Rare Earth Fluorescent Composite Hydrogel with Controllable Color Photoluminescence for Information Encryption
Abstract
1. Introduction
2. Materials and Methods
2.1. Material Preparation
2.2. Preparation of PVA/PEG/Ln Hydrogel
2.3. Characterization
3. Results and Discussion
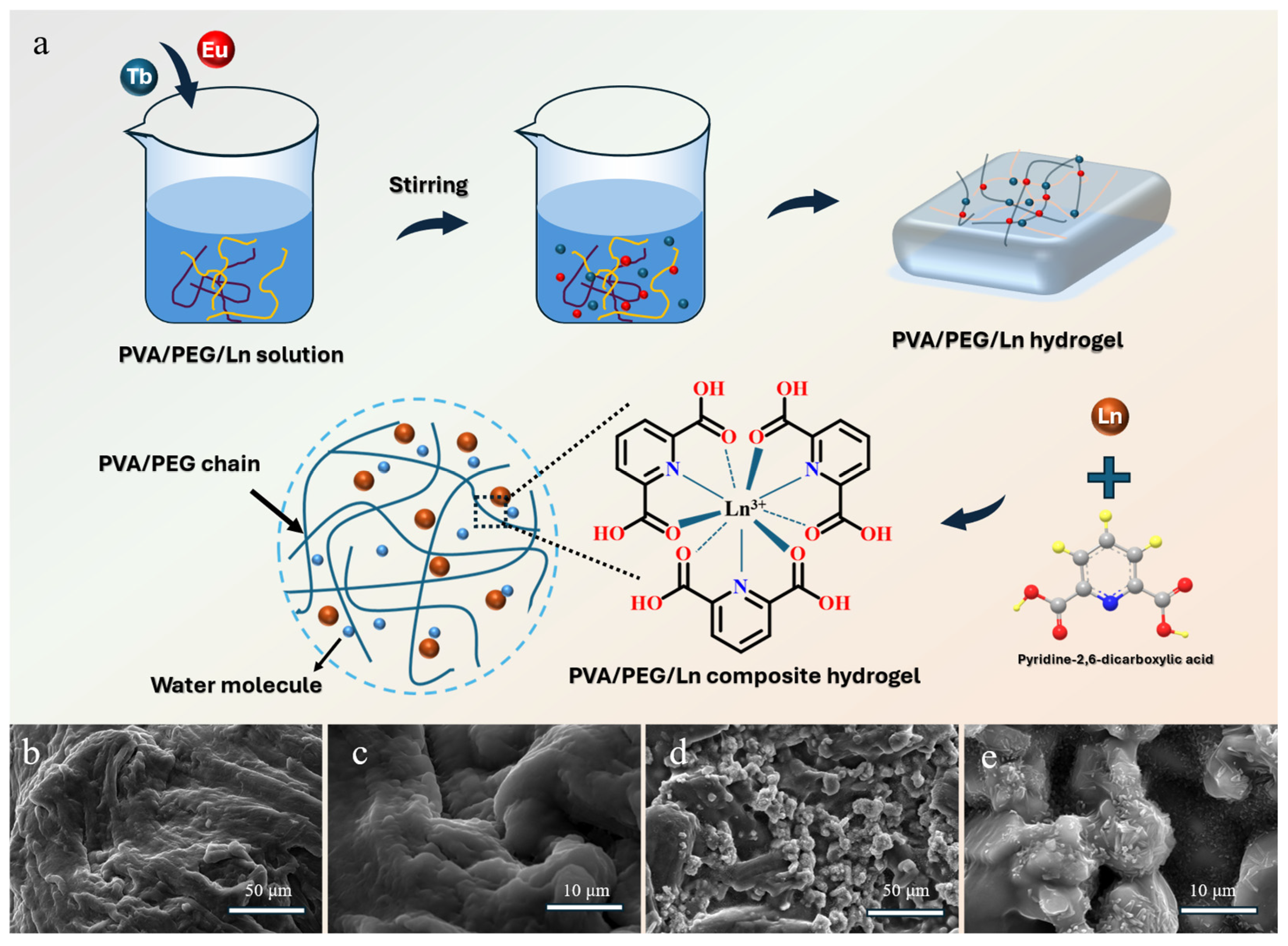
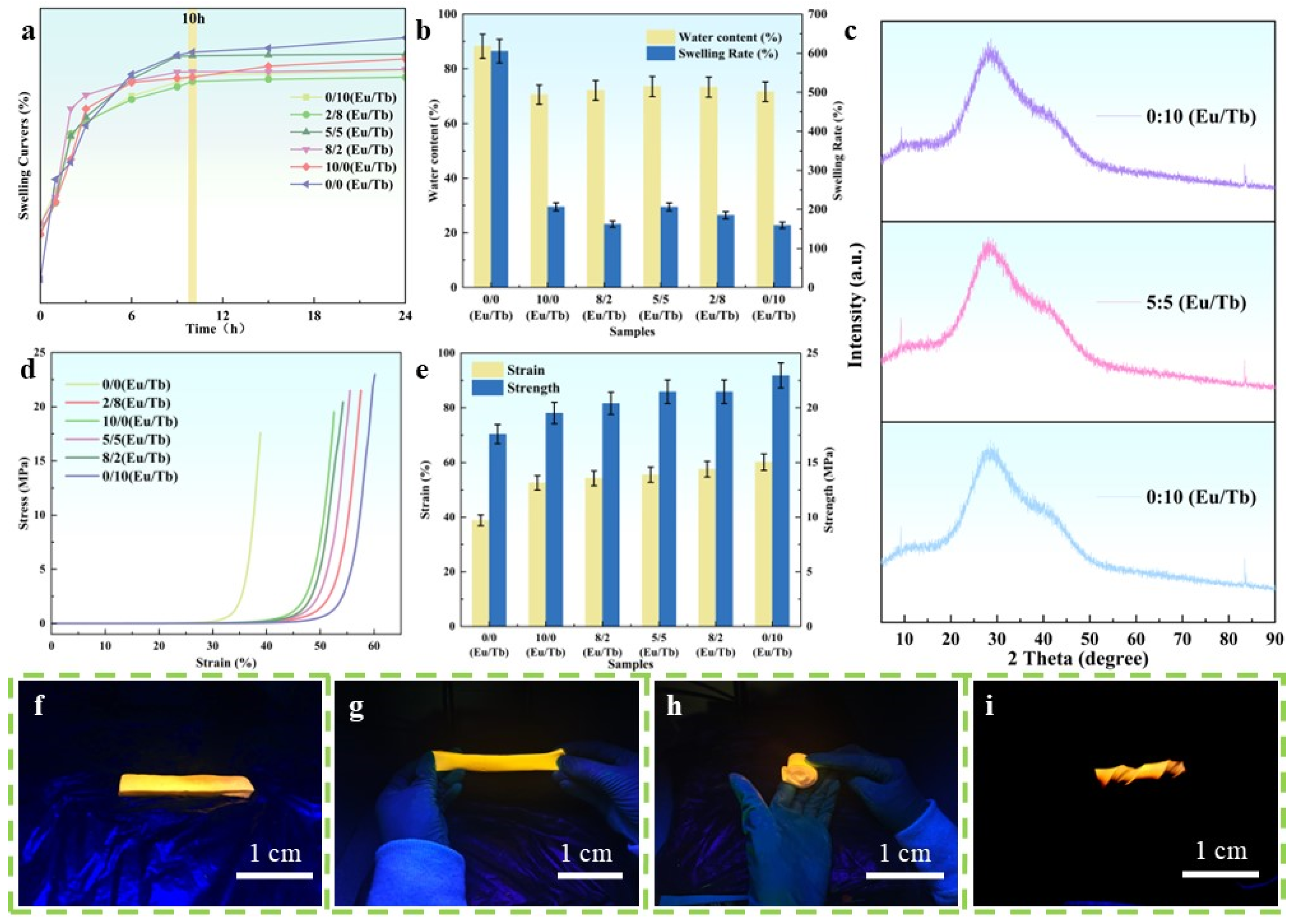
3.1. Structure and Morphology of PVA/PEG/Ln Composite Hydrogel Material
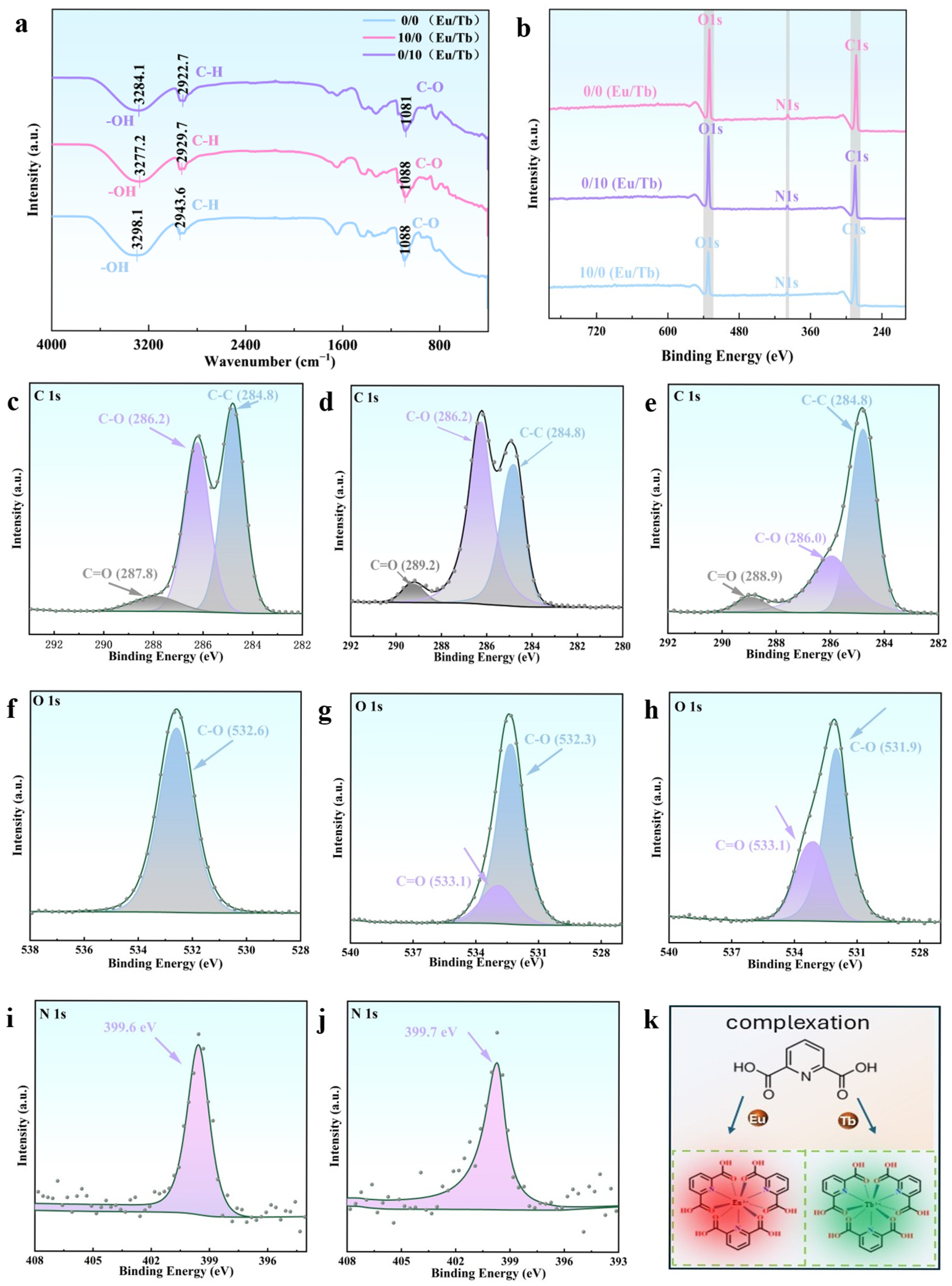
3.2. Characterization of PVA/PEG/Ln Composite Hydrogel Material
3.3. Photoluminescence Properties of PVA/PEG/Ln Composite Hydrogel Materials
3.4. The Application of Information Encryption
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Li, Z.; Chen, H.; Li, B.; Xie, Y.; Gong, X.; Liu, X.; Li, H.; Zhao, Y. Photoresponsive Luminescent Polymeric Hydrogels for Reversible Information Encryption and Decryption. Adv. Sci. 2019, 6, 1901529. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, C.; Zhao, S.; Huang, X.; Jiang, Y.; Li, J.; Fu, D.-Y. Environment-friendly luminescent inks and films based on lanthanides toward advanced anti-counterfeiting. J. Mol. Liq. 2023, 376, 121442. [Google Scholar] [CrossRef]
- Wu, L.; Chen, G.; Li, Z. Layered Rare-Earth Hydroxide/Polyacrylamide Nanocomposite Hydrogels with Highly Tunable Photoluminescence. Small 2017, 13, 1604070. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Han, Y.; Liu, R.; Duan, C.; Li, H. Excitation-Controlled Host-Guest Multicolor Luminescence in Lanthanide-Doped Calcium Zirconate for Information Encryption. Molecules 2023, 28, 7623. [Google Scholar] [CrossRef]
- Lian, X.; Chang, R.; Huang, G.; Peng, Y.; Wang, K.; Zhang, J.; Yao, B.; Niu, H. Multicolor Fluorescent Inks Based on Lanthanide Hybrid Organogels for Anticounterfeiting and Logic Circuit Design. ACS Appl. Mater. Interfaces 2024, 16, 6133–6142. [Google Scholar] [CrossRef]
