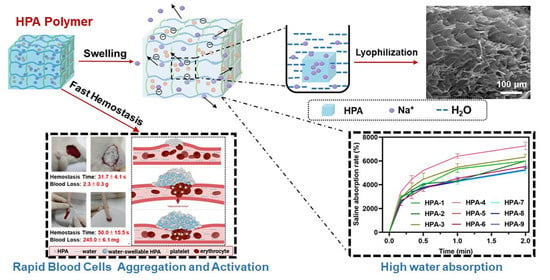
Highly Efficient Hemostatic Cross-Linked Polyacrylate Polymer Dressings for Immediate Hemostasis
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
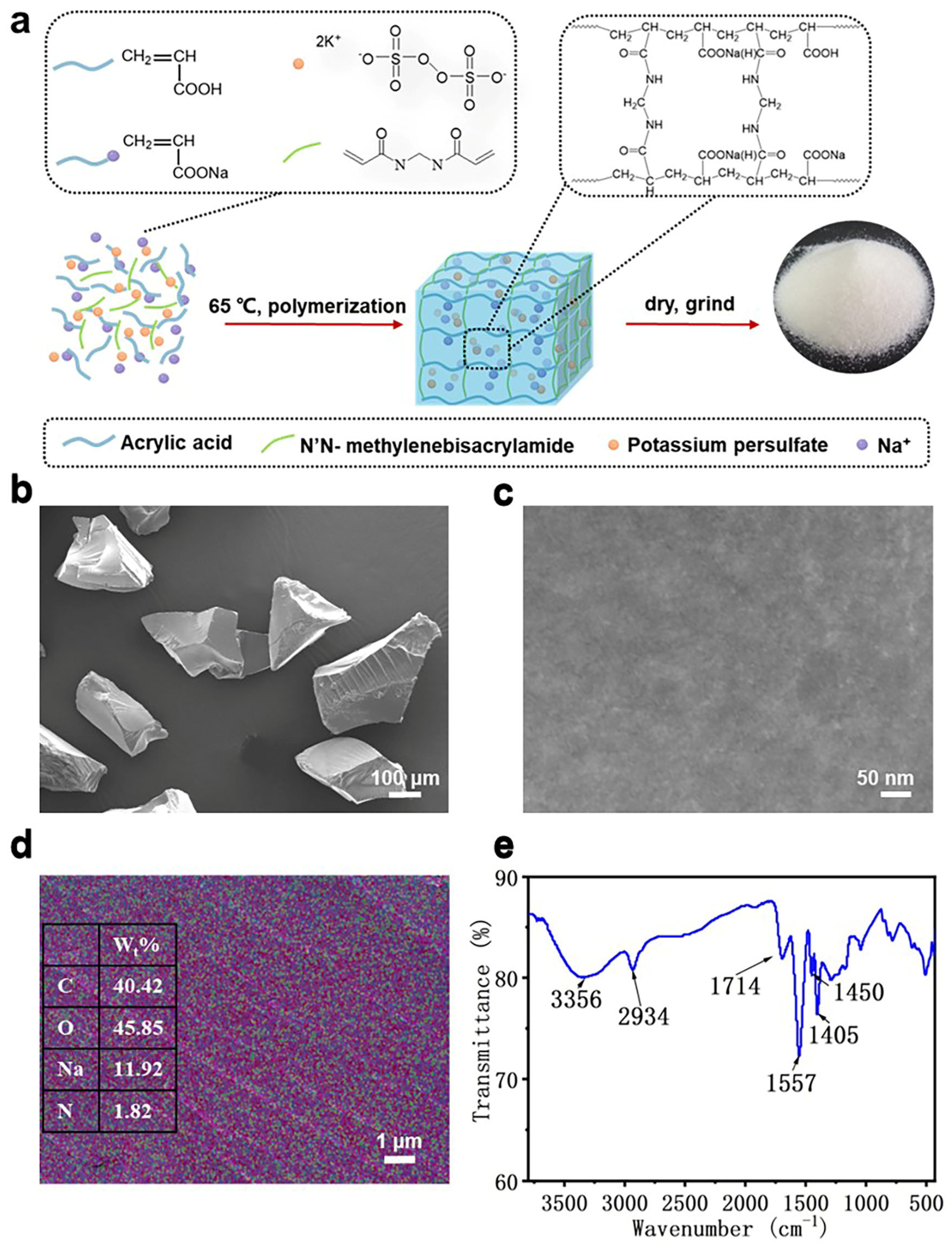
2.2. HPA Synthesis
2.3. Structural Characterization
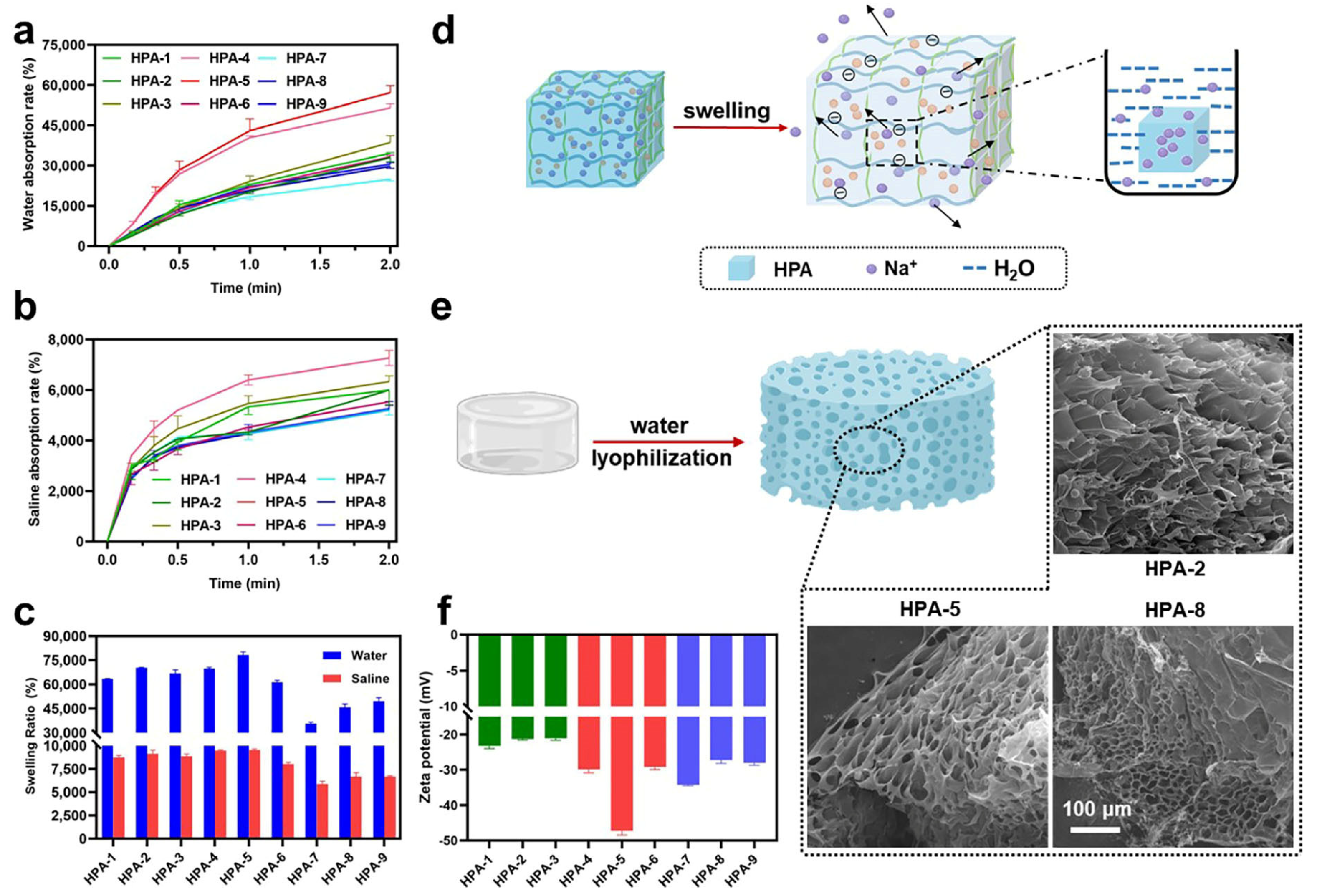
2.4. Water/Saline Absorption Ratio and Swelling Ratio Measurement
2.5. In Vitro Hemostatic Assay of HPA
2.6. Cytotoxicity Assay
2.7. Hemolysis Assay
2.8. Acute Toxicity Assay
2.9. Adhesion Property Test
2.10. In Vivo Hemostatic Assay
2.11. Tail Arteriovenous Injury
2.12. Femoral Arteriovenous Complete Shear
2.13. Preparation of Platelet-Rich Plasma (PRP) and Platelet-Poor Plasma (PPP)
2.14. Morphology of Adsorbed Platelets and Erythrocytes
2.15. Adherent Platelet Quantitative Analysis
2.16. Platelet Activation Was Measured Using Flow Cytometry
2.17. Adherent Erythrocyte Quantitative Analysis
2.18. Thromboelastogram (TEG) Analysis
2.19. Activated Partial Thromboplastin Time (APTT) and Prothrombin Time (PT) Analysis
2.20. Determination of Coagulation FXII Factors
2.21. Statistical Analysis
3. Results and Discussion
3.1. Characteristics of HPA
3.2. Water and Saline Absorption and Swelling Abilities of HPA
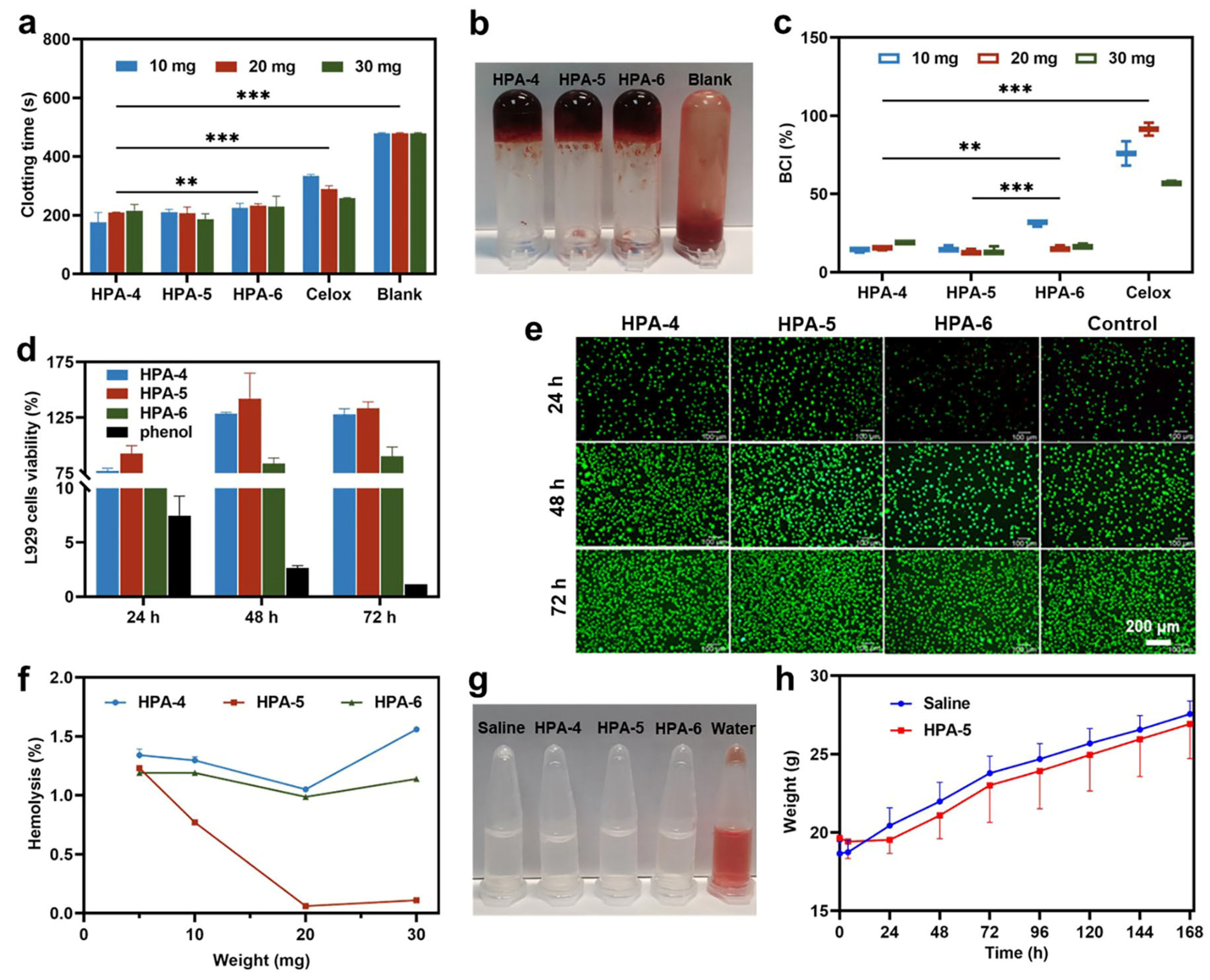
3.3. In Vitro Hemostatic Ability of HPA4–6
3.4. Biocompatibility of HPA-5
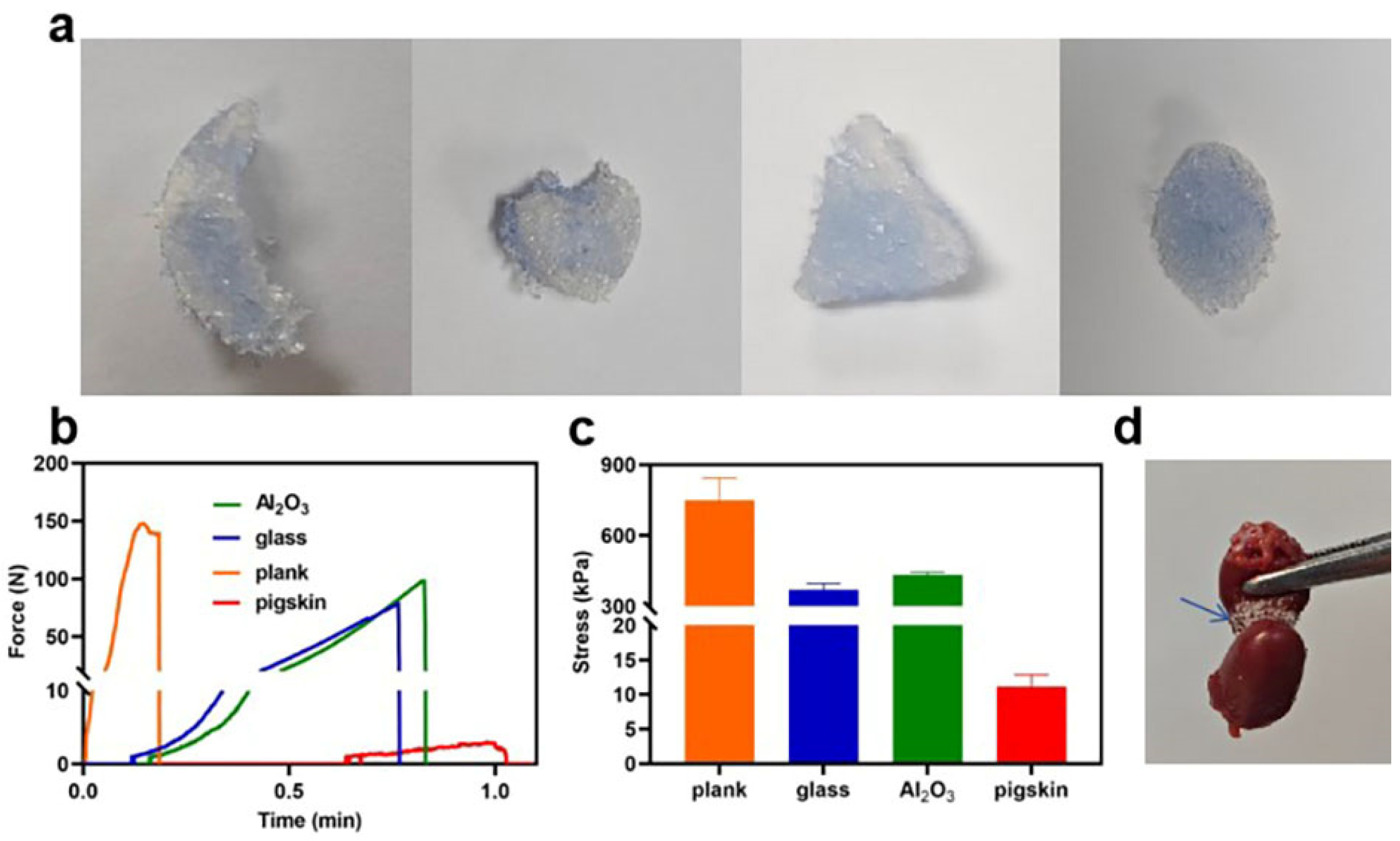
3.5. Adhesion Property of HPA-5
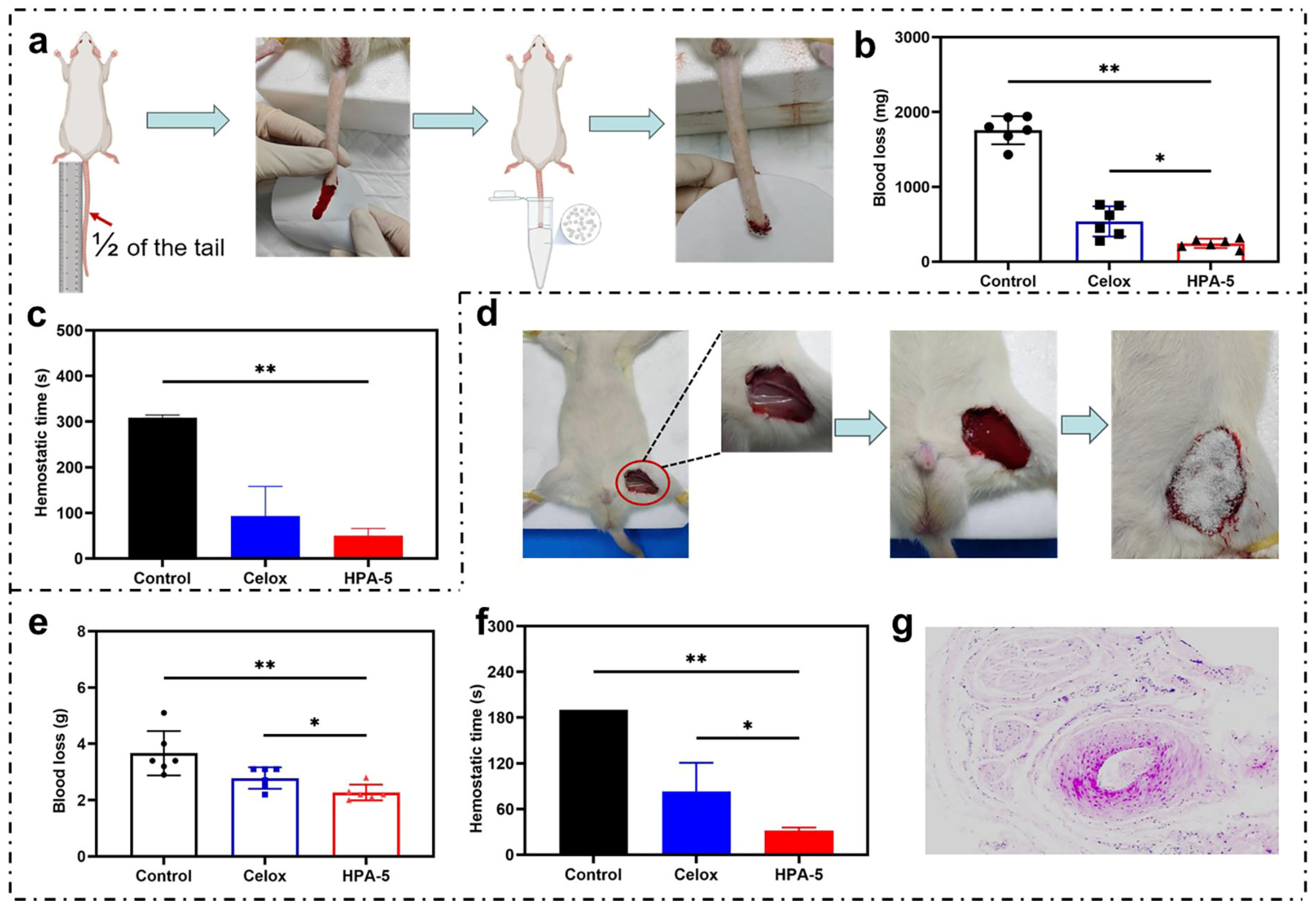
3.6. In Vivo Hemostatic Properties of HPA-5
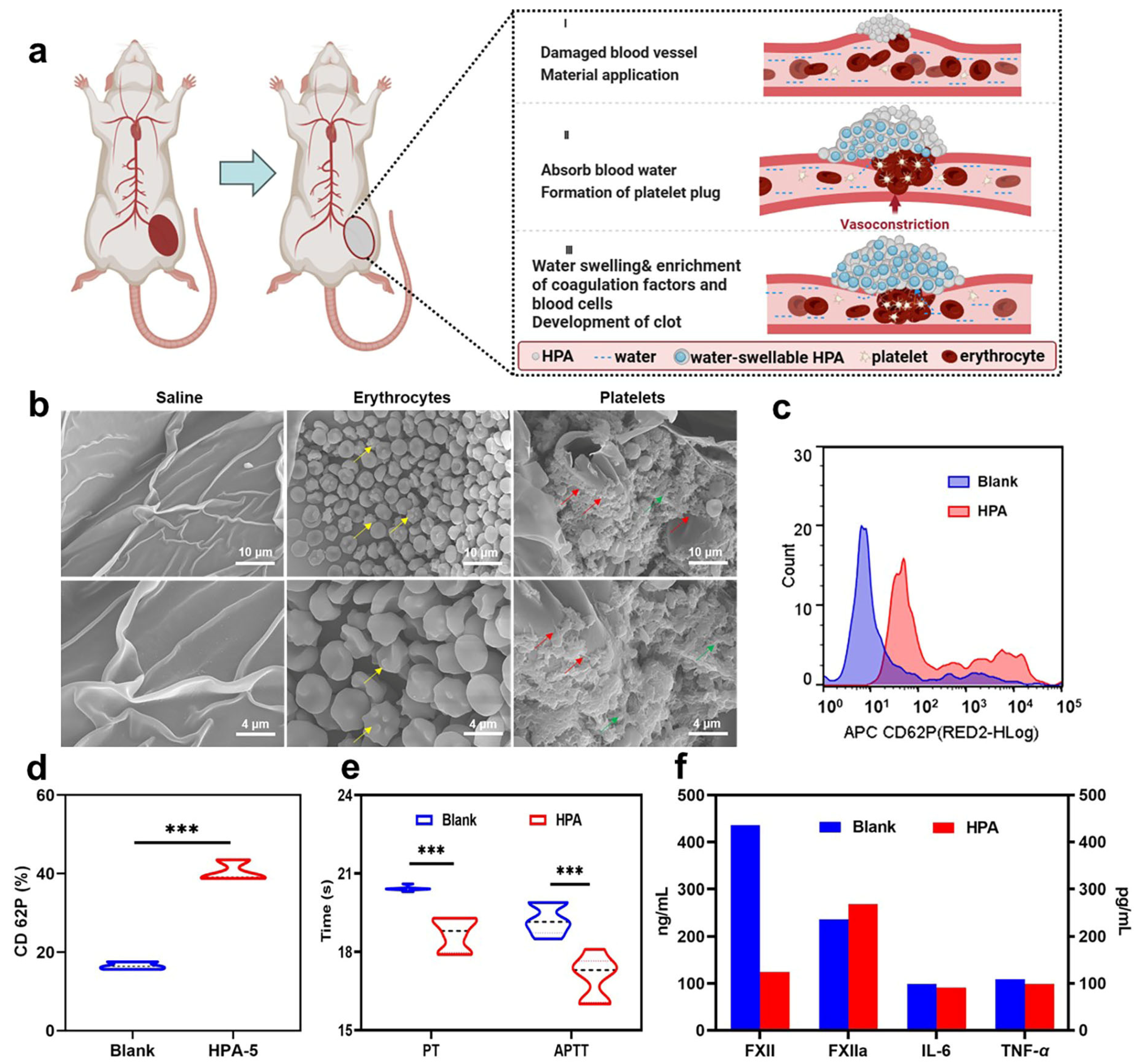
3.7. Hemostatic Mechanism Based on Multiple Comprehensive Interactions of HPA-5
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Littlejohn, L.F.; Devlin, J.J.; Kircher, S.S.; Lueken, R.; Melia, M.R.; Johnson, A.S. Comparison of Celox-A, ChitoFlex, WoundStat, and Combat Gauze Hemostatic Agents Versus Standard Gauze Dressing in Control of Hemorrhage in a Swine Model of Penetrating Trauma. Acad. Emerg. Med. 2011, 18, 340–350. [Google Scholar] [CrossRef]
