Toward Sustainable Polyurethane Alternatives: A Review of the Synthesis, Applications, and Lifecycle of Non-Isocyanate Polyurethanes (NIPUs)
Abstract
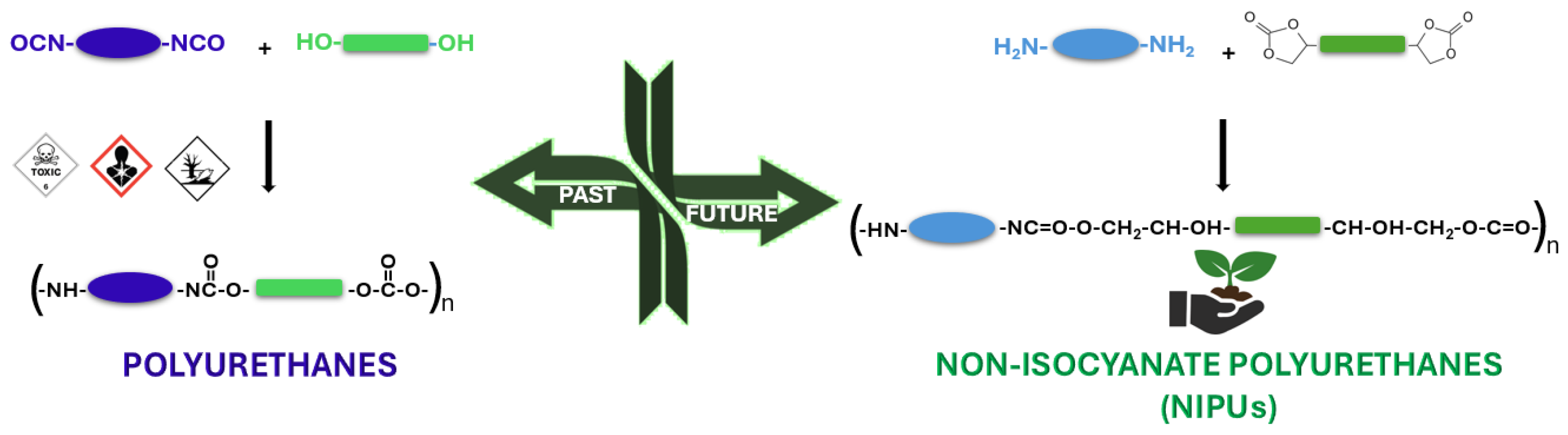
1. Introduction
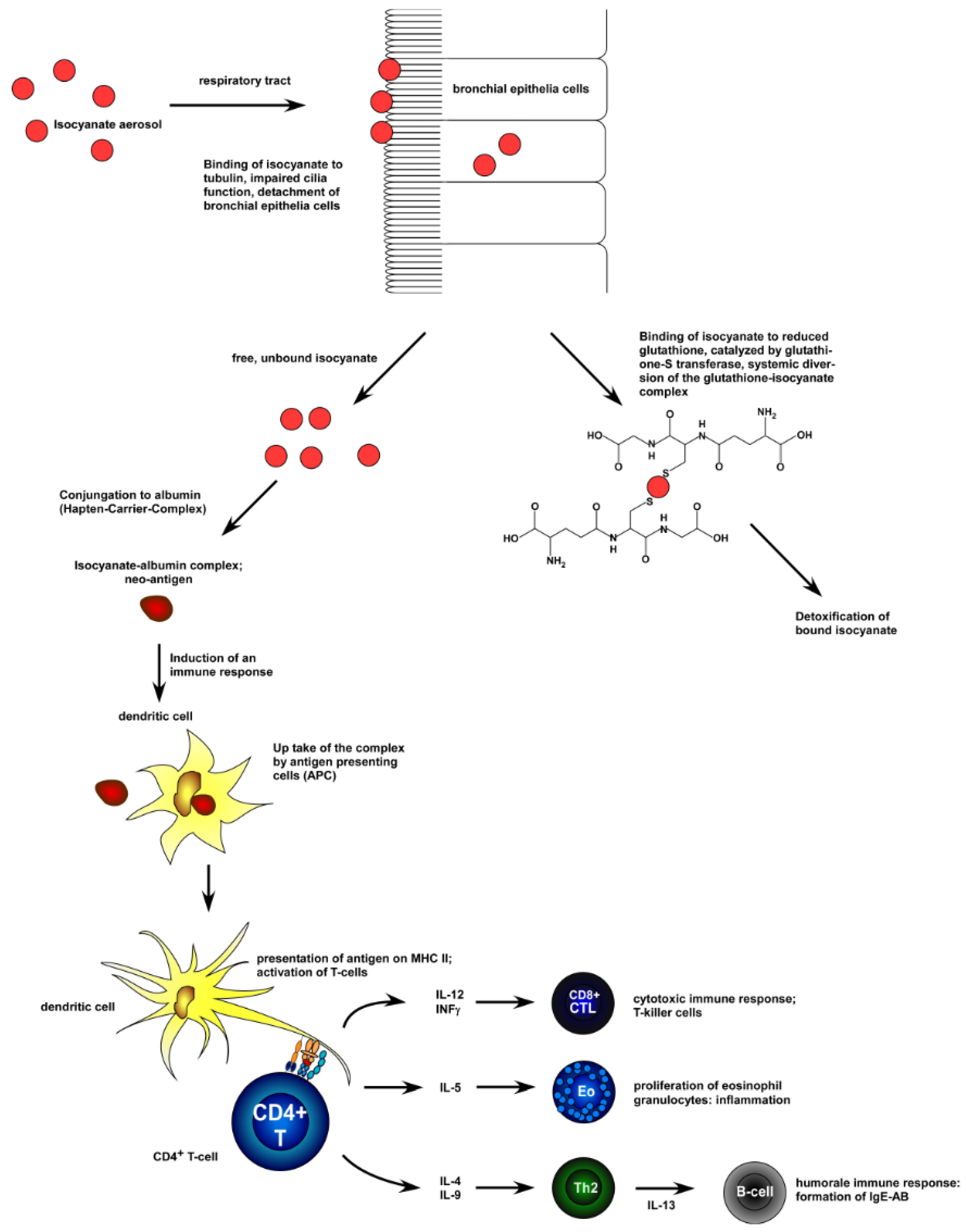
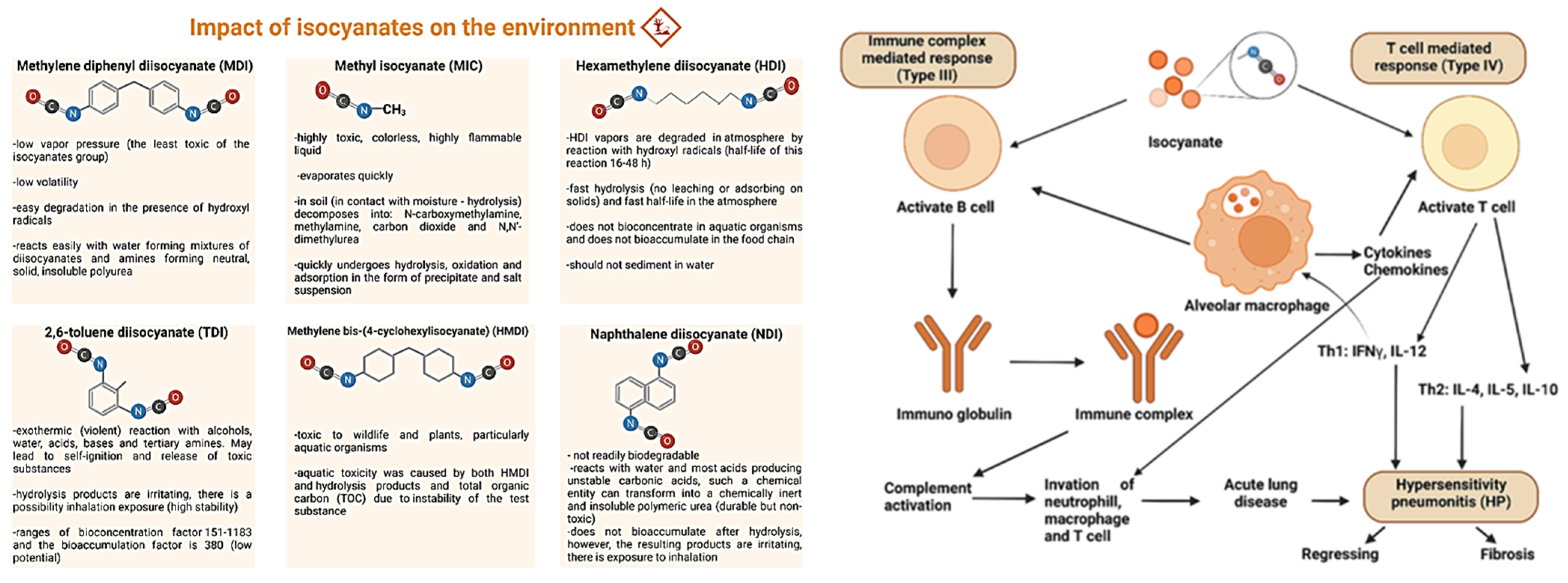
2. Toxicology and Health Impact Issues of Isocyanates
3. Investigation of Potential NIPU Toxicity
4. Synthetic Routes of NIPUs
4.1. Polycondensation Reactions (Transurethanization Pathway)
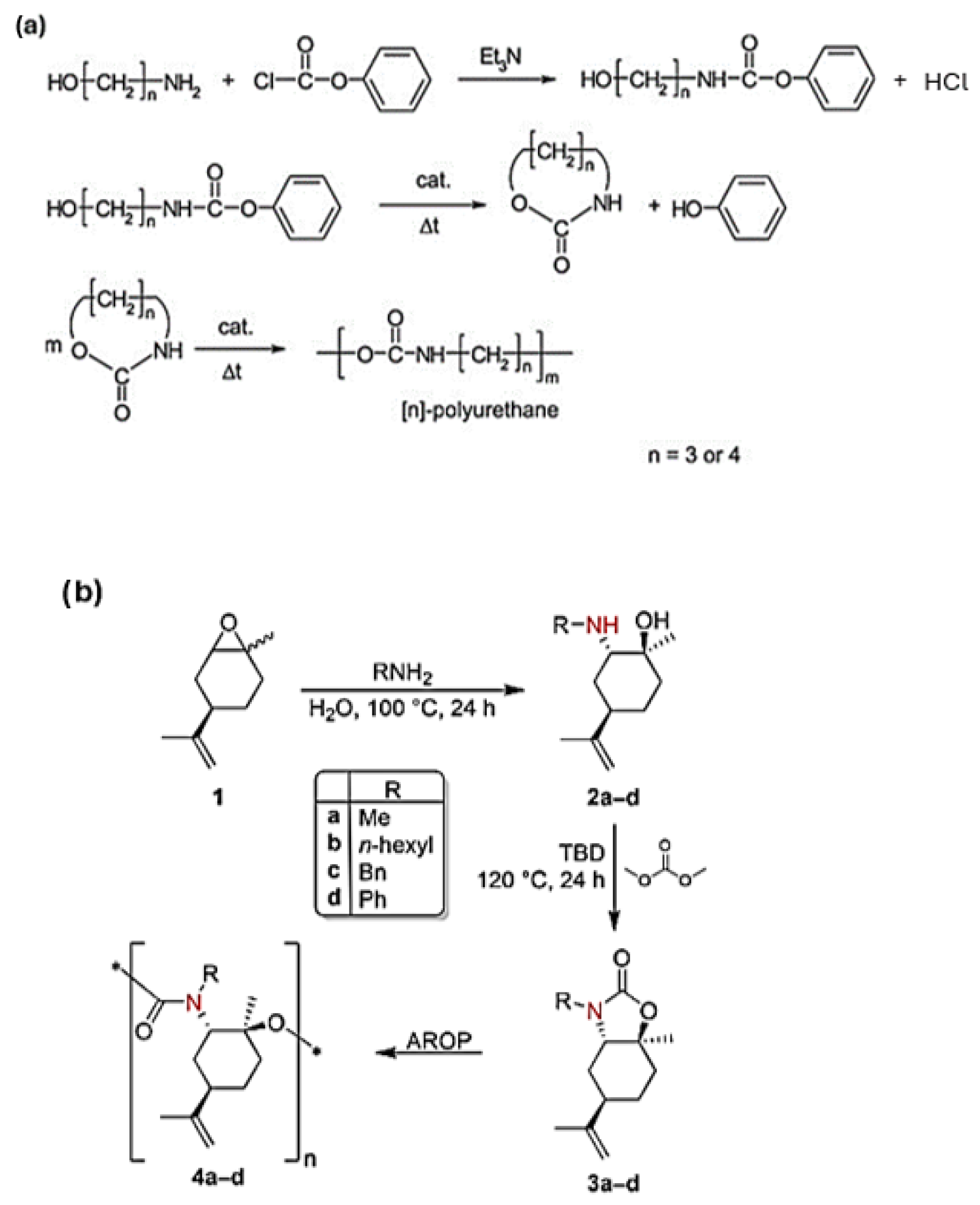
4.2. Ring-Opening Polymerization of Cyclic Carbamates
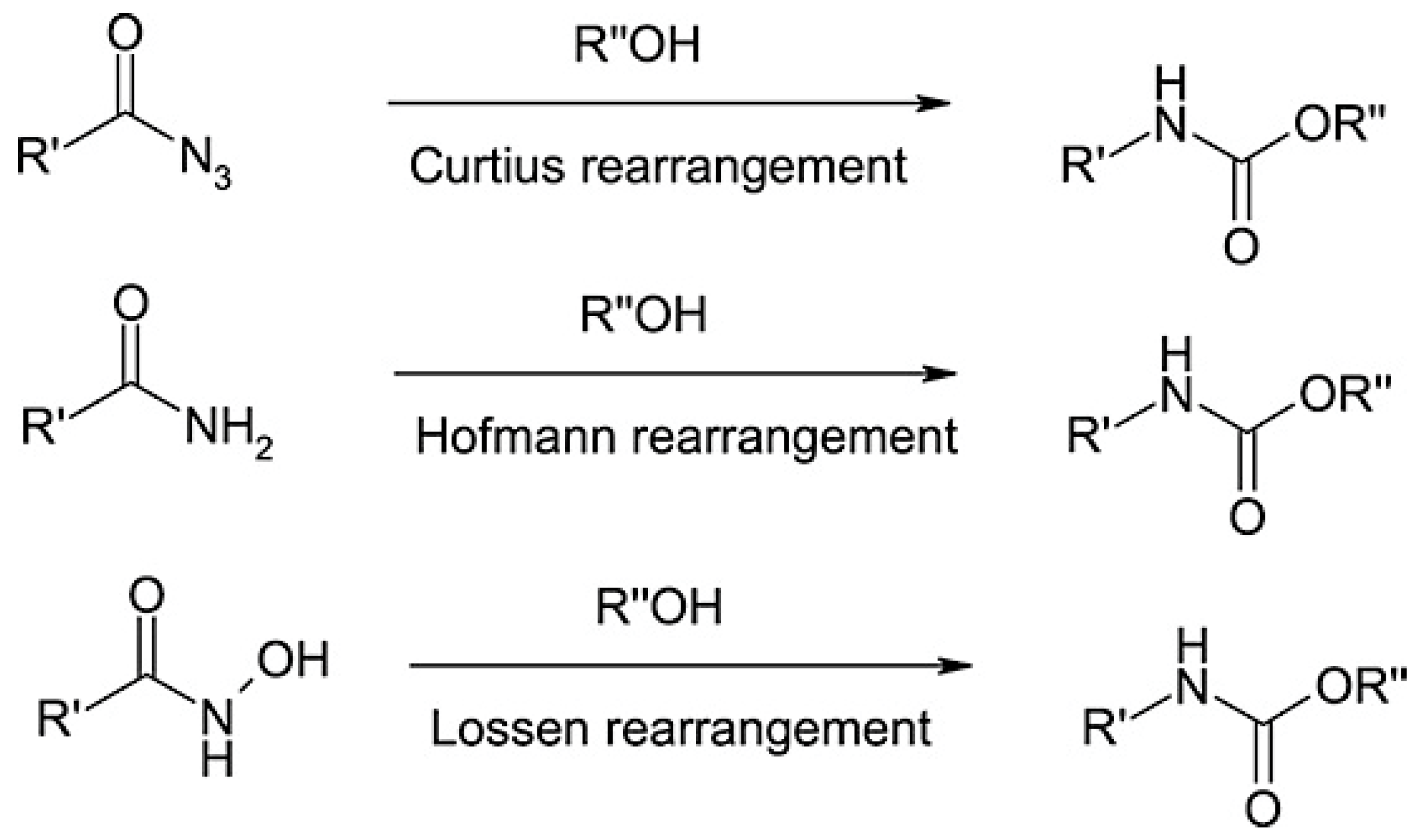
4.3. Rearrangement Reactions
4.4. Polyaddition of Cyclic Carbonates and Amines
5. Bio-Based Synthesis of NIPU
5.1. Bio-Based Synthetic Routes for Cyclic Carbonates
5.2. Bio-Based Synthetic Routes for Polyamines
6. Circular Economy in NIPUs: A Lifecycle Perspective
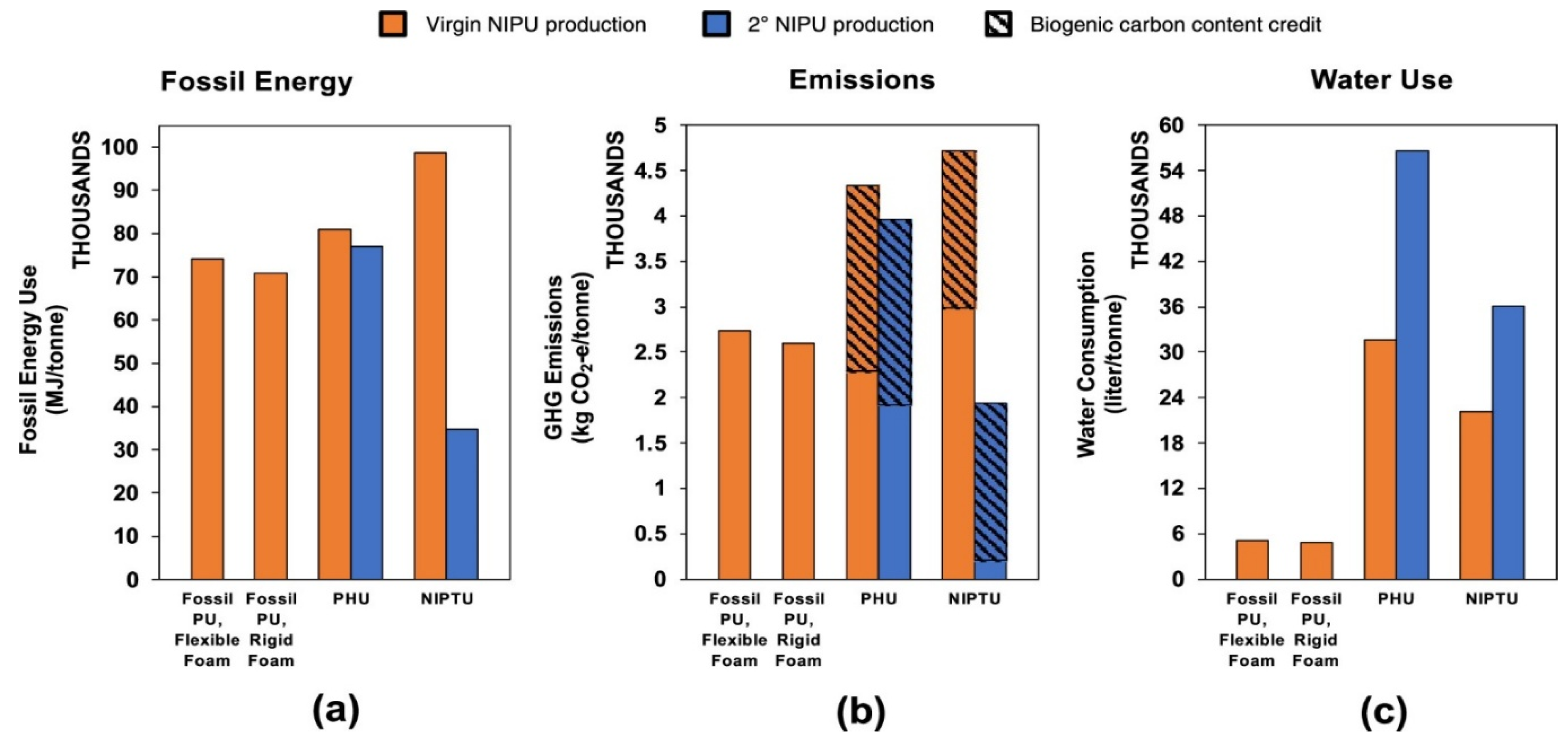
7. Sustainability and Environmental Impacts
8. End of Life of PUs and NIPUs
9. Application Fields of NIPUs
9.1. NIPUs for Coatings
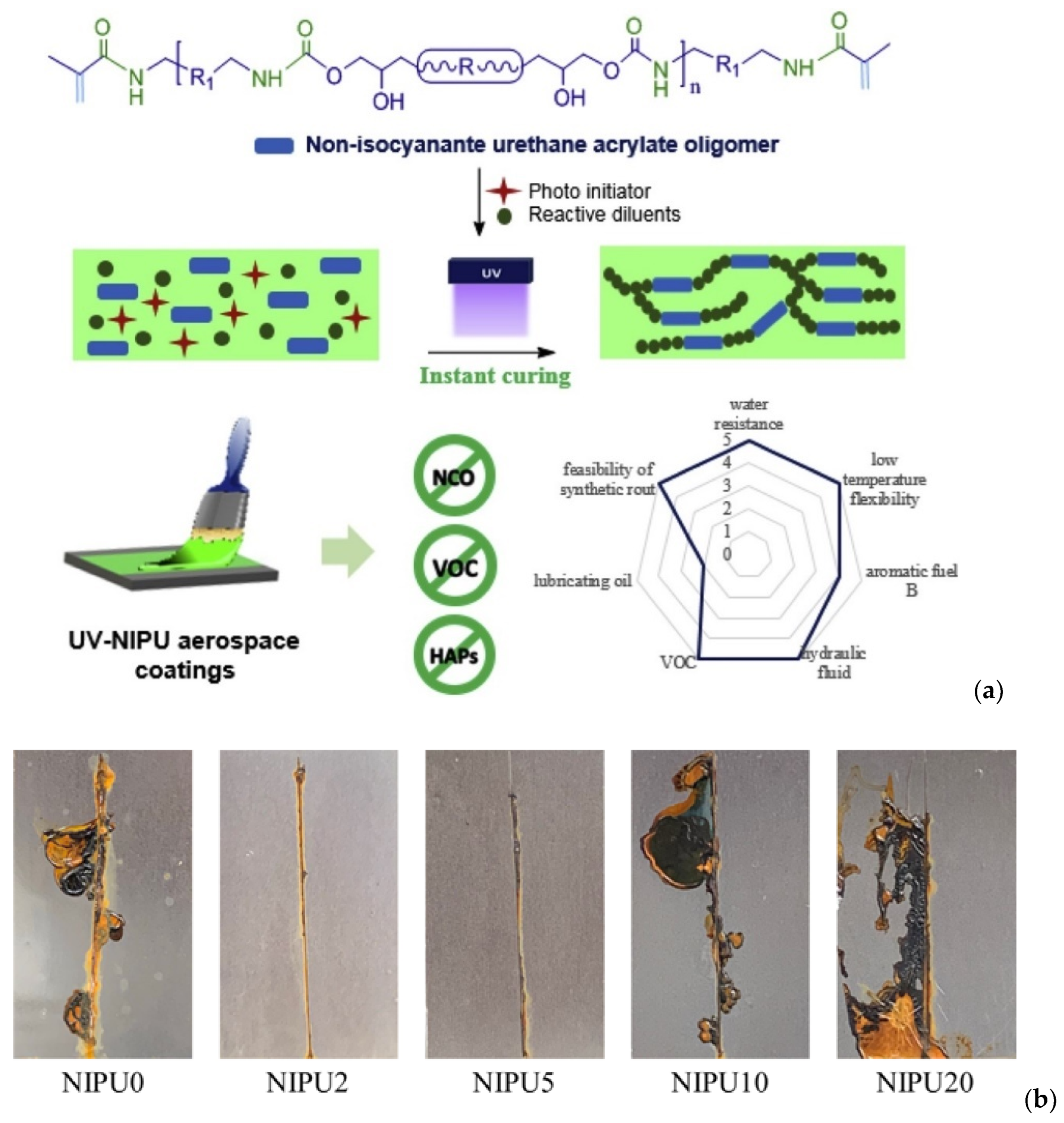
9.2. NIPUs for Adhesives
9.3. NIPUs for Foams
9.4. NIPUs for Biomedical Applications
9.5. Additional NIPU Applications
10. Challenges and Opportunities in Scaling Up NIPU Production
11. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bayer, O. Das Di-Isocyanat-Polyadditionsverfahren (Polyurethane). Angew. Chem. 1947, 59, 257–272. [Google Scholar] [CrossRef]
- Suryawanshi, Y.; Sanap, P.; Wani, V. Advances in the Synthesis of Non-Isocyanate Polyurethanes. Polym. Bull. 2019, 76, 3233–3246. [Google Scholar] [CrossRef]
- De Souza, F.M.; Kahol, P.K.; Gupta, R.K. Introduction to Polyurethane Chemistry. ACS Symp. Ser. 2021, 1380, 1–24. [Google Scholar] [CrossRef]
