Improving the 3D Printability and Mechanical Performance of Biorenewable Soybean Oil-Based Photocurable Resins
Abstract
1. Introduction
2. Experimental Section/Methods
2.1. Materials and Samples Preparation
2.2. Sample Characterization
3. Results
3.1. Viscosity of the Liquid Formulations
3.2. Characterization of Printed Formulations
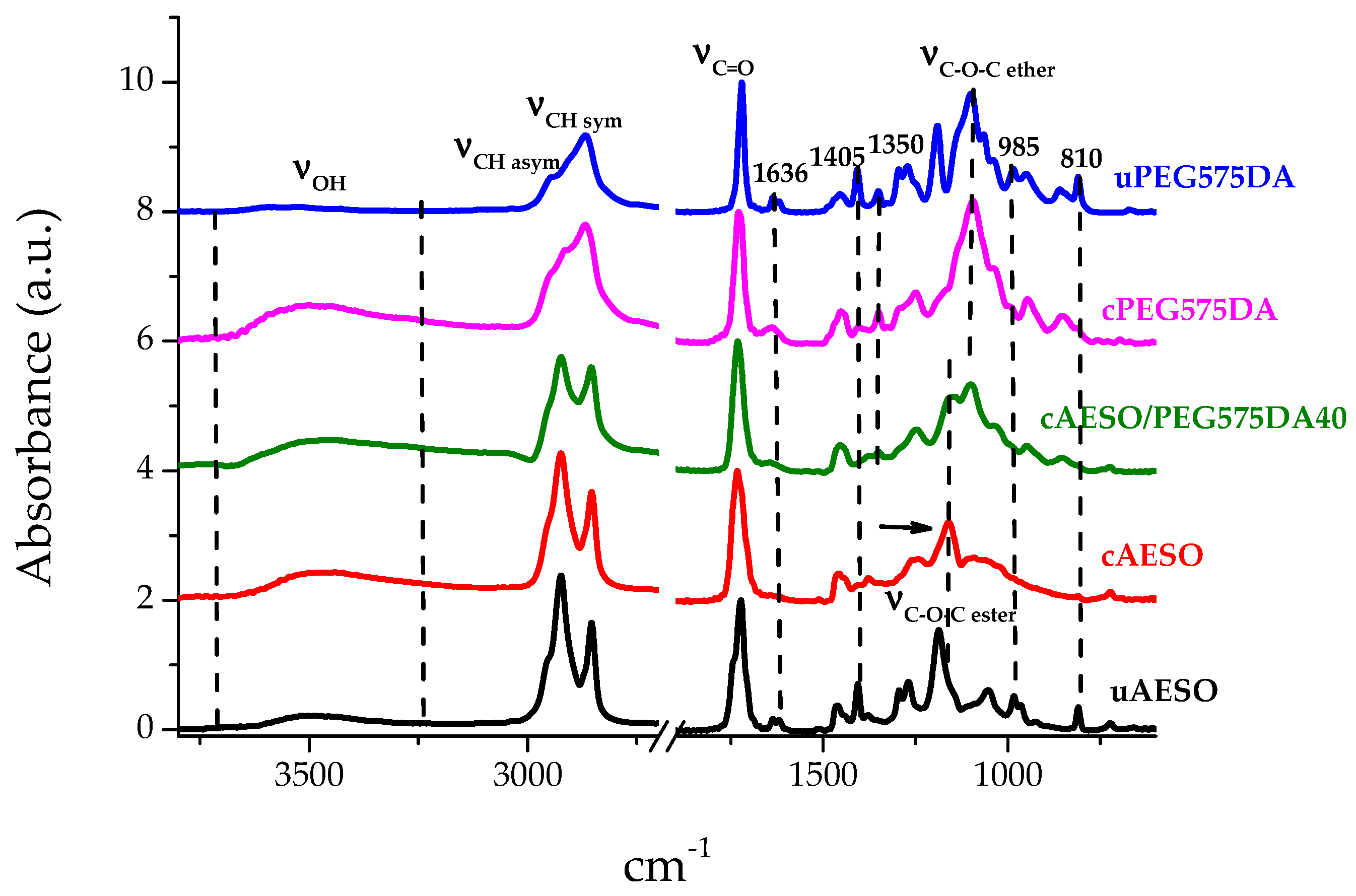
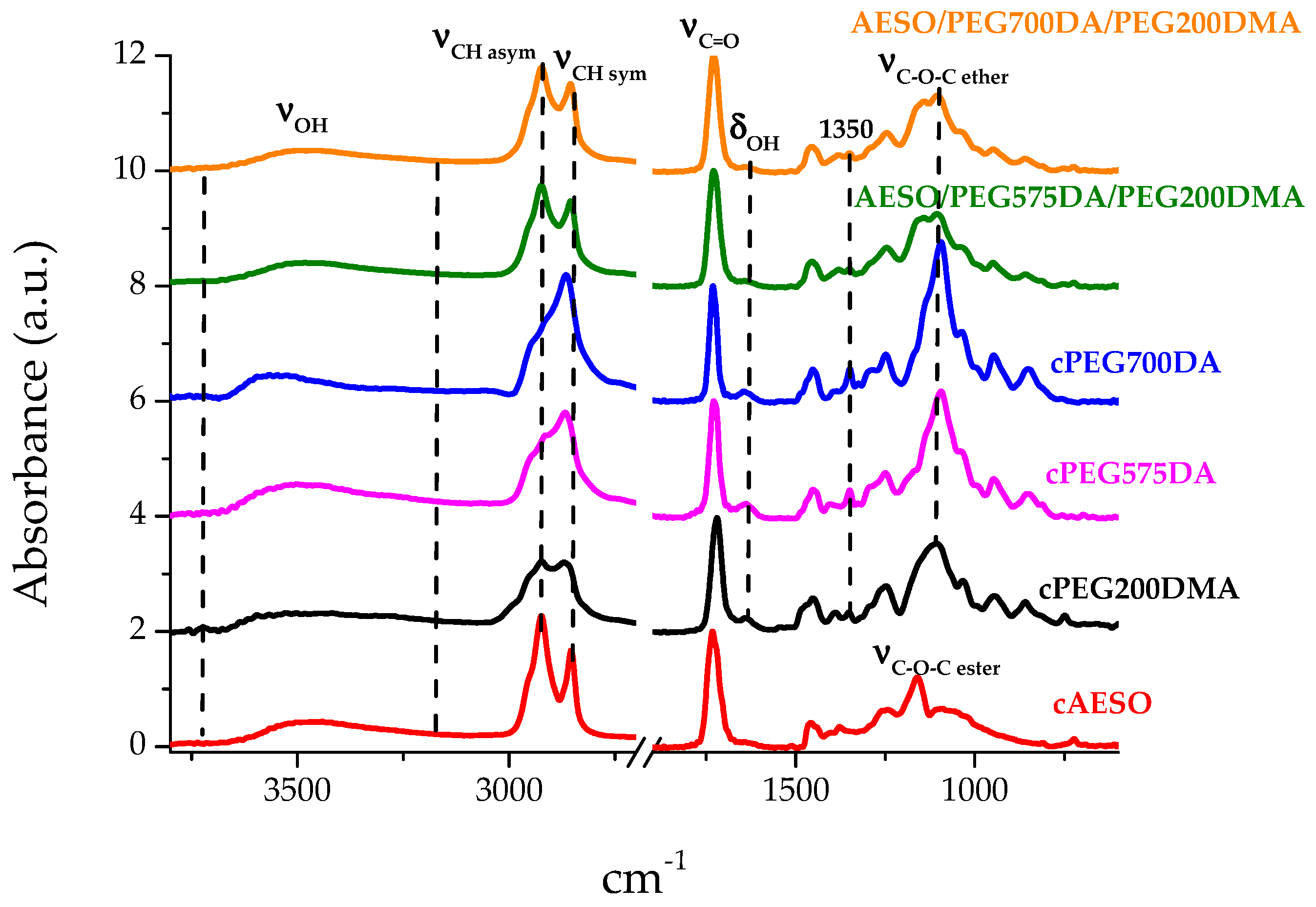
3.2.1. ATR Analysis
3.2.2. Mechanical Properties
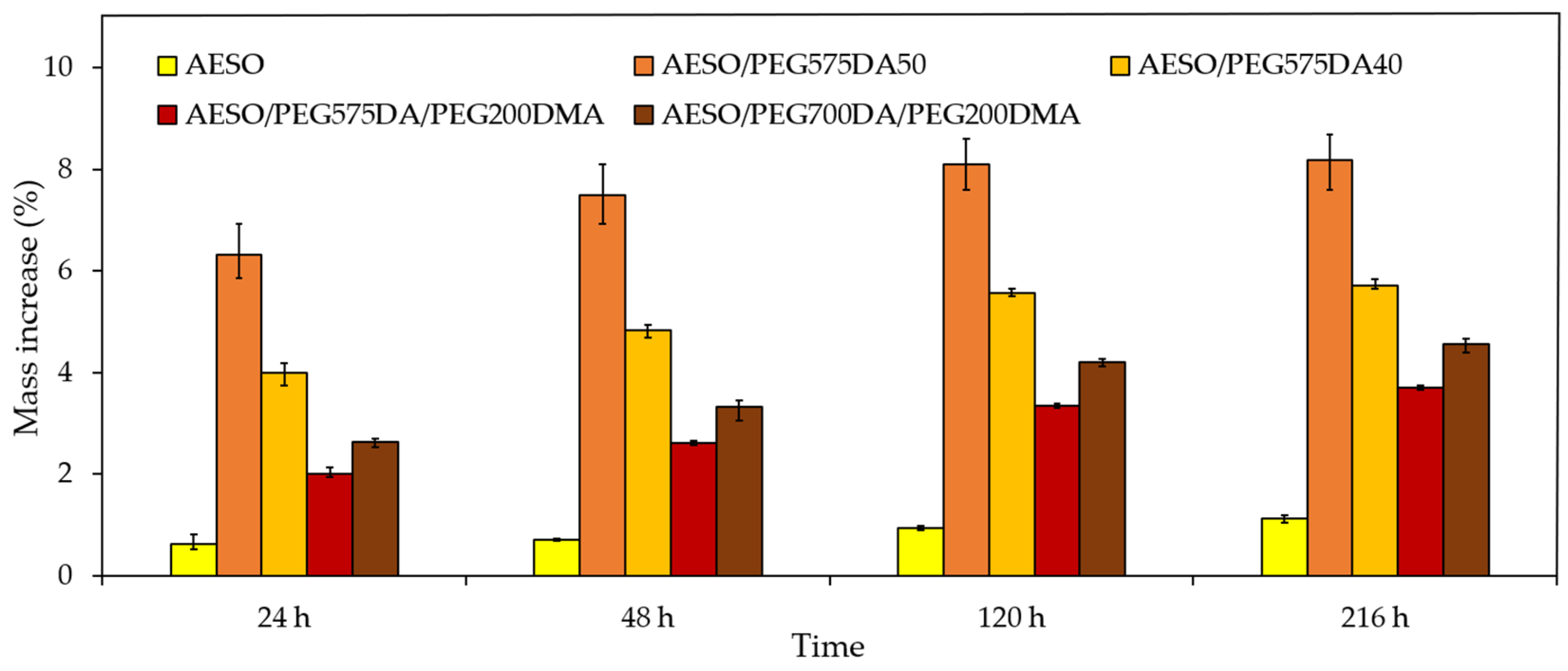
3.2.3. Hydrophobic/Hydrophilic Properties
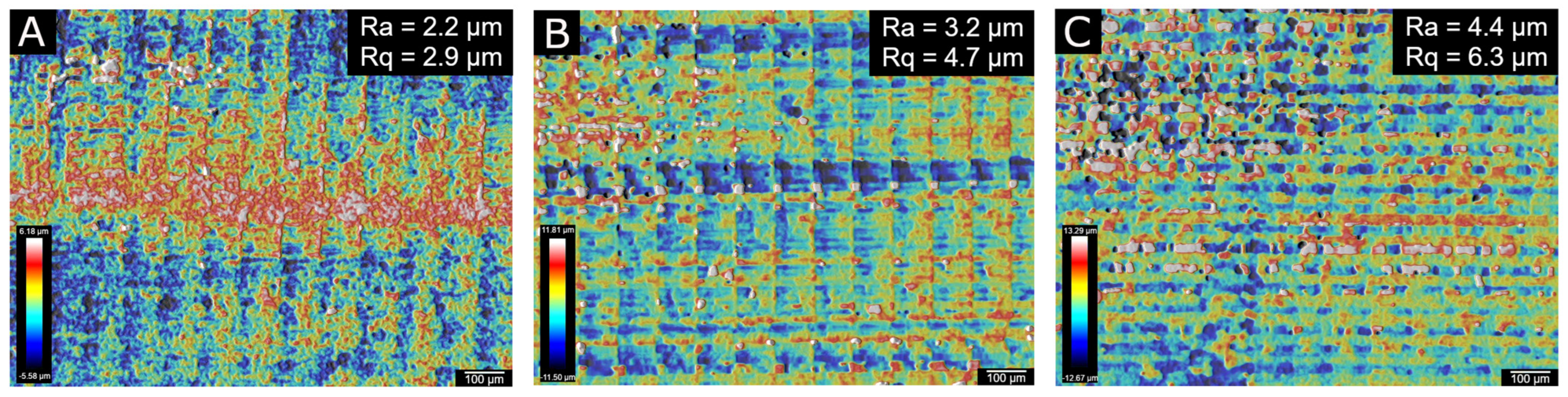
3.3. Printing Performance of AESO: Polyethylene Glycol Composites
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Voet, V.S.D.; Guit, J.; Loos, K. Sustainable Photopolymers in 3D Printing: A Review on Biobased, Biodegradable, and Recyclable Alternatives. Macromol. Rapid Commun. 