Theoretical DFT Analysis of a Polyacrylamide/Amylose Copolymer for the Removal of Cd(II), Hg(II), and Pb(II) from Aqueous Solutions
Abstract
1. Introduction
2. Materials and Methods
Computational Details
3. Results
3.1. Choice of Method
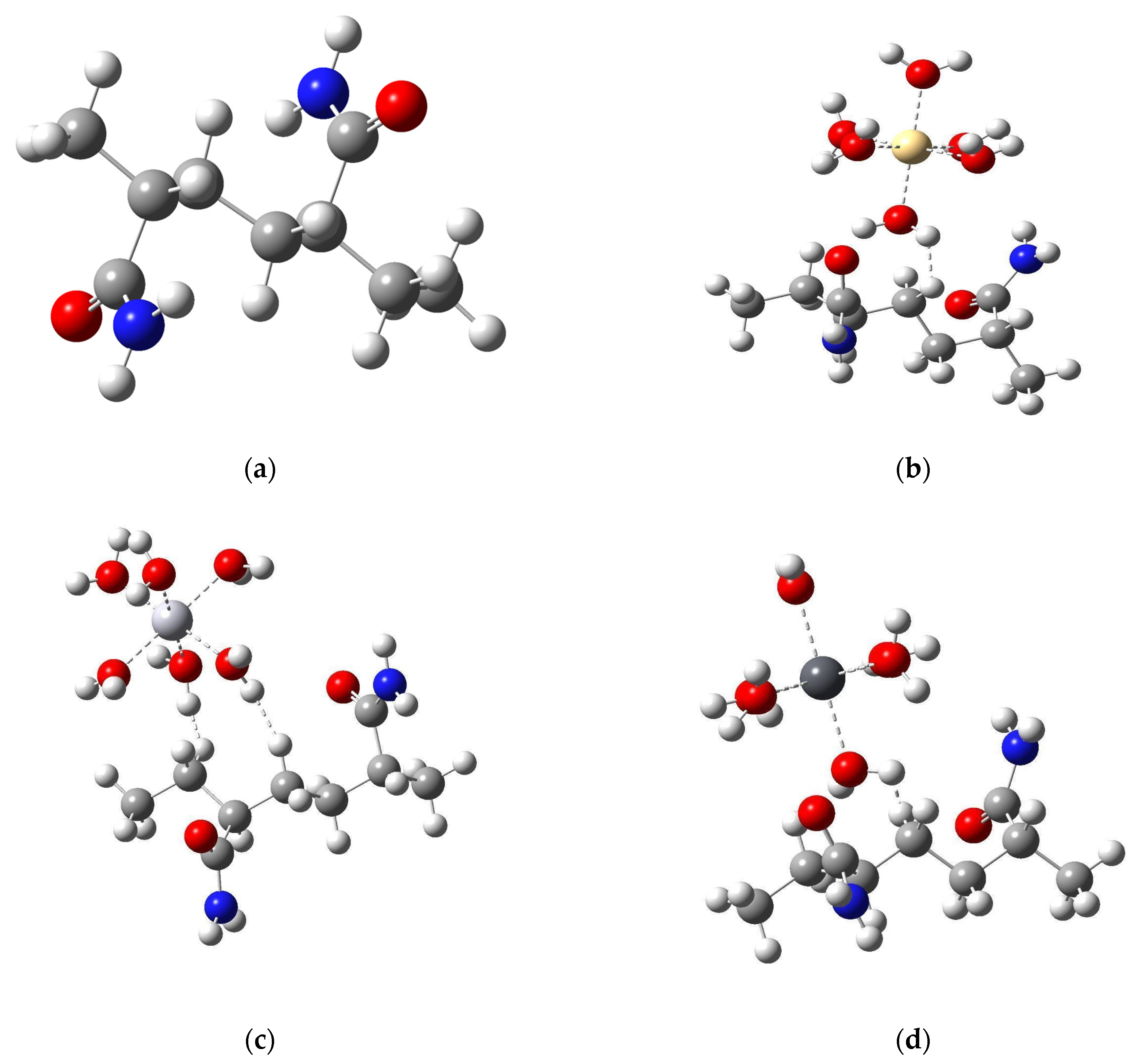
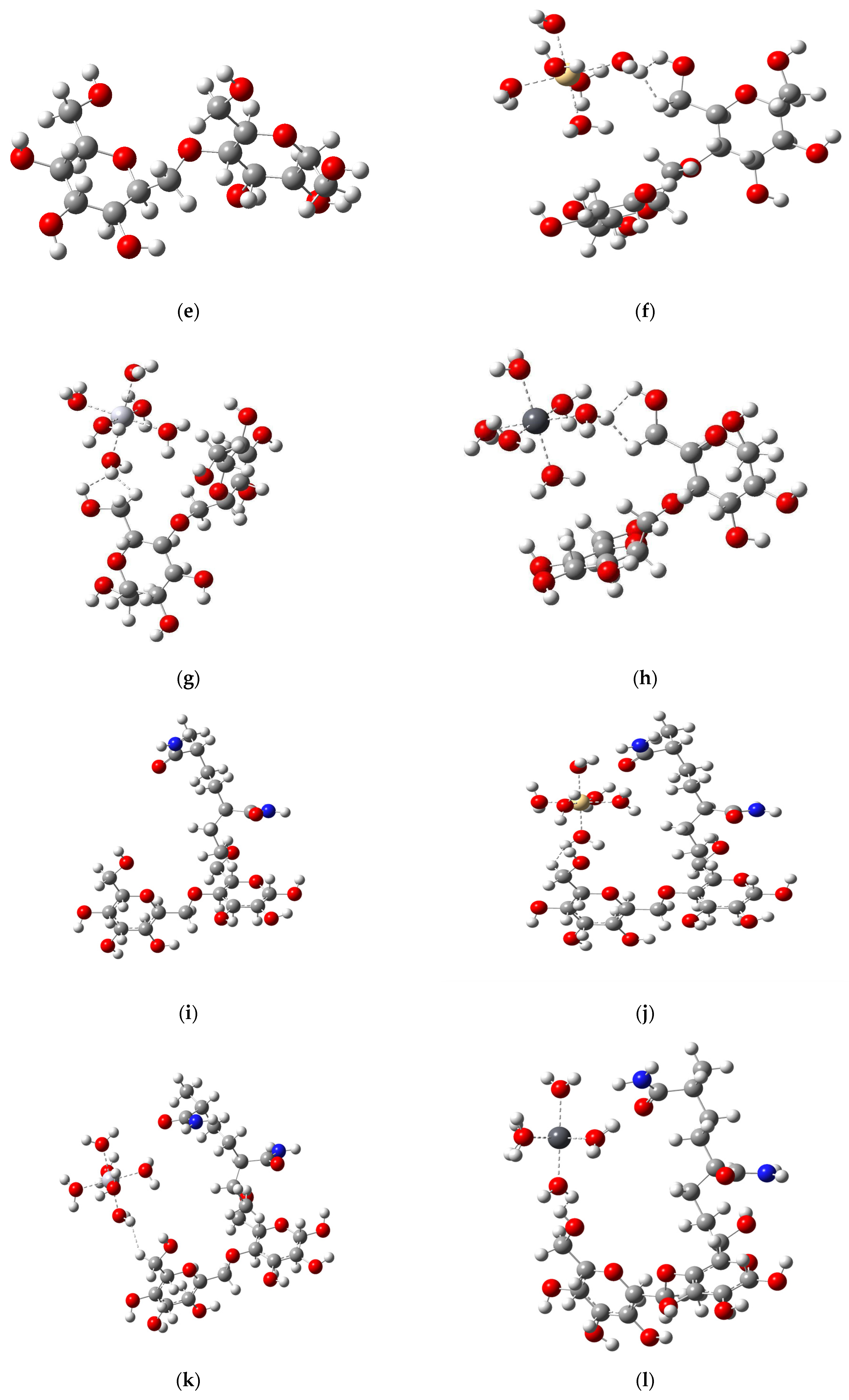
3.2. Model Structures
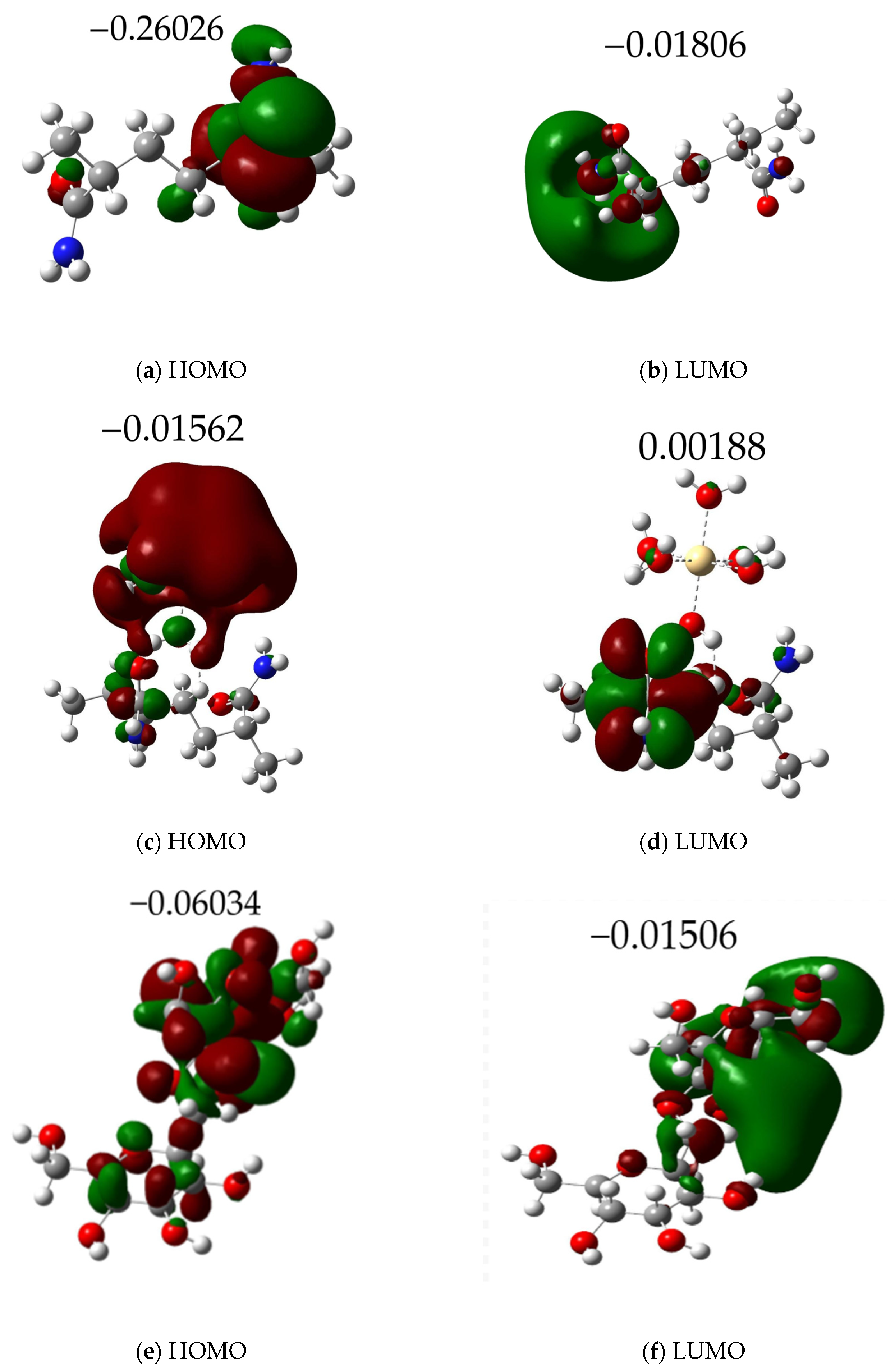
3.3. HOMO-LUMO Calculations
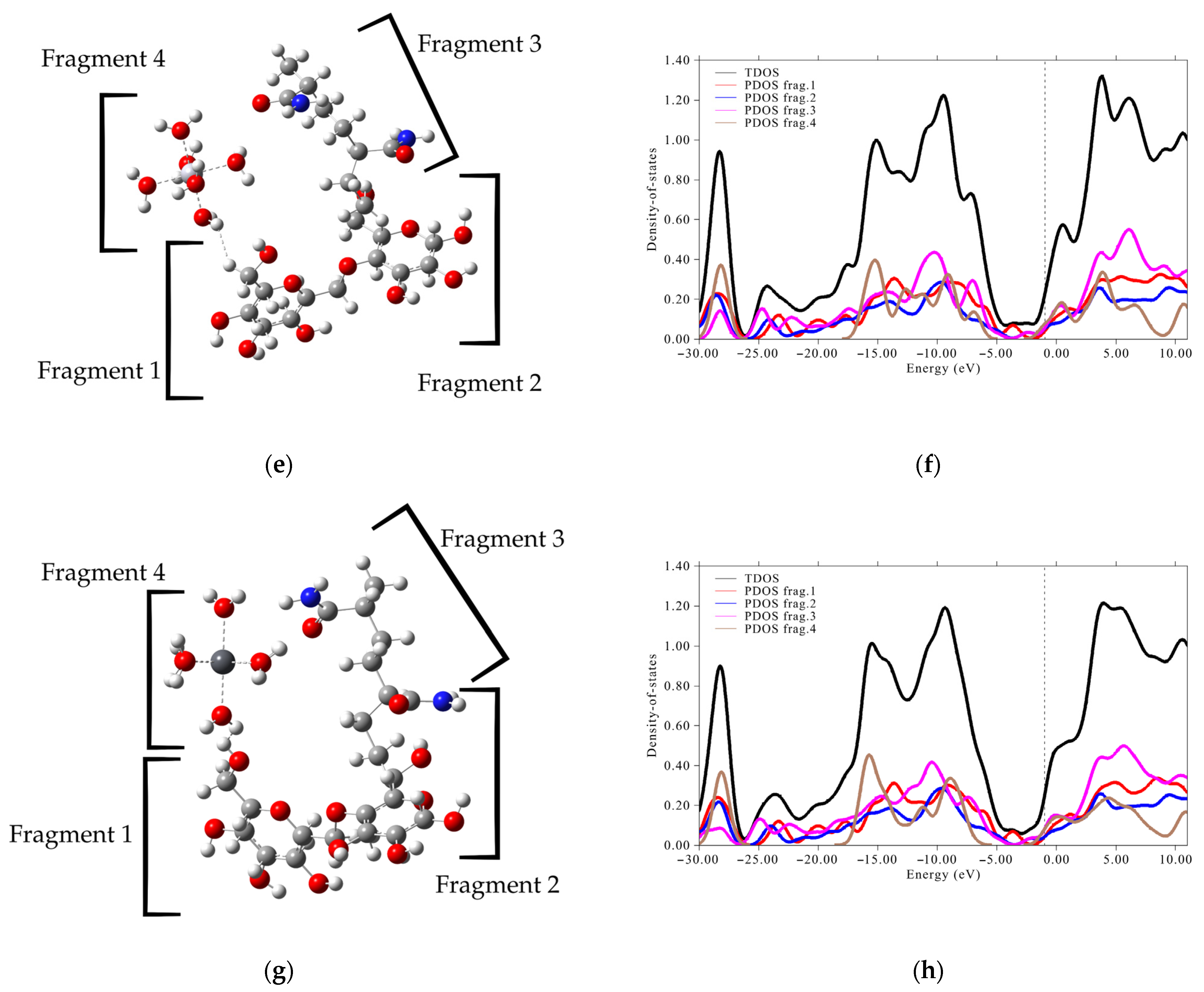
3.4. DOS Analysis
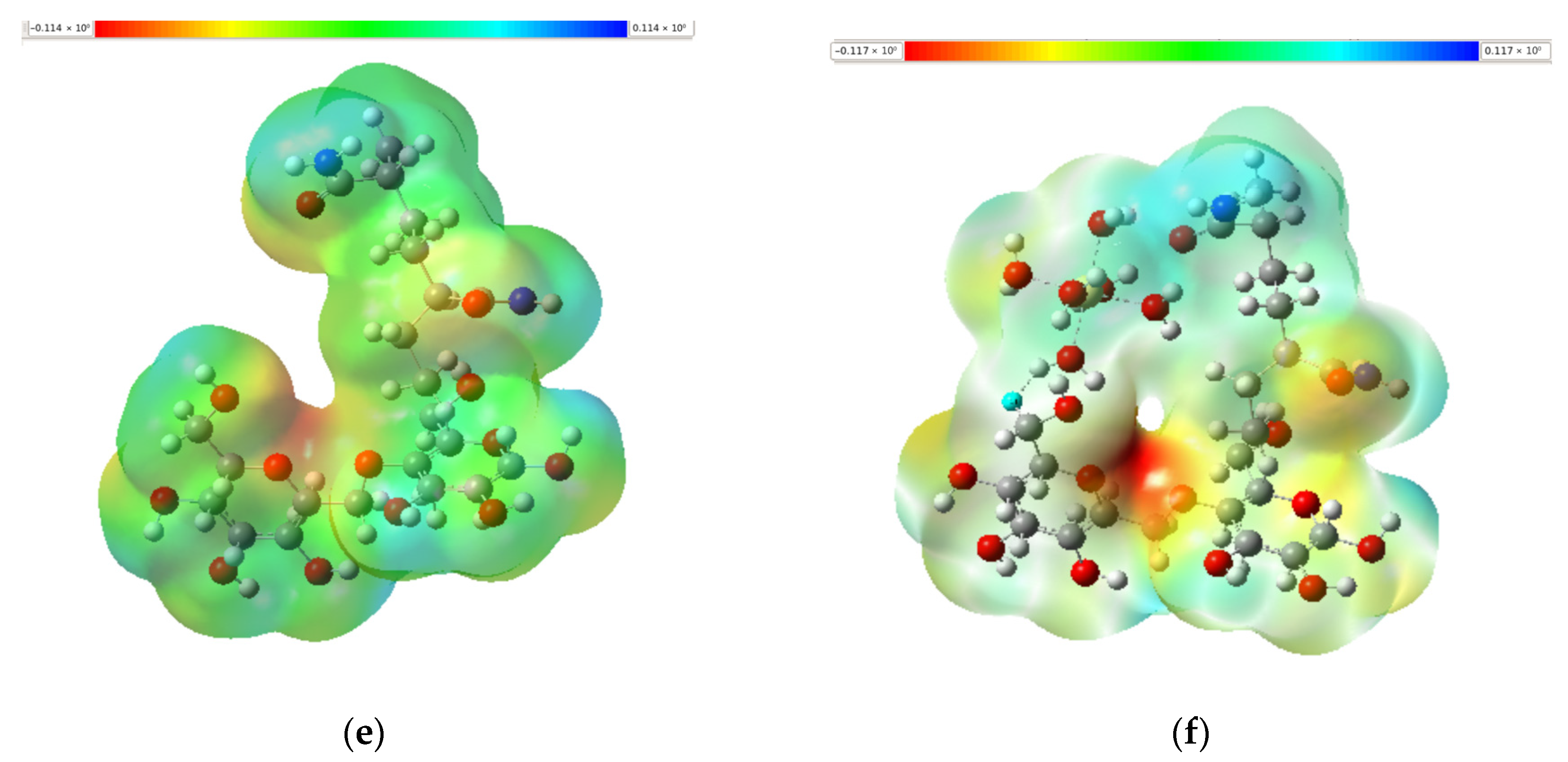
3.5. Molecular Electrostatic Potential
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AM | Acrylamide |
| Amy | Amilose |
| AM/Amy | Acrylamide-Amilose copolymer |
| DFT | Density Functional Theory |
| DOS | Destiny of States |
| HOMO | Highest Occupied Molecular Orbital |
| LUMO | Lowest Unoccupied Molecular Orbital |
| TDM | Total Dipole Moment |
| MEP | Molecular Electrostatic Potential |
References
- Edo, G.I.; Samuel, P.O.; Oloni, G.O.; Ezekiel, G.O.; Ikpekoro, V.O.; Obasohan, P.; Ongulu, J.; Otunuya, C.F.; Opiti, A.R.; Ajakaye, R.S.; et al. Environmental Persistence, Bioaccumulation, and Ecotoxicology of Heavy Metals. Chem. Ecol. 2024, 40, 322–349. [Google Scholar] [CrossRef]
- Sall, M.L.; Diaw, A.K.D.; Gningue-Sall, D.; Aaron, S.E.; Aaron, J.-J. Toxic Heavy Metals: Impact on the Environment and Human Health, and Treatment with Conducting Organic Polymers, a Review. Environ. Sci. Pollut. Res. 2020, 27, 29927–29942. [Google Scholar] [CrossRef] [PubMed]
- Kiran, N.; Bharti, R.; Sharma, R. Effect of Heavy Metals: An Overview. Mater. Today Proc. 2021, 51, 880–885. [Google Scholar] [CrossRef]
- Jomova, K.; Alomar, S.Y.; Nepovimova, E.; Kuca, K.; Valko, M. Heavy Metals: Toxicity and Human Health Effects. Arch. Toxicol. 2024, 99, 153–209. [Google Scholar] [CrossRef] [PubMed]
- Fatima, G.; Raza, A.M.; Hadi, N.; Nigam, N.; Mahdi, A.A. Cadmium in Human Diseases: It’s More than Just a Mere Metal. Indian J. Clin. Biochem. 2019, 34, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-S.; Osman, A.I.; Hosny, M.; Elgarahy, A.M.; Eltaweil, A.S.; Rooney, D.W.; Chen, Z.; Rahim, N.S.; Sekar, M.; Gopinath, S.C.B.; et al. The Toxicity of Mercury and Its Chemical Compounds: Molecular Mechanisms and Environmental and Human Health Implications: A Comprehensive Review. ACS Omega 2024, 9, 5100–5126. [Google Scholar] [CrossRef] [PubMed]
