Highly Electroconductive Metal-Polymer Hybrid Foams Based on Silver Nanowires: Manufacturing and Characterization
Abstract
1. Introduction
2. Materials and Methods
2.1. Silver Nanowire Manufacturing
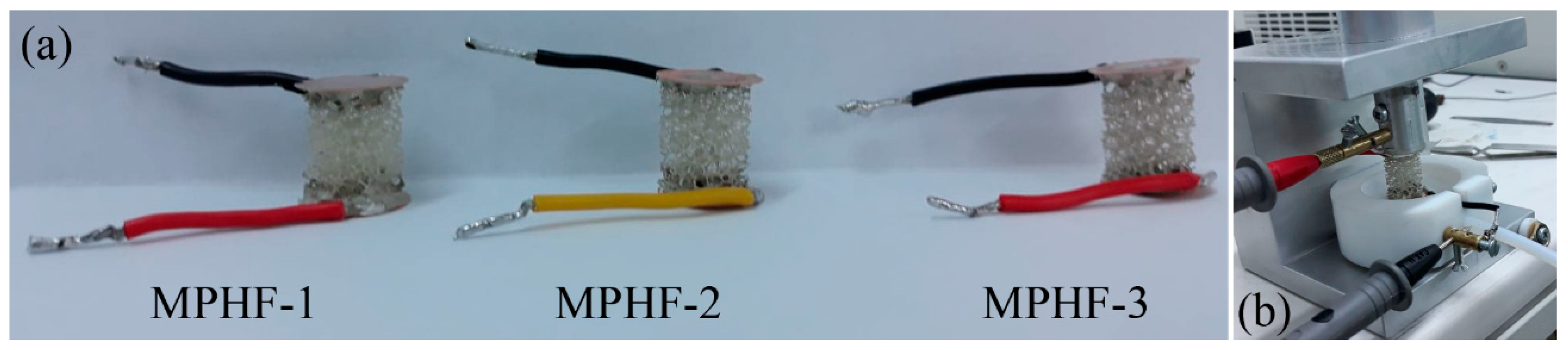
2.2. Metal-Polymer Hybrid Foam Manufacturing
2.3. Experimental Setup
3. Results and Discussion
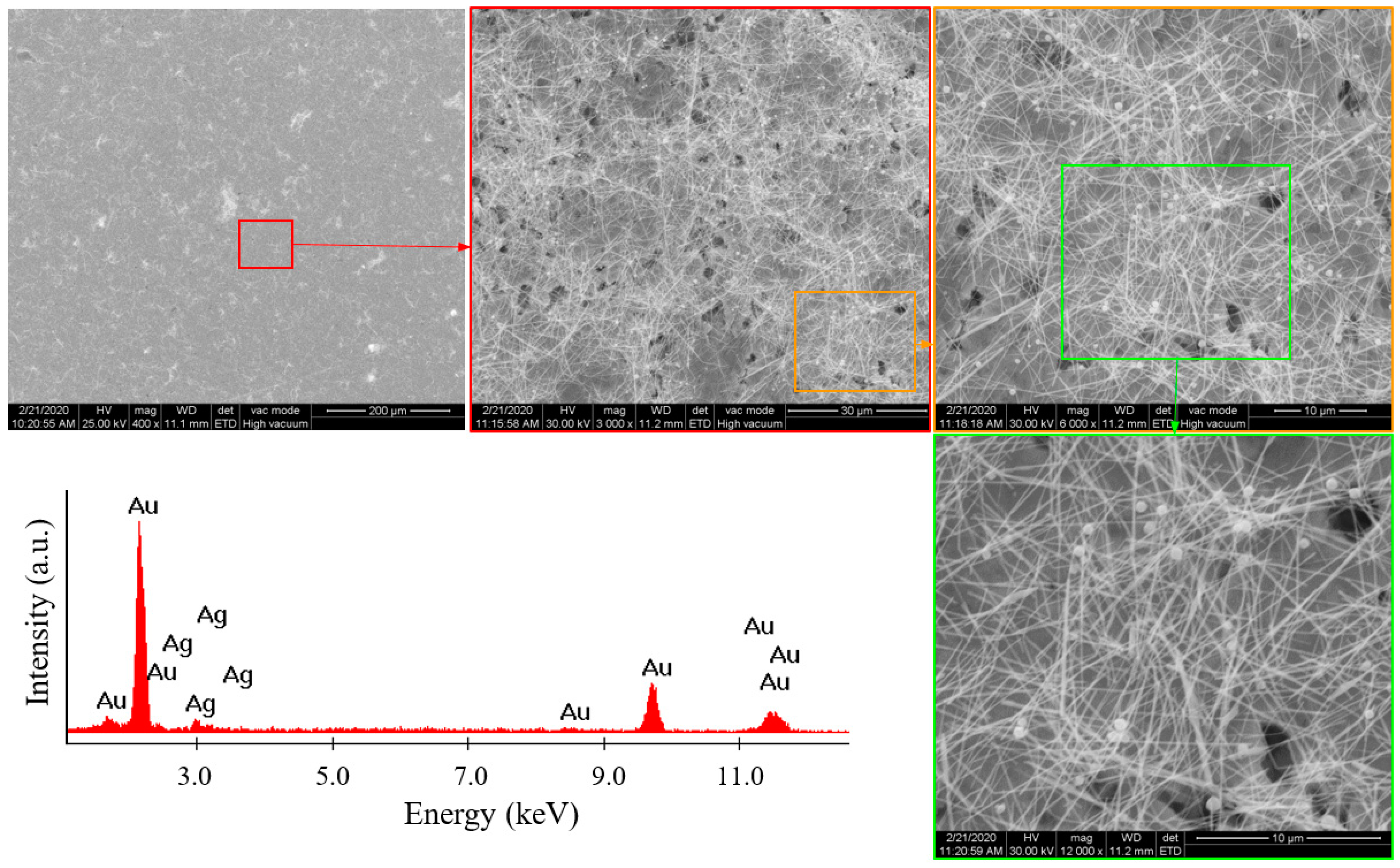
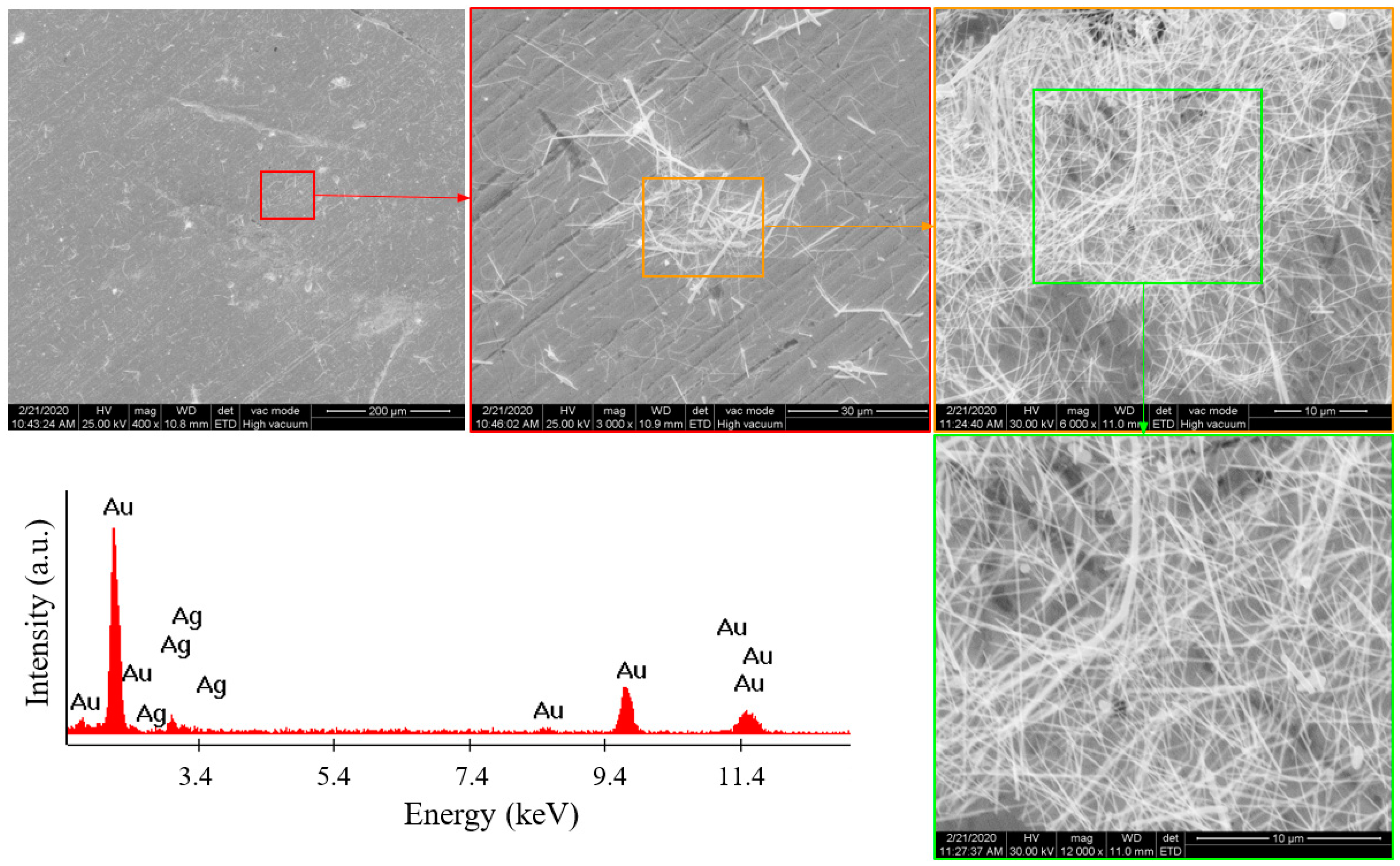
3.1. Silver Nanowire (AgNW) Characterization
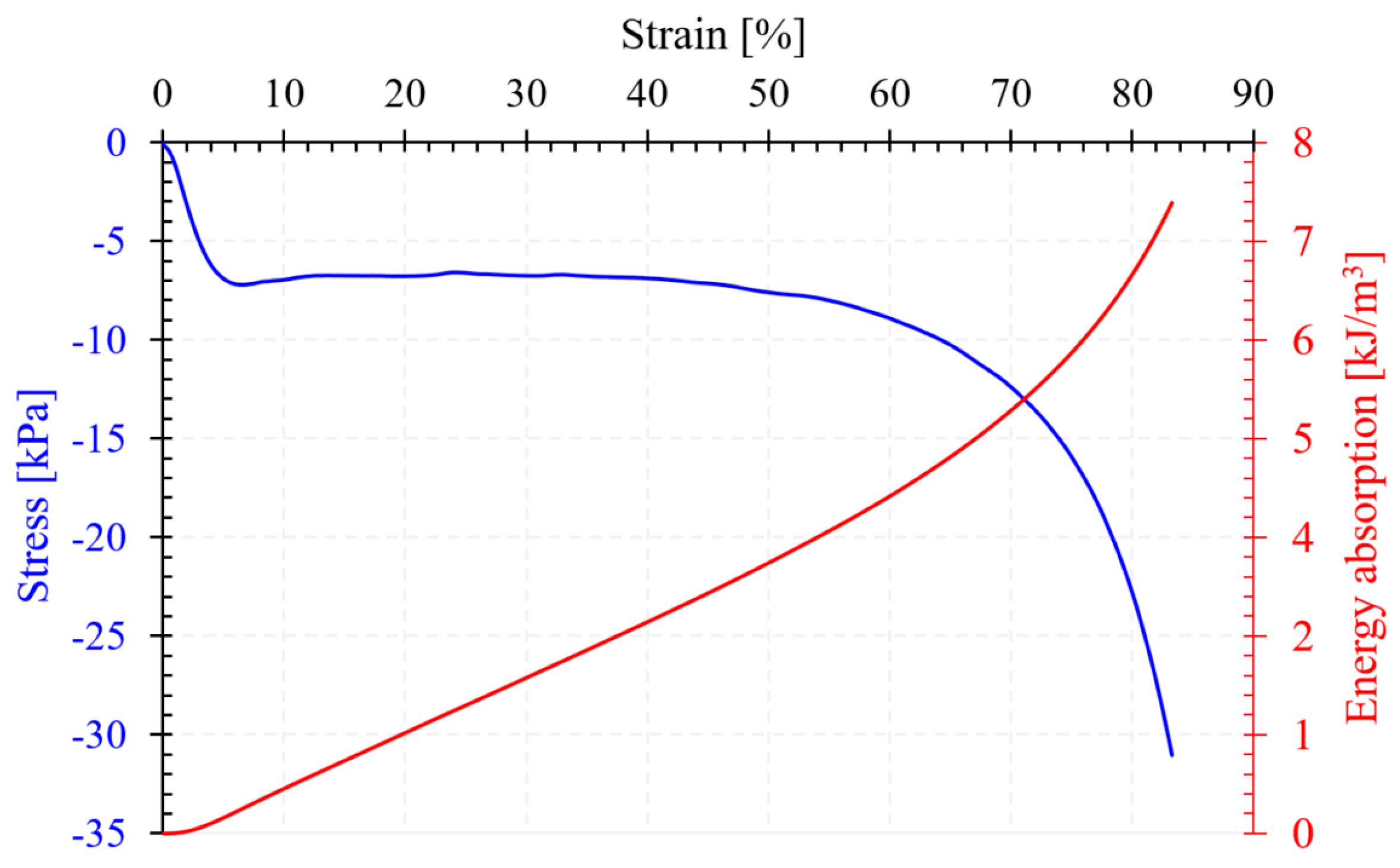
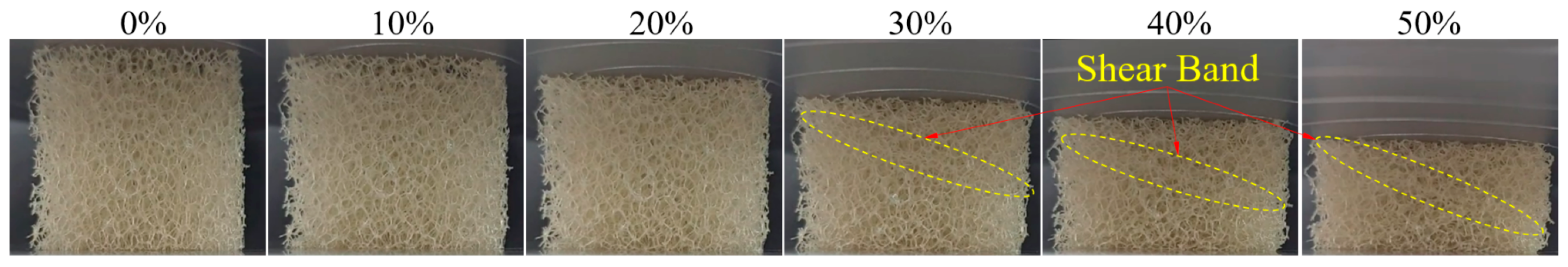
3.2. Flexible Open-Cell Polyurethane Foam (PUF) Characterization
3.3. Metal-Polymer Hybrid Foam (MPHF) Characterization
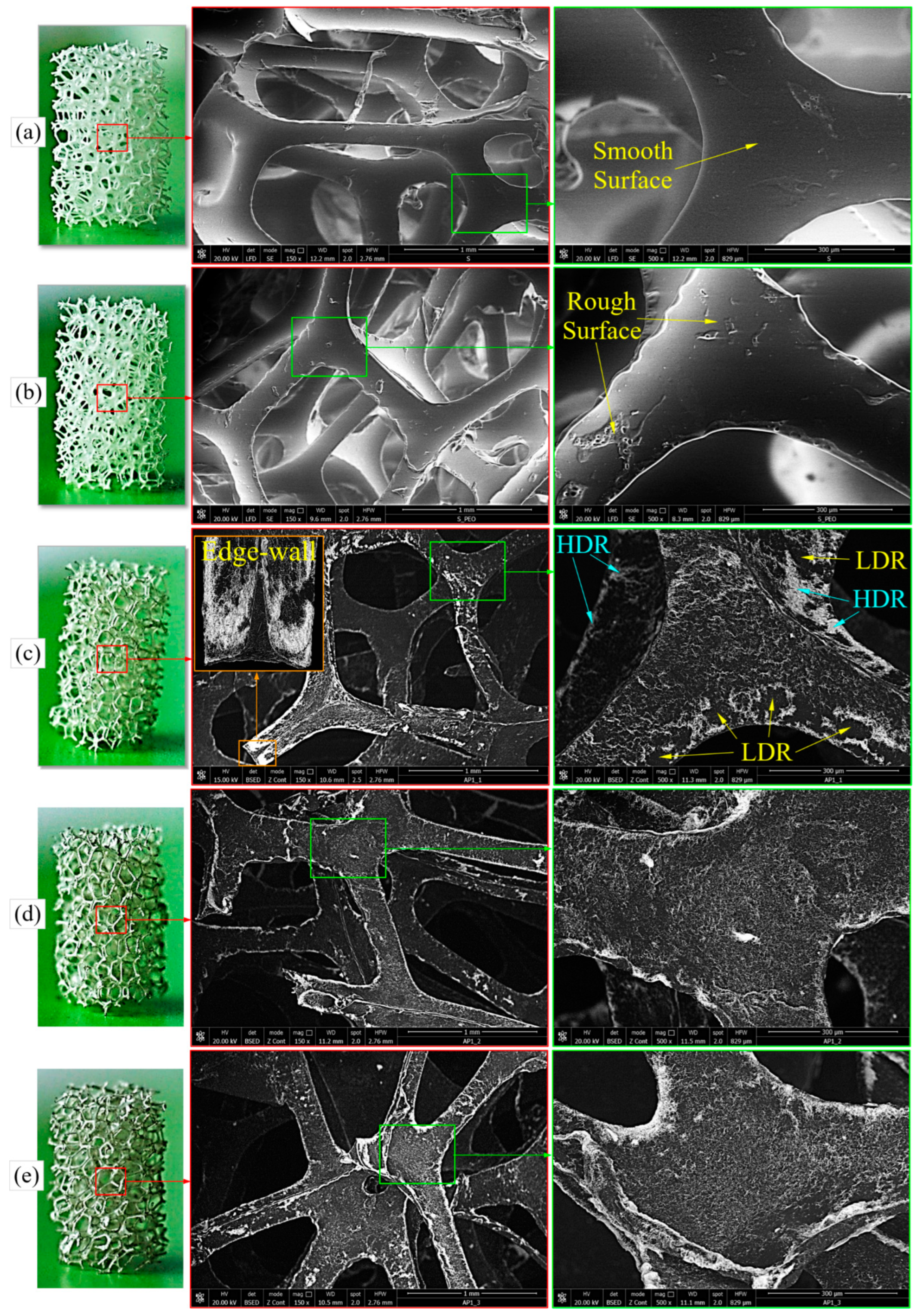
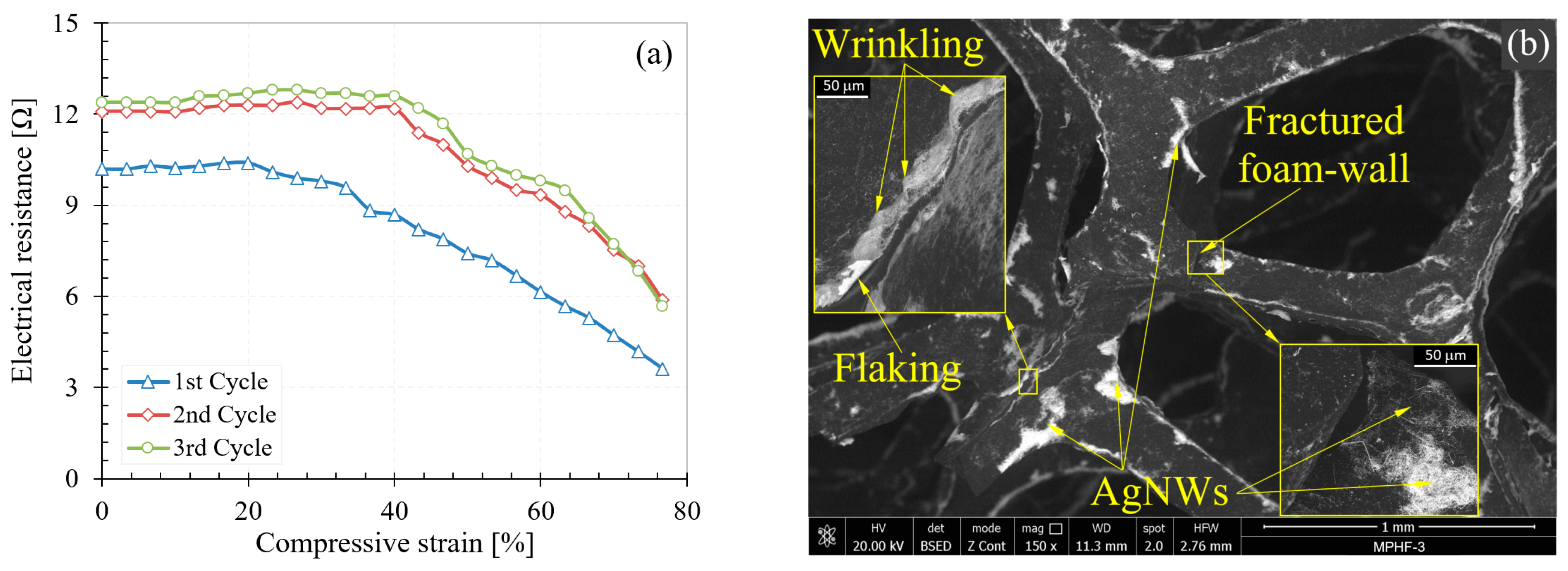
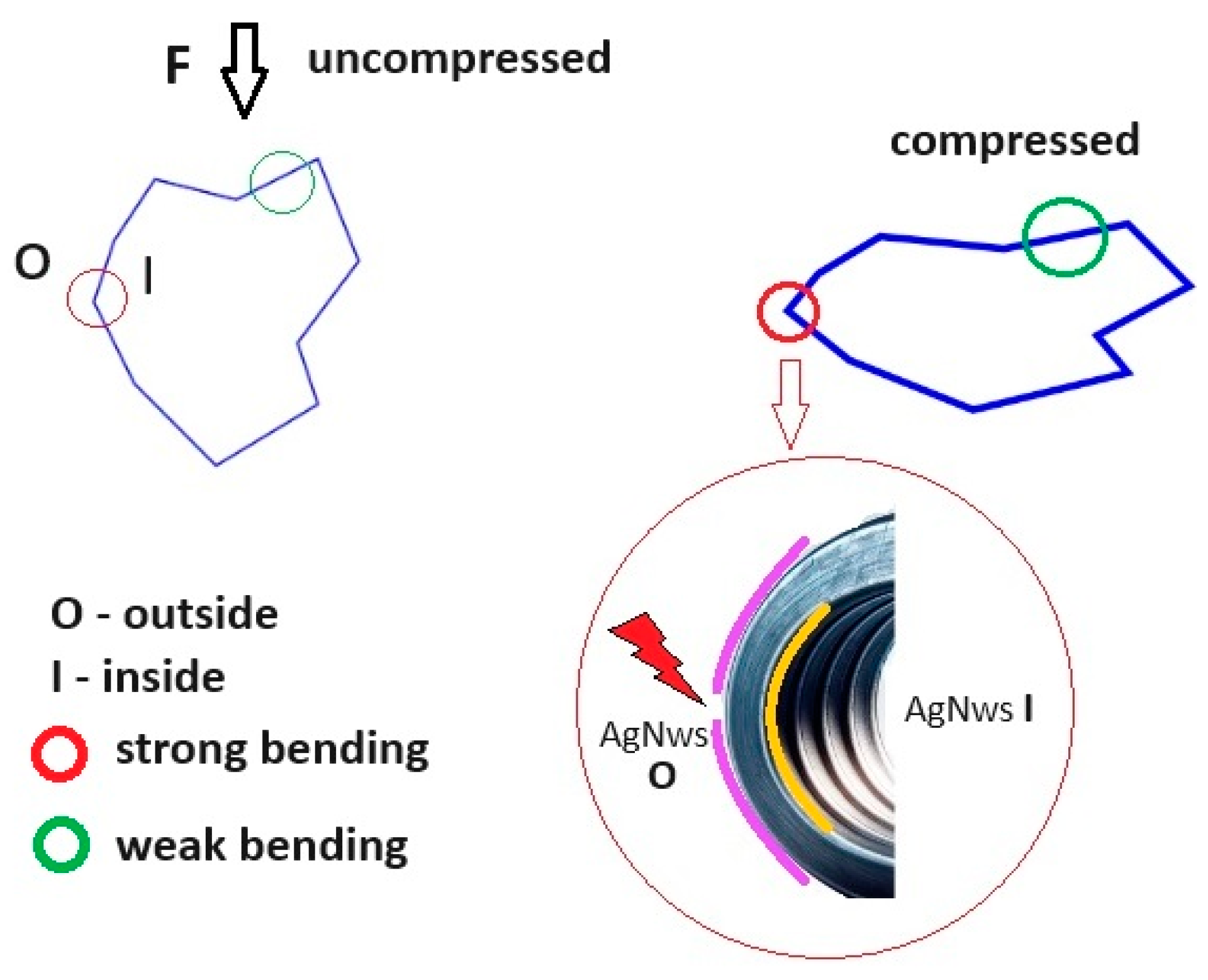
3.3.1. SEM Analysis
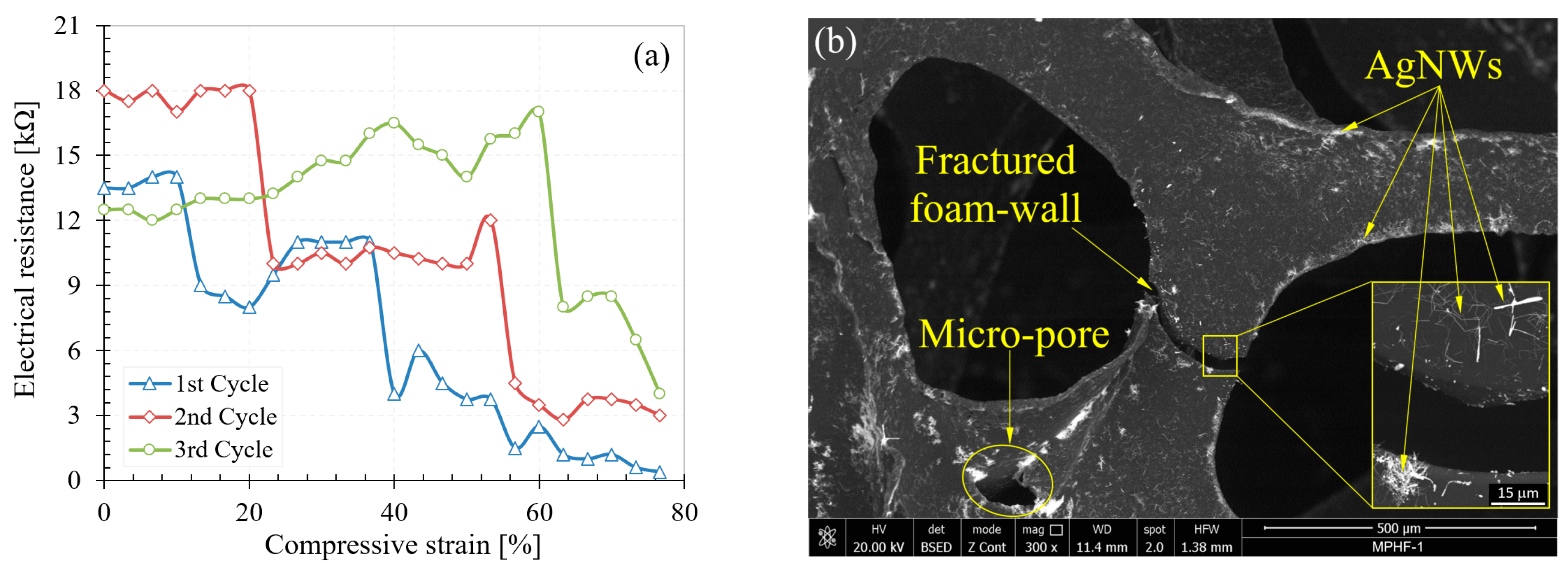
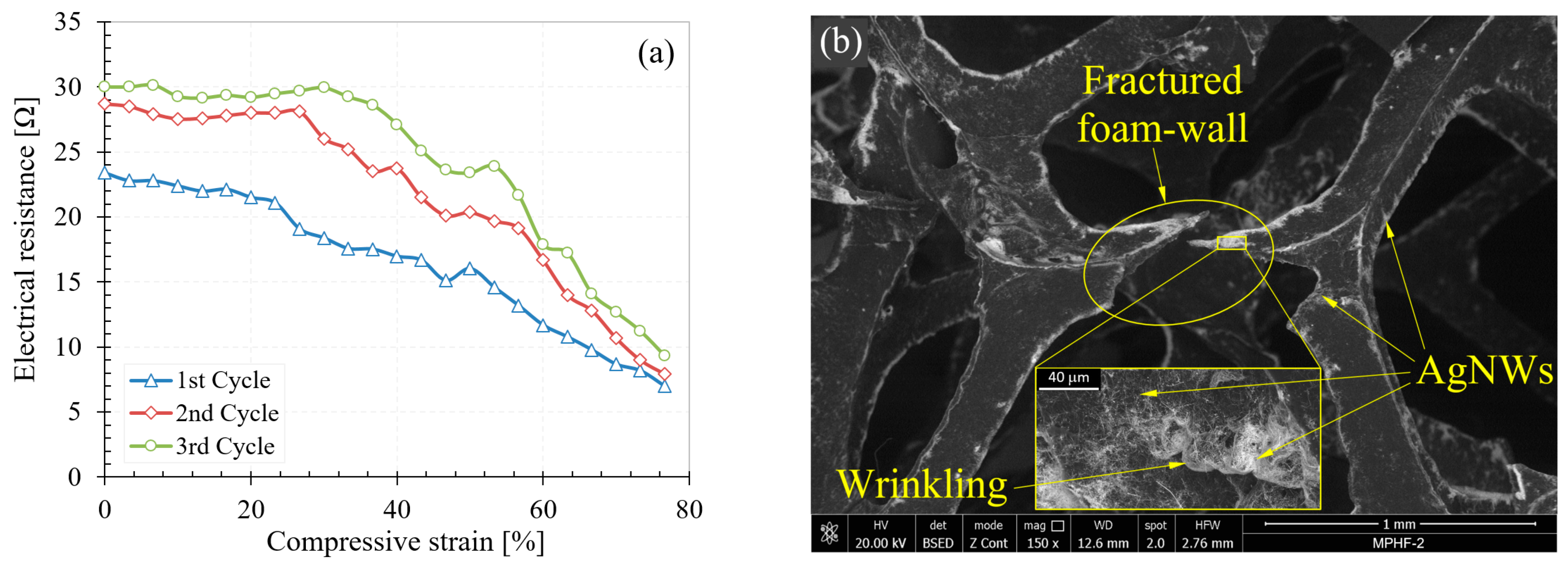
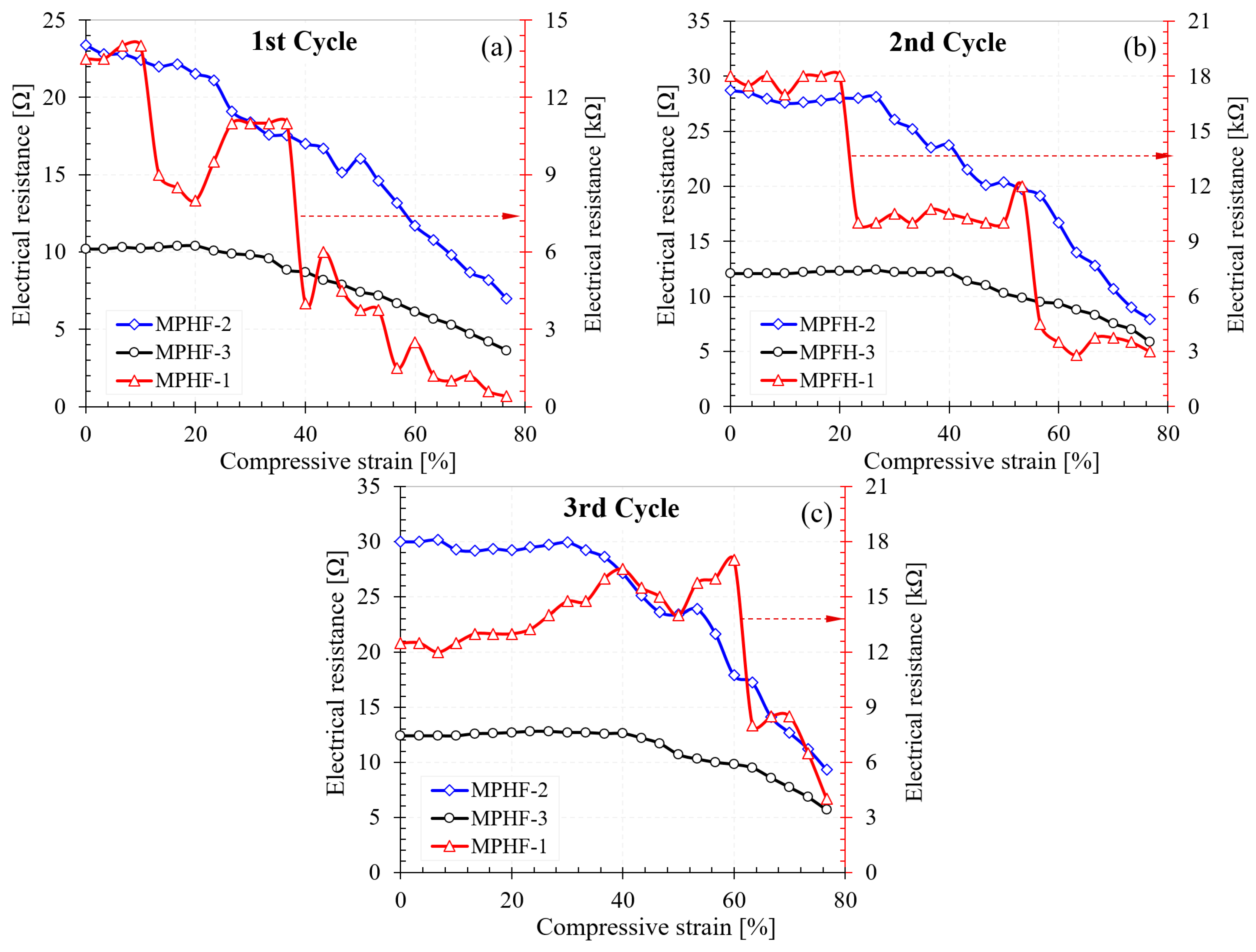
3.3.2. Electrical Behavior
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Hao, T.; Zhang, L.; Ji, H.; Zhou, Q.; Feng, T.; Song, S.; Wang, B.; Liu, D.; Ren, Z.; Liu, W.; et al. A Stretchable, Transparent, and Mechanically Robust Silver Nanowire–Polydimethylsiloxane Electrode for Electrochromic Devices. Polymers 2023, 15, 2640. [Google Scholar] [CrossRef]
- Jin, I.S.; Lee, H.D.; Hong, S.I.; Lee, W.; Jung, J.W. Facile Post Treatment of Ag Nanowire/Polymer Composites for Flexible Transparent Electrodes and Thin Film Heaters. Polymers 2021, 13, 586. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yu, J.; Bai, D.; Li, Z.; Liu, H.; Li, Y.; Chen, S.; Cheng, J.; Li, L. Biodegradable, Flexible, and Transparent Conducting Silver Nanowires/Polylactide Film with High Performance for Optoelectronic Devices. Polymers 2020, 12, 604. [Google Scholar] [CrossRef]
- Yu, K.; He, T. Silver-Nanowire-Based Elastic Conductors: Preparation Processes and Substrate Adhesion. Polymers 2023, 15, 1545. [Google Scholar] [CrossRef]
