Preparation and Application of Cellulose-Based Materials with Selective Adsorption of Dyes
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. The Preparation Principle and Process of Polymers
2.3. Characterization
2.4. Adsorption Performance Testing of Copolymers
2.4.1. The Influence of Dye Types on Adsorption Performance
2.4.2. The Influence of Adsorption Time on Adsorption Performance
2.4.3. The Influence of Initial Concentration of Dye Solution on Adsorption Performance
2.4.4. The Influence of Polymer Dosage on Adsorption Performance
2.4.5. The Influence of Environmental Temperature on Adsorption Performance
2.4.6. The Influence of the Acid–Base Environment on Adsorption Performance
2.5. Degradation Performance Testing of Copolymers
3. Results and Discussion
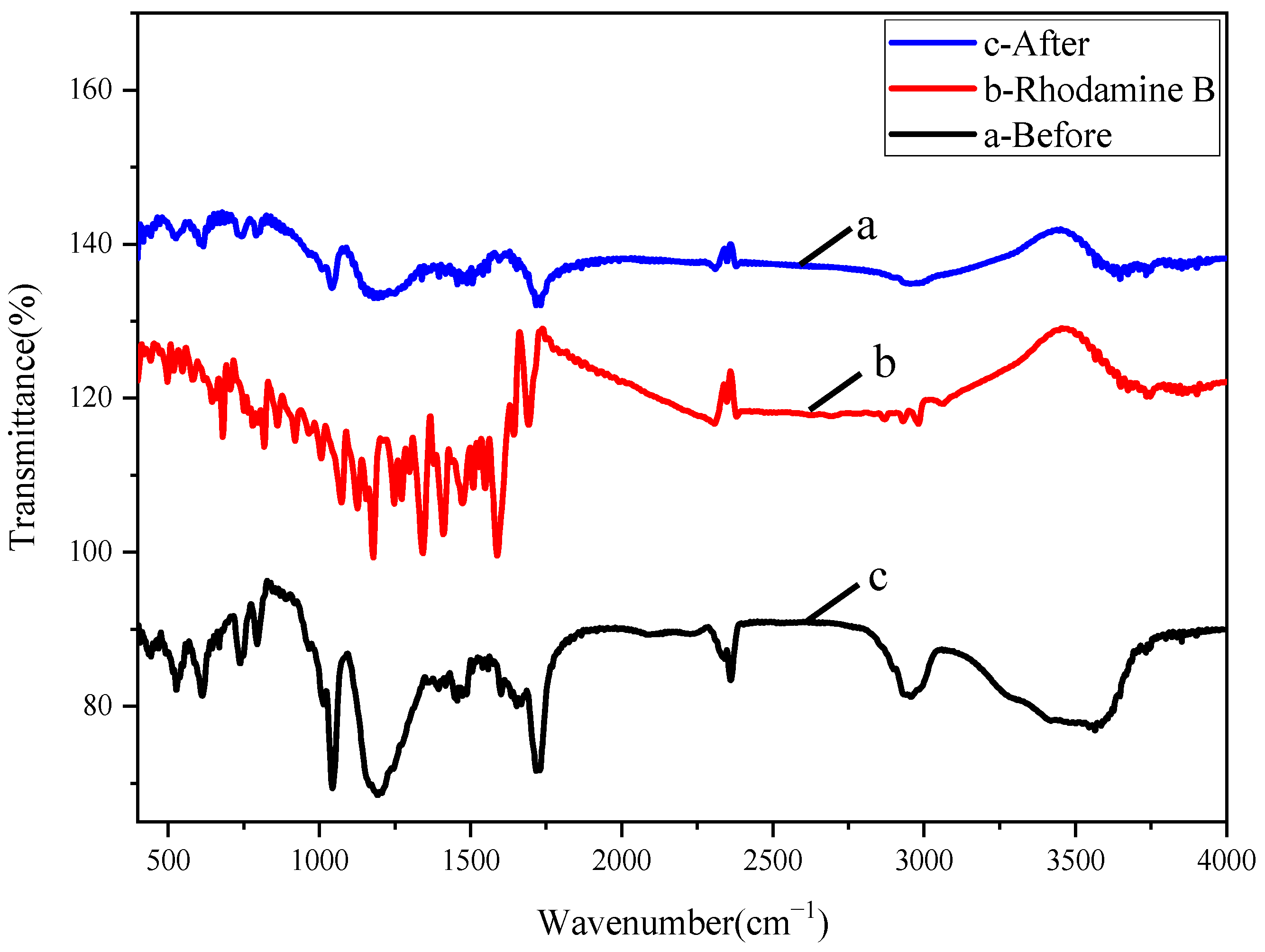
3.1. Characterizations of Structure
3.2. Thermal Properties
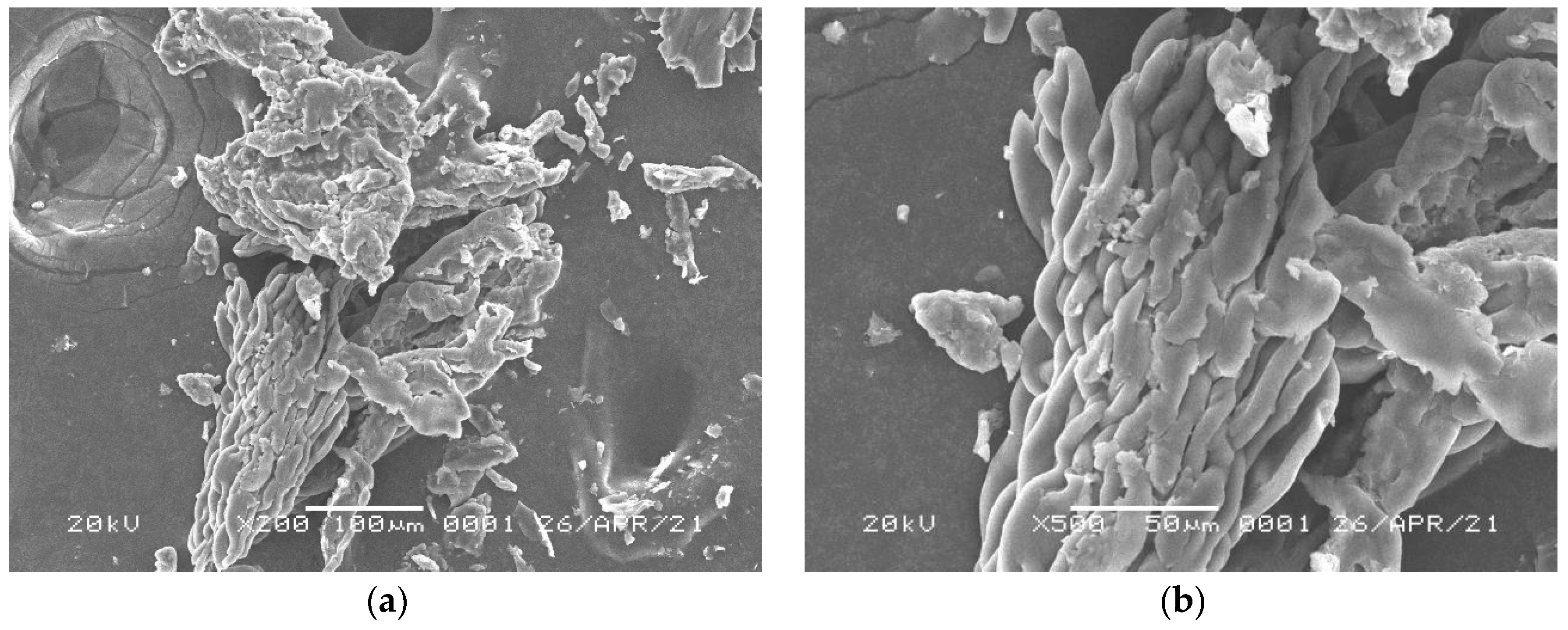
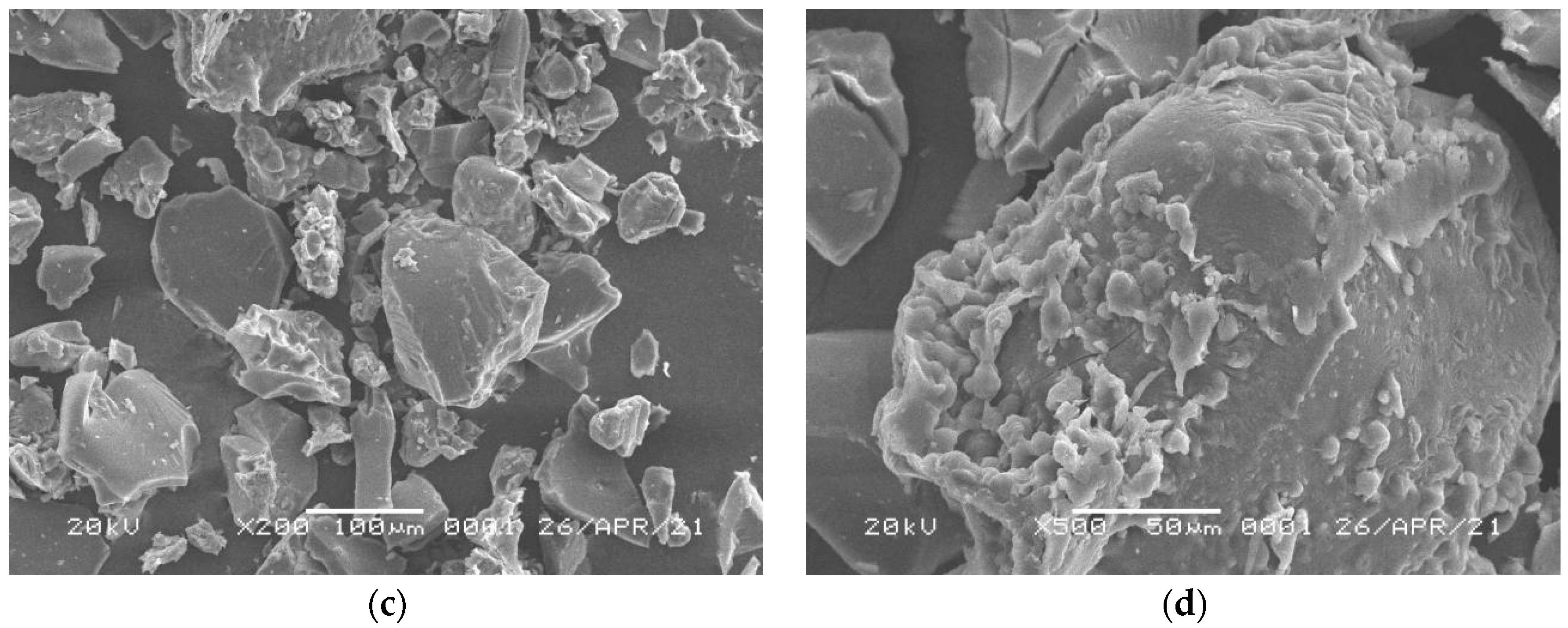
3.3. Characterizations of Surface Morphology
3.4. Adsorption Performance of Copolymers
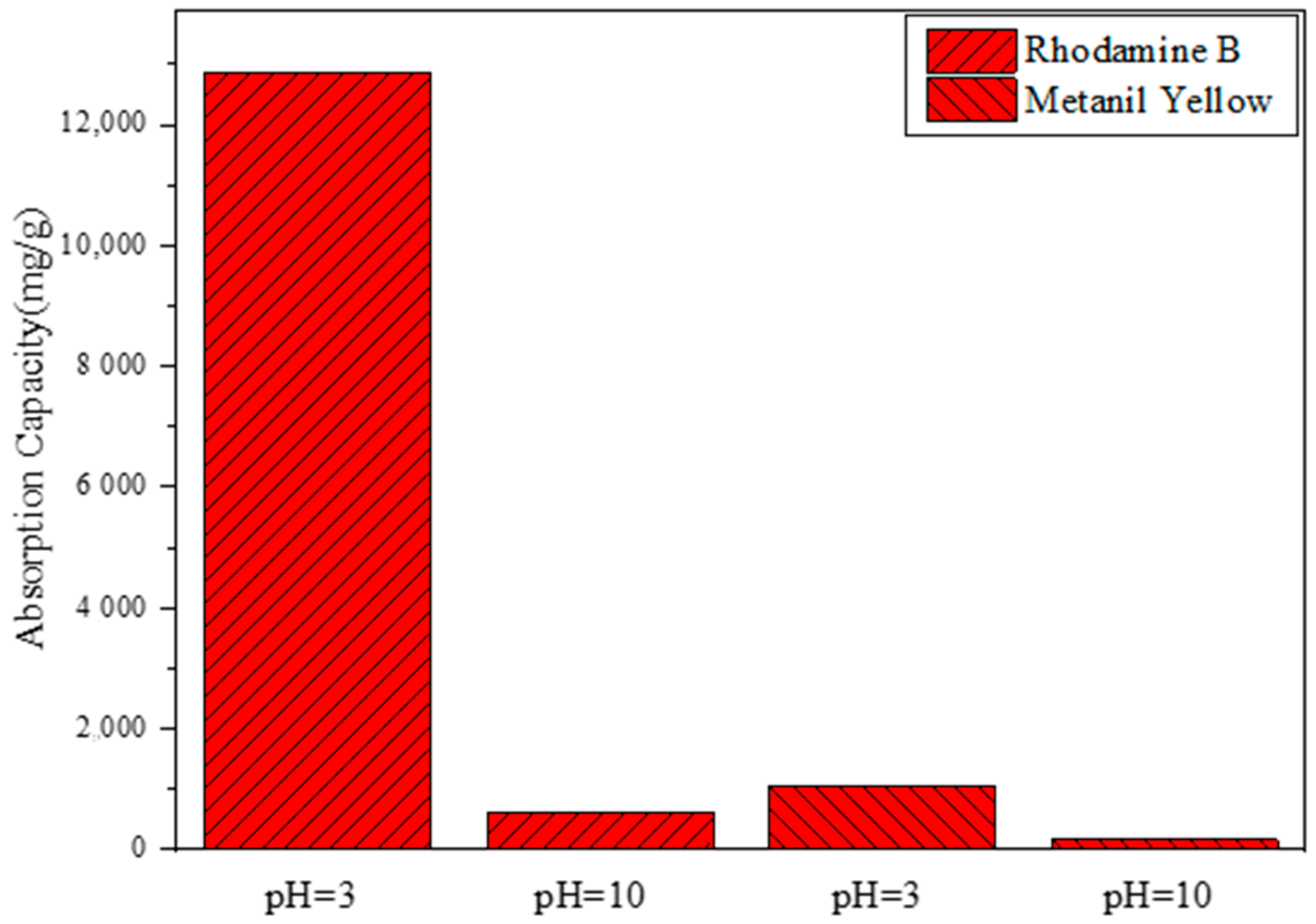
3.4.1. The Influence of Dye Types on Adsorption Performance
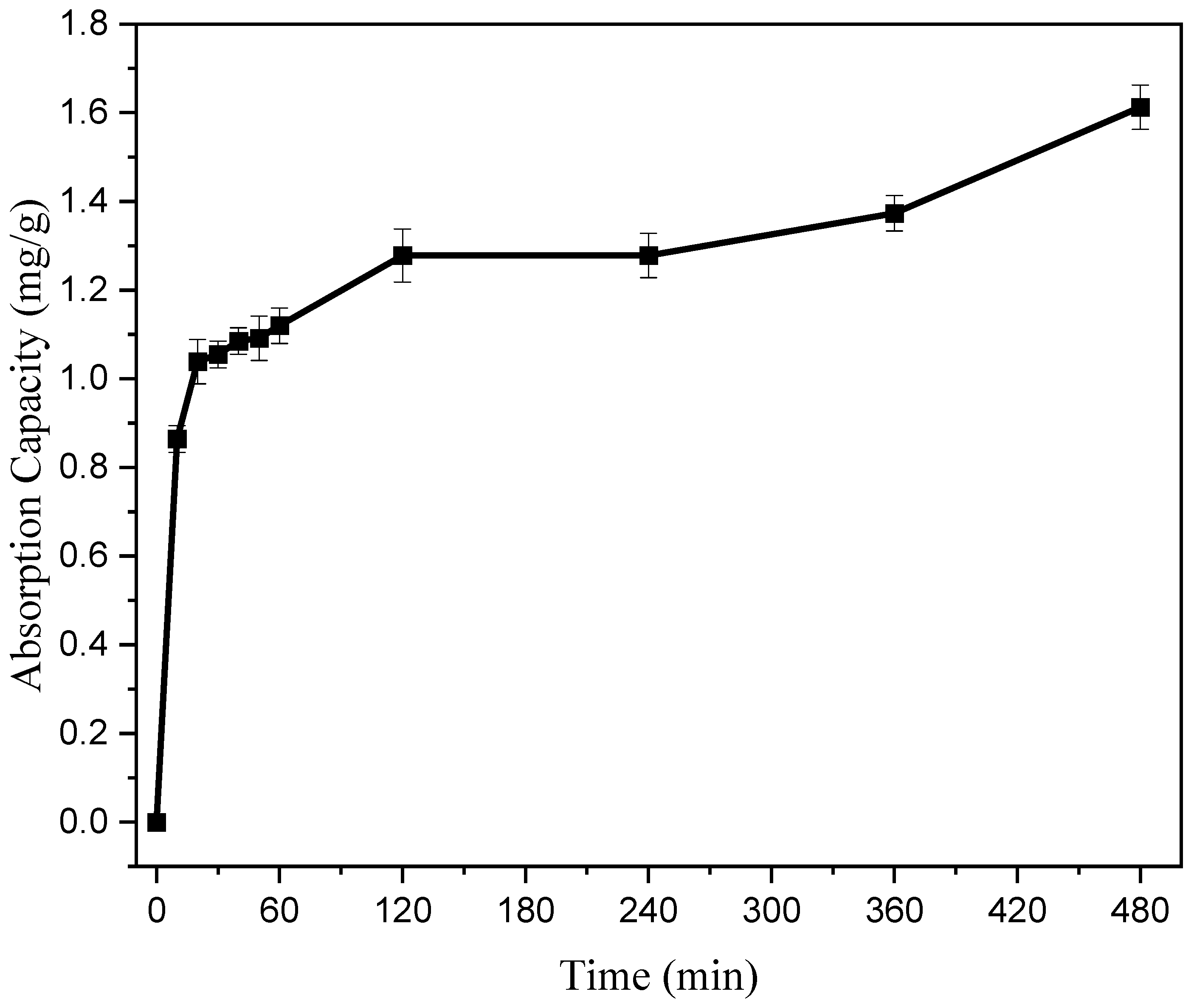
3.4.2. The Influence of Reaction Time on Adsorption Performance
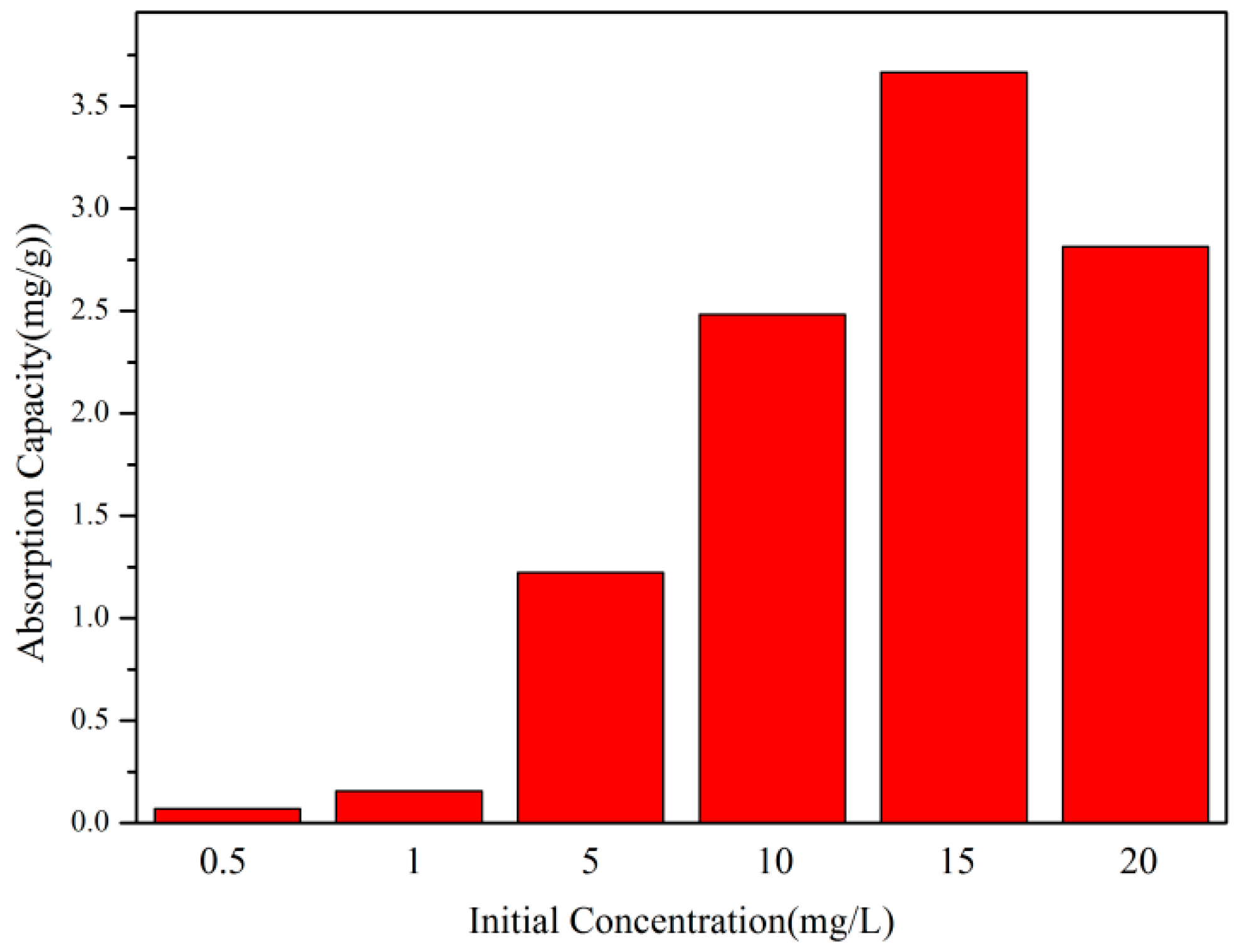
