Three-Dimensional Printed Porous PLA Scaffolds with Dual Functionality: Cell Proliferation Enhancement and Antibacterial Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Surface Fabrication
2.2. Mechanical Test (Tensile Test)
2.3. Biological Tests
2.3.1. Cell Culture
2.3.2. AlamarBlue Assay
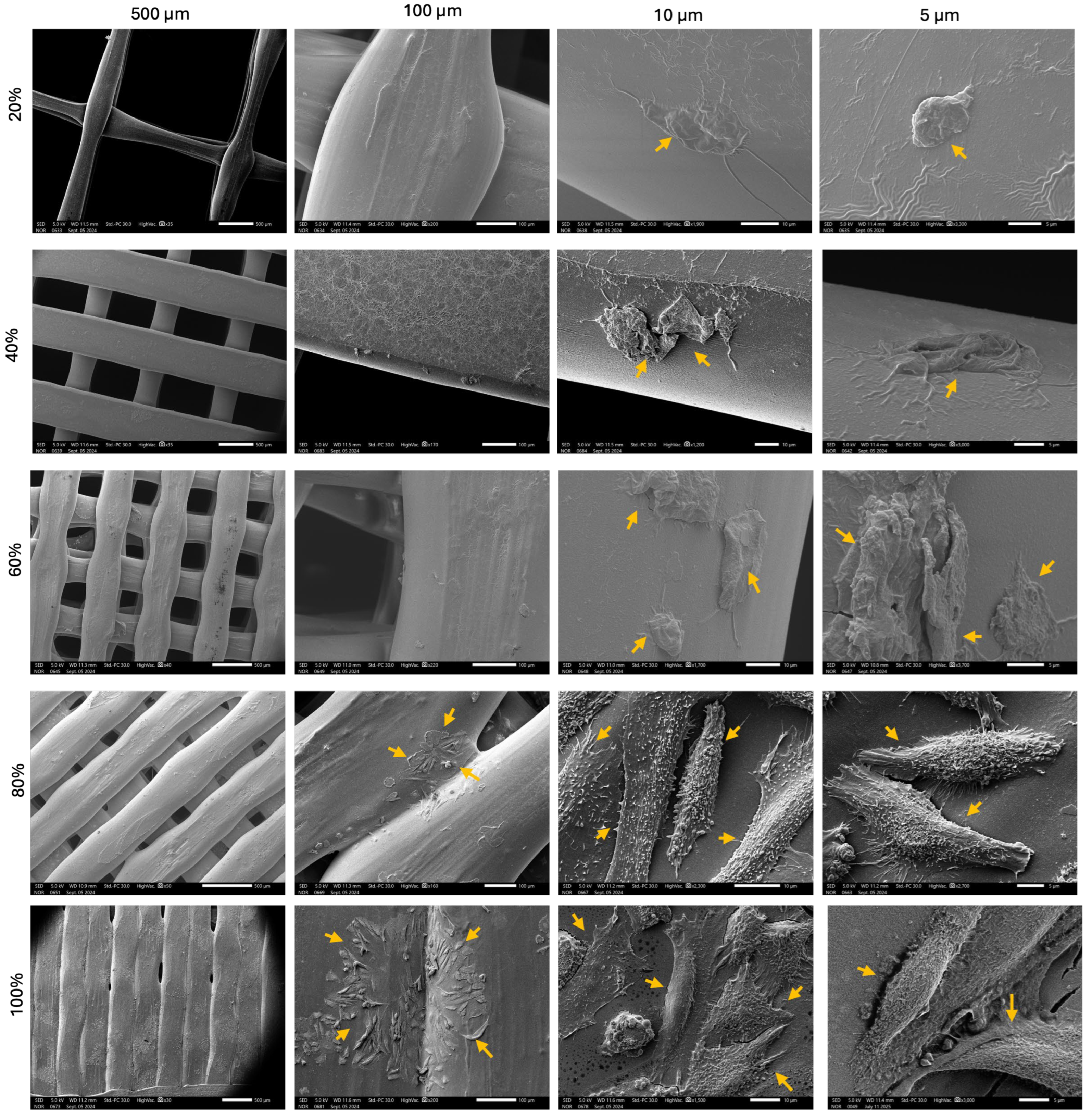
2.3.3. Scanning Electron Microscope (SEM)
2.3.4. Bacterial Culture
2.3.5. Bacterial Metabolomic Activity Measurement (ATP Release)
2.4. Statistical Analysis
3. Results and Discussion
3.1. Effect of Varying Levels of Porosity on the Mechanical Strength of the 3D-Printed Samples
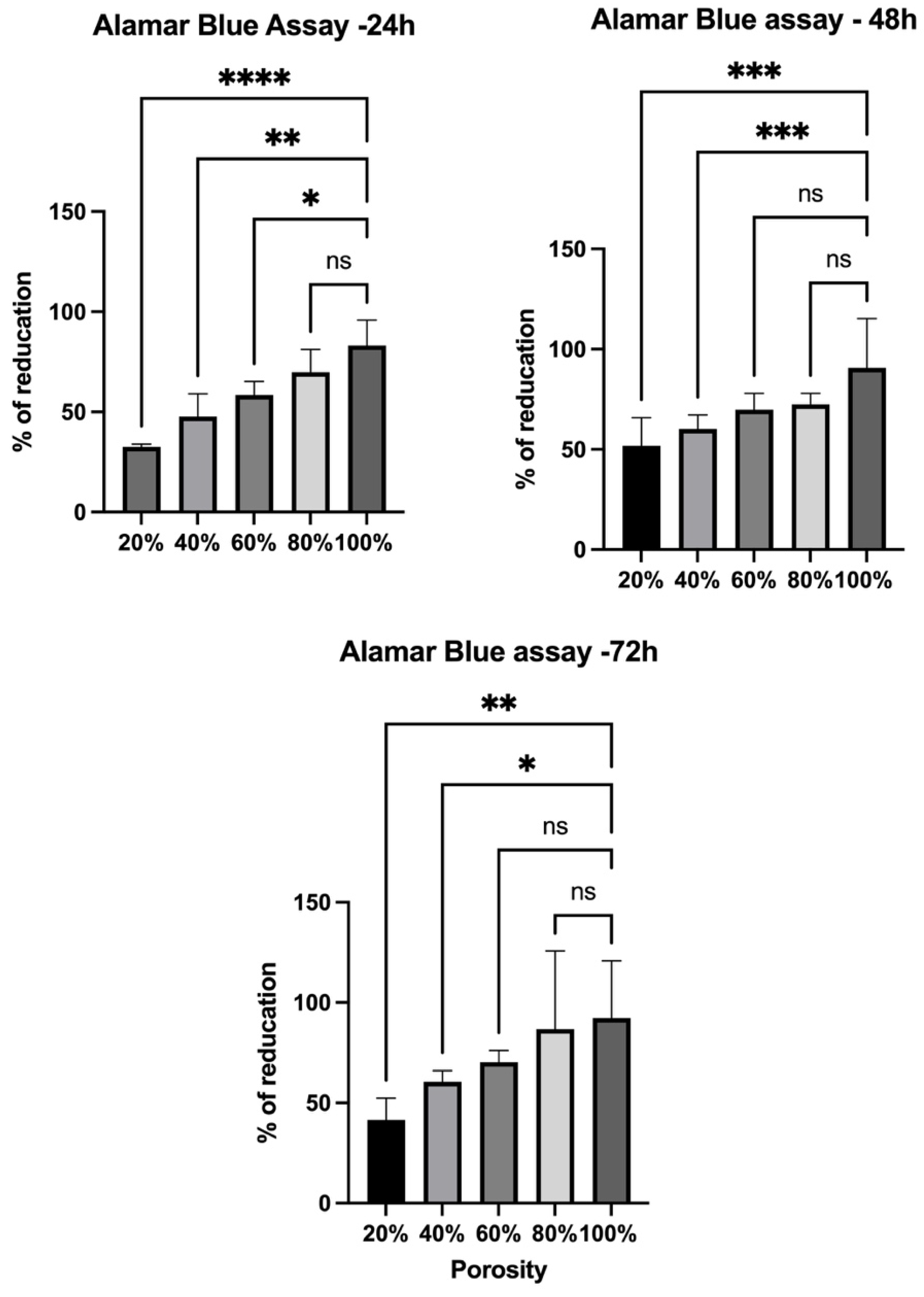
3.2. Cell Culture and Visualization
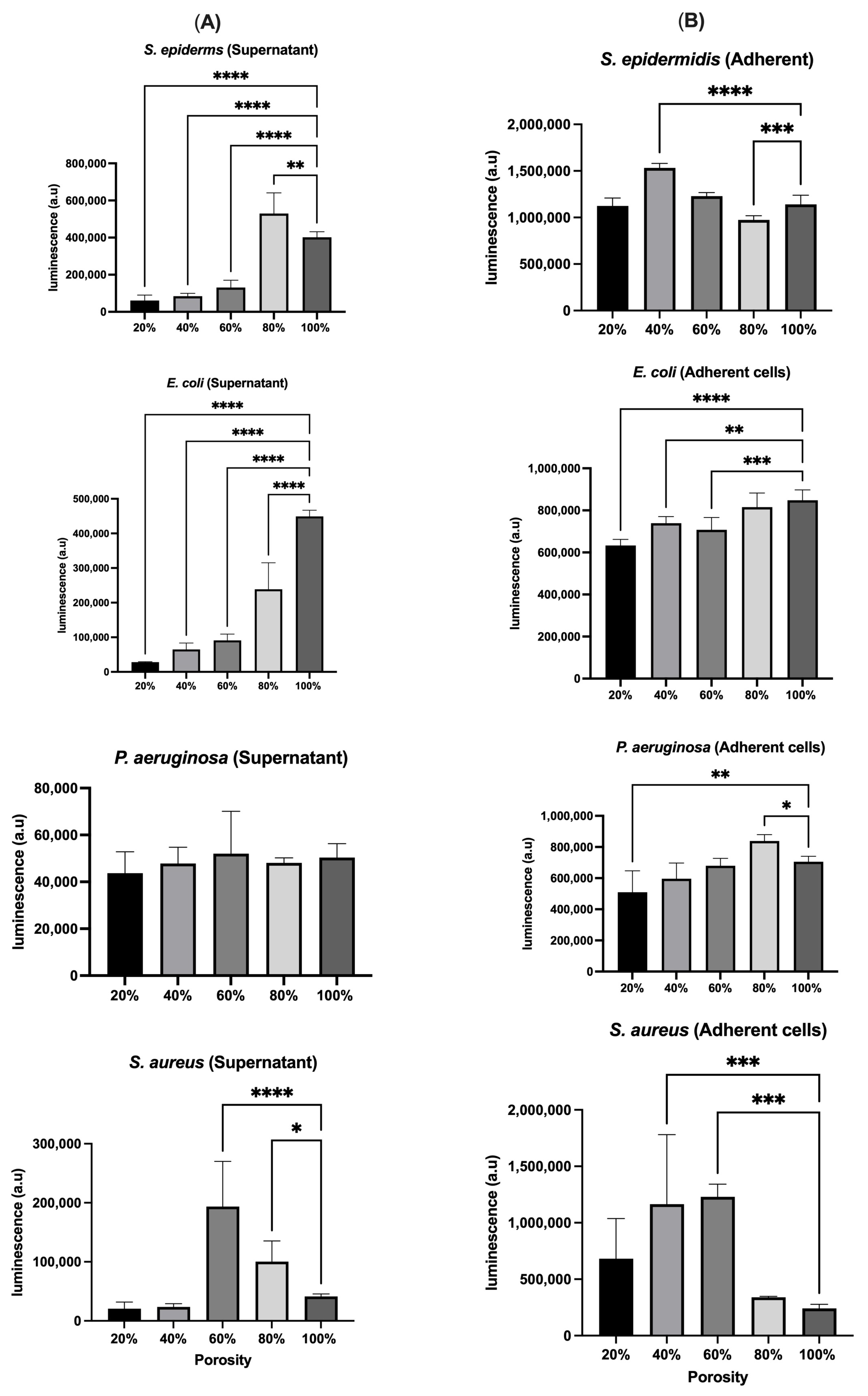
3.3. Bacterial Culture
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PLA | Poly(lactic acid) |
| HSF | Human skin fibroblast |
| ASTM | American Society for Testing and Materials |
| ECM | Extracellular matrix |
| NEAA | Non-essential amino acid |
| RT | Room temperature |
| PBS | Phosphate-buffer saline |
| CFU | Colony-forming unit |
| OD | Optical density |
| PCL | Polycaprolactone |
References
- Ramezani, R.; Alizadeh, R.; Labbaf, S. 3D-printed PLA/Fe3O4/MgO hybrid composite scaffolds with improved properties. Bioprinting 2025, 47, e00398. [Google Scholar] [CrossRef]
- Do, A.-V.; Khorsand, B.; Geary, S.M.; Salem, A.K. 3D Printing of Scaffolds for Tissue Regeneration Applications. Adv. Healthc. Mater. 2015, 4, 1742–1762. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yan, C. 3D Printing of Scaffolds for Tissue Engineering. In 3D Printing; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef]
- Damiati, L.A.; El-Yaagoubi, M.; Damiati, S.A.; Kodzius, R.; Sefat, F.; Damiati, S. Role of Polymers in Microfluidic Devices. Polymers 2022, 14, 5132. [Google Scholar] [CrossRef]