2021, 42, 2000475. [Google Scholar] [CrossRef]
- Palucci Rosa, R.; Rosace, G. Nanomaterials for 3D Printing of Polymers via Stereolithography: Concept, Technologies, and Applications. Macromol. Mater. Eng. 2021, 306, 2100345. [Google Scholar] [CrossRef]
- Elomaa, L.; Teixeira, S.; Hakala, R.; Korhonen, H.; Grijpma, D.W.; Seppälä, J.V. Preparation of Poly(ε-Caprolactone)-Based Tissue Engineering Scaffolds by Stereolithography. Acta Biomater. 2011, 7, 3850–3856. [Google Scholar] [CrossRef]
- Chan, V.; Zorlutuna, P.; Jeong, J.H.; Kong, H.; Bashir, R. Three-Dimensional Photopatterning of Hydrogels Using Stereolithography for Long-Term Cell Encapsulation. Lab. Chip 2010, 10, 2062–2070. [Google Scholar] [CrossRef]
- Lai, H.; Zhang, J.; Xiao, P. Renewable Photopolymers: Transformation of Biomass Resources into Value-Added Products Under Light. ACS Sustain. Chem. Eng. 2023, 11, 16365–16406. [Google Scholar] [CrossRef]
- Marriam, F.; Irshad, A.; Umer, I.; Asghar, M.A.; Atif, M. Vegetable Oils as Bio-Based Precursors for Epoxies. Sustain. Chem. Pharm. 2023, 31, 100935. [Google Scholar] [CrossRef]
- Cui, Y.; Yang, J.; Lei, D.; Su, J. 3D Printing of a Dual-Curing Resin with Cationic Curable Vegetable Oil. Ind. Eng. Chem. Res. 2020, 59, 11381–11388. [Google Scholar] [CrossRef]
- Oilseeds: World Markets and Trade; Foreign Agricultural Service. Global Market Analysis; United States Department of Agriculture: Washington, DC, USA, 2024.
