Intrinsically Microporous Polyimides Derived from 2,2′-Dibromo-4,4′,5,5′-bipohenyltetracarboxylic Dianhydride for Gas Separation Membranes
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Polymer Synthesis
2.3. Membrane Casting
2.4. Characterization
3. Results and Discussion
3.1. Synthesis of Polymers
3.2. Microstructural Properties
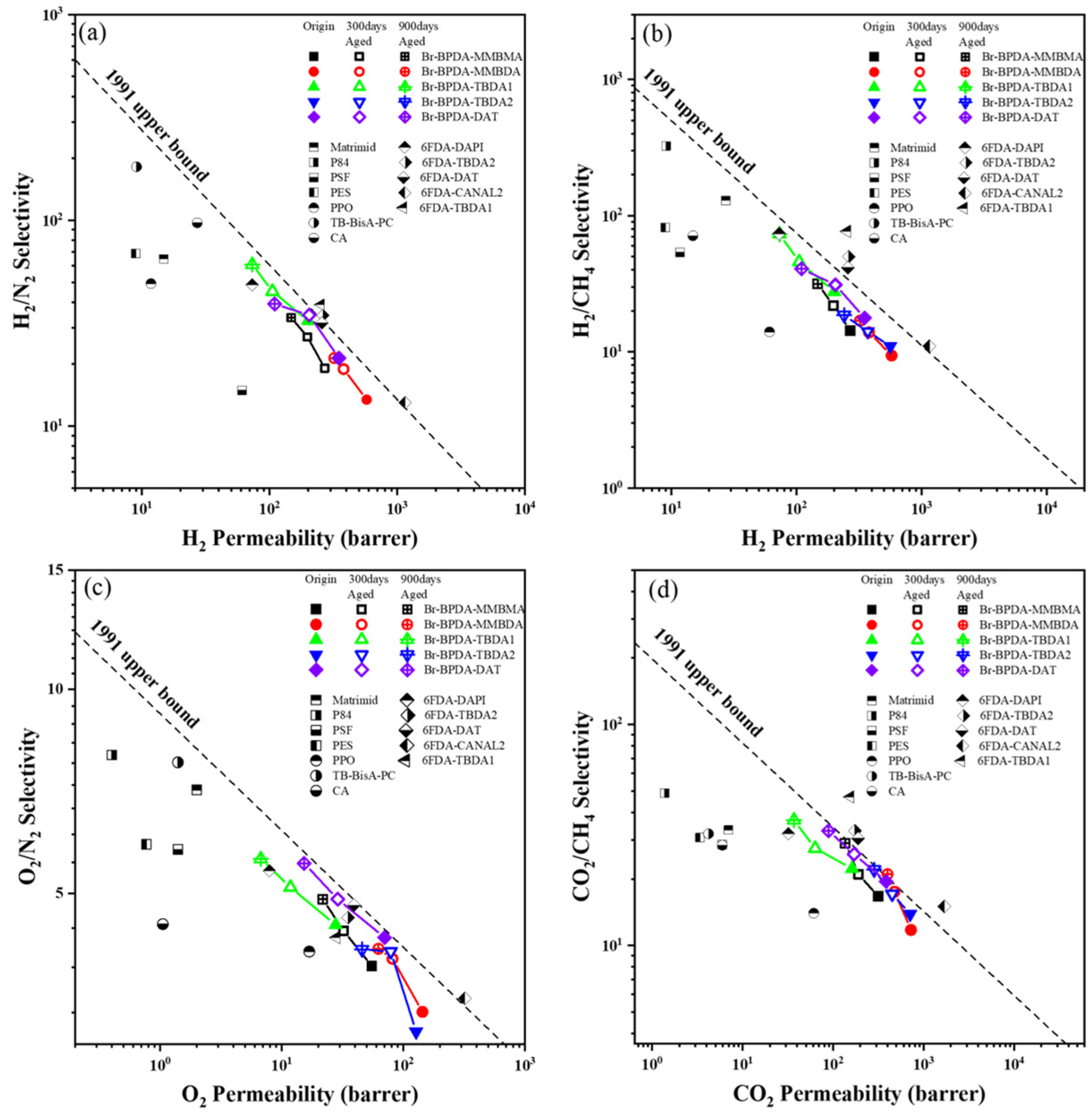
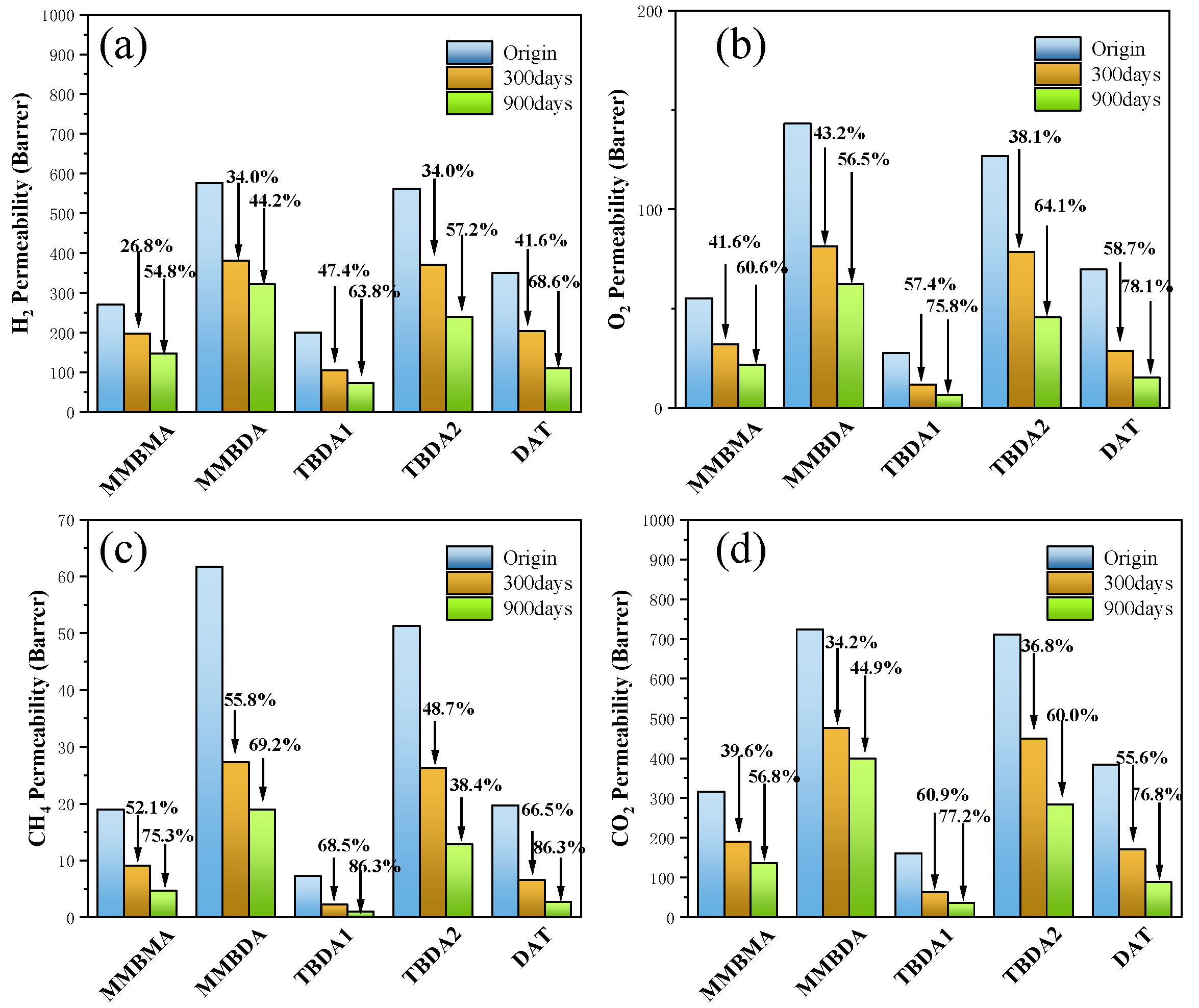
3.3. Gas Separation Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Hosseini, S.S.; Chung, T.S. Carbon membranes from blends of PBI and polyimides for N2/CH4 and CO2/CH4 separation and hydrogen purification. J. Membr. Sci. 2009, 328, 174–185. [Google Scholar] [CrossRef]
- Lu, Y.; Hu, X.; Pang, Y.; Miao, J.; Zhao, J.; Nie, W.; Wang, Z.; Yan, J. Intrinsically microporous polyimides derived from norbornane-2-spiro-α-cyclopentanone-α′-spiro-2″-norbornane-5,5″,6,6″-tetracarboxylic dianhydride. Polymer 2021, 228, 123955. [Google Scholar] [CrossRef]
- Koros, W.J.; Fleming, G.K.; Jordan, S.M.; Kim, T.H.; Hoehn, H.H. Polymeric membrane materials for solution-diffusion based permeation separations. Prog. Polym. Sci. 1988, 13, 339–401. [Google Scholar] [CrossRef]
- Robeson, L.M. The upper bound revisited. J. Membr. Sci. 2008, 320, 390–400. [Google Scholar] [CrossRef]
- Carta, M.; Malpass-Evans, R.; Croad, M.; Rogan, Y.; Jansen, J.C.; Bernardo, P.; Bazzarelli, F.; McKeown, N.B. An efficient polymer molecular sieve for membrane gas separations. Science 2013, 339, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Guiver, M.D.; Lee, Y.M. Materials science. Polymer rigidity improves microporous membranes. Science 2013, 339, 284–285. [Google Scholar] [CrossRef]
- Park, H.B.; Jung, C.H.; Lee, Y.M.; Hill, A.J.; Pas, S.J.; Mudie, S.T.; Van Wagner, E.; Freeman, B.D.; Cookson, D.J. Polymers with cavities tuned for fast selective transport of small molecules and ions. Science 2007, 318, 254–258. [Google Scholar] [CrossRef]
- Low, Z.X.; Budd, P.M.; McKeown, N.B.; Patterson, D.A. Gas permeation properties, physical aging, and its mitigation in high free volume glassy polymers. Chem. Rev. 2018, 118, 5871–5911. [Google Scholar] [CrossRef] [PubMed]