- Ding, X.; Xie, S.; Zhang, W.; Zhu, Y.; Xu, D.; Xian, S.; Sun, H.; Guo, X.; Li, Y.; Lu, J.; et al. Current application of tissue-engineered dermal scaffolds mimicking the extracellular matrix microenvironment in wound healing. Regen. Ther. 2025, 28, 371–382. [Google Scholar] [CrossRef]
- Miranda-Buendia, E.; González-Gómez, G.H.; Maciel-Cerda, A.; González-Torres, M. In Vitro Culture of Human Dermal Fibroblasts on Novel Electrospun Polylactic Acid Fiber Scaffolds Loaded with Encapsulated Polyepicatechin Physical Gels. Gels 2024, 10, 601. [Google Scholar] [CrossRef]
- Karabay, U.; Husemoglu, R.B.; Egrilmez, M.Y.; Aydemiïr, S.; BAYKARA, B.; CagïrAl, S.; Havitçioğlu, H. Comparison of the Biological Behaviour of Human Dermal Fibroblasts seeded on 3D Printed Polylactic acid, Polycaprolactone and Polyethylene Terephthalate Scaffolds in vitro. J. Med. Innov. Technol. 2021, 3, 7–13. [Google Scholar] [CrossRef]
- Kayadurmus, H.M.; Rezaei, A.; Ilhan, E.; Cesur, S.; Sahin, A.; Gunduz, O.; Kalaskar, D.M.; Ekren, N. Whey protein-loaded 3D-printed poly (lactic) acid scaffolds for wound dressing applications. Biomed Mater 2024, 19, 045045. [Google Scholar] [CrossRef]
- Dubey, A.; Vahabi, H.; Kumaravel, V. Antimicrobial and Biodegradable 3D Printed Scaffolds for Orthopedic Infections. ACS Biomater. Sci. Eng. 2023, 9, 4020–4044. [Google Scholar] [CrossRef]
- Pugliese, R.; Beltrami, B.; Regondi, S.; Lunetta, C. Polymeric biomaterials for 3D printing in medicine: An overview. Ann. 3D Print. Med. 2021, 2, 100011. [Google Scholar] [CrossRef]
- Arefin, A.M.E.; Khatri, N.R.; Kulkarni, N.; Egan, P.F. Polymer 3D printing review: Materials, process, and design strategies for medical applications. Polymers 2021, 13, 1499. [Google Scholar] [CrossRef]
- Damiati, L.A.; Tsimbouri, M.P.; Hernandez, V.-L.; Jayawarna, V.; Ginty, M.; Childs, P.; Xiao, Y.; Burgess, K.; Wells, J.; Sprott, M.R.; et al. Materials-driven fibronectin assembly on nanoscale topography enhances mesenchymal stem cell adhesion, protecting cells from bacterial virulence factors and preventing biofilm formation. Biomaterials 2022, 280, 121263. [Google Scholar] [CrossRef] [PubMed]
