Valorisation of Sub-Products from Pyrolysis of Carbon Fibre-Reinforced Plastic Waste: Catalytic Recovery of Chemicals from Liquid and Gas Phases
Abstract
1. Introduction
2. Materials and Methods
2.1. Carbon Fibre Composite
2.2. Catalysts and Reactor Bed Material
2.3. Pyrolysis Installation and Experiments
2.4. Analytical Techniques
2.5. Characterisation of Catalysts
2.5.1. Chemical Analysis
2.5.2. Physical Adsorption/Desorption with Nitrogen
2.5.3. Chemical Adsorption of CO
2.5.4. Reduction at Programmed Temperature with H2
2.5.5. Programmed Thermal Desorption with NH3
2.5.6. X-ray Diffraction
3. Results and Discussion
3.1. Catalysts Characterisation
3.1.1. Surface Area, Porosity and Chemical Analysis
3.1.2. Active Surface and Metal Dispersion
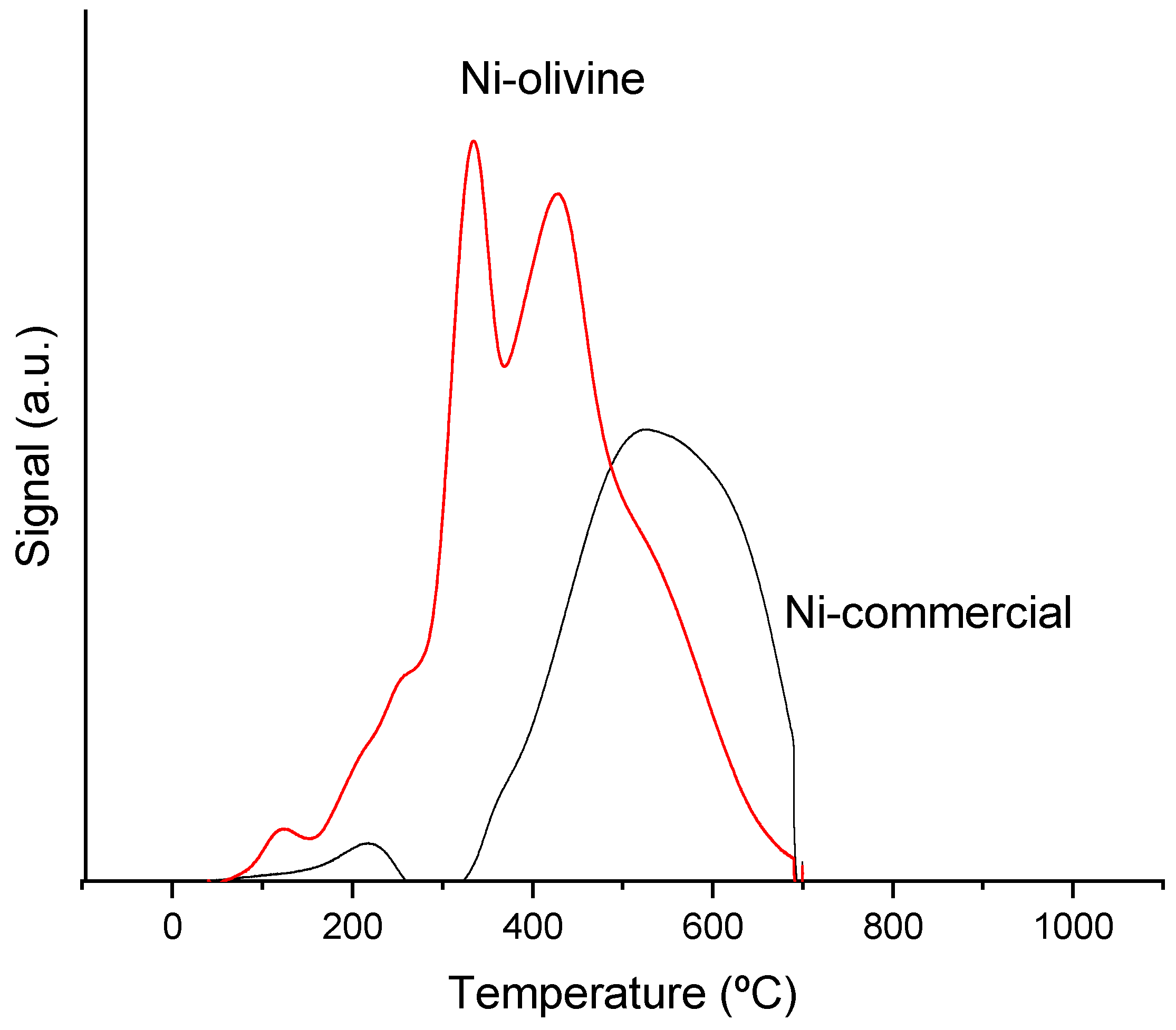
3.1.3. Reduction Temperature
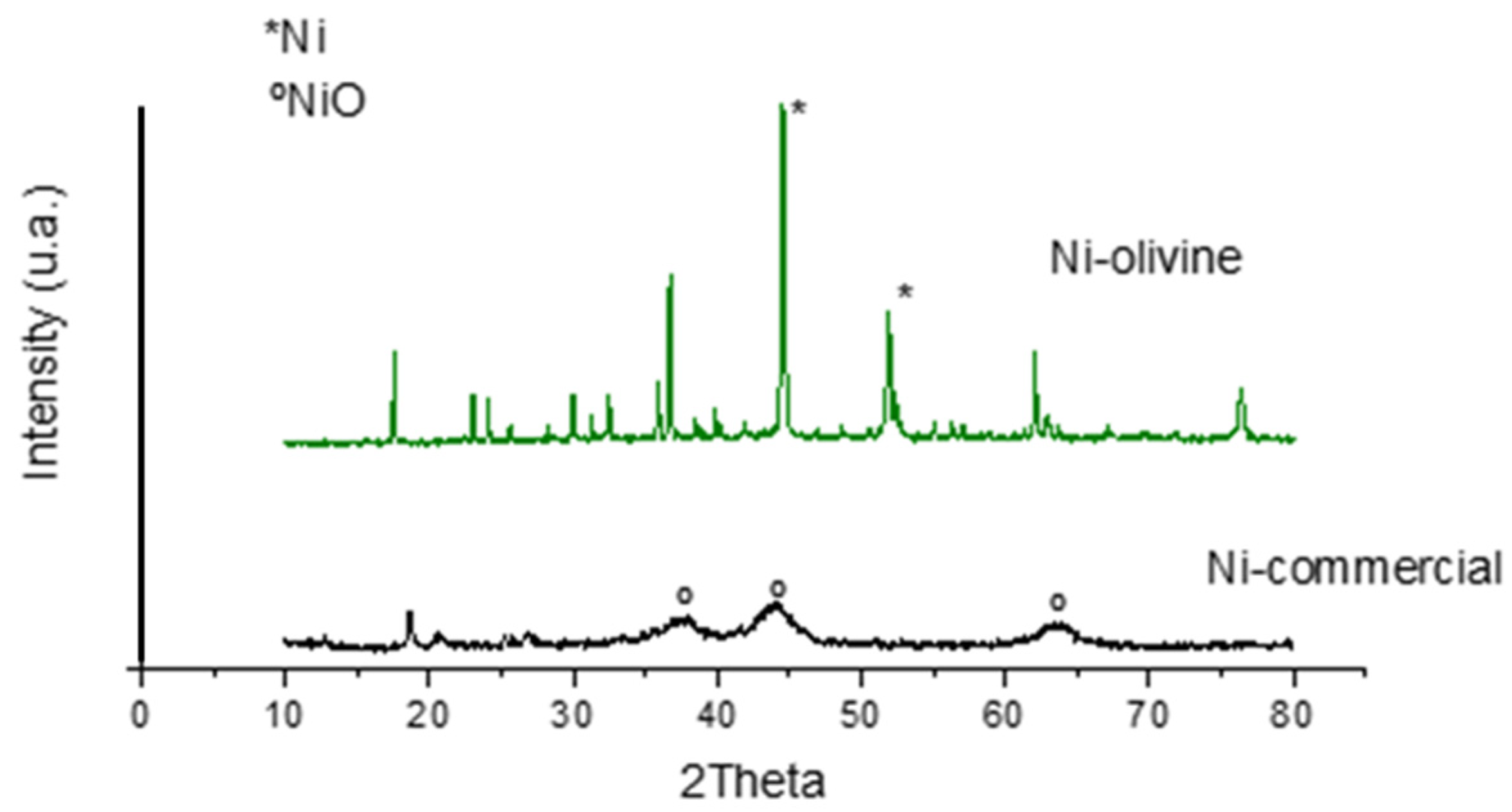
3.1.4. Crystallinity and Crystalline Particle Size
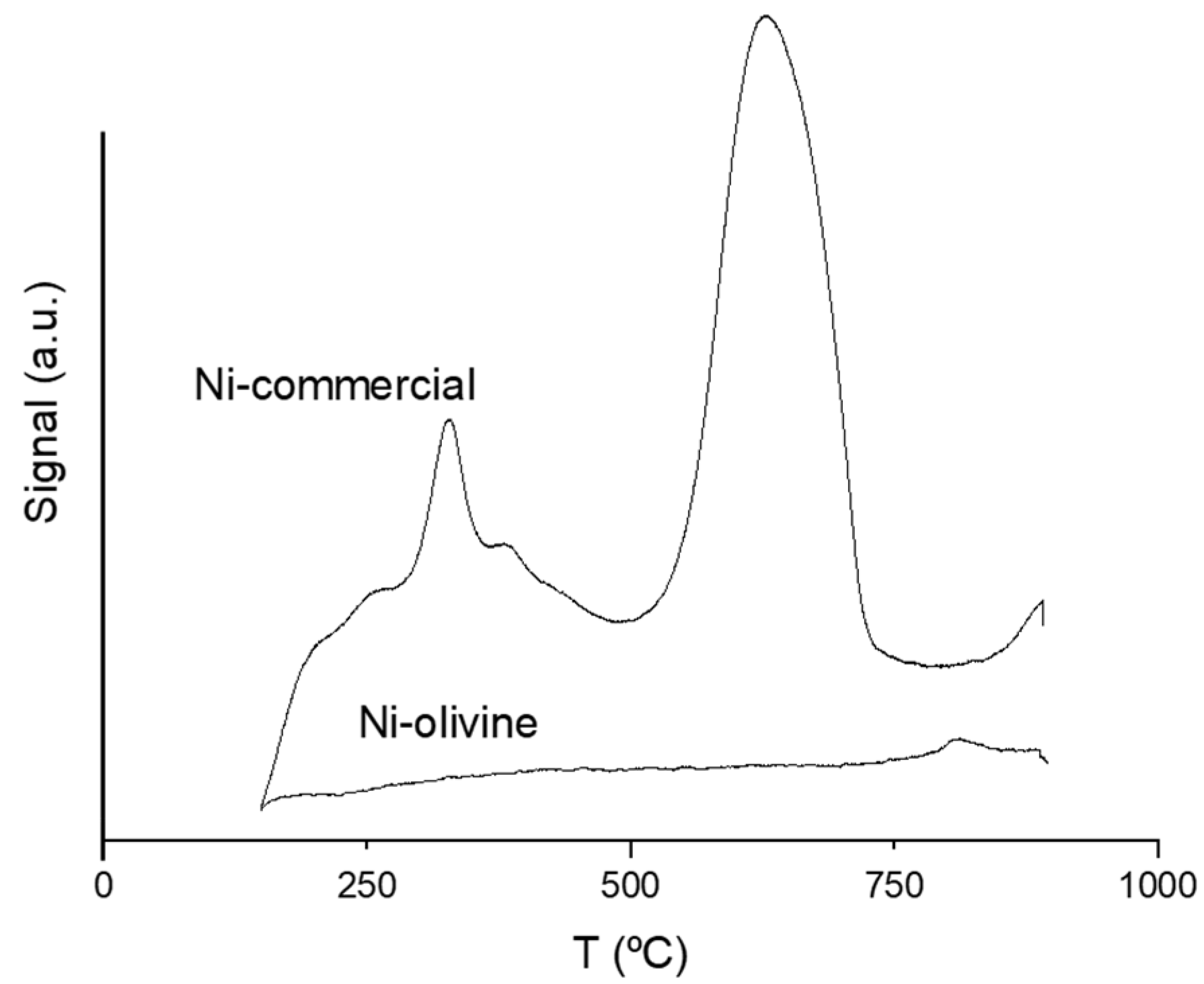
3.1.5. Acidity
3.2. Pyrolysis Results
3.2.1. Influence of Thermal Treatment in Pyrolysis Vapours
3.2.2. Catalytic Treatment of Pyrolysis Vapours
4. Conclusions
- The problem that remains to be solved in the pyrolysis treatment of fibre-reinforced plastic is mainly the liquid stream.
- In order to obtain useful liquids and gases, it is necessary to place a thermal or thermo-catalytic vapour treatment reactor in series with the pyrolysis reactor.
