Supercritical Impregnation of Olive Leaf Extract in Poly(L-lactic acid-co-caprolactone) Filaments: An Environmentally Friendly Approach to Obtaining Active Biomedical Materials
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials and Chemical Reagents
2.2. High-Pressure Extraction
2.3. Characterization of the Extracts
2.3.1. Antioxidant Activity
2.3.2. Anti-Inflammatory Capacity
2.3.3. Identification and Quantification of Major Phenolic Compounds via High-Performance Liquid Chromatography Coupled with a Diode Array Detector (HPLC-DAD)
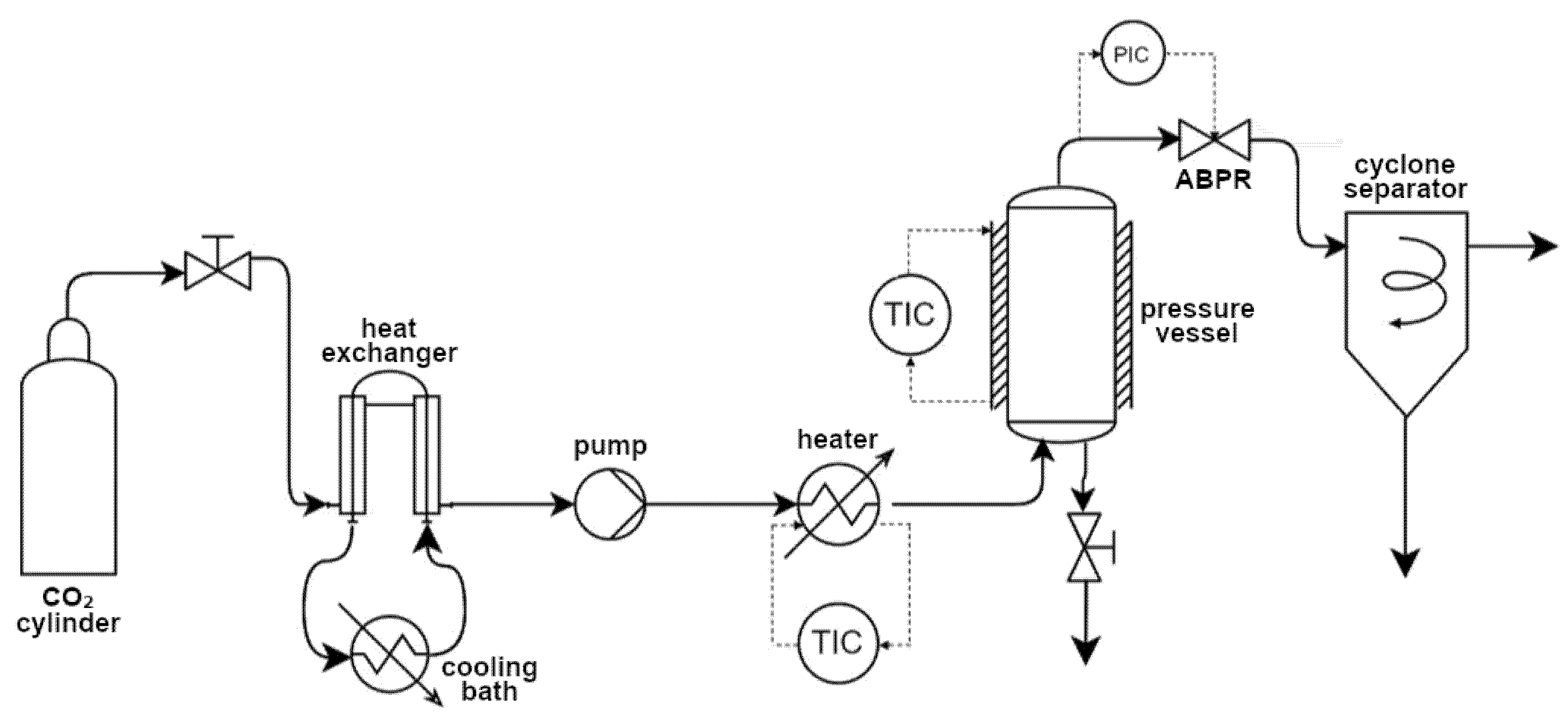
2.4. Supercritical Solvent Impregnation
2.5. Characterization of the Impregnated Filaments
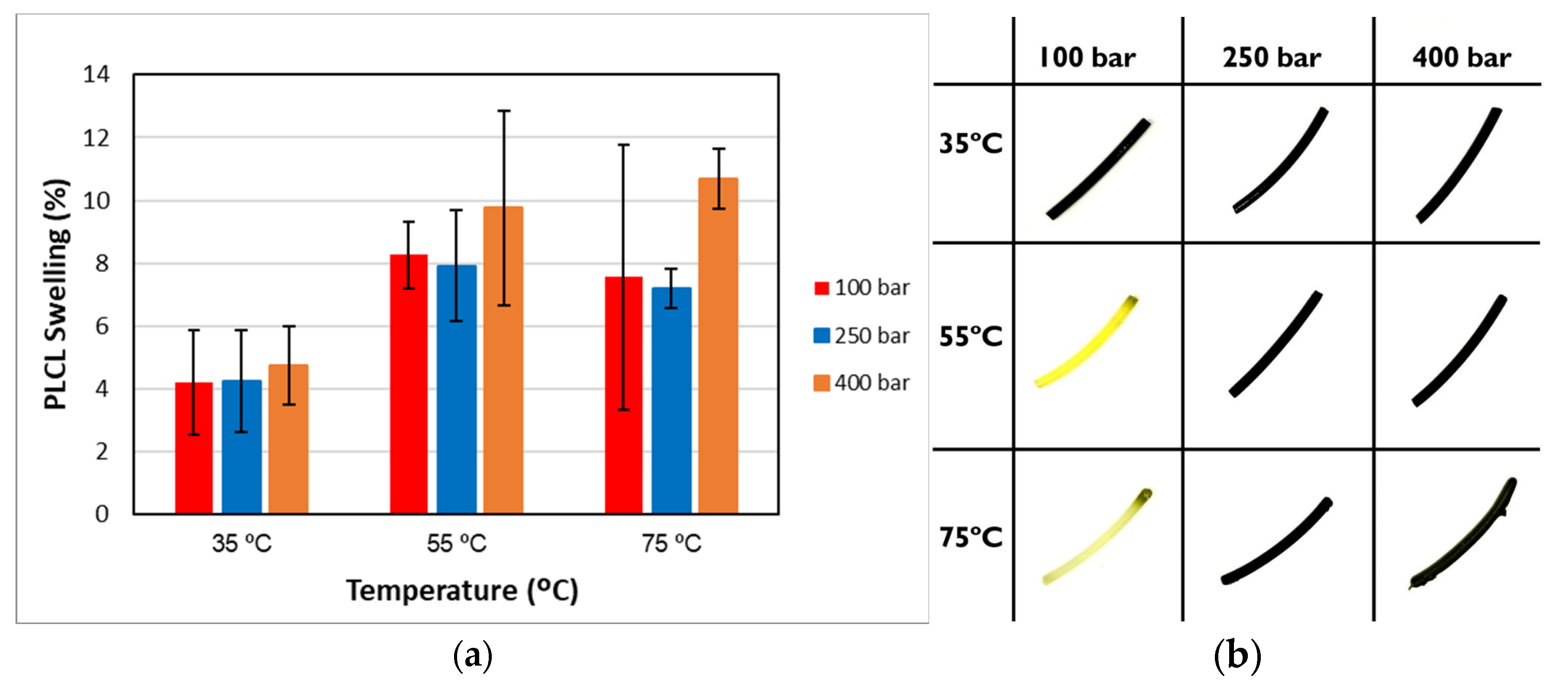
2.5.1. Swelling of the Impregnated Filaments
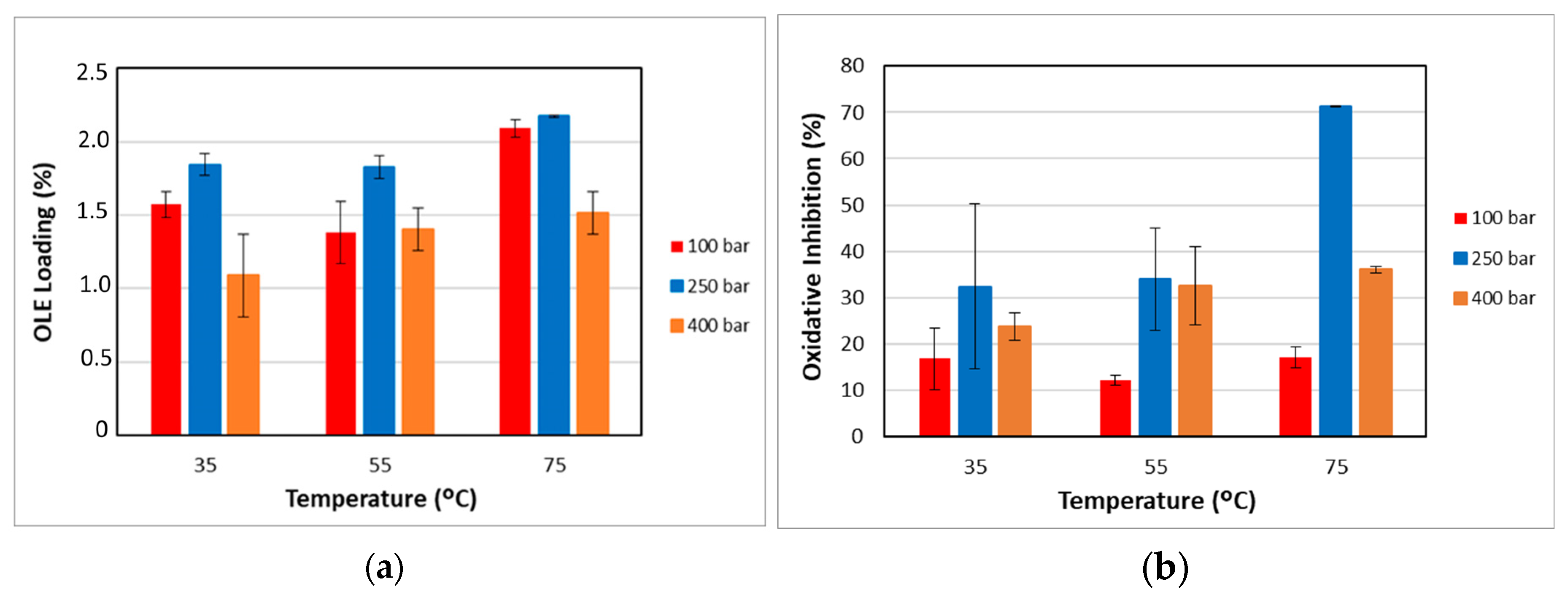
2.5.2. OLE Loading of the Impregnated Filaments
2.5.3. Antioxidant Capacity of Impregnated Filaments
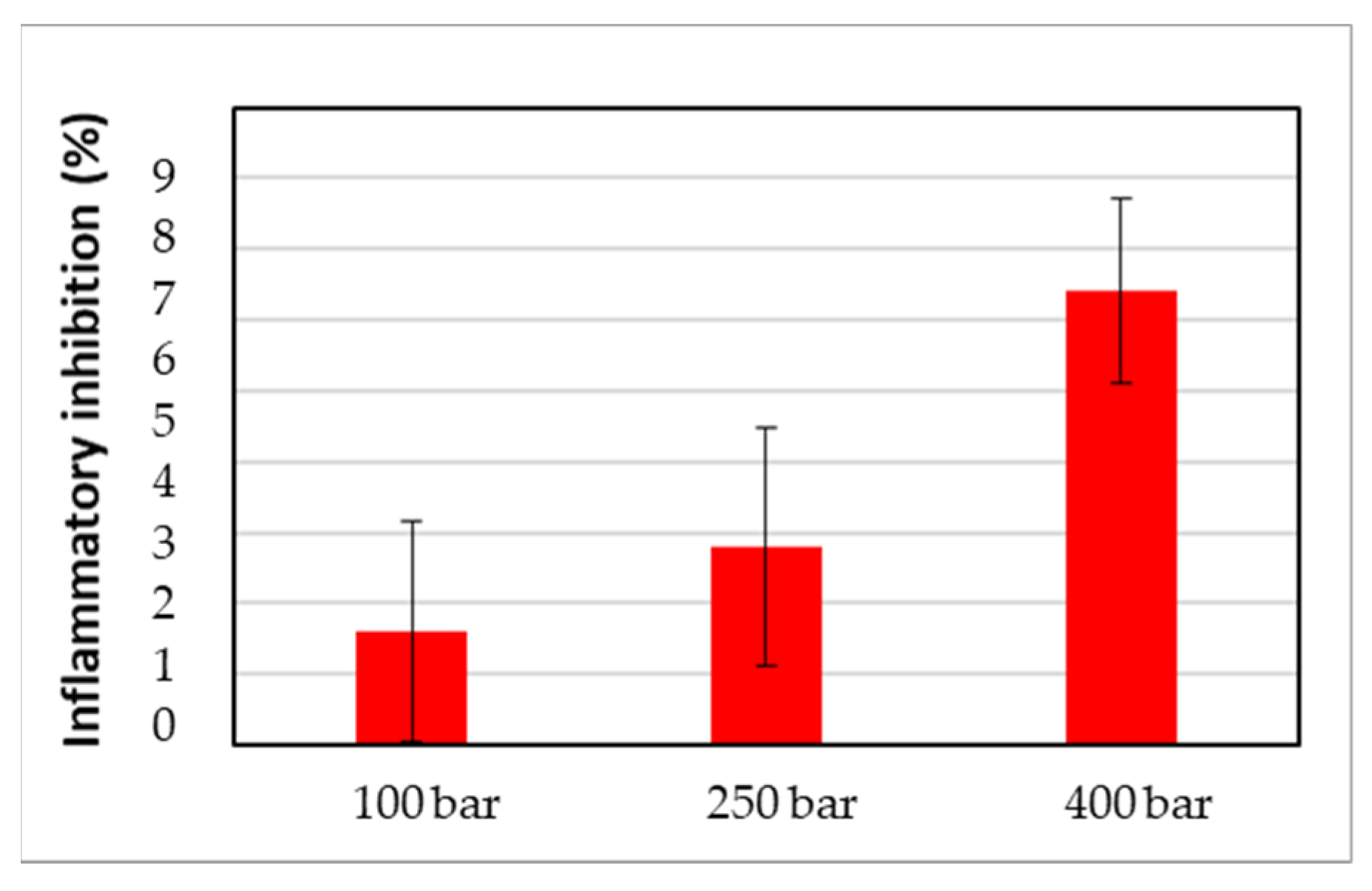
2.5.4. Anti-Inflammatory Capacity of Impregnated Filaments
2.5.5. Identification and Quantification of OLE Polyphenols in Impregnated Filaments
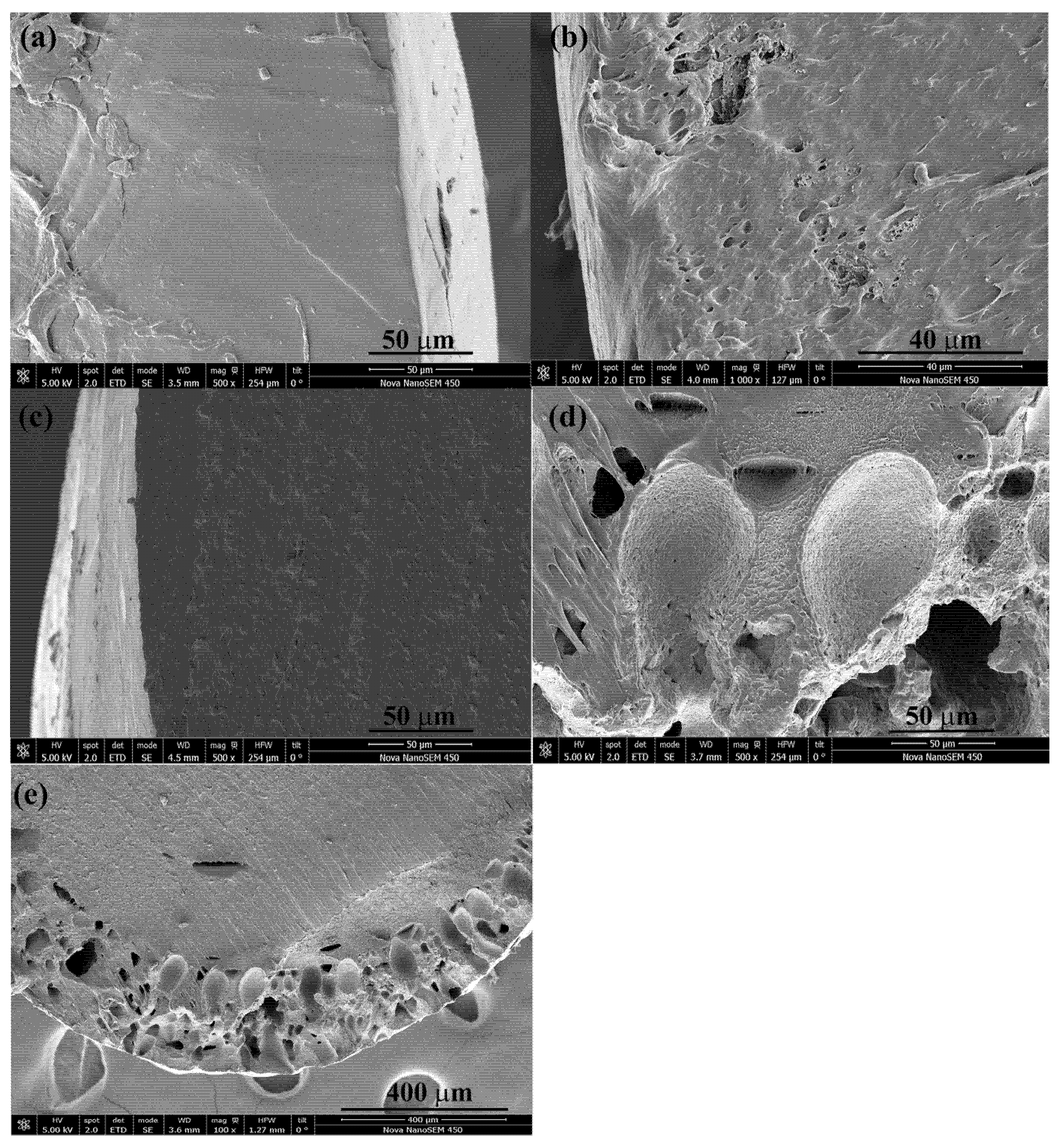
2.5.6. Scanning Electron Microscopy (SEM) of Impregnated Filaments
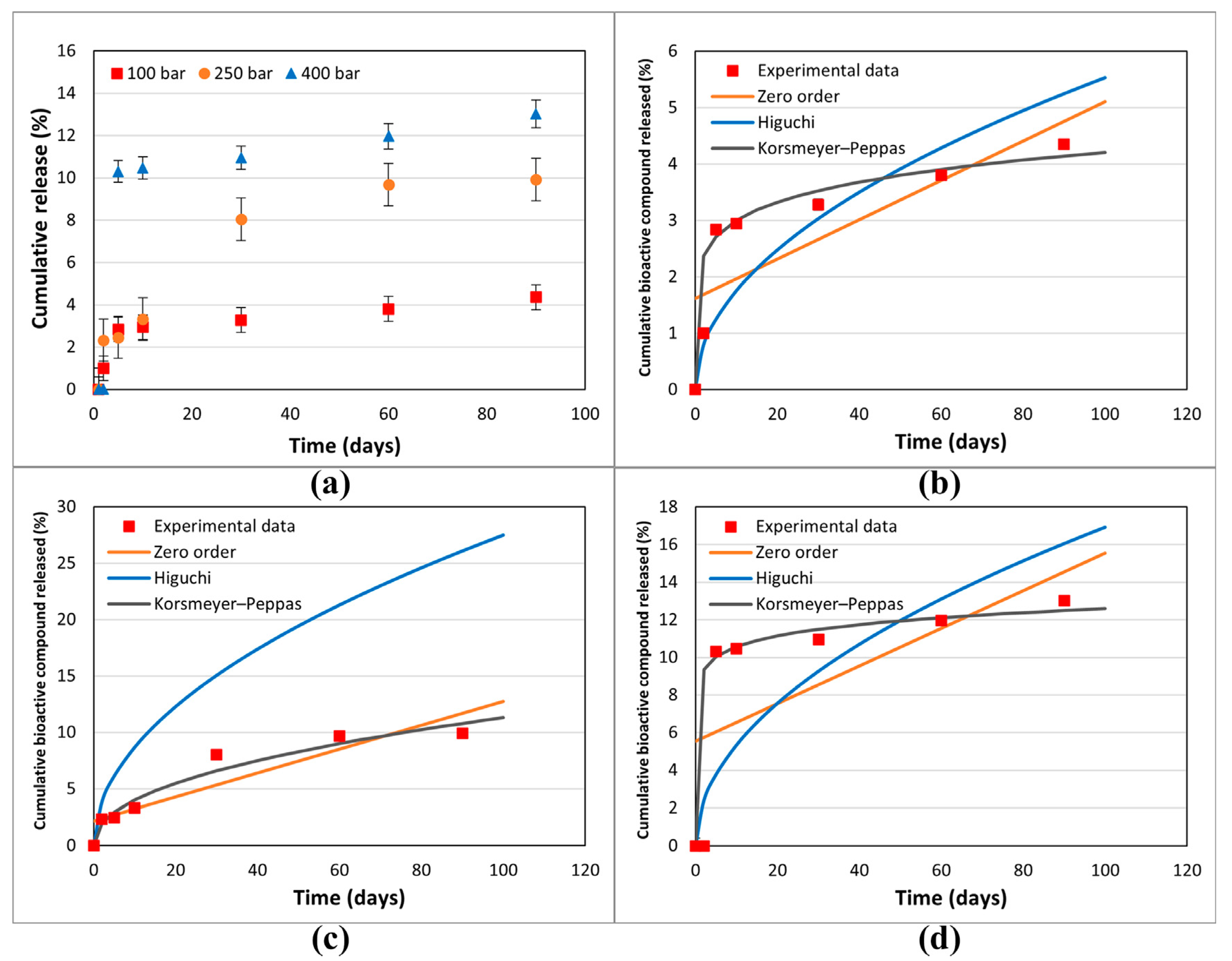
2.6. In Vitro Release Studies
Application of Compound Release Data to Mathematical Models
2.7. Statistical Analysis
3. Results and Discussion
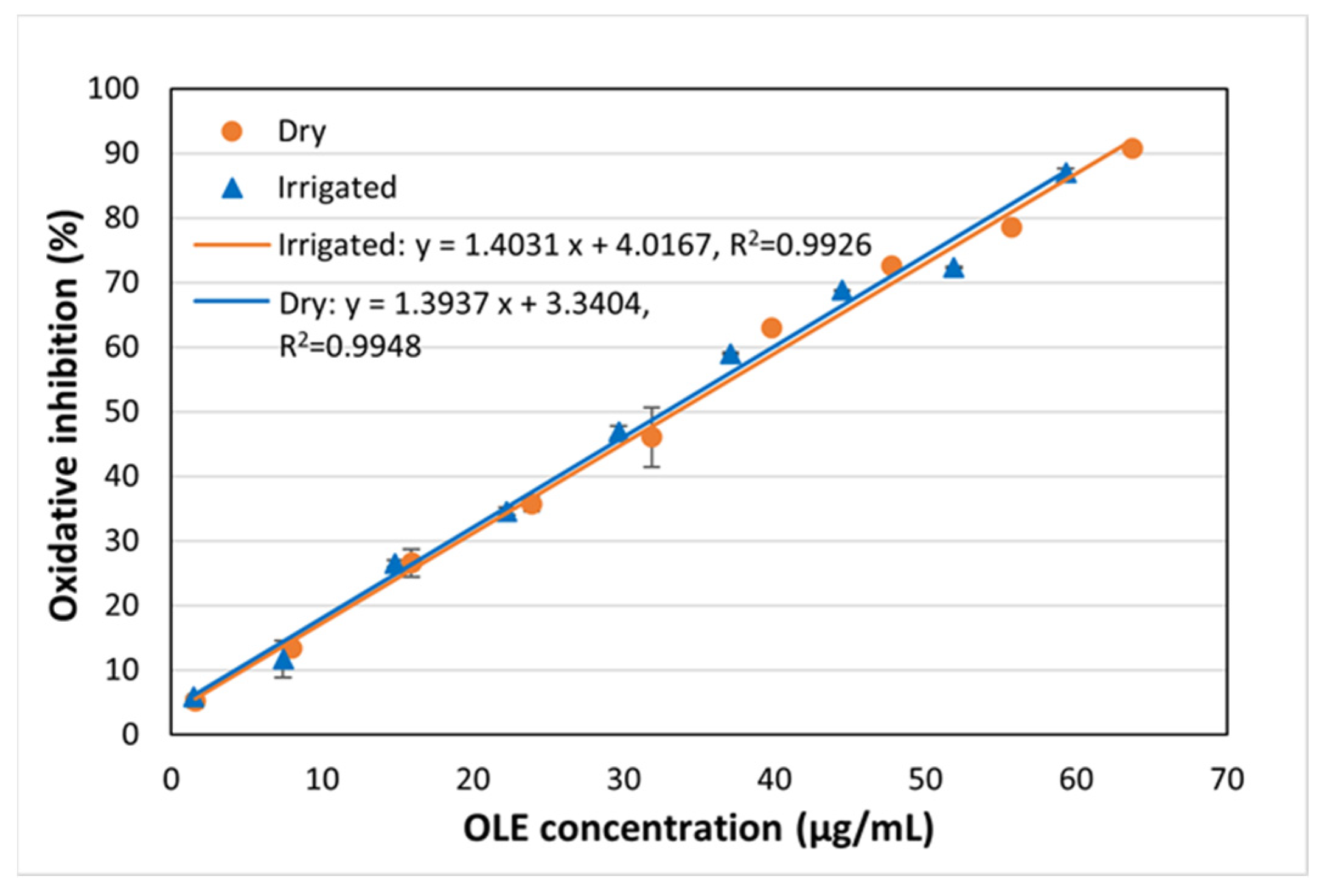
3.1. Enhance Solvent Extraction
3.2. Impregnation into the PLCL Filaments
3.3. In Vitro Release Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Székács, A. Environmental and ecological aspects in the overall assessment of bioeconomy. J. Agric. Environ. Ethics 2017, 30, 153–170. [Google Scholar] [CrossRef]
- Duque-Soto, C.; Quirantes-Piné, R.; Borrás-Linares, I.; Segura-Carretero, A.; Lozano-Sánchez, J. Characterization and Influence of Static In Vitro Digestion on Bioaccessibility of Bioactive Polyphenols from an Olive Leaf Extract. Foods 2022, 11, 743. [Google Scholar] [CrossRef]
- Abaza, L.; Taamalli, A.; Nsir, H.; Zarrouk, M. Olive Tree (Olea europeae L.) Leaves: Importance and Advances in the Analysis of Phenolic Compounds. Antioxidants 2015, 4, 682–698. [Google Scholar] [CrossRef]
- Zhang, C.; Xin, X.; Zhang, J.; Zhu, S.; Niu, E.; Zhou, Z.; Liu, D. Comparative Evaluation of the Phytochemical Profiles and Antioxidant Potentials of Olive Leaves from 32 Cultivars Grown in China. Molecules 2022, 27, 1292. [Google Scholar] [CrossRef]
- Herrera, M.; Pérez-Guerrero, C.; Marhuenda, E.; Ruiz-Gutiérrez, V. Effects of dietary oleic-rich oils (virgin olive and high-oleic-acid sunflower) on vascular reactivity in Wistar-Kyoto and spontaneously hypertensive rats. Br. J. Nutr. 2001, 86, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Malik, N.S.; Bradford, J.M. Recovery and stability of oleuropein and other phenolic compounds during extraction and processing of olive (Olea europaea L.) leaves. J. Food. Agric. Environ. 2008, 6, 8–13. [Google Scholar]