3.4.3. The Influence of the Initial Concentration of Dye Solution on Adsorption Performance
3.4.4. The Influence of Polymer Dosage on Adsorption Performance
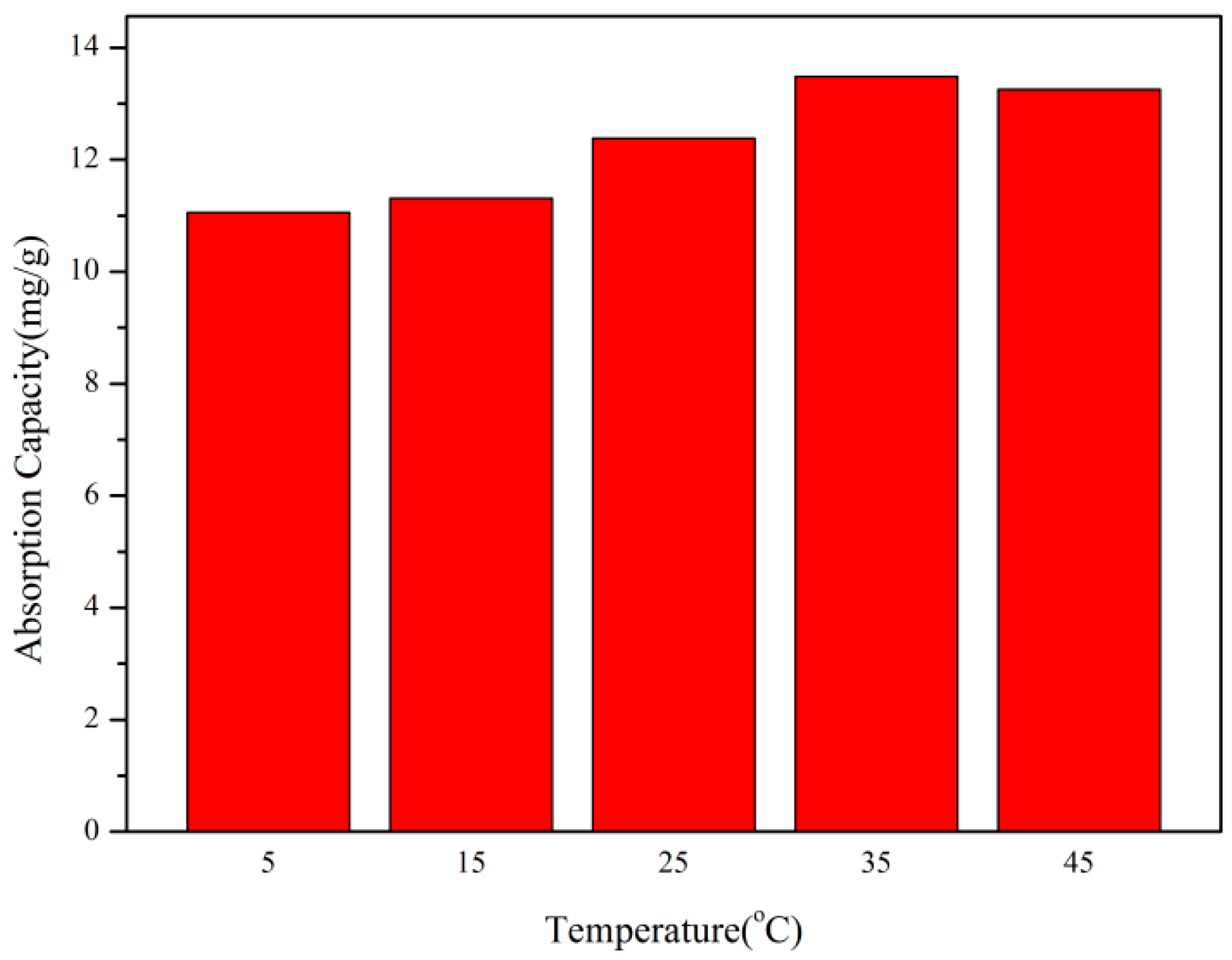
3.4.5. The Influence of Environmental Temperature on Adsorption Performance
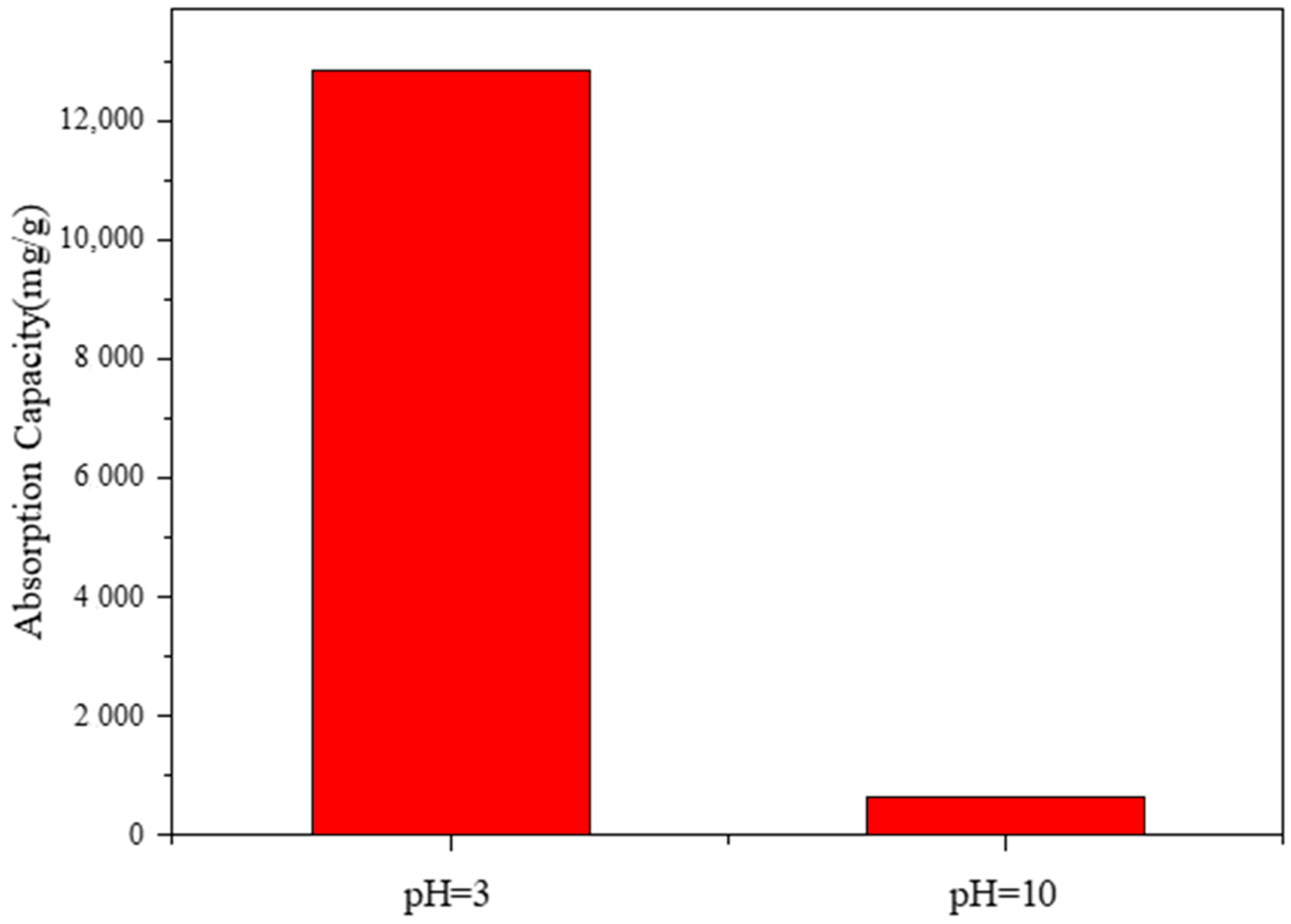
3.4.6. The Influence of the Acid–Base Environment on Adsorption Performance
3.5. Degradation Performance of Copolymers
3.6. Comparison of Various Adsorbents
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhao, R.; Wang, Y.; Li, X.; Sun, B.; Wang, C. Synthesis of Β-Cyclodextrin-Based Electrospun Nanofiber Membranes for Highly Efficient Adsorption and Separation of Methylene Blue. ACS Appl. Mater. Interfaces 2015, 7, 26649–26657. [Google Scholar] [CrossRef] [PubMed]
- Koedsiri, Y.; Amornpitoksuk, P.; Randorn, C.; Rattana, T.; Tandorn, S.; Suwanboon, S. S-Schematic Cuwo4/Zno Nanocomposite Boosted Photocatalytic Degradation of Organic Dye Pollutants. Mater. Sci. Semicond. Process. 2024, 177, 108385. [Google Scholar] [CrossRef]
- Alothman, A.A.; Ahmad, N.; Albaqami, M.D.; Alothman, Z.A.; Alqahtani, K.N.; Rizwan Khan, M. Bismuth-Doped Lanthanum Ferrite Perovskites: A Promising Approach for Enhancing Photo-Fenton Degradation of Organic Dye Pollutants. Ceram. Int. 2024, 50, 15867–15878. [Google Scholar] [CrossRef]
