Glycopolypeptoids as Novel Biomimetic Antifreeze Agents: Structural Design, Synthesis, and Antifreeze Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
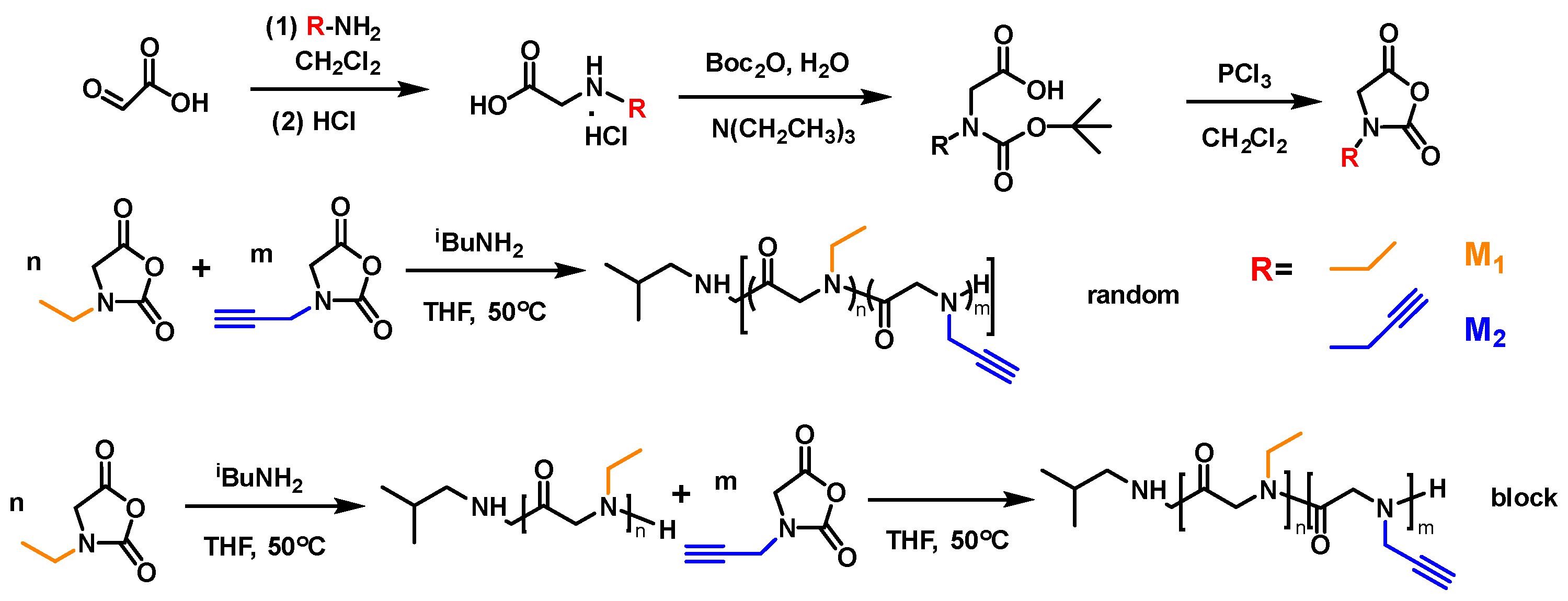
2.2. Synthesis of Polypeptoids
2.2.1. Synthesis of Ethyl-NCA (M1)
2.2.2. Synthesis of Propargyl-NCA (M2)
2.2.3. Synthetic Procedure for the Poly[(N-ethyl glycine)-r-(N-propargyl glycine)] Random Copolymer PNEG-r-PNPG
2.2.4. Synthetic Procedure for the Poly[(N-ethyl glycine)-b-(N-propargyl glycine)] Block Copolymer PNEG-b-PNPG
2.2.5. Synthetic Procedure for the Glycopolypeptoids
2.3. Characterization of Polypeptoids and Glycopolypeptoids
2.4. Characterization of Antifreeze Activity
2.4.1. Nanoliter Osmometry Experiment
2.4.2. Ice Recrystallization Inhibition (IRI) Experiment
2.5. Cytotoxicity Test
3. Results and Discussion
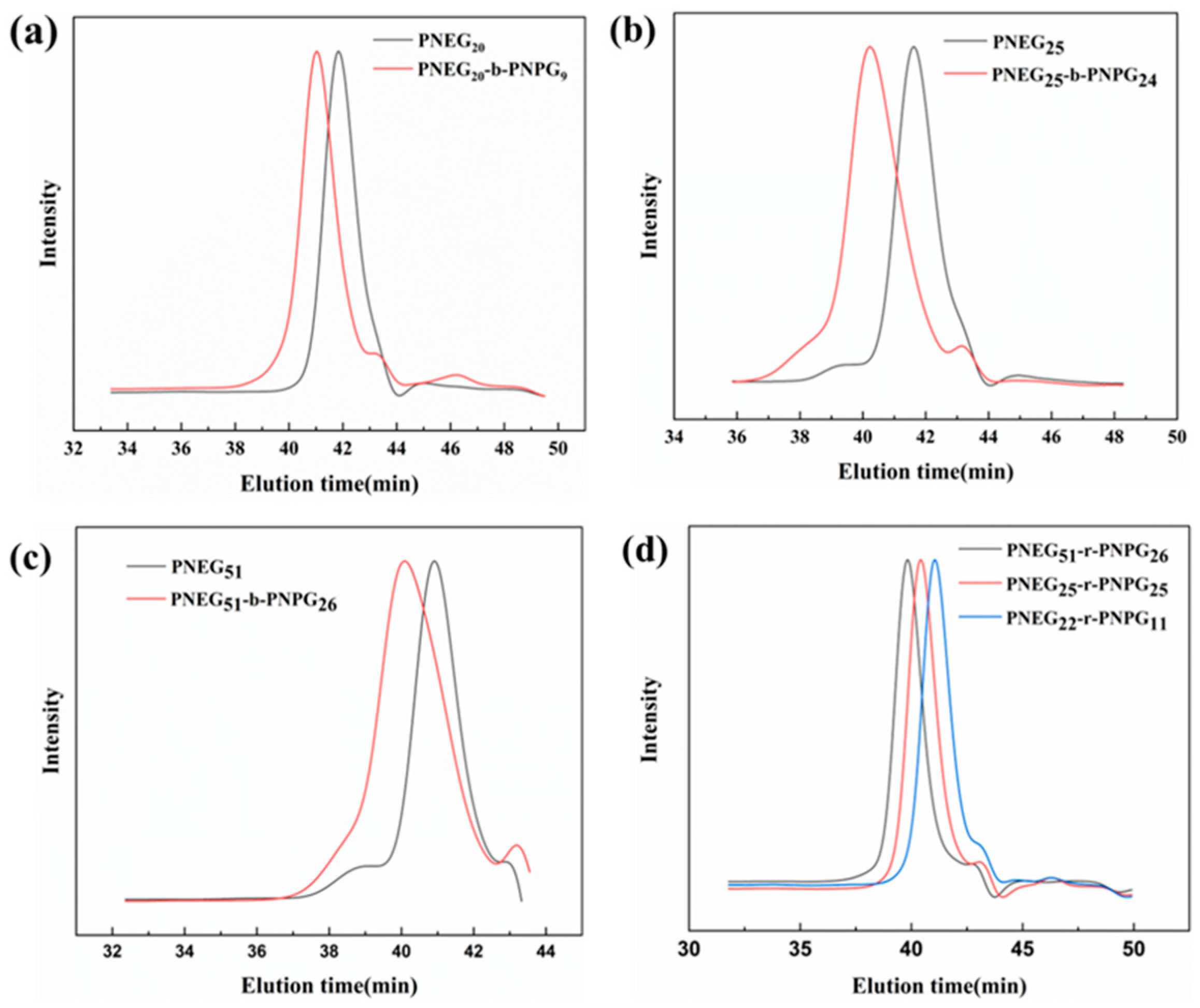
3.1. Results of the Characterization of Polypeptoids and Glycopolypeptoids
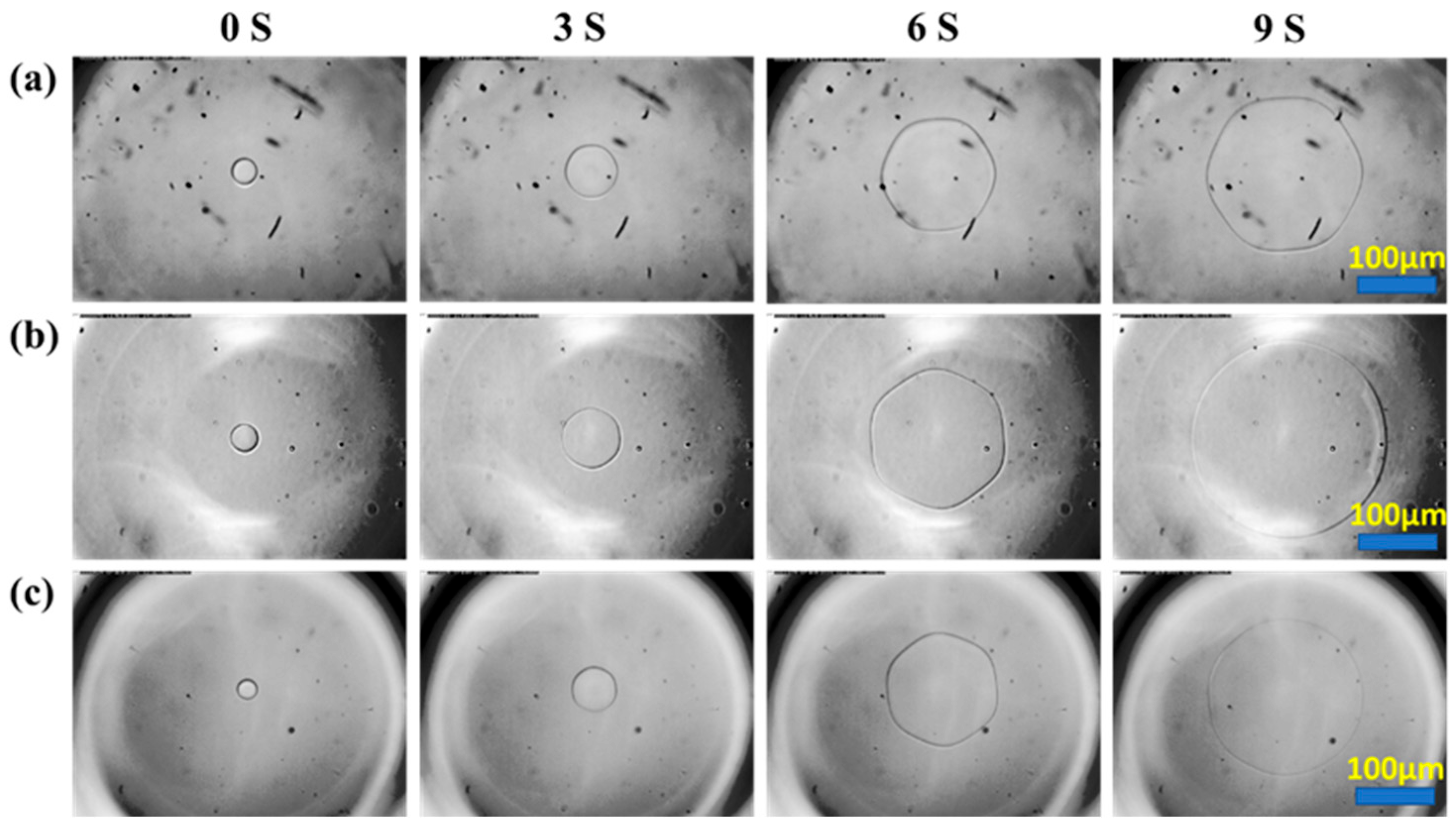
3.2. Effect of Glycopolypeptoids on Ice Crystal Morphology
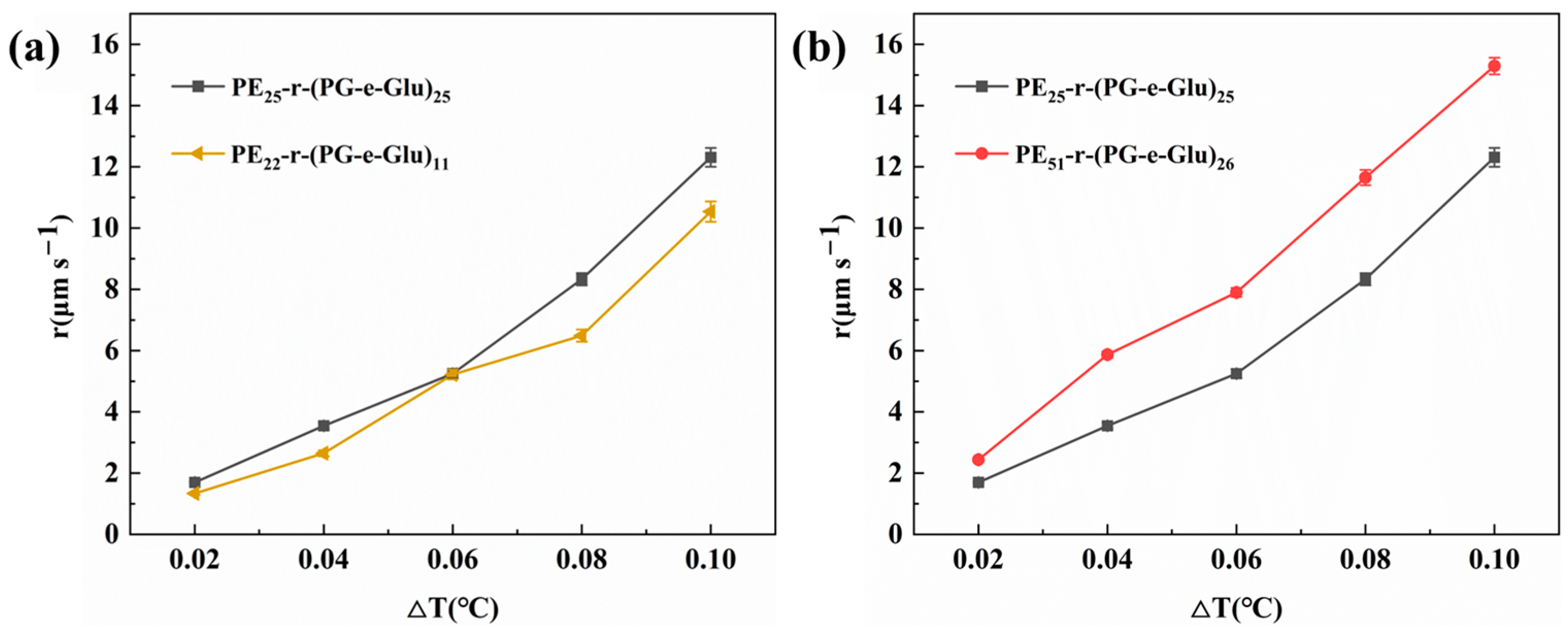
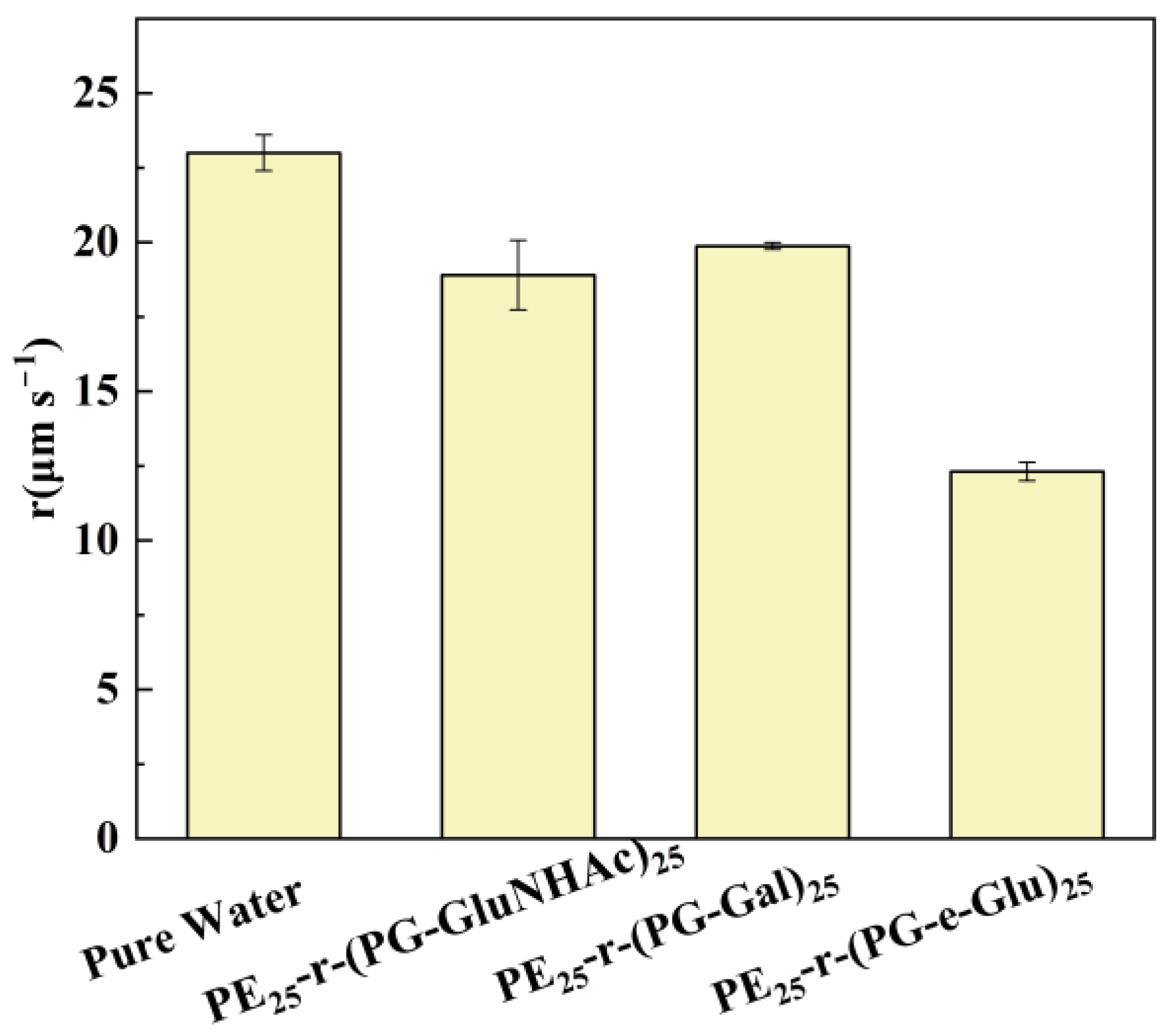
3.3. Effect of Glycopolypeptoids on Ice Crystal Growth Rate
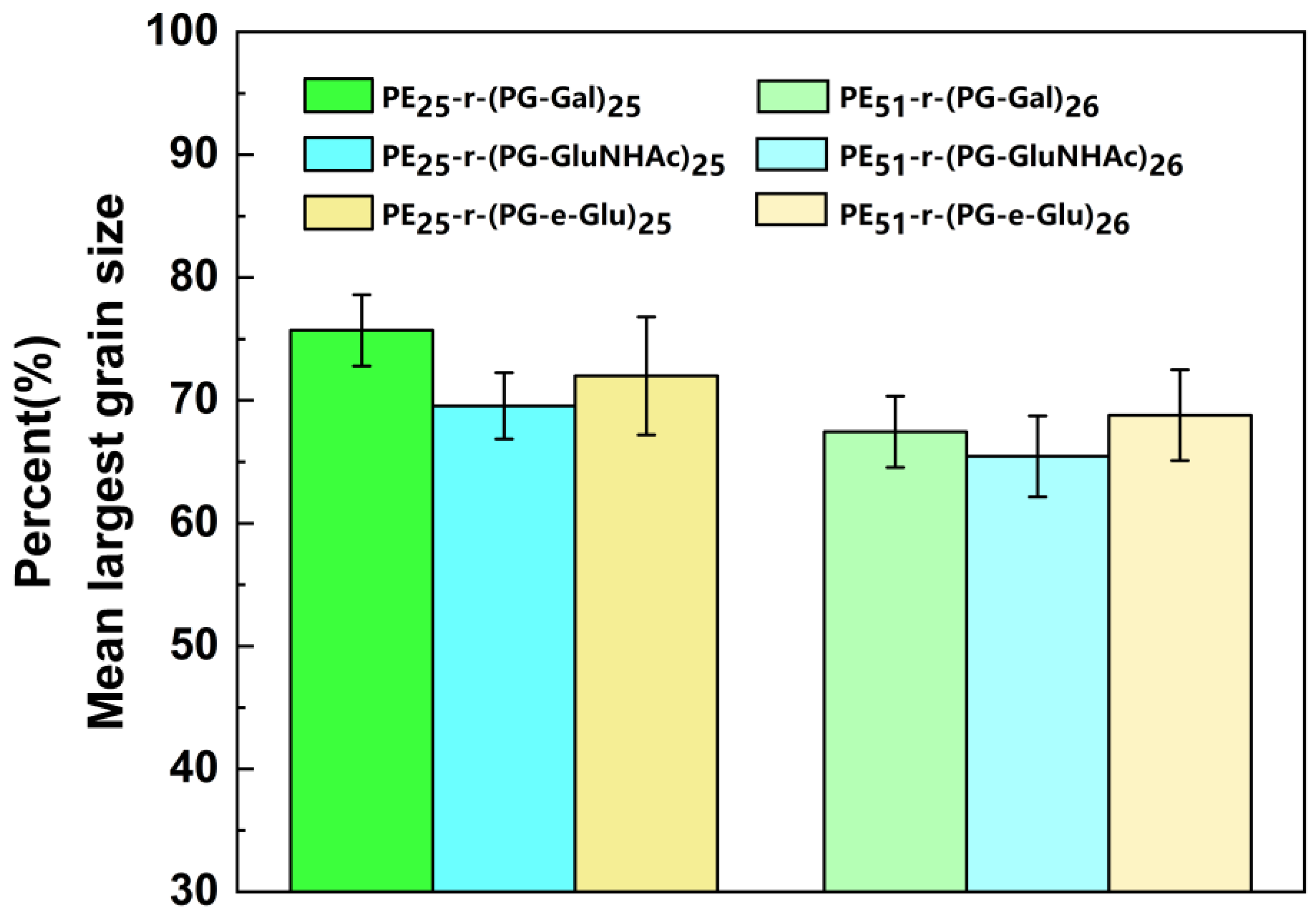
3.4. Effect of Glycopolypeptoids on Ice Recrystallization Inhibition (IRI) Activity
3.5. Cytotoxicity Tests of Glycopolypeptoids
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, L.; He, M.; Wu, T.; Yang, K.; Wang, Y.; Zhang, Y.; Gu, Y.; Deng, K. Screening of the freeze-drying protective agent for high-quality milk beer yeast (Kluyveromyces marxianus) and optimization of freeze-drying process conditions. J. Food Process. Preserv. 2021, 45, e16016. [Google Scholar] [CrossRef]