- Mao, H.; Guo, Y.; Han, Q.; He, X.; Zheng, C. Environmentally Friendly Non-Isocyanate Polyurethane Binder for Pigment Dyeing. Macromol. Res. 2025, 33, 407–414. [Google Scholar] [CrossRef]
- Dhore, N.; Prasad, E.; Narayan, R.; Rao, C.R.K.; Palanisamy, A. Studies on Biobased Non-Isocyanate Polyurethane Coatings with Potential Corrosion Resistance. Sustain. Chem. 2023, 4, 95–109. [Google Scholar] [CrossRef]
- Sahoo, S.; Mohanty, S.; Nayak, S.K. Biobased Polyurethane Adhesive over Petroleum Based Adhesive: Use of Renewable Resource. J. Macromol. Sci. Part A Pure Appl. Chem. 2018, 55, 36–48. [Google Scholar] [CrossRef]
- Zhang, L.; Luo, X.; Qin, Y.; Li, Y. A Novel 2,5-Furandicarboxylic Acid-Based Bis(Cyclic Carbonate) for the Synthesis of Biobased Non-Isocyanate Polyurethanes. RSC Adv. 2017, 7, 37–46. [Google Scholar] [CrossRef]
- Morales-Cerrada, R.; Tavernier, R.; Caillol, S. Fully Bio-Based Thermosetting Polyurethanes from Bio-Based Polyols and Isocyanates. Polymers 2021, 13, 1255. [Google Scholar] [CrossRef]
- Fereidoon, A.; Ghasemi-Ghalebahman, A.; Amini-Nejad, R.; Golshan-Ebrahimi, N. Experimental Investigation on Self-Activated Healing Performance of Thermosetting Polyurethane Prepared by Tungsten (VI) Chloride Catalyst. Mater. Res. Express 2020, 7, 35705. [Google Scholar] [CrossRef]
- Tao, Y.; Liang, X.; Zhang, J.; Lei, I.M.; Liu, J. Polyurethane Vitrimers: Chemistry, Properties and Applications. J. Polym. Sci. 2023, 61, 2233–2253. [Google Scholar] [CrossRef]
- Armanasco, F.; D’hers, S.; Chiacchiarelli, L.M. Kinetic and Chemorheological Modeling of Thermosetting Polyurethanes Obtained from an Epoxidized Soybean Oil Polyol Crosslinked with Glycerin. J. Appl. Polym. Sci. 2022, 139, e53194. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, H.; Xie, Y.; Bao, X.; Pan, L.; Zhao, D.; Chen, J.; Zou, M.; Tian, T.; Li, R. Strain Effect on Dielectricity of Elastic Thermoplastic Polyurethanes. Polymers 2024, 16, 1465. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.S.; Phan, A.; Olikagu, C.; Lee, T.; Loy, D.A.; Kwon, M.; Paik, H.J.; Hong, S.J.; Bang, J.; Parker, W.O.; et al. Segmented Polyurethanes and Thermoplastic Elastomers from Elemental Sulfur with Enhanced Thermomechanical Properties and Flame Retardancy. Angew. Chem.-Int. Ed. 2021, 60, 22900–22907. [Google Scholar] [CrossRef] [PubMed]
- Kohári, A.; Bárány, T. Sustainable Thermoplastic Elastomers Based on Thermoplastic Polyurethane and Ground Tire Rubber. J. Appl. Polym. Sci. 2024, 141, e56157. [Google Scholar] [CrossRef]
- Datta, J.; Kasprzyk, P. Thermoplastic Polyurethanes Derived from Petrochemical or Renewable Resources: A Comprehensive Review. Polym. Eng. Sci. 2018, 58, E14–E35. [Google Scholar] [CrossRef]
- Vaishali, M.; Gopal, S.; Sreeram, K.J. A Facile Approach towards Recycling of Polyurethane Coated PET Fabrics. RSC Sustain. 2024, 2, 2324–2334. [Google Scholar] [CrossRef]
- Simón, D.; Borreguero, A.M.; de Lucas, A.; Rodríguez, J.F. Recycling of Polyurethanes from Laboratory to Industry, a Journey towards the Sustainability. Waste Manag. 2018, 76, 147–171. [Google Scholar] [CrossRef]
- Nees, M.; Adeel, M.; Pazdur, L.; Porters, M.; Vande Velde, C.M.L.; Billen, P. Polyurethane Waste Recycling: Thermolysis of the Carbamate Fraction. ACS Omega 2024, 9, 43438–43446. [Google Scholar] [CrossRef]
- Wieczorek, K.; Bukowski, P.; Stawiński, K.; Ryłko, I. Recycling of Polyurethane Foams via Glycolysis: A Review. Materials 2024, 17, 4617. [Google Scholar] [CrossRef]
- Zarezadeh, E.; Tangestani, M.; Jafari, A.J. A Systematic Review of Methodologies and Solutions for Recycling Polyurethane Foams to Safeguard the Environment. Heliyon 2024, 10, e40724. [Google Scholar] [CrossRef]
- ATSDR. ATSDR MMG for Phosgene; ATSDR: Atlanta, GA, USA, 2002.
