The Role of Reduced Graphene Oxide in Enhancing the Mechanical and Thermal Properties of a Rubber Cover Joint
Abstract
1. Introduction
2. Experimental Section
2.1. Chemicals
2.2. Prescription
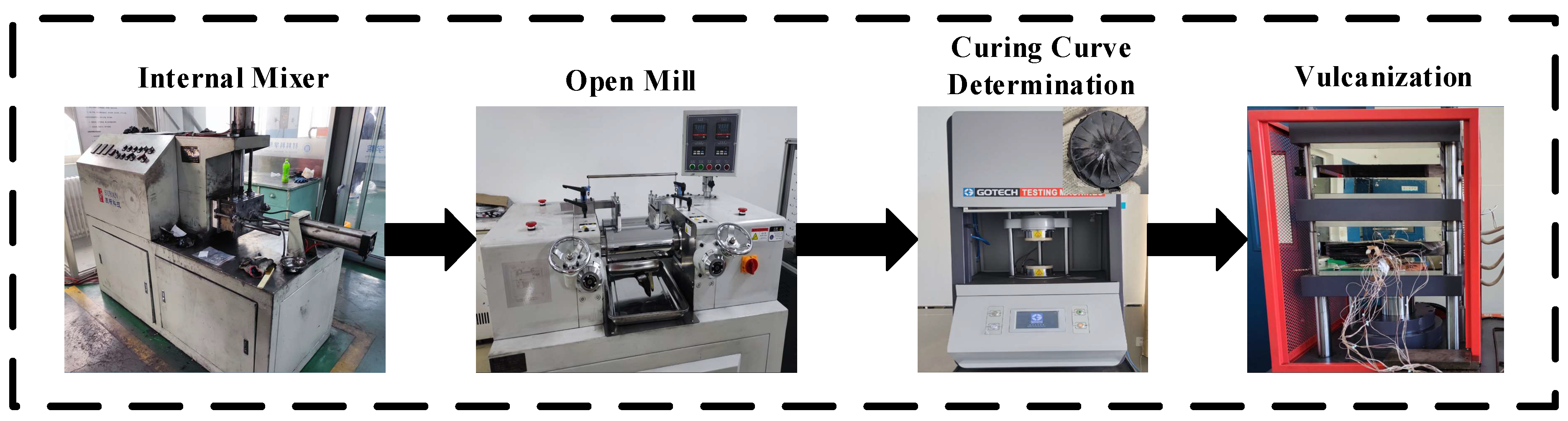
2.3. Equipment and Instruments
2.4. Preparation of rGO/NR Raw Rubber
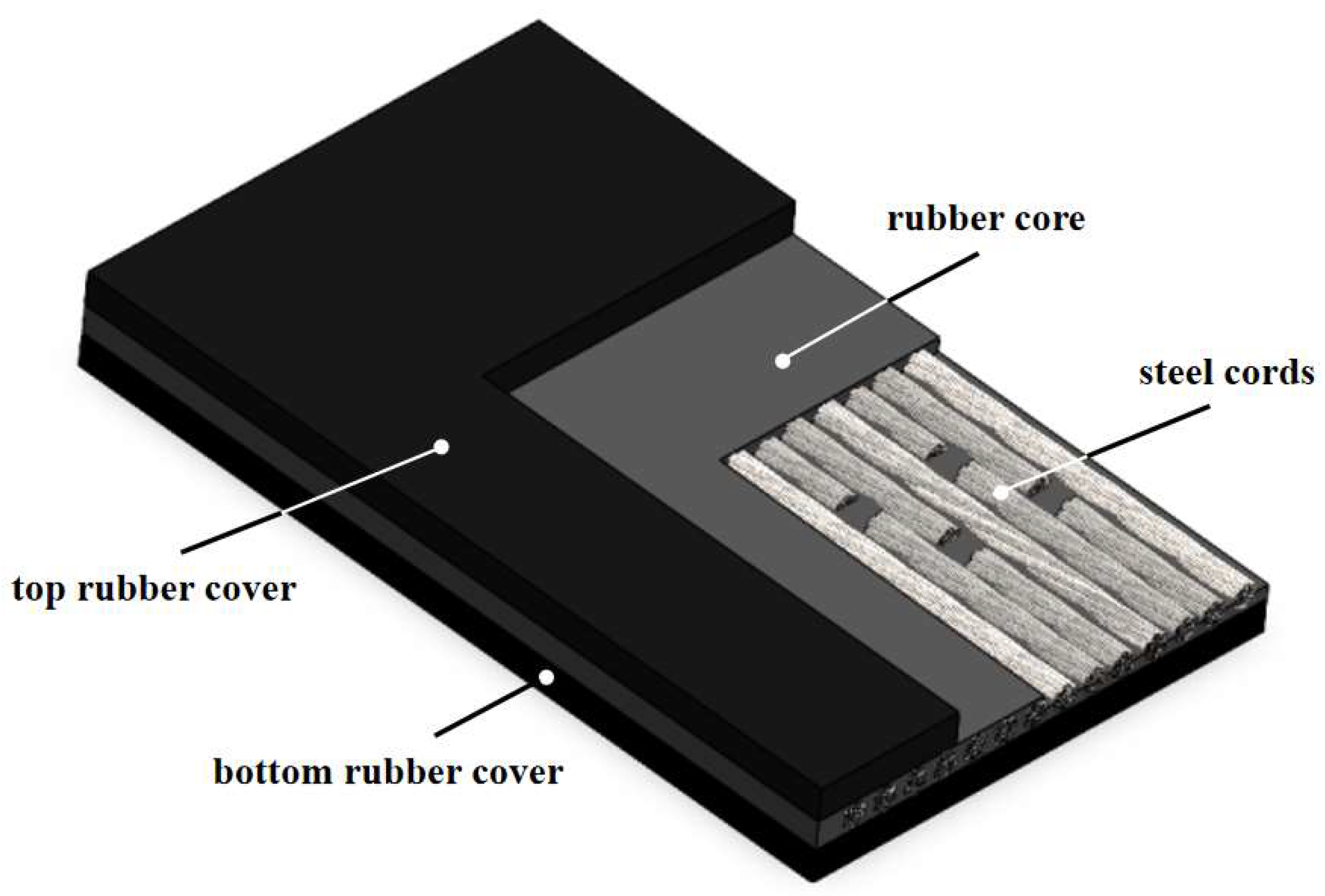
2.5. Preparation of rGO Rubber Cover Joint
2.6. Characterization
2.6.1. XRD Analysis
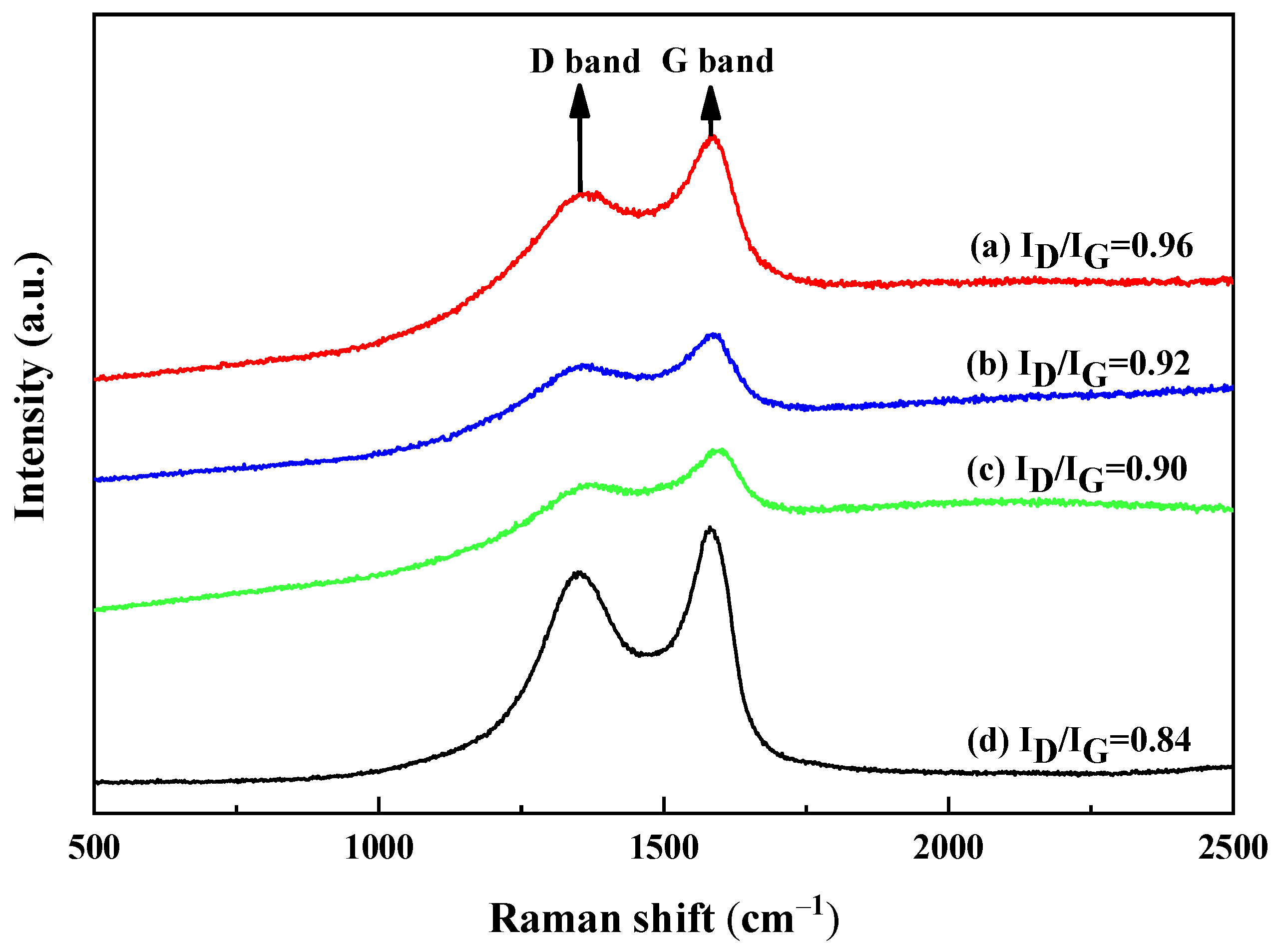
2.6.2. Raman Analysis
2.6.3. SEM Analysis
2.6.4. Mechanical Performance Testing
2.6.5. Adhesion Strength Testing
2.6.6. Thermal Conductivity Testing
2.6.7. Thermal Aging Testing
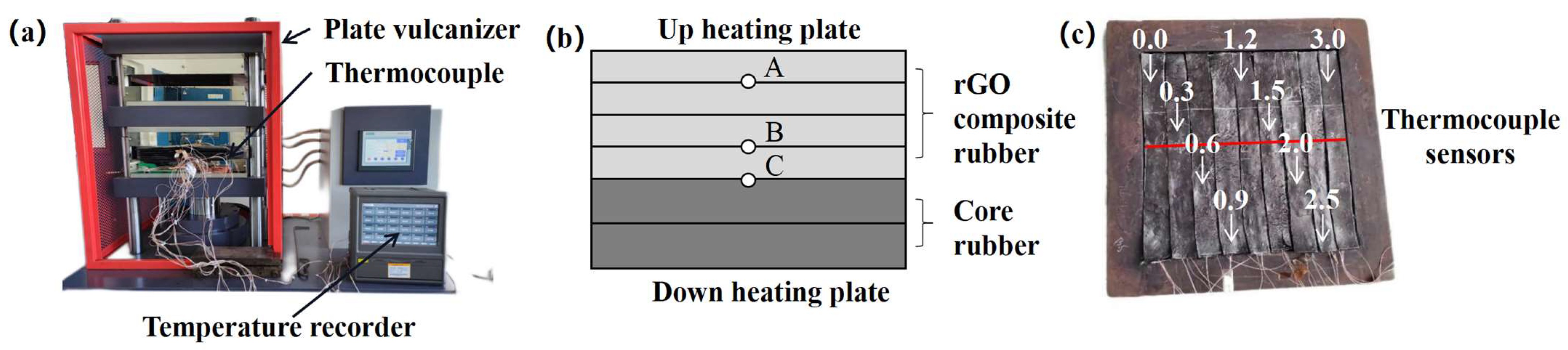
2.6.8. Conveyor Belt Vertical Temperature Conduction Testing
3. Results and Discussion
3.1. Characterization of the rGO/NR Rubber
3.2. Microstructure Analysis of the rGO Rubber Cover Joint
3.3. Analysis of Vulcanization Characteristics
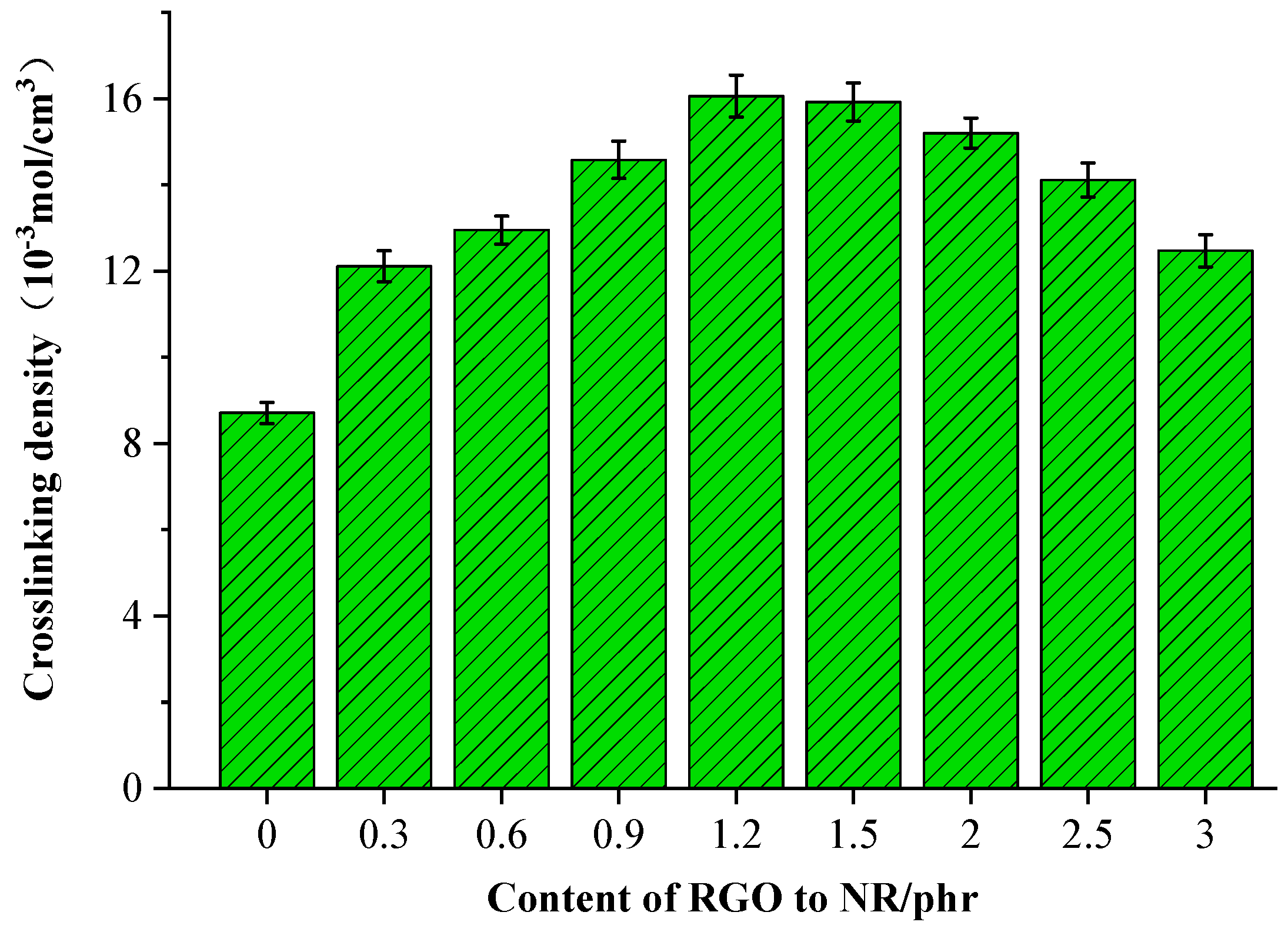
3.4. Cross-Linking Density
3.5. Physical Characteristics
3.6. Adhesion Properties
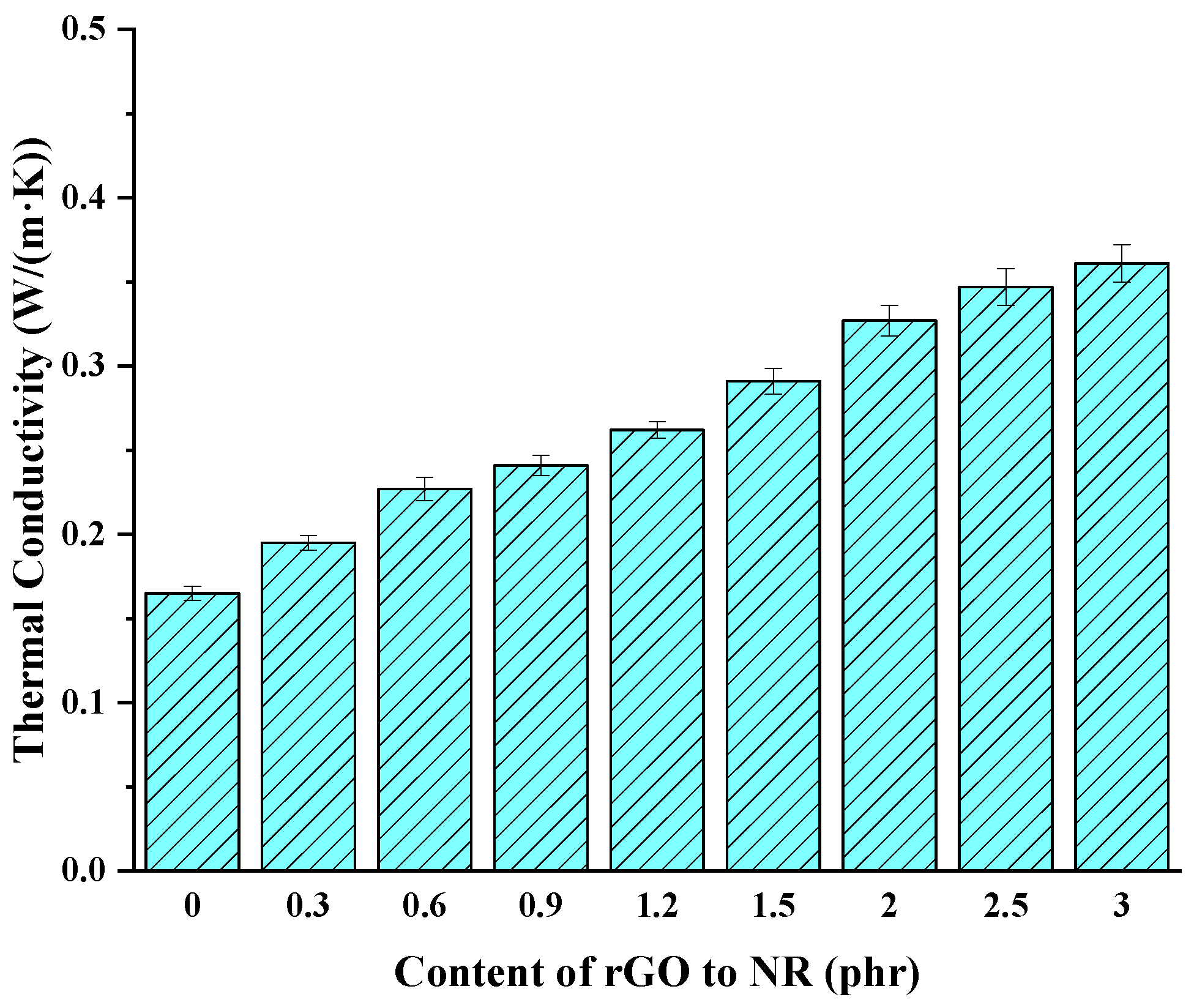
3.7. Thermal Conductivity
3.8. Thermal Aging
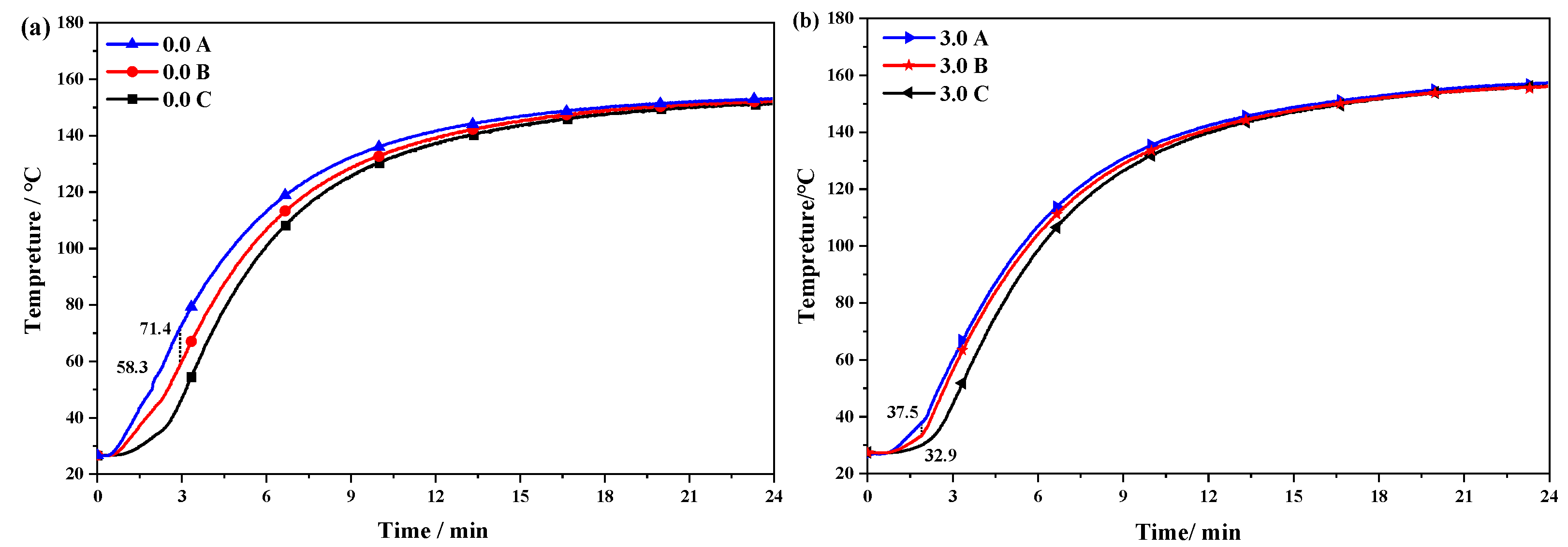
3.9. Actual Thermal Conductivity Performance of rGO on Conveyor Belts
4. Conclusions
- (1)
- The rGO dispersed uniformly in the rubber composites at a low dosage, but when 3.0 phr rGO was added, it agglomerated in the rubber composite.
- (2)
