Preparation and Properties of Low-Dielectric Polyimide Films Containing Tert-Butyl
Abstract
1. Introduction
2. Experiment Section
2.1. Materials
2.2. Characterization
2.3. Synthesis of 4,4′-Diamino-3,5-Ditert Butyl Biphenyl Ether
2.4. Preparation of Tert-Butyl PI Films
2.5. Preparation of Traditional PI Film without Tert-Butyl Group
2.6. Density Functional Theory (DFT) Calculations
3. Results and Discussion
3.1. Structure Characterization of 4,4′-Diamino-3,5-Ditert Butyl Biphenyl Ether
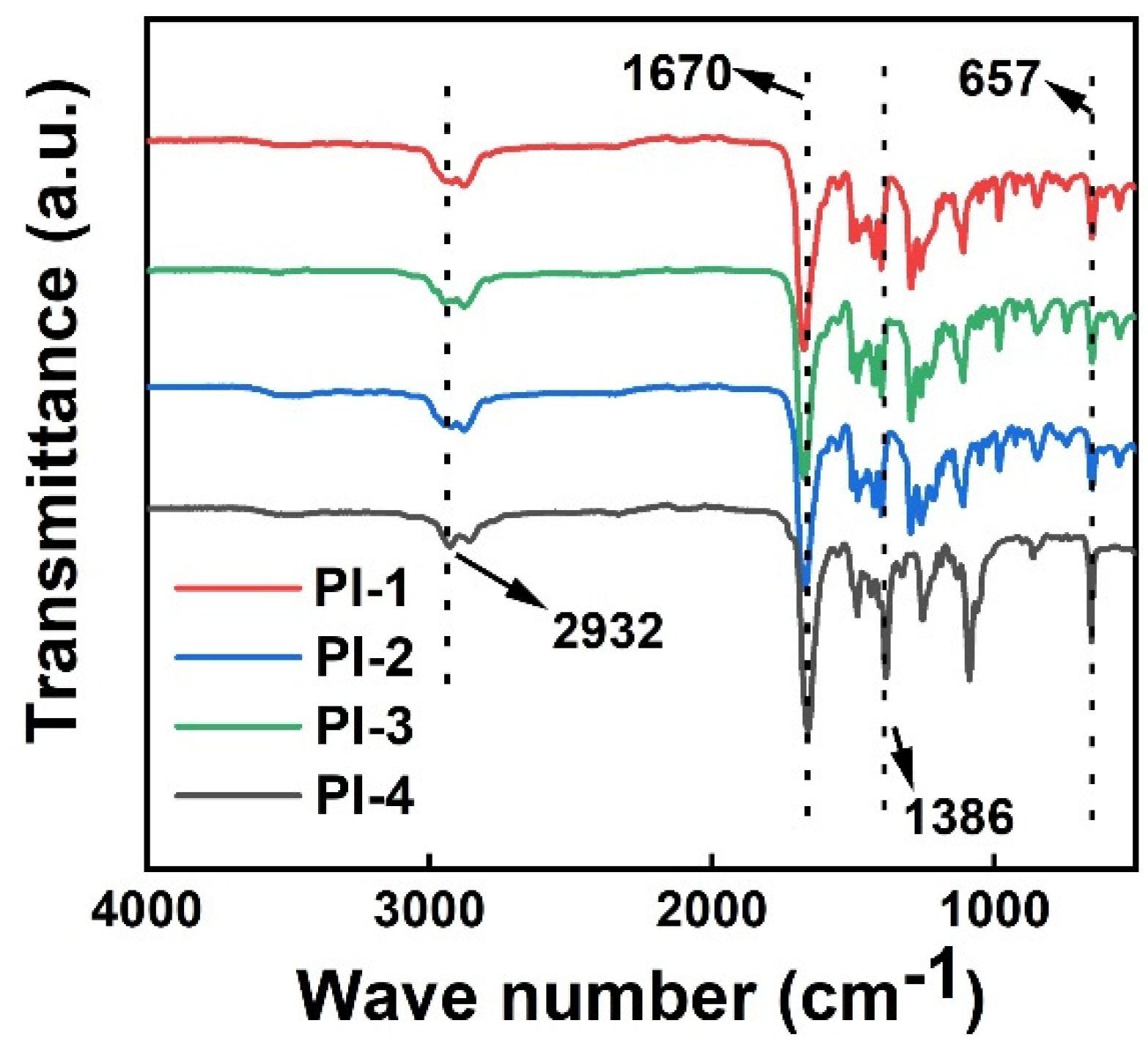
3.2. Structure Characterization of Tert-Butyl PI Films
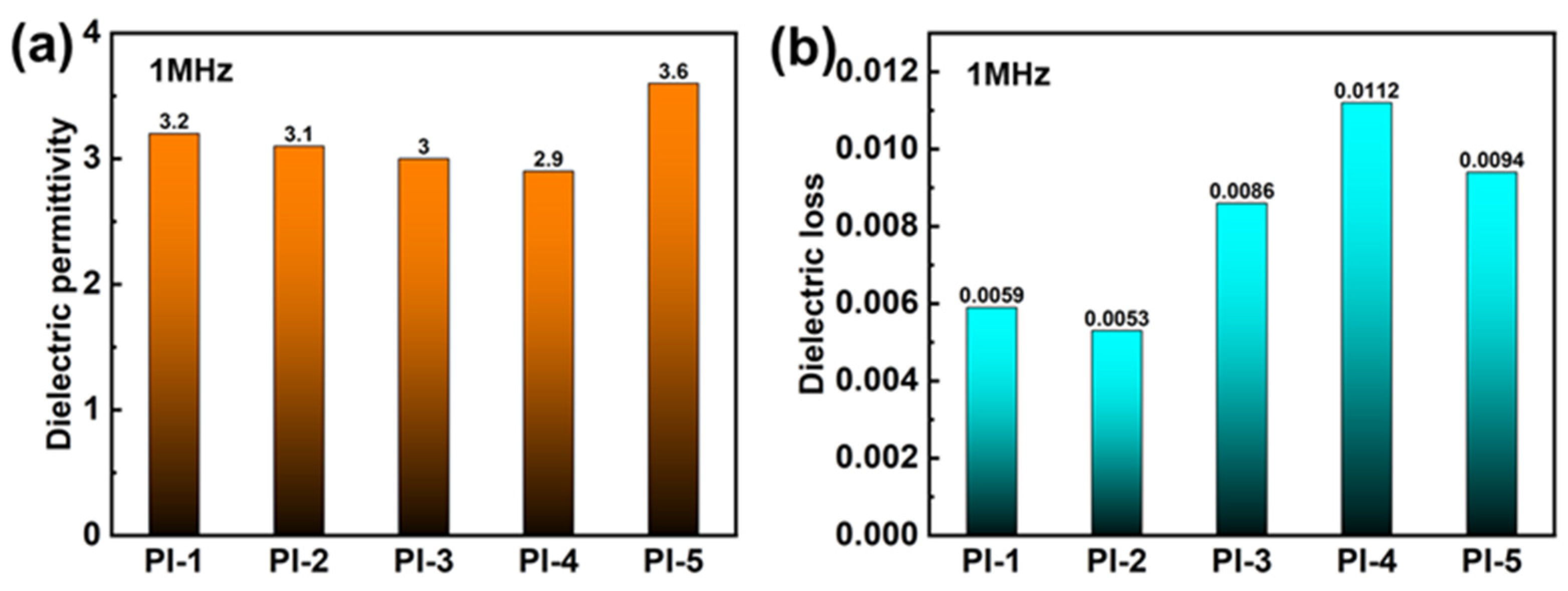
3.3. Study of Dielectric Properties of Tert-Butyl PI Films
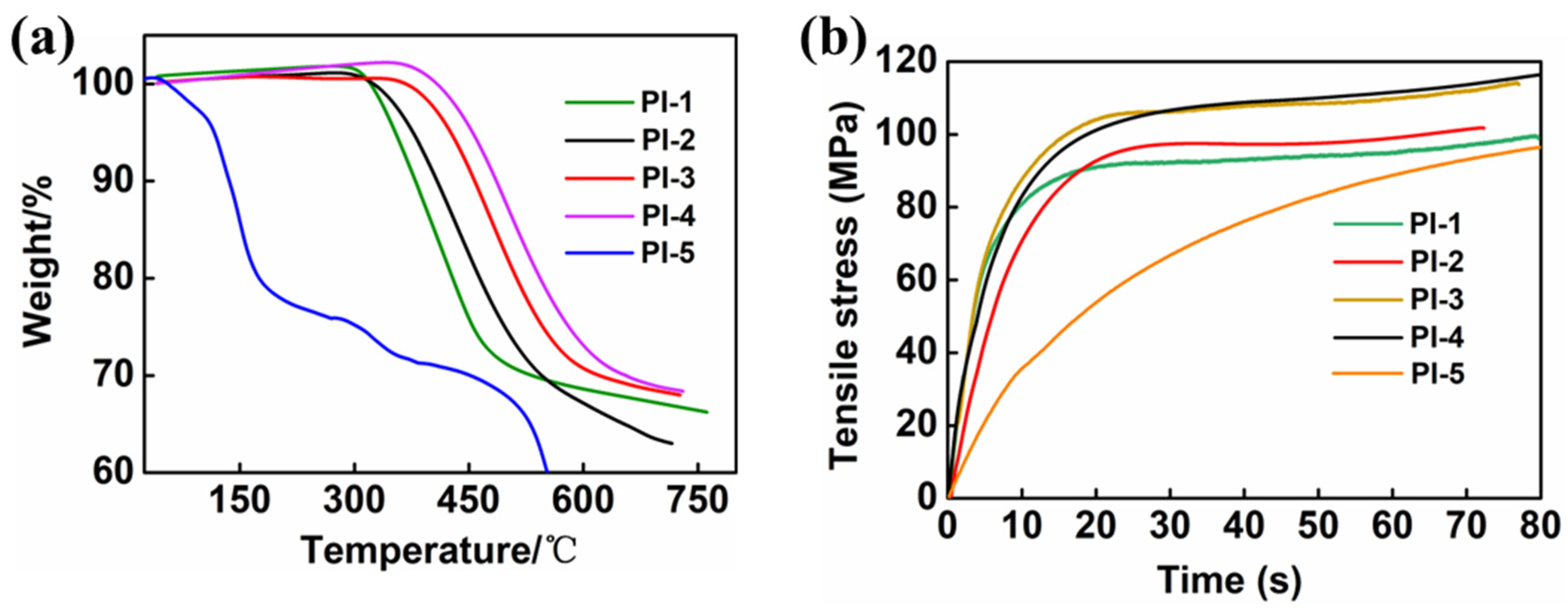
3.4. Study of Thermal Performance of Tert-Butyl PI Films
3.5. Study of Mechanical Performance of Tert-Butyl PI Films
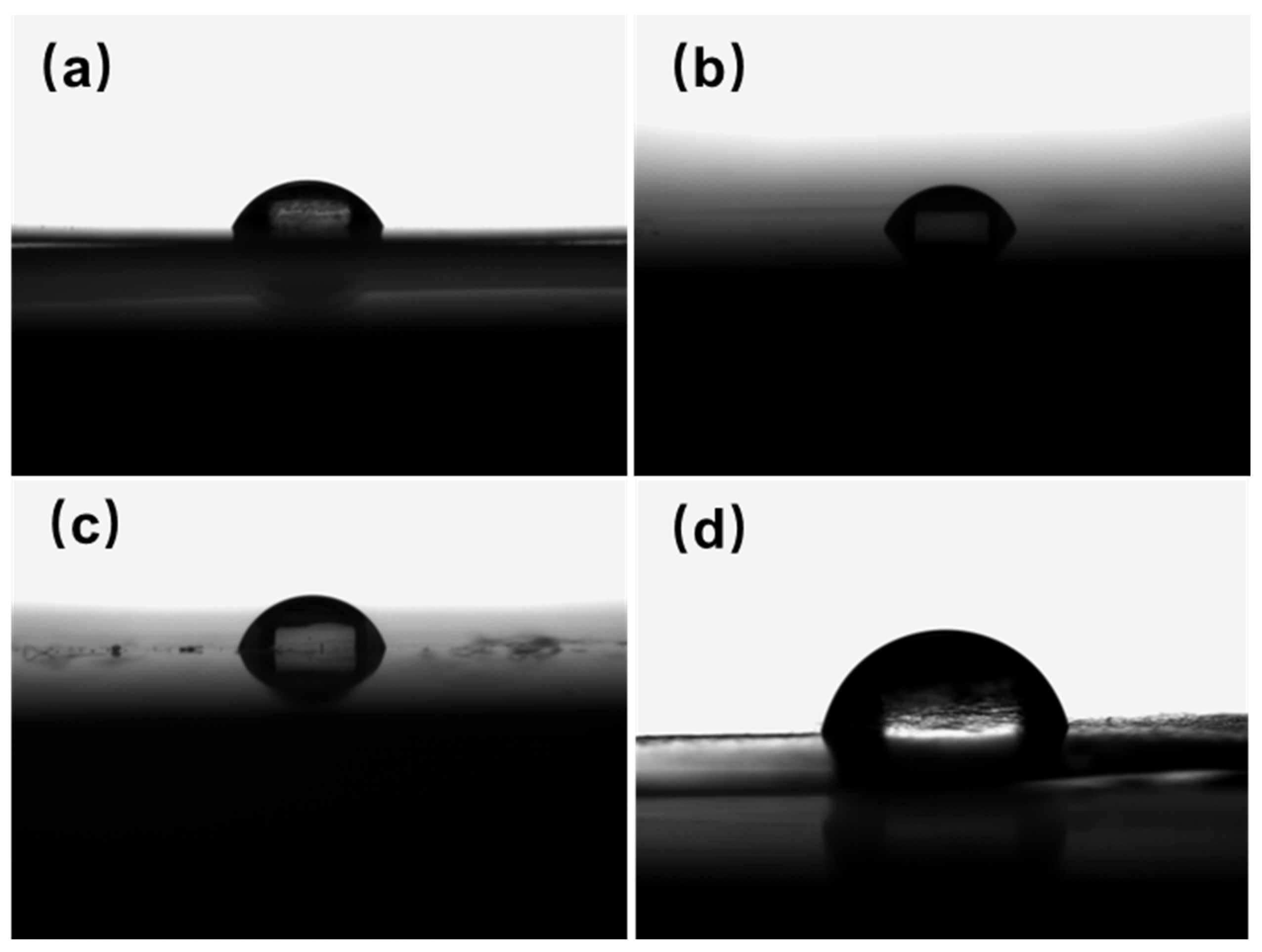
3.6. Study of Hydrophobic Performance of Tert-Butyl PI Films
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Gao, M.Y.; Wang, C.O.; Jia, Y.; Zhai, L.; Mo, S.; He, M.H.; Fang, L. Research Progress in Anisotropic Thermal Conduction Behavior of Polyimide Films. Acta Polym. Sin. 2021, 52, 1283–1297. [Google Scholar] [CrossRef]
- Li, Y.H.; Sun, G.H.; Zhou, Y.; Liu, G.M.; Wang, J.; Han, S.H. Progress in low dielectric polyimide film—A review. Prog. Org. Coat. 2022, 172, 107103. [Google Scholar] [CrossRef]
- Wang, X.; Liu, S.Y.; Han, H.Y.; Liu, X.Y.; Wang, X. Research progress in insulating and thermal conductivity of fluorinated graphene and its polyimide composites. IET Nanodielectr. 2023. [Google Scholar] [CrossRef]
- Zha, J.W.; Wang, F. Research progress of high thermal conductivity polyimide dielectric films. Acta Phys. Sin. 2022, 71, 233601. [Google Scholar] [CrossRef]
- Zhou, Z.H.; Zhou, N.; Jia, X.L.; Liu, N.; Shi, B.L.; Jin, R.Z.; Qu, L.J.; Xu, B.S. Research progress of filled-type high-thermal-conductivity flexible polyimide composites: A review. J. Mater. Sci. 2023, 58, 15973–16001. [Google Scholar] [CrossRef]