- Collin, M.S.; Venkatraman, S.K.; Vijayakumar, N.; Kanimozhi, V.; Arbaaz, S.M.; Stacey, R.G.S.; Anusha, J.; Choudhary, R.; Lvov, V.; Tovar, G.I.; et al. Bioaccumulation of Lead (Pb) and Its Effects on Human: A Review. J. Hazard. Mater. Adv. 2022, 7, 100094. [Google Scholar] [CrossRef]
- Meirelles, M.R.; Malafatti, J.O.D.; Escote, M.T.; Pinto, A.H.; Paris, E.C. Magnetic Adsorbent Based on Faujasite Zeolite Decorated with Magnesium Ferrite Nanoparticles for Metal Ion Removal. Magnetochemistry 2023, 9, 136. [Google Scholar] [CrossRef]
- Xiang, H.; Min, X.; Tang, C.-J.; Sillanpää, M.; Zhao, F. Recent Advances in Membrane Filtration for Heavy Metal Removal from Wastewater: A Mini Review. J. Water Process Eng. 2022, 49, 103023. [Google Scholar] [CrossRef]
- Tran, T.-K.; Chiu, K.-F.; Lin, C.-Y.; Leu, H.-J. Electrochemical Treatment of Wastewater: Selectivity of the Heavy Metals Removal Process. Int. J. Hydrogen Energy 2017, 42, 27741–27748. [Google Scholar] [CrossRef]
- Chen, Q.; Yao, Y.; Li, X.; Lu, J.; Zhou, J.; Huang, Z. Comparison of Heavy Metal Removals from Aqueous Solutions by Chemical Precipitation and Characteristics of Precipitates. J. Water Process Eng. 2018, 26, 289–300. [Google Scholar] [CrossRef]
- Darban, Z.; Shahabuddin, S.; Gaur, R.; Ahmad, I.; Sridewi, N. Hydrogel-Based Adsorbent Material for the Effective Removal of Heavy Metals from Wastewater: A Comprehensive Review. Gels 2022, 8, 263. [Google Scholar] [CrossRef] [PubMed]
- Du, B.; Chai, L.; Zheng, Q.; Liu, Y.; Wang, X.; Chen, X.; Zhai, S.; Zhou, J.; Sun, R.-C. Designed Synthesis of Multifunctional Lignin-Based Adsorbent for Efficient Heavy Metal Ions Removal and Electromagnetic Wave Absorption. Int. J. Biol. Macromol. 2023, 234, 123668. [Google Scholar] [CrossRef] [PubMed]
- Rashid, R.; Shafiq, I.; Akhter, P.; Iqbal, M.J.; Hussain, M. A State-of-the-Art Review on Wastewater Treatment Techniques: The Effectiveness of Adsorption Method. Environ. Sci. Pollut. Res. 2021, 28, 9050–9066. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, S.; Priya, A.K.; Kumar, P.S.; Hoang, T.K.A.; Sekar, K.; Chong, K.Y.; Khoo, K.S.; Ng, H.S.; Show, P.L. A Critical and Recent Developments on Adsorption Technique for Removal of Heavy Metals from Wastewater-A Review. Chemosphere 2022, 303, 135146. [Google Scholar] [CrossRef] [PubMed]