- Donate, R.; Monzón, M.; Alemán-Domínguez, M.E. Additive manufacturing of PLA-based scaffolds intended for bone regeneration and strategies to improve their biological properties. e-Polymers 2020, 20, 571–599. [Google Scholar] [CrossRef]
- Souness, A.; Zamboni, F.; Walker, G.M.; Collins, M.N. Influence of scaffold design on 3D printed cell constructs. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 533–545. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Vijayavenkataraman, S.; Lu, W.F.; Fuh, J.Y.H. A review on the use of computational methods to characterize, design, and optimize tissue engineering scaffolds, with a potential in 3D printing fabrication. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 107, 1329–1351. [Google Scholar] [CrossRef]
- Loh, Q.L.; Choong, C. Three-dimensional scaffolds for tissue engineering applications: Role of porosity and pore size. Tissue Eng. Part B Rev. 2013, 19, 485–502. [Google Scholar] [CrossRef]
- Huang, D.; Li, Z.; Li, G.; Zhou, F.; Wang, G.; Ren, X.; Su, J. Biomimetic structural design in 3D-printed scaffolds for bone tissue engineering. Mater. Today Bio 2025, 32, 101664. [Google Scholar] [CrossRef]
- Liu, S.; Qin, S.; He, M.; Zhou, D.; Qin, Q.; Wang, H. Current applications of poly(lactic acid) composites in tissue engineering and drug delivery. Compos. B Eng. 2020, 199, 108238. [Google Scholar] [CrossRef]
- Popelka, A.; Abdulkareem, A.; Mahmoud, A.A.; Nassr, M.G.; Al-Ruweidi, M.K.A.; Mohamoud, K.J.; Hussein, M.K.; Lehocky, M.; Vesela, D.; Humpolíček, P.; et al. Antimicrobial modification of PLA scaffolds with ascorbic and fumaric acids via plasma treatment. Surf. Coat. Technol. 2020, 400, 126216. [Google Scholar] [CrossRef]
