Preparation and Characterization of Guaiacol-Furfuramine Benzoxazine and Its Modification of Bisphenol A-Aniline Oxazine Resin
Abstract
1. Introduction
2. Materials and Methods
2.1. Material Preparation
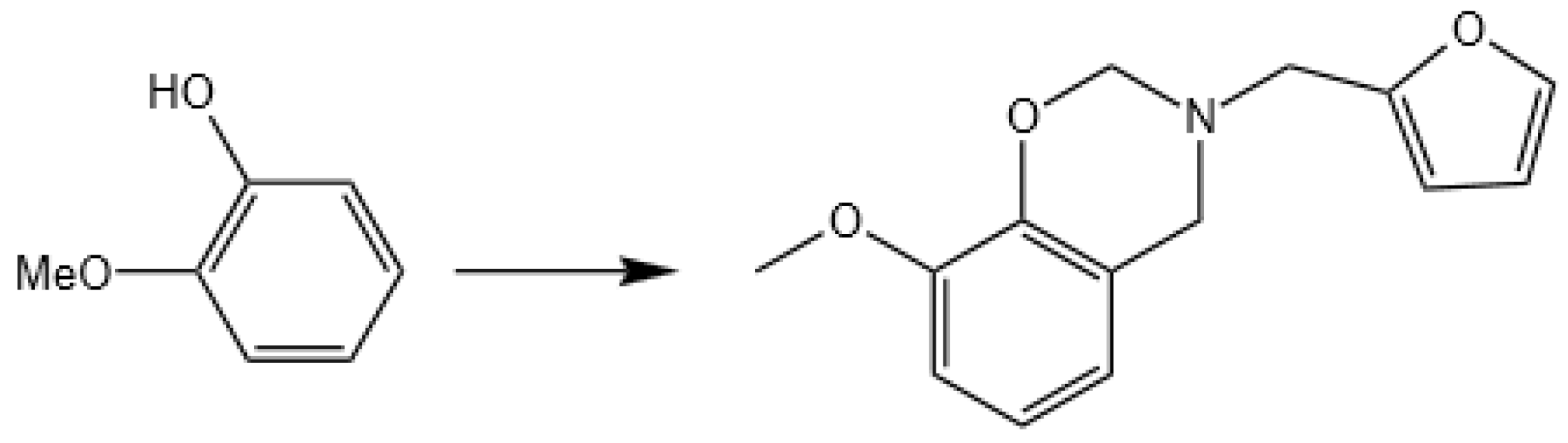
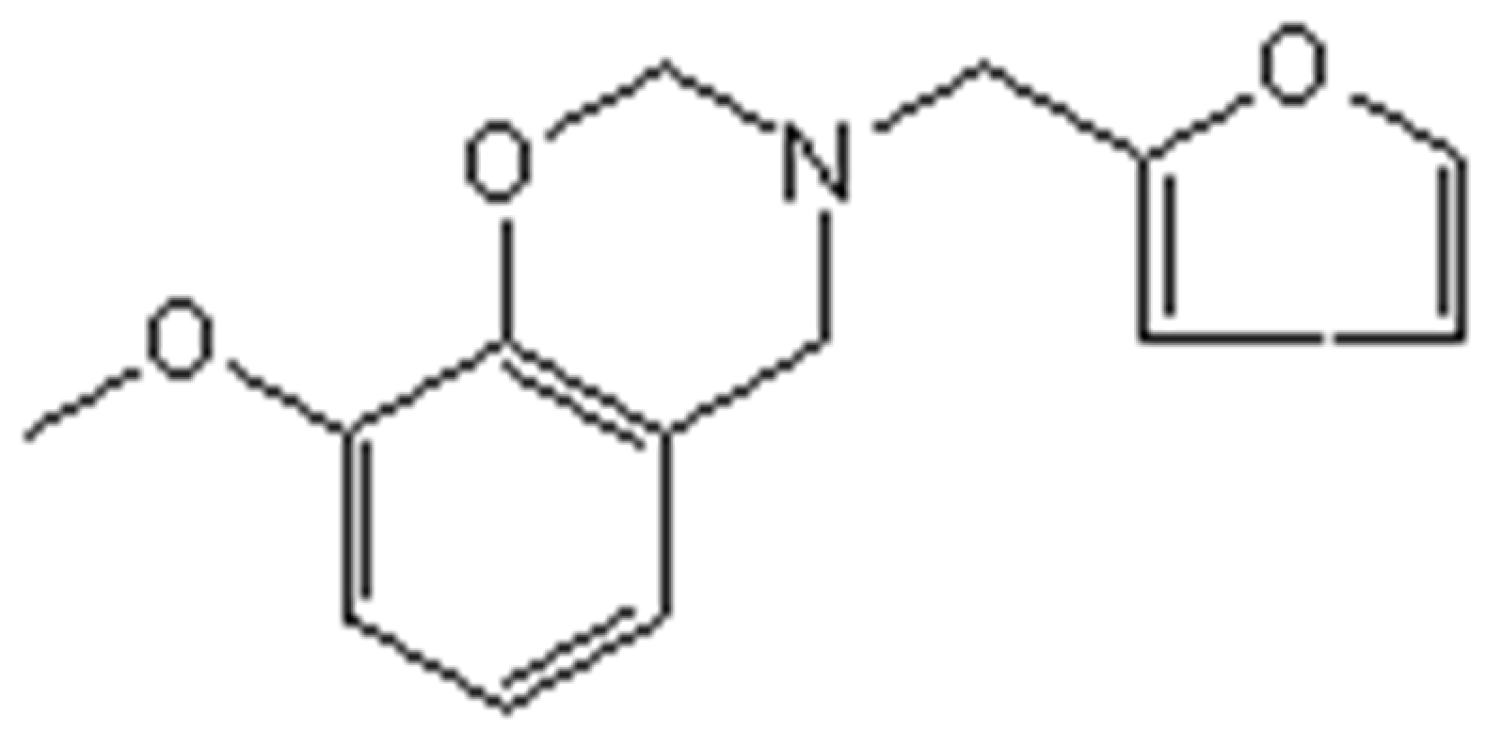
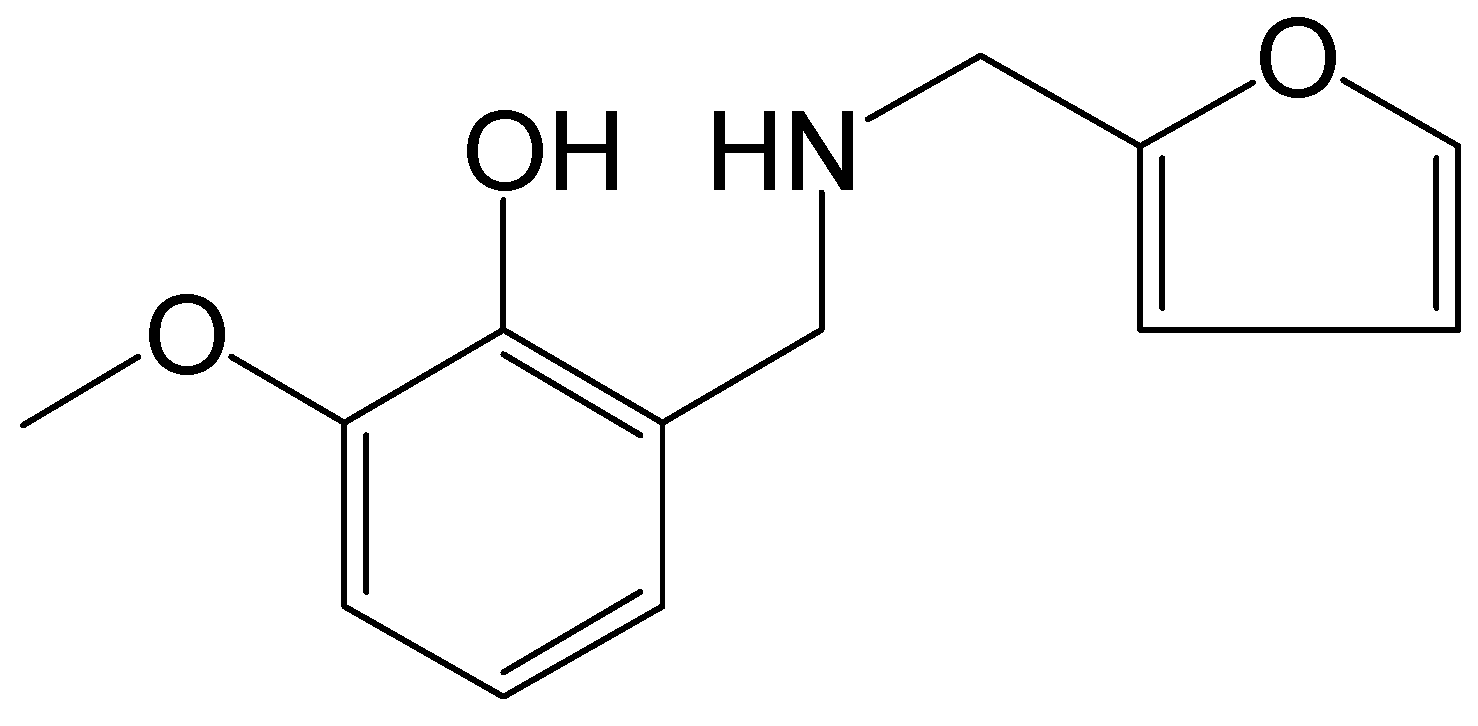
2.1.1. Preparation of GFZ
2.1.2. Preparation of BAZ
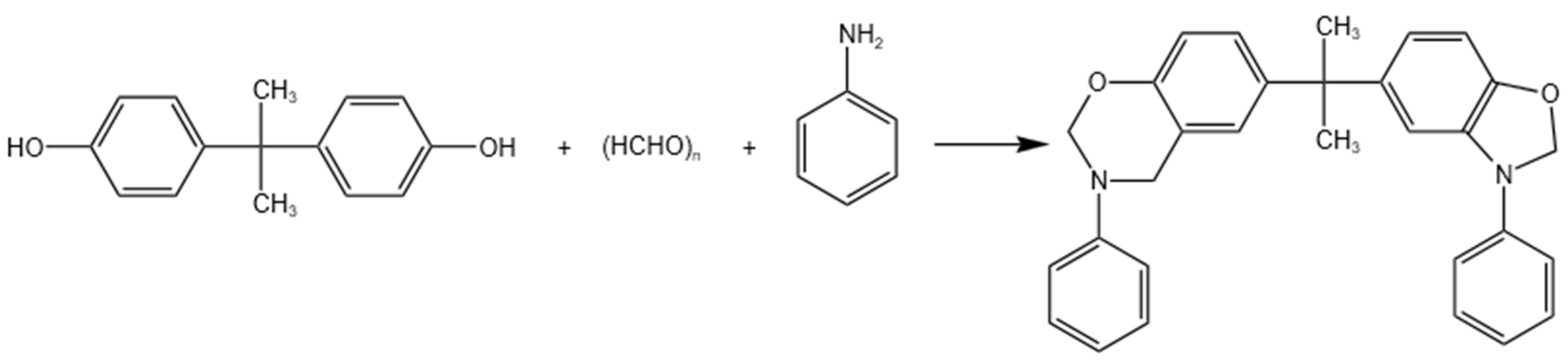
2.1.3. The Co-Curing of GFZ/BAZ Copolymer
2.2. Characterization
3. Results and Discussion
3.1. Determination of Synthesis Conditions
3.1.1. The Effect of Solution on Yield
3.1.2. The Effect of Aldehyde Types on Yield
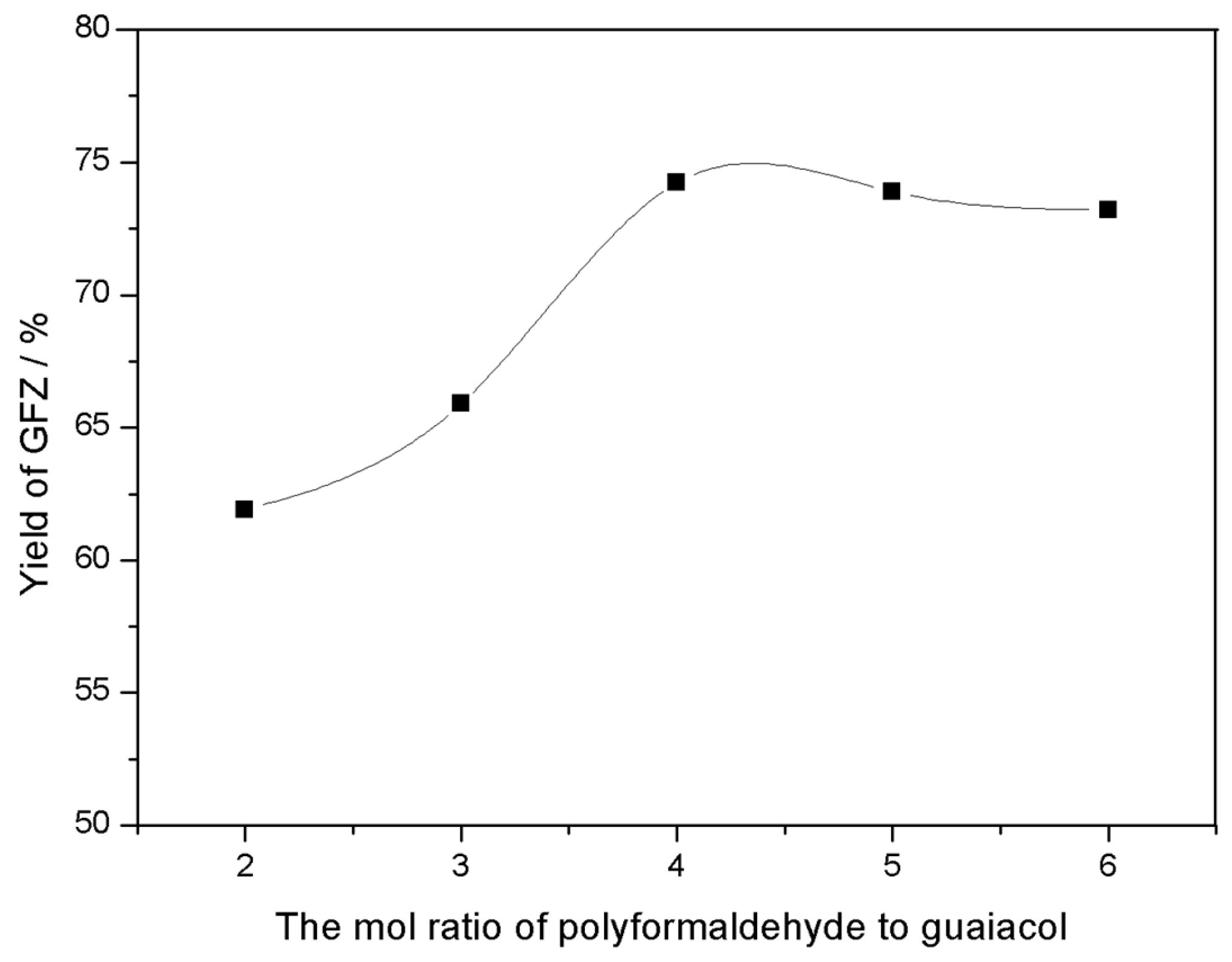
3.1.3. The Effect of Aldehyde Amount on Yield
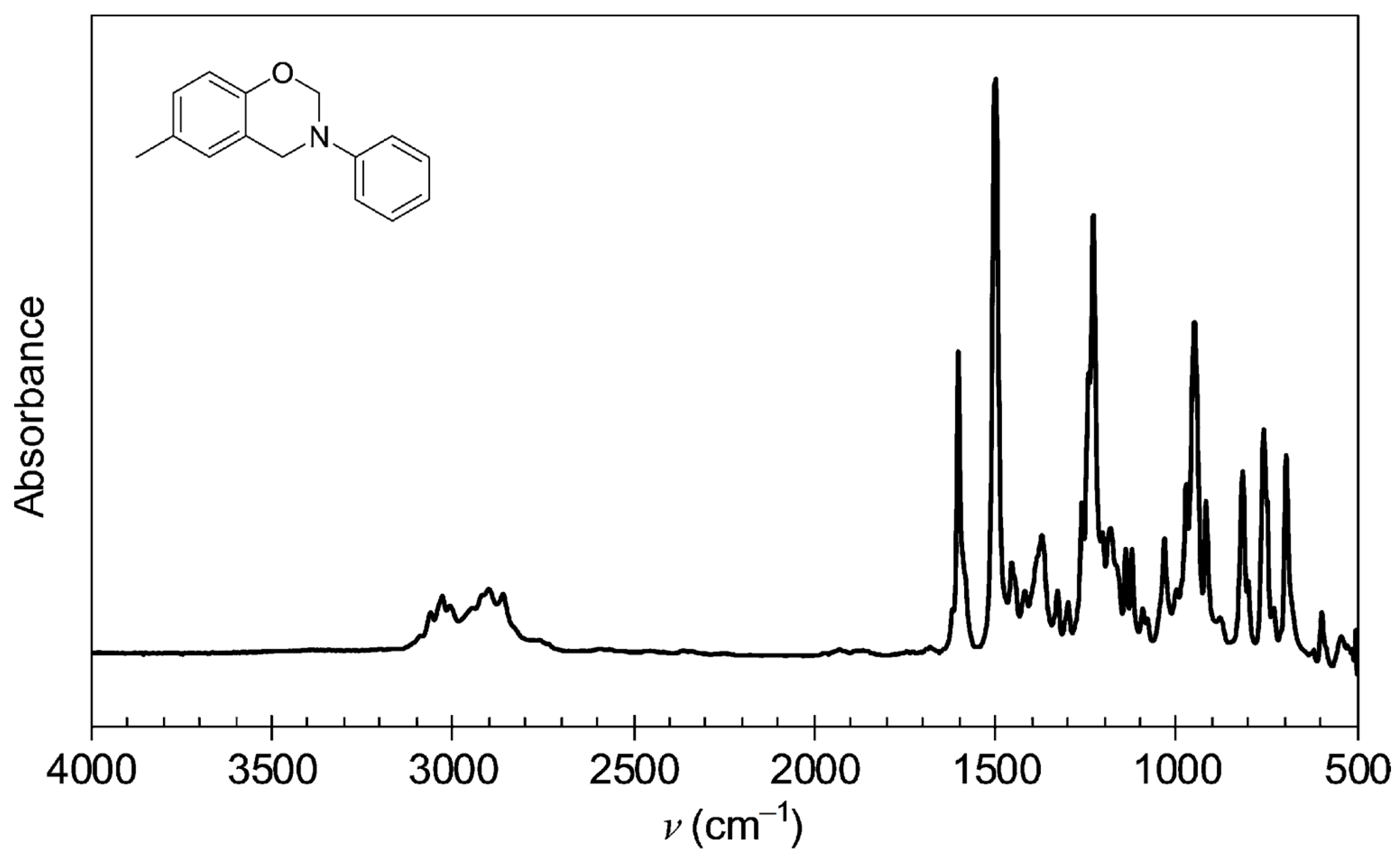
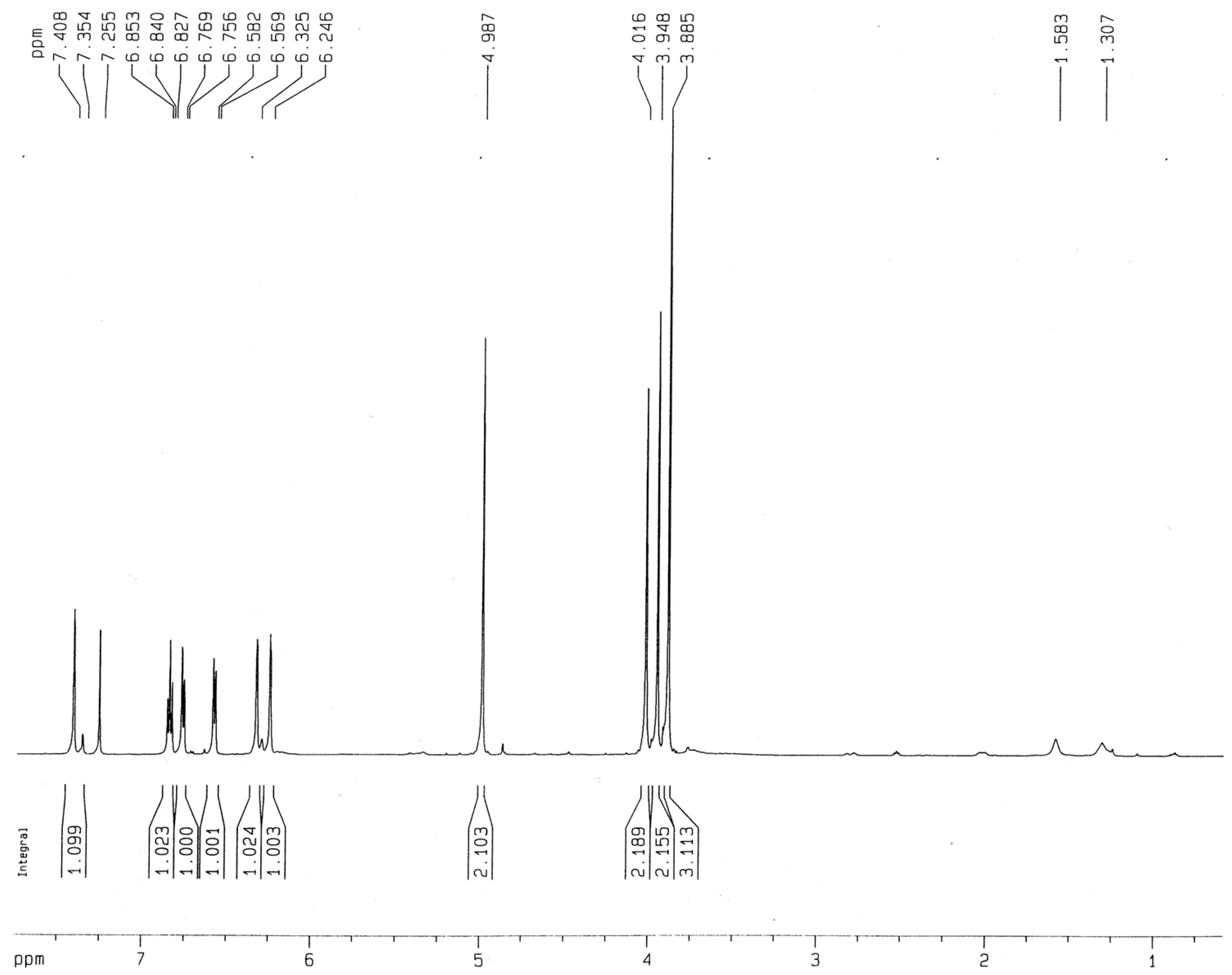
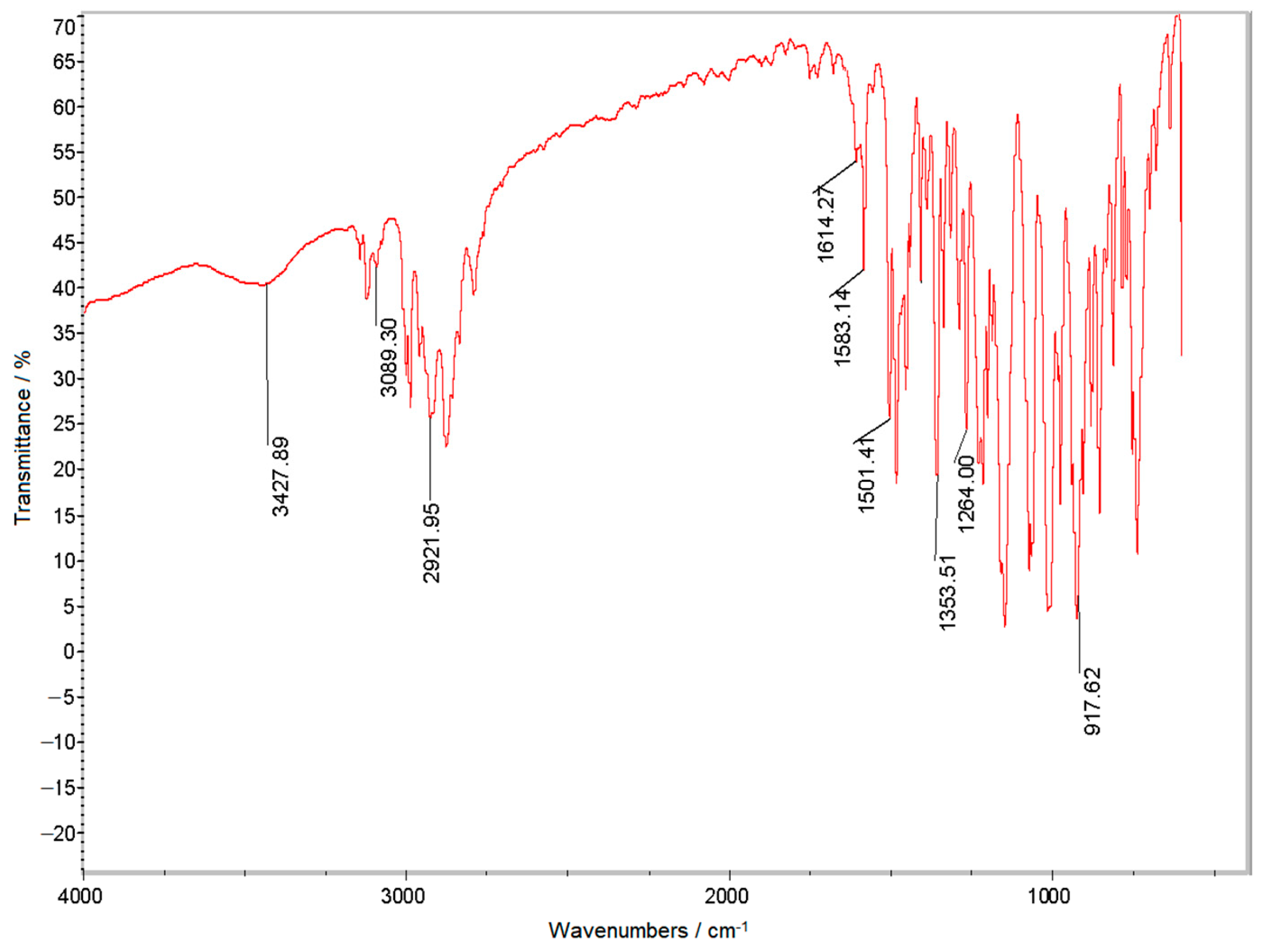
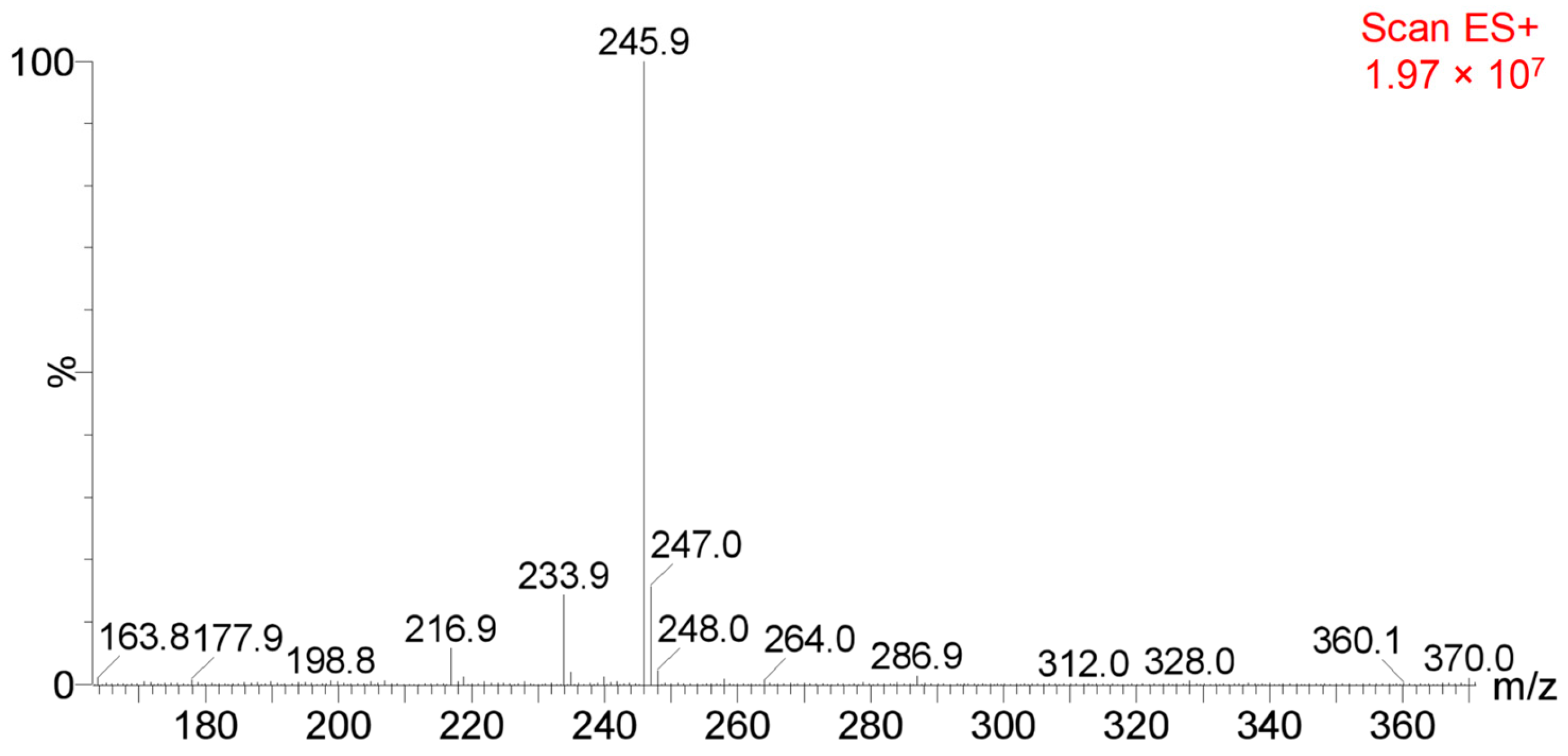
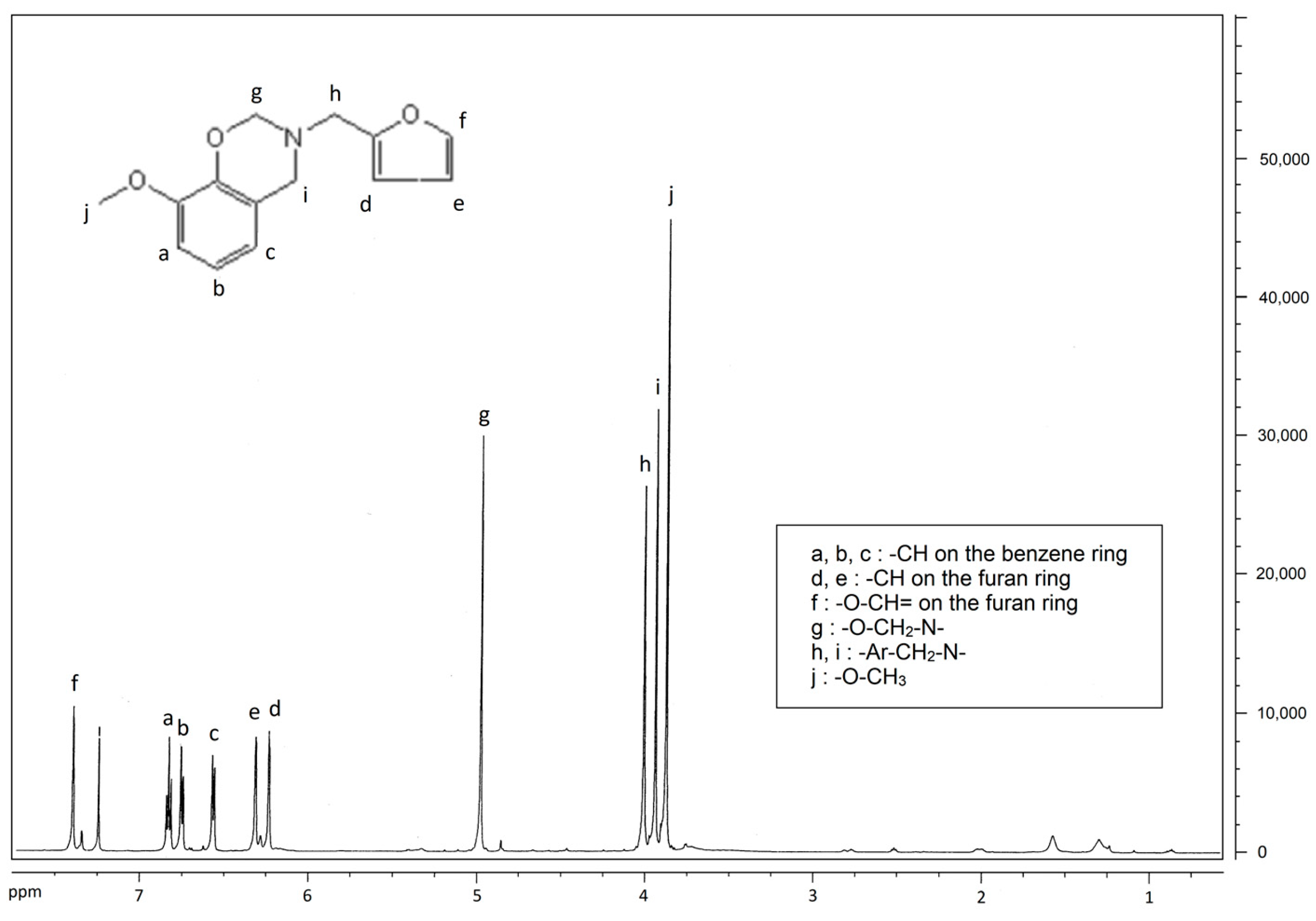
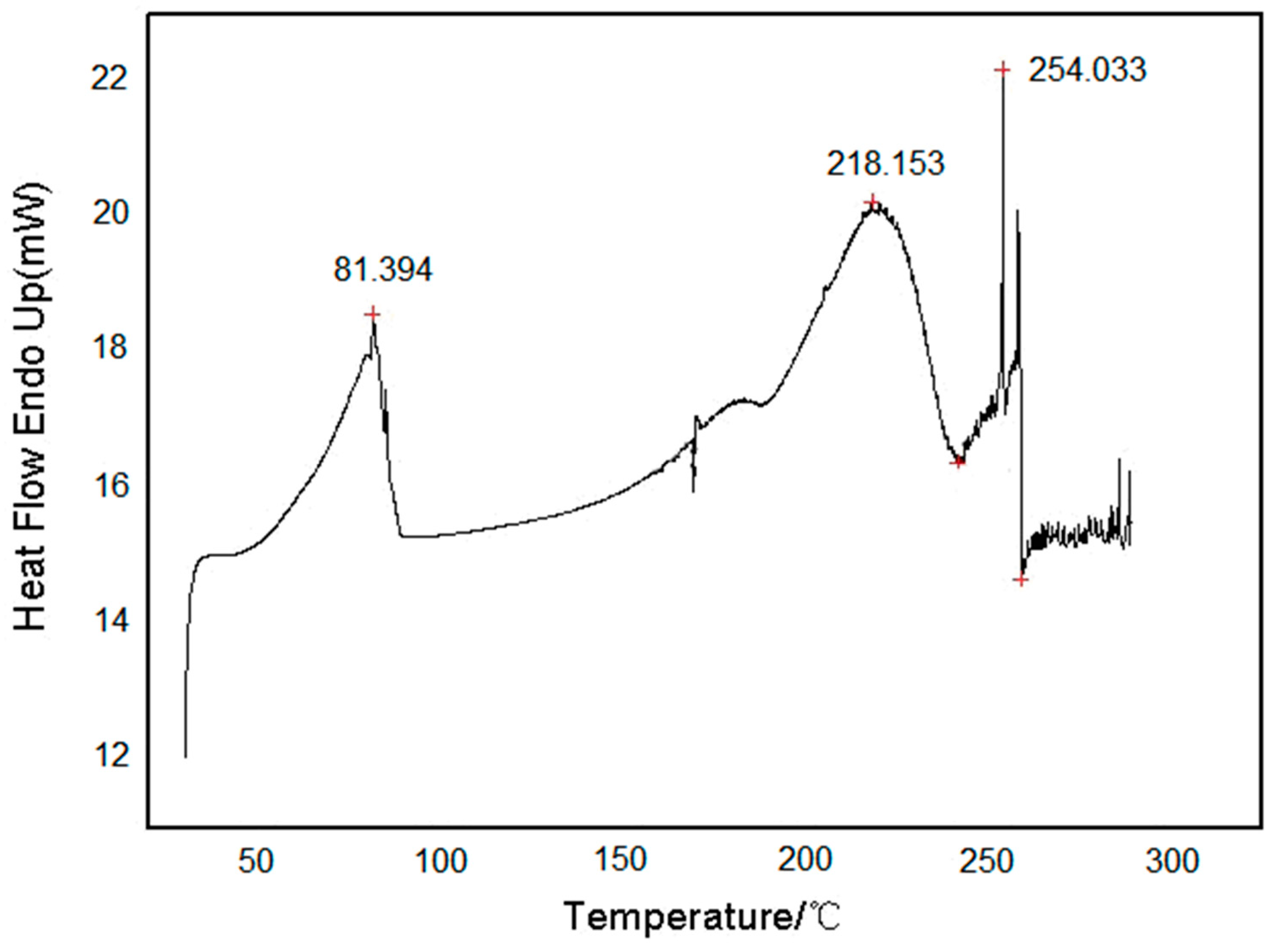
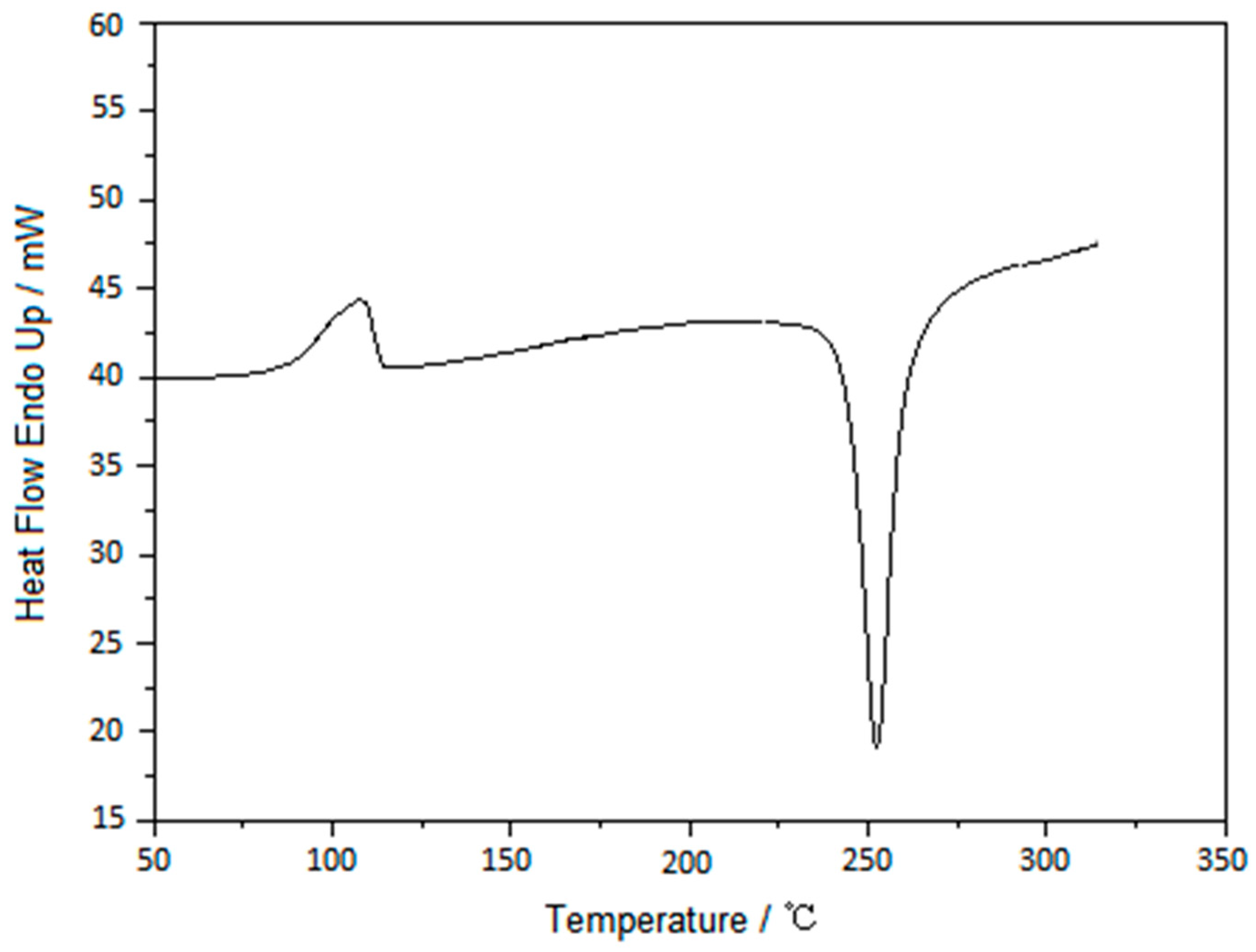