- Swaidan, R.; Ghanem, B.; Litwiller, E.; Pinnau, I. Physical aging, plasticization and their effects on gas permeation in “rigid” polymers of intrinsic microporosity. Macromolecules 2015, 48, 6553–6561. [Google Scholar] [CrossRef]
- Hu, X.; Lee, W.H.; Bae, J.Y.; Zhao, J.; Kim, J.S.; Wang, Z.; Yan, J.; Lee, Y.M. Highly permeable polyimides incorporating Tröger’s base (TB) units for gas separation membranes. J. Membr. Sci. 2020, 615, 118533. [Google Scholar] [CrossRef]
- Ghanem, B.S.; McKeown, N.B.; Budd, P.M.; Selbie, J.D.; Fritsch, D. High-performance membranes from polyimides with intrinsic microporosity. Adv. Mater. 2008, 20, 2766–2771. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, D.; Zhang, F.; Jin, J. Troger’s base-based microporous polyimide membranes for high-performance gas separation. ACS Macro Lett. 2014, 3, 597–601. [Google Scholar] [CrossRef] [PubMed]
- Tsui, N.T.; Paraskos, A.J.; Torun, L.; Swager, T.M.; Thomas, E.L. Minimization of internal molecular free volume: A mechanism for the simultaneous enhancement of polymer stiffness, strength, and ductility. Macromolecules 2006, 39, 3350–3358. [Google Scholar] [CrossRef]
- Swaidan, R.; Ghanem, B.; Pinnau, I. Fine-tuned intrinsically ultramicroporous polymers redefine the permeability/selectivity upper bounds of membrane-based air and hydrogen separations. ACS Macro Lett. 2015, 4, 947–951. [Google Scholar] [CrossRef]
- Comesaña-Gándara, B.; Chen, J.; Bezzu, C.G.; Carta, M.; Rose, I.; Ferrari, M.-C.; Esposito, E.; Fuoco, A.; Jansen, J.C.; McKeown, N.B. Redefining the Robeson upper bounds for CO2/CH4 and CO2/N2 separations using a series of ultrapermeable benzotriptycene-based polymers of intrinsic microporosity. Energy Environ. Sci. 2019, 12, 2733–2740. [Google Scholar] [CrossRef]
- Liaw, D.J.; Wang, K.L.; Huang, Y.C.; Lee, K.R.; Lai, J.Y.; Ha, C.S. Advanced polyimide materials: Syntheses, physical properties and applications. Prog. Polym. Sci. 2012, 37, 907–974. [Google Scholar] [CrossRef]