- Meng, D.; Zhao, T.; Busko, D.; Cosgun Ergene, A.; Richards, B.S.; Howard, I.A. Tb and Eu in MOF-76: Elucidating the Mechanisms Responsible for the Divergent Excellent and Poor Photoluminescence Quantum Yields. Adv. Opt. Mater. 2024, 12, 2300867. [Google Scholar] [CrossRef]
- Yang, Y.; Huang, H.; Wang, Y.; Qiu, F.; Feng, Y.; Song, X.; Tang, X.; Zhang, G.; Liu, W. A family of mixed-lanthanide metal-organic framework thermometers in a wide temperature range. Dalton Trans. 2018, 47, 13384–13390. [Google Scholar] [CrossRef]
- Yu, B.; Zheng, B.; Xia, H.; Wang, J.; Song, H.; Chen, B. Tunable emission and temperature sensing performance in novel oxyfluoride borosilicate glass ceramics containing Eu3+/Tb3+: KY3F10 nanocrystals. Ceram. Int. 2021, 47, 9668–9678. [Google Scholar] [CrossRef]
- Wang, M.; Kitagawa, Y.; Hasegawa, Y. Current development of lanthanide complexes for biomedical applications. Chem. Asian J. 2024, 19, e202400038. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Y.; Zheng, J.; Liu, L.; Zhang, Q. Three-Dimensional Printing Self-Healing Dynamic/Photocrosslinking Gelatin-Hyaluronic Acid Double-Network Hydrogel for Tissue Engineering. ACS Omega 2022, 7, 12076–12088. [Google Scholar] [CrossRef]
- Micale, N.; Citarella, A.; Molonia, M.S.; Speciale, A.; Cimino, F.; Saija, A.; Cristani, M. Hydrogels for the Delivery of Plant-Derived (Poly)Phenols. Molecules 2020, 25, 3254. [Google Scholar] [CrossRef] [PubMed]
- Adelnia, H.; Ensandoost, R.; Shebbrin Moonshi, S.; Gavgani, J.N.; Vasafi, E.I.; Ta, H.T. Freeze/thawed polyvinyl alcohol hydrogels: Present, past and future. Eur. Polym. J. 2022, 164, 110974. [Google Scholar] [CrossRef]
- Guo, B.; Dong, R.; Liang, Y.; Li, M. Haemostatic materials for wound healing applications. Nat. Rev. Chem. 2021, 5, 773–791. [Google Scholar] [CrossRef] [PubMed]