3.2. Testing and Characterization of GFZ
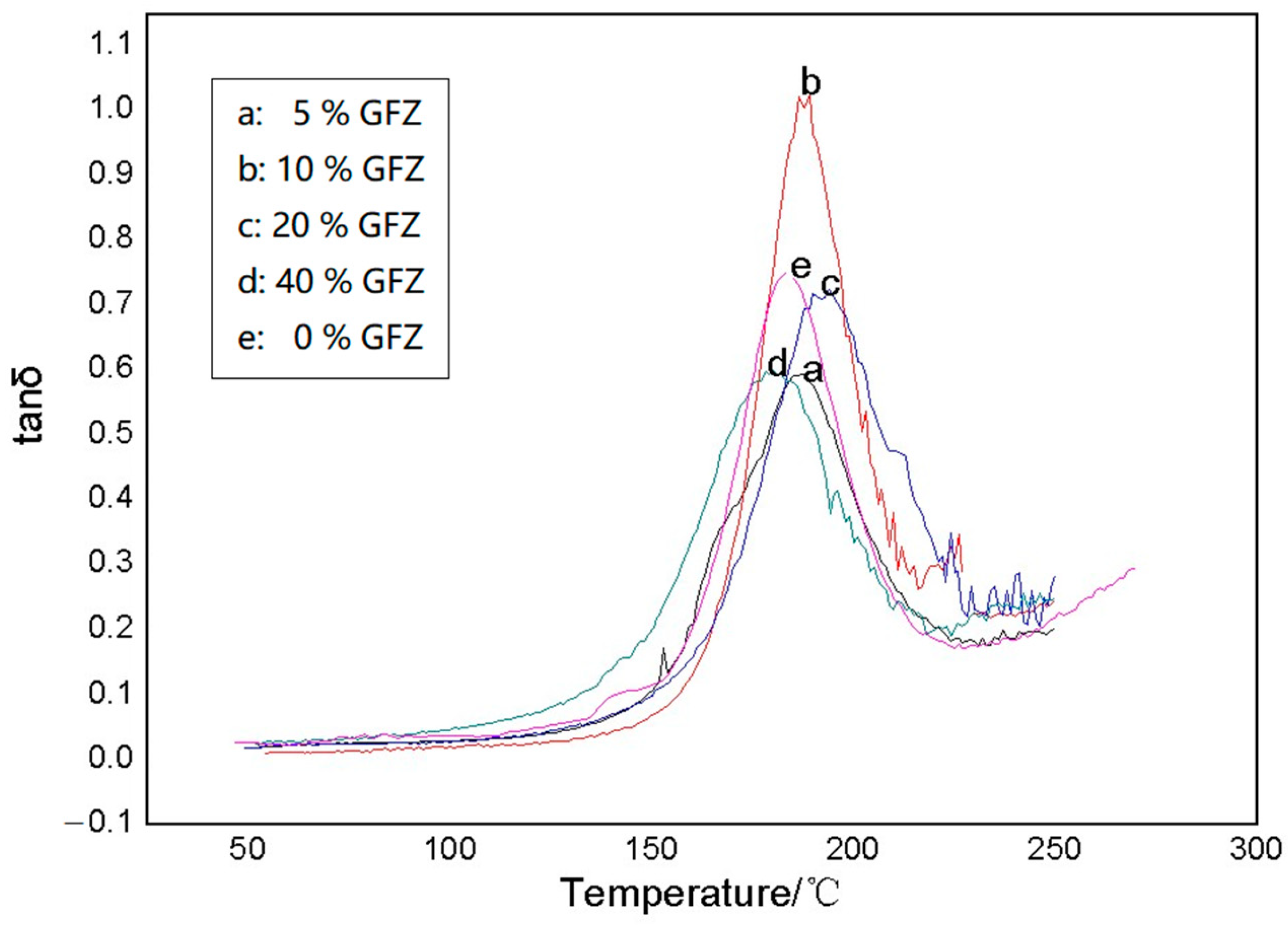
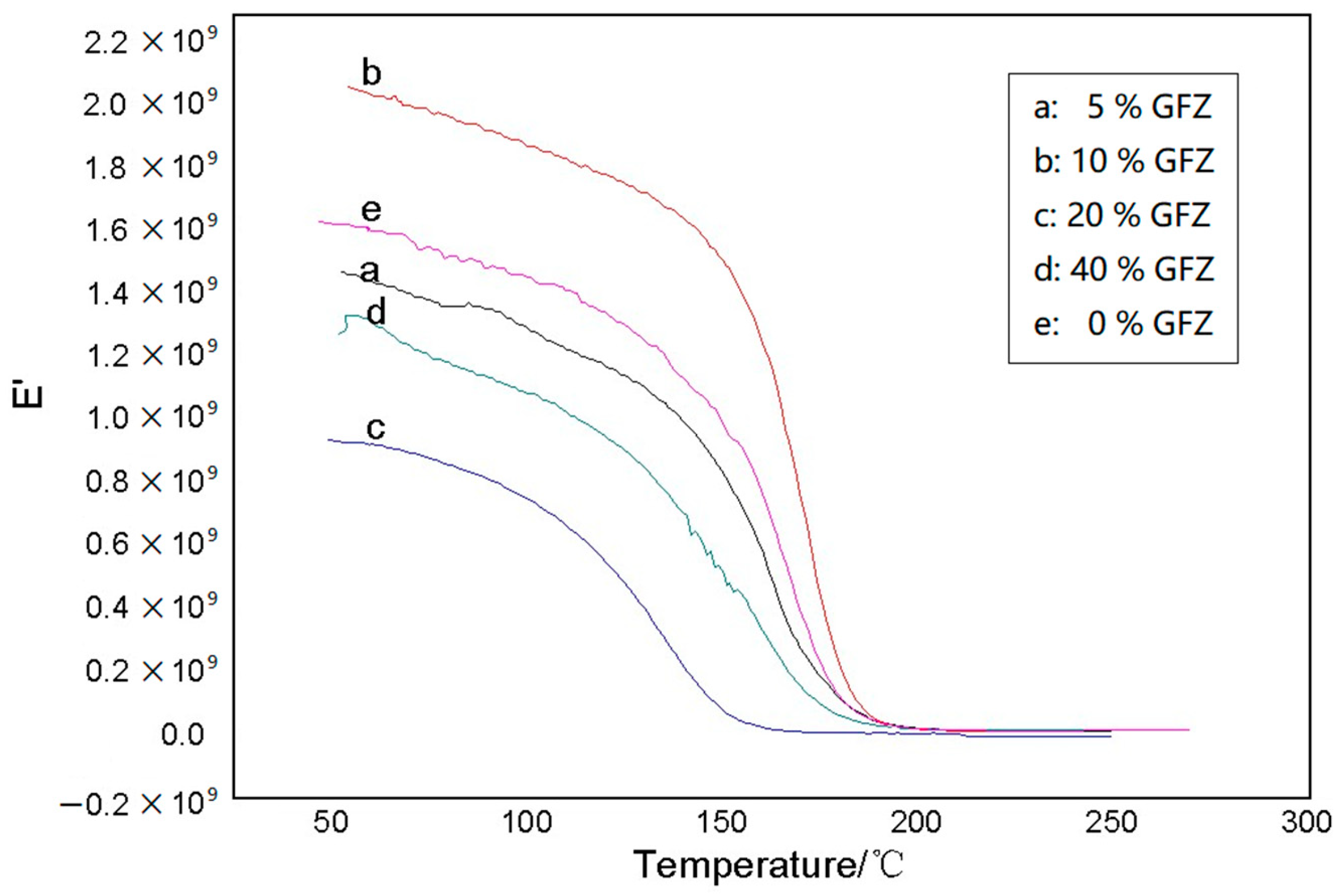
3.3. Testing and Characterization of GFZ/BAZ Copolymer
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A

References
- Ning, X.; Ishida, H.J. Phenolic materials via ring-opening polymerization of benzoxazines: Effect of molecular structure on mechanical and dynamic mechanical properties. J. Polym. Sci. Part B Polym. Phys. 1994, 32, 921–927. [Google Scholar] [CrossRef]
- Li, S.F.; Fu, J.F.; Wang, L.L. Research Development of Polybenzoxazines. Adv. Fine Petrochem. 2004, 5, 47–52. [Google Scholar]
- Ishida, H.; Hong, L. A study on the volumetric expansion of the benzoxazines-based phonelic resin. Macromolecular 1997, 30, 1099–1106. [Google Scholar] [CrossRef]
- Ishida, H.; Ohba, S. Synthesis and characterization of maleimide and norbornene functionalized benzoxazines. Polymer 2005, 46, 5588–5595. [Google Scholar] [CrossRef]