- Lama-Muñoz, A.; Del Mar Contreras, M.; Espínola, F.; Moya, M.; Romero, I.; Castro, E. Content of Phenolic Compounds and Mannitol in Olive Leaves Extracts from Six Spanish Cultivars: Extraction with the Soxhlet Method and Pressurized Liquids. Food Chem. 2020, 320, 126626. [Google Scholar] [CrossRef] [PubMed]
- Alcántara, C.; Žugčić, T.; Abdelkebir, R.; García-Pérez, J.V.; Jambrak, A.R.; Lorenzo, J.M.; Collado, M.C.; Granato, D.; Barba, F.J. Effects of Ultrasound-Assisted Extraction and Solvent on the Phenolic Profile, Bacterial Growth, and Anti-Inflammatory/Antioxidant Activities of Mediterranean Olive and Fig Leaves Extracts. Molecules 2020, 25, 1718. [Google Scholar] [CrossRef]
- Fiorito, S.; Collevecchio, C.; Spogli, R.; Epifano, F.; Genovese, S. Novel procedures for olive leaves extracts processing: Selective isolation of oleuropein and elenolic acid. Food Chem. 2024, 447, 139038. [Google Scholar] [CrossRef]
- Fernández Ponce, M.T.; Parjikolaei, B.R.; Lari, H.N.; Casas, L.; Mantell, C.; Martínez de la Ossa, E.J. Pilot-plant scale extraction of phenolic compounds from mango leaves using different green techniques: Kinetic and scale up study. Chem. Eng. J. 2016, 299, 420–430. [Google Scholar] [CrossRef]
- Burgos-Briones, G.A.; Verano-Naranjo, L.; Cejudo-Bastante, C.; Dueñas-Rivadeneira, A.A.; Mantell-Serrano, C.; Casas-Cardoso, L. Extraction of Bioactive Compounds from Prestonia mollis Leaves and Their Impregnation into Polylactic Acid Using High-Pressure Technologies: Potential for Biomedical Application. Antioxidants 2023, 12, 1864. [Google Scholar] [CrossRef] [PubMed]
- Ratnam, D.V.; Ankola, D.; Bhardwaj, V.; Sahana, D.; Kumar, M.R. Role of antioxidants in prophylaxis and therapy: A pharmaceutical perspective. J. Control. Release 2006, 113, 189–207. [Google Scholar] [CrossRef] [PubMed]
- Ratner, B.D. Biomaterials: Been there, done that, and evolving into the future. Annu. Rev. Biomed. Eng. 2019, 21, 171–191. [Google Scholar] [CrossRef] [PubMed]
- Holban, A.N.; Grumezescu, A. Materials for Biomedical Engineering: Nanobiomaterials in Tissue Engineering, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2009. [Google Scholar]
- Guney, O.; Akgerman, A. Synthesis of controlled-release products in supercritical medium. AIChE J. 2002, 48, 856–866. [Google Scholar] [CrossRef]
- Gadekar, V.; Borade, Y.; Kannaujia, S.; Rajpoot, K.; Anup, N.; Tambe, V.; Kalia, K.; Tekade, R.K. Nanomedicines accessible in the market for clinical interventions. J. Control Release 2021, 330, 372–397. [Google Scholar] [CrossRef]
- Lu, T.; Li, Y.; Chen, T. Techniques for fabrication and construction of three-dimensional scaffolds for tissue engineering. Int. J. Nanomed. 2013, 8, 337–350. [Google Scholar] [CrossRef]
- Clifford, A.; Williams, J.R. Introduction to Supercritical Fluids and Their Applications. In Supercritical Fluid. Methods and Protocols; Humana Press Inc.: Totowa, NJ, USA, 2000; pp. 1–16. [Google Scholar]