- Barik, B.; Kumar, A.; Nayak, P.S.; Achary, L.S.K.; Rout, L.; Dash, P. Ionic Liquid Assisted Mesoporous Silica-Graphene Oxide Nanocomposite Synthesis and Its Application for Removal of Heavy Metal Ions from Water. Mater. Chem. Phys. 2019, 239, 122028. [Google Scholar] [CrossRef]
- Pet, I.; Sanad, M.N.; Farouz, M.; ElFaham, M.M.; El-Hussein, A.; El-Sadek, M.S.A.; Althobiti, R.A.; Ioanid, A. Review: Recent Developments in the Implementation of Activated Carbon as Heavy Metal Removal Management. Water Conserv. Sci. Eng. 2024, 9, 62. [Google Scholar] [CrossRef]
- Upadhyay, U.; Sreedhar, I.; Singh, S.A.; Patel, C.M.; Anitha, K.L. Recent Advances in Heavy Metal Removal by Chitosan Based Adsorbents. Carbohydr. Polym. 2020, 251, 117000. [Google Scholar] [CrossRef] [PubMed]
- Noor, N.M.; Othman, R.; Mubarak, N.M.; Abdullah, E.C. Agricultural Biomass-Derived Magnetic Adsorbents: Preparation and Application for Heavy Metals Removal. J. Taiwan Inst. Chem. Eng. 2017, 78, 168–177. [Google Scholar] [CrossRef]
- Zhao, G.; Huang, X.; Tang, Z.; Huang, Q.; Niu, F.; Wang, X. Polymer-Based Nanocomposites for Heavy Metal Ions Removal from Aqueous Solution: A Review. Polym. Chem. 2018, 9, 3562–3582. [Google Scholar] [CrossRef]
- Shalla, A.H.; Yaseen, Z.; Bhat, M.A.; Rangreez, T.A.; Maswal, M. Recent Review for Removal of Metal Ions by Hydrogels. Sep. Sci. Technol. 2018, 54, 89–100. [Google Scholar] [CrossRef]
- Naahidi, S.; Jafari, M.; Logan, M.; Wang, Y.; Yuan, Y.; Bae, H.; Dixon, B.; Chen, P. Biocompatibility of Hydrogel-Based Scaffolds for Tissue Engineering Applications. Biotechnol. Adv. 2017, 35, 530–544. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.-C.; Chang, C.-C.; Chan, H.-P.; Chung, T.-W.; Shu, C.-W.; Chuang, K.-P.; Duh, T.-H.; Yang, M.-H.; Tyan, Y.-C. Hydrogels: Properties and Applications in Biomedicine. Molecules 2022, 27, 2902. [Google Scholar] [CrossRef] [PubMed]
- Vo, T.S.; Hossain, M.M.; Jeong, H.M.; Kim, K. Heavy Metal Removal Applications Using Adsorptive Membranes. Nano Converg. 2020, 7, 26. [Google Scholar] [CrossRef] [PubMed]
- Thang, N.H.; Chien, T.B.; Cuong, D.X. Polymer-Based Hydrogels Applied in Drug Delivery: An Overview. Gels 2023, 9, 523. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; He, J.; Guo, B. Functional Hydrogels as Wound Dressing to Enhance Wound Healing. ACS Nano 2021, 15, 12687–12722. [Google Scholar] [CrossRef] [PubMed]
