Green Fabrication of Phosphorus-Containing Chitosan Derivatives via One-Step Protonation for Multifunctional Flame-Retardant, Anti-Dripping, and Antibacterial Coatings on Polyester Fabrics
Abstract
1. Introduction
2. Experiment Section
2.1. Materials
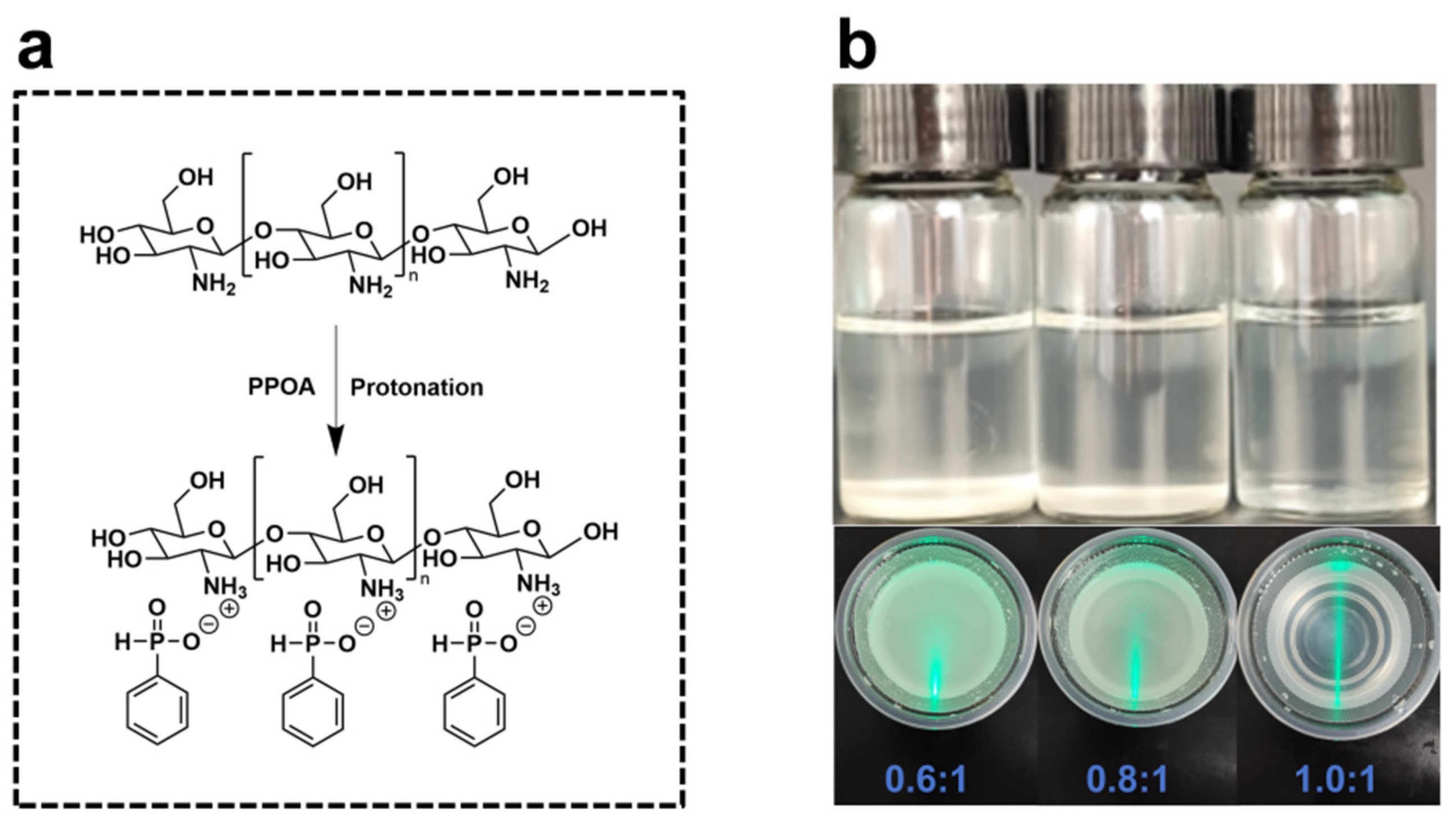
2.2. Preparation of CS-PPOA
2.3. Fabrication of Chitosan Derivative Films
2.4. Preparation of Flame-Retardant Coated PET Fabrics
2.5. Characterization
3. Results and Discussion
3.1. Dissolution Behavior and Structural Characterization
3.2. Rheological Properties and Processability of CS-PPOA Solution
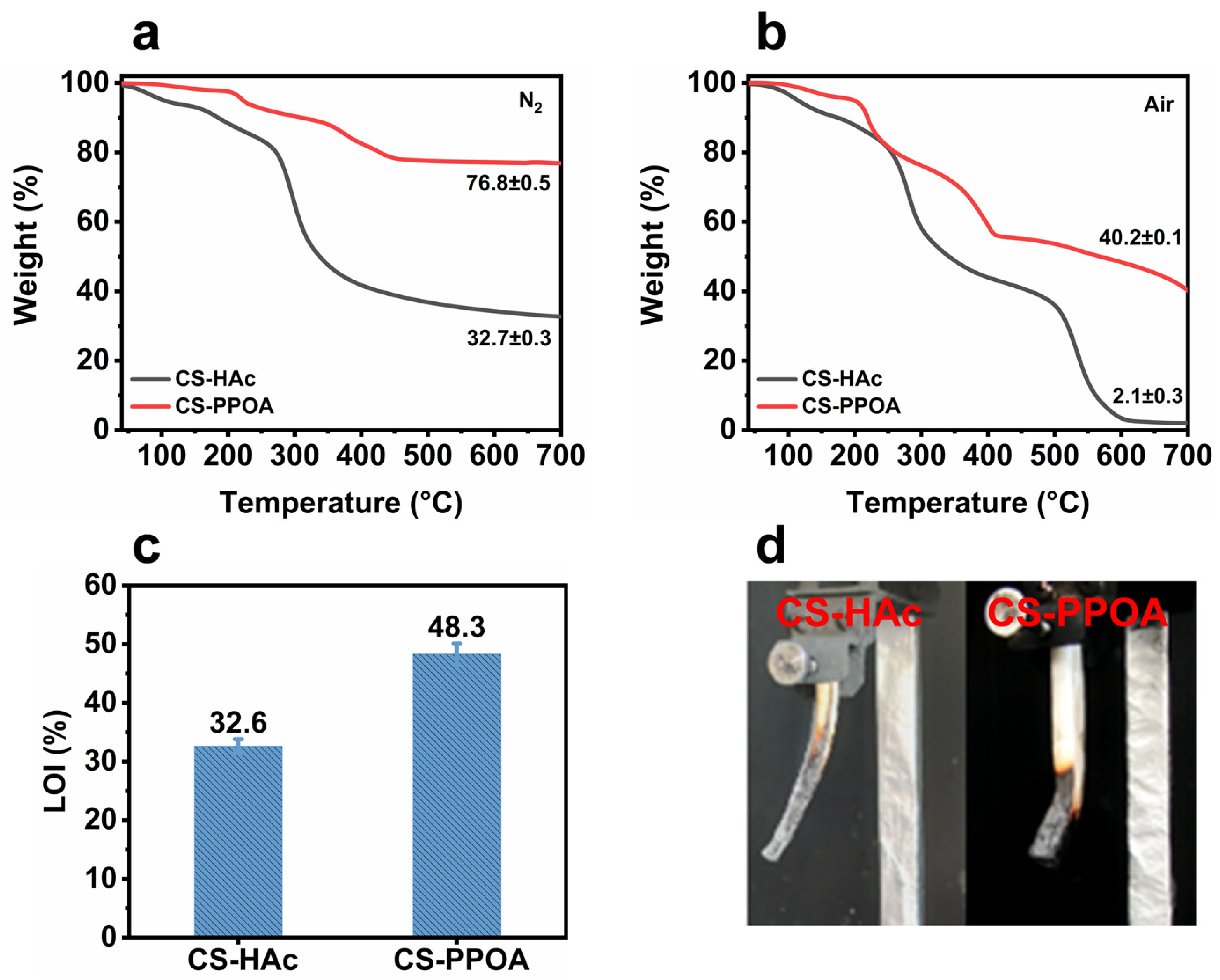
3.3. Thermal Stability and Charring Performance
3.4. Potential Evaluation of CS-PPOA as a Flame Retardant
3.5. The Flame-Retardant Property of Terylene/Px Fabric
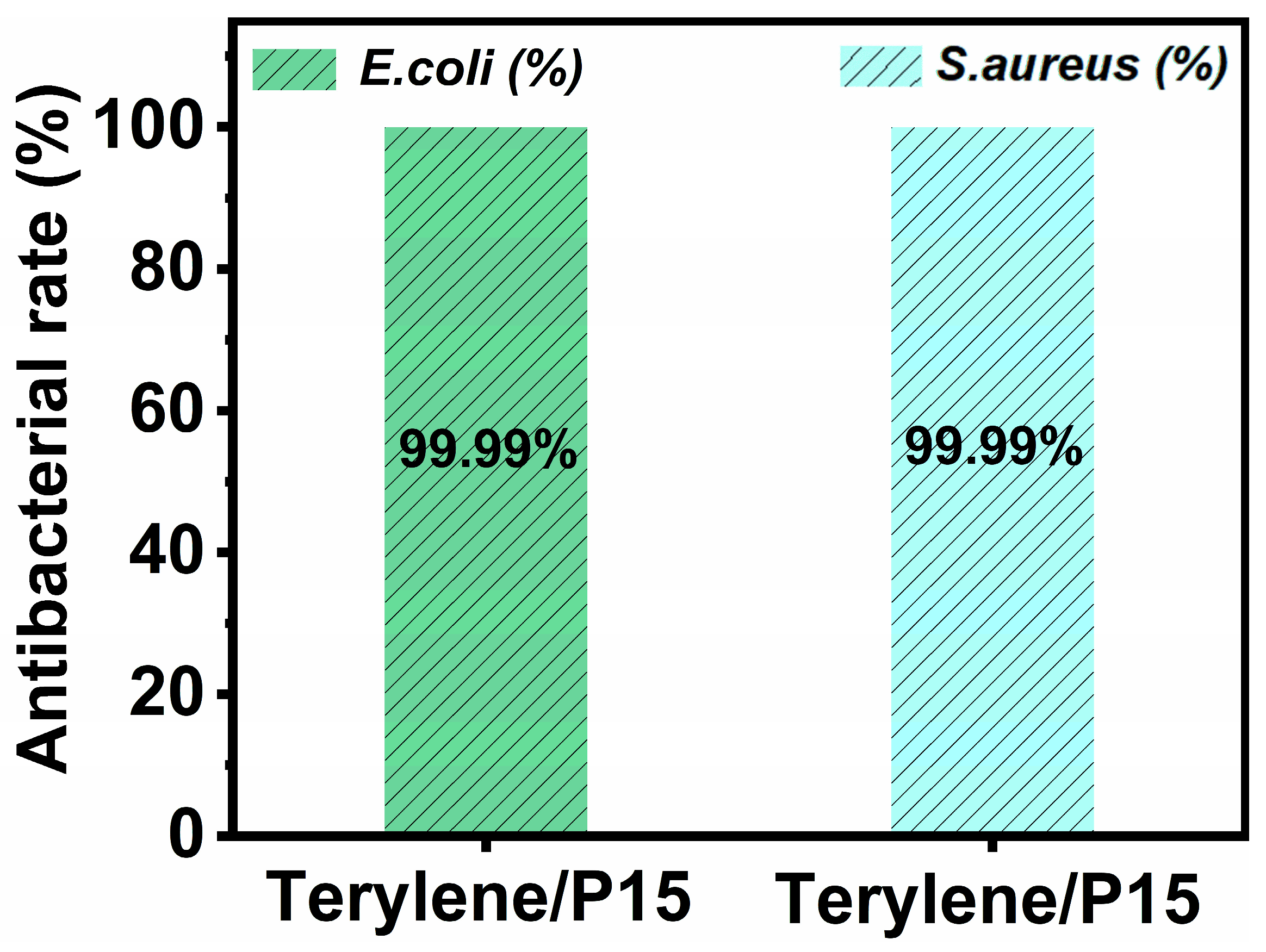
3.6. Antimicrobial Property of Terylene/P15 Fabric
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Tu, Z.; Ou, H.; Ran, Y.; Xue, H.; Zhu, F. Chitosan-based biopolyelectrolyte complexes intercalated montmorillonite: A strategy for green flame retardant and mechanical reinforcement of polypropylene composites. Int. J. Biol. Macromol. 2024, 277, 134316. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Liu, H.; Yan, Q.; Chen, Q.; Hong, M.; Zhou, Z.-X.; Fu, H. Straightforward synthesis of novel chitosan bio-based flame retardants and their application to epoxy resin flame retardancy. Compos. Commun. 2024, 48, 101949. [Google Scholar] [CrossRef]
- Zhu, G.; Wang, J.; Gao, J.; Lin, X.; Zhu, Z. Simple preparation, big effect: Chitosan-based flame retardant towards simultaneous enhancement of flame retardancy, antibacterial, crystallization and mechanical properties of PLA. Int. J. Biol. Macromol. 2025, 303, 140668. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, Y.; Dong, C.; Li, R.; Zhang, X.; Wang, T.; Zhang, K. Transparent, thermal stable, water resistant and high gas barrier films from cellulose nanocrystals prepared by reactive deep eutectic solvents. Int. J. Biol. Macromol. 2024, 276, 134107. [Google Scholar] [CrossRef]
