. Cell Adhesion and Morphology
3.7.2. Biocompatibility of the Composite PCL/SBA3 Materials
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Culbreath, C.J.; Taylor, M.S.; McCullen, S.D.; Mefford, O.T. A Review of Additive Manufacturing in Tissue Engineering and Regenerative Medicine. Biomed. Mater. Devices 2025, 3, 237–258. [Google Scholar] [CrossRef]
- Fukuba, S.; Okada, M.; Nohara, K.; Iwata, T. Alloplastic Bone Substitutes for Periodontal and Bone Regeneration in Dentistry: Current Status and Prospects. Materials 2021, 14, 1096. [Google Scholar] [CrossRef]
- Ho-Shui-Ling, A.; Bolander, J.; Rustom, L.E.; Johnson, A.W.; Luyten, F.P.; Picart, C. Bone Regeneration Strategies: Engineered Scaffolds, Bioactive Molecules and Stem Cells Current Stage and Future Perspectives. Biomaterials 2018, 180, 143–162. [Google Scholar] [CrossRef]
- Amini, A.R.; Laurencin, C.T.; Nukavarapu, S.P. Bone Tissue Engineering: Recent Advances and Challenges. Crit. Rev. Biomed. Eng. 2012, 40, 363–408. [Google Scholar] [CrossRef]
- Pugliese, R.; Beltrami, B.; Regondi, S.; Lunetta, C. Polymeric Biomaterials for 3D Printing in Medicine: An Overview. Ann. 3D Print. Med. 2021, 2, 100011. [Google Scholar] [CrossRef]
- Guzzi, E.A.; Tibbitt, M.W. Additive Manufacturing of Precision Biomaterials. Adv. Mater. 2020, 32, 1901994. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Zhang, P.; Qin, Q.; Jiang, K.; Luo, Y.; Xiang, C.; He, J.; Chen, L.; Jiang, D.; Cui, W.; et al. 3D-Printed Bone Regeneration Scaffolds Modulate Bone Metabolic Homeostasis through Vascularization for Osteoporotic Bone Defects. Biomaterials 2024, 311, 122699. [Google Scholar] [CrossRef] [PubMed]
- Di Maro, M.; Pedraza, R.; Mosca Balma, A.; Gomez d’Ayala, G.; Poggetto, G.D.; Malucelli, G.; Roato, I.; Duraccio, D.; Mussano, F.; Faga, M.G. Influence of Dry-Mixing and Solvent Casting Blending Techniques on the Mechanical and Biological Behavior of Novel Biocompatible Poly(ε-Caprolactone)/Alumina-Toughened Zirconia Scaffolds Obtained by 3D Printing. J. Compos. Sci. 2024, 8, 194. [Google Scholar] [CrossRef]
- Catauro, M.; Raucci, M.G.; De Gaetano, F.; Marotta, A. Sol-Gel Synthesis, Characterization and Bioactivity of Polycaprolactone/SiO2 Hybrid Material. J. Mater. Sci. 2003, 38, 3097–3102. [Google Scholar] [CrossRef]
- Catauro, M.; Raucci, M.G.; Continenza, M.A.; Marotta, A. Biocompatibility Tests with Fibroblasts of CaO Rich Calcium Silicate Glasses. J. Mater. Sci. 2004, 39, 373–375. [Google Scholar] [CrossRef]
- Fiume, E.; Barberi, J.; Verné, E.; Baino, F. Bioactive Glasses: From Parent 45S5 Composition to Scaffold-Assisted Tissue-Healing Therapies. JFB 2018, 9, 24. [Google Scholar] [CrossRef]