- Tan, X.; Zheng, J. A Novel Porous PDMS-AgNWs-PDMS (PAP)-Sponge-Based Capacitive Pressure Sensor. Polymers 2022, 14, 1495. [Google Scholar] [CrossRef] [PubMed]
- Park, K.; Kang, S.; Park, J.; Hwang, J. Fabrication of silver nanowire coated fibrous air filter medium via a two-step process of electrospinning and electrospray for anti-bioaerosol treatment. J. Hazard. Mater. 2021, 411, 125043. [Google Scholar] [CrossRef]
- Shi, G.; Liu, T.; Kopecki, Z.; Cowin, A.; Lee, I.; Pai, J.-H.; Lowe, S.E.; Zhong, Y.L. A Multifunctional Wearable Device with a Graphene/Silver Nanowire Nanocomposite for Highly Sensitive Strain Sensing and Drug Delivery. C J. Carbon Res. 2019, 5, 17. [Google Scholar] [CrossRef]
- Haghniaz, R.; Gangrade, A.; Montazerian, H.; Zarei, F.; Ermis, M.; Li, Z.; Du, Y.; Khosravi, S.; de Barros, N.R.; Mandal, K.; et al. An All-In-One Transient Theranostic Platform for Intelligent Management of Hemorrhage. Adv. Sci. 2023, 10, 2301406. [Google Scholar] [CrossRef]
- Lee, H.; Kim, M.; Kim, I.; Lee, H. Flexible and Stretchable Optoelectronic Devices using Silver Nanowires and Graphene. Adv. Mater. 2016, 28, 4541–4548. [Google Scholar] [CrossRef]
- Huang, S.; Liu, Y.; Yang, F.; Wang, Y.; Yu, T.; Ma, D. Metal nanowires for transparent conductive electrodes in flexible chromatic devices: A review. Environ. Chem Lett. 2022, 20, 3005–3037. [Google Scholar] [CrossRef]
- Joe, Y.H.; Park, D.H.; Hwang, J. Evaluation of Ag nanoparticle coated air filter against aerosolized virus: Anti-viral efficiency with dust loading. J. Hazard. Mater. 2016, 301, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Joe, Y.H.; Woo, K.; Hwang, J. Fabrication of an anti-viral air filter with SiO2–Ag nanoparticles and performance evaluation in a continuous airflow condition. J. Hazard. Mater. 2014, 280, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Jiang, J.; Zhang, W.; Zhou, J.; Wang, T.; Huang, C.-H.; Xie, X. Silver Nanowire-Modified Filter with Controllable Silver Ion Release for Point-of-Use Disinfection. Environ. Sci. Technol. 2019, 53, 7504–7512. [Google Scholar] [CrossRef] [PubMed]