- Bao, Y.; Ertbjerg, P.; Estévez, M.; Yuan, L.; Gao, R. Freezing of meat and aquatic food: Underlying mechanisms and implications on protein oxidation. Compr. Rev. Food Sci. Food Saf. 2021, 20, 5548–5569. [Google Scholar] [CrossRef]
- Zhou, H.; Song, M.; Zhang, X.; Ke, T.; Shi, G.; Wu, Y.; Geng, H. Mechanism Unraveling of Scalable Antifreeze Oligopeptides for Enhanced Cryopreservation. Langmuir 2025, 41, 9532–9541. [Google Scholar] [CrossRef]
- Yeh, Y.; Feeney, R.E. Antifreeze proteins: Structures and mechanisms of function. Chem. Rev. 1996, 96, 601–618. [Google Scholar] [CrossRef]
- Mangiagalli, M.; Bar-Dolev, M.; Tedesco, P.; Natalello, A.; Kaleda, A.; Brocca, S.; Pascale, D.; Pucciarelli, S.; Miceli, C.; Braslavsky, I.; et al. Cryo-protective effect of an ice-binding protein derived from Antarctic bacteria. FEBS J. 2017, 284, 163–177. [Google Scholar] [CrossRef]
- Surís-Valls, R.; Voets, I.K. Peptidic antifreeze materials: Prospects and challenges. Int. J. Mol. Sci. 2019, 20, 5149. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Qi, H.; Zhang, L. Advances in Antifreeze Molecules: From Design and Mechanisms to Applications. Ind. Eng. Chem. Res. Rep. Exp. 2023, 62, 7839–7858. [Google Scholar] [CrossRef]
- Eickhoff, L.; Dreischmeier, K.; Zipori, A.; Sirotinskaya, V.; Adar, C.; Reicher, N.; Braslavsky, I.; Rudich, Y.; Koop, T. Contrasting behavior of antifreeze proteins: Ice growth inhibitors and ice nucleation promoters. J. Phys. Chem. Lett. 2019, 10, 966–972. [Google Scholar] [CrossRef]
