Assessment of the Interactions Between Hemicellulose Xylan and Kaolinite Clay: Structural Characterization and Adsorptive Behavior
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Extraction of Xylan Hemicelluloses
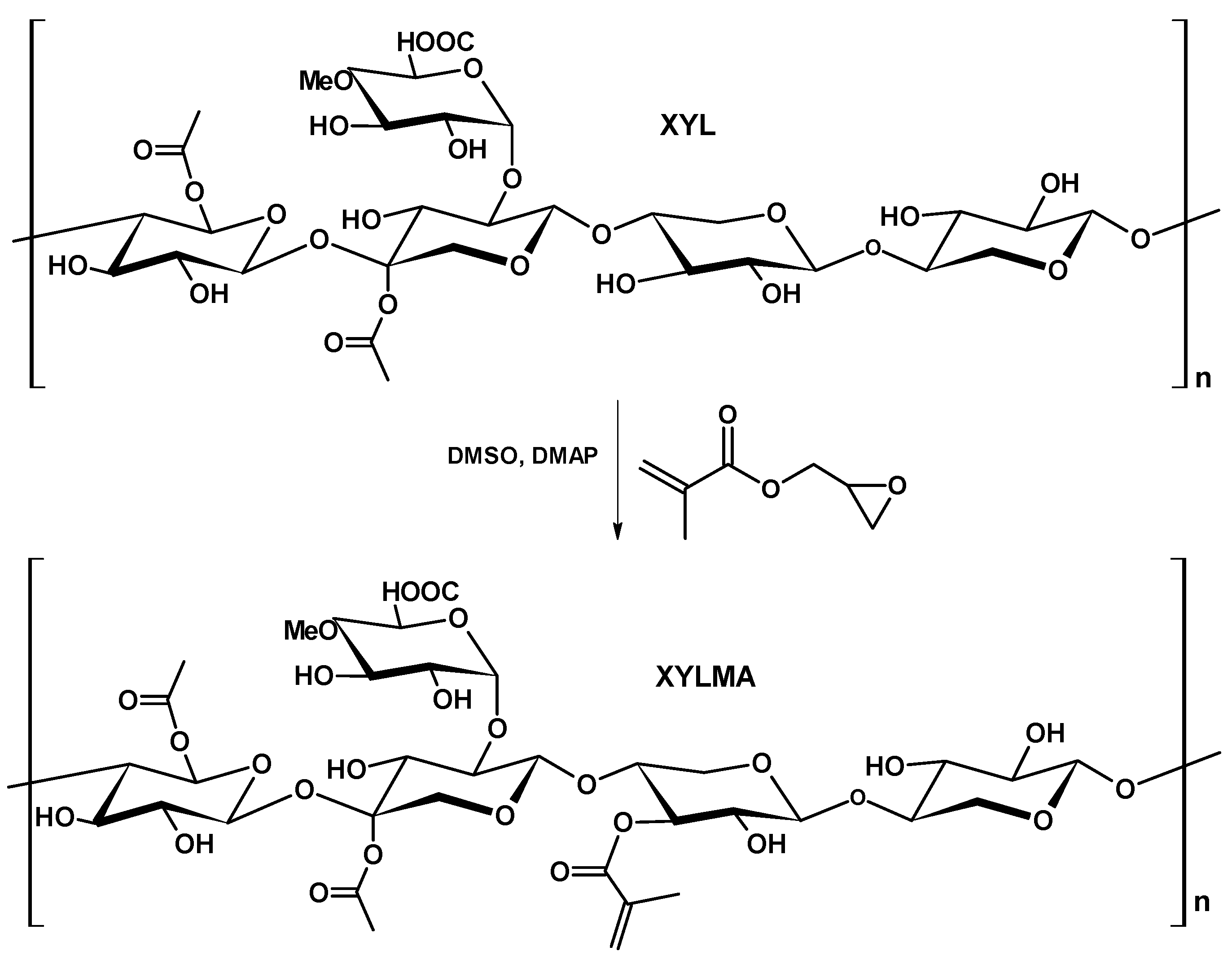
2.3. Xylan Modification
2.4. XYL and XYLMA Characterizations
2.5. Adsorption Tests
3. Results and Discussion
3.1. XYL and XYLMA Characterizations
3.2. Adsorption of XYL and XYLMA on Kaolinite
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, C.; Wu, Z.; Wu, Z.; Chen, X.; Cao, Y. Effect of the Interaction between Clays and Cations on Froth Rheology in Flotation. Minerals 2024, 14, 706. [Google Scholar] [CrossRef]
- Estrada, D.; Echeverry, L.; Ramirez, A.; Gutierrez, L. Molybdenite flotation in the presence of a polyacrylamide of low anionicity subjected to different conditions of mechanical shearing. Minerals 2020, 10, 895. [Google Scholar] [CrossRef]
- Raabe, D. The Materials Science behind Sustainable Metals and Alloys. Chem. Rev. 2023, 123, 2436–2608. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Atkins, B.; Williams, S.; Yin, H.; Reinhart, B.; Herndon, E. Sorption and Oxidative Degradation of Small Organic Molecules on Mn-Oxides─Effects of pH and Mineral Structures. ACS Earth Space Chem. 2024, 8, 2067–2077. [Google Scholar] [CrossRef]
- Ren, Z.; Chao, C.; Krishnamoorthy, P.; Asselin, E.; Dixon, D.G.; Mora, N. The overlooked mechanism of chalcopyrite passivation. Acta Mater. 2022, 236, 118111. [Google Scholar] [CrossRef]
- Torres, D.; Ayala, L.; Jeldres, R.I.; Cerecedo-Sáenz, E.; Salinas-Rodríguez, E.; Robles, P.; Toro, N. Leaching Chalcopyrite with High MnO2 and Chloride Concentrations. Metals 2020, 10, 107. [Google Scholar] [CrossRef]
- Jolin, W.C.; Richard, A.; Vasudevan, D.; Gascón, J.A.; MacKay, A.A. Aluminosilicate Mineralogy and the Sorption of Organic Cations: Interplay between Electrostatic Barriers and Compound Structural Features. Environ. Sci. Technol. 2020, 54, 1623–1633. [Google Scholar] [CrossRef]
- Sigmund, G.; Arp, H.P.H.; Aumeier, B.M.; Bucheli, T.D.; Chefetz, B.; Chen, W.; Droge, S.T.; Endo, S.; Escher, B.I.; Hale, S.E.; et al. Sorption and Mobility of Charged Organic Compounds: How to Confront and Overcome Limitations in Their Assessment. Environ. Sci. Technol. 2022, 56, 4702–4710. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, S. Enhanced copper extraction in the chalcopyrite bioleaching system assisted by microbial fuel cells and catalyzed by silver-bearing ores. J. Environ. Chem. Eng. 2022, 10, 108827. [Google Scholar] [CrossRef]