- Evans, J.A.; van Wessem, K.J.P.; McDougall, D.; Lee, K.A.; Lyons, T.; Balogh, Z.J. Epidemiology of Traumatic Deaths: Comprehensive Population-Based Assessment. World J. Surg. 2010, 34, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.S.; Satahoo, S.S.; Butler, F.K.; Dermer, H.; Naranjo, D.; Julien, K.; Van Haren, R.M.; Namias, N.; Blackbourne, L.H.; Schulman, C.I. An Analysis of Prehospital Deaths: Who Can We Save? J. Trauma Acute. Care 2014, 77, 213–218. [Google Scholar] [CrossRef]
- Hickman, D.A.; Pawlowski, C.L.; Sekhon, U.D.S.; Marks, J.; Gupta, A.S. Biomaterials and Advanced Technologies for Hemostatic Management of Bleeding. Adv. Mater. 2018, 30, 1700859. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Sarode, A.; Kokoroskos, N.; Ukidve, A.; Zhao, Z.; Guo, S.; Flaumenhaft, R.; Gupta, A.S.; Saillant, N.; Mitragotri, S. A Polymer-Based Systemic Hemostatic Agent. Sci. Adv. 2020, 6, eaba0588. [Google Scholar] [CrossRef]
- Khoshmohabat, H.; Paydar, S.; Kazemi, H.M.; Dalfardi, B. Overview of Agents Used for Emergency Hemostasis. Trauma Mon. 2016, 21, e26023. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.A.; Ochoa, J.E.; Wong, S.; Beatty, S.; Elder, J.; Guidry, C.; McGrew, P.; McGinness, C.; Duchesne, J.; Schroll, R. Prehospital Tourniquet Use in Penetrating Extremity Trauma: Decreased Blood Transfusions and Limb Complications. J. Trauma Acute Care Surg. 2019, 86, 43–51. [Google Scholar] [CrossRef]
- Pedowitz, R.A.; Nordborg, C.; Rosenqvist, A.-L.; Rydevik, B.L. Nerve Function and Structure Beneath and Distal to a Pneumatic Tourniquet Applied to Rabbit Hindlimbs. Scand. J. Plast. Recons. 1991, 25, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Déry, R.; Pelletier, J.; Jacques, A.; Clavet, M.; Houde, J.J. Metabolic Changes Induced in the Limb during Tourniquet Ischaemla. Can. J. Anesth. 1965, 12, 367–378. [Google Scholar] [CrossRef]
- Taboada, G.M.; Yang, K.; Pereira, M.J.N.; Liu, S.S.; Hu, Y.; Karp, J.M.; Artzi, N.; Lee, Y. Overcoming the Translational Barriers of Tissue Adhesives. Nat. Rev. Mater. 2020, 5, 310–329. [Google Scholar] [CrossRef]
- Hong, C.; Olsen, B.D.; Hammond, P.T. A Review of Treatments for Non-Compressible Torso Hemorrhage (NCTH) and Internal Bleeding. Biomaterials 2022, 283, 121432. [Google Scholar] [CrossRef]