- Bahcelioglu, E.; Doganay, D.; Coskun, S.; Unalan, H.E.; Erguder, T.H. A Point-of-Use (POU) Water Disinfection: Silver Nanowire Decorated Glass Fiber Filters. J. Water Process Eng. 2020, 38, 101616. [Google Scholar] [CrossRef]
- Wang, L.; Wang, H.; Wan, Q.; Gao, J. Recent development of conductive polymer composite-based strain sensors. J. Polym. Sci. 2023, 61, 3167–3185. [Google Scholar] [CrossRef]
- Fierascu, R.C.; Lungulescu, E.-M.; Fierascu, I.; Stan, M.S.; Voinea, I.C.; Dumitrescu, S.I. Metal and Metal Oxide Nanoparticle Incorporation in Polyurethane Foams: A Solution for Future Antimicrobial Materials? Polymers 2023, 15, 4570. [Google Scholar] [CrossRef]
- Yuan, Z.; Rayess, N.; Dukhan, N. Modeling of the Mechanical Properties of a Polymer-metal Foam Hybrid. Procedia Mater. Sci. 2014, 4, 215–219. [Google Scholar] [CrossRef]
- Kulshreshtha, A.; Dhakad, S.K. Preparation of metal foam by different methods: A review. Mater. Today Proc. 2020, 26, 1784–1790. [Google Scholar] [CrossRef]
- Madgule, M.; Sreenivasa, C.G.; Borgaonkar, A.V. Aluminium metal foam production methods, properties and applications—A review. Mater. Today Proc. 2023, 77, 673–679. [Google Scholar] [CrossRef]
- Miyoshi, T.; Itoh, M.; Mukai, T.; Kanahashi, H.; Kohzu, H.; Tanabe, S.; Higashi, K. Enhancement of energy absorption in a closed-cell aluminum by the modification of cellular structures. Scr. Mater. 1999, 41, 1055–1060. [Google Scholar] [CrossRef]
- Tammaro, D.; Gatta, R.D.; Villone, M.M.; Maffettone, P.L. Continuous 3D Printing of Hierarchically Structured Microfoamed Objects. Adv. Eng. Mater. 2022, 24, 2101226. [Google Scholar] [CrossRef]