- Yue, S.; He, H.; Li, B.; Hou, T. Hydrogel as a Biomaterial for Bone Tissue Engineering: A Review. Nanomaterials 2020, 10, 1511. [Google Scholar] [CrossRef] [PubMed]
- Alonso, J.M.; Del Olmo, J.A.; Gonzalez, R.P.; Saez-Martinez, V. Injectable Hydrogels: From Laboratory to Industrialization. Polymers 2021, 13, 650. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, K.; Shi, X.; Fukazawa, K.; Yamaoka, T.; Yao, G.; Wu, J.Y. Biomimetic-Engineered Silicone Hydrogel Contact Lens Materials. ACS Appl. Bio Mater. 2023, 6, 3600–3616. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Zhong, J.; Sun, F.; Liu, B.; Peng, Z.; Lian, J.; Wu, X.; Li, L.; Hao, M.; Zhang, T. Hydrogel Sensors for Biomedical Electronics. Chem. Eng. J. 2023, 481, 148317. [Google Scholar] [CrossRef]
- Badsha, M.A.H.; Khan, M.; Wu, B.; Kumar, A.; Lo, I.M.C. Role of Surface Functional Groups of Hydrogels in Metal Adsorption: From Performance to Mechanism. J. Hazard. Mater. 2020, 408, 124463. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Ye, Q.; Yu, S.; Akhavan, B. Poly Ethylene Glycol (PEG)-based Hydrogels for Drug Delivery in Cancer Therapy. Adv. Healthc. Mater. 2023, 12, 2300105. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Wu, Z.; Zhao, W.; He, M.; Guo, N.; Weng, L.; Lin, Z.; Taleb, M.F.A.; Ibrahim, M.M.; Singh, M.V.; et al. Strategies in the Preparation of Conductive Polyvinyl Alcohol Hydrogels for Applications in Flexible Strain Sensors, Flexible Supercapacitors, and Triboelectric Nanogenerator Sensors: An Overview. Adv. Compos. Hybrid Mater. 2023, 6, 203. [Google Scholar] [CrossRef]
- Andreazza, R.; Morales, A.; Pieniz, S.; Labidi, J. Gelatin-Based Hydrogels: Potential Biomaterials for Remediation. Polymers 2023, 15, 1026. [Google Scholar] [CrossRef] [PubMed]
- Suyambulingam, I.; Gangadhar, L.; Sana, S.S.; Divakaran, D.; Siengchin, S.; Kurup, L.A.; Iyyadurai, J.; Noble, K.E.A.B. Chitosan Biopolymer and Its Nanocomposites: Emerging Material as Adsorbent in Wastewater Treatment. Adv. Mater. Sci. Eng. 2023, 2023, 1–20. [Google Scholar] [CrossRef]