- Shang, X.; Chen, H.; Castagnola, V.; Liu, K.; Boselli, L.; Petseva, V.; Yu, L.; Xiao, L.; He, M.; Wang, F.; et al. Unusual Zymogen Activation Patterns in the Protein Corona of Ca-Zeolites. Nat. Catal. 2021, 4, 607–614. [Google Scholar] [CrossRef]
- Liu, W.; Yang, C.; Gao, R.; Zhang, C.; Ou-Yang, W.; Feng, Z.; Zhang, C.; Pan, X.; Huang, P.; Kong, D.; et al. Polymer Composite Sponges with Inherent Antibacterial, Hemostatic, Inflammation-Modulating and Proregenerative Performances for Methicillin-Resistant Staphylococcus Aureus-Infected Wound Healing. Adv. Health. Mater. 2021, 10, 2101247. [Google Scholar] [CrossRef]
- Yuk, H.; Wu, J.; Sarrafian, T.L.; Mao, X.; Varela, C.E.; Roche, E.T.; Griffiths, L.G.; Nabzdyk, C.S.; Zhao, X. Rapid and Coagulation-Independent Haemostatic Sealing by a Paste Inspired by Barnacle Glue. Nat. Biomed. Eng. 2021, 5, 1131–1142. [Google Scholar] [CrossRef]
- Zhao, X.; Sun, Y.; Meng, Z.; Yang, Z.; Fan, S.; Ye, T.; Yang, L.; Li, T.; Gu, R.; Wu, Z.; et al. Preparation and Characterization of Tranexamic Acid Modified Porous Starch and Its Application as a Hemostatic Agent. Int. J. Biol. Macromol. 2022, 200, 273–284. [Google Scholar] [CrossRef]
- Bogdan, Y.; Helfet, D.L. Use of Tourniquets in Limb Trauma Surgery. Orthop. Clin. N. Am. 2018, 49, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Beaman, H.T.; Shepherd, E.; Satalin, J.; Blair, S.; Ramcharran, H.; Serinelli, S.; Gitto, L.; Dong, K.S.; Fikhman, D.; Nieman, G.; et al. Hemostatic Shape Memory Polymer Foams with Improved Survival in a Lethal Traumatic Hemorrhage Model. Acta. Biomater. 2022, 137, 112–123. [Google Scholar] [CrossRef] [PubMed]
- Duggan, M.; Rago, A.; Sharma, U.; Zugates, G.; Freyman, T.; Busold, R.; Caulkins, J.; Pham, Q.; Chang, Y.; Mejaddam, A.; et al. Self-Expanding Polyurethane Polymer Improves Survival in a Model of Noncompressible Massive Abdominal Hemorrhage. J. Trauma Acute Care 2013, 74, 1462–1467. [Google Scholar] [CrossRef]