- Bengtström, L.; Salden, M.; Stec, A.A. The Role of Isocyanates in Fire Toxicity. Fire Sci. Rev. 2016, 5, 4. [Google Scholar] [CrossRef]
- Pawlak, M.; Pobłocki, K.; Drzeżdżon, J.; Gawdzik, B.; Jacewicz, D. Isocyanates and Isocyanides—Life-Threatening Toxins or Essential Compounds? Sci. Total Environ. 2024, 934, 173250. [Google Scholar] [CrossRef] [PubMed]
- Karol, M.H. Respiratory Effects of Inhaled Isocyanates. Crit. Rev. Toxicol. 1986, 16, 349–379. [Google Scholar] [CrossRef] [PubMed]
- Varma, D.R.; Guest, I. The Bhopal Accident and Methyl Isocyanate Toxicity. J. Toxicol. Environ. Health 1993, 40, 513–529. [Google Scholar] [CrossRef]
- Vidakis, N.; Petousis, M.; Mountakis, N.; David, C.N.; Sagris, D.; Das, S.C. Thermomechanical Response of Thermoplastic Polyurethane Used in MEX Additive Manufacturing over Repetitive Mechanical Recycling Courses. Polym. Degrad. Stab. 2023, 207, 110232. [Google Scholar] [CrossRef]
- He, P.; Lu, H.; Ruan, H.; Wang, C.; Zhang, Q.; Huang, Z.; Liu, J. Mechanochemistry: An Efficient Way to Recycle Thermoset Polyurethanes. Polymers 2022, 14, 3277. [Google Scholar] [CrossRef]
- Rossignolo, G.; Malucelli, G.; Lorenzetti, A. Recycling of Polyurethanes: Where We Are and Where We Are Going. Green Chem. 2024, 26, 1132–1152. [Google Scholar] [CrossRef]
- Ugarte, L.; Calvo-Correas, T.; Gonzalez-Gurrutxaga, I.; Peña, C.; Etxeberria, O.; Corcuera, M.; Eceiza, A. Towards Circular Economy: Different Strategies for Polyurethane Waste Recycling and the Obtaining of New Products. Proceedings 2018, 2, 1490. [Google Scholar] [CrossRef]
- Components, P.; Mark, F.; Kamprath, A. End-of-Life Vehicles Recovery and Recycling Polyurethane Seat Cushion Recycling Options Analysis. SAE Tech. Pap. 2004. [Google Scholar] [CrossRef]
- Heiran, R.; Ghaderian, A.; Reghunadhan, A.; Sedaghati, F.; Thomas, S.; Haghighi, A. hossein. Glycolysis: An Efficient Route for Recycling of End of Life Polyurethane Foams. J. Polym. Res. 2021, 28, 22. [Google Scholar] [CrossRef]
- Wu, C.-H.; Chang, C.-Y.; Li, J.-K. Glycolysis of Rigid Polyurethane from Waste Refrigerators. Polym. Degrad. Stab. 2002, 75, 413–421. [Google Scholar] [CrossRef]
- Bhatti, H.; Ahmad, I. Methods for Polyurethane and Polyurethane Composites, Recycling and Recovery: A Review. React. Funct. Polym. 2007, 67, 675–692. [Google Scholar] [CrossRef]
- Mahoney, L.R.; Weiner, S.A.; Ferris, F.C. Hydrolysis of Polyurethane Foam Waste. Environ. Sci. Technol. 1974, 8, 135–139. [Google Scholar] [CrossRef]
- Cornille, A.; Auvergne, R.; Figovsky, O.; Boutevin, B.; Caillol, S. A Perspective Approach to Sustainable Routes for Non-Isocyanate Polyurethanes. Eur. Polym. J. 2017, 87, 535–552. [Google Scholar] [CrossRef]
- Pathak, R.; Kathalewar, M.; Wazarkar, K.; Sabnis, A. Progress in Organic Coatings Non-Isocyanate Polyurethane (NIPU) from Tris-2-Hydroxy Ethyl Isocyanurate Modified Fatty Acid for Coating Applications. Prog. Org. Coat. 2015, 89, 160–169. [Google Scholar] [CrossRef]
- Catalá, J.; Guerra, I.; García-Vargas, J.M.; Ramos, M.J.; García, M.T.; Rodríguez, J.F. Tailor-Made Bio-Based Non-Isocyanate Polyurethanes (NIPUs). Polymers 2023, 15, 1589. [Google Scholar] [CrossRef]
- Rayung, M.; Ghani, N.A.; Hasanudin, N. A Review on Vegetable Oil-Based Non Isocyanate Polyurethane: Towards a Greener and Sustainable Production Route. RSC Adv. 2024, 14, 9273–9299. [Google Scholar] [CrossRef]
- Guan, J.; Song, Y.; Lin, Y.; Yin, X.; Zuo, M.; Zhao, Y.; Tao, X.; Zheng, Q. Progress in Study of Non-Isocyanate Polyurethane Progress in Study of Non-Isocyanate Polyurethane. Ind. Eng. Chem. 2017, 50, 6517–6527. [Google Scholar] [CrossRef]
- Stachak, P.; Łukaszewska, I.; Hebda, E.; Pielichowski, K. Recent Advances in Fabrication of Non-Isocyanate Polyurethane-Based Composite Materials. Materials 2021, 14, 3497. [Google Scholar] [CrossRef]
- Cornille, A.; Dworakowska, S.; Bogdal, D.; Boutevin, B.; Caillol, S. A New Way of Creating Cellular Polyurethane Materials: NIPU Foams. Eur. Polym. J. 2015, 66, 129–138. [Google Scholar] [CrossRef]
- Fisseler-Eckhoff, A.; Bartsch, H.; Zinsky, R.; Schirren, J. Environmental Isocyanate-Induced Asthma: Morphologic and Pathogenetic Aspects of an Increasing Occupational Disease. Int. J. Environ. Res. Public Health 2011, 8, 3672–3687. [Google Scholar] [CrossRef] [PubMed]
- Mundo, F.; Caillol, S.; Ladmiral, V.; Meier, M.A.R. On Sustainability Aspects of the Synthesis of Five-Membered Cyclic Carbonates. ACS Sustain. Chem. Eng. 2024, 12, 6452–6466. [Google Scholar] [CrossRef]
- Alves, M.; Grignard, B.; Mereau, R.; Jerome, C.; Tassaing, T.; Detrembleur, C. Organocatalyzed Coupling of Carbon Dioxide with Epoxides for the Synthesis of Cyclic Carbonates: Catalyst Design and Mechanistic Studies. Catal. Sci. Technol. 2017, 7, 2651–2684. [Google Scholar] [CrossRef]
- Nikje, M.M.A.; Garmarudi, A.B.; Idris, A.B. Polyurethane Waste Reduction and Recycling: From Bench to Pilot Scales. Des. Monomers Polym. 2011, 14, 395–421. [Google Scholar] [CrossRef]
- Dyer, E.; Scott, H. The Preparation of Polymeric and Cyclic Urethans and Ureas from Ethylene Carbonate and Amines. J. Am. Chem. Soc. 1957, 79, 672–675. [Google Scholar] [CrossRef]
- Nowick, J.S.; Powell, N.A.; Nguyen, T.M.; Noronha, G. An Improved Method for the Synthesis of Enantiomerically Pure Amino Acid Ester Isocyanates. J. Org. Chem. 1992, 57, 7364–7366. [Google Scholar] [CrossRef]
- Chaturvedi, D. Perspectives on the Synthesis of Organic Carbamates. Tetrahedron 2012, 68, 15–45. [Google Scholar] [CrossRef]
- Qin, F.; Li, Q.; Wang, J.; Feng, Y.; Kang, M.; Zhu, Y.; Wang, X. One Pot Synthesis of Methyl N Phenyl Carbamate from Aniline, Urea and Methanol. Catal. Lett. 2008, 126, 419–425. [Google Scholar] [CrossRef]
- Shi, F.; Deng, Y. Polymer-Immobilized Gold Catalysts for the Efficient and Clean Syntheses of Carbamates and Symmetric Ureas by Oxidative Carbonylation of Aniline and Its Derivatives. J. Catal. 2002, 211, 548–551. [Google Scholar] [CrossRef]
- Peterson, S.L.; Stucka, S.M.; Dinsmore, C.J. Parallel Synthesis of Ureas and Carbamates from Amines and CO2 under Mild Conditions. Org. Lett. 2010, 12, 1340–1343. [Google Scholar] [CrossRef]
- Wołosz, D. Non-Isocyanate Aliphatic–Aromatic Poly(Carbonate-Urethane)s—An Insight into Transurethanization Reactions and Structure–Property Relationships. Int. J. Mol. Sci. 2022, 23, 999. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Zhang, Y.; Fan, Y.; Rager, M.; Bouteiller, L.; Li, M.; Thomas, C.M. Polymerization of Cyclic Carbamates: A Practical Route to Aliphatic Polyurethanes. Macromolecules 2019, 52, 2719–2724. [Google Scholar] [CrossRef]
- Neffgen, S.; Keul, H. Ring-Opening Polymerization of Cyclic Urethanes and Ring-Closing Depolymerization of the Respective Polyurethanes. Macromol. Rapid Commun. 1996, 17, 373–382. [Google Scholar] [CrossRef]
- Ihata, O.; Kayaki, Y.; Ikariya, T. Synthesis of Thermoresponsive Polyurethane from 2-Methylaziridine and Supercritical Carbon Dioxide. Angew. Chem.-Int. Ed. 2004, 43, 717–719. [Google Scholar] [CrossRef]
- Rokicki, G.; Parzuchowski, P.G.; Mazurek, M. Non-Isocyanate Polyurethanes: Synthesis, Properties, and Applications. Polym. Adv. Technol. 2015, 26, 707–761. [Google Scholar] [CrossRef]
- Mcelroy, C.R.; Aricò, F.; Benetollo, F.; Tundo, P.; Foscari, C.; Ambientali, S. Cyclization Reaction of Amines with Dialkyl Carbonates to Yield 1,3-Oxazinan-2-Ones. Pure Appl. Chem. 2012, 84, 707–719. [Google Scholar] [CrossRef]