- Torres, C.M.; Ghorbani, Y.; Hernández, P.C.; Justel, F.J.; Aravena, M.I.; Herreros, O.O. Cupric and chloride ions: Leaching of chalcopyrite concentrate with low chloride concentration media. Minerals 2019, 9, 639. [Google Scholar] [CrossRef]
- Huang, Z.; Cao, S.; Yilmaz, E. Microstructure and mechanical behavior of cemented gold/tungsten mine tailings-crushed rock backfill: Effects of rock gradation and content. J. Environ. Manag. 2023, 339, 117897. [Google Scholar] [CrossRef] [PubMed]
- Castellón, C.I.; Toro, N.; Gálvez, E.; Robles, P.; Leiva, W.H.; Jeldres, R.I. Froth Flotation of Chalcopyrite/Pyrite Ore: A Critical Review. Materials 2022, 15, 6536. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Qiu, T.; Qiu, X.; Yan, H.; Jiao, Q.; Ding, K.; Zhao, G. Pullulan Polysaccharide as an Eco-Friendly Depressant for Flotation Separation of Chalcopyrite and Molybdenite. ACS Omega 2024, 9, 29557–29565. [Google Scholar] [CrossRef]
- Neisiani, A.A.; Saneie, R.; Mohammadzadeh, A.; Wonyen, D.G.; Chelgani, S.C. Polysaccharides-based pyrite depressants for green flotation separation: An overview. Int. J. Min. Sci. Technol. 2023, 33, 1229–1241. [Google Scholar] [CrossRef]
- Xing, Q.; Ming, P.; Wang, X.; Li, F.; Wang, Z.; Zhao, K. Fenugreek Polysaccharide Gum as a Depressant in the Flotation Separation of Gold Ore with a High Content of Clay Minerals. Colloids Interfaces 2025, 9, 21. [Google Scholar] [CrossRef]
- Liang, G.; Chimonyo, W.; Lv, J.; Peng, Y. Differential depression of calcium lignosulfonate on chalcopyrite and molybdenite flotation with collector kerosene. Miner. Eng. 2023, 201, 108192. [Google Scholar] [CrossRef]
- Castillo, I.; Gutierrez, L.; Hernandez, V.; Diaz, E.; Ramirez, A. Hemicelluloses monosaccharides and their effect on molybdenite flotation. Powder Technol. 2020, 373, 758–764. [Google Scholar] [CrossRef]
- Hernandez, V.A.; Ulloa, A.; Gutierrez, L. Use of wood hemicelluloses to improve copper recovery from high clay Cu-Mo ores. Miner. Eng. 2017, 111, 198–200. [Google Scholar] [CrossRef]
- Pan, G.; Shi, Q.; Zhang, G.; Huang, G. Selective depression of talc in chalcopyrite flotation by xanthan gum: Flotation response and adsorption mechanism. Colloids Surf. A Physicochem. Eng. Asp. 2020, 600, 124902. [Google Scholar] [CrossRef]
- Gao, Z.Y.; Jiang, Z.Y.; Sun, W.; Gao, Y.S. Typical roles of metal ions in mineral flotation: A review. Trans. Nonferr. Met. Soc. China 2021, 31, 2081–2101. [Google Scholar] [CrossRef]
- Darvanjooghi, M.H.K.; Davoodi, S.M.; Dursun, A.Y.; Ehsani, M.R.; Karimpour, I.; Ameri, E. Application of treated eggplant peel as a low-cost adsorbent for water treatment toward elimination of Pb2+: Kinetic modeling and isotherm study. Adsorpt. Sci. Technol. 2018, 36, 1112–1143. [Google Scholar] [CrossRef]