- Zhang, C.; Garrison, T.F.; Madbouly, S.A.; Kessler, M.R. Recent Advances in Vegetable Oil-Based Polymers and Their Composites. Prog. Polym. Sci. 2017, 71, 91–143. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, Q.; Yang, Z.; Bian, Y.; Chen, G.; Li, D.; Zheng, W.; Wei, Y.; Bi, Y.; Ding, K.; et al. Fabrication and Characterization of Light-Curing Soybean Oil-Based Epoxy Resin Applied for LCD Additive Manufacturing. Ind. Crops Prod. 2023, 202, 117037. [Google Scholar] [CrossRef]
- Lebedevaite, M.; Ostrauskaite, J. Influence of Photoinitiator and Temperature on Photocross-Linking Kinetics of Acrylated Epoxidized Soybean Oil and Properties of the Resulting Polymers. Ind. Crops Prod. 2021, 161, 113210. [Google Scholar] [CrossRef]
- Guit, J.; Tavares, M.B.L.; Hul, J.; Ye, C.; Loos, K.; Jager, J.; Folkersma, R.; Voet, V.S.D. Photopolymer Resins with Biobased Methacrylates Based on Soybean Oil for Stereolithography. ACS Appl. Polym. Mater. 2020, 2, 949–957. [Google Scholar] [CrossRef]
- Lebedevaite, M.; Ostrauskaite, J.; Skliutas, E.; Malinauskas, M. Photoinitiator Free Resins Composed of Plant-Derived Monomers for the Optical μ-3D Printing of Thermosets. Polymers 2019, 11, 116. [Google Scholar] [CrossRef]
- Mendes-Felipe, C.; Oliveira, J.; Costa, P.; Ruiz-Rubio, L.; Iregui, A.; González, A.; Vilas, J.L.; Lanceros-Mendez, S. Stimuli Responsive UV Cured Polyurethane Acrylated/Carbon Nanotube Composites for Piezoresistive Sensing. Eur. Polym. J. 2019, 120, 109226. [Google Scholar] [CrossRef]
- Kousaalya, A.B.; Ayalew, B.; Pilla, S. Photopolymerization of Acrylated Epoxidized Soybean Oil: A Photocalorimetry-Based Kinetic Study. ACS Omega 2019, 4, 21799–21808. [Google Scholar] [CrossRef]
- Lebedevaite, M.; Talacka, V.; Ostrauskaite, J. High Biorenewable Content Acrylate Photocurable Resins for DLP 3D Printing. J. Appl. Polym. Sci. 2021, 138, 50233. [Google Scholar] [CrossRef]
- Kim, H.M.; Kim, H.R.; Kim, B.S. Soybean Oil-Based Photo-Crosslinked Polymer Networks. J. Polym. Environ. 2010, 18, 291–297. [Google Scholar] [CrossRef]
- Grauzeliene, S.; Kazlauskaite, B.; Skliutas, E.; Malinauskas, M.; Ostrauskaite, J. Photocuring and Digital Light Processing 3D Printing of Vitrimer Composed of 2-Hydroxy-2-Phenoxypropyl Acrylate and Acrylated Epoxidized Soybean Oil. Express Polym. Lett. 2023, 17, 54–68. [Google Scholar] [CrossRef]
- Mendes-Felipe, C.; Costa, P.; Roppolo, I.; Sangermano, M.; Lanceros-Mendez, S. Bio-Based Piezo- and Thermoresistive Photocurable Sensing Materials from Acrylated Epoxidized Soybean Oil. Macromol. Mater. Eng. 2022, 307, 2100934. [Google Scholar] [CrossRef]
- Noè, C.; Cosola, A.; Tonda-Turo, C.; Sesana, R.; Delprete, C.; Chiappone, A.; Hakkarainen, M.; Sangermano, M. DLP-Printable Fully Biobased Soybean Oil Composites. Polymer 2022, 247, 124779. [Google Scholar] [CrossRef]
- Bragaglia, M.; Sciarretta, F.; Filetici, P.; Lettieri-Barbato, D.; Dassatti, L.; Nicoletti, F.; Sibilia, D.; Aquilano, K.; Nanni, F. Soybean Oil-Based 3D Printed Mesh Designed for Guided Bone Regeneration (GBR) in Oral Surgery. Macromol. Biosci. 2024, 2300458. [Google Scholar] [CrossRef]
- Zhu, G.; Zhang, J.; Huang, J.; Yu, X.; Cheng, J.; Shang, Q.; Hu, Y.; Liu, C.; Hu, L.; Zhou, Y. High-Performance 3D Printing UV-Curable Resins Derived from Soybean Oil and Gallic Acid. Green Chem. 2021, 23, 5911–5923. [Google Scholar] [CrossRef]
- Lebedevaite, M.; Gineika, A.; Talacka, V.; Baltakys, K.; Ostrauskaite, J. Development and Optical 3D Printing of Acrylated Epoxidized Soybean Oil-Based Composites with Functionalized Calcium Silicate Hydrate Filler Derived from Aluminium Fluoride Production Waste. Compos. Part A Appl. Sci. Manuf. 2022, 157, 106929. [Google Scholar] [CrossRef]
- Bergoglio, M.; Najmi, Z.; Cochis, A.; Miola, M.; Vernè, E.; Sangermano, M. UV-Cured Bio-Based Acrylated Soybean Oil Scaffold Reinforced with Bioactive Glasses. Polymers 2023, 15, 4089. [Google Scholar] [CrossRef] [PubMed]
- Barkane, A.; Platnieks, O.; Jurinovs, M.; Kasetaite, S.; Ostrauskaite, J.; Gaidukovs, S.; Habibi, Y. Uv-Light Curing of 3d Printing Inks from Vegetable Oils for Stereolithography. Polymers 2021, 13, 1195. [Google Scholar] [CrossRef]
- Mondal, D.; Willett, T.L. Mechanical Properties of Nanocomposite Biomaterials Improved by Extrusion during Direct Ink Writing. J. Mech. Behav. Biomed. Mater. 2020, 104, 103653. [Google Scholar] [CrossRef] [PubMed]
- Mondal, D.; Hashemi, S.S.; Diederichs, E.; Patil, H.; Willett, T.L. 3D Printing of Biopolymer Composites and Nanocomposites. In Additive Manufacturing of Biopolymers: Handbook of Materials, Techniques, and Applications; Elsevier: Amsterdam, The Netherlands, 2023; pp. 135–166. ISBN 9780323951517. [Google Scholar]
- Mondal, D.; Haghpanah, Z.; Huxman, C.J.; Tanter, S.; Sun, D.; Gorbet, M.; Willett, T.L. MSLA-Based 3D Printing of Acrylated Epoxidized Soybean Oil—Nano-Hydroxyapatite Composites for Bone Repair. Mater. Sci. Eng. C 2021, 130, 112456. [Google Scholar] [CrossRef] [PubMed]
- Rosa, R.P.; Rosace, G.; Arrigo, R.; Malucelli, G. Preparation and Characterization of a Fully Biobased Resin System for 3d-Printing, Suitable for Replacing Fossil-Based Acrylates. J. Polym. Res. 2023, 30, 139. [Google Scholar] [CrossRef]
- Veith, C.; Diot-Néant, F.; Miller, S.A.; Allais, F. Synthesis and Polymerization of Bio-Based Acrylates: A Review. Polym. Chem. 2020, 11, 7452–7470. [Google Scholar] [CrossRef]
- León, A.; Reuquen, P.; Garín, C.; Segura, R.; Vargas, P.; Zapata, P.; Orihuela, P.A. FTIR and Raman Characterization of TiO2 Nanoparticles Coated with Polyethylene Glycol as Carrier for 2-Methoxyestradiol. Appl. Sci. 2017, 7, 49. [Google Scholar] [CrossRef]
- Bernhard, M.; Eubeler, J.P.; Zok, S.; Knepper, T.P. Aerobic Biodegradation of Polyethylene Glycols of Different Molecular Weights in Wastewater and Seawater. Water Res. 2008, 42, 4791–4801. [Google Scholar] [CrossRef]
- ISO 527; Determination of Tensile Properties. AENOR. Asociación Española de Normalización y Certificación: Madrid, Spain, 2014.