- Rubino, L.; Patamia, V.; Rescifina, A.; Galimberti, M. Cyclic Carbamates Based on (R)-(+)-Limonene Oxide for Ring-Opening Polymerization. Sci. Rep. 2024, 14, 23521. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Sarkar, A.; Brindisi, M. The Curtius Rearrangement: Mechanistic Insight and Recent Applications in Natural Product Syntheses. Org. Biomol. Chem. 2018, 16, 2006–2027. [Google Scholar] [CrossRef]
- Strotman, N.A.; Ortiz, A.; Savage, S.A.; Wilbert, C.R.; Ayers, S.; Kiau, S. Revisiting a Classic Transformation: A Lossen Rearrangement Initiated by Nitriles and “Pseudo-Catalytic” in Isocyanate. J. Org. Chem. 2017, 82, 4044–4049. [Google Scholar] [CrossRef]
- Kreye, O.; Wald, S.; Meier, M. ChemInform Abstract: Introducing Catalytic Lossen Rearrangements: Sustainable Access to Carbamates and Amines. Adv. Synth. Catal. 2013, 2013, 81. [Google Scholar] [CrossRef]
- Groszos, S.J.; Drechsel, E.K. Method of Preparing a Polyurethane. U.S. Patent US2802022A, 6 August 1957. [Google Scholar]
- Gomez-Lopez, A.; Panchireddy, S.; Grignard, B.; Calvo, I.; Jerome, C.; Detrembleur, C.; Sardon, H. Poly(Hydroxyurethane) Adhesives and Coatings: State-of-the-Art and Future Directions. ACS Sustain. Chem. Eng. 2021, 9, 9541–9562. [Google Scholar] [CrossRef] [PubMed]
- Kathalewar, M.S.; Joshi, P.B.; Sabnis, A.S.; Malshe, V.C. Non-Isocyanate Polyurethanes: From Chemistry to Applications. RSC Adv. 2013, 3, 4110–4129. [Google Scholar] [CrossRef]
- Carré, C.; Ecochard, Y.; Caillol, S.; Avérous, L. From the Synthesis of Biobased Cyclic Carbonate to Polyhydroxyurethanes: A Promising Route towards Renewable Non-Isocyanate Polyurethanes. ChemSusChem 2019, 12, 3410–3430. [Google Scholar] [CrossRef] [PubMed]
- Blattmann, H.; Fleischer, M.; Bähr, M.; Mülhaupt, R. Isocyanate- and Phosgene-Free Routes to Polyfunctional Cyclic Carbonates and Green Polyurethanes by Fixation of Carbon Dioxide. Macromol. Rapid Commun. 2014, 35, 1238–1254. [Google Scholar] [CrossRef]
- Wu, Z.; Tang, L.; Dai, J.; Qu, J. Synthesis and Properties of Fluorinated Non-Isocyanate Polyurethanes Coatings with Good Hydrophobic and Oleophobic Properties. J. Coat. Technol. Res. 2019, 16, 1233–1241. [Google Scholar] [CrossRef]
- Valette, V.; Kébir, N.; Burel, F.; Lecamp, L. Design of Biobased Non-Isocyanate Polyurethane (NIPU) Foams Blown with Water and/or Ethanol. Express Polym. Lett. 2023, 17, 974–990. [Google Scholar] [CrossRef]
- Tamami, B.; Sohn, S.; Wilkes, G.L. Incorporation of Carbon Dioxide into Soybean Oil and Subsequent Preparation and Studies of Nonisocyanate Polyurethane Networks. J. Appl. Polym. Sci. 2004, 92, 883–891. [Google Scholar] [CrossRef]
- Lopes, E.J.C.; Ribeiro, A.P.C.; Martins, L.M.D.R.S. New Trends in the Conversion of CO2 to Cyclic Carbonates. Catalysts 2020, 10, 479. [Google Scholar] [CrossRef]
- Vol, P.L.; Carbon, C.D. Copolymerization of Carbon Dioxide. J. Polym. Sci. 1969, 7, 287–292. [Google Scholar]
- Tanaka, S.; Nakashima, T.; Maeda, T.; Ratanasak, M.; Hasegawa, J.Y.; Kon, Y.; Tamura, M.; Sato, K. Quaternary Alkyl Ammonium Salt-Catalyzed Transformation of Glycidol to Glycidyl Esters by Transesterification of Methyl Esters. ACS Catal. 2018, 8, 1097–1103. [Google Scholar] [CrossRef]
- Tanaka, S.; Nakajima, Y.; Ogawa, A.; Kuragano, T.; Kon, Y.; Tamura, M.; Sato, K.; Copéret, C. DNP NMR Spectroscopy Enabled Direct Characterization of Polystyrene-Supported Catalyst Species for Synthesis of Glycidyl Esters by Transesterification. Chem. Sci. 2022, 13, 4490–4497. [Google Scholar] [CrossRef] [PubMed]
- Alves, L.R.; Carriello, G.M.; Pegoraro, G.M.; Gomes, D.R.; de Lourdes Rezende, M.; de Menezes, A.J. Development and Characterization of a Simple and Fast Castor Oil-Based Polyurethane Coating. Polym. Bull. 2024, 81, 17049–17074. [Google Scholar] [CrossRef]
- Pouladi, J.; Mirabedini, S.M.; Eivaz Mohammadloo, H.; Rad, N.G. Synthesis of Novel Plant Oil-Based Isocyanate-Free Urethane Coatings and Study of Their Anti-Corrosion Properties. Eur. Polym. J. 2021, 153, 110502. [Google Scholar] [CrossRef]
- Michel, T.; Cokoja, M.; Kühn, F.E. Journal of Molecular Catalysis A: Chemical Catalytic Epoxidation of Camphene Using Methyltrioxorhenium (VII) as Catalyst. J. Mol. Catal. A Chem. 2013, 368–369, 145–151. [Google Scholar] [CrossRef]
- Martínez, J.; de la Cruz-Martínez, F.; Martínez de Sarasa Buchaca, M.; Fernández-Baeza, J.; Sánchez-Barba, L.F.; North, M.; Castro-Osma, J.A.; Lara-Sánchez, A. Efficient Synthesis of Cyclic Carbonates from Unsaturated Acids and Carbon Dioxide and Their Application in the Synthesis of Biobased Polyurethanes. Chempluschem 2021, 86, 460–468. [Google Scholar] [CrossRef]
- Fache, M.; Darroman, E.; Besse, V.; Auvergne, R.; Caillol, S.; Boutevin, B. Vanillin, a Promising Biobased Building-Block for Monomer Synthesis. Green Chem. 2014, 16, 1987–1998. [Google Scholar] [CrossRef]
- Fache, M.; Auvergne, R.; Boutevin, B.; Caillol, S. New Vanillin-Derived Diepoxy Monomers for the Synthesis of Biobased Thermosets. Eur. Polym. J. 2015, 67, 527–538. [Google Scholar] [CrossRef]
- Aouf, C.; Benyahya, S.; Esnouf, A.; Caillol, S.; Boutevin, B.; Fulcrand, H. Tara Tannins as Phenolic Precursors of Thermosetting Epoxy Resins. Eur. Polym. J. 2014, 55, 186–198. [Google Scholar] [CrossRef]
- Pescarmona, P.P. Cyclic Carbonates Synthesised from CO2: Applications, Challenges and Recent Research Trends. Curr. Opin. Green Sustain. Chem. 2021, 29, 100457. [Google Scholar] [CrossRef]
- Pfister, D.P.; Xia, Y.; Larock, R.C. Recent Advances in Vegetable Oil-Based Polyurethanes. ChemSusChem 2011, 4, 703–717. [Google Scholar] [CrossRef]
- Bähr, M.; Mülhaupt, R. Linseed and Soybean Oil-Based Polyurethanes Prepared via the Non-Isocyanate Route and Catalytic Carbon Dioxide Conversion. Green Chem. 2012, 14, 483–489. [Google Scholar] [CrossRef]
- Marcovich, N.E.; Kurańska, M.; Prociak, A.; Malewska, E.; Kulpa, K. Open Cell Semi-Rigid Polyurethane Foams Synthesized Using Palm Oil-Based Bio-Polyol. Ind. Crops Prod. 2017, 102, 88–96. [Google Scholar] [CrossRef]
- Pradhan, S.; Pandey, P.; Mohanty, S.; Nayak, S.K. Insight on the Chemistry of Epoxy and Its Curing for Coating Applications: A Detailed Investigation and Future Perspectives. Polym.-Plast. Technol. Eng. 2016, 55, 862–877. [Google Scholar] [CrossRef]
- Yang, Z.; Peng, H.; Wang, W.; Liu, T. Crystallization Behavior of Poly(ε-Caprolactone)/Layered Double Hydroxide Nanocomposites. J. Appl. Polym. Sci. 2010, 116, 2658–2667. [Google Scholar] [CrossRef]
- Werlinger, F.; Caballero, M.P.; Trofymchuk, O.S.; Flores, M.E.; Moreno-Villoslada, I.; Cruz-Martínez, F.D.L.; Castro-Osma, J.A.; Tejeda, J.; Martínez, J.; Lara-Sánchez, A. Turning Waste into Resources. Efficient Synthesis of Biopolyurethanes from Used Cooking Oils and CO2. J. CO2 Util. 2024, 79, 102659. [Google Scholar] [CrossRef]
- Ng, F.; Couture, G.; Philippe, C.; Boutevin, B.; Caillol, S. Bio-Based Aromatic Epoxy Monomers for Thermoset Materials. Molecules 2017, 22, 149. [Google Scholar] [CrossRef]
- Chen, Q.; Gao, K.; Peng, C.; Xie, H.; Zhao, Z.K.; Bao, M. Preparation of Lignin/Glycerol-Based Bis(Cyclic Carbonate) for the Synthesis of Polyurethanes. Green Chem. 2015, 17, 4546–4551. [Google Scholar] [CrossRef]
- Janvier, M.; Ducrot, P.H.; Allais, F. Isocyanate-Free Synthesis and Characterization of Renewable Poly(Hydroxy)Urethanes from Syringaresinol. ACS Sustain. Chem. Eng. 2017, 5, 8648–8656. [Google Scholar] [CrossRef]