- Yuan, N.; Shao, K.; Huang, S.; Chen, C. Chitosan, Alginate, Hyaluronic Acid and Other Novel Multifunctional Hydrogel Dressings for Wound Healing: A Review. Int. J. Biol. Macromol. 2023, 240, 124321. [Google Scholar] [CrossRef] [PubMed]
- Hernández, J.A.J.; Ortiz, L.S.; Ibáñez, M.V.; Cabrera, L.I.F.; Francisco, J.B.P.; Loranca, B.E.J. Efecto Del Glutaraldehído En Las Propiedades Viscoelásticas de Hidrogeles de Carboximetilcelulosa. Ing. Investig. Tecnol. 2024, 25, 1–7. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, H.J.; Liu, X.; Liu, Y.; Zhu, X.; Liu, X.; You, X. A Physically Cross-Linked Sodium Alginate–Gelatin Hydrogel with High Mechanical Strength. ACS Appl. Polym. Mater. 2021, 3, 3197–3205. [Google Scholar] [CrossRef]
- Cui, C.; Jia, Y.; Sun, Q.; Yu, M.; Ji, N.; Dai, L.; Wang, Y.; Qin, Y.; Xiong, L.; Sun, Q. Recent Advances in the Preparation, Characterization, and Food Application of Starch-Based Hydrogels. Carbohydr. Polym. 2022, 291, 119624. [Google Scholar] [CrossRef] [PubMed]
- Abt, E.; Robin, L.P.; McGrath, S.; Srinivasan, J.; DiNovi, M.; Adachi, Y.; Chirtel, S. Acrylamide Levels and Dietary Exposure from Foods in the United States, an Update Based on 2011-2015 Data. Food Addit. Contam. Part A 2019, 36, 1475–1490. [Google Scholar] [CrossRef] [PubMed]
- Xiong, B.; Loss, R.D.; Shields, D.; Pawlik, T.; Hochreiter, R.; Zydney, A.L.; Kumar, M. Polyacrylamide Degradation and Its Implications in Environmental Systems. Npj Clean Water 2018, 1, 9. [Google Scholar] [CrossRef]
- Sennakesavan, G.; Mostakhdemin, M.; Dkhar, L.K.; Seyfoddin, A.; Fatihhi, S.J. Acrylic Acid/Acrylamide Based Hydrogels and Its Properties–A Review. Polym. Degrad. Stab. 2020, 180, 109308. [Google Scholar] [CrossRef]
- Kaşgöz, H.; Özgümüş, S.; Orbay, M. Modified Polyacrylamide Hydrogels and Their Application in Removal of Heavy Metal Ions. Polymer 2003, 44, 1785–1793. [Google Scholar] [CrossRef]
- Compart, J.; Singh, A.; Fettke, J.; Apriyanto, A. Customizing Starch Properties: A Review of Starch Modifications and Their Applications. Polymers 2023, 15, 3491. [Google Scholar] [CrossRef] [PubMed]
- Da Costa, T.B.; Camani, P.H.; Ferreira, R.R.; Barbosa, R.F.S.; Rosa, D.D.S. Enhancing Corn Starch Hydrogels for Effective Sorption of Potentially Toxic Metals: The Role of Amylose and Amylopectin Content. J. Polym. Environ. 2025, 33, 1615–1635. [Google Scholar] [CrossRef]