- Ishida, H.; Allen, D.J. Physcial and mechanical characterization of near-zero shrinkage poly benzoxazine. J. Polym. Sci. Part B Polym. Phys. 1996, 34, 1019–1030. [Google Scholar] [CrossRef]
- Burke, W.J.; Bishop, J.L.; Mortensen, E.L.; Bauer, W.N., Jr. A new aminoalky-lation reaction. Condensation of phenols with dihydro-l,3-aroxazines. J. Org. Chem. 1965, 30, 3423–3427. [Google Scholar] [CrossRef]
- Oliveira, J.R.; Kotzebue, L.R.; Ribeiro, F.W.; Mota, B.C.; Zampieri, D.; Mazzetto, S.E.; Ishida, H.; Lomonaco, D. Microwave-assisted solvent-free synthesis of novel benzoxazines: A faster and environmentally friendly route to the development of bio-based thermosetting resins. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 3534–3544. [Google Scholar] [CrossRef]
- Ishida, H.; Dunkers, J. Reaction of benzoxazine-based phenolic resins with strong and weak carboxyl in acids an dphenols as catalysts. J. Polym. Sci. Part B Polym. Phys. 1999, 37, l913–l921. [Google Scholar]
- Hyun, J.K.; Zdenka, B.; Ishida, H. Dynamic mechanical analysis on highly thermally stable polybenzoxazines with an acetylene functional group. J. Appl. Polym. Sci. 1999, 73, 857–862. [Google Scholar]