- The analysis of vulcanization characteristics showed that adding rGO can prolong the curing scorch period of a rubber compound and thus improve the safety of the rubber vulcanization process. With the increase in the rGO content, the mechanical properties of the rubber composites first improved and then worsened. When 1.2 phr was added, the mechanical properties of the rubber were highest. Compared with the original formula, the cross-linking density increased by 80.6%, the tensile strength increased by 49.7%, the elongation at break increased by 23.6%, and the adhesion strength between the rGO rubber cover joint and the original rubber cover, as well as the rubber core, increased by approximately 12.4%. The tensile strength of the rGO rubber cover joint still maintained 72.5% of its pre-thermal aging value. In addition, the wear resistance and thermal conductivity of the rubber increased as more rGO was added. When 3.0 phr rGO was added, the wear resistance of the rubber improved by 32.9%, and the thermal conductivity increased by 118.8%.
- (3)
- The thermal conduction test of the rGO rubber cover joint on the conveyor belt shows that the addition of rGO can improve the uniformity of the internal and external temperatures of rubber during vulcanization. The temperature difference was reduced from 4.5 °C to 1.8 °C, improving the vulcanization quality. Therefore, this work provides important information regarding the industrial production of high-strength steel wire rope core conveyor belts.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Li, X.; Long, X.; Shen, Z.; Miao, C. Analysis of Strength Factors of Steel Cord Conveyor Belt Splices Based on the Fem. Adv. Mater. Sci. Eng. 2019, 2019, 6926413. [Google Scholar] [CrossRef]