- Ali, N.; Khan, F.; Nawaz, A.; Khan, A.; Amin, F.; Ali, N. Visible Light-Triggered Chitosan Supported Ternary Ferrite for Efficient Removal of Organic Dye Pollutants from Wastewater. Top. Catal. 2025, 68, 759–779. [Google Scholar] [CrossRef]
- Salama, A.; Shukry, N.; El-Sakhawy, M. Carboxymethyl Cellulose-G-Poly(2-(Dimethylamino) Ethyl Methacrylate) Hydrogel as Adsorbent for Dye Removal. Int. J. Biol. Macromol. 2015, 73, 72–75. [Google Scholar] [CrossRef]
- Yao, G.; Bi, W.; Liu, H. Ph-Responsive Magnetic Graphene Oxide/Poly(Nvi-Co-Aa) Hydrogel as an Easily Recyclable Adsorbent for Cationic and Anionic Dyes. Colloids Surf. A Physicochem. Eng. Asp. 2020, 588, 124393. [Google Scholar] [CrossRef]
- Pirkarami, A.; Olya, M.E. Removal of Dye from Industrial Wastewater with an Emphasis on Improving Economic Efficiency and Degradation Mechanism. J. Saudi Chem. Soc. 2017, 21, S179–S186. [Google Scholar] [CrossRef]
- Lafi, R.; Gzara, L.; Lajimi, R.H.; Hafiane, A. Treatment of Textile Wastewater by a Hybrid Ultrafiltration/Electrodialysis Process. Chem. Eng. Process.-Process Intensif. 2018, 132, 105–113. [Google Scholar] [CrossRef]