- Wang, Y.; Yuehong, S.; Wang, W.; Fang, Y.; Riffat, S.B.; Jiang, F. The advances of polysaccharide-based aerogels: Preparation and potential application. Carbohidr. Polym. 2019, 226, 115242. [Google Scholar] [CrossRef]
- Machado, N.D.; Cejudo-Bastante, C.; Goñi, M.L.; Gañán, N.A.; Casas-Cardoso, L.; Mantell-Serrano, C. Screening of the Supercritical Impregnation of Olea europaea Leaves Extract into Filaments of Thermoplastic Polyurethane (TPU) and Polylactic Acid (PLA) Intended for Biomedical Applications. Antioxidants 2022, 11, 1170. [Google Scholar] [CrossRef]
- Machado, N.D.; Mosquera, J.E.; Cejudo-Bastante, C.; Goñi, M.L.; Martini, R.E.; Gañán, N.A.; Mantell-Serrano, C.; Casas-Cardoso, L. Supercritical Impregnation of PETG with Olea europaea Leaf Extract: Influence of Operational Parameters on Expansion Degree, Antioxidant and Mechanical Properties. Polymers 2024, 16, 1567. [Google Scholar] [CrossRef]
- García-Casas, I.; Valor, D.; Elayoubi, H.; Montes, A.; Pereyra, C. Morphological 3D Analysis of PLGA/Chitosan Blend Polymer Scaffolds and Their Impregnation with Olive Pruning Residues via Supercritical CO2. Polymers 2024, 16, 1451. [Google Scholar] [CrossRef]
- Köse, M.; Gümüş Işik, Ş.; Bayraktar, O. Olive Leaf Polyphenols Loaded Mucoadhesive Oral Films. J. Eng. Sci. Des. 2021, 9, 366–380. [Google Scholar] [CrossRef]
- Perego, G.; Cella, G.D.; Aldini, N.N.; Fini, M.; Giardino, R. Preparation of a new nerve guide from a poly(L-lactide-co-6-caprolactone). Biomaterials 1994, 15, 189–193. [Google Scholar] [CrossRef]
- Aliev, G.; Toms, R.; Melnikov, P.; Gervald, A.; Glushchenko, L.; Sedush, N.; Chvalun, S. Synthesis of L-Lactide from Lactic Acid and Production of PLA Pellets: Full-Cycle Laboratory-Scale Technology. Polymers 2024, 16, 624. [Google Scholar] [CrossRef]
- Azimi, B.; Nourpanah, P.; Rabiee, M.; Arbab, S. Poly (ε-caprolactone) Fiber: An Overview. J. Eng. Fibers Fabr. 2014, 9, 74–90. [Google Scholar] [CrossRef]
- Cingolani, A.; Casalini, T.; Caimi, S.; Klaue, A.; Sponchioni, M.; Rossi, F.; Perale, G. A Methodologic Approach for the Selection of Bio-Resorbable Polymers in the Development of Medical Devices: The Case of Poly(l-lactide-co-ε-caprolactone). Polymers 2018, 10, 851. [Google Scholar] [CrossRef] [PubMed]
- Sriyai, M.; Tasati, J.; Molloy, R.; Meepowpan, P.; Somsunan, R.; Worajittiphon, P.; Daranarong, D.; Meerak, J.; Punyodom, W. Development of an Antimicrobial-Coated Absorbable Monofilament Suture from a Medical-Grade Poly(l-lactide-co-ε-caprolactone) Copolymer. ACS Omega 2021, 6, 28788–28803. [Google Scholar] [CrossRef]
- Kwon, I.; Matsuda, T. Co-Electrospun Nanofiber Fabrics of Poly(l-lactide-co-ε-caprolactone) with Type I Collagen or Heparin. Biomacromolecules 2005, 6, 2096–2105. [Google Scholar] [CrossRef]
- Fan, R.; Chuan, D.; Hou, H.; Chen, H.; Xu, J.; Guo, G. Development and evaluation of a novel biodegradable implants with excellent inflammatory response suppression effect by hot-melt extrusion. Eur. J. Pharm. Sci. 2021, 166, 105981. [Google Scholar] [CrossRef]