- Pei, D.F.; Cheng, D.; Gu, Y.; Cai, X. Benzoxazin Compounds and their Phenolic Resins Synthesized by Ring-opening Polymerization. Thermosetting Resin 1998, 13, 39–42. [Google Scholar]
- Xiang, H.; Ling, H.; Wang, J.; Song, L.; Gu, Y. A novel high performance RTM resin based on benzoxazine. Polym. Compos. 2005, 26, 563–571. [Google Scholar] [CrossRef]
- Kasapoglu, F.; Cianga, I.; Yagci, Y.; Takeichi, T. Photoinitiated cationic polymerization of monofunctional benzoxazine. J. Polym. Sci. Part A Polym. Chem. 2003, 41, 3320–3328. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, R.; Li, T.; Zhu, P.; Zhuang, Q. Novel fully biobased benzoxazines from rosin: Synthesis and properties. ACS Sustain. Chem. Eng. 2017, 5, 10682–10692. [Google Scholar] [CrossRef]
- Grabarnic, I.G. Hardening of phenol-formaIdehyde and arenephenol-formaldehyde oligomers without evolution of volatilematter. Izv. Akad. Nauk Kaz. SSR Ser. Khim. 1989, 3, 64–70. [Google Scholar]
- Jiang, H.; Wang, J.; Wu, S.; Yuan, Z.; Hu, Z.; Wu, R.; Liu, Q. The pyrolysis mechanism of phenol formaldehyde resin. Polym. Degrad. Stab. 2012, 97, 1527–1533. [Google Scholar] [CrossRef]
- Ishida, H.; Lee, Y.H. Synergism observed in polybenzoxazine and poly(ε-caprolactone) blends by dynamic mechanical and thermogravimetric analysis. Polymer 2001, 42, 6971–6979. [Google Scholar] [CrossRef]
- Hai, X.; Yi, G. Research Progress in Polymer Blend Modification of Benzoxazine Resins. Mater. Rep. 2004, 18, 54–56. [Google Scholar]
- Yang, L.; Zhou, W.; Seshan, K.; Li, Y. Green and efficient syn thesis route of catechol from guaiacol. J. Mol. Catal. A Chem. 2013, 368, 61–65. [Google Scholar]
- Thirukumaran, P.; Parveen, A.S.; Sarojadevi, M. Synthesis and copolymerization of fully biobased benzoxazines from renewable resources. ACS Sustain. Chem. Eng. 2014, 2, 2790–2801. [Google Scholar] [CrossRef]