- Tammaro, D.; Villone, M.M.; Maffettone, P.L. Microfoamed Strands by 3D Foam Printing. Polymers 2022, 14, 3214. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wu, M.; Ma, W.; Zhou, X.; Chen, J.; Ren, Q.; Li, S.; Xiao, P.; Wang, L.; Zheng, W. Development of Eco-Friendly and High-Strength Foam Sensors Based on Segregated Elastomer Composites with a Large Work Range and High Sensitivity. ACS Appl. Mater. Interfaces 2023, 15, 57613–57625. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, S.; Wu, M.; Weng, Z.; Ren, Q.; Xiao, P.; Wang, L.; Zheng, W. Multifunctional polyether block amides/carbon nanostructures piezoresistive foams with largely linear range, enhanced and humidity-regulated microwave shielding. Chem. Eng. J. 2023, 455, 140860. [Google Scholar] [CrossRef]
- Yu, Y.; Zeng, J.F.; Chen, C.J. Three-Dimensional Compressible and Stretchable Conductive Composites. Adv. Mater. 2014, 26, 810–815. [Google Scholar] [CrossRef]
- Joo, Y.; Byun, J.; Seong, N.; Ha, J.; Kim, H.; Kim, S.; Hong, Y. Silver nanowire-embedded PDMS with a multiscale structure for a highly sensitive and robust flexible pressure sensor. Nanoscale 2015, 7, 6208–6215. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, J.G.; Muhammad, T.; Kim, S. A silver nanowire-based flexible pressure sensor to measure the non-nutritive sucking power of neonates. Micro Nano Syst. Lett. 2020, 8, 18. [Google Scholar] [CrossRef]
- Ko, Y.; Kim, D.; Kwon, G.; You, J. High-Performance Resistive Pressure Sensor Based on Elastic Composite Hydrogel of Silver Nanowires and Poly(ethylene glycol). Micromachines 2018, 9, 438. [Google Scholar] [CrossRef]
- Vimala, K.; Varaprasad, K.; Sadiku, R.; Ramam, K.; Kanny, K. Development of novel protein–Ag nanocomposite for drug delivery and inactivation of bacterial applications. Int. J. Biol. Macromol. 2014, 63, 75–82. [Google Scholar] [CrossRef]
- De Mori, A.; Hafidh, M.; Mele, N.; Yusuf, R.; Cerri, G.; Gavini, E.; Tozzi, G.; Barbu, E.; Conconi, M.; Draheim, R.R.; et al. Sustained Release from Injectable Composite Gels Loaded with Silver Nanowires Designed to Combat Bacterial Resistance in Bone Regeneration Applications. Pharmaceutics 2019, 11, 116. [Google Scholar] [CrossRef]