- Huang, A.P.-H.; Lai, D.-M.; Hsu, Y.-H.; Tsai, H.-H.; Su, C.-Y.; Hsu, S. An Anti-Inflammatory Gelatin Hemostatic Agent with Biodegradable Polyurethane Nanoparticles for Vulnerable Brain Tissue. Mat. Sci. Eng. C-Mater. 2021, 121, 111799. [Google Scholar] [CrossRef]
- Liang, Y.; Xu, C.; Liu, F.; Du, S.; Li, G.; Wang, X. Eliminating Heat Injury of Zeolite in Hemostasis via Thermal Conductivity of Graphene Sponge. ACS Appl. Mater. Inter. 2019, 11, 23848–23857. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.W.; Liu, C.C.; Cherng, J.H.; Lin, C.S.; Chang, S.J.; Hong, Z.J.; Liu, C.C.; Chiu, Y.K.; Hsu, S.D.; Chang, H. Biological Effects of Chitosan-Based Dressing on Hemostasis Mechanism. Polymers 2019, 11, 1906. [Google Scholar] [CrossRef] [PubMed]
- Hooshmand, S.; Mollazadeh, S.; Akrami, N.; Ghanad, M.; El-Fiqi, A.; Baino, F.; Nazarnezhad, S.; Kargozar, S. Mesoporous Silica Nanoparticles and Mesoporous Bioactive Glasses for Wound Management: From Skin Regeneration to Cancer Therapy. Materials 2021, 14, 3337. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Li, J.; Leng, F.; Lv, S.; Huang, W.; Sun, W.; Jiang, X. Degradable Porous Carboxymethyl Chitin Hemostatic Microspheres. J. Biomat. Sci.-Polym. Ed. 2020, 31, 1369–1384. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, Z.; Liu, J.; Dong, P.; Tian, F.; Li, F.; Meng, X. Electrospun Kaolin-Loaded Chitosan/PEO Nanofibers for Rapid Hemostasis and Accelerated Wound Healing. Int. J. Biol. Macromol. 2022, 217, 998–1011. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Shang, X.; Chen, H.; Xiao, L.; Zhu, Y.; Fan, J. A Tightly-Bonded and Flexible Mesoporous Zeolite-Cotton Hybrid Hemostat. Nat. Commun. 2019, 10, 1932. [Google Scholar] [CrossRef] [PubMed]
- Slezak, P.; Keibl, C.; Redl, H.; Labahn, D.; Gulle, H. An Efficacy Comparison of Two Hemostatic Agents in a Porcine Liver Bleeding Model: Gelatin/Thrombin Flowable Matrix versus Collagen/Thrombin Powder. J. Investig. Surg. 2020, 33, 828–838. [Google Scholar] [CrossRef]
- Cao, X.; Zhang, Z.; Sun, L.; Luo, Z.; Zhao, Y. Multifunctional Fish Gelatin Hydrogel Inverse Opal Films for Wound Healing. J. Nanobiotechnol. 2022, 20, 355. [Google Scholar] [CrossRef]