- Feng, X.; Wang, Y.H.; Zhao, Y.X.; Yu, X.L.; Zhang, P.B.; Cui, J.; Guo, M.J. Preparation of Colorless Transparent Polyimide Films with High Comprehensive Properties. Acta Polym. Sin. 2023, 54, 1219–1228. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Liang, B.; Long, J.P. Preparation and characteristics of composite films with functionalized graphene/polyimide. J. Appl. Polym. Sci. 2023, 141, e55045. [Google Scholar] [CrossRef]
- Mo, S.; Zhai, L.; Liu, Y.; Han, G.; Jia, Y.; He, M.H.; Fan, L. Siloxane-containing polyimide films with high heat resistance and low dielectric constant. IET Nanodielectr. 2023, 7, 33–45. [Google Scholar] [CrossRef]
- Chu, N.; Li, Y.M.; Luo, C.J.; Li, L.X.; Zhan, J.C.; Chao, M. Preparation and properties of MWCNTs-ODA/Ag/polyimide composite films. Cailiao Gongcheng J. Mater. Eng. 2023, 51, 75–82. [Google Scholar] [CrossRef]
- Jiang, T.; Hu, W.D.; Cui, J.; Liu, J.N.; Sun, X.Y.; Zhang, P.B.; Wang, C.M.; Li, Y.C. Insights into Entropy Change of β2 Relaxation in Retraction of Uniaxially Stretched Polyimide Films. Macromol. Chem. Phys. 2023, 224, 2300309. [Google Scholar] [CrossRef]
- Li, Y.Q.; Cao, Y.J.; Wu, S.L.; Ju, Y.L.; Zhang, X.H.; Lu, C.H.; Sun, W. Manipulative pore-formation of polyimide film for tuning the dielectric property via breath figure method. Polymer 2023, 269, 125731. [Google Scholar] [CrossRef]
- Qu, C.H.; Shan, L.; Zhang, G.P.; Sun, R. Preparation and dielectric properties research of a novel kind of intrinsic silane-containing polyimide. Polymer 2023, 285, 126361. [Google Scholar] [CrossRef]
- Song, Q.Y.; Wang, M.Q.; Lai, Y.M.; Wang, Q.H.; Yin, X.Y.; Hou, L.X. Preparation and properties of branch-leaf-like polyimide composite films with high thermal conductivity and low dielectric loss. J. Appl. Polym. Sci. 2023, 140, e54520. [Google Scholar] [CrossRef]
- Wang, R.Z.; Hou, X.B.; Ma, B.; Zhang, R.B. Preparation of flexible and ultralow dielectric polyimide porous films by a low-cost and environmentally friendly non-solvent phase separation. Mater. Lett. 2023, 344, 134417. [Google Scholar] [CrossRef]