- Cejudo-Bastante, C.; Casas-Cardoso, L.; Mantell-Serrano, C.; Martínez de la Osa, E.J. Supercritical impregnation of food packaging films to provide antioxidant properties. J. Supercrit. Fluids 2017, 128, 200–207. [Google Scholar] [CrossRef]
- Zeković, Z.; Bušić, A.; Komes, D.; Vladić, J.; Adamović, D.; Pavlić, B. Coriander seeds processing: Sequential extraction of non-polar and polar fractions using supercritical carbon dioxide extraction and ultrasound-assisted extraction. Food Bioprod. Process. 2015, 95, 218–227. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C.L.W.T. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Scherer, R.; Godoy, H. Antioxidant activity index (AAI) by the 2,2-diphenyl-1-picrylhydrazyl method. Food Chem. 2009, 112, 654–658. [Google Scholar] [CrossRef]
- Pinto, M.; Tejeda, A.; Duque, A.L.; Macías, P. Determination of Lipoxygenase Activity in Plant Extracts Using a Modified Ferrous Oxidation−Xylenol Orange Assay. J. Agric. Food Chem. 2007, 55, 5956–5959. [Google Scholar] [CrossRef]
- León-Marcos, L.; Montes, A.; Valor, A.; García-Casas, I.; Machado, N.; Pereyra, C. Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHB-HV) impregnated with mango leaves extracts using supercritical solvent impregnation. J. CO2 Util. 2025, 95, 103062. [Google Scholar] [CrossRef]
- Span, R.; Wagner, W. A New Equation of State for Carbon Dioxide Covering the Fluid Region from the Triple-Point Temperature to 1100 K at Pressures up to 800 MPa. J. Phys. Chem. Ref. Data 1996, 25, 1509–1596. [Google Scholar] [CrossRef]
- Directorate for the Quality of Medicines & HealthCare of the Council of Europe. European Pharmacopoeia, 8th ed.; Council of Europe: Strasbourg, France, 2013. [Google Scholar]
- Talevi, A.; Ruiz, M. Korsmeyer-Peppas, Peppas-Sahlin, and Brazel-Peppas: Models of Drug Release. In The ADME Encyclopedia; Talevi, A., Ed.; Springer Nature: Cham, Switzerland, 2022; pp. 413–418. [Google Scholar]
- Verano Naranjo, L.; Cejudo Bastante, C.; Casas Cardoso, L.; Mantell Serrano, C.; Martínez de la Ossa Fernández, E.J. Supercritical Impregnation of Ketoprofen into Polylactic Acid for Biomedical Application: Analysis and Modeling of the Release Kinetic. Polymers 2021, 13, 1982. [Google Scholar] [CrossRef]
- Ritger, P.L.; Peppas, N.A. A simple equation for description of solute release II. Fickian and anomalous release from swellable devices. J. Control. Release 1987, 5, 37–42. [Google Scholar] [CrossRef]
- Xynos, N.; Papaefstathiou, G.; Psychis, M.; Argyropoulou, A.; Aligiannis, N.; Skaltsounis, A.-L. Development of a green extraction procedure with super/subcritical fluids to produce extracts enriched in oleuropein from olive leaves. J. Supercrit. Fluids 2012, 67, 89–93. [Google Scholar] [CrossRef]