- Chen, R.; Luo, Z.; Yu, X.; Tang, H.; Zhou, Y.; Zhou, H. Synthesis of chitosan-based flame retardant and its fire resistance in epoxy resin. Carbohydr. Polym. 2020, 245, 116530. [Google Scholar] [CrossRef]
- Cho, W.; Shields, J.R.; Dubrulle, L.; Wakeman, K.; Bhattarai, A.; Zammarano, M.; Fox, D.M. Ion—Complexed chitosan formulations as effective fire-retardant coatings for wood substrates. Polym. Degrad. Stab. 2022, 197, 109870. [Google Scholar] [CrossRef]
- Mahaninia, M.H.; Wang, Z.; Rajabi-Abhari, A.; Yan, N. Self-healing, flame-retardant, and antimicrobial chitosan-based dynamic covalent hydrogels. Int. J. Biol. Macromol. 2023, 252, 126422. [Google Scholar] [CrossRef]
- Matsushita, Y.; Hirano, D.; Aoki, D.; Yagami, S.; Takagi, Y.; Fukushima, K. A Biobased Flame-Retardant Resin Based on Lignin. Adv. Sustain. Syst. 2017, 1, 1700073. [Google Scholar] [CrossRef]
- Niu, D.; Yu, W.; Yang, W.; Xu, P.; Liu, T.; Wang, Z.; Yan, X.; Ma, P. Flame-retardant and UV-shielding poly (lactic acid) composites with preserved mechanical properties by incorporating cyclophosphazene derivative and phosphorus-modified lignin. Chem. Eng. J. 2023, 474, 145753. [Google Scholar] [CrossRef]
- Weng, S.; Li, Z.; Bo, C.; Song, F.; Xu, Y.; Hu, L.; Zhou, Y.; Jia, P. Design lignin doped with nitrogen and phosphorus for flame retardant phenolic foam materials. React. Funct. Polym. 2023, 185, 105535. [Google Scholar] [CrossRef]
- Chen, B.; Wu, D.; Wang, T.; Liu, Q.; Jia, D. Porous carbon generation by burning starch-based intumescent flame retardants for supercapacitors. Chem. Eng. J. 2024, 486, 150353. [Google Scholar] [CrossRef]