- Rocha, U.G.; Chalhub, D.J.N.M.; Mangiavacchi, N.; Pimenta, A.R.; Miguel, M.A.L.; Diniz, M.G. Bactericidal Effectiveness of 3D Printed PLA Surfaces Coated with Sealant Containing Silver Nitrate. Braz. Arch. Biol. Technol. 2024, 67, e24231023. [Google Scholar] [CrossRef]
- Daskalova, A.; Angelova, L.; Filipov, E.; Aceti, D.; Mincheva, R.; Carrete, X.; Kerdjoudj, H.; Dubus, M.; Chevrier, J.; Trifonov, A.; et al. Biomimetic hierarchical structuring of pla by ultra-short laser pulses for processing of tissue engineered matrices: Study of cellular and antibacterial behavior. Polymers 2021, 13, 2577. [Google Scholar] [CrossRef]
- Uchida, D.T.; Bruschi, M.L. 3D Printing as a Technological Strategy for the Personalized Treatment of Wound Healing. Aaps Pharmscitech 2023, 24, 41. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.; Sousa, D.; Teixeira, J.A.; Ferreira-Santos, P.; Botelho, C.M. Polymeric biomaterials for wound healing. Front. Bioeng. Biotechnol. 2023, 11, 1136077. [Google Scholar] [CrossRef]
- Tabriz, A.G.; Douroumis, D. Recent advances in 3D printing for wound healing: A systematic review. J. Drug Deliv. Sci. Technol. 2022, 74, 103564. [Google Scholar] [CrossRef]
- Damiati, L.A.; Alsudir, S.A.; Mohammed, R.Y.; Majrashi, M.A.; Albrahim, S.H.; Algethami, A.; Alghamdi, F.O.; Alamari, H.A.; Alzaydi, M.M. 4D printing in skin tissue engineering: A revolutionary approach to enhance wound healing and combat infections. Bioprinting 2025, 45, e00386. [Google Scholar] [CrossRef]
- Murphy, S.V.; Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef]
- Ogundeji, K.D.; Risenga, P.R.; Thupayagale-Tshweneagae, G. Cost of wound dressing: Implication for enrollment into the National Health Insurance scheme, Nigeria. Curationis 2023, 46, 2390. [Google Scholar] [CrossRef]
- Chen, H.; Shi, Q.; Shui, H.; Wang, P.; Chen, Q.; Li, Z. Degradation of 3D-Printed Porous Polylactic Acid Scaffolds Under Mechanical Stimulus. Front. Bioeng. Biotechnol. 2021, 9, 691834. [Google Scholar] [CrossRef]