- McKeown, N.B. The synthesis of polymers of intrinsic microporosity (PIMs). Sci. China Chem. 2017, 60, 1023–1032. [Google Scholar] [CrossRef]
- Ghanem, B.S.; McKeown, N.B.; Budd, P.M.; Al-Harbi, N.M.; Fritsch, D.; Heinrich, K.; Starannikova, L.; Tokarev, A.; Yampolskii, Y. Synthesis, characterization, and gas permeation properties of a novel group of polymers with intrinsic microporosity: PIM-polyimides. Macromolecules 2009, 42, 7881–7888. [Google Scholar] [CrossRef]
- Marjani, A.; Nakhjiri, A.T.; Taleghani, A.S.; Shirazian, S. Mass transfer modeling absorption using nanofluids in porous polymeric membranes. J. Mol. Liq. 2020, 318, 114115. [Google Scholar] [CrossRef]
- Rostami, A.; Baghban, A.; Shirazian, S. On the evaluation of density of ionic liquids: Towards a comparative study. Chem. Eng. Res. Des. 2019, 147, 648–663. [Google Scholar] [CrossRef]
- Friess, K.; Izak, P.; Karaszova, M.; Pasichnyk, M.; Lanc, M.; Nikolaeva, D.; Luis, P.; Jansen, J.C. A review on ionic liquid gas separation membranes. Membranes 2021, 11, 97. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Hu, X.; Lee, W.H.; Bae, J.Y.; Zhao, J.; Nie, W.; Wang, Z.; Yan, J.; Lee, Y.M. Effects of bulky 2,2′-substituents in dianhydrides on the microstructures and gas transport properties of thermally rearranged polybenzoxazoles. J. Membr. Sci. 2021, 639, 119777. [Google Scholar] [CrossRef]
- Tanaka, K.; Kita, H.; Okamoto, K.; Nakamura, A.; Kusuki, Y. Gas permeability and permselectivity in polyimides based on 3,3’,4,4’-biphenyltetracarboxylic dianhydride. J. Membr. Sci. 1989, 47, 203–215. [Google Scholar] [CrossRef]
- Li, F.; Ge, J.J.; Honigfort, P.S.; Fang, S.; Chen, J.-C.; Harris, F.W.; Cheng, S.Z.D. Dianhydride architectural effects on the relaxation behaviors and thermal and optical properties of organo-soluble aromatic polyimide films. Polymer 1999, 40, 4987–5002. [Google Scholar] [CrossRef]
- Li, F.; Fang, S.; Ge, J.J.; Honigfort, P.S.; Chen, J.C.; Harris, F.W.; Cheng, S.Z.D. Diamine architecture effects on glass transitions, relaxation processes and other material properties in organo-soluble aromatic polyimide films. Polymer 1999, 40, 4571–4583. [Google Scholar] [CrossRef]