- Min, F.; Lou, A.; Wei, Q. Design and Experiment of Dynamic Measurement Method for Bulk Material of Large Volume Belt Conveyor Based on Laser Triangulation Method. IOP Conf. Ser. Mater. Sci. Eng. 2020, 735, 012029. [Google Scholar] [CrossRef]
- Ryba, T.; Bzinkowski, D.; Siemiątkowski, Z.; Rucki, M.; Stawarz, S.; Caban, J.; Samociuk, W. Monitoring of Rubber Belt Material Performance and Damage. Materials 2024, 17, 765. [Google Scholar] [CrossRef] [PubMed]
- Wen, S.; Zhang, R.; Xu, Z. Effect of the Topology of Carbon-Based Nanofillers on the Filler Networks and Gas Barrier Properties of Rubber Composites. Materials 2020, 13, 5416. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Yang, L.; Guo, H.; Hu, W.; Du, A. The effect of silica modified by deep eutectic solvents on the properties of nature rubber/silica composites. J. Elastomers Plast. 2022, 54, 111–122. [Google Scholar] [CrossRef]
- Murugesan, T.M.; Palanisamy, S.; Santulli, C.; Palaniappan, M. Mechanical characterization of alkali treated Sansevieria cylindrica fibers–Natural rubber composites. Mater. Today Proc. 2022, 62, 5402–5406. [Google Scholar] [CrossRef]
- Xing, W.; Tang, M.; Wu, J. Multifunctional properties of graphene/rubber nanocomposites fabricated by a modified latex compounding method. Compos. Sci. Technol. 2014, 99, 67–74. [Google Scholar] [CrossRef]
- Zhang, S.; Zheng, L.; Liu, D. Improved mechanical and fatigue properties of graphene oxide/silica/SBR composites. RSC Adv. 2017, 7, 40813–40818. [Google Scholar] [CrossRef]