- Huang, W.; Ju, T.; Li, R.; Duan, Y.; Duan, Y.; Wei, J.; Zhu, L. High-κ and High-Temperature Dipolar Glass Polymers Based on Sulfonylated and Cyanolated Poly(Arylene Ether)s for Capacitive Energy Storage. Adv. Electron. Mater. 2022, 9, 2200414. [Google Scholar] [CrossRef]
- Wu, Z.Q.; Peng, Y.W.; Guo, Q.; Zhou, H.H.; Gong, L.; Liu, Z.G.; Zhang, Q.Y.; Chen, Y.H. Multilayer heterogeneous dielectric films with simultaneously improved dielectric constant and breakdown strength. Mater. Today Commun. 2022, 32, 103857. [Google Scholar] [CrossRef]
- Chang, E.C.; Tseng, L.Y.; Liu, Y.; Chen, C.K.; Kuo, C.C.; Ueda, M.; Lin, Y.C.; Chen, W.C. Investigating the structure-sensitivity relationship of photosensitive polyimide formulated by using a photobase generator. J. Polym. Sci. 2023, 61, 2122–2132. [Google Scholar] [CrossRef]
- Haoliang, T.; Changliang, W.; Mengqiu, G.; Yongjing, C.; Junguo, G.; Zhihui, T.; Yi, L.; Cong, S.; Hao, W.; Guo, J.; et al. Study on process and performance of thermal protective coating on polyimide resin matrix composite. Ceram. Int. 2020, 46, 12744–12758. [Google Scholar] [CrossRef]
- Han, M.; Zhang, S.; Cao, Y.; Han, C.; Li, X.; Zhang, Y.; Yang, Z.; Sun, J. A one-for-all strategy of polyimide coating layer for resolving the comprehensive issues of phosphorus anode. J. Energy Chem. 2022, 70, 276–282. [Google Scholar] [CrossRef]
- Kwac, L.K.; Kim, H.G.; Chang, J.H. Comparison of Properties of Colorless and Transparent Polyimide Nanocomposites Containing Different Diamine Monomers. Acs Omega 2021, 6, 19006–19016. [Google Scholar] [CrossRef]
- Li, B.Y.; Jiang, S.J.; Yu, S.W.; Chen, Y.; Tang, X.L.; Wu, X.D.; Zhong, Y.; Shen, X.D.; Cui, S. Co-polyimide aerogel using aromatic monomers and aliphatic monomers as mixing diamines. J. Sol-Gel Sci. Technol. 2018, 88, 386–394. [Google Scholar] [CrossRef]