- Esmaeili, N.; Zohuriaan-Mehr, M.J.; Salimi, A.; Vafayan, M.; Meyer, W. Tannic Acid Derived Non-Isocyanate Polyurethane Networks: Synthesis, Curing Kinetics, Antioxidizing Activity and Cell Viability. Thermochim. Acta 2018, 664, 64–72. [Google Scholar] [CrossRef]
- Fidalgo, D.M.; Kolender, A.A.; Varela, O. Stereoregular Poly-O-Methyl[m,n]-Polyurethanes Derived from D-Mannitol. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 463–470. [Google Scholar] [CrossRef]
- Mazurek-Budzyńska, M.M.; Rokicki, G.; Drzewicz, M.; Guńka, P.A.; Zachara, J. Bis(Cyclic Carbonate) Based on D-Mannitol, D-Sorbitol and Di(Trimethylolpropane) in the Synthesis of Non-Isocyanate Poly(Carbonate-Urethane)s. Eur. Polym. J. 2016, 84, 799–811. [Google Scholar] [CrossRef]
- Furtwengler, P.; Avérous, L. From D-Sorbitol to Five-Membered Bis(Cyclo-Carbonate) as a Platform Molecule for the Synthesis of Different Original Biobased Chemicals and Polymers. Sci. Rep. 2018, 8, 9134. [Google Scholar] [CrossRef] [PubMed]
- Aouf, C.; Nouailhas, H.; Fache, M.; Caillol, S.; Boutevin, B.; Fulcrand, H. Multi-Functionalization of Gallic Acid. Synthesis of a Novel Bio-Based Epoxy Resin. Eur. Polym. J. 2013, 49, 1185–1195. [Google Scholar] [CrossRef]
- Schmidt, S.; Ritter, B.S.; Kratzert, D.; Bruchmann, B.; Mülhaupt, R. Isocyanate-Free Route to Poly(Carbohydrate-Urethane) Thermosets and 100% Bio-Based Coatings Derived from Glycerol Feedstock. Macromolecules 2016, 49, 7268–7276. [Google Scholar] [CrossRef]
- Bähr, M.; Bitto, A.; Mülhaupt, R. Cyclic Limonene Dicarbonate as a New Monomer for Non-Isocyanate Oligo- and Polyurethanes (NIPU) Based upon Terpenes. Green Chem. 2012, 14, 1447–1454. [Google Scholar] [CrossRef]
- Kathalewar, M.; Sabnis, A.; D’Mello, D. Isocyanate Free Polyurethanes from New CNSL Based Bis-Cyclic Carbonate and Its Application in Coatings. Eur. Polym. J. 2014, 57, 99–108. [Google Scholar] [CrossRef]
- Dworakowska, S.; Cornille, A.; Bogdal, D.; Boutevin, B.; Caillol, S. Formulation of Bio-Based Epoxy Foams from Epoxidized Cardanol and Vegetable Oil Amine. Eur. J. Lipid Sci. Technol. 2015, 117, 1893–1902. [Google Scholar] [CrossRef]
- Garipov, R.M.; Mikheev, V.V.; Deberdeev, T.R.; Irzhak, V.I.; Berlin, A.A. Study of the Curing Kinetics for Modified Epoxy Amine Systems Using Model Compounds. Dokl. Phys. Chem. 2003, 392, 268–271. [Google Scholar] [CrossRef]
- Ghasemlou, M.; Daver, F.; Ivanova, E.P.; Adhikari, B. Bio-Based Routes to Synthesize Cyclic Carbonates and Polyamines Precursors of Non-Isocyanate Polyurethanes: A Review. Eur. Polym. J. 2019, 118, 668–684. [Google Scholar] [CrossRef]
- Nohra, B.; Candy, L.; Blanco, J.-F.; Raoul, Y.; Mouloungui, Z. Aminolysis Reaction of Glycerol Carbonate in Organic and Hydroorganic Medium. J. Am. Oil Chem. Soc. 2012, 89, 1125–1133. [Google Scholar] [CrossRef]
- Mao, H.; Chen, C.-W.; Yan, H.; Rwei, S. Synthesis and Characteristics of Nonisocyanate Polyurethane Composed of Bio-based Dimer Diamine for Supercritical CO2 Foaming Applications. J. Appl. Polym. Sci. 2022, 139, e52841. [Google Scholar] [CrossRef]
- Shoaib, M.; Bahadur, A. Synthesis of Thermally and Mechanically Improved Polyurethane-Urea Elastomers by Using Novel Diamines as Chain Extenders. E-Polymers 2016, 16, 411–418. [Google Scholar] [CrossRef]
- Froidevaux, V.; Negrell, C.; Caillol, S.; Pascault, J.-P.; Boutevin, B. Biobased Amines: From Synthesis to Polymers; Present and Future. Chem. Rev. 2016, 116, 14181–14224. [Google Scholar] [CrossRef] [PubMed]
- Médici, R.; De María, P.D.; Otten, L.G.; Straathof, A.J.J. A High-Throughput Screening Assay for Amino Acid Decarboxylase Activity. Adv. Synth. Catal. 2011, 353, 2369–2376. [Google Scholar] [CrossRef]
- Biermann, U.; Fuermeier, S.; Metzger, J.O. ChemInform Abstract: Some Carbon—Nitrogen and Carbon—Oxygen Bond Forming Additions to Unsaturated Fatty Compounds. ChemInform 1998, 29, 236–246. [Google Scholar] [CrossRef]
- Cok, B.; Tsiropoulos, I.; Roes, A.L.; Patel, M.K. Succinic Acid Production Derived from Carbohydrates: An Energy and Greenhouse Gas Assessment of a Platform Chemical toward a Bio-Based Economy. Biofuels Bioprod. Biorefining 2014, 8, 16–29. [Google Scholar] [CrossRef]
- Pelckmans, M.; Renders, T.; Van De Vyver, S.; Sels, B.F. Bio-Based Amines through Sustainable Heterogeneous Catalysis. Green Chem. 2017, 19, 5303–5331. [Google Scholar] [CrossRef]
- Niemeier, J.; Engel, R.V.; Rose, M. Is Water a Suitable Solvent for the Catalytic Amination of Alcohols? Green Chem. 2017, 19, 2839–2845. [Google Scholar] [CrossRef]
- Wendels, S.; Avérous, L. Biobased Polyurethanes for Biomedical Applications. Bioact. Mater. 2021, 6, 1083–1106. [Google Scholar] [CrossRef]
- Wray, H.E.; Luzzi, S.; D’Arrigo, P.; Griffini, G. Life Cycle Environmental Impact Considerations in the Design of Novel Biobased Polyurethane Coatings. ACS Sustain. Chem. Eng. 2023, 11, 8065–8074. [Google Scholar] [CrossRef]
- Liang, C.; Jadidi, Y.; Chen, Y.; Gracida-Alvarez, U.; Torkelson, J.M.; Hawkins, T.R.; Dunn, J.B. Techno-Economic Analysis and Life Cycle Assessment of Biomass-Derived Polyhydroxyurethane and Nonisocyanate Polythiourethane Production and Reprocessing. ACS Sustain. Chem. Eng. 2024, 12, 12161–12170. [Google Scholar] [CrossRef] [PubMed]
- Al-Salem, S.M.; Lettieri, P.; Baeyens, J. Recycling and Recovery Routes of Plastic Solid Waste (PSW): A Review. Waste Manag. 2009, 29, 2625–2643. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Dong, Q.; Liu, S.; Xie, H.; Liu, L.; Li, J. Recycling and Disposal Methods for Polyurethane Foam Wastes. Procedia Environ. Sci. 2012, 16, 167–175. [Google Scholar] [CrossRef]
- Munzmay, T.; Meckel, W.; Liman, U.; Nefzger, H.; Rasshofer, W.; Dorner, K.-H.; Ruckes, A. Process for the Production of Hydroxyfunctional Compounds from (Polyurethane) Polyurea Waste. U.S. Patent US6020386A, 1 February 2000. [Google Scholar]
- Naber, B.; Lezius, M. Preparation of Recyclate Polyols. U.S. Patent No. 5,556,889, 17 September 1996. [Google Scholar]
- Sendijarevic, V. Process for Chemical Recycling of Polyurethane-Containing Scrap. U.S. Patent US 6,750,260 B2, 15 June 2004. [Google Scholar]
- Choong, P.S.; Chong, N.X.; Wai Tam, E.K.; Seayad, A.M.; Seayad, J.; Jana, S. Biobased Nonisocyanate Polyurethanes as Recyclable and Intrinsic Self-Healing Coating with Triple Healing Sites. ACS Macro Lett. 2021, 10, 635–641. [Google Scholar] [CrossRef]
- Liu, J.; Miao, P.; Leng, X.; Che, J.; Wei, Z.; Li, Y. Chemically Recyclable Biobased Non-Isocyanate Polyurethane Networks from CO2-Derived Six-Membered Cyclic Carbonates. Macromol. Rapid Commun. 2023, 44, 2300263. [Google Scholar] [CrossRef]
- Mucci, V.L.; Hormaiztegui, M.E.V.; Amalvy, J.I.; Aranguren, M.I. Formulation, Structure and Properties of Waterborne Polyurethane Coatings: A Brief Review. J. Adhes. Sci. Technol. 2024, 38, 489–516. [Google Scholar] [CrossRef]
- Figovsky, O.L.; Shapovalov, L.D. Nonisocyanate Polyurethanes for Adhesives and Coatings. In Proceedings of the First International IEEE Conference on Polymers and Adhesives in Microelectronics and Photonics. Incorporating POLY, PEP & Adhesives in Electronics, Potsdam, Germany, 21–24 October 2001; pp. 257–264. [Google Scholar] [CrossRef]
- Błażek, K.; Datta, J. Renewable Natural Resources as Green Alternative Substrates to Obtain Bio-Based Non-Isocyanate Polyurethanes-Review. Crit. Rev. Environ. Sci. Technol. 2019, 49, 173–211. [Google Scholar] [CrossRef]