- Qiu, Z.; Chen, G.; Zhang, Q.; Zhang, S. Synthesis and gas transport property of polyimide from 2,2′-disubstituted biphenyltetracarboxylic dianhydrides (BPDA). Eur. Polym. J. 2007, 43, 194–204. [Google Scholar] [CrossRef]
- Kim, H.-S.; Kim, Y.-H.; Ahn, S.-K.; Kwon, S.-K. Synthesis and characterization of highly soluble and oxygen permeable new polyimides bearing a noncoplanar twisted biphenyl unit containing tert-butylphenyl or trimethylsilyl phenyl groups. Macromolecules 2003, 36, 2327–2332. [Google Scholar] [CrossRef]
- Zhao, W.; Zhang, J.; Liu, C.; Ma, Y.; Li, K.; Guo, M.; Jiao, L.; Ma, X.; Yang, L.; Yang, S.; et al. Fine-tuning gas separation performance of intrinsic microporous polyimide by the regulation of atomic-level halogen substitution. J. Membr. Sci. 2024, 692, 122317. [Google Scholar] [CrossRef]
- Zhao, W.; Li, K.; Ma, Y.; Gao, Y.; Zhang, J.; Zhou, L.; Wang, W.; Wang, J.; Ma, Y.; Guo, M.; et al. Simultaneously enhanced gas separation and anti-aging performance of intrinsic microporous polyimide by dibromo substitution. J. Membr. Sci. 2023, 687, 122081. [Google Scholar] [CrossRef]
- Harris, F.W.; Lin, S.-H.; Li, F.; Cheng, S.Z.D. Organo-soluble polyimides: Synthesis and polymerization of 2,2′-disubstituted-4,4′,5,5′-biphenyltetracarboxylic dianhydrides. Polymer 1996, 37, 5049–5057. [Google Scholar] [CrossRef]
- Hu, X.; Pang, Y.; Mu, H.; Meng, X.; Wang, X.; Wang, Z.; Yan, J. Synthesis and gas separation performances of intrinsically microporous polyimides based on 4-methylcatechol-derived monomers. J. Membr. Sci. 2021, 620, 118825. [Google Scholar] [CrossRef]
- Zhang, C.; Fu, L.; Tian, Z.; Cao, B.; Li, P. Post-crosslinking of triptycene-based Tröger’s base polymers with enhanced natural gas separation performance. J. Membr. Sci. 2018, 556, 277–284. [Google Scholar] [CrossRef]
- Hu, X.; Lee, W.H.; Zhao, J.; Kim, J.S.; Wang, Z.; Yan, J.; Zhuang, Y.; Lee, Y.M. Thermally rearranged polymer membranes containing highly rigid biphenyl ortho-hydroxyl diamine for hydrogen separation. J. Membr. Sci. 2020, 604, 118053. [Google Scholar] [CrossRef]