- Lu, Y.; Zhao, P.; Chen, Y.; Huang, T.; Liu, Y.; Ding, D.; Zhang, G. A bio-based macromolecular phosphorus-containing active cotton flame retardant synthesized from starch. Carbohydr. Polym. 2022, 298, 120076. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Chai, W.; Liu, Y.; Su, X.; Liao, C.; Gao, M.; Li, Y.; Zheng, Z. Facile fabrication of starch-based, synergistic intumescent and halogen-free flame retardant strategy with expandable graphite in enhancing the fire safety of polypropylene. Ind. Crops Prod. 2022, 184, 115002. [Google Scholar] [CrossRef]
- Liu, L.; Yao, M.; Zhang, H.; Zhang, Y.; Feng, J.; Fang, Z.; Song, P. Aqueous Self-Assembly of Bio-Based Flame Retardants for Fire-Retardant, Smoke-Suppressive, and Toughened Polylactic Acid. ACS Sustain. Chem. Eng. 2022, 10, 16313–16323. [Google Scholar] [CrossRef]
- Chen, Y.; Yin, N.; Wang, Y.; Gao, S.; Shen, C. A novel bio-based charcoal-forming agent based on chitosan and phytate@Mg to improve the flame retardancy of poly(lactic acid). J. Appl. Polym. Sci. 2024, 141, e55074. [Google Scholar] [CrossRef]
- Luo, Q.; Tang, Z.-H.; Zhou, Y.-W.; Zhong, C.-Z.; Zhang, J.-J.; Ding, C.-J.; Li, W.-D.; Wang, L.; Xu, S. Fabrication of nickel–cobalt bimetallic hydroxide modified by chitosan and benzenephosphonic acid with high catalytic carbonization as a novel flame retardant for polylactic acid. J. Mater. Sci. 2024, 59, 17747–17768. [Google Scholar] [CrossRef]
- Natolino, A.; Voce, S.; Alò, E.; Comuzzo, P. Exploring an eco-friendlier strategy for chitosan production and valuable compounds recovery from mushroom by-products with modified subcritical water. Innov. Food Sci. Emerg. Technol. 2025, 100, 103923. [Google Scholar] [CrossRef]