- Khromiak, U.; Levytskyi, V.; Stepova, K.; Tarnawsky, A. Synthesis and Properties of Adhesive Polymer-Methylmethacrylate Materials. Int. J. Polym. Sci. 2018, 2018, 4905304. [Google Scholar] [CrossRef]
- Jafarzadeh, S.; Johansson, M.; Sundell, P.E.; Claudino, M.; Pan, J.; Claesson, P.M. UV-Curable Acrylate-Based Nanocomposites: Effect of Polyaniline Additives on the Curing Performance. Polym. Adv. Technol. 2013, 24, 668–678. [Google Scholar] [CrossRef]
- UNE-EN ISO 868; Determinación de La Dureza de In-Dentación Por Medio de Un Durómetro (Dureza Shore). AENOR. Asociación Española de Normalización y Certificación: Madrid, Spain, 2003.
- ISO 62:2008; Determination of Water Absorption. AENOR. Asociación Española de Normalización y Certificación: Madrid, Spain, 2008.
- ASME_B46.1-2019; Surface Texture. American Society of Mechanical Engineers: New York, NY, USA, 2019.
- Voet, V.S.D.; Strating, T.; Schnelting, G.H.M.; Dijkstra, P.; Tietema, M.; Xu, J.; Woortman, A.J.J.; Loos, K.; Jager, J.; Folkersma, R. Biobased Acrylate Photocurable Resin Formulation for Stereolithography 3D Printing. ACS Omega 2018, 3, 1403–1408. [Google Scholar] [CrossRef] [PubMed]
- Briede, S.; Jurinovs, M.; Nechausov, S.; Platnieks, O.; Gaidukovs, S. State-of-the-Art UV-Assisted 3D Printing via a Rapid Syringe-Extrusion Approach for Photoactive Vegetable Oil Acrylates Produced in One-Step Synthesis. Mol. Syst. Des. Eng. 2022, 7, 1434–1448. [Google Scholar] [CrossRef]
- Zubrzycka, P.; Radecka, M.; Graule, T.; Stuer, M. Debinding of Additively Manufactured Parts from Spinel Powders with Particle Sizes below 200 Nm. Ceram. Int. 2023, 49, 11355–11367. [Google Scholar] [CrossRef]
- Miao, S.; Zhu, W.; Castro, N.J.; Nowicki, M.; Zhou, X.; Cui, H.; Fisher, J.P.; Zhang, L.G. 4D Printing Smart Biomedical Scaffolds with Novel Soybean Oil Epoxidized Acrylate. Sci. Rep. 2016, 6, 27226. [Google Scholar] [CrossRef]
- Danish, M.; Vijay Anirudh, P.; Karunakaran, C.; Rajamohan, V.; Mathew, A.T.; Koziol, K.; Thakur, V.K.; Kannan, C.; Balan, A.S.S. 4D Printed Stereolithography Printed Plant-Based Sustainable Polymers: Preliminary Investigation and Optimization. J. Appl. Polym. Sci. 2021, 138, 50903. [Google Scholar] [CrossRef]