- Hu, X.; Lee, W.H.; Bae, J.Y.; Kim, J.S.; Jung, J.T.; Wang, H.H.; Park, H.J.; Lee, Y.M. Thermally rearranged polybenzoxazole copolymers incorporating Tröger’s base for high flux gas separation membranes. J. Membr. Sci. 2020, 612, 118437. [Google Scholar] [CrossRef]
- Bondi, A. Van der Waals Volumes and Radii. J. Phys. Chem. 2002, 68, 441–451. [Google Scholar] [CrossRef]
- Jung, C.H.; Lee, Y.M. Gas permeation properties of hydroxyl-group containing polyimide membranes. Macromol. Res. 2008, 16, 555–560. [Google Scholar] [CrossRef]
- Hao, G.P.; Li, W.C.; Qian, D.; Lu, A.H. Rapid synthesis of nitrogen-doped porous carbon monolith for CO2 capture. Adv. Mater. 2010, 22, 853–857. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Lee, W.H.; Zhao, J.; Bae, J.Y.; Kim, J.S.; Wang, Z.; Yan, J.; Zhuang, Y.; Lee, Y.M. Tröger’s Base (TB)-containing polyimide membranes derived from bio-based dianhydrides for gas separations. J. Membr. Sci. 2020, 610, 118255. [Google Scholar] [CrossRef]
- Lee, W.M. Selection of barrier materials from molecular structure. Polym. Eng. Sci. 2004, 20, 65–69. [Google Scholar] [CrossRef]
- Turner-Jones, A. Development of the γ-crystal form in random copolymers of propylene and their analysis by dsc and X-ray methods. Polymer 1971, 12, 487–508. [Google Scholar] [CrossRef]
- Huo, H.; Jiang, S.; An, L.; Feng, J. Influence of shear on crystallization behavior of the β phase in isotactic polypropylene with β-nucleating agent. Macromolecules 2004, 37, 2478–2483. [Google Scholar] [CrossRef]
- Rodriguez, M.K.; Lin, S.; Wu, A.X.; Storme, K.R.; Joo, T.; Grosz, A.F.; Roy, N.; Syar, D.; Benedetti, F.M.; Smith, Z.P. Penetrant-induced plasticization in microporous polymer membranes. Chem. Soc. Rev. 2024, 53, 2435–2529. [Google Scholar] [CrossRef]
- Seong, J.G.; Zhuang, Y.; Kim, S.; Do, Y.S.; Lee, W.H.; Guiver, M.D.; Lee, Y.M. Effect of methanol treatment on gas sorption and transport behavior of intrinsically microporous polyimide membranes incorporating Tröger’s base. J. Membr.Sci. 2015, 480, 104–114. [Google Scholar] [CrossRef]