- Maya, M.G.; Abraham, J.; Moni, G.; George, J.J.; George, S.C.; Thomas, S. A comprehensive study on the impact of rGO/MWCNT hybrid filler reinforced polychloroprene rubber multifunctional nanocomposites. Polym. Test. 2020, 87, 106525. [Google Scholar] [CrossRef]
- Sethulekshmi, S.; Jayan, J.; Saritha, A. Recent developments in natural rubber nanocomposites containing graphene derivatives and its hybrids. Ind. Crops Prod. 2022, 177, 114529. [Google Scholar] [CrossRef]
- Zhu, H.; Wang, Z.; Huang, X.; Wang, F.; Kong, L.; Guo, B.; Ding, T. Enhanced comprehensive performance of SSBR/BR with self-assembly reduced graphene oxide/silica nanocomposites. Compos. Part B Eng. 2019, 175, 107027. [Google Scholar] [CrossRef]
- Ma, Z.; Lu, X.; Wang, Z.; Fei, G.; Xia, H. Preparation and properties of butyl rubber/reduced graphene oxide composites. Polym. Mater. Sci. Eng. 2022, 2022, 38. [Google Scholar]
- Yan, N.; Buonocore, G.; Lavorgna, M.; Kaciulis, S.; Balijepalli, S.K.; Zhan, Y.; Xia, H.; Ambrosio, L. The role of reduced graphene oxide on chemical, mechanical and barrier properties of natural rubber composites. Compos. Sci. Technol. 2014, 102, 74–81. [Google Scholar] [CrossRef]
- Zxa, B.; Long, Z.; Swa, B. Graphene oxide-supported zinc oxide nanoparticles for chloroprene rubber with improved crosslinking network and mechanical properties. Compos. Part A Appl. Sci. Manuf. 2019, 124, 105492. [Google Scholar]