- Cao, X.; Huang, Y.-Y.; Tian, X.-Y.; Ni, Y.-P.; Wang, Y.-Z. Facile and atom-economical synthesis of highly efficient chitosan-based flame retardants towards fire-retarding and antibacterial multifunctional coatings on cotton fabrics. Int. J. Biol. Macromol. 2025, 300, 140205. [Google Scholar] [CrossRef]
- Rathod, N.B.; Bangar, S.P.; Šimat, V.; Ozogul, F. Chitosan and gelatine biopolymer-based active/biodegradable packaging for the preservation of fish and fishery products. Int. J. Food Sci. Technol. 2023, 58, 854–861. [Google Scholar] [CrossRef]
- Khan, I.; Ali, N.; Jing, Z.; Khan, A.; Ali, F.; Hhan, F.; Kareem, A.; Sun, Y.; Al Balushi, R.A.; Al-Hinaai, M.M.; et al. Biopolymer-carbonaceous composites, progress, and adsorptive mitigation of water pollutants. Int. J. Biol. Macromol. 2024, 274, 133379. [Google Scholar] [CrossRef]
- Xue, C.; Xu, X.; Zhang, L.; Liu, Y.; Liu, S.; Liu, Z.; Wu, M.; Shuai, Q. Self-healing/pH-responsive/inherently antibacterial polysaccharide-based hydrogel for a photothermal strengthened wound dressing. Colloids Surf. B Biointerfaces 2022, 218, 112738. [Google Scholar] [CrossRef] [PubMed]
- Jogaiah, S.; Mujtaba, A.G.; Mujtaba, M.; Archana; De Britto, S.; Geetha, N.; Belorkar, S.A.; Shetty, H.S. Chitosan-metal and metal oxide nanocomposites for active and intelligent food packaging; a comprehensive review of emerging trends and associated challenges. Carbohydr. Polym. 2025, 357, 123459. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhou, C.; Dai, S.; Wang, J.; Pan, Z.; Zhou, H. Function of chitosan in aDOPO-based flame retardant modified epoxy resin. J. Appl. Polym. Sci. 2022, 139, 51593. [Google Scholar] [CrossRef]
- Ding, H.; Li, B.; Jiang, Y.; Liu, G.; Pu, S.; Feng, Y.; Jia, D.; Zhou, Y. pH-responsive UV crosslinkable chitosan hydrogel via “thiol-ene” click chemistry for active modulating opposite drug release behaviors. Carbohydr. Polym. 2021, 251, 117101. [Google Scholar] [CrossRef]