- Liao, Y.; Liu, C.; Coppola, B.; Barra, G.; Di Maio, L.; Incarnato, L.; Lafdi, K. Effect of Porosity and Crystallinity on 3D Printed PLA Properties. Polymers 2019, 11, 1487. [Google Scholar] [CrossRef]
- Mandal, B.B.; Kundu, S.C. Cell proliferation and migration in silk fibroin 3D scaffolds. Biomaterials 2009, 30, 2956–2965. [Google Scholar] [CrossRef]
- Santoro, M.; Shah, S.R.; Walker, J.L.; Mikos, A.G. Poly(lactic acid) nanofibrous scaffolds for tissue engineering. Adv. Drug Deliv. Rev. 2016, 107, 206–212. [Google Scholar] [CrossRef]
- Sundaramurthi, D.; Krishnan, U.M.; Sethuraman, S. Electrospun nanofibers as scaffolds for skin tissue engineering. Polym. Rev. 2014, 54, 348–376. [Google Scholar] [CrossRef]
- Lanno, G.-M.; Ramos, C.; Preem, L.; Putrinš, M.; Laidmäe, I.; Tenson, T.; Kogermann, K. Antibacterial porous electrospun fibers as skin scaffolds for wound healing applications. ACS Omega 2020, 5, 30011–30022. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, H.; Lu, W.; Guo, Y. Antibacterial porous coaxial drug-carrying nanofibers for sustained drug-releasing applications. Nanomaterials 2021, 11, 1316. [Google Scholar] [CrossRef]
- Mira, P.; Yeh, P.; Hall, B.G. Estimating microbial population data from optical density. PLoS ONE 2022, 17, e0276040. [Google Scholar] [CrossRef]
- Beal, J.; Farny, N.G.; Haddock-Angelli, T.; Selvarajah, V.; Baldwin, G.S.; Buckley-Taylor, R.; Gershater, M.; Kiga, D.; Marken, J.; Sanchania, V.; et al. Robust estimation of bacterial cell count from optical density. Commun. Biol. 2020, 3, 512. [Google Scholar] [CrossRef]
- Stevenson, K.; McVey, A.F.; Clark, I.B.N.; Swain, P.S.; Pilizota, T. General calibration of microbial growth in microplate readers. Sci. Rep. 2016, 6, 38828. [Google Scholar] [CrossRef]