- Pandey, S.; Son, N.; Kang, M. Synergistic Sorption Performance of Karaya Gum Crosslink Poly(Acrylamide-Co-Acrylonitrile) @ Metal Nanoparticle for Organic Pollutants. Int. J. Biol. Macromol. 2022, 210, 300–314. [Google Scholar] [CrossRef]
- Tie, L.; Ke, Y.; Gong, Y.; Zhang, W.; Deng, Z. Nanocellulose Fine-Tuned Poly(Acrylic Acid) Hydrogel for Enhanced Diclofenac Removal. Int. J. Biol. Macromol. 2022, 213, 1029–1036. [Google Scholar] [CrossRef]
- Kim, J.; Krishna-Subbaiah, N.; Wu, Y.; Ko, J.; Shiva, A.; Sitti, M. Enhanced Flexible Mold Lifetime for Roll-to-Roll Scaled-Up Manufacturing of Adhesive Complex Microstructures. Adv. Mater. 2023, 35, 2207257. [Google Scholar] [CrossRef]
- Song, Z.; Liu, X.; Ding, J.; Liu, J.; Han, X.; Deng, Y.; Zhong, C.; Hu, W. Poly(Acrylic Acid)-Based Composite Gel Polymer Electrolytes with High Mechanical Strength and Ionic Conductivity toward Flexible Zinc–Air Batteries with Long Cycling Lifetime. ACS Appl. Mater. Inter. 2022, 14, 49801–49810. [Google Scholar] [CrossRef]
- Xu, H.-G.; Liang, Q.-L.; Li, L.; Qi, G.-F.; Wang, L.; Zhan, L.-N.; Ding, M.-R.; Zhang, K.; Cui, X. Biomimetic Peptide Nanoparticles Participate in Natural Coagulation for Hemostasis and Wound Healing. Biomater. Sci. 2022, 10, 2628–2637. [Google Scholar] [CrossRef]
- Zhang, M.; Yang, Q.; Hu, T.; Tang, L.; Ni, Y.; Chen, L.; Wu, H.; Huang, L.; Ding, C. Adhesive, Antibacterial, Conductive, Anti-UV, Self-Healing, and Tough Collagen-Based Hydrogels from a Pyrogallol-Ag Self-Catalysis System. ACS Appl. Mater. Inter. 2022, 14, 8728–8742. [Google Scholar] [CrossRef]
- Ma, C.; Zhao, J.; Zhu, C.; Jiang, M.; Ma, P.; Mi, Y.; Fan, D. Oxidized Dextran Crosslinked Polysaccharide/Protein/Polydopamine Composite Cryogels with Multiple Hemostatic Efficacies for Noncompressible Hemorrhage and Wound Healing. Int. J. Biol. Macromol. 2022, 215, 675–690. [Google Scholar] [CrossRef]
- Yu, X.; Gao, Z.; Mu, J.; Lian, H.; Meng, Z. Gelatin/Calcium Chloride Electrospun Nanofibers for Rapid Hemostasis. Biomater. Sci. 2023, 11, 2158–2166. [Google Scholar] [CrossRef]