- Dauber, C.; Carreras, T.; González, L.; Gámbaro, A.; Valdés, A.; Ibañez, E.; Vieitez, I. Characterization and incorporation of extracts from olive leaves obtained through maceration and supercritical extraction in Canola oil: Oxidative stability evaluation. LWT—Food Sci. Technol. 2022, 160, 113274. [Google Scholar] [CrossRef]
- Romero-Márquez, J.M.; Navarro-Hortal, M.D.; Forbes-Hernández, T.Y.; Varela-López, A.; Puentes, J.G.; Sánchez-González, C.; Sumalla-Cano, S.; Battino, M.; García-Ruiz, R.; Sánchez, S.; et al. Effect of olive leaf phytochemicals on the anti-acetylcholinesterase, anti-cyclooxygenase-2 and ferric reducing antioxidant capacity. Food Chem. 2024, 444, 138516. [Google Scholar] [CrossRef]
- Şahin, S.; Bilgin, M.; Dramur, M.U. Investigation of oleuropein content in olive leaf extract obtained by supercritical fluid extraction and soxhlet methods. Sep. Sci. Technol. 2011, 46, 1829–1837. [Google Scholar] [CrossRef]
- de Lucas, A.; Martinez de la Ossa, E.J.; Rincón, J.; Blanco, M.A.; Gracia, I. Supercritical fluid extraction of tocopherol concentrates from olive tree leaves. J. Supercrit. Fluids 2002, 22, 221–228. [Google Scholar] [CrossRef]
- Kyriakoudi, A.; Mourtzinos, I.; Tyśkiewicz, K.; Milovanovic, S. An Eco-Friendly Supercritical CO2 Recovery of Value-Added Extracts from Olea europaea Leaves. Foods 2024, 13, 1836. [Google Scholar] [CrossRef] [PubMed]
- Milovanovic, S.; Lukic, I.; Kamiński, P.; Dębczak, A.; Klimkowska, K.; Tyśkiewicz, K.; Konkol, M. Green manufacturing of high-value extracts from milk thistle seeds: Parameters that affect the supercritical CO2 extraction process. J. CO2 Util. 2022, 63, 102134. [Google Scholar] [CrossRef]
- Borjan, D.; Leitgeb, M.; Knez, Ž.; Hrnčič, M.K. Microbiological and antioxidant activity of phenolic compounds in olive leaf extract. Molecules 2020, 25, 5946. [Google Scholar] [CrossRef]
- Cejudo Bastante, C.; Casas Cardoso, L.; Fernández Ponce, M.; Mantell Serrano, C.; Martínez de la Osa Fernández, E.J. Characterization of olive leaf extract polyphenols loaded by supercritical solvent impregnation into PET/PP food packaging films. J. Supercrit. Fluids 2018, 140, 196–206. [Google Scholar] [CrossRef]
- Le Floch, F.; Tena, M.T.; Ríos, A.; Valcárcel, M. Supercritical fluid extraction of phenol compounds from olive leaves. Talanta 1998, 46, 1123–1130. [Google Scholar] [CrossRef]
- Sánchez-Gomar, I.; Benítez-Camacho, J.; Cejudo-Bastante, C.; Casas-Cardoso, L.; Moreno-Luna, R.; Mantell-Serrano, C.; Durán Ruiz, M. Pro-Angiogenic Effects of Natural Antioxidants Extracted from Mango Leaf, Olive Leaf and Red Grape Pomace over Endothelial Colony-Forming Cells. Antioxidants 2022, 11, 851. [Google Scholar] [CrossRef] [PubMed]
- Olszewska, M.A.; Kolodziejczyk-Czepas, J.; Rutkowska, M.; Magiera, A.; Michel, P.; Rejman, M.W.; Nowak, P.; Owczarek, A. The Effect of Standardised Flower Extracts of Sorbus aucuparia L. on Proinflammatory Enzymes, Multiple Oxidants, and Oxidative/Nitrative Damage of Human Plasma Components In Vitro. Oxid. Med. Cell. Longev. 2019, 2019, 9746358. [Google Scholar] [CrossRef]