- Nazari, L.; Xu, C.; Ray, M.B. (Eds.) Resource Utilization of Agricultural/Forestry Residues via Fractionation into Cellulose, Hemicellulose and Lignin. In Advanced and Emerging Technologies for Resource Recovery from Wastes; Springer: Singapore, 2021; pp. 179–204. [Google Scholar] [CrossRef]
- Mathura, S.R.; Landázuri, A.C.; Mathura, F.; Andrade Sosa, A.G.; Orejuela-Escobar, L.M. Hemicelluloses from bioresidues and their applications in the food industry-towards an advanced bioeconomy and a sustainable global value chain of chemicals and materials. Sustain. Food Technol. 2024, 2, 1183–1205. [Google Scholar] [CrossRef]
- Abik, F.; Palasingh, C.; Bhattarai, M.; Leivers, S.; Ström, A.; Westereng, B.; Mikkonen, K.S.; Nypelo, T. Potential of Wood Hemicelluloses and Their Derivates as Food Ingredients. J. Agric. Food Chem. 2023, 71, 2667–2683. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Martínez, I.; Pfrengle, F. On the structure, conformation and reactivity of β-1,4-linked plant cell wall glycans: Why are xylan polysaccharides or furanosyl substituents easier to hydrolyze than cellulose? Cellulose 2025, 32, 2145–2165. [Google Scholar] [CrossRef]
- Lehuedé, L.; Henríquez, C.; Carú, C.; Córdova, A.; Mendonça, R.T.; Salazar, O. Xylan extraction from hardwoods by alkaline pretreatment for xylooligosaccharide production: A detailed fractionation analysis. Carbohydr. Polym. 2023, 302, 120381. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Madrid, A.; Irribarra, K.; Gutierrez, L.; Vega-Garcia, D. Effect of sodium silicate modified with Fe2+ and Al3+ as dispersant on flotation of molybdenite and chalcopyrite in presence of kaolinite and seawater. Miner. Eng. 2024, 207, 108551. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, K.; Yang, Y.; Kim, M.S.; Lee, C.H.; Zhang, R.; Xu, T.; Choi, S.-E.; Si, C. Hemicellulose-based hydrogels for advanced applications. Front. Bioeng. Biotechnol. 2023, 10, 1110004. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Peng, X.; Zhong, L.; Sun, R. Multiresponsive Hydrogels Based on Xylan-Type Hemicelluloses and Photoisomerized Azobenzene Copolymer as Drug Delivery Carrier. J. Agric. Food Chem. 2014, 62, 10000–10007. [Google Scholar] [CrossRef]
- Andrade, D.; Moya, C.; Olate, F.; Gatica, N.; Sanchez, S.; Díaz, E.; Elgueta, E.; Parra, M.; Dahrouch, M. Soft amphiphilic polyesters obtained from PEGs and silicon fatty compounds: Structural characterizations and self-assembly studies. RSC Adv. 2016, 6, 38505–38514. [Google Scholar] [CrossRef]