- Xiang, J.; Wang, Y.; Yang, L.; Zhang, X.; Hong, Y.; Shen, L. A Novel Hydrogel Based on Bletilla Striata Polysaccharide for Rapid Hemostasis: Synthesis, Characterization and Evaluation. Int. J. Biol. Macromol. 2022, 196, 1–12. [Google Scholar] [CrossRef]
- Yu, J.; Qin, Y.; Yang, Y.; Zhao, X.; Zhang, Z.; Zhang, Q.; Su, Y.; Zhang, Y.; Cheng, Y. Robust Hydrogel Adhesives for Emergency Rescue and Gastric Perforation Repair. Bioact. Mater. 2023, 19, 703–716. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Xu, G.; He, Y.; Mao, H.; Kong, D.; Luo, K.; Tang, W.; Liu, R.; Gu, Z. A Dual-Bioinspired Tissue Adhesive Based on Peptide Dendrimer with Fast and Strong Wet Adhesion. Adv. Health. Mater. 2022, 11, 2200874. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, R.G.; De Bon, F.; Pereira, P.; Carvalho, F.M.; Freitas, M.; Tavakoli, M.; Serra, A.C.; Fonseca, A.C.; Coelho, J.F.J. Photo-Degradable, Tough and Highly Stretchable Hydrogels. Mater. Today Bio. 2022, 15, 100325. [Google Scholar] [CrossRef] [PubMed]
- Matthay, Z.A.; Fields, A.T.; Nunez-Garcia, B.; Park, J.J.; Jones, C.; Leligdowicz, A.; Hendrickson, C.M.; Callcut, R.A.; Matthay, M.A.; Kornblith, L.Z. Importance of Catecholamine Signaling in the Development of Platelet Exhaustion after Traumatic Injury. J. Thromb. Haemost. 2022, 20, 2109–2118. [Google Scholar] [CrossRef] [PubMed]
- Jungsinyatam, P.; Suwanakood, P.; Saengsuwan, S. Multicomponent Biodegradable Hydrogels Based on Natural Biopolymers as Environmentally Coating Membrane for Slow-Release Fertilizers: Effect of Crosslinker Type. Sci. Total Environ. 2022, 843, 157050. [Google Scholar] [CrossRef] [PubMed]
- Roig-Sanchez, S.; Kam, D.; Malandain, N.; Sachyani-Keneth, E.; Shoseyov, O.; Magdassi, S.; Laromaine, A.; Roig, A. One-Step Double Network Hydrogels of Photocurable Monomers and Bacterial Cellulose Fibers. Carbohyd. Polym. 2022, 294, 119778. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, T.; Colombani, T.; Alade, T.; Bencherif, S.A.; Memić, A. Injectable Lignin-Co-Gelatin Cryogels with Antioxidant and Antibacterial Properties for Biomedical Applications. Biomacromolecules 2021, 22, 4110–4121. [Google Scholar] [CrossRef]