- Di Maio, E.; Kiran, E. Foaming of Polymers with Supercritical Fluids and Perspectives on the Current Knowledge Gaps and Challenges. J. Supercrit. Fluids 2018, 134, 157–166. [Google Scholar] [CrossRef]
- Masmoudi, Y.; Ben Azzouk, L.; Forzano, O.; Andre, J.-M.; Badens, E. Supercritical impregnation of intraocular lenses. J. Supercrit. Fluids 2011, 60, 98–105. [Google Scholar] [CrossRef]
- Yáñez, F.; Martikainen, L.; Braga, M.; Álvarez-Lorenzo, C.; Concheiro, A.; Duarte, C.; Gil, M.H.; de Sousa, H.C. Supercritical fluid-assisted preparation of imprinted contact lenses for drug delivery. Acta Biomater. 2011, 7, 1019–1030. [Google Scholar] [CrossRef]
- Belizón, M.; Fernández Ponce, M.; Casas Cardoso, L.; Mantell Serrano, C.; Mantínez de la Ossa Fernández, E.J. Supercritical Impregnation of Antioxidant Mango Polyphenols into a Multilayer PET/PP Food-Grade Film. J. CO2 Util. 2018, 25, 56–67. [Google Scholar] [CrossRef]
- Santos de Araujo, E.J.; Martínez, J. Scenarios, prospects, and challenges related to supercritical fluid impregnation in the food industry: A scoping review (2018–2023). Biofuels Bioprod. Bioref. 2024, 18, 2091–2115. [Google Scholar] [CrossRef]
- Rosales, J.; Cejudo Bastante, C.; Verano Naranjo, L.; Casas Cardoso, L.; Mantell Serrano, C.; Martínez de la Ossa, E.J. Supercritical Impregnation of PLA Filaments with Mango Leaf Extract to Manufacture Functionalized Biomedical Devices by 3D Printing. Polymers 2021, 13, 2125. [Google Scholar] [CrossRef]
- Sanchez-Sanchez, J.; Fernández-Ponce, M.T.; Casas, L.; Mantell, C.; Martínez de la Ossa, E.J. Impregnation of Mango Leaf Extract into a Polyester Textile Using Supercritical Carbon Dioxide. J. Supercrit. Fluids 2017, 128, 208–217. [Google Scholar] [CrossRef]
- Longo, R.; Raimondo, M.; Vertuccio, L.; Ciardulli, M.C.; Sirignano, M.; Mariconda, A.; Della Porta, G.; Guadagno, L. Bottom-Up Strategy to Forecast the Drug Location and Release Kinetics in Antitumoral Electrospun Drug Delivery Systems. Int. J. Mol. Sci. 2023, 24, 1507. [Google Scholar] [CrossRef]
- Altun, E.; Yuca, E.; Ekren, N.; Kalaskar, D.M.; Ficai, D.; Dolete, G.; Ficai, A.; Gunduz, O. Kinetic Release Studies of Antibiotic Patches for Local Transdermal Delivery. Pharmaceutics 2021, 13, 613. [Google Scholar] [CrossRef] [PubMed]
- Cejudo-Bastante, C.; Verano-Naranjo, L.; Toro-Barrios, N.; Pereyra, C.; Mantell, C.; Casas, L. Structural Modification of Polymers Functionalized with Mango Leaf Extract by Supercritical Impregnation: Approaching of Further Food and Biomedical Applications. Polymers 2022, 14, 2413. [Google Scholar] [CrossRef]
- Sabbatier, G.; Larrañaga, A.; Guay-Bégin, A.-A.; Fernandez, J.; Diéval, F.; Durand, B.; Sarasua, J.-R.; Laroche, G. Design, Degradation Mechanism and Long-Term Cytotoxicity of Poly(l-lactide) and Poly(Lactide-co-ϵ-Caprolactone) Terpolymer Film and Air-Spun Nanofiber Scaffold. Macromol. Biosci. 2015, 15, 1392–1410. [Google Scholar] [CrossRef]