- Saeed, M.B.; Zhan, M.S. Effects of monomer structure and imidization degree on mechanical properties and viscoelastic behavior of thermoplastic polyimide films. Eur. Polym. J. 2006, 42, 1844–1854. [Google Scholar] [CrossRef]
- Tachibana, S.; Hashimoto, K.; Mizuno, H.; Ueno, K.; Watanabe, M. Effects of polyimide sequence and monomer structures on CO2 permeation and mechanical properties of sulfonated polyimide/ionic liquid composite membranes. Polymer 2022, 241, 124533. [Google Scholar] [CrossRef]
- Tan, J.H.; Chen, Y.F.; Huang, J.; Fei, L.F.; Jiang, L.B.; Wu, D.; Sun, W.; Zhang, H.L.; Liu, Y.W. Effect of monomer moieties on the aggregate structure and gas transport properties of polyimides: Insights from experiments and simulations. J. Appl. Polym. Sci. 2023, 140, e54091. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, X.Y.; Wang, Y.; Nie, Z.; Wang, X.; Tan, J.X.; Zhuang, Y.B.; Liu, X.Y.; Wang, X. Increasing Twist Rigidity to Prepare Polyimides with Low Dielectric Constants and Dissipation Factors over a Wide Temperature Range. Macromolecules 2023, 56, 9379–9388. [Google Scholar] [CrossRef]
- Patidar, R.; Gupta, H.; Savita, A. Computational insight towards the binding affinity and participation of aliphatic unsaturated sidearms of aza-18-crown-6 extractants for Sr<SUP>2+</SUP> encapsulation in different solvent medium. J. Mol. Model. 2023, 29, 294. [Google Scholar] [CrossRef] [PubMed]
- Bei, R.X.; Qian, C.; Zhang, Y.; Chi, Z.G.; Liu, S.W.; Chen, X.D.; Xu, J.R.; Aldred, M.P. Intrinsic low dielectric constant polyimides: Relationship between molecular structure and dielectric properties. J. Mater. Chem. C 2017, 5, 12807–12815. [Google Scholar] [CrossRef]
- Zuo, H.-T.; Gan, F.; Dong, J.; Zhang, P.; Zhao, X.; Zhang, Q.-H. Highly Transparent and Colorless Polyimide Film with Low Dielectric Constant by Introducing Meta-Substituted Structure and Trifluoromethyl Groups. Chin. J. Polym. Sci. 2020, 39, 455–464. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, S.; Zhao, J. Simultaneously Improving the Optical, Dielectric, and Solubility Properties of Fluorene-Based Polyimide with Silyl Ether Side Groups. ACS Omega 2022, 7, 11939–11945. [Google Scholar] [CrossRef]