- Klimaschewski, S.F.; Küpperbusch, J.; Kunze, A.; Vehse, M. Material Investigations on Poly(Ethylene Glycol) Diacrylate-Based Hydrogels for Additive Manufacturing Considering Different Molecular Weights. J. Mech. Energy Eng. 2022, 6, 33–42. [Google Scholar] [CrossRef]
- Rosace, G.; Palucci Rosa, R.; Arrigo, R.; Malucelli, G. Photosensitive Acrylates Containing Bio-Based Epoxy-Acrylate Soybean Oil for 3D Printing Application. J. Appl. Polym. Sci. 2021, 138, 51292. [Google Scholar] [CrossRef]
- Stillman, Z.; Jarai, B.M.; Raman, N.; Patel, P.; Fromen, C.A. Degradation Profiles of Poly(Ethylene Glycol)Diacrylate (PEGDA)-Based Hydrogel Nanoparticles. Polym. Chem. 2020, 11, 568–580. [Google Scholar] [CrossRef]
- Jacobs, P.F. Rapid Prototyping & Manufacturing: Fundamentals of Stereolithography; Society of Manufacturing Engineers: Southfield, MI, USA, 1992; pp. 1–434. [Google Scholar]
- Arias-Ferreiro, G.; Ares-Pernas, A.; Lasagabáster-Latorre, A.; Aranburu, N.; Guerrica-Echevarria, G.; Dopico-García, M.S.; Abad, M.J. Printability Study of a Conductive Polyaniline/Acrylic Formulation for 3D Printing. Polymers 2021, 13, 2068. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Shan, M.; Zhang, H.; Sheng, J.; Zhou, J.; Cui, C.; Wei, J.; Zhu, W.; Lu, J. 4D Printing of Soybean Oil Based Shape Memory Polymer and Its Magnetic-Sensitive Composite via Digital Light Processing. Polym.-Plast. Technol. Mater. 2022, 61, 923–936. [Google Scholar] [CrossRef]
- Narewska, J.; Lassila, L.; Fardim, P. Preparation and Characterization of New Mouldable Cellulose-AESO Biocomposites. Cellulose 2014, 21, 1769–1780. [Google Scholar] [CrossRef]
- Pasek-Allen, J.L.; Wilharm, R.K.; Bischof, J.C.; Pierre, V.C. NMR Characterization of Polyethylene Glycol Conjugates for Nanoparticle Functionalization. ACS Omega 2023, 8, 4331–4336. [Google Scholar] [CrossRef] [PubMed]
- Melchels, F.P.W.; Feijen, J.; Grijpma, D.W. A Review on Stereolithography and Its Applications in Biomedical Engineering. Biomaterials 2010, 31, 6121–6130. [Google Scholar] [CrossRef] [PubMed]
- Kowsari, K.; Zhang, B.; Panjwani, S.; Chen, Z.; Hingorani, H.; Akbari, S.; Fang, N.X.; Ge, Q. Photopolymer Formulation to Minimize Feature Size, Surface Roughness, and Stair-Stepping in Digital Light Processing-Based Three-Dimensional Printing. Addit. Manuf. 2018, 24, 627–638. [Google Scholar] [CrossRef]