- Todorova, M.; Milusheva, M.; Kaynarova, L.; Georgieva, D.; Delchev, V.; Simeonova, S.; Pilicheva, B.; Nikolova, S. Drug-Loaded Silver Nanoparticles—A Tool for Delivery of a Mebeverine Precursor in Inflammatory Bowel Diseases Treatment. Biomedicines 2023, 11, 1593. [Google Scholar] [CrossRef] [PubMed]
- Shahzadi, K.; Wu, L.; Ge, X.; Zhao, F.; Li, H.; Pang, S.; Mu, X. Preparation and characterization of bio-based hybrid film containing chitosan and silver nanowires. Carbohydr. Polym. 2016, 137, 732–738. [Google Scholar] [CrossRef]
- Wickham, A.; Vagin, M.; Khalaf, H.; Bertazzo, S.; Hodder, P.; Dånmark, S.; Aili, D. Electroactive biomimetic collagen-silver nanowire composite scaffolds. Nanoscale 2016, 8, 14146–14155. [Google Scholar] [CrossRef] [PubMed]
- An, J.; Ji, Z.; Wang, D.; Luo, Q.; Li, X. Preparation and characterization of uniform-sized chitosan/silver microspheres with antibacterial activities. Mater. Sci. Eng. C 2014, 36, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Paladini, F.; Pollini, M. Antimicrobial Silver Nanoparticles for Wound Healing Application: Progress and Future Trends. Materials 2019, 12, 2540. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Fang, L.; Huang, X.; Jiang, P. Three-Dimensional Highly Conductive Graphene–Silver Nanowire Hybrid Foams for Flexible and Stretchable Conductors. ACS Appl. Mater. Interfaces 2014, 6, 21026–21034. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Chen, M.; Pei, Y.; Seyed Shahabadi, S.I.; Che, B.; Wang, P.; Lu, X. Ultralight and Flexible Polyurethane/Silver Nanowire Nanocomposites with Unidirectional Pores for Highly Effective Electromagnetic Shielding. ACS Appl. Mater. Interfaces 2017, 9, 32211–32219. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.F.; Li, Y.; Zeng, C. Stretchable and compressible piezoresistive sensors from auxetic foam and silver nanowire. Mater. Chem. Phys. 2019, 229, 167–173. [Google Scholar] [CrossRef]