- Raina, N.; Pahwa, R.; Thakur, V.K.; Gupta, M. Polysaccharide-based hydrogels: New insights and futuristic prospects in wound healing. Int. J. Biol. Macromol. 2022, 223, 1586–1603. [Google Scholar] [CrossRef]
- Zhou, L.; Min, T.; Bian, X.; Dong, Y.; Zhang, P.; Wen, Y. Rational Design of Intelligent and Multifunctional Dressing to Promote Acute/Chronic Wound Healing. ACS Appl. Bio Mater. 2022, 5, 4055–4085. [Google Scholar] [CrossRef]
- Dong, R.; Guo, B. Smart wound dressings for wound healing. Nano Today 2021, 41, 101290. [Google Scholar] [CrossRef]
- Zeng, Z.; Zhu, M.; Chen, L.; Zhang, Y.; Lu, T.; Deng, Y.; Ma, W.; Xu, J.; Huang, C.; Xiong, R. Design the molecule structures to achieve functional advantages of hydrogel wound dressings: Advances and strategies. Compos. Part B Eng. 2022, 247, 110313. [Google Scholar] [CrossRef]
- Teodorescu, M.; Bercea, M.; Morariu, S. Biomaterials of PVA and PVP in medical and pharmaceutical applications: Perspectives and challenges. Biotechnol. Adv. 2019, 37, 109–131. [Google Scholar] [CrossRef]
- El Halal, S.L.M.; Fonseca, L.M.; do Evangelho, J.A.; Bruni, G.P.; dos Santos Hackbart, H.C.; da Rosa Zavareze, E.; Dias, A.R.G. Electrospun Ultrafine Fibers from Black Bean Protein Concentrates and Polyvinyl Alcohol. Food Biophys. 2019, 14, 446–455. [Google Scholar] [CrossRef]
- Xu, Y.; Pei, M.; Du, J.; Yang, R.; Pan, Y.; Zhang, D.; Qin, S. A tough, anticorrosive hydrogel consisting of bio-friendly resources for conductive and electromagnetic shielding materials. New J. Chem. 2023, 47, 13721–13728. [Google Scholar] [CrossRef]
- Takara, E.A.; Pereira, S.V.; Scala-Benuzzi, M.L.; Fernández-Baldo, M.A.; Raba, J.; Messina, G.A. Novel electrochemical sensing platform based on a nanocomposite of PVA/PVP/RGO applied to IgG anti- Toxoplasma gondii antibodies quantitation. Talanta 2019, 195, 699–705. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Guo, J.; Wang, Y.; Chen, L.; Cai, J.; Zhang, L. Creation of the tunable color light emission of cellulose hydrogels consisting of primary rare-earth compounds. Carbohydr. Polym. 2017, 161, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Gao, M.; Li, J. Lanthanides-based luminescent hydrogels applied as luminescent inks for anti-counterfeiting. J. Lumin. 2021, 236, 118128. [Google Scholar] [CrossRef]
- Kim, T.; Jeon, H.; Lee, J.-R.; Kim, D. Magnetic separation-enhanced photoluminescence detection of dipicolinic acid and quenching detection of Cu (II) ions. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2024, 305, 123501. [Google Scholar] [CrossRef]
- Tugsuz, T.; Yüksel, D.; Gökoğlu, E.; Ateş, S. A Study on the Luminescent Terbium (III) and Pyridine 2, 6 Dicarboxylate Complexes by Experimental and TD-DFT Approaches. J. Fluoresc. 2023, 33, 1057–1065. [Google Scholar] [CrossRef]
- Xu, Y.; Zhan, X.; Du, J.; Wu, Z.; Zhang, D. Fluorescent hydrogel with high toughness response based on lanthanide Metals: Material Adhesion, multicolor Modulation, information encryption. Chem. Eng. J. 2024, 489, 151303. [Google Scholar] [CrossRef]
- Zhang, M.Y.; Yi, F.Y.; Guo, Q.Z.; Luo, F.L.; Liu, L.J.; Guo, J.F. A ratiometric luminescence sensing platform based on lanthanide-based silica nanoparticles for selective and sensitive detection of Fe(3+) and Cu(2+) ions. Dalton Trans. 2023, 52, 3300–3307. [Google Scholar] [CrossRef]
- Chen, K.; Liu, J.; Yang, X.; Zhang, D. Preparation, optimization and property of PVA-HA/PAA composite hydrogel. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 78, 520–529. [Google Scholar] [CrossRef]
- Xu, Y.; Pei, M.; Zhan, X.; Wang, H.; Zhang, D.; Qin, S. Mechanical, robust and conductive eco-friendly self-assembling hydrogel: A novel material for electromagnetic shielding. New J. Chem. 2023, 47, 21475–21484. [Google Scholar] [CrossRef]