- Kahraman, E.; Hayri-Senel, T.; Nasun-Saygili, G. Kinetics and Optimization Studies of Controlled 5-Fluorouracil Release from Graphene Oxide Incorporated Vegetable Oil-Based Polyurethane Composite Film. ACS Omega 2024, 9, 47395–47409. [Google Scholar] [CrossRef] [PubMed]



| Factor | Variable | Levels | Response Variable | Fixed Parameters |
|---|---|---|---|---|
| A | Pressure (bar) | 100, 250, 400 | Swelling (% S) | Impregnation time = 1 h |
| B | Temperature (°C) | 35, 55, 75 | High depressurization rate = 2 bar/seg | |
| A | Pressure (bar) | 100, 250, 400 | Swelling (% S) OLE loading (% L) DPPH antioxidant activity (% I) | Impregnation time = 1 h Low depressurization rate = 0.278 bar/min |
| B | Temperature (°C) | 35, 55, 75 | ||
| Dry Land | Irrigated Land | |||
|---|---|---|---|---|
| scCO2 | ESE (CO2/ethanol 1:1 v/v) | scCO2 | ESE (CO2/ethanol 1:1 v/v) | |
| Extraction yield (%) | 0.93 ± 0.12 | 25.5 ± 1.2 | 0.89 ± 0.11 | 22.8 ± 1.6 |
| Extract concentration (g/L) | 71.7 ± 12.4 | 68.4 ± 0.2 | 77.7 ± 6.6 | 77.0 ± 2.3 |
| Luteolin-7-glucoside concentration (mg/100 g OLE) | n.d. * | 45 ± 4 | n.d. * | 38 ± 3 |
| Oleuropein concentration (mg/100 g OLE) | n.d. * | 1480 ± 20 | n.d. * | 1750 ± 50 |
| Hydroxytyrosol concentration (mg/100 g OLE) | n.d. * | 7 ± 2 | n.d. * | 19 ± 2 |
| DPPH IC50 (µg/mL)/(AAI) | 578/0.052 | 33.5/1.07 | 447/0.074 | 32.8/1.10 |
| Anti-inflammatory capacity (IC50 for 12.7 µM of linoleic acid, µg/mL) | n.d. * | 819 | n.d. * | 734 |
| Sum Sq | Df | Mean Sq | F-Value | p-Value | |
|---|---|---|---|---|---|
| OLE Concentration | 1.35359 | 1 | 1.35359 | 2242.91 | 0.0000 |
| y-intercept | 0.00041853 | 1 | 0.00041853 | 0.69 | 0.4190 |
| slope | 0.0000151678 | 1 | 0.0000151678 | 0.03 | 0.8763 |
| Model | 1.35402 | 3 |
| Sum Sq | Df | Mean Sq | F-Value | p-Value | |
|---|---|---|---|---|---|
| OLE Concentration | 0.597813 | 1 | 0.597813 | 667.08 | 0.0000 |
| y-intercept | 0.00346884 | 1 | 0.00346884 | 3.87 | 0.0847 |
| slope | 0.0015048 | 1 | 0.0015048 | 1.68 | 0.2312 |
| Model | 0.602787 | 3 |
| Sum Sq | Df | Mean Sq | F-Value | p-Value | ||
|---|---|---|---|---|---|---|
| A: Temperature | 399.997 | 1 | 399.997 | 79.09 | 0.0000 | 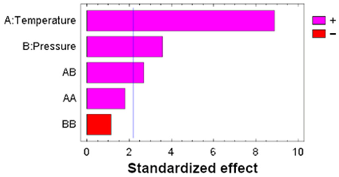 |
| B: Pressure | 65.1934 | 1 | 65.1934 | 12.89 | 0.0042 | |
| AA | 16.4295 | 1 | 16.4295 | 3.25 | 0.0989 | |
| AB | 36.6796 | 1 | 36.6796 | 7.25 | 0.0209 | |
| BB | 6.51100 | 1 | 6.51100 | 1.29 | 0.2806 | |
| Blocks | 0.30681 | 1 | 0.30681 | 0.06 | 0.8100 | |
| Error | 55.6294 | 11 | 5.05721 | |||
| Total | 580.726 | 17 |
| Sum Sq | Df | Mean Sq | F-Value | p-Value | ||
|---|---|---|---|---|---|---|
| A: Temperature | 49.8984 | 1 | 49.8984 | 28.96 | 0.0002 |  |
| B: Pressure | 8.89241 | 1 | 8.89241 | 5.16 | 0.0442 | |
| AA | 19.5217 | 1 | 19.5217 | 11.33 | 0.0063 | |
| AB | 3.31531 | 1 | 3.31531 | 1.92 | 0.1928 | |
| BB | 4.71614 | 1 | 4.71614 | 2.74 | 0.1262 | |