- Barrak, F.N.; Li, S.; Mohammed, A.A.; Myant, C.; Jones, J.R. Anti-Inflammatory Properties of S53P4 Bioactive Glass Implant Material. J. Dent. 2022, 127, 104296. [Google Scholar] [CrossRef]
- Elhamouly, Y.; El Backly, R.M.; Talaat, D.M.; Omar, S.S.; El Tantawi, M.; Dowidar, K.M.L. Tailored 70S30C Bioactive Glass Induces Severe Inflammation as Pulpotomy Agent in Primary Teeth: An Interim Analysis of a Randomised Controlled Trial. Clin. Oral Investig. 2021, 25, 3775–3787. [Google Scholar] [CrossRef]
- Vujović, S.; Desnica, J.; Stanišić, D.; Ognjanović, I.; Stevanovic, M.; Rosic, G. Applications of Biodegradable Magnesium-Based Materials in Reconstructive Oral and Maxillofacial Surgery: A Review. Molecules 2022, 27, 5529. [Google Scholar] [CrossRef]
- Brauer, D.S. Bioactive Glasses—Structure and Properties. Angew. Chem. Int. Ed. 2015, 54, 4160–4181. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Van Le, Q. Bioactive Glass Coated Zirconia for Dental Implants: A Review. J. Compos. Compd. 2020, 2, 10–17. [Google Scholar] [CrossRef]
- Motameni, A.; Çardaklı, İ.S.; Gürbüz, R.; Alshemary, A.Z.; Razavi, M.; Farukoğlu, Ö.C. Bioglass-Polymer Composite Scaffolds for Bone Tissue Regeneration: A Review of Current Trends. Int. J. Polym. Mater. Polym. Biomater. 2024, 73, 600–619. [Google Scholar] [CrossRef]
- Fendi, F.; Abdullah, B.; Suryani, S.; Usman, A.N.; Tahir, D. Development and Application of Hydroxyapatite-Based Scaffolds for Bone Tissue Regeneration: A Systematic Literature Review. Bone 2024, 183, 117075. [Google Scholar] [CrossRef]
- ShiraliPour, F.; Shafiei, S.S.; Nikakhtar, Y. Three-dimensional Porous Poly(ε-Caprolactone)/Beta-tricalcium Phosphate Microsphere-aggregated Scaffold for Bone Tissue Engineering. Int. J. Appl. Ceram. Technol. 2021, 18, 1442–1456. [Google Scholar] [CrossRef]
- Gritsch, L.; Perrin, E.; Chenal, J.-M.; Fredholm, Y.; Maçon, A.L.; Chevalier, J.; Boccaccini, A.R. Combining Bioresorbable Polyesters and Bioactive Glasses: Orthopedic Applications of Composite Implants and Bone Tissue Engineering Scaffolds. Appl. Mater. Today 2021, 22, 100923. [Google Scholar] [CrossRef]
- Piatti, E.; Miola, M.; Liverani, L.; Verné, E.; Boccaccini, A.R. Poly(ε-Caprolactone)/Bioactive Glass Composite Electrospun Fibers for Tissue Engineering Applications. J. Biomed. Mater. Res. 2023, 111, 1692–1709. [Google Scholar] [CrossRef]
- Liverani, L.; Lacina, J.; Roether, J.A.; Boccardi, E.; Killian, M.S.; Schmuki, P.; Schubert, D.W.; Boccaccini, A.R. Incorporation of Bioactive Glass Nanoparticles in Electrospun PCL/Chitosan Fibers by Using Benign Solvents. Bioact. Mater. 2018, 3, 55–63. [Google Scholar] [CrossRef]
- Bernardo, M.P.; Da Silva, B.C.R.; Hamouda, A.E.I.; De Toledo, M.A.S.; Schalla, C.; Rütten, S.; Goetzke, R.; Mattoso, L.H.C.; Zenke, M.; Sechi, A. PLA/Hydroxyapatite Scaffolds Exhibit in Vitro Immunological Inertness and Promote Robust Osteogenic Differentiation of Human Mesenchymal Stem Cells without Osteogenic Stimuli. Sci. Rep. 2022, 12, 2333. [Google Scholar] [CrossRef]