- Gao, M.; Li, J.; Lu, X.; Li, R.; Hong, C.; Zhao, S.; Li, G. Lanthanides-based invisible multicolor luminescent hydrogels and films for anti-counterfeiting. Inorganica Chim. Acta 2024, 560, 121813. [Google Scholar] [CrossRef]
- Li, H.; Guo, J.; Ren, J.; Li, Y.; Yu, X. Carbon nanodot-induced Eu3+-based fluorescent polymeric hydrogel for excellent phase-separation absorption of VOC. J. Mater. Chem. A 2022, 10, 7941–7947. [Google Scholar] [CrossRef]
- Trikkaliotis, D.G.; Christoforidis, A.K.; Mitropoulos, A.C.; Kyzas, G.Z. Adsorption of copper ions onto chitosan/poly(vinyl alcohol) beads functionalized with poly(ethylene glycol). Carbohydr. Polym. 2020, 234, 115890. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Zhang, Q.; Cheng, H.; Liu, Y.; Shu, Y.; Geng, Y.; Zheng, Y.; Qin, B.; Zhou, Y.; Chen, S.; et al. Role of Ions in Hydrogels with an Ionic Seebeck Coefficient of 52.9 mV K−1. J. Phys. Chem. Lett. 2022, 13, 4621–4627. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, Y.-C.; Lee, L.-C.; Lin, Y.-T.; Hong, S.-H.; Wang, K.-C.; Tung, S.-H.; Liu, C.-L. Stretchable polyvinyl alcohol and sodium alginate double network ionic hydrogels for low-grade heat harvesting with ultrahigh thermopower. Mater. Today Energy 2023, 37, 101383. [Google Scholar] [CrossRef]
- Zhan, Y.; Wan, X.; He, S.; Yang, Q.; He, Y. Design of durable and efficient poly(arylene ether nitrile)/bioinspired polydopamine coated graphene oxide nanofibrous composite membrane for anionic dyes separation. Chem. Eng. J. 2018, 333, 132–145. [Google Scholar] [CrossRef]
- Gouthaman, A.; Azarudeen, R.S.; Thirumarimurugan, M. A strategic approach towards thermal crosslinking of the electrospun PVA membrane using o-phenylene diamine: Superhydrophilic platform to grow PANI for simultaneous cationic and anionic dye rejections. J. Membr. Sci. 2024, 695, 122476. [Google Scholar] [CrossRef]