- Moreno-Sader, K.; García-Padilla, A.; Realpe, A.; Acevedo-Morantes, M.; Soares, J.B.P. Removal of Heavy Metal Water Pollutants (CO2+ and NI2+) Using Polyacrylamide/Sodium Montmorillonite (PAM/NA-MMT) Nanocomposites. ACS Omega 2019, 4, 10834–10844. [Google Scholar] [CrossRef] [PubMed]
- Hasan, I.; Khan, R.A.; Alharbi, W.; Alharbi, K.H.; Alsalme, A. In Situ Copolymerized Polyacrylamide Cellulose Supported FE3O4 Magnetic Nanocomposites for Adsorptive Removal of PB(II): Artificial Neural Network Modeling and Experimental Studies. Nanomaterials 2019, 9, 1687. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, I.H.S.; Reis, D.T.; Pereira, D.H. A DFT-Based Analysis of Adsorption of Cd2+, Cr3+, Cu2+, Hg2+, Pb2+, and Zn2+, on Vanillin Monomer: A Study of the Removal of Metal Ions from Effluents. J. Mol. Model. 2019, 25, 267. [Google Scholar] [CrossRef] [PubMed]
- Zaferani, S.P.G.; Amiri, M.K.; Amooey, A.A. Computational AI to Predict and Optimize the Relationship between Dye Removal Efficiency and Gibbs Free Energy in the Adsorption Process Utilizing TiO2/Chitosan-Polyacrylamide Composite. Int. J. Biol. Macromol. 2024, 264, 130738. [Google Scholar] [CrossRef] [PubMed]
- Parr, R.G. Density Functional Theory of Atoms and Molecules. In Springer eBooks; Springer: Berlin/Heidelberg, Germany, 1980; pp. 5–15. [Google Scholar]
- Zhao, Y.; Schultz, N.E.; Truhlar, D.G. Design of Density Functionals by Combining the Method of Constraint Satisfaction with Parametrization for Thermochemistry, Thermochemical Kinetics, and Noncovalent Interactions. J. Chem. Theory Comput. 2006, 2, 364–382. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Truhlar, D.G. The M06 Suite of Density Functionals for Main Group Thermochemistry, Thermochemical Kinetics, Noncovalent Interactions, Excited States, and Transition Elements: Two New Functionals and Systematic Testing of Four M06-Class Functionals and 12 Other Functionals. Theor. Chem. Acc. 2007, 120, 215–241. [Google Scholar] [CrossRef]
- Wu, H.; Zhong, S.; Bin, Y.; Jiang, X.; Cui, H. Ni-Decorated WS2-WSe2 Heterostructure as a Novel Sensing Candidate upon C2H2 and C2H4 in Oil-Filled Transformers: A First-Principles Investigation. Mol. Phys. 2025, e2492391. [Google Scholar] [CrossRef]
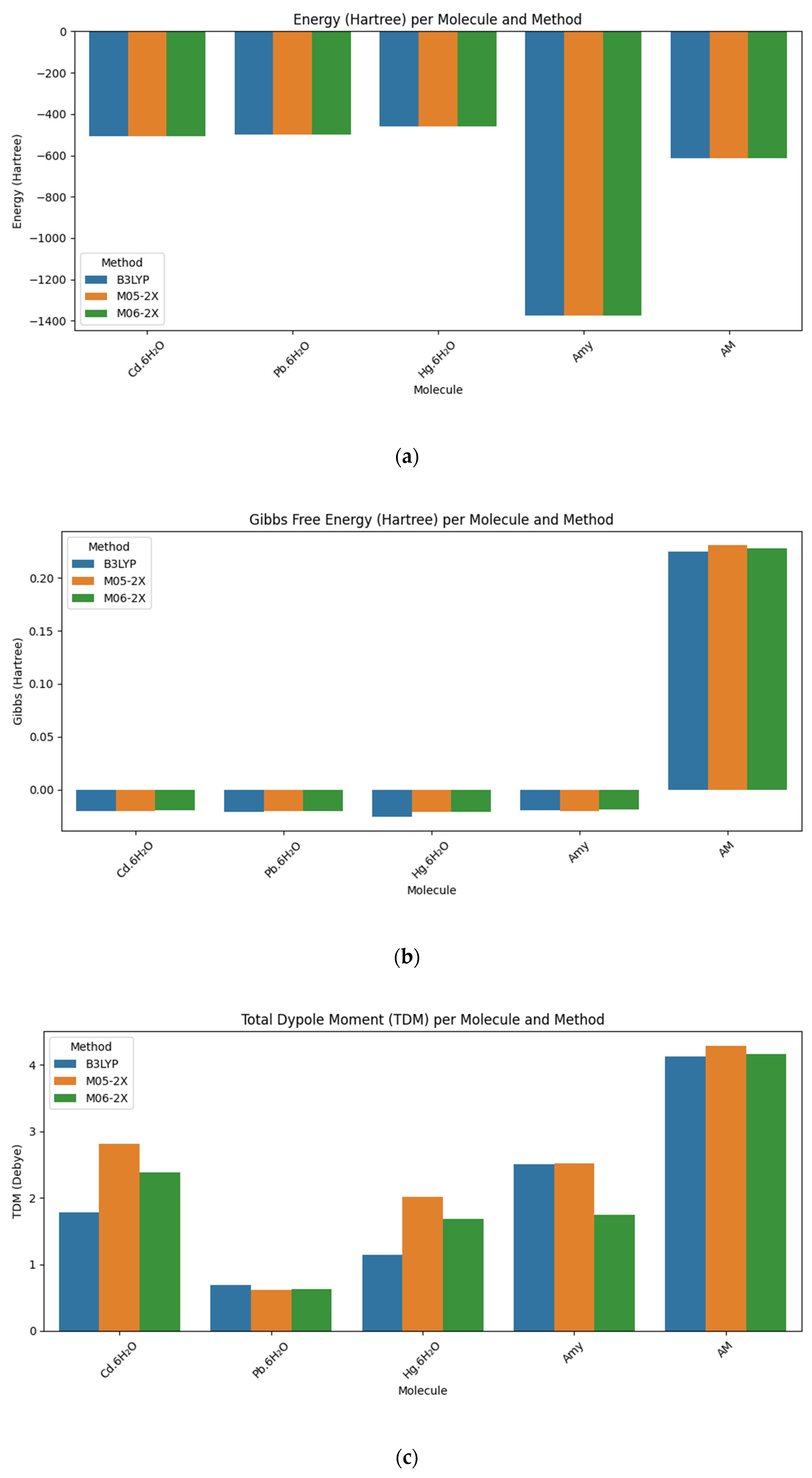
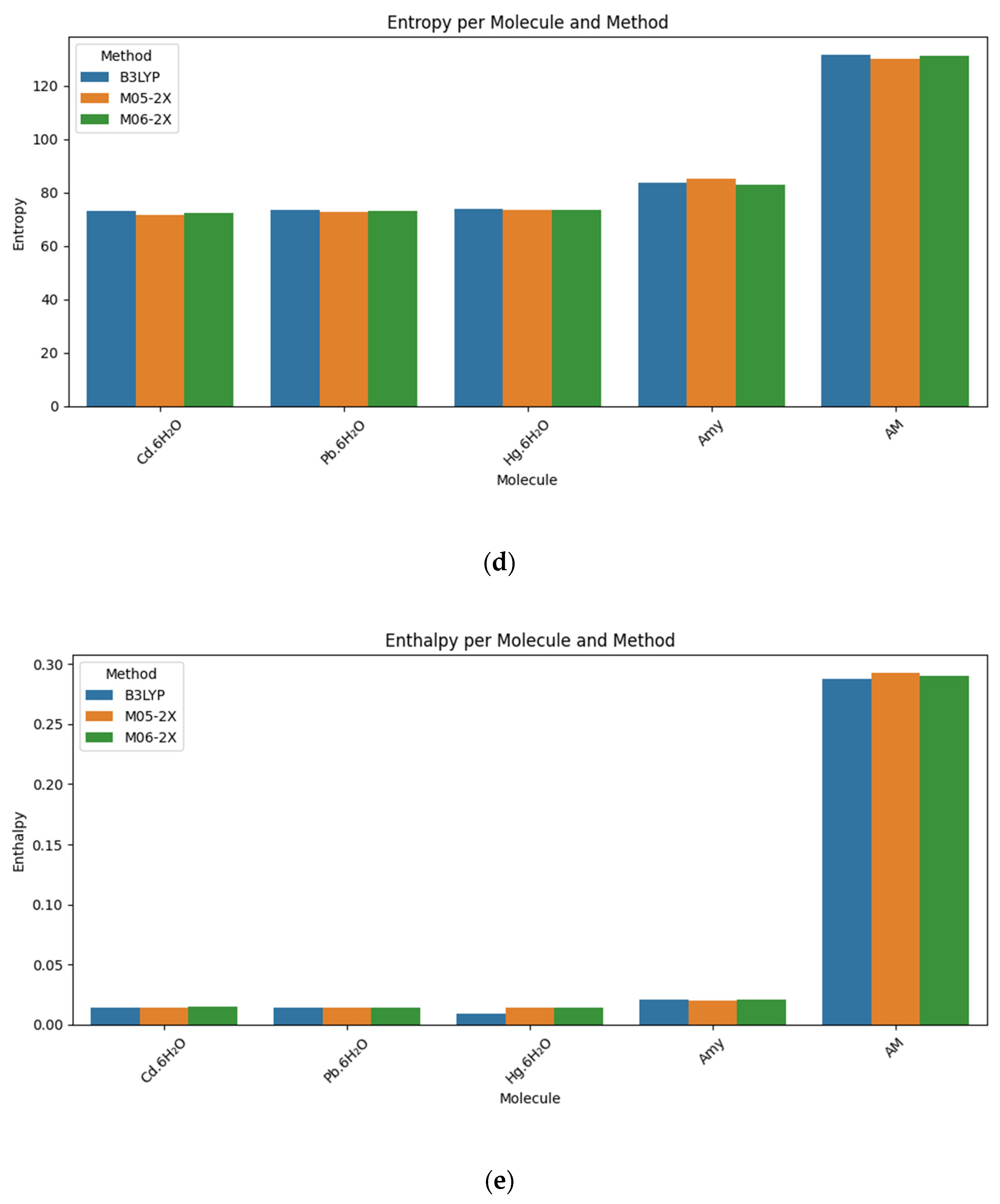