- Rahman, A.T.; Arai, T.; Yamauchi, A.; Miura, A.; Kondo, H.; Ohyama, Y.; Tsuda, S. Ice recrystallization is strongly inhibited when antifreeze proteins bind to multiple ice planes. Sci. Rep. 2019, 9, 2212. [Google Scholar] [CrossRef]
- Surís-Valls, R.; Voets, I.K. The impact of salts on the ice recrystallization inhibition activity of antifreeze (glyco)proteins. Biomolecules 2019, 9, 347. [Google Scholar] [CrossRef]
- Perez, A.E.; Taing, K.R.; Quon, J.C.; Flores, A.; Ba, Y. Effect of type I antifreeze proteins on the freezing and melting processes of cryoprotective solutions studied by site-directed spin labeling technique. Crystals 2019, 9, 352. [Google Scholar] [CrossRef] [PubMed]
- Bar Dolev, M.; Bernheim, R.; Guo, S.Q.; Davies, P.L.; Braslavsky, I. Putting life on ice: Bacteria that bind to frozen water. J. R. Soc. Interface 2016, 13, 20160210. [Google Scholar] [CrossRef]
- Raymond, J.A. Algal ice-binding proteins change the structure of sea ice. Proc. Natl. Acad. Sci. USA 2011, 108, E198. [Google Scholar] [CrossRef] [PubMed]
- Ben, R.N.; Eniade, A.A.; Hauer, L. Synthesis of a C-linked antifreeze glycoprotein (AFGP) mimic: Probes for investigating the mechanism of action. Org. Lett. 1999, 1, 1759–1762. [Google Scholar] [CrossRef]
- Capicciotti, C.J.; Trant, J.F.; Leclère, M.; Ben, R.N. Synthesis of C-linked triazole-containing AFGP analogues and their ability to inhibit ice recrystallization. Bioconjugate Chem. 2011, 22, 605–616. [Google Scholar] [CrossRef]