- Li, X.; McKenna, G.B. Considering viscoelastic micromechanics for the reinforcement of graphene polymer nanocomposites. ACS Macro Lett. 2012, 1, 388–391. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Abdala, A.A.; Macosko, C.W. Graphene/polymer nanocomposites. Macromolecules 2010, 43, 6515–6530. [Google Scholar] [CrossRef]
- Wang, H. Simultaneous reduction and surface functionalization of graphene oxide and the application for rubber composites. J. Appl. Polym. Sci. 2019, 136, 15–16. [Google Scholar] [CrossRef]
- Zxa, B.; Sj, C.; Hao, G. Influence of graphene oxide and carbon nanotubes on the fatigue properties of silica/styrene-butadiene rubber composites under uniaxial and multiaxial cyclic loading—ScienceDirect. Int. J. Fatigue 2020, 131, 105388. [Google Scholar]
- Yu, J.; Yan, S.; Zhao, X.; Wang, X. Cationic PS Microspheres Modified GO and its effect on the properties of SBR/BR Rubber Composite materials. J. Polym. Res. 2023, 30, 460. [Google Scholar] [CrossRef]
- Fang, Z.; Yin, J.; Zhang, Y. Manufacture and properties of reduced graphene oxide/natural rubber-nitrile butadiene rubber composites. Fuhe Cailiao Xuebao/Acta Mater. Compos. Sin. 2018, 35, 1253–1259. [Google Scholar]
- Yang, X.; Zhang, Y.; Xu, Y.; Gao, S.; Guo, S. Study on calcium fluoride modified graphene/brominated butyl rubber nanocomposites. Polym. Bull. 2017, 74, 4959–4972. [Google Scholar] [CrossRef]