- Sahmani, S.; Khandan, A.; Esmaeili, S.; Saber-Samandari, S.; Ghadiri Nejad, M.; Aghdam, M.M. Calcium Phosphate-PLA Scaffolds Fabricated by Fused Deposition Modeling Technique for Bone Tissue Applications: Fabrication, Characterization and Simulation. Ceram. Int. 2020, 46, 2447–2456. [Google Scholar] [CrossRef]
- Rahaman, M.N.; Day, D.E.; Sonny Bal, B.; Fu, Q.; Jung, S.B.; Bonewald, L.F.; Tomsia, A.P. Bioactive Glass in Tissue Engineering. Acta Biomater. 2011, 7, 2355–2373. [Google Scholar] [CrossRef] [PubMed]
- Negut, I.; Ristoscu, C. Bioactive Glasses for Soft and Hard Tissue Healing Applications—A Short Review. Appl. Sci. 2023, 13, 6151. [Google Scholar] [CrossRef]
- Elahpour, N.; Niesner, I.; Abdellaoui, N.; Holzapfel, B.M.; Gritsch, L.; Jallot, E.; Mayer-Wagner, S.; Lao, J. Antibacterial Therapeutic Ions Incorporation into Bioactive Glasses as a Winning Strategy against Antibiotic Resistance. Adv. Mater. Interfaces 2024, 11, 2400068. [Google Scholar] [CrossRef]
- Kargozar, S.; Baino, F.; Hamzehlou, S.; Hill, R.G.; Mozafari, M. Bioactive Glasses: Sprouting Angiogenesis in Tissue Engineering. Trends Biotechnol. 2018, 36, 430–444. [Google Scholar] [CrossRef]
- Miola, M.; Massera, J.; Cochis, A.; Kumar, A.; Rimondini, L.; Vernè, E. Tellurium: A New Active Element for innovative Multifunctional bioactive Glasses. Mater. Sci. Eng. C 2021, 123, 111957. [Google Scholar] [CrossRef]
- Kargozar, S.; Hooshmand, S.; Hosseini, S.A.; Gorgani, S.; Kermani, F.; Baino, F. Antioxidant Effects of Bioactive Glasses (BGs) and Their Significance in Tissue Engineering Strategies. Molecules 2022, 27, 6642. [Google Scholar] [CrossRef]
- Chen, Y.-Y.; Chiou, Y.-J.; Chang, P.-J.; Chang, W.-M.; Yeh, Y.-C.; Chen, C.-Y.; Chang, Y.-K.; Lin, C.-K. In Vitro Evaluation of Electrospun PCL Bioscaffold with Zinc-Doped Bioactive Glass Powder Addition. Polymers 2024, 16, 2811. [Google Scholar] [CrossRef]
- Augusto, T.A.; Crovace, M.C.; Pinto, L.A.; Costa, L.C. Polycaprolactone/F18 Bioactive Glass Scaffolds Obtained via Fused Filament Fabrication. ACS Appl. Polym. Mater. 2025, 7, 2359–2370. [Google Scholar] [CrossRef]
- Miola, M.; Verné, E. Bioactive and Antibacterial Glass Powders Doped with Copper by Ion-Exchange in Aqueous Solutions. Materials 2016, 9, 405. [Google Scholar] [CrossRef] [PubMed]
- Lallukka, M.; Miola, M.; Najmi, Z.; Cochis, A.; Spriano, S.; Rimondini, L.; Verné, E. Cu-Doped Bioactive Glass with Enhanced in Vitro Bioactivity and Antibacterial Properties. Ceram. Int. 2024, 50, 5091–5103. [Google Scholar] [CrossRef]