- Zhang, C.G.; He, X.J.; Lu, Q.H. Polyimide films with ultralow dielectric loss for 5G applications: Influence and mechanism of ester groups in molecular chains. Eur. Polym. J. 2023, 200, 112544. [Google Scholar] [CrossRef]
- Ding, C.; Chen, C.; Yuan, Z.; Chen, L.X. Molecular simulation of the effect of plasma modification on the microscopic properties of polyimide. Front. Mater. 2022, 9, 1018882. [Google Scholar] [CrossRef]
- Hougham, G.; Tesoro, G.; Viehbeck, A. Influence of free volume change on the relative permittivity and refractive index in fluoropolyimides. Macromolecules 1996, 29, 3453–3456. [Google Scholar] [CrossRef]
- Li, X.L.; Lei, H.Y.; Guo, J.C.; Wang, J.H.; Qi, S.L.; Tian, G.F.; Wu, D.Z. Composition design and properties investigation of BPDA/PDA/TFDB co-polyimide films with low dielectric permittivity. J. Appl. Polym. Sci. 2019, 136, 47989. [Google Scholar] [CrossRef]
- Wang, L.; Yang, J.; Cheng, W.H.; Zou, J.J.; Zhao, D. Progress on Polymer Composites with Low Dielectric Constant and Low Dielectric Loss for High-Frequency Signal Transmission. Front. Mater. 2021, 8, 774843. [Google Scholar] [CrossRef]
- Park, H.M.; Ismael, M.; Takaba, H.; Lee, Y.T. Acid-resistant thin-film composite nanofiltration membrane prepared from polyamide-polyurea and the behavior of density functional theory study. J. Membr. Sci. 2022, 645, 120175. [Google Scholar] [CrossRef]
- Wu, D.; Zhao, X.; Li, X.; Dong, J.; Zhang, Q. Polyimide film containing sulfone groups with high dielectric properties. Polymer 2022, 256, 125221. [Google Scholar] [CrossRef]
- Huang, X.W.; Han, Z.Y.; Wang, J.; Li, Q.M. An MD-based physical characteristics analysis on the PI/EP duo-layer composites, impacting of diamino groups. Mater. Today Commun. 2023, 35, 105482. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, W.L.; Lin, J.Q.; Liu, X.M.; Sun, H.G.; Yang, Y.; He, X.J. Constructing high-polarization silica for polyimide-based nanocomposites with enhanced dielectric permittivity and mechanical performances: Molecular dynamics simulations. J. Appl. Polym. Sci. 2023, 140, 54321. [Google Scholar] [CrossRef]