- Kim, S.; Lee, Y.M. Rigid and microporous polymers for gas separation membranes. Prog. Polym. Sci. 2015, 43, 1–32. [Google Scholar] [CrossRef]
- Li, J.; Wang, S.; Nagai, K.; Nakagawa, T.; Mau, A.W.H. Effect of polyethyleneglycol (PEG) on gas permeabilities and permselectivities in its cellulose acetate (CA) blend membranes. J. Membr. Sci. 1998, 138, 143–152. [Google Scholar] [CrossRef]
- Huang, Z.; Li, Y.; Wen, R.; May Teoh, M.; Kulprathipanja, S. Enhanced gas separation properties by using nanostructured PES-Zeolite 4A mixed matrix membranes. J. Appl. Polym. Sci. 2006, 101, 3800–3805. [Google Scholar] [CrossRef]
- Alghunaimi, F.; Ghanem, B.; Alaslai, N.; Swaidan, R.; Litwiller, E.; Pinnau, I. Gas permeation and physical aging properties of iptycene diamine-based microporous polyimides. J. Membr. Sci. 2015, 490, 321–327. [Google Scholar] [CrossRef]
- Muruganandam, N.; Koros, W.J.; Paul, D.R. Gas sorption and transport in substituted polycarbonates. J. Polym. Sci. B Polym. Phys. 1987, 25, 1999–2026. [Google Scholar] [CrossRef]
- Sridhar, S.; Veerapur, R.S.; Patil, M.B.; Gudasi, K.B.; Aminabhavi, T.M. Matrimid polyimide membranes for the separation of carbon dioxide from methane. J. Appl. Polym. Sci. 2007, 106, 1585–1594. [Google Scholar] [CrossRef]
- Abdulhamid, M.A.; Lai, H.W.H.; Wang, Y.; Jin, Z.; Teo, Y.C.; Ma, X.; Pinnau, I.; Xia, Y. Microporous polyimides from ladder diamines synthesized by facile catalytic arene–norbornene annulation as high-performance membranes for gas separation. Chem. Mater. 2019, 31, 1767–1774. [Google Scholar] [CrossRef]
- Ahn, J.; Chung, W.-J.; Pinnau, I.; Guiver, M.D. Polysulfone/silica nanoparticle mixed-matrix membranes for gas separation. J. Membr. Sci. 2008, 314, 123–133. [Google Scholar] [CrossRef]
- Abdulhamid, M.A.; Ma, X.; Miao, X.; Pinnau, I. Synthesis and characterization of a microporous 6FDA-polyimide made from a novel carbocyclic pseudo Tröger’s base diamine: Effect of bicyclic bridge on gas transport properties. Polymer 2017, 130, 182–190. [Google Scholar] [CrossRef]