| Blocks | 22.6240 | 1 | 22.6240 | 13.13 | 0.0040 | |
| Error | 18.9503 | 11 | 1.72275 | |||
| Total | 127.918 | 17 |
| Sum Sq | Df | Mean Sq | F-Value | p-Value | ||
|---|---|---|---|---|---|---|
| A: Temperature | 0.53763 | 1 | 0.53763 | 14.40 | 0.0030 | 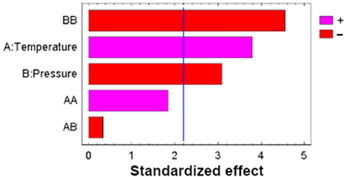 |
| B: Pressure | 0.35708 | 1 | 0.35708 | 9.57 | 0.0102 | |
| AA | 0.12721 | 1 | 0.12721 | 3.41 | 0.0919 | |
| AB | 0.00405 | 1 | 0.00405 | 0.11 | 0.7480 | |
| BB | 0.77147 | 1 | 0.77147 | 20.67 | 0.0008 | |
| Blocks | 0.00080 | 1 | 0.00080 | 0.02 | 0.8863 | |
| Error | 0.41061 | 11 | 0.03733 | |||
| Total | 2.20884 | 17 |
| Sum Sq | Df | Mean Sq | F-Value | p-Value | ||
|---|---|---|---|---|---|---|
| A: Temperature | 877.230 | 1 | 877.230 | 5.60 | 0.0374 | 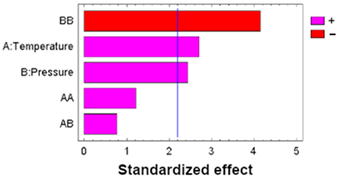 |
| B: Pressure | 711.480 | 1 | 711.480 | 4.54 | 0.0565 | |
| AA | 397.338 | 1 | 397.338 | 2.54 | 0.1396 | |
| AB | 71.4013 | 1 | 71.4013 | 0.46 | 0.5136 | |
| BB | 1510.62 | 1 | 1510.62 | 9.64 | 0.0100 | |
| Blocks | 115.520 | 1 | 115.520 | 0.74 | 0.4089 | |
| Error | 1723.87 | 11 | 156.716 | |||
| Total | 5407.46 | 17 |
| Zero Order | Higuchi | Korsmeyer–Peppas | |||||
|---|---|---|---|---|---|---|---|
| K0 | R2 | KH | R2 | kKP | n | R2 | |
| 100 bar/75 °C | 0.0349 | 0.6004 | 0.5528 | 0.7079 | 2.1419 | 0.1464 | 0.9108 |
| 250 bar/75 °C | 0.1058 | 0.8054 | 2.748 | 0.8952 | 1.4231 | 0.4403 | 0.9362 |
| 400 bar/75 °C | 0.1007 | 0.4521 | 1.6929 | 0.6163 | 8.8746 | 0.0759 | 0.8901 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montes-Lobato, J.R.; Machado, N.D.; Cejudo-Bastante, C.; Mantell-Serrano, C.; Casas-Cardoso, L. Supercritical Impregnation of Olive Leaf Extract in Poly(L-lactic acid-co-caprolactone) Filaments: An Environmentally Friendly Approach to Obtaining Active Biomedical Materials. Polymers 2025, 17, 1464. https://doi.org/10.3390/polym17111464
Montes-Lobato JR, Machado ND, Cejudo-Bastante C, Mantell-Serrano C, Casas-Cardoso L. Supercritical Impregnation of Olive Leaf Extract in Poly(L-lactic acid-co-caprolactone) Filaments: An Environmentally Friendly Approach to Obtaining Active Biomedical Materials. Polymers. 2025; 17(11):1464. https://doi.org/10.3390/polym17111464
Chicago/Turabian StyleMontes-Lobato, Juan Ramón, Noelia D. Machado, Cristina Cejudo-Bastante, Casimiro Mantell-Serrano, and Lourdes Casas-Cardoso. 2025. "Supercritical Impregnation of Olive Leaf Extract in Poly(L-lactic acid-co-caprolactone) Filaments: An Environmentally Friendly Approach to Obtaining Active Biomedical Materials" Polymers 17, no. 11: 1464. https://doi.org/10.3390/polym17111464
APA StyleMontes-Lobato, J. R., Machado, N. D., Cejudo-Bastante, C., Mantell-Serrano, C., & Casas-Cardoso, L. (2025). Supercritical Impregnation of Olive Leaf Extract in Poly(L-lactic acid-co-caprolactone) Filaments: An Environmentally Friendly Approach to Obtaining Active Biomedical Materials. Polymers, 17(11), 1464. https://doi.org/10.3390/polym17111464