- Peng, X.; Ren, J.; Zhong, L.; Sun, R.; Shi, W.; Hu, B. Glycidyl methacrylate derivatized xylan-rich hemicelluloses: Synthesis and characterizations. Cellulose 2012, 19, 1361–1372. [Google Scholar] [CrossRef]
- Elgueta, E.; Becerra, Y.; Martínez, A.; Pereira, M.; Carrillo-Varela, I.; Sanhueza, F.; Nuñez, D.; Rivas, B.L. Adsorbents Derived from Xylan Hemicellulose with Removal Properties of Pollutant Metals. Chin. J. Polym. Sci. 2023, 41, 874–886. [Google Scholar] [CrossRef]
- Lowman, D.W. Characterization of Cellulose Esters by Solution-State and Solid-State NMR Spectroscopy. In Cellulose Derivatives; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 1998; Volume 688, pp. 131–162. [Google Scholar] [CrossRef]
- El Hariri El Nokab, M.; Habib, M.H.; Alassmy, Y.A.; Abduljawad, M.M.; Alshamrani, K.M.; Sebakhy, K.O. Solid State NMR a Powerful Technique for Investigating Sustainable/Renewable Cellulose-Based Materials. Polymers 2022, 14, 1049. [Google Scholar] [CrossRef]
- Kallakas, H.; Kattamanchi, T.; Kilumets, C.; Tarasova, E.; Krasnou, I.; Savest, N.; Ahmadian, I.; Kers, J.; Krumme, A. Tensile and Surface Wettability Properties of the Solvent Cast Cellulose Fatty Acid Ester Films. Polymers 2023, 15, 2677. [Google Scholar] [CrossRef] [PubMed]
- Apaydın Varol, E.; Mutlu, Ü. TGA-FTIR Analysis of Biomass Samples Based on the Thermal Decomposition Behavior of Hemicellulose, Cellulose, and Lignin. Energies 2023, 16, 3674. [Google Scholar] [CrossRef]
- Gao, C.; Ren, J.; Kong, W.; Sun, R.; Chen, Q. Comparative study on temperature/pH sensitive xylan-based hydrogels: Their properties and drug controlled release. RSC Adv. 2015, 5, 90671–90681. [Google Scholar] [CrossRef]
- Wu, Z.; Li, H.; Zhao, X.; Ye, F.; Zhao, G. Hydrophobically modified polysaccharides and their self-assembled systems: A review on structures and food applications. Carbohydr. Polym. 2022, 84, 119182. [Google Scholar] [CrossRef]
- Bickmore, B.R.; Nagy, K.L.; Sandlin, P.E.; Crater, T.S. Quantifying surface areas of clays by atomic force microscopy. Am. Mineral. 2002, 87, 780–783. [Google Scholar] [CrossRef]
- Guo, Y.; Yu, X. Characterizing the surface charge of clay minerals with Atomic Force Microscope (AFM). AIMS Mater. Sci. 2017, 4, 582–593. [Google Scholar] [CrossRef]
- Gupta, V.; Miller, J.D. Surface force measurements at the basal planes of ordered kaolinite particles. J. Colloid Interface Sci. 2010, 344, 362–371. [Google Scholar] [CrossRef]
- Awad, A.M.; Shaikh, S.M.R.; Jalab, R.; Gulied, M.H.; Nasser, M.S.; Benamor, A.; Adham, S. Adsorption of organic pollutants by natural and modified clays: A comprehensive review. Sep. Purif. Technol. 2019, 228, 115719. [Google Scholar] [CrossRef]