- Jia, L.; Zhang, B.; Xu, J.; Zhu, T.; Chen, R.; Zhou, F. Chameleon Luminophore for Erasable Encrypted and Decrypted Devices: From Dual-Channel, Programmable, Smart Sensory Lanthanide Hydrogel to Logic Devices. ACS Appl. Mater. Interfaces 2020, 12, 19955–19964. [Google Scholar] [CrossRef]
- Li, Z.; Wang, J.; Chen, M.; Wang, Y. Lanthanide Luminescence Improvement by Using a Functional Poly(Ionic Liquid) as Matrix and Co-ligand. Chem. Asian J. 2016, 11, 745–749. [Google Scholar] [CrossRef]
- Shao, B.; Zhang, X.; Sang, S.; Guo, A.; Cui, F.; Yang, X. A novel layered rare-earth hydroxides/polyvinyl alcohol hydrogel with multicolor photoluminescence behavior. Eur. Polym. J. 2021, 147, 110324. [Google Scholar] [CrossRef]
- Zhang, Y.; Lin, L.; Wu, Y.; Gong, C.; Feng, Z.; Wang, Z.; Huang, Y.; Zheng, Z. A ligand-free hydrogel as a visual fluorescence sensor for detection of rare-earth ions. Opt. Commun. 2024, 570, 130885. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, J.; Zhang, D.; Zhou, T.; Diao, K.; Lei, Z. Rare Earth Fluorescent Composite Hydrogel with Controllable Color Photoluminescence for Information Encryption. Polymers 2025, 17, 1534. https://doi.org/10.3390/polym17111534
Du J, Zhang D, Zhou T, Diao K, Lei Z. Rare Earth Fluorescent Composite Hydrogel with Controllable Color Photoluminescence for Information Encryption. Polymers. 2025; 17(11):1534. https://doi.org/10.3390/polym17111534
Chicago/Turabian StyleDu, Jiajia, Daohai Zhang, Teng Zhou, Kunlan Diao, and Zhi Lei. 2025. "Rare Earth Fluorescent Composite Hydrogel with Controllable Color Photoluminescence for Information Encryption" Polymers 17, no. 11: 1534. https://doi.org/10.3390/polym17111534
APA StyleDu, J., Zhang, D., Zhou, T., Diao, K., & Lei, Z. (2025). Rare Earth Fluorescent Composite Hydrogel with Controllable Color Photoluminescence for Information Encryption. Polymers, 17(11), 1534. https://doi.org/10.3390/polym17111534