- Yu, J.; Li, Y.; Lu, Q.; Zheng, J.; Yang, S.; Jin, F.; Wang, Q.; Yang, W. Synthesis, Characterization and Adsorption of Cationic Dyes by Cs/P(Amps-Co-Am) Hydrogel Initiated by Glow-Discharge-Electrolysis Plasma. Iran. Polym. J. 2016, 25, 423–435. [Google Scholar] [CrossRef]
- Milošević, K.; Lončarević, D.; Kalagasidis Krušić, M.; Hadnađev-Kostić, M.; Dostanić, J. Eco-Friendly G-C3n4/Carboxymethyl Cellulose/Alginate Composite Hydrogels for Simultaneous Photocatalytic Degradation of Organic Dye Pollutants. Int. J. Mol. Sci. 2024, 25, 7896. [Google Scholar] [CrossRef]
- Sarkar, N.; Sahoo, G.; Swain, S.K. Reduced Graphene Oxide Decorated Superporous Polyacrylamide Based Interpenetrating Network Hydrogel as Dye Adsorbent. Mater. Chem. Phys. 2020, 250, 123022. [Google Scholar] [CrossRef]
- Qin, J.-S.; Zhang, S.-R.; Du, D.-Y.; Shen, P.; Bao, S.-J.; Lan, Y.-Q.; Su, Z.-M. A Microporous Anionic Metal–Organic Framework for Sensing Luminescence of Lanthanide(Iii) Ions and Selective Absorption of Dyes by Ionic Exchange. Chem.-A Eur. J. 2014, 20, 5625–5630. [Google Scholar] [CrossRef]
- Zinadini, S.; Zinatizadeh, A.A.; Rahimi, M.; Vatanpour, V.; Zangeneh, H.; Beygzadeh, M. Novel High Flux Antifouling Nanofiltration Membranes for Dye Removal Containing Carboxymethyl Chitosan Coated Fe3o4 Nanoparticles. Desalination 2014, 349, 145–154. [Google Scholar] [CrossRef]