| Molecule | Method/Base Set | Energy (Hartree) | Gibbs Free Energy (Hartree) | TDM (Debye) | Entropy (Cal/Mol-Kelvin) | Enthalpy (Hartree) |
|---|---|---|---|---|---|---|
| Cd·6H2O | B3LYP/6-311++G(d,p) | −506.402385 | −0.020369 | 1.7821 | 73.214 | 0.014417 |
| M05-2X/6-31++G(d,p) | −506.122292 | −0.020156 | 2.8165 | 71.839 | 0.013977 | |
| M06-2X/6-311+G(d,p) | −506.094871 | −0.019112 | 2.3847 | 72.427 | 0.015300 | |
| Pb·6H2O | B3LYP/6-311++G(d,p) | −500.978698 | −0.020813 | 0.6991 | 73.486 | 0.014102 |
| M05-2X/6-31++G(d,p) | −500.706202 | −0.020537 | 0.6189 | 72.850 | 0.014077 | |
| M06-2X/6-311+G(d,p) | −500.660737 | −0.020567 | 0.6241 | 73.257 | 0.01424 | |
| Hg·6H2O | B3LYP/6-311++G(d,p) | −461.812930 | −0.025824 | 1.1458 | 73.841 | 0.009260 |
| M05-2X/6-31++G(d,p) | −461.596211 | −0.021032 | 2.0222 | 73.433 | 0.013858 | |
| M06-2X/6-311+G(d,p) | −461.494978 | −0.020732 | 1.6838 | 73.462 | 0.014172 | |
| Amy | B3LYP/6-311++G(d,p) | −1376.086739 | −0.019303 | 2.5127 | 83.814 | 0.02052 |
| M05-2X/6-31+G(d,p) | −1376.481552 | −0.020062 | 2.5264 | 85.329 | 0.020480 | |
| M06-2X/6-311++G(d,p) | −1375.525214 | −0.018545 | 1.7550 | 83.108 | 0.020942 | |
| AM | B3LYP/6-311++G(d,p) | −613.991554 | 0.224943 | 4.1389 | 131.765 | 0.287548 |
| M05-2X/6-31+G(d,p) | −613.760652 | 0.230967 | 4.2997 | 130.003 | 0.292735 | |
| M06-2X/6-311++G(d,p) | −613.714295 | 0.228123 | 4.1722 | 131.258 | 0.290488 |
| Molecule | Metal | Lumo (eV) | Homo (eV) | ΔE (eV) | TDM (Debye) |
|---|---|---|---|---|---|
| Amy | Cd·6H2O | −0.01506 | −0.06034 | 0.04528 | 4.32257 |
| Hg·6H2O | −0.01267 | −0.06237 | 0.04970 | 4.08872 | |
| Pb·6H2O | −0.01018 | −0.03631 | 0.02613 | 7.17751 | |
| AM | Cd·6H2O | 0.00188 | −0.01562 | 0.01750 | 5.77141 |
| Hg·6H2O | 0.00503 | −0.02259 | 0.02762 | 5.41831 | |
| Pb·6H2O | 0.02341 | −0.00195 | 0.02536 | 5.82425 | |
| AM/Amy | Cd·6H2O | −0.01637 | −0.03633 | 0.01996 | 11.00673 |
| Hg·6H2O | −0.01169 | −0.02541 | 0.01372 | 8.76457 | |
| Pb·6H2O | −0.01149 | −0.02541 | 0.01392 | 10.79232 |
| Molecule | Metal | Chemical Potential (μ) | Ionization Potential (I) | Electronegativity (χ) | Electronic Affinity (A) | Electrophilicity (ω) | Hardness (η) | Softness (S) |
|---|---|---|---|---|---|---|---|---|
| Amy | - | −0.10320 | 0.16829 | 0.10320 | 0.03811 | 0.0003466 | 0.06509 | 15.3633 |