- Mohammed, I.; Al Shehri, D.; Mahmoud, M.; Kamal, M.S.; Alade, O.S. A Surface Charge Approach to Investigating the Influence of Oil Contacting Clay Minerals on Wettability Alteration. ACS Omega 2021, 6, 12841–12852. [Google Scholar] [CrossRef] [PubMed]
- Tombácz, E.; Szekeres, M. Surface charge heterogeneity of kaolinite in aqueous suspension in comparison with montmorillonite. Appl. Clay Sci. 2006, 34, 105–124. [Google Scholar] [CrossRef]
- Zhang, H.; Liang, Y.; Wu, J.; Hua, Q.; Wan, X.; Renneckar, S. Carboxylated xylan nanoparticles prepared by sequential periodate-chlorite oxidation and their application as a highly effective bio-based adsorbent. Int. J. Biol. Macromol. 2025, 308, 142423. [Google Scholar] [CrossRef]
- Shen, F.; Ling, H.; Ge, W.; Yang, Y.; Wang, X.; Ren, J.; Wang, X. Self-assembly behavior and conformation of amphiphilic hemicellulose-graft-fatty acid micelles. Carbohydr. Polym. 2021, 261, 117886. [Google Scholar] [CrossRef] [PubMed]
- Silva, T.C.F.; Filpponen, I.; Habibi, Y.; Colodette, J.L.; Lucia, L.A. A Facile Approach for the Synthesis of Xylan-Derived Hydrogels. In Functional Materials from Renewable Sources; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2012; Volume 1107, pp. 257–270. [Google Scholar] [CrossRef]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díaz, E.; Gutiérrez, L.; Elgueta, E.; Núñez, D.; Carrillo-Varela, I.; Hernández, V.A. Assessment of the Interactions Between Hemicellulose Xylan and Kaolinite Clay: Structural Characterization and Adsorptive Behavior. Polymers 2025, 17, 1958. https://doi.org/10.3390/polym17141958
Díaz E, Gutiérrez L, Elgueta E, Núñez D, Carrillo-Varela I, Hernández VA. Assessment of the Interactions Between Hemicellulose Xylan and Kaolinite Clay: Structural Characterization and Adsorptive Behavior. Polymers. 2025; 17(14):1958. https://doi.org/10.3390/polym17141958
Chicago/Turabian StyleDíaz, Enzo, Leopoldo Gutiérrez, Elizabeth Elgueta, Dariela Núñez, Isabel Carrillo-Varela, and Vicente A. Hernández. 2025. "Assessment of the Interactions Between Hemicellulose Xylan and Kaolinite Clay: Structural Characterization and Adsorptive Behavior" Polymers 17, no. 14: 1958. https://doi.org/10.3390/polym17141958
APA StyleDíaz, E., Gutiérrez, L., Elgueta, E., Núñez, D., Carrillo-Varela, I., & Hernández, V. A. (2025). Assessment of the Interactions Between Hemicellulose Xylan and Kaolinite Clay: Structural Characterization and Adsorptive Behavior. Polymers, 17(14), 1958. https://doi.org/10.3390/polym17141958