- Zúñiga, C.; Larrechi, M.S.; Lligadas, G.; Ronda, J.C.; Galià, M.; Cádiz, V. Polybenzoxazines from renewable diphenolic acid. J. Polym. Sci. Part A Polym. Chem. 2011, 49, 1219–1227. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Q.; Yu, J.; Xu, R.; Wang, C.; Xiong, J. Synthesis and Characterization of Benzoxazine Resin Based on Furfurylamine. Materials 2022, 23, 8364. [Google Scholar] [CrossRef]
- Li, L.; Dong, F.Y.; Li, Y.; Gao, Y.Z. Research on the Ring-Opening Polymerization Reaction of 3,4-D ihydro-1,3-Benzoxazine Resin. J. North Univ. China 2008, 29, 69–73. [Google Scholar]
- Chernykh, A.; Liu, J.P.; Ishida, H. Synthesis and properties of a new crosslinkable polymer containing benzoxazine moiety in the main chain. Polymer 2006, 47, 7664–7669. [Google Scholar] [CrossRef]
- Ohashi, S.; Zhang, K.; Ran, Q.; Arza, C.R. Preparation of High Purity samples, Effect of Purity on Properties, and FTIR, Raman, 1H and 13C NMR, and DSC Data of Highly Purified Benzoxazine Monomers. In Advanced and Emerging Polybenzoxazine Science and Technology; Elsevier: Amsterdam, The Netherlands, 2017; ISBN 9780128041703. [Google Scholar]