- Mosca Balma, A.; Pedraza, R.; Orrico, C.; Meinardi, S.; Genova, T.; Gautier Di Confiengo, G.; Faga, M.G.; Roato, I.; Mussano, F. Poly(ε-Caprolactone)/Sodium Bicarbonate/β-Tricalcium Phosphate Composites: Surface Characterization and Early Biological Response. Materials 2025, 18, 2600. [Google Scholar] [CrossRef] [PubMed]
- Pedraza, R.; Mosca Balma, A.; Roato, I.; Orrico, C.; Genova, T.; Baima, G.; Berta, G.N.; Giura, A.; Ribotta, L.; Duraccio, D.; et al. Early Biological Response to Poly(ε-Caprolactone)/Alumina-Toughened Zirconia Composites Obtained by 3D Printing for Peri-Implant Application. Polymers 2024, 16, 2521. [Google Scholar] [CrossRef] [PubMed]
- Waldner, C.; Hirn, U. Modeling Liquid Penetration into Porous Materials Based on Substrate and Liquid Surface Energies. J. Colloid Interface Sci. 2023, 640, 445–455. [Google Scholar] [CrossRef]
- Pachitariu, M.; Rariden, M.; Stringer, C. Cellpose-SAM: Superhuman Generalization for Cellular Segmentation. bioRxiv 2025, 65, 1001. [Google Scholar] [CrossRef]
- Dietrich, C.F. Uncertainty, Calibration and Probability: The Statistics of Scientific and Industrial Measurement, 1st ed.; Routledge: New York, NY, USA, 2017; ISBN 978-0-203-73475-9. [Google Scholar]
- Vecchio, G.; Darcos, V.; Grill, S.L.; Brouillet, F.; Coppel, Y.; Duttine, M.; Pugliara, A.; Combes, C.; Soulié, J. Spray-Dried Ternary Bioactive Glass Microspheres: Direct and Indirect Structural Effects of Copper-Doping on Acellular Degradation Behavior. Acta Biomater. 2024, 181, 453–468. [Google Scholar] [CrossRef]
- Piatti, E.; Verné, E.; Miola, M. Synthesis and Characterization of Sol-Gel Bioactive Glass Nanoparticles Doped with Boron and Copper. Ceram. Int. 2022, 48, 13706–13718. [Google Scholar] [CrossRef]
- Khajehmohammadi, M.; Azizi Tafti, R.; Nikukar, H. Effect of Porosity on Mechanical and Biological Properties of Bioprinted Scaffolds. J. Biomed. Mater. Res. 2023, 111, 245–260. [Google Scholar] [CrossRef] [PubMed]
- Soufivand, A.A.; Abolfathi, N.; Hashemi, A.; Lee, S.J. The Effect of 3D Printing on the Morphological and Mechanical Properties of Polycaprolactone Filament and Scaffold. Polym. Adv. Technol. 2020, 31, 1038–1046. [Google Scholar] [CrossRef]
- Carballeira, P.; Haupert, F. Toughening Effects of Titanium Dioxide Nanoparticles on TiO2 /Epoxy Resin Nanocomposites. Polym. Compos. 2010, 31, 1241–1246. [Google Scholar] [CrossRef]
- Mumm, D.R.; Faber, K.T. Interfacial Debonding and Sliding in Brittle-Matrix Composites Measured Using an Improved Fiber pullout Technique. Acta Metall. Et Mater. 1995, 43, 1259–1270. [Google Scholar] [CrossRef]
- Cai, M.; Takagi, H.; Nakagaito, A.N.; Li, Y.; Waterhouse, G.I.N. Effect of Alkali Treatment on Interfacial Bonding in Abaca Fiber-Reinforced Composites. Compos. Part A Appl. Sci. Manuf. 2016, 90, 589–597. [Google Scholar] [CrossRef]