- Soriano-Ortiz, J.A.; Rueda-Morales, G.; Martínez-Guitiérrez, H.; Rojas-Trigos, J.B.; Ortega-Cervantez, G.; Ortiz-López, J. Thermal and electrical properties enhancement of a nanocomposite of industrial silicone rubber filled with reduced graphene oxide. Fuller. Nanotub. Carbon Nanostruct. 2021, 30, 221–231. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, H.; Song, S. Improving thermal conductivity of styrene-butadiene rubber composites by incorporating mesoporous silica@solvothermal reduced graphene oxide hybrid nanosheets with low graphene content. Compos. Sci. Technol. 2017, 150, 174–180. [Google Scholar] [CrossRef]
- Lin, G.; Wang, H.; Yu, B.; Liu, S.; Liang, Z.; Liu, F.; Zeng, R.; Chen, S.; Kuang, T.; Yu, K.; et al. Incorporation and optimization of rGO and GO in SSBR/NR composites expands their applicability. Polym. Polym. Compos. 2021, 29 (Suppl. S9), S411–S421. [Google Scholar] [CrossRef]
- Tao, W.; Zeng, S.; Xu, Y. 3D Graphene—Sponge skeleton reinforced polysulfide rubber nanocomposites with improved electrical and thermal conductivity. Compos. Part A. Appl. Sci. Manuf. 2021, 143, 105534. [Google Scholar] [CrossRef]
- GB/T 528-2009; Rubber, Vulcanized or Thermoplastic—Determination of Tensile Stress-Strain Properties. Standardization Administration of the P.R.C. China: Beijing, China, 2009.
- GBT529-2008; Rubber, Vulcanized or Thermoplastic—Determination of Tear Strength (Trouser, Angle and Crescent Test Pieces). Standardization Administration of the P.R.C. China: Beijing, China, 2008.
- GBT2411-2008; Plastics and Ebonite—Determination of Indentation Hardness by Means of a Duronmeter (Shore Hardness). Standardization Administration of the P.R.C. China: Beijing, China, 2008.