- Llevot, A.; Meier, M. Perspective: Green Polyurethane Synthesis for Coating Applications. Polym. Int. 2019, 68, 826–831. [Google Scholar] [CrossRef]
- Zareanshahraki, F.; Asemani, H.R.; Skuza, J.; Mannari, V. Synthesis of Non-Isocyanate Polyurethanes and Their Application in Radiation-Curable Aerospace Coatings. Prog. Org. Coat. 2020, 138, 105394. [Google Scholar] [CrossRef]
- Liu, G.; Wu, G.; Chen, J.; Huo, S.; Jin, C.; Kong, Z. Synthesis and Properties of POSS-Containing Gallic Acid-Based Non-Isocyanate Polyurethanes Coatings. Polym. Degrad. Stab. 2015, 121, 247–252. [Google Scholar] [CrossRef]
- Ling, Z.; Zhou, Q. Synthesis and Properties of Linseed Oil-Based Waterborne Non-Isocyanate Polyurethane Coating. Green Chem. 2023, 25, 10082–10090. [Google Scholar] [CrossRef]
- Liu, C.; Wu, J.; Zhou, X.; Zhou, X.; Wu, Z.; Qu, J. Synthesis and Properties of Poly(Dimethylsiloxane)-Based Non-Isocyanate Polyurethanes Coatings with Good Anti-Smudge Properties. Prog. Org. Coat. 2022, 163, 106690. [Google Scholar] [CrossRef]
- Zhang, C.; Huang, K.C.; Wang, H.; Zhou, Q. Anti-Corrosion Non-Isocyanate Polyurethane Polysiloxane Organic/Inorganic Hybrid Coatings. Prog. Org. Coat. 2020, 148, 105855. [Google Scholar] [CrossRef]
- Boisaubert, P.; Kébir, N.; Schuller, A.S.; Burel, F. Photo-Crosslinked Coatings from an Acrylate Terminated Non-Isocyanate Polyurethane (NIPU) and Reactive Diluent. Eur. Polym. J. 2020, 138, 109961. [Google Scholar] [CrossRef]
- Khatoon, H.; Iqbal, S.; Irfan, M.; Darda, A.; Rawat, N.K. A Review on the Production, Properties and Applications of Non-Isocyanate Polyurethane: A Greener Perspective. Prog. Org. Coat. 2021, 154, 106124. [Google Scholar] [CrossRef]
- Panchireddy, S.; Grignard, B.; Thomassin, J.M.; Jerome, C.; Detrembleur, C. Catechol Containing Polyhydroxyurethanes as High-Performance Coatings and Adhesives. ACS Sustain. Chem. Eng. 2018, 6, 14936–14944. [Google Scholar] [CrossRef]
- Xi, X.; Wu, Z.; Pizzi, A.; Gerardin, C.; Lei, H.; Zhang, B.; Du, G. Non-Isocyanate Polyurethane Adhesive from Sucrose Used for Particleboard. Wood Sci. Technol. 2019, 53, 393–405. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, B.; Pan, J.; Zhang, G.; Ma, C.; Zhang, G. Ultrastrong and Versatile Nonisocyanate Polyurethane Adhesive under Extreme Conditions. Chem. Mater. 2023, 35, 7730–7740. [Google Scholar] [CrossRef]
- Chen, X.; Pizzi, A.; Xi, X.; Zhou, X.; Fredon, E.; Gerardin, C. Soy Protein Isolate Non-Isocyanates Polyurethanes (NIPU) Wood Adhesives. J. Renew. Mater. 2021, 9, 1045–1057. [Google Scholar] [CrossRef]
- Saražin, J.; Pizzi, A.; Amirou, S.; Schmiedl, D.; Šernek, M. Organosolv Lignin for Non-Isocyanate Based Polyurethanes (Nipu) as Wood Adhesive. J. Renew. Mater. 2021, 9, 881–907. [Google Scholar] [CrossRef]
- Chen, X.; Pizzi, A.; Fredon, E.; Gerardin, C.; Zhou, X.; Zhang, B.; Du, G. Low Curing Temperature Tannin-Based Non-Isocyanate Polyurethane (NIPU) Wood Adhesives: Preparation and Properties Evaluation. Int. J. Adhes. Adhes. 2022, 112, 103001. [Google Scholar] [CrossRef]
- Zhang, P.; Guo, H.; Qin, C.; Yuan, H.; Cao, Y.; Wang, Z.; Jin, C. Solvent-Free Synthesis of Bio-Based Non-Isocyanate Polyurethane (NIPU) with Robust Adhesive Property and Resistance to Low Temperature. Polym. Test. 2024, 140, 108616. [Google Scholar] [CrossRef]
- Chen, X.; Pizzi, A.; Essawy, H.; Fredon, E.; Gerardin, C.; Guigo, N.; Sbirrazzuoli, N. Non-Furanic Humins-Based Non-Isocyanate Polyurethane (NIPU) Thermoset Wood Adhesives. Polymers 2021, 13, 372. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Lopez, A.; Grignard, B.; Calvo, I.; Detrembleur, C.; Sardon, H. Synergetic Effect of Dopamine and Alkoxysilanes in Sustainable Non-Isocyanate Polyurethane Adhesives. Macromol. Rapid Commun. 2021, 42, 2000538. [Google Scholar] [CrossRef]
- Horizon Databook. Polyurethane Foam Market Size, Share & Trends Analysis Report by Product (Rigid Foam, Flexible Foam), by Application (Bedding & Furniture, Transportation, Packaging, Construction, Electronics, Footwear), by Region, and Segment Forecasts, 2024–2030; Horizon Databook: San Francisco, CA, USA, 2023. [Google Scholar]
- Blattmann, H.; Lauth, M.; Mülhaupt, R. Flexible and Bio-Based Nonisocyanate Polyurethane (NIPU) Foams. Macromol. Mater. Eng. 2016, 301, 944–952. [Google Scholar] [CrossRef]
- Cornille, A.; Guillet, C.; Benyahya, S.; Negrell, C.; Boutevin, B.; Caillol, S. Room Temperature Flexible Isocyanate-Free Polyurethane Foams. Eur. Polym. J. 2016, 84, 873–888. [Google Scholar] [CrossRef]
- Pizzi, A.P. Tannin-Based Biofoams—A Review. J. Renew. Mater. 2019, 7, 477–492. [Google Scholar] [CrossRef]
- Xi, X.; Pizzi, A.; Gerardin, C.; Du, G. Glucose-Biobased Non-Isocyanate Polyurethane Rigid Foams. J. Renew. Mater. 2019, 7, 301–312. [Google Scholar] [CrossRef]
- Ei-Kawy, O.; Hashem, A.; Amin, M.; Ei-Wetery, A. Preparation and Evaluation of [125I]Troxacitabine: L-Nucleoside Model of a Potential Agent for Tumor Diagnosis and Radiotherapy. J. Label. Comp. Radiopharm. 2011, 54, 98–104. [Google Scholar] [CrossRef]
- Yang, T.; Pizzi, A.; Xi, X.; Zhou, X.; Zhang, Q. Preparation and Characterization of Glucose-Based Self-Blowing Non-Isocyanate Polyurethane (NIPU) Foams with Different Acid Catalysts. Polymers 2024, 16, 2899. [Google Scholar] [CrossRef]
- Azadeh, E.; Chen, X.; Pizzi, A.; Gérardin, C.; Gérardin, P.; Essawy, H. Self-Blowing Non-Isocyanate Polyurethane Foams Based on Hydrolysable Tannins. J. Renew. Mater. 2022, 10, 3217–3227. [Google Scholar] [CrossRef]
- Chen, X.; Xi, X.; Pizzi, A.; Fredon, E.; Zhou, X.; Li, J.; Gerardin, C.; Du, G. Preparation and Characterization of Condensed Tannin Non-Isocyanate Polyurethane (NIPU) Rigid Foams by Ambient Temperature Blowing. Polymers 2020, 12, 750. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.L.; Rodriguez-Melendez, D.; Cotton, S.M.; Quan, Y.; Wang, Q.; Grunlan, J.C. Non-Isocyanate Polyurethane Bio-Foam with Inherent Heat and Fire Resistance. Polymers 2022, 14, 5019. [Google Scholar] [CrossRef] [PubMed]
- Choong, P.S.; Hui, Y.L.E.; Lim, C.C. CO2-Blown Nonisocyanate Polyurethane Foams. ACS Macro Lett. 2023, 12, 1094–1099. [Google Scholar] [CrossRef]
- Grignard, B.; Thomassin, J.M.; Gennen, S.; Poussard, L.; Bonnaud, L.; Raquez, J.M.; Dubois, P.; Tran, M.P.; Park, C.B.; Jerome, C.; et al. CO2-Blown Microcellular Non-Isocyanate Polyurethane (NIPU) Foams: From Bio- and CO2-Sourced Monomers to Potentially Thermal Insulating Materials. Green Chem. 2016, 18, 2206–2215. [Google Scholar] [CrossRef]
- Monie, F.; Grignard, B.; Detrembleur, C. Divergent Aminolysis Approach for Constructing Recyclable Self-Blown Nonisocyanate Polyurethane Foams. ACS Macro Lett. 2022, 11, 236–242. [Google Scholar] [CrossRef]
- Medical, L. TecoflexTM TPU. Available online: https://www.lubrizol.com/Health/Medical/Polymers/Tecoflex-TPU (accessed on 1 February 2025).
- Medical, L. Pellethane® TPU. Available online: https://www.lubrizol.com/Health/Medical/Polymers/Pellethane-TPU#:~:text=Pellethane%20%C2%AE%20thermoplastic%20polyurethanes%20%28TPUs%29%20offer%20a%20wide,for%20their%20flexibility%20and%20wide%20range%20of%20hardnesses (accessed on 1 February 2025).
- Medical, L. CarbothaneTM TPU. Available online: https://www.lubrizol.com/Health/Medical/Polymers/Carbothane-TPU (accessed on 1 February 2025).