- Moon, I.K.; Yoon, S.; Oh, J. 3D Highly Conductive Silver Nanowire@PEDOT:PSS Composite Sponges for Flexible Conductors and Their All-Solid-State Supercapacitor Applications. Adv. Mater. Interfaces 2017, 4, 1700860. [Google Scholar] [CrossRef]
- Wan, Y.; Yang, S.; Wang, J.; Gan, D.; Gama, M.; Yang, Z.; Luo, H. Scalable synthesis of robust and stretchable composite wound dressings by dispersing silver nanowires in continuous bacterial cellulose. Compos. Part B Eng. 2020, 199, 108259. [Google Scholar] [CrossRef]
- Daiyan, R.; Lu, X.; Ng, Y.H.; Amal, R. Highly Selective Conversion of CO2to CO Achieved by a Three-Dimensional Porous Silver Electrocatalyst. Chem. Sel. 2017, 2, 879–884. [Google Scholar] [CrossRef]
- Murgunde, B.K.; Mulla, R.; Rabinal, M.K. A rapid synthesis of silver nanoparticle foam by ultrasonication. J. Porous Mater. 2020, 27, 1727–1733. [Google Scholar] [CrossRef]
- Cherevko, S.; Xing, X.; Chung, C.H. Electrodeposition of three-dimensional porous silver foams. Electrochem. Commun. 2010, 12, 467–470. [Google Scholar] [CrossRef]
- Custodio, V.; Herrera, F.; López, G.; Moreno, J. A Review on Architectures and Communications Technologies for Wearable Health-Monitoring Systems. Sensors 2012, 12, 13907–13946. [Google Scholar] [CrossRef] [PubMed]
- Coosemans, J.; Hermans, B.; Puers, R. Integrating wireless ECG monitoring in textiles. Sens. Actuators A Phys. 2006, 130–131, 48–53. [Google Scholar] [CrossRef]
- Linz, T.; Gourmelon, L.; Langereis, G. Contactless EMG sensors embroidered onto textile. In Proceedings of the 4th International Workshop on Wearable and Implantable Body Sensor Networks (BSN 2007), Aachen, Germany, 26–28 March 2007; IFMBE Proceedings. Leonhardt, S., Falck, T., Mähönen, P., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; Volume 13, pp. 29–34. [Google Scholar] [CrossRef]
- Löfhede, J.; Seoane, F.; Thordstein, M. Textile Electrodes for EEG Recording—A Pilot Study. Sensors 2012, 12, 16907–16919. [Google Scholar] [CrossRef] [PubMed]