- McBirney, S.E.; Trinh, K.; Wong-Beringer, A.; Armani, A.M. Wavelength-normalized spectroscopic analysis of Staphylococcus aureus and Pseudomonas aeruginosa growth rates. Biomed. Opt. Express 2016, 7, 4034. [Google Scholar] [CrossRef]





| Bacteria | OD (Planktonic) | OD (Adherent) | Luminescence (Planktonic) | Luminescence (Adherent) | Conclusion |
|---|---|---|---|---|---|
| S. epidermidis | High at 20%, low at 80% | Increases with porosity, peaks at 100% | High at 80%, low at 20% | High at 40%, low at 20% | OD may include non-viable cells; luminescence confirms viability at 80% |
| E. coli | Consistent trend, slight increase at 80% | Increases with porosity, peaks at 100% | Highest at 100% | Generally matches OD, higher at low porosity | High metabolic activity at high porosity |
| P. aeruginosa | Consistent across porosities | Sharp increase at 80%, drops at 100% | Follows OD, peak at 60% | Consistent trend, peak at 80% | 80% porosity optimal for attachment and viability |
| S. aureus | High at 60%, low at 20% | Highest at 40–60%, lowest at 100% | Mismatch with OD, lower at 40% and 100% | Aligns with OD | Discrepancy between OD and viability at extreme porosities |
| Scaffolds/Odification | Bacterial Response | Cellular Response | Study |
|---|---|---|---|
| PLA + PEC (electrospun) | Not reported | Improved HDF viability and metabolic activity | [6] |
| Three-dimensional-printed PLA vs. PCL and PET | PLA superior to PCL and PET (general biocompatibility) | Higher HDF viability and collagen expression on PLA | [7] |
| PLA + WPC | Not specified | Enhanced viability, swelling, and degradability | [8] |
| PLA + silver coating | 64x CFU reduction in E. coli and S. aureus | Not reported | [20] |
| Laser-structured PLA surface | Increased S. aureus adhesion | Promoted stem cell adhesion and orientation | [21] |