- Sumii, Y.; Hibino, H.; Saidalimu, I.; Kawahara, H.; Shibata, N. Design and synthesis of galactose-conjugated fluorinated and non-fluorinated proline oligomers: Towards antifreeze molecules. Chem. Commun. 2018, 54, 9749–9752. [Google Scholar] [CrossRef] [PubMed]
- Qin, Q.; Zhao, L.; Liu, Z.; Liu, T.; Qu, J.; Zhang, X. Addition to “bioinspired l-proline oligomers for the cryopreservation of oocytes via controlling ice growth”. ACS Appl. Mater. Interfaces 2020, 12, 56661. [Google Scholar] [CrossRef]
- Anna, C.D.; Simranpreet, S.S.; Alexander, C.W. Synthetic antifreeze glycoproteins with potent ice-binding activity. Chem. Mater. 2024, 36, 3424–3434. [Google Scholar]
- Zuckermann, R.N. Peptoid origins. Biopolymers 2011, 96, 545–555. [Google Scholar] [CrossRef]
- Baldauf, C.; Günther, R.; Hofmann, H.J. Helices in peptoids of α-and β-peptides. Phys. Biol. 2006, 3, S1–S9. [Google Scholar] [CrossRef]
- Chan, B.A.; Xuan, S.T.; Li, A.; Simpson, J.M.; Sternhagen, G.L.; Yu, T.Y.; Darvish, O.A.; Jiang, N.S.; Zhang, D.H. Polypeptoid polymers: Synthesis, characterization, and properties. Biopolymers 2018, 109, e23070. [Google Scholar] [CrossRef] [PubMed]
- Burkoth, T.S.; Beausoleil, E.; Kaur, S.; Tang, D.Z.; Cohen, F.E.; Zuckermann, R.N. Toward the synthesis of artificial proteins: The discovery of an amphiphilic helical peptoid assembly. Chem. Biol. 2002, 9, 647–654. [Google Scholar] [CrossRef]
- Li, Z.; Cai, B.; Yang, W.; Chen, C. Hierarchical Nanomaterials Assembled from Peptoids and Other Sequence-Defined Synthetic Polymers. Chem. Rev. 2021, 121, 14031–14087. [Google Scholar] [CrossRef]