- You, Y.; Zheng, A.; Wei, D. A small addition of reduced graphene oxide to protect fluorosilicone rubber from thermal oxidative degradation. Polym. Adv. Technol. 2022, 33, 3718–3727. [Google Scholar] [CrossRef]
- GB/T 1689-2014; Rubber Vulcanized―Determination of Abrasion Resistance (Akron Machine). Standardization Administration of the P.R.C. China: Beijing, China, 2014.
- Shahamatifard, F.; Rodrigue, D.; Park, K. Preparation and Characterization of Reduced Graphene Oxide Based Natural Rubber Nanocomposites. Int. Polym. Process 2020, 35, 493–502. [Google Scholar] [CrossRef]
- GB/T 30691-2014; Conveyor Belts—Test Atmospheres and Conditioning Periods. Standardization Administration of the P.R.C. China: Beijing, China, 2014.
- ISO 6133; Rubber and Plastics—Analysis of Multipeak Traces Obtained in Determination of Tear Strength and Adhesion Strength. German Institute for Standardization: Berlin, Germany, 2004.
- GB/T 3512-2014; Rubber, Vulcanized or Thermoplastic―Accelerated Ageing and Heat Resistance Tests. Standardization Administration of the P.R.C. China: Beijing, China, 2014.
- Cheng, S.; Duan, X.; Cui, Y. Facile strategy for the preparation of green graphene rubber with enhanced interfacial interaction and thermal management capability. J. Appl. Polym. Sci. 2022, 139, e52882. [Google Scholar] [CrossRef]
- Maya, M.G.; George, S.C.; Jose, T.; Kailas, L.; Thomas, S. Development of a flexible and conductive elastomeric composite based on chloroprene rubber. Polym. Test. Lond. 2018, 65, 256–263. [Google Scholar] [CrossRef]
- Lim, L.; Juan, J.; Huang, N. Enhanced tensile strength and thermal conductivity of natural rubber graphene composite properties via rubber-graphene interaction. Mater. Sci. Eng. 2019, 246, 112–119. [Google Scholar] [CrossRef]
- Nwosu, C.; Iliut, M.; Vijayaraghavan, A. Graphene and water-based elastomer nanocomposites—A review. Nanoscale 2021, 13, 9505–9540. [Google Scholar] [CrossRef] [PubMed]
- Kalat, M.; Razzaghi-Kashani, M. The role of reduced graphene oxide as a secondary filler in improving the performance of silica-filled styrene-butadiene rubber compounds. Polym. J. 2021, 54, 355–365. [Google Scholar] [CrossRef]
- Sarath, P.; Moni, G.; George, J. A study on the influence of reduced graphene oxide on the mechanical, dynamic mechanical and tribological properties of silicone rubber nanocomposites. Compos. Mater. 2021, 55, 2011–2024. [Google Scholar] [CrossRef]
- Capezza, A.; Andersson, R.; Ström, V. Preparation and Comparison of Reduced Graphene Oxide and Carbon Nanotubes as Fillers in Conductive Natural Rubber for Flexible Electronics. ACS Omega 2019, 4, 3458–3468. [Google Scholar] [CrossRef] [PubMed]