- Iqbal, Y.; Ahmed, I.; Irfan, M.F.; Chatha, S.A.S.; Zubair, M.; Ullah, A. Recent advances in chitosan-based materials; The synthesis, modifications and biomedical applications. Carbohydr. Polym. 2023, 321, 121318. [Google Scholar] [CrossRef]
- Kundu, C.K.; Wang, X.; Hou, Y.; Hu, Y. Construction of flame retardant coating on polyamide 6.6 via UV grafting of phosphorylated chitosan and sol–gel process of organo-silane. Carbohydr. Polym. 2018, 181, 833–840. [Google Scholar] [CrossRef]
- Li, X.-L.; Shi, X.-H.; Chen, M.-J.; Liu, Q.-Y.; Li, Y.-M.; Li, Z.; Huang, Y.-H.; Wang, D.-Y. Biomass-based coating from chitosan for cotton fabric with excellent flame retardancy and improved durability. Cellulose 2022, 29, 5289–5303. [Google Scholar] [CrossRef]
- GB/T 1040.2-2022; Plastics-Determination of Tensile Properties-Part 2: Test Conditions for Moulding and Extruding Plastics. China Standard Press: Beijing, China, 2022.
- GB/T 5455-2014; Textiles-Determination of Vertical Flame Resistance of Fabric-Part 1: Procedure for Specimens Subjected to Small Flames. China Standard Press: Beijing, China, 2014.
- GB/T 20944.3-2008; Antibacterial Performance of Textiles-Part 3: Test Method by Vibration. China Standard Press: Beijing, China, 2008.
- Yan, N.; Wan, X.-F.; Chai, X.-S. Rapid determination of degree of deacetylation of chitosan by a headspace analysis based Titrimetric technique. Polym. Test. 2019, 76, 340–343. [Google Scholar] [CrossRef]
- Zhang, Q.; Cao, X.; Ni, Y.-P.; Zhang, L.-P.; Ding, Z.; Xu, Y.-J.; Wang, Y.-Z. Fully recyclable flame-retardant PLA composites with balanced performance based on a phosphorus/nitrogen-containing zwitterion. Chem. Eng. J. 2025, 511, 162161. [Google Scholar] [CrossRef]