- Fiddian-Green, R.G.; Silen, W. Mechanisms of Disposal of Acid and Alkali in Rabbit Duodenum. Am. J. Physiol. 1975, 229, 1641–1648. [Google Scholar] [CrossRef]
- Nukala, S.G.; Kong, I.; Patel, V.I.; Kakarla, A.B.; Kong, W.; Buddrick, O. Development of Biodegradable Composites Using Polycaprolactone and Bamboo Powder. Polymers 2022, 14, 4169. [Google Scholar] [CrossRef]
- Vallée, A.; Faga, M.G.; Mussano, F.; Catalano, F.; Tolosano, E.; Carossa, S.; Altruda, F.; Martra, G. Alumina–Zirconia Composites Functionalized with Laminin-1 and Laminin-5 for Dentistry: Effect of Protein Adsorption on Cellular Response. Colloids Surf. B Biointerfaces 2014, 114, 284–293. [Google Scholar] [CrossRef]
- Dziadek, M.; Dziadek, K.; Checinska, K.; Zagrajczuk, B.; Golda-Cepa, M.; Brzychczy-Wloch, M.; Menaszek, E.; Kopec, A.; Cholewa-Kowalska, K. PCL and PCL/Bioactive Glass Biomaterials as Carriers for Biologically Active Polyphenolic Compounds: Comprehensive Physicochemical and Biological Evaluation. Bioact. Mater. 2021, 6, 1811–1826. [Google Scholar] [CrossRef]
- Wu, X.; Wang, C.; Hao, P.; He, F.; Yao, Z.; Zhang, X. Adsorption Properties of Albumin and Fibrinogen on Hydrophilic/Hydrophobic TiO2 Surfaces: A Molecular Dynamics Study. Colloids Surf. B Biointerfaces 2021, 207, 111994. [Google Scholar] [CrossRef]
- Kapp, M.; Li, C.; Xu, Z.; Boccaccini, A.R.; Zheng, K. Protein Adsorption on SiO2-CaO Bioactive Glass Nanoparticles with Controllable Ca Content. Nanomaterials 2021, 11, 561. [Google Scholar] [CrossRef]
- Lim, J.Y.; Shaughnessy, M.C.; Zhou, Z.; Noh, H.; Vogler, E.A.; Donahue, H.J. Surface Energy Effects on Osteoblast Spatial Growth and Mineralization. Biomaterials 2008, 29, 1776–1784. [Google Scholar] [CrossRef] [PubMed]
- Iwata, R.; Suk-In, P.; Hoven, V.P.; Takahara, A.; Akiyoshi, K.; Iwasaki, Y. Control of Nanobiointerfaces Generated from Well-Defined Biomimetic Polymer Brushes for Protein and Cell Manipulations. Biomacromolecules 2004, 5, 2308–2314. [Google Scholar] [CrossRef] [PubMed]
- Majhy, B.; Priyadarshini, P.; Sen, A.K. Effect of Surface Energy and Roughness on Cell Adhesion and Growth–Facile Surface Modification for Enhanced Cell Culture. RSC Adv. 2021, 11, 15467–15476. [Google Scholar] [CrossRef] [PubMed]
- Gittens, R.A.; Scheideler, L.; Rupp, F.; Hyzy, S.L.; Geis-Gerstorfer, J.; Schwartz, Z.; Boyan, B.D. A Review on the Wettability of Dental Implant Surfaces II: Biological and Clinical Aspects. Acta Biomater. 2014, 10, 2907–2918. [Google Scholar] [CrossRef]
- Zuk, P.A.; Zhu, M.; Ashjian, P.; De Ugarte, D.A.; Huang, J.I.; Mizuno, H.; Alfonso, Z.C.; Fraser, J.K.; Benhaim, P.; Hedrick, M.H. Human Adipose Tissue Is a Source of Multipotent Stem Cells. Mol. Biol. Cell 2002, 13, 4279–4295. [Google Scholar] [CrossRef]