- Wei, W.C.; Zhang, Y.Y.; Chen, H.Q.; Xu, C.Q.; Zha, J.W.; Nie, S.X. Molecular dynamics simulations guided the preparation of nano-silica/ polyimide/cellulose composite insulating paper. Mater. Des. 2023, 233, 112176. [Google Scholar] [CrossRef]
- Lei, H.Y.; Li, X.L.; Wang, J.L.; Song, Y.H.; Tian, G.F.; Huang, M.J.; Wu, D.Z. DFT and molecular dynamic simulation for the dielectric property analysis of polyimides. Chem. Phys. Lett. 2022, 786, 139131. [Google Scholar] [CrossRef]
- Jiao, L.; Du, Z.; Dai, X.; Wang, H.; Yao, H.; Qiu, X. Multifunctional polyimide films with superheat-resistance, low coefficient of thermal expansion and fluorescence performance. Polymer 2022, 247, 124792. [Google Scholar] [CrossRef]
- Li, S.e.; Zhu, H.; Bao, F.; Lan, X.; Li, H.; Li, Y.; Ji, M.; Wang, M.; Zhu, C.; Xu, J. Polyimide resins with superior thermal stability, dielectric properties, and solubility obtained by introducing trifluoromethyl and diphenylpyridine with different bulk pendant groups. Polymer 2023, 283, 126245. [Google Scholar] [CrossRef]
- Liu, T.-Q.; Zheng, F.; Ma, X.; Ding, T.-M.; Chen, S.; Jiang, W.; Zhang, S.-Y.; Lu, Q. High heat-resistant polyimide films containing quinoxaline moiety for flexible substrate applications. Polymer 2020, 209, 122963. [Google Scholar] [CrossRef]
- Wu, X.M.; Shu, C.; He, X.Q.; Wang, S.B.; Fan, X.; Yu, Z.H.; Yan, D.Y.; Huang, W. Optically Transparent and Thermal-Stable Polyimide Films Derived from a Semi-Aliphatic Diamine: Synthesis and Properties. Macromol. Chem. Phys. 2020, 221, 1900506. [Google Scholar] [CrossRef]
- Lin, D.; Li, R.; Liu, Y.; Qi, S.; Wu, D. Clarifying the effect of moisture absorption and high-temperature thermal aging on structure and properties of polyimide film at molecular dynamic level. Polymer 2021, 214, 123251. [Google Scholar] [CrossRef]