- Nurhafizah, M.D.; Sabar, I. The study of reduced graphene oxide/activated char from rubber seed shell composites and the capacitive behaviour of its electrodes. Int. J. Nanotechnol. 2020, 17, 768. [Google Scholar] [CrossRef]
- Song, S.; Thamyongkit, P.; Poompradub, S. Natural rubber/reduced-graphene oxide composite materials: Morphological and oil adsorption properties for treatment of oil spills. J. Adv. Res. 2019, 20, 79–89. [Google Scholar] [CrossRef]
- Maya; Abrahann, M.; George, J.; Soney, C.; Thomas, S. Exploring the filler-polymer interaction and solvent transport behavior of nanocomposites derived from reduced graphene oxide and polychloroprene rubber. J. Appl. Polym. Sci. 2019, 136, 48168. [Google Scholar] [CrossRef]





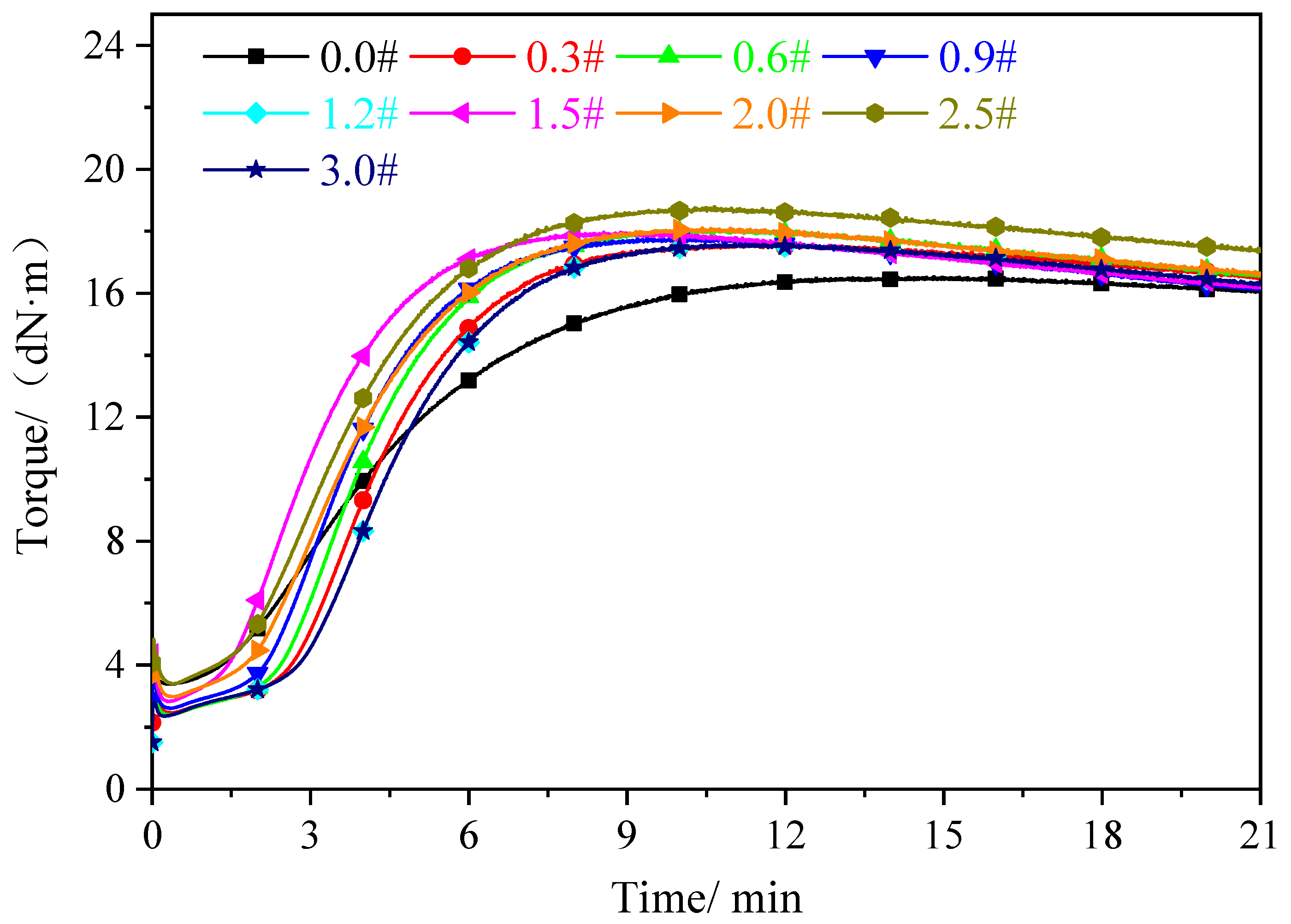



| Sample | MH | ML | TC10/min | TC90/min | MH-ML | TC90-TC10/min |
|---|---|---|---|---|---|---|
| 0.0 # | 14.70 | 2.74 | 01:30 | 04:07 | 13.76 | 2:37 |
| 0.3 # | 17.56 | 1.88 | 01:37 | 04:19 | 15.68 | 2:42 |
| 0.6 # | 17.31 | 1.18 | 01:43 | 04:40 | 13.13 | 2:57 |
| 0.9 # | 17.76 | 1.98 | 01:47 | 04:54 | 15.78 | 3:07 |
| 1.2 # | 17.54 | 1.69 | 02:03 | 05:15 | 15.85 | 3:12 |
| 1.5 # | 17.91 | 2.16 | 02:08 | 05:25 | 15.75 | 3:17 |
| 2.0 # | 18.04 | 2.31 | 02:21 | 05:55 | 15.73 | 3:34 |
| 2.5 # | 18.72 | 2.67 | 02:30 | 06:21 | 16.05 | 3:51 |
| 3.0 # | 19.16 | 4.07 | 02:49 | 06:45 | 16.12 | 3:56 |
| Sample | 0 # | 0.3 # | 0.6 # | 0.9 # | 1.2 # | 1.5 # | 2.0 # | 2.5 # | 3.0 # |
|---|---|---|---|---|---|---|---|---|---|
| tA/°C | 154.4 | 154.3 | 154.2 | 154.3 | 153.9 | 153.9 | 154.0 | 153.7 | 153.5 |
| tB/°C | 150.7 | 150.9 | 151.2 | 151.2 | 151.4 | 15.1.3 | 152.0 | 152.4 | 152.9 |
| tC/°C | 149.9 | 149.9 | 150.3 | 151.0 | 150.6 | 151.2 | 151.4 | 151.7 | 151.7 |
| ΔtAC/°C | 4.5 | 4.4 | 3.9 | 3.3 | 3.3 | 2.7 | 2.6 | 2.0 | 1.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Li, J.; Fan, W. The Role of Reduced Graphene Oxide in Enhancing the Mechanical and Thermal Properties of a Rubber Cover Joint. Polymers 2024, 16, 1143. https://doi.org/10.3390/polym16081143
Zhang H, Li J, Fan W. The Role of Reduced Graphene Oxide in Enhancing the Mechanical and Thermal Properties of a Rubber Cover Joint. Polymers. 2024; 16(8):1143. https://doi.org/10.3390/polym16081143
Chicago/Turabian StyleZhang, Hongyu, Junxia Li, and Wenrui Fan. 2024. "The Role of Reduced Graphene Oxide in Enhancing the Mechanical and Thermal Properties of a Rubber Cover Joint" Polymers 16, no. 8: 1143. https://doi.org/10.3390/polym16081143
APA StyleZhang, H., Li, J., & Fan, W. (2024). The Role of Reduced Graphene Oxide in Enhancing the Mechanical and Thermal Properties of a Rubber Cover Joint. Polymers, 16(8), 1143. https://doi.org/10.3390/polym16081143