- Ades, E.W.; Candal, F.J.; Swerlick, R.A.; George, V.G.; Summers, S.; Bosse, D.C.; Lawley, T.J. HMEC-1: Establishment of an Immortalized Human Microvascular Endothelial Cell Line. J. Investig. Dermatol. 1992, 99, 683–690. [Google Scholar] [CrossRef]
- Roato, I.; Genova, T.; Duraccio, D.; Ruffinatti, F.A.; Zanin Venturini, D.; Di Maro, M.; Mosca Balma, A.; Pedraza, R.; Petrillo, S.; Chinigò, G.; et al. Mechanical and Biological Characterization of PMMA/Al2O3 Composites for Dental Implant Abutments. Polymers 2023, 15, 3186. [Google Scholar] [CrossRef]
- Ruffinatti, F.A.; Genova, T.; Mussano, F.; Munaron, L. MORPHEUS: An Automated Tool for Unbiased and Reproducible Cell Morphometry. J. Cell. Physiol. 2020, 235, 10110–10115. [Google Scholar] [CrossRef]
- Lin, Z.; Cao, Y.; Zou, J.; Zhu, F.; Gao, Y.; Zheng, X.; Wang, H.; Zhang, T.; Wu, T. Improved Osteogenesis and Angiogenesis of a Novel Copper Ions Doped Calcium Phosphate Cement. Mater. Sci. Eng. C 2020, 114, 111032. [Google Scholar] [CrossRef]
- Romero-Sánchez, L.B.; Marí-Beffa, M.; Carrillo, P.; Medina, M.Á.; Díaz-Cuenca, A. Copper-Containing Mesoporous Bioactive Glass Promotes Angiogenesis in an in Vivo Zebrafish Model. Acta Biomater. 2018, 68, 272–285. [Google Scholar] [CrossRef]
- Cao, B.; Zheng, Y.; Xi, T.; Zhang, C.; Song, W.; Burugapalli, K.; Yang, H.; Ma, Y. Concentration-Dependent Cytotoxicity of Copper Ions on Mouse Fibroblasts in Vitro: Effects of Copper Ion Release from TCu380A vs TCu220C Intra-Uterine Devices. Biomed. Microdevices 2012, 14, 709–720. [Google Scholar] [CrossRef]
- Akdere, Ö.E.; Shikhaliyeva, İ.; Gümüşderelioğlu, M. Boron Mediated 2D and 3D Cultures of Adipose Derived Mesenchymal Stem Cells. Cytotechnology 2019, 71, 611–622. [Google Scholar] [CrossRef]
- Renaud, M.; Bousquet, P.; Macias, G.; Rochefort, G.Y.; Durand, J.-O.; Marsal, L.F.; Cuisinier, F.; Cunin, F.; Collart-Dutilleul, P.-Y. Allogenic Stem Cells Carried by Porous Silicon Scaffolds for Active Bone Regeneration In Vivo. Bioengineering 2023, 10, 852. [Google Scholar] [CrossRef]
- Collart-Dutilleul, P.-Y.; Secret, E.; Panayotov, I.; Deville De Périère, D.; Martín-Palma, R.J.; Torres-Costa, V.; Martin, M.; Gergely, C.; Durand, J.-O.; Cunin, F.; et al. Adhesion and Proliferation of Human Mesenchymal Stem Cells from Dental Pulp on Porous Silicon Scaffolds. ACS Appl. Mater. Interfaces 2014, 6, 1719–1728. [Google Scholar] [CrossRef]



| Sample | PCL (g) | SBA3 (g) | SBA3_Cu (g) |
|---|---|---|---|
| Neat PCL | 6 | / | / |
| PCL/SBA3 90/10 | 5.4 | 0.6 | / |
| PCL/SBA3_Cu 90/10 | 5.4 | / | 0.6 |
| Liquid | γ (mN/m) | γP (mN/m) | γD (mN/m) |
|---|---|---|---|
| Water | 72.8 | 43.7 | 29.1 |
| Di-iodomethane | 50 | 2.6 | 47.4 |
| Sample | E (MPa) | εy (%) | σy (MPa) | εb (%) | σb (MPa) |