- Banica, R.; Taranu, B.; Ladasiu, C.; Hulka, I.; Linul, P. Three-dimensional porous electrode based on silver nanowires for hydrogen sulfide detection. Mater. Lett. 2021, 304, 130720. [Google Scholar] [CrossRef]
- Jerz, J.; Mináriková, N.; Marsavina, L.; Linul, E. Scaling of compression strength in disordered solids: Metallic foams. Frat. Ed Integrita Strutt. 2016, 10, 55–62. [Google Scholar] [CrossRef]
- Șerban, D.A.; Linul, E. Fatigue behaviour of closed-cell polyurethane rigid foams. Eng. Fail. Anal. 2023, 154, 107728. [Google Scholar] [CrossRef]
- ASTM D1621; Standard Test Method for Compressive Properties of Rigid Cellular Plastics. ASTM International: West Conshohocken, PA, USA, 2000.
- Linul, E.; Khezrzadeh, O. Axial crashworthiness performance of foam-based composite structures under extreme temperature conditions. Compos. Struct. 2021, 271, 114156. [Google Scholar] [CrossRef]
- Bouchahdane, K.; Ouelaa, N.; Belaadi, A. Static and fatigue compression behaviour of conventional and auxetic open-cell foam. Mech. Adv. Mater. Struct. 2022, 29, 6154–6167. [Google Scholar] [CrossRef]
- Olszewski, A.; Kosmela, P.; Piasecki, A.; Żukowska, W.; Szczepański, M.; Wojtasz, P.; Barczewski, M.; Barczewski, R.; Hejna, A. Comprehensive Investigation of Stoichiometry–Structure–Performance Relationships in Flexible Polyurethane Foams. Polymers 2022, 14, 3813. [Google Scholar] [CrossRef]
- Pietras, D.; Linul, E.; Sadowski, T.; Rusinek, A. Out-of-plane crushing response of aluminum honeycombs in-situ filled with graphene-reinforced polyurethane foam. Compos. Struct. 2020, 249, 112548. [Google Scholar] [CrossRef]
- Voiconi, T.; Linul, E.; Marşavina, L.; Sadowski, T.; Kneć, M. Determination of flexural properties of rigid PUR foams using digital image correlation. Solid State Phenom. 2014, 216, 116–121. [Google Scholar] [CrossRef]
- Gama, N.V.; Ferreira, A.; Barros-Timmons, A. Polyurethane Foams: Past, Present, and Future. Materials 2018, 11, 1841. [Google Scholar] [CrossRef]