- Rekhate, C.V.; Shrivastava, J.K. Decolorization of Azo Dye Solution by Ozone Based Advanced Oxidation Processes: Optimization Using Response Surface Methodology and Neural Network. Ozone: Sci. Eng. 2020, 42, 492–506. [Google Scholar] [CrossRef]
- Ali, H. Biodegradation of Synthetic Dyes—A Review. Water Air Soil Pollut. 2010, 213, 251–273. [Google Scholar] [CrossRef]
- Punzi, M.; Anbalagan, A.; Aragão Börner, R.; Svensson, B.-M.; Jonstrup, M.; Mattiasson, B. Degradation of a Textile Azo Dye Using Biological Treatment Followed by Photo-Fenton Oxidation: Evaluation of Toxicity and Microbial Community Structure. Chem. Eng. J. 2015, 270, 290–299. [Google Scholar] [CrossRef]
- Yagub, M.T.; Sen, T.K.; Afroze, S.; Ang, H.M. Dye and Its Removal from Aqueous Solution by Adsorption: A Review. Adv. Colloid Interface Sci. 2014, 209, 172–184. [Google Scholar] [CrossRef]
- Can, O.T.; Kobya, M.; Demirbas, E.; Bayramoglu, M. Treatment of the Textile Wastewater by Combined Electrocoagulation. Chemosphere 2006, 62, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Mohan, N.; Balasubramanian, N.; Basha, C.A. Electrochemical Oxidation of Textile Wastewater and Its Reuse. J. Hazard. Mater. 2007, 147, 644–651. [Google Scholar] [CrossRef]