| Porous chloramphenicol-loaded PCL microfibers | Porous fibers showed highest antibiofilm activity and better elasticity than nonporous fibers | Not reported | [33] |
| Coaxial PCL/PLA porous nanofibers loaded with Roxithromycin | Porous nanofibers showed good antibacterial zones (1.70–1.73 cm) against S. aureus | Not reported | [34] |
| Three-dimensional-printed PLA scaffolds with varying porosities | Porosity influenced bacterial adhesion and viability; OD alone is insufficient for accuracy; luminescence revealed metabolic activity more accurately | HSF viability increased over time and was highest on scaffolds with intermediate to high porosity (60–80%) | Current study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
AlQurashi, R.N.; Bataweel, N.M.; AlQriqri, M.A.; Alqahtani, S.H.; Basalah, A.A.; Damiati, L.A. Three-Dimensional Printed Porous PLA Scaffolds with Dual Functionality: Cell Proliferation Enhancement and Antibacterial Properties. Polymers 2025, 17, 1928. https://doi.org/10.3390/polym17141928
AlQurashi RN, Bataweel NM, AlQriqri MA, Alqahtani SH, Basalah AA, Damiati LA. Three-Dimensional Printed Porous PLA Scaffolds with Dual Functionality: Cell Proliferation Enhancement and Antibacterial Properties. Polymers. 2025; 17(14):1928. https://doi.org/10.3390/polym17141928
Chicago/Turabian StyleAlQurashi, Renad N., Noora M. Bataweel, Mehal Atallah AlQriqri, Sarah H. Alqahtani, Ahmad A. Basalah, and Laila A. Damiati. 2025. "Three-Dimensional Printed Porous PLA Scaffolds with Dual Functionality: Cell Proliferation Enhancement and Antibacterial Properties" Polymers 17, no. 14: 1928. https://doi.org/10.3390/polym17141928
APA StyleAlQurashi, R. N., Bataweel, N. M., AlQriqri, M. A., Alqahtani, S. H., Basalah, A. A., & Damiati, L. A. (2025). Three-Dimensional Printed Porous PLA Scaffolds with Dual Functionality: Cell Proliferation Enhancement and Antibacterial Properties. Polymers, 17(14), 1928. https://doi.org/10.3390/polym17141928