- Zhang, D.; Lahasky, S.H.; Guo, L.; Lee, C.U.; Lavan, M. Polypeptoid materials: Current status and future perspectives. Macromolecules 2012, 45, 5833–5841. [Google Scholar] [CrossRef]
- Zhu, L.; Simpson, J.M.; Xu, X.; He, H.; Zhang, D.; Yin, L. Cationic polypeptoids with optimized molecular characteristics toward efficient nonviral gene delivery. ACS Appl. Mater. Interfaces 2017, 9, 23476–23486. [Google Scholar] [CrossRef] [PubMed]
- Herlan, C.; Feser, D.; Schepers, U.; Bräse, S. Bio-instructive materials on-demand—Combinatorial chemistry of peptoids, foldamers, and beyond. Chem. Commun. 2021, 57, 11131–11152. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Ehre, D.; Jiang, Q.; Hu, C.; Kirshenbaum, K.; Ward, M.D. Biomimetic peptoid oligomers as dual-action antifreeze agents. Proc. Natl. Acad. Sci. USA 2012, 109, 19922–19927. [Google Scholar] [CrossRef]
- Yang, W.; Yin, Q.; Chen, C. Designing Sequence-Defined Peptoids for Biomimetic Control over Inorganic Crystallization. Chem. Mater. 2021, 33, 3047–3065. [Google Scholar] [CrossRef]
- Hua, W.; Wang, Y.; Guo, C.; Wang, J.; Li, S.; Guo, L. Ice recrystallization inhibition activity of protein mimetic peptoids. J. Inorg. Organomet. Polym. Mater. 2020, 31, 203–208. [Google Scholar] [CrossRef]
- Zhang, M.; Qiu, Z.; Yang, K.; Zhou, W.; Liu, W.; Lu, J.; Guo, L. Design, synthesis and antifreeze properties of biomimetic peptoid oligomers. Chem. Commun. 2023, 59, 7028–7031. [Google Scholar] [CrossRef]
- Yang, K.; Liu, D.; Feng, L.; Xu, L.; Jiang, Y.; Shen, X.; Ali, A.; Lu, J.; Guo, L. Preparation of peptoid antifreeze agents and their structure–property relationship. Polymers 2024, 16, 990. [Google Scholar] [CrossRef] [PubMed]