- Ahmed Basha, C.; Bhadrinarayana, N.S.; Anantharaman, N.; Meera Sheriffa Begum, K.M. Heavy Metal Removal from Copper Smelting Effluent Using Electrochemical Cylindrical Flow Reactor. J. Hazard. Mater. 2008, 152, 71–78. [Google Scholar] [CrossRef]
- Daneshvar, N.; Ashassi Sorkhabi, H.; Kasiri, M.B. Decolorization of Dye Solution Containing Acid Red 14 by Electrocoagulation with a Comparative Investigation of Different Electrode Connections. J. Hazard. Mater. 2004, 112, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Bayramoglu, M.; Kobya, M.; Can, O.T.; Sozbir, M. Operating Cost Analysis of Electrocoagulation of Textile Dye Wastewater. Sep. Purif. Technol. 2004, 37, 117–125. [Google Scholar] [CrossRef]
- Gu, J.; Yuan, S.; Shu, W.; Jiang, W.; Tang, S.; Liang, B.; Pehkonen, S.O. Pvbc Microspheres Tethered with Poly(3-Sulfopropyl Methacrylate) Brushes for Effective Removal of Pb(Ii) Ions from Aqueous Solution. Colloids Surf. A Physicochem. Eng. Asp. 2016, 498, 218–230. [Google Scholar] [CrossRef]
- Karim, Z.; Mathew, A.P.; Grahn, M.; Mouzon, J.; Oksman, K. Nanoporous Membranes with Cellulose Nanocrystals as Functional Entity in Chitosan: Removal of Dyes from Water. Carbohydr. Polym. 2014, 112, 668–676. [Google Scholar] [CrossRef]
- Monier, M.; Abdel-Latif, D.A. Synthesis and Characterization of Ion-Imprinted Resin Based on Carboxymethyl Cellulose for Selective Removal of Uo22+. Carbohydr. Polym. 2013, 97, 743–752. [Google Scholar] [CrossRef] [PubMed]