- Dose, M.E.; Chwatko, M.; Hubacek, I.; Lynd, N.A.; Paul, D.R.; Freeman, B.D. Thermally cross-linked diaminophenylindane (DAPI) containing polyimides for membrane based gas separations. Polymer 2019, 161, 16–26. [Google Scholar] [CrossRef]
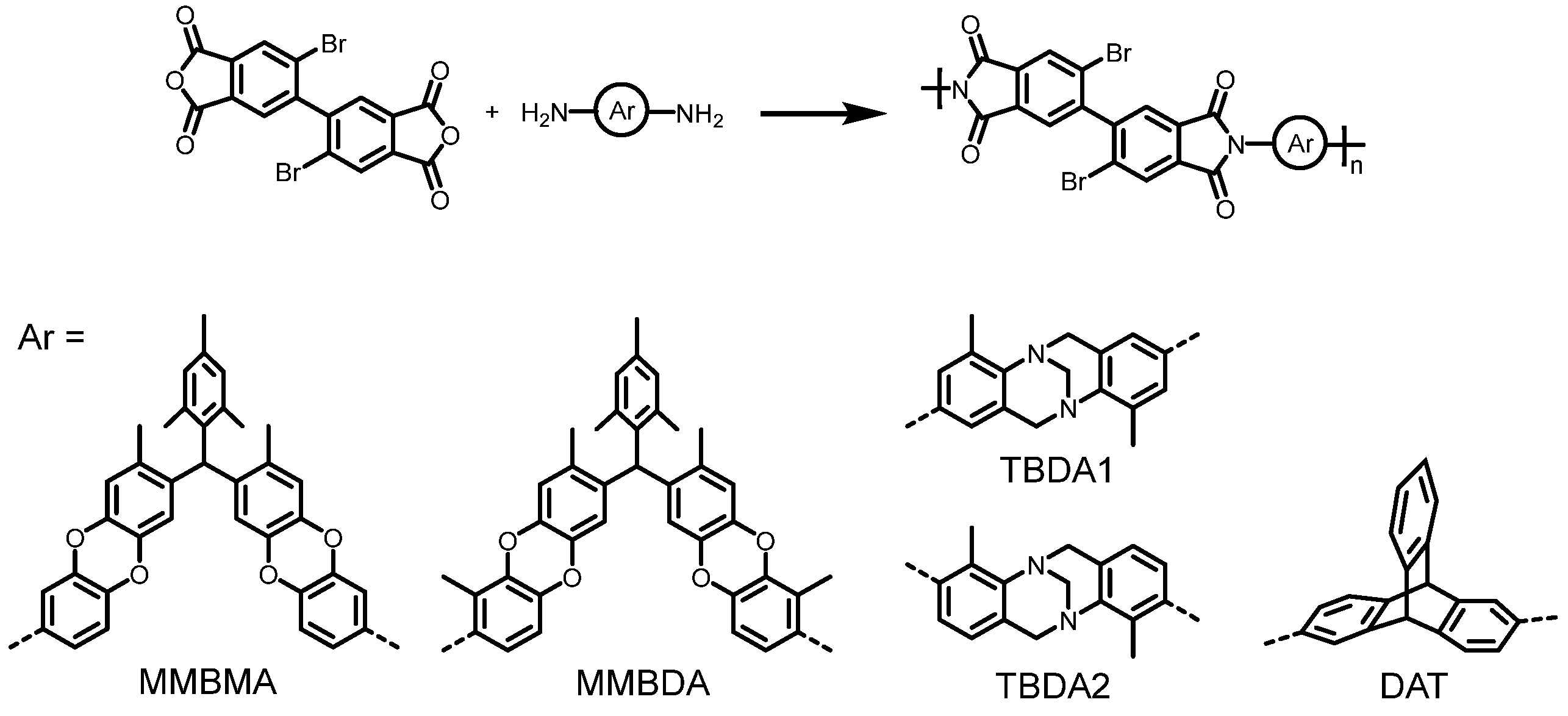
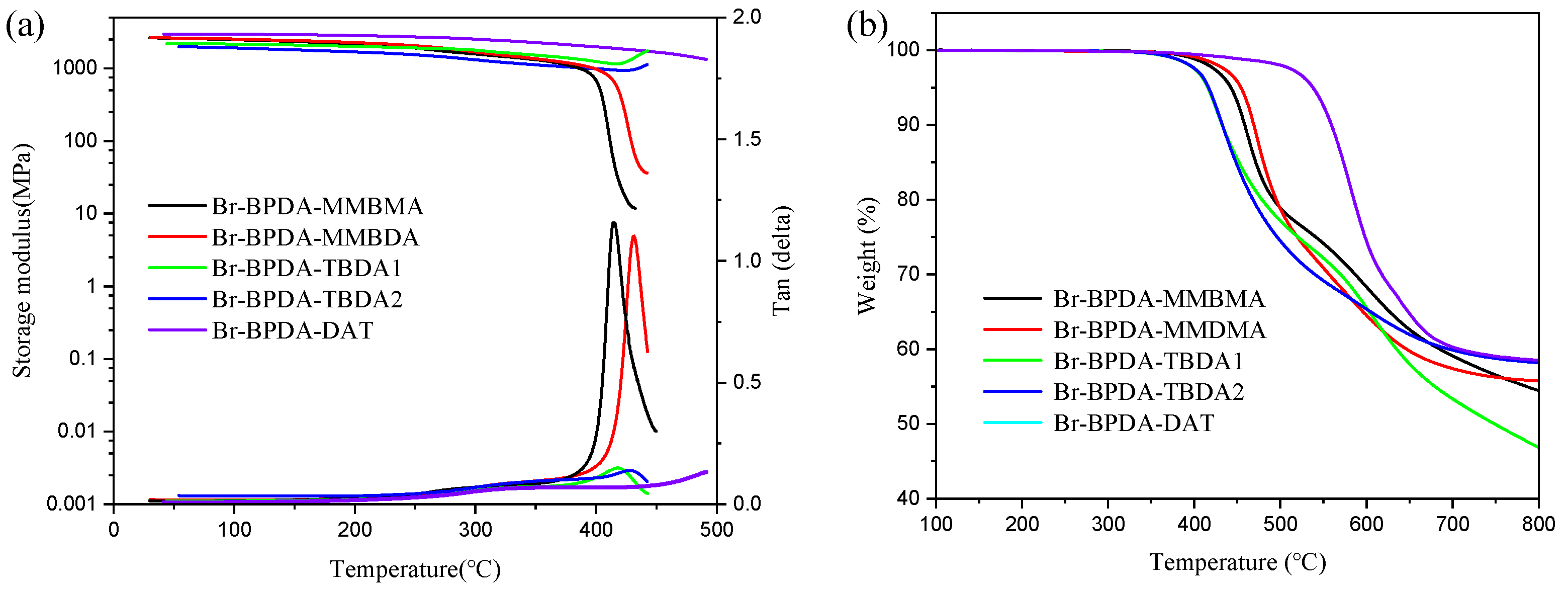
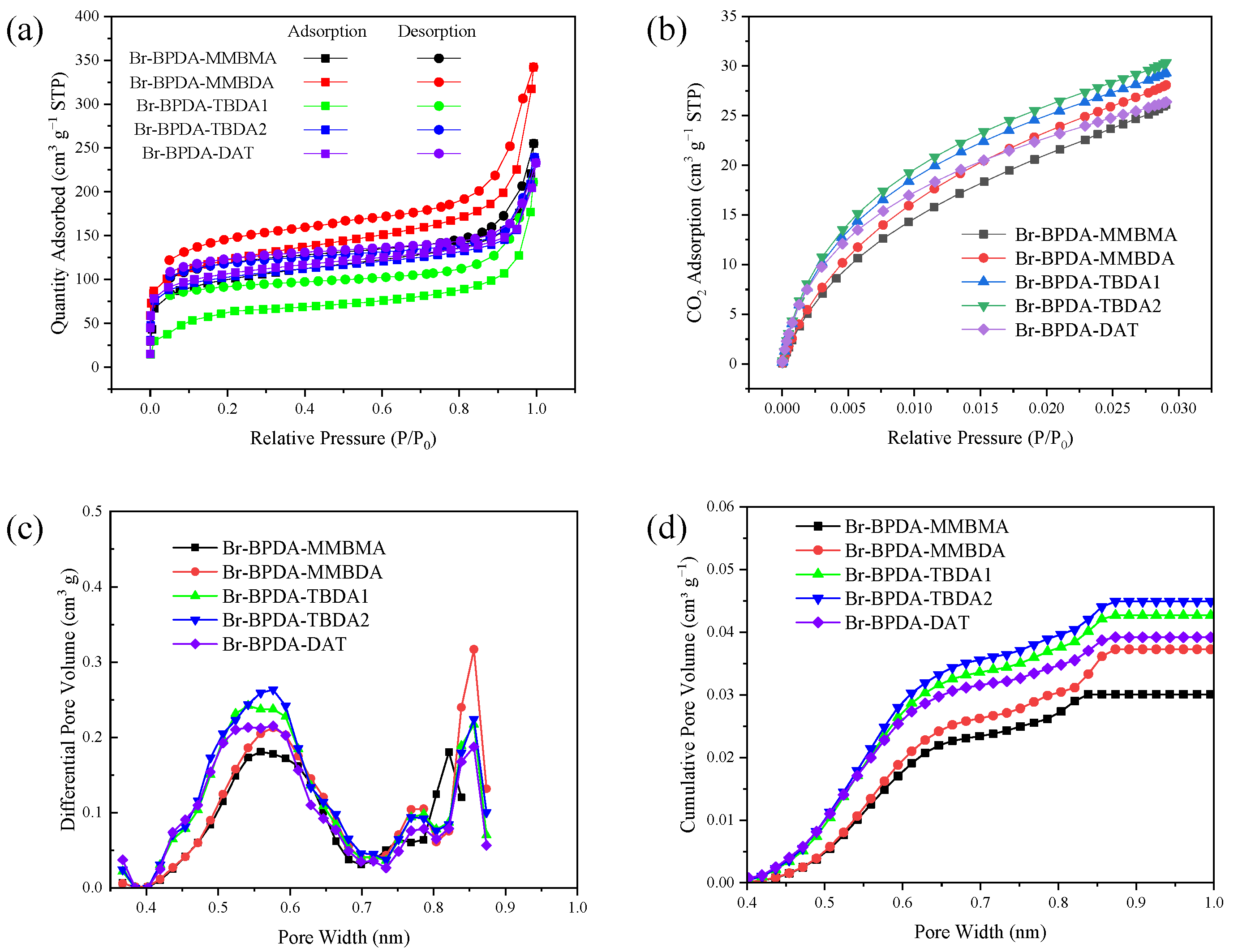
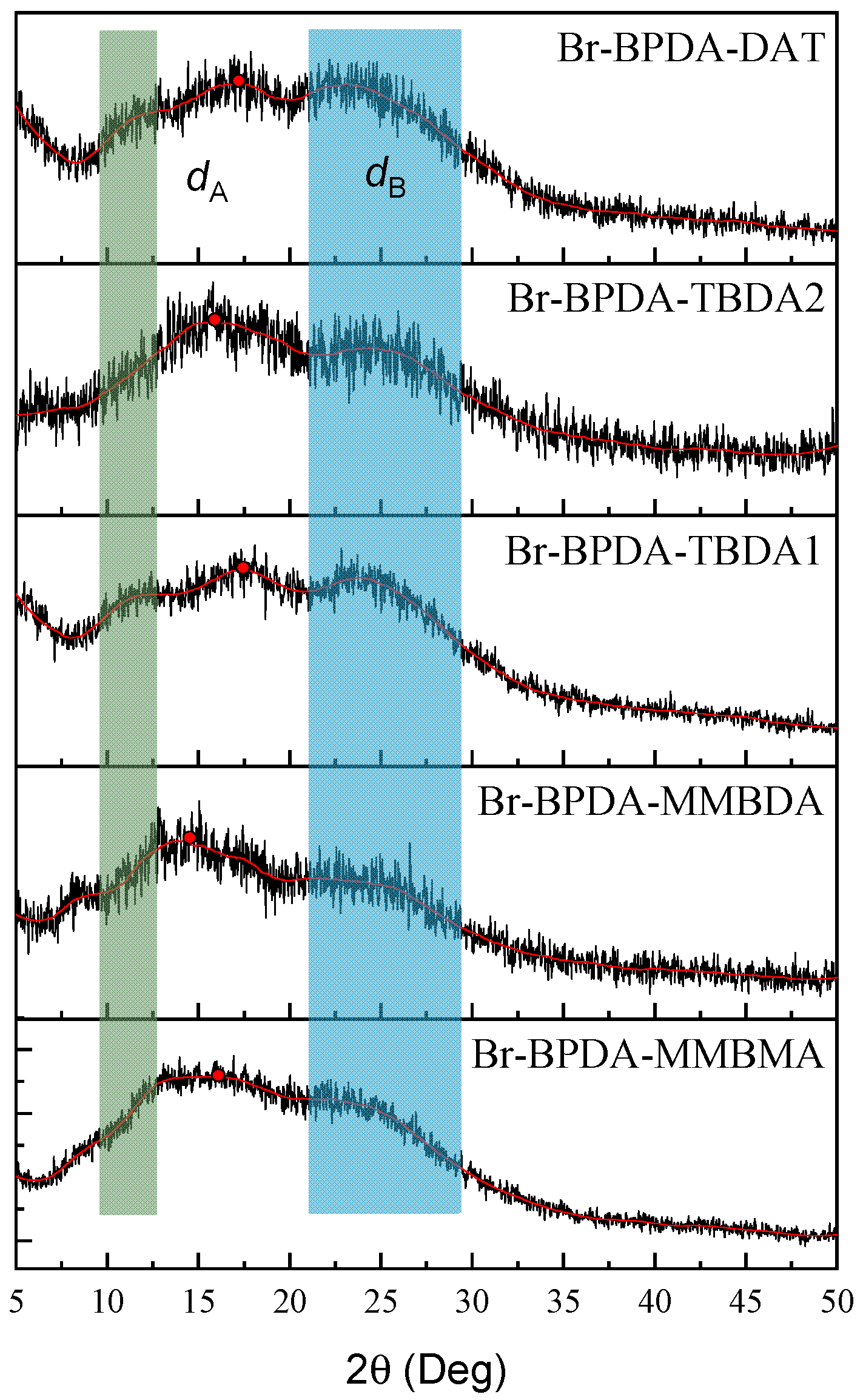
| Polymer | Tg (°C) | Td5 (°C) | Mw (kg mol−1) | PDI | Tensile Strength (MPa) | Modulus (GPa) | Elongation at Break (%) |
|---|---|---|---|---|---|---|---|
| Br-BPDA-MMBMA | 416 | 443 | 81.0 | 1.9 | 59.2 ± 7.0 | 2.1 ± 0.1 | 3.4 ± 0.6 |
| Br-BPDA-MMBDA | 432 | 454 | 42.0 | 1.9 | 63.1 ± 0.5 | 2.0 ± 0.1 | 3.7 ± 0.2 |
| Br-BPDA-TBDA1 | 419 | 416 | 45.0 | 1.9 | 78.5 ± 3.6 | 2.2 ± 0.1 | 4.9 ± 0.1 |
| Br-BPDA-TBDA2 | 428 | 417 | 31.0 | 1.8 | 75.8 ± 5.6 | 1.8 ± 0.1 | 7.4 ± 1.4 |
| Br-BPDA-DAT | >500 | 538 | 52.0 | 3.8 | 109.3 ± 7.0 | 2.1 ± 0.1 | 12.3 ± 1.1 |
| Polymer | SBET (m2 g−1) | CO2 Uptake (cm3 g−1) | D 1 (nm) | VM 2 (cm3 g−1) | dA (Å) | dB (Å) | Vw 3 (cm3 mol−1) | ρ (g cm−3) | FFV |
|---|---|---|---|---|---|---|---|---|---|
| Br-BPDA-MMBMA | 255 | 26.1 | 0.56/0.75/0.82 | 0.030 | 5.51 | 3.76 | 456.57 | 1.328 | 0.190 |
| Br-BPDA-MMBDA | 342 | 28.1 | 0.58/0.78/0.86 | 0.037 | 5.88 | 3.94 | 470.17 | 1.283 | 0.216 |
| Br-BPDA-TBDA1 | 211 | 29.3 | 0.54/0.78/0.86 | 0.043 | 4.92 | 3.75 | 304.35 | 1.463 | 0.169 |
| Br-BPDA-TBDA2 | 239 | 30.4 | 0.58/0.77/0.86 | 0.045 | 5.69 | 3.92 | 304.35 | 1.448 | 0.177 |
| Br-BPDA-DAT | 232 | 26.4 | 0.58/0.79/0.86 | 0.039 | 5.23 | 3.76 | 301.42 | 1.449 | 0.189 |
| Permeability (Barrer 1) | Selectivity | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Polymer | H2 | CO2 | O2 | N2 | CH4 | H2/CH4 | H2/N2 | O2/N2 | CO2/N2 | CO2/CH4 |
| Br-BPDA-MMBMA | 270.0 | 315.5 | 55.1 | 14.1 | 19.0 | 14.2 | 19.1 | 3.90 | 22.3 | 16.6 |
| 300 days aged | 198.0 | 190.7 | 32.2 | 7.3 | 9.1 | 21.8 | 27.1 | 4.4 | 26.1 | 21.0 |
| 900 days aged | 147.9 | 136.3 | 21.7 | 4.4 | 4.7 | 31.5 | 33.6 | 4.9 | 31.0 | 29.0 |
| Br-BPDA-MMBDA | 576.5 | 724.5 | 143.2 | 42.9 | 61.7 | 9.4 | 13.4 | 3.3 | 16.9 | 11.8 |