| Solvent | Dielectric Constant (20 °C) | Polarity | Yield/% | Product |
|---|---|---|---|---|
| toluene | 2.240 | polar | 58.56 | light yellow crystalline solid |
| chloroform | 4.900 | non-polar | - | - |
| n-butanol | 17.84 | polar | - | - |
| DMF | 36.71 | polar | - | - |
| Aldehyde Types | Theoretical Yield/g | Actual Yield/g | Yield/% |
|---|---|---|---|
| polyformaldehyde | 21.5 | 13.31 | 61.91 |
| trimeric formaldehyde | 21.5 | - | - |
| formaldehyde aqueous solution | 21.5 | 12.59 | 58.56 |
| Aldehydes Amount (Polyformaldehyde) | Theoretical Yield/g | Actual Yield/g | Yield/% |
|---|---|---|---|
| 0.2 mol | 21.5 | 13.31 | 61.91 |
| 0.3 mol | 21.5 | 14.17 | 65.90 |
| 0.4 mol | 21.5 | 15.96 | 74.23 |
| 0.5 mol | 21.5 | 15.89 | 73.90 |
| 0.6 mol | 21.5 | 15.74 | 73.21 |
| Different Proportions (GFZ:BAZ) | Experiment 1 | Experiment 2 | Experiment 3 |
|---|---|---|---|
| Impact Strength/KJ/m2 | |||
| 0:100 | 0.81 | 0.81 | 0.81 |
| 5:95 | 2.15 | 2.07 | 1.98 |
| 10:90 | 2.68 | 2.54 | 2.73 |
| 20:80 | 2.38 | 2.30 | 2.32 |
| 40:60 | 2.26 | 2.18 | 2.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Xu, R. Preparation and Characterization of Guaiacol-Furfuramine Benzoxazine and Its Modification of Bisphenol A-Aniline Oxazine Resin. Polymers 2024, 16, 783. https://doi.org/10.3390/polym16060783
Wang J, Xu R. Preparation and Characterization of Guaiacol-Furfuramine Benzoxazine and Its Modification of Bisphenol A-Aniline Oxazine Resin. Polymers. 2024; 16(6):783. https://doi.org/10.3390/polym16060783
Chicago/Turabian StyleWang, Jing, and Riwei Xu. 2024. "Preparation and Characterization of Guaiacol-Furfuramine Benzoxazine and Its Modification of Bisphenol A-Aniline Oxazine Resin" Polymers 16, no. 6: 783. https://doi.org/10.3390/polym16060783
APA StyleWang, J., & Xu, R. (2024). Preparation and Characterization of Guaiacol-Furfuramine Benzoxazine and Its Modification of Bisphenol A-Aniline Oxazine Resin. Polymers, 16(6), 783. https://doi.org/10.3390/polym16060783