- Feng, C.; Li, J.; Wu, G.S.; Mu, Y.Z.; Kong, M.; Jiang, C.Q.; Cheng, X.J.; Liu, Y.; Chen, X.G. Chitosan-Coated Diatom Silica as Hemostatic Agent for Hemorrhage Control. ACS Appl. Mater. Inter. 2016, 8, 34234–34243. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Bai, Q.; Wu, W.; Han, K.; Zeng, Q.; Dong, K.; Zhang, Y.; Lu, T. Study on Hemostatic Effect and Mechanism of Starch-Based Nano-Microporous Particles. Int. J. Biol. Macromol. 2021, 179, 507–518. [Google Scholar] [CrossRef]
- Zheng, C.; Zeng, Q.; Pimpi, S.; Wu, W.; Han, K.; Dong, K.; Lu, T. Research Status and Development Potential of Composite Hemostatic Materials. J. Mater. Chem. B 2020, 8, 5395–5410. [Google Scholar] [CrossRef]
- Zhang, Z.; Kuang, G.; Zong, S.; Liu, S.; Xiao, H.; Chen, X.; Zhou, D.; Huang, Y. Sandwich-Like Fibers/Sponge Composite Combining Chemotherapy and Hemostasis for Efficient Postoperative Prevention of Tumor Recurrence and Metastasis. Adv. Mater. 2018, 30, 1803217. [Google Scholar] [CrossRef]
- Huang, Y.; Fan, C.; Liu, Y.; Yang, L.; Hu, W.; Liu, S.; Wang, T.; Shu, Z.; Li, B.; Xing, M.; et al. Nature-Derived Okra Gel as Strong Hemostatic Bioadhesive in Human Blood, Liver, and Heart Trauma of Rabbits and Dogs. Adv. Healthc. Mater. 2022, 11, 2200939. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, Y.; Qiao, L.; Zou, F.; Xie, Y.; Zheng, Y.; Chao, Y.; Yang, Y.; He, W.; Yang, S. Cellulose Fibers-Reinforced Self-Expanding Porous Composite with Multiple Hemostatic Efficacy and Shape Adaptability for Uncontrollable Massive Hemorrhage Treatment. Bioact. Mater. 2021, 6, 2089–2104. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Xu, X.; Deng, Y.; Xie, X.; Xu, L.; Xu, X.; Yuan, W.; Yang, B.; Yang, X.; Xia, X.; et al. Ultrafast Self-Gelling and Wet Adhesive Powder for Acute Hemostasis and Wound Healing. Adv. Funct. Mater. 2021, 31, 2102583. [Google Scholar] [CrossRef]
- Du, X.; Wu, L.; Yan, H.; Jiang, Z.; Li, S.; Li, W.; Bai, Y.; Wang, H.; Cheng, Z.; Kong, D.; et al. Microchannelled Alkylated Chitosan Sponge to Treat Noncompressible Hemorrhages and Facilitate Wound Healing. Nat. Commun. 2021, 12, 4733. [Google Scholar] [CrossRef]