| 300 days aged | 380.6 | 476.9 | 81.3 | 20.1 | 27.3 | 13.9 | 18.9 | 4.0 | 23.7 | 17.5 |
| 900 days aged | 321.9 | 399.0 | 62.3 | 15.1 | 19.0 | 16.9 | 21.3 | 4.1 | 26.4 | 21.0 |
| Br-BPDA-TBDA1 | 200.4 | 161.6 | 27.7 | 6.2 | 7.3 | 27.6 | 32.5 | 4.5 | 26.2 | 22.2 |
| 300 days aged | 105.3 | 63.2 | 11.8 | 2.3 | 2.3 | 45.8 | 45.8 | 5.1 | 27.5 | 27.5 |
| 900 days aged | 73.2 | 36.9 | 6.7 | 1.2 | 1.0 | 73.2 | 61.0 | 5.6 | 30.8 | 36.9 |
| Br-BPDA-TBDA2 | 561.6 | 711.0 | 127.0 | 40.7 | 51.3 | 11.0 | 13.8 | 3.1 | 17.5 | 13.9 |
| 300 days aged | 370.9 | 449.1 | 78.6 | 19.3 | 26.3 | 14.1 | 19.2 | 4.1 | 23.3 | 17.1 |
| 900 days aged | 240.5 | 284.1 | 45.6 | 11.0 | 12.9 | 18.6 | 21.9 | 4.1 | 25.8 | 22.0 |
| Br-BPDA-DAT | 349.8 | 384.4 | 69.8 | 16.3 | 19.7 | 17.7 | 21.4 | 4.3 | 23.5 | 19.5 |
| 300 days aged | 204.3 | 170.7 | 28.8 | 5.9 | 6.6 | 31.0 | 34.6 | 4.9 | 28.9 | 25.9 |
| 900 days aged | 109.8 | 89.0 | 15.3 | 2.8 | 2.7 | 40.7 | 39.2 | 5.5 | 31.8 | 33.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Lu, Y.; Tian, C.; Wang, Z.; Yan, J. Intrinsically Microporous Polyimides Derived from 2,2′-Dibromo-4,4′,5,5′-bipohenyltetracarboxylic Dianhydride for Gas Separation Membranes. Polymers 2024, 16, 1198. https://doi.org/10.3390/polym16091198
Li Y, Lu Y, Tian C, Wang Z, Yan J. Intrinsically Microporous Polyimides Derived from 2,2′-Dibromo-4,4′,5,5′-bipohenyltetracarboxylic Dianhydride for Gas Separation Membranes. Polymers. 2024; 16(9):1198. https://doi.org/10.3390/polym16091198
Chicago/Turabian StyleLi, Yongle, Yao Lu, Chun Tian, Zhen Wang, and Jingling Yan. 2024. "Intrinsically Microporous Polyimides Derived from 2,2′-Dibromo-4,4′,5,5′-bipohenyltetracarboxylic Dianhydride for Gas Separation Membranes" Polymers 16, no. 9: 1198. https://doi.org/10.3390/polym16091198
APA StyleLi, Y., Lu, Y., Tian, C., Wang, Z., & Yan, J. (2024). Intrinsically Microporous Polyimides Derived from 2,2′-Dibromo-4,4′,5,5′-bipohenyltetracarboxylic Dianhydride for Gas Separation Membranes. Polymers, 16(9), 1198. https://doi.org/10.3390/polym16091198