| AM | - | −0.13916 | 0.26026 | 0.13916 | 0.01806 | 0.0011726 | 0.1211 | 8.2576 |
| AM/Amy | - | −0.06857 | 0.10128 | 0.06857 | 0.03586 | 0.0000769 | 0.03271 | 30.5717 |
| Amy | Cd·6H2O | −0.03770 | 0.06034 | 0.03770 | 0.01506 | 0.0000161 | 0.02264 | 44.1696 |
| Hg·6H2O | −0.02325 | 0.03631 | 0.02325 | 0.01018 | 0.0000035 | 0.013065 | 76.5404 | |
| Pb·6H2O | −0.03752 | 0.06237 | 0.03752 | 0.01267 | 0.0000175 | 0.02485 | 40.2414 | |
| AM | Cd·6H2O | −0.00687 | 0.01562 | 0.00687 | −0.00188 | 0.0000002 | 0.00875 | 114.2857 |
| Hg·6H2O | 0.01073 | 0.00195 | −0.01073 | −0.02341 | 0.0000007 | 0.01268 | 78.8644 | |
| Pb·6H2O | −0.00878 | 0.02259 | 0.00878 | −0.00503 | 0.0000005 | 0.01381 | 72.4113 | |
| AM/Amy | Cd·6H2O | −0.02635 | 0.03633 | 0.02635 | 0.01637 | 0.0000035 | 0.00998 | 100.2004 |
| Hg·6H2O | −0.01845 | 0.02541 | 0.01845 | 0.01149 | 0.0000012 | 0.00696 | 143.6782 | |
| Pb·6H2O | −0.01855 | 0.02541 | 0.01855 | 0.01169 | 0.0000012 | 0.00686 | 145.7726 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernandez-Fernandez, J.; Maldonado-Morales, Y.; Gonzalez-Cuello, R.; Villabona-Ortíz, Á.; Ortega-Toro, R. Theoretical DFT Analysis of a Polyacrylamide/Amylose Copolymer for the Removal of Cd(II), Hg(II), and Pb(II) from Aqueous Solutions. Polymers 2025, 17, 1943. https://doi.org/10.3390/polym17141943
Hernandez-Fernandez J, Maldonado-Morales Y, Gonzalez-Cuello R, Villabona-Ortíz Á, Ortega-Toro R. Theoretical DFT Analysis of a Polyacrylamide/Amylose Copolymer for the Removal of Cd(II), Hg(II), and Pb(II) from Aqueous Solutions. Polymers. 2025; 17(14):1943. https://doi.org/10.3390/polym17141943
Chicago/Turabian StyleHernandez-Fernandez, Joaquin, Yuly Maldonado-Morales, Rafael Gonzalez-Cuello, Ángel Villabona-Ortíz, and Rodrigo Ortega-Toro. 2025. "Theoretical DFT Analysis of a Polyacrylamide/Amylose Copolymer for the Removal of Cd(II), Hg(II), and Pb(II) from Aqueous Solutions" Polymers 17, no. 14: 1943. https://doi.org/10.3390/polym17141943
APA StyleHernandez-Fernandez, J., Maldonado-Morales, Y., Gonzalez-Cuello, R., Villabona-Ortíz, Á., & Ortega-Toro, R. (2025). Theoretical DFT Analysis of a Polyacrylamide/Amylose Copolymer for the Removal of Cd(II), Hg(II), and Pb(II) from Aqueous Solutions. Polymers, 17(14), 1943. https://doi.org/10.3390/polym17141943