- Zhao, X.; Liang, Y.; Guo, B.; Yin, Z.; Zhu, D.; Han, Y. Injectable Dry Cryogels with Excellent Blood-Sucking Expansion and Blood Clotting to Cease Hemorrhage for Lethal Deep-Wounds, Coagulopathy and Tissue Regeneration. Chem. Eng. J. 2021, 403, 126329. [Google Scholar] [CrossRef]
- Liu, C.; Liu, X.; Liu, C.; Wang, N.; Chen, H.; Yao, W.; Sun, G.; Song, Q.; Qiao, W. A Highly Efficient, in Situ Wet-Adhesive Dextran Derivative Sponge for Rapid Hemostasis. Biomaterials 2019, 205, 23–37. [Google Scholar] [CrossRef]
- Sparkenbaugh, E.M.; Henderson, M.W.; Miller-Awe, M.; Abrams, C.; Ilich, A.; Trebak, F.; Ramadas, N.; Vital, S.; Bohinc, D.; Bane, K.L.; et al. Factor XII Contributes to Thrombotic Complications and Vaso-Occlusion in Sickle Cell Disease. Blood 2023, 141, 1871–1883. [Google Scholar] [CrossRef] [PubMed]
| KPSa (0.05%) | KPSb (0.10%) | KPSc (0.25%) | |
|---|---|---|---|
| MBA1 (0.03%) | HPA-1 | HPA-2 | HPA-3 |
| MBA2 (0.06%) | HPA-4 | HPA-5 | HPA-6 |
| MBA3 (0.12%) | HPA-7 | HPA-8 | HPA-9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, T.; Yang, Z.; Hao, R.; Guo, J.; Dou, G.; Meng, Z.; Liu, S.; Gu, R.; Wu, Z.; Sun, Y.; et al. Highly Efficient Hemostatic Cross-Linked Polyacrylate Polymer Dressings for Immediate Hemostasis. Polymers 2024, 16, 863. https://doi.org/10.3390/polym16060863
Ye T, Yang Z, Hao R, Guo J, Dou G, Meng Z, Liu S, Gu R, Wu Z, Sun Y, et al. Highly Efficient Hemostatic Cross-Linked Polyacrylate Polymer Dressings for Immediate Hemostasis. Polymers. 2024; 16(6):863. https://doi.org/10.3390/polym16060863
Chicago/Turabian StyleYe, Tong, Zhiyuan Yang, Ruolin Hao, Jinnan Guo, Guifang Dou, Zhiyun Meng, Shuchen Liu, Ruolan Gu, Zhuona Wu, Yunbo Sun, and et al. 2024. "Highly Efficient Hemostatic Cross-Linked Polyacrylate Polymer Dressings for Immediate Hemostasis" Polymers 16, no. 6: 863. https://doi.org/10.3390/polym16060863
APA StyleYe, T., Yang, Z., Hao, R., Guo, J., Dou, G., Meng, Z., Liu, S., Gu, R., Wu, Z., Sun, Y., Han, P., Jin, Y., & Gan, H. (2024). Highly Efficient Hemostatic Cross-Linked Polyacrylate Polymer Dressings for Immediate Hemostasis. Polymers, 16(6), 863. https://doi.org/10.3390/polym16060863