- Ampaw, A.; Charlton, T.A.; Briard, J.G.; Ben, R.N. Designing the next generation of cryoprotectants-from proteins to small molecules. Pept. Sci. 2019, 111, e24086. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, Y.; Zheng, X.; Jin, S.; Liu, S.; He, Z.; Xiang, J.; Wang, J. Bioinspired crowding inhibits explosive ice growth in antifreeze protein solutions. Biomacromolecules 2021, 22, 2614–2624. [Google Scholar] [CrossRef] [PubMed]
- Gibson, M.I. Slowing the growth of ice with synthetic macromolecules: Beyond antifreeze(glyco) proteins. Polym. Chem. 2010, 1, 1141–1152. [Google Scholar] [CrossRef]
- Budke, C.; Heggemann, C.; Koch, M.; Sewald, N.; Koop, T. Ice recrystallization kinetics in the presence of synthetic antifreeze glycoprotein analogues using the framework of LSW theory. J. Phys. Chem. B 2009, 113, 2865–2873. [Google Scholar] [CrossRef]
- Biggs, C.I.; Stubbs, C.; Graham, B.; Fayter, A.E.R.; Hasan, M.; Gibson, M.I. Mimicking the ice recrystallization activity of biological antifreezes. When is a new polymer “Active”? Macromol. Biosci. 2019, 19, 1900082. [Google Scholar] [CrossRef]
- Knight, C.A.; Wen, D.; Laursen, R.A. Nonequilibrium antifreeze peptides and the recrystallization of ice. Cryobiology 1995, 32, 23–34. [Google Scholar] [CrossRef]
- Graham, L.A.; Agrawal, P.; Oleschuk, R.D.; Davies, P.L. High-capacity ice-recrystallization endpoint assay employing superhydrophobic coatings that is equivalent to the ‘splat’ assay. Cryobiology 2018, 81, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Shi, K.; Yu, K.; Xing, F.; Lai, H.; Zhou, Y.; Xiao, P. Degradable hydrogel adhesives with enhanced tissue adhesion, superior self-healing, cytocompatibility, and antibacterial property. Adv. Healthc. Mater. 