- DSM. Bionate® Thermoplastic Polycarbonate Polyurethane (PCU); DSM: Calhoun, MI, USA, 2021. [Google Scholar]
- DSM. CarboSil® Thermoplastic Silicone Polycarbonate Polyurethane (TSPCU); DSM: Calhoun, MI, USA, 2021. [Google Scholar]
- La Fuente, C.I.A.; Maniglia, B.C.; Tadini, C.C. Biodegradable Polymers: A Review about Biodegradation and Its Implications and Applications. Packag. Technol. Sci. 2022, 36, 81–95. [Google Scholar] [CrossRef]
- Bourguignon, M.; Thomassin, J.-M.; Grignard, B.; Jérôme, C.; Detrembleur, C. Fast and Facile One-Pot One-Step Preparation of Nonisocyanate Polyurethane Hydrogels in Water at Room Temperature. ACS Sustain. Chem. Eng. 2019, 7, 12601–12610. [Google Scholar] [CrossRef]
- Pramanik, S.K.; Sreedharan, S.; Singh, H.; Khan, M.; Tiwari, K.; Shiras, A.; Smythe, C.; Thomas, J.A.; Das, A. Mitochondria Targeting Non-Isocyanate-Based Polyurethane Nanocapsules for Enzyme-Triggered Drug Release. Bioconjug. Chem. 2018, 29, 3532–3543. [Google Scholar] [CrossRef]
- Aduba, D.C.; Zhang, K.; Kanitkar, A.; Sirrine, J.M.; Verbridge, S.S.; Long, T.E. Electrospinning of Plant Oil-Based, Non-Isocyanate Polyurethanes for Biomedical Applications. J. Appl. Polym. Sci. 2018, 135, 46464. [Google Scholar] [CrossRef]
- Visser, D.; Bakhshi, H.; Rogg, K.; Fuhrmann, E.; Wieland, F.; Schenke-Layland, K.; Meyer, W.; Hartmann, H. Green Chemistry for Biomimetic Materials: Synthesis and Electrospinning of High-Molecular-Weight Polycarbonate-Based Nonisocyanate Polyurethanes. ACS Omega 2022, 7, 39772–39781. [Google Scholar] [CrossRef] [PubMed]
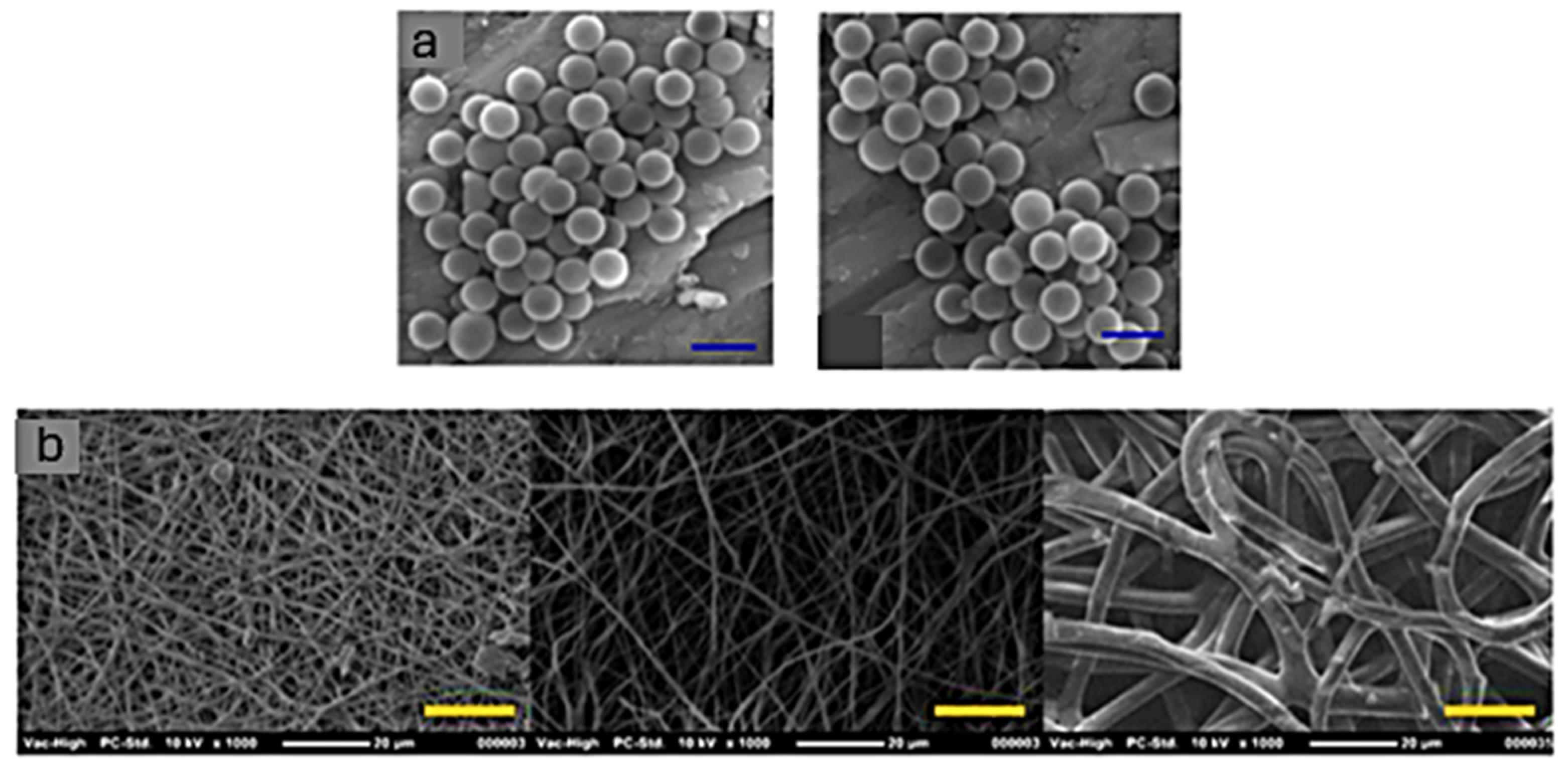
- Melo, S.F.; Nondonfaz, A.; Aqil, A.; Pierrard, A.; Hulin, A.; Delierneux, C.; Ditkowski, B.; Gustin, M.; Legrand, M.; Tullemans, B.M.E.; et al. Design, Manufacturing and Testing of a Green Non-Isocyanate Polyurethane Prosthetic Heart Valve. Biomater. Sci. 2024, 12, 2149–2164. [Google Scholar] [CrossRef] [PubMed]
- Pierrard, A.; Melo, S.F.; Thijssen, Q.; Van Vlierberghe, S.; Lancellotti, P.; Oury, C.; Detrembleur, C.; Jérôme, C. Design of 3D-Photoprintable, Bio-, and Hemocompatible Nonisocyanate Polyurethane Elastomers for Biomedical Implants. Biomacromolecules 2024, 25, 1810–1824. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zheng, Z.; Pathak, J.L.; Feng, W.; Wu, W.; Yang, C.; Wu, L.; Zheng, H. Fabrication and Characterization of Photosensitive Non-Isocyanate Polyurethane Acrylate Resin for 3D Printing of Customized Biocompatible Orthopedic Surgical Guides. Int. J. Bioprint. 2023, 9, 80–93. [Google Scholar] [CrossRef]
- Zhao, Y.; Xia, X.; Zhou, J.; Huang, Z.; Lei, F.; Tan, X.; Yu, D.; Zhu, Y.; Xu, H. Thermoresponsive Behavior of Non-Isocyanate Poly(Hydroxyl)Urethane for Biomedical Composite Materials. Adv. Compos. Hybrid Mater. 2022, 5, 843–852. [Google Scholar] [CrossRef]
- Lam, K.Y.; Lee, C.S.; Pichika, M.R.; Cheng, S.F.; Tan, R.Y.H. Non-Isocyanate Polyurethane-Acrylate as UV- and Thermo-Responsive Plasticizer for Thermoplastic Elastomer. J. Appl. Polym. Sci. 2024, 141, e55703. [Google Scholar] [CrossRef]
- Li, J.; Xu, X.; Ma, X.; Cui, M.; Wang, X.; Chen, J.; Zhu, J.; Chen, J. Antimicrobial Nonisocyanate Polyurethane Foam Derived from Lignin for Wound Healing. ACS Appl. Bio Mater. 2024, 7, 1301–1310. [Google Scholar] [CrossRef]
- Laurén, I.; Farzan, A.; Teotia, A.; Lindfors, N.C.; Seppälä, J. Direct Ink Writing of Biocompatible Chitosan/Non-Isocyanate Polyurethane/Cellulose Nanofiber Hydrogels for Wound-Healing Applications. Int. J. Biol. Macromol. 2024, 259, 129321. [Google Scholar] [CrossRef]
- Gholami, H.; Yeganeh, H. Soybean Oil-Derived Non-Isocyanate Polyurethanes Containing Azetidinium Groups as Antibacterial Wound Dressing Membranes. Eur. Polym. J. 2021, 142, 110142. [Google Scholar] [CrossRef]
- Yu, A.Z.; Setien, R.A.; Sahouani, J.M.; Docken, J.; Webster, D.C. Catalyzed Non-Isocyanate Polyurethane (NIPU) Coatings from Bio-Based Poly(Cyclic Carbonates). J. Coat. Technol. Res. 2019, 16, 41–57. [Google Scholar] [CrossRef]
- Mustapa, S.; Aung, M.M.; Rayung, M. Physico-Chemical, Thermal, and Electrochemical Analysis of Solid Polymer Electrolyte From Vegetable Oil-Based Polyurethane. Polymers 2020, 13, 132. [Google Scholar] [CrossRef]
- Momodu, A.S.; Aransiola, E.F.; Okunade, I.D.; Ogunlusi, G.O.; Awokoya, K.N.; Ogundari, I.O.; Falope, O.T.; Makinde, O.W.; Akinbami, J.F.K. Greening Nigeria’s Economy for Industrial and Environmental Sustainability: Polyurethane Production as a Test Case. Nat. Resour. Forum 2019, 43, 73–81. [Google Scholar] [CrossRef]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balla, E.; Bikiaris, D.N.; Pardalis, N.; Bikiaris, N.D. Toward Sustainable Polyurethane Alternatives: A Review of the Synthesis, Applications, and Lifecycle of Non-Isocyanate Polyurethanes (NIPUs). Polymers 2025, 17, 1364. https://doi.org/10.3390/polym17101364
Balla E, Bikiaris DN, Pardalis N, Bikiaris ND. Toward Sustainable Polyurethane Alternatives: A Review of the Synthesis, Applications, and Lifecycle of Non-Isocyanate Polyurethanes (NIPUs). Polymers. 2025; 17(10):1364. https://doi.org/10.3390/polym17101364
Chicago/Turabian StyleBalla, Evangelia, Dimitrios N. Bikiaris, Nikolaos Pardalis, and Nikolaos D. Bikiaris. 2025. "Toward Sustainable Polyurethane Alternatives: A Review of the Synthesis, Applications, and Lifecycle of Non-Isocyanate Polyurethanes (NIPUs)" Polymers 17, no. 10: 1364. https://doi.org/10.3390/polym17101364
APA StyleBalla, E., Bikiaris, D. N., Pardalis, N., & Bikiaris, N. D. (2025). Toward Sustainable Polyurethane Alternatives: A Review of the Synthesis, Applications, and Lifecycle of Non-Isocyanate Polyurethanes (NIPUs). Polymers, 17(10), 1364. https://doi.org/10.3390/polym17101364