- Bei, R.X.; Chen, K.J.; He, Y.W.; Li, C.Y.; Chi, Z.G.; Liu, S.W.; Xu, J.R.; Zhang, Y. A systematic study of the relationship between the high-frequency dielectric dissipation factor and water adsorption of polyimide films. J. Mater. Chem. C 2023, 11, 10274–10281. [Google Scholar] [CrossRef]
- Pan, Z.; Wang, H.L.; Qi, H.R.; Gao, Y.S.; Wang, X.L.; Zhi, X.X.; Zhang, Y.; Ren, X.; Liu, J.G. Molecular Design, Preparation, and Characterization of Fluoro-Containing Polyimide Ultrafine Fibrous Membranes with High Whiteness, High Thermal Stability, and Good Hydrophobicity. Molecules 2022, 27, 5447. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, Y.; Guo, W.; Sun, X.; Feng, Y.; Sun, D.; Liu, Y.; Yan, K.; Wu, Z.; Su, B.; et al. Dielectric and mechanical properties of polyimide composite films reinforced with graphene nanoribbon. Surf. Coat. Technol. 2017, 320, 497–502. [Google Scholar] [CrossRef]
- Zhao, G.; Mu, X.; Ma, D.; Wang, S.; Pan, J.; Cui, J.; Qi, M. Dielectric and mechanical properties of TiO2/polyimide composites with low dielectric constant. Polym. Eng. Sci. 2023, 63, 1953–1960. [Google Scholar] [CrossRef]
- Li, R.Y.; Ding, C.C.; Yu, J.; Wang, X.D.; Huang, P. Enhanced thermal conductivity of polyimide composite film filled with hybrid fillers. High Perform. Polym. 2021, 33, 905–913. [Google Scholar] [CrossRef]
- Zhang, T.; Huang, Y.W.; Sun, Y.; Tang, P.P.; Hu, C.Y. Improved mechanical, thermal properties and ideal dielectric properties of polyimide composite films by incorporation of boron nitride nanosheets and aramid nanofibers. Polym. Adv. Technol. 2022, 33, 2123–2136. [Google Scholar] [CrossRef]
- Zhou, H.; Lei, H.; Wang, J.; Qi, S.; Tian, G.; Wu, D. Breaking the mutual restraint between low permittivity and low thermal expansion in polyimide films via a branched crosslink structure. Polymer 2019, 162, 116–120. [Google Scholar] [CrossRef]





| Polymer Code | PI-1 | PI-2 | PI-3 | PI-4 | PI-1 * | PI-2 * | PI-3 * | PI-4 * |