- Lee, K.W.; Wang, S.; Fox, B.C.; Ritman, E.L.; Yaszemski, M.J.; Lu, L. Poly(Propylene Fumarate) Bone Tissue Engineering Scaffold Fabrication Using Stereolithography: Effects of Resin Formulations and Laser Parameters. Biomacromolecules 2007, 8, 1077–1084. [Google Scholar] [CrossRef] [PubMed]
- Skliutas, E.; Lebedevaite, M.; Kasetaite, S.; Rekštytė, S.; Lileikis, S.; Ostrauskaite, J.; Malinauskas, M. A Bio-Based Resin for a Multi-Scale Optical 3D Printing. Sci. Rep. 2020, 10, 9758. [Google Scholar] [CrossRef]
- Cano, L.; Builes, D.H.; Carrasco-Hernandez, S.; Gutierrez, J.; Tercjak, A. Quantitative Nanomechanical Property Mapping of Epoxy Thermosetting System Modified with Poly(Ethylene Oxide-b-Propylene Oxide-b-Ethylene Oxide) Triblock Copolymer. Polym. Test. 2017, 57, 38–41. [Google Scholar] [CrossRef]
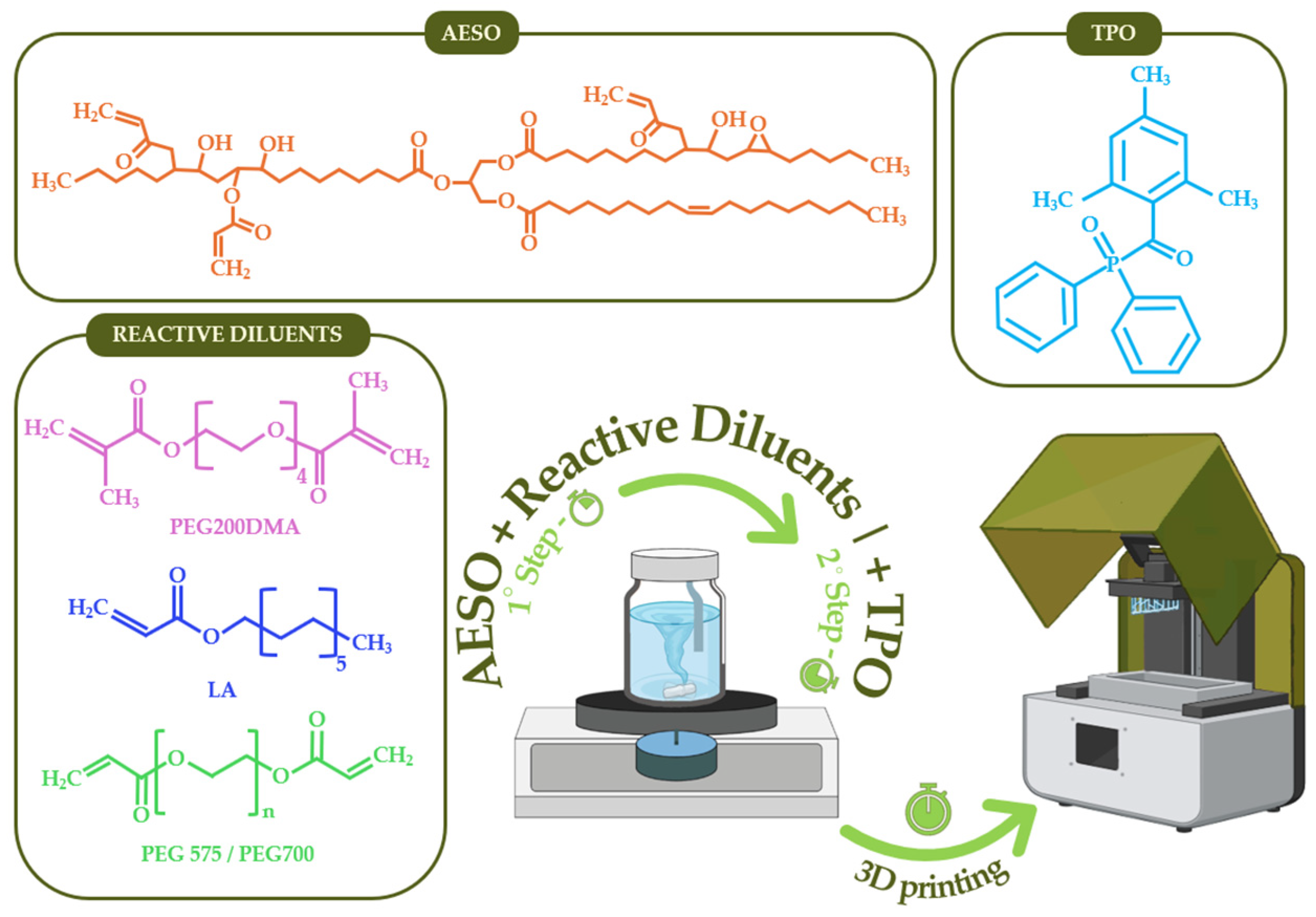



| Resin | AESO (wt.%) | RD1 (wt.%) | RD2 (wt.%) | BRC (%) | η (Pa·s) |
|---|---|---|---|---|---|
| AESO * | 100 | - | - | 86.0 | 10.31 ± 0.21 |
| AESO/LA10 * | 90 | 10 | - | 85.4 | 1.71 ± 0.21 |
| AESO/LA20 * | 80 | 20 | - | 84.8 | 0.79 ± 0.09 |
| AESO/LA30 * | 70 | 30 | - | 84.2 | 0.36 ± 0.04 |
| AESO/LA40 * | 60 | 40 | - | 83.6 | 0.24 ± 0.27 |
| AESO/LA50 * | 50 | 50 | - | 83.0 | 0.14 ± 0.15 |
| LA * | - | 100 | - | 80.0 | 0.012 ± 0.003 |
| AESO/PEG575DA20 | 80 | 20 | - | 68.8 | 1.064 ± 0.23 |
| AESO/PEG575DA30 | 70 | 30 | - | 60.2 | 0.95 ± 0.14 |
| AESO/PEG575DA40 * | 60 | 40 | - | 51.6 | 0.46 ± 0.04 |
| AESO/PEG575DA50 * | 50 | 50 | - | 43.0 | 0.23 ± 0.04 |
| PEG575DA * | - | 100 | - | 0 | 0.031 ± 0.011 |
| AESO/PEG200DMA20 | 80 | 20 | - | 83.8 | 1.18 ± 0.05 |
| AESO/PEG200DMA30 | 70 | 30 | - | 82.7 | 0.42 ± 0.15 |
| AESO/PEG200DMA40 | 60 | 40 | - | 81.6 | 0.21 ± 0.02 |
| AESO/PEG200DMA50 | 50 | 50 | - | 80.5 | 0.11 ± 0.04 |
| AESO/PEG200DMA60 | 40 | 60 | - | 79.4 | 0.06 ± 0.006 |
| PEG200DMA * | - | 100 | - | 75.0 | 0.031 ± 0.014 |
| AESO/PEG575DA/PEG200DMA * | 60 | 20 | 20 | 66.6 | 0.25 ± 0.02 |
| AESO/PEG700DA/PEG200DMA * | 60 | 20 | 20 | 66.6 | 0.24 ± 0.04 |
| PEG700DA * | - | 100 | - | 0 | 0.064 ± 0.022 |
| Sample | DBC % | Hardness Shore D (°Sh) | E (MPa) | σ (MPa) | ε (%) |
|---|---|---|---|---|---|
| AESO | 92.6 ± 0.8 | 50.0 ± 1.5 | 44.5 ± 5.5 | 3.61 ± 1.04 | 15.5 ± 2.5 |