2022, 11, 2101504. [Google Scholar] [CrossRef]
- Mo, X.; Wu, F.; Yu, B.; Wang, W.; Cai, X. Folate-PG modified halloysite nanotube for enhancing tumor targeting and anticancer efficacy. Appl. Clay Sci. 2020, 193, 105664. [Google Scholar] [CrossRef]
- Tao, Y.; Wang, S.; Zhang, X.; Wang, Z.; Tao, Y.; Wang, X. Synthesis and properties of alternating polypeptoids and polyampholytes as protein-resistant polymers. Biomacromolecules 2018, 19, 936–942. [Google Scholar] [CrossRef] [PubMed]
- Robinson, J.W.; Secker, C.; Weidner, S.; Schlaad, H. Thermoresponsive poly(N-C3 glycine)s. Macromolecules 2013, 46, 580–587. [Google Scholar] [CrossRef]
- Carpenter, J.F.; Hansen, T.N. Antifreeze protein modulates cell survival during cryopreservation: Mediation through influence on ice crystal growth. Proc. Natl. Acad. Sci. USA 1992, 89, 8953–8957. [Google Scholar] [CrossRef]
- Matsumoto, S.; Matsusita, M.; Morita, T.; Kamachi, H.; Tsukiyama, S.; Furukawa, Y.; Koshida, S.; Tachibana, Y.; Nishimura, S.; Todo, S. Effects of synthetic antifreeze glycoprotein analogue on islet cell survival and function during cryopreservation. Cryobiology 2006, 52, 90–98. [Google Scholar] [CrossRef]
- Dirain, C.O.; Karnani, D.N.; Antonelli, P.J. Cytotoxicity of ear drop excipients in human and mouse tympanic membrane fibroblasts. Otolaryngol.–Head Neck Surg. 2019, 162, 204–210. [Google Scholar] [CrossRef]