|---|---|---|---|---|---|
| Neat PCL | 51 ± 16 | 7.5 ± 0.8 | 14.3 ± 0.8 | 693 ± 60 | 23.4 ± 3.8 |
| PCL/SBA3 90/10 | 252 ± 35 | 14.9 ± 3.2 | 12.7 ± 1.3 | 647 ± 48 | 17.8 ± 2.2 |
| PCL/SBA3_Cu 90/10 | 369 ± 46 | 14.7 ± 1.5 | 13.9 ± 1.1 | 656 ± 47 | 21.1 ± 1.6 |
| Sample | Ra [µm] | Rz [µm] | Sa [µm] |
|---|---|---|---|
| Neat PCL | 0.266 ± 0.034 | 0.157 ± 0.029 | 0.077 ± 0.009 |
| PCL/SBA3 90/10 | 0.218 ± 0.049 | 0.074 ± 0.022 | 0.076 ± 0.015 |
| PCL/SBA3_Cu 90/10 | 0.211 ± 0.028 | 0.055 ± 0.006 | 0.063 ± 0.006 |
| Sample | Surface Energy: Total [mN/m] | Surface Energy: Polar [mN/m] | Surface Energy: Dispersive [mN/m] |
|---|---|---|---|
| Neat PCL | 42.7 | 2.7 | 40 |
| PCL/SBA3 90/10 | 39.2 | 17.6 | 21.5 |
| PCL/SBA3_Cu 90/10 | 38.8 | 9.9 | 28.9 |
| Sample | B [mg/L] | Si [mg/L] | Cu [mg/L] |
|---|---|---|---|
| Alpha-MEM | 0.17 ± 0.03 | 0.23 ± 0.01 | N.A. |
| PCL/SBA3 90/10 | |||
| 1 day | 0.85 ± 0.06 | 46.3 ± 0.3 | N.A. |
| 3 days | 1.16 ± 0.03 | 53.8 ± 0.4 | N.A. |
| 7 days | 2.14 ± 0.06 | 62.6 ± 0.2 | N.A. |
| PCL/SBA3_Cu 90/10 | |||
| 1 day | 0.36 ± 0.04 | 9.5 ± 0.1 | 1.33 ± 0.01 |
| 3 days | 0.81 ± 0.03 | 23.0 ± 0.1 | 2.58 ± 0.02 |
| 7 days | 1.63 ± 0.03 | 31.2 ± 0.2 | 3.39 ± 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mosca Balma, A.; Pedraza, R.; Roato, I.; Orrico, C.; Meinardi, S.; Bertinetti, S.; Genova, T.; Gautier di Confiengo, G.; Faga, M.G.; Duraccio, D.; et al. Early Biological Response to Poly(ε-caprolactone) PCL—Bioactive Glass Composites Obtained by 3D Printing as Bone Substitutes. Polymers 2025, 17, 2229. https://doi.org/10.3390/polym17162229
Mosca Balma A, Pedraza R, Roato I, Orrico C, Meinardi S, Bertinetti S, Genova T, Gautier di Confiengo G, Faga MG, Duraccio D, et al. Early Biological Response to Poly(ε-caprolactone) PCL—Bioactive Glass Composites Obtained by 3D Printing as Bone Substitutes. Polymers. 2025; 17(16):2229. https://doi.org/10.3390/polym17162229
Chicago/Turabian StyleMosca Balma, Alessandro, Riccardo Pedraza, Ilaria Roato, Clarissa Orrico, Sara Meinardi, Stefano Bertinetti, Tullio Genova, Giovanna Gautier di Confiengo, Maria Giulia Faga, Donatella Duraccio, and et al. 2025. "Early Biological Response to Poly(ε-caprolactone) PCL—Bioactive Glass Composites Obtained by 3D Printing as Bone Substitutes" Polymers 17, no. 16: 2229. https://doi.org/10.3390/polym17162229
APA StyleMosca Balma, A., Pedraza, R., Roato, I., Orrico, C., Meinardi, S., Bertinetti, S., Genova, T., Gautier di Confiengo, G., Faga, M. G., Duraccio, D., Malucelli, G., Miola, M., Verné, E., & Mussano, F. (2025). Early Biological Response to Poly(ε-caprolactone) PCL—Bioactive Glass Composites Obtained by 3D Printing as Bone Substitutes. Polymers, 17(16), 2229. https://doi.org/10.3390/polym17162229