- Germani, R.; Mancinelli, M.; Roselli, A.; Tiecco, M.; Fantacci, S.; di Bona, S.; Del Giacco, T. Kinetic Effects of Cationic Surfactants on the Photocatalytic Degradation of Anionic Dyes in Aqueous Tio2 Dispersions. New J. Chem. 2023, 47, 392–401. [Google Scholar] [CrossRef]
- Salama, A. Preparation of Cmc-G-P(Spma) Super Adsorbent Hydrogels: Exploring Their Capacity for Mb Removal from Waste Water. Int. J. Biol. Macromol. 2018, 106, 940–946. [Google Scholar] [CrossRef]
- Dong, C.; Zhang, F.; Pang, Z.; Yang, G. Efficient and Selective Adsorption of Multi-Metal Ions Using Sulfonated Cellulose as Adsorbent. Carbohydr. Polym. 2016, 151, 230–236. [Google Scholar] [CrossRef]
- Halouane, F.; Oz, Y.; Meziane, D.; Barras, A.; Juraszek, J.; Singh, S.K.; Kurungot, S.; Shaw, P.K.; Sanyal, R.; Boukherroub, R.; et al. Magnetic Reduced Graphene Oxide Loaded Hydrogels: Highly Versatile and Efficient Adsorbents for Dyes and Selective Cr(Vi) Ions Removal. J. Colloid Interface Sci. 2017, 507, 360–369. [Google Scholar] [CrossRef]
- Li, Y.; Yue, Q.-Y.; Gao, B.-Y. Effect of Humic Acid on the Cr(Vi) Adsorption onto Kaolin. Appl. Clay Sci. 2010, 48, 481–484. [Google Scholar] [CrossRef]