- Linul, E.; Marşavina, L. Prediction of fracture toughness for open cell polyurethane foams by finite-element micromechanical analysis. Iran. Polym. J. 2011, 20, 735–746. [Google Scholar]
- Losio, S.; Cifarelli, A.; Vignali, A.; Tomaselli, S.; Bertini, F. Flexible Polyurethane Foams from Bio-Based Polyols: Prepolymer Synthesis and Characterization. Polymers 2023, 15, 4423. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Jiang, P.; Liu, D.F.; Yuan, H.J.; Yan, X.Q.; Zhou, Z.P.; Wang, J.X.; Song, L.; Liu, L.F.; Zhou, W.Y.; et al. Evidence for the Monolayer Assembly of Poly(vinylpyrrolidone) on the Surfaces of Silver Nanowires. J. Phys. Chem. B 2004, 108, 12877–12881. [Google Scholar] [CrossRef]
- Ha, H.; Amicucci, C.; Matteini, P.; Hwang, B. Mini review of synthesis strategies of silver nanowires and their applications. Colloid Interface Sci. Commun. 2022, 50, 100663. [Google Scholar] [CrossRef]
- Available online: https://www.cheaptubes.com/product-category/nanowires/ (accessed on 4 February 2024).

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Linul, P.; Bănică, R.; Grad, O.; Linul, E.; Vaszilcsin, N. Highly Electroconductive Metal-Polymer Hybrid Foams Based on Silver Nanowires: Manufacturing and Characterization. Polymers 2024, 16, 608. https://doi.org/10.3390/polym16050608
Linul P, Bănică R, Grad O, Linul E, Vaszilcsin N. Highly Electroconductive Metal-Polymer Hybrid Foams Based on Silver Nanowires: Manufacturing and Characterization. Polymers. 2024; 16(5):608. https://doi.org/10.3390/polym16050608
Chicago/Turabian StyleLinul, Petrică, Radu Bănică, Oana Grad, Emanoil Linul, and Nicolae Vaszilcsin. 2024. "Highly Electroconductive Metal-Polymer Hybrid Foams Based on Silver Nanowires: Manufacturing and Characterization" Polymers 16, no. 5: 608. https://doi.org/10.3390/polym16050608
APA StyleLinul, P., Bănică, R., Grad, O., Linul, E., & Vaszilcsin, N. (2024). Highly Electroconductive Metal-Polymer Hybrid Foams Based on Silver Nanowires: Manufacturing and Characterization. Polymers, 16(5), 608. https://doi.org/10.3390/polym16050608