| PEG575DA | 93.0 ± 2.8 | - | 18.8 ± 1.0 | 3.1 ± 1.8 | 18.8 ± 1.0 |
| PEG700DA | 98.8 ± 0.2 | - | 13.6 ± 0.4 | 2.46 ± 0.62 | 18.9 ± 4.5 |
| PEG200DMA | 94.2 ± 4.4 | 77.0 ± 2.0 | 521.1 ± 60 | 48.5 ± 2.5 | 27.7 ± 4.9 |
| AESO/LA20 | 87.9 ± 1.8 | 44.0 ± 0.5 | 16.9 ± 2.4 | 2.37 ± 0.25 | 20.3 ± 1.9 |
| AESO/LA30 | 89.9 ± 7.0 | 33.0 ± 0.5 | 8.8 ± 1.0 | 1.20 ± 0.22 | 18.3 ± 2.9 |
| AESO/LA40 | 94.7 ± 3.0 | 21.0 ± 1.0 | 4.9 ± 0.4 | 0.30 ± 0.26 | 9.1 ± 4.2 |
| AESO/LA50 | 96.4 ± 2.8 | 25.0 ± 1.0 | 1.4 ± 0.2 | 0.22 ± 0.20 | 8.2 ± 2.0 |
| AESO/PEG575DA40 | 97.3 ± 0.7 | 42.0 ± 1.0 | 23.5 ± 2.0 | 1.75 ± 0.45 | 10.7 ± 2.0 |
| AESO/PEG575DA50 | 98.8 ± 0.1 | 41.0 ± 1.0 | 22.8 ± 1.0 | 1.40 ± 0.23 | 8.3 ± 1.7 |
| AESO/PEG575DA/PEG200DMA | 94.7 ± 2.0 | 52.0 ± 1.5 | 51.7 ± 1.9 | 5.9 ± 0.8 | 18.7 ± 3.1 |
| AESO/PEG700DA/PEG200DMA | 96.6 ± 3.0 | 50.5 ± 1.5 | 41.0 ± 4.2 | 3.7 ± 1.1 | 13.4 ± 5.0 |
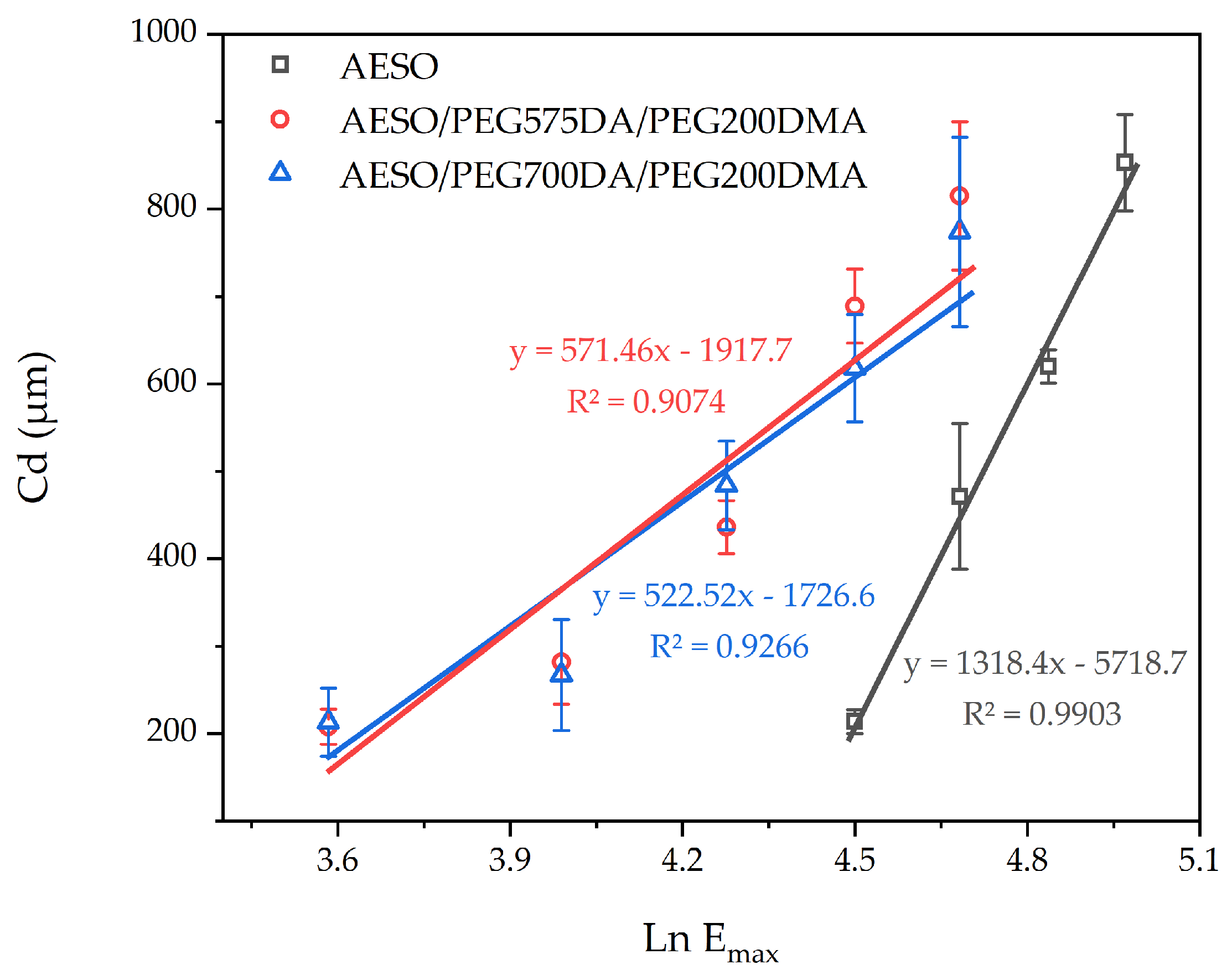
| Sample | Dp (µm) | Ec (mJ/cm2) |
|---|---|---|
| AESO | 1318 | 77 |
| AESO/PEG575DA/PEG200DMA | 571 | 29 |
| AESO/PEG700DA/PEG200DMA | 523 | 27 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bodor, M.; Lasagabáster-Latorre, A.; Arias-Ferreiro, G.; Dopico-García, M.S.; Abad, M.-J. Improving the 3D Printability and Mechanical Performance of Biorenewable Soybean Oil-Based Photocurable Resins. Polymers 2024, 16, 977. https://doi.org/10.3390/polym16070977
Bodor M, Lasagabáster-Latorre A, Arias-Ferreiro G, Dopico-García MS, Abad M-J. Improving the 3D Printability and Mechanical Performance of Biorenewable Soybean Oil-Based Photocurable Resins. Polymers. 2024; 16(7):977. https://doi.org/10.3390/polym16070977
Chicago/Turabian StyleBodor, Marius, Aurora Lasagabáster-Latorre, Goretti Arias-Ferreiro, María Sonia Dopico-García, and María-José Abad. 2024. "Improving the 3D Printability and Mechanical Performance of Biorenewable Soybean Oil-Based Photocurable Resins" Polymers 16, no. 7: 977. https://doi.org/10.3390/polym16070977
APA StyleBodor, M., Lasagabáster-Latorre, A., Arias-Ferreiro, G., Dopico-García, M. S., & Abad, M.-J. (2024). Improving the 3D Printability and Mechanical Performance of Biorenewable Soybean Oil-Based Photocurable Resins. Polymers, 16(7), 977. https://doi.org/10.3390/polym16070977