- Zhang, L.-P.; Zhao, Z.-G.; Huang, Y.-Y.; Cao, X.; Tian, X.-Y.; Ni, Y.-P.; Wang, Y.-Z. Synergetic improvement of flame retardancy and heat resistance of cationic waterborne polyurethanes by introducing a DOPO-containing flame retardant. Prog. Org. Coat. 2024, 194, 108613. [Google Scholar] [CrossRef]
- Wang, T.-C.; Jia, M.-H.; Xu, N.-T.; Hu, W.; Jiang, Z.; Zhao, B.; Ni, Y.-P.; Shao, Z.-B. Facile fabrication of adenosine triphosphate/chitosan/polyethyleneimine coating for high flame-retardant lyocell fabrics with outstanding antibacteria. Int. J. Biol. Macromol. 2024, 260, 129599. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-Y.; Zhang, L.-P.; Cao, X.; Tian, X.-Y.; Ni, Y.-P. Facile Fabrication of Highly Efficient Chitosan-Based Multifunctional Coating for Cotton Fabrics with Excellent Flame-Retardant and Antibacterial Properties. Polymers 2024, 16, 1409. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.-P.; Zhao, Z.-G.; Huang, Y.-Y.; Zhu, C.-J.; Cao, X.; Ni, Y.-P. Robust, Flame-Retardant, and Anti-Corrosive Waterborne Polyurethane Enabled by a PN Synergistic Flame-Retardant Containing Benzimidazole and Phosphinate Groups. Polymers 2023, 15, 2400. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Huang, W.; Chen, Y.; Zhou, Z.; Liu, H.; Zhang, W.; Huang, J. Facile Preparation of Chitosan-Based Composite Film with Good Mechanical Strength and Flame Retardancy. Polymers 2022, 14, 1337. [Google Scholar] [CrossRef]
- Panda, P.K.; Fu, H.-Y.; Tsai, T.-P.; Chu, C.-Y.; Dash, P.; Hsieh, C.-T. Development of hexagonal boron nitride and zinc oxide nanocomposite for fire retardant and anti-electromagnetic construction coatings. J. Indian Chem. Soc. 2025, 102, 101647. [Google Scholar] [CrossRef]