- Tiwari, J.N.; Mahesh, K.; Le, N.H.; Kemp, K.C.; Timilsina, R.; Tiwari, R.N.; Kim, K.S. Reduced Graphene Oxide-Based Hydrogels for the Efficient Capture of Dye Pollutants from Aqueous Solutions. Carbon 2013, 56, 173–182. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, S.; Ding, D.; Chen, J.; Yang, Y.; Lei, Z.; Feng, C.; Zhang, Z. Effective Adsorption of Cr (Vi) from Aqueous Solution Using Natural Akadama Clay. J. Colloid Interface Sci. 2013, 395, 198–204. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, L.; Chen, Y.; Ma, H.; Meng, X. Preparation and Application of Cellulose-Based Materials with Selective Adsorption of Dyes. Polymers 2025, 17, 1653. https://doi.org/10.3390/polym17121653
Bai L, Chen Y, Ma H, Meng X. Preparation and Application of Cellulose-Based Materials with Selective Adsorption of Dyes. Polymers. 2025; 17(12):1653. https://doi.org/10.3390/polym17121653
Chicago/Turabian StyleBai, Linlin, Yuxing Chen, Huiting Ma, and Xu Meng. 2025. "Preparation and Application of Cellulose-Based Materials with Selective Adsorption of Dyes" Polymers 17, no. 12: 1653. https://doi.org/10.3390/polym17121653
APA StyleBai, L., Chen, Y., Ma, H., & Meng, X. (2025). Preparation and Application of Cellulose-Based Materials with Selective Adsorption of Dyes. Polymers, 17(12), 1653. https://doi.org/10.3390/polym17121653