| Polymer | [M1]0:[M2]0:[I]0 a | Mn (NMR) (kg mol−1) b | Mn (GPC) (kg mol−1) c | PDI c | Yield |
|---|---|---|---|---|---|
| PNEG20-b-PNPG9 | 20:10:01 | 2.6 | 35.9 | 1.06 | 80% |
| PNEG25-b-PNPG24 | 25:25:01 | 4.4 | 48.1 | 1.09 | 85% |
| PNEG51-b-PNPG22 | 50:25:01 | 6.5 | 55 | 1.1 | 81% |
| PNEG22-r-PNPG11 | 20:10:01 | 3 | 35.5 | 1.05 | 86% |
| PNEG25-r-PNPG25 | 25:25:01 | 4.5 | 44.8 | 1.05 | 80% |
| PNEG51-r-PNPG26 | 50:25:01 | 6.9 | 55.8 | 1.04 | 88% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, L.; Pi, J.; Feng, L.; Wen, J.; Zhao, M.; Ali, A.; Lu, J.; Guo, L. Glycopolypeptoids as Novel Biomimetic Antifreeze Agents: Structural Design, Synthesis, and Antifreeze Properties. Polymers 2025, 17, 1600. https://doi.org/10.3390/polym17121600
Xu L, Pi J, Feng L, Wen J, Zhao M, Ali A, Lu J, Guo L. Glycopolypeptoids as Novel Biomimetic Antifreeze Agents: Structural Design, Synthesis, and Antifreeze Properties. Polymers. 2025; 17(12):1600. https://doi.org/10.3390/polym17121600
Chicago/Turabian StyleXu, Liugen, Junwei Pi, Lei Feng, Junhao Wen, Minghai Zhao, Amjad Ali, Jianwei Lu, and Li Guo. 2025. "Glycopolypeptoids as Novel Biomimetic Antifreeze Agents: Structural Design, Synthesis, and Antifreeze Properties" Polymers 17, no. 12: 1600. https://doi.org/10.3390/polym17121600
APA StyleXu, L., Pi, J., Feng, L., Wen, J., Zhao, M., Ali, A., Lu, J., & Guo, L. (2025). Glycopolypeptoids as Novel Biomimetic Antifreeze Agents: Structural Design, Synthesis, and Antifreeze Properties. Polymers, 17(12), 1600. https://doi.org/10.3390/polym17121600