|---|---|---|---|---|---|---|---|---|
| EHOMO (eV) | −4.696 | −4.542 | −4.562 | −4.647 | −4.88 | −4.764 | −4.661 | −4.701 |
| ELOMO (eV) | −3.825 | −3.466 | −3.292 | −3.717 | −3.895 | −3.48 | −3.366 | −3.702 |
| Dielectric constant | 4.12 | 3.6 | 3.42 | 3.13 | 6.24 | 5.69 | 5.03 | 4.64 |
| Polymer Code | Thermal Weight Loss a | ||
|---|---|---|---|
| 5% | 10% | 20% | |
| PI-1 | 354 | 381 | 428 |
| PI-2 | 376 | 412 | 463 |
| PI-3 | 423 | 460 | 512 |
| PI-4 | 454 | 480 | 542 |
| PI-5 | 117 | 135 | 175 |
| Polymer Code | Tensile Stress (MPa) | Modulus (MPa/mm/mm) |
|---|---|---|
| PI-1 | 99.70 | 2619.68 |
| PI-2 | 102.00 | 2589.72 |
| PI-3 | 114.26 | 2576.72 |
| PI-4 | 117.40 | 2570.01 |
| PI-5 | 106.29 | 2517.49 |
| Polymer Code | Left Contact Angle | Right Contact Angle |
|---|---|---|
| PI-1 | 74.67 | 74.29 |
| PI-2 | 75.26 | 75.18 |
| PI-3 | 79.12 | 78.66 |
| PI-4 | 79.57 | 80.16 |
| PI-5 | 34.13 | 33.96 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Zheng, R.; Wang, C.; Chang, H.; Chen, S.; Wang, L.; Cui, X.; Liu, Y.; Li, J.; Yu, G.; et al. Preparation and Properties of Low-Dielectric Polyimide Films Containing Tert-Butyl. Polymers 2024, 16, 984. https://doi.org/10.3390/polym16070984
Li X, Zheng R, Wang C, Chang H, Chen S, Wang L, Cui X, Liu Y, Li J, Yu G, et al. Preparation and Properties of Low-Dielectric Polyimide Films Containing Tert-Butyl. Polymers. 2024; 16(7):984. https://doi.org/10.3390/polym16070984
Chicago/Turabian StyleLi, Xin, Rongrong Zheng, Cheng Wang, Haiyang Chang, Shuwu Chen, Liyan Wang, Xue Cui, Yutao Liu, Junhao Li, Guangning Yu, and et al. 2024. "Preparation and Properties of Low-Dielectric Polyimide Films Containing Tert-Butyl" Polymers 16, no. 7: 984. https://doi.org/10.3390/polym16070984
APA StyleLi, X., Zheng, R., Wang, C., Chang, H., Chen, S., Wang, L., Cui, X., Liu, Y., Li, J., Yu, G., & Shi, J. (2024). Preparation and Properties of Low-Dielectric Polyimide Films Containing Tert-Butyl. Polymers, 16(7), 984. https://doi.org/10.3390/polym16070984