- Feng, J.; Xu, S.; Pan, G.; Yao, L.; Guan, Y.; Zhou, L.; Cui, L.; Yang, Z. Clean preparation of washable antibacterial polyester fibers by high temperature and high pressure hydrothermal self-assembly. Nanotechnol. Rev. 2021, 10, 1740–1751. [Google Scholar] [CrossRef]
- Guo, H.; Qiao, B.; Ji, X.; Wang, X.; Zhu, E. Antifungal activity and possible mechanisms of submicron chitosan dispersions against Alteraria alternata. Postharvest Biol. Technol. 2020, 161, 110883. [Google Scholar] [CrossRef]



| Samples | Z-Average Size (nm) | PDI | Zeta Potential (mV) |
|---|---|---|---|
| CS-HAc | 177 | 0.433 | 38 |
| CS-PPOA | 220 | 0.295 | 41 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Z.-G.; Huang, Y.-Y.; Tian, X.-Y.; Ni, Y.-P. Green Fabrication of Phosphorus-Containing Chitosan Derivatives via One-Step Protonation for Multifunctional Flame-Retardant, Anti-Dripping, and Antibacterial Coatings on Polyester Fabrics. Polymers 2025, 17, 1531. https://doi.org/10.3390/polym17111531
Zhao Z-G, Huang Y-Y, Tian X-Y, Ni Y-P. Green Fabrication of Phosphorus-Containing Chitosan Derivatives via One-Step Protonation for Multifunctional Flame-Retardant, Anti-Dripping, and Antibacterial Coatings on Polyester Fabrics. Polymers. 2025; 17(11):1531. https://doi.org/10.3390/polym17111531
Chicago/Turabian StyleZhao, Zhen-Guo, Yuan-Yuan Huang, Xin-Yu Tian, and Yan-Peng Ni. 2025. "Green Fabrication of Phosphorus-Containing Chitosan Derivatives via One-Step Protonation for Multifunctional Flame-Retardant, Anti-Dripping, and Antibacterial Coatings on Polyester Fabrics" Polymers 17, no. 11: 1531. https://doi.org/10.3390/polym17111531
APA StyleZhao, Z.-G., Huang, Y.-Y., Tian, X.-Y., & Ni, Y.-P. (2025). Green Fabrication of Phosphorus-Containing Chitosan Derivatives via One-Step Protonation for Multifunctional Flame-Retardant, Anti-Dripping, and Antibacterial Coatings on Polyester Fabrics. Polymers, 17(11), 1531. https://doi.org/10.3390/polym17111531