- Among the thermal treatments of pyrolysis vapours, the most interesting results have been obtained at 900 °C: 95.5 vol% of synthesis gas, 75.0 vol% of H2 and 92.1 area% of the collected liquid being water.
- The main effect of the catalysts have been on the composition of the liquids, not so much on the yields.
- Among the thermo-catalytic treatments of pyrolysis vapours, the most interesting results have been obtained at 800 °C with the Ni commercial catalyst: 93.2 vol% of synthesis gas, 72.3 vol% of H2 and 97.5 area% of the collected liquids being water.
- It is proposed that the best experimental conditions are with the tubular reactor at 700 °C with the Ni commercial catalyst, as long as aniline can be recovered from the liquid and the gas is 93.1 vol% H2. If aniline from the liquid phase cannot be recovered and used, we propose as the best experimental conditions the tubular reactor at 800 °C with the Ni commercial catalyst, because the liquid is almost entirely water, so it can be an inert stream to be managed without economic or environmental cost. At these conditions, the obtained gas flow will have a 72.3 vol% of H2 and a 93.2 vol% of syngas.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, J.; Lin, G.; Vaidya, U.; Wang, H. Past, present and future prospective of global carbon fibre composite developments and applications. Compos. Part B Eng. 2023, 250, 110463. [Google Scholar] [CrossRef]
- Bachmann, J.; Hidalgo, C.; Bricout, S. Environmental analysis of innovative sustainable composites with potential use in aviation sector—A life cycle assessment review. Sci. China Technol. Sci. 2017, 60, 1301–1317. [Google Scholar] [CrossRef]
- JEC Observer. JEC Observer: Current Trends in the Global Composites Industry 2020–2025; JEC Observer: Paris, France, 2021. [Google Scholar]
- Branfoot, C.; Folkvord, H.; Keith, M.; Leeke, G.A. Recovery of chemical recyclates from fibre-reinforced composites: A review of progress. Polym. Degrad. Stab. 2023, 215, 110447. [Google Scholar] [CrossRef]
- Ma, W.; Zhu, Y.; Cai, N.; Wang, X.; Chen, Y.; Yang, H.; Chen, H. Preparation of carbon nanotubes by catalytic pyrolysis of dechlorinated PVC. Waste Manag. 2023, 169, 62–69. [Google Scholar] [CrossRef]
- Almushaikeh, A.M.; Alaswad, S.O.; Alsuhybani, M.S.; AlOtaibi, B.M.; Alarifi, I.M.; Alqahtani, N.B.; Aldosari, S.M.; Alsaleh, S.S.; Haidyrah, A.S.; Alolyan, A.A.; et al. Manufacturing of carbon fiber reinforced thermoplastics and its recovery of carbon fiber: A review. Polym. Test. 2023, 122, 108029. [Google Scholar] [CrossRef]
- Wang, Y.; Li, A.Y.; Zhang, S.H.; Guo, B.B.; Niu, D.T. A review on new methods of recycling waste carbon fiber and its application in construction and industry. Constr. Build. Mater. 2023, 367, 130301. [Google Scholar] [CrossRef]
- European Techonology & Innovation Platform on Wind Energy. How Wind is Going Circular; European Commission: Brussels, Belgium, 2019. [Google Scholar]
- Yang, W.; Kim, K.H.; Lee, J. Upcycling of decommissioned wind turbine blades through pyrolysis. J. Clean. Prod. 2022, 376, 134292. [Google Scholar] [CrossRef]
- Gastelu, N.; Lopez-Urionabarrenechea, A.; Acha, E.; Caballero, B.M.; de Marco, I. Evaluation of HZSM-5 Zeolite as Cracking Catalyst for Upgrading the Vapours Generated in the Pyrolysis of an Epoxy-Carbon Fibre Waste Composite. Top. Catal. 2019, 62, 479–490. [Google Scholar] [CrossRef]
- Lopez-Urionabarrenechea, A.; Gastelu, N.; Acha, E.; Caballero, B.M.; de Marco, I. Production of hydrogen-rich gases in the recycling process of residual carbon fiber reinforced polymers by pyrolysis. Waste Manag. 2021, 128, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Gastelu, N.; Lopez-Urionabarrenechea, A.; Solar, J.; Acha, E.; Caballero, B.M.; López, F.A.; De Marco, I. Thermo-catalytic treatment of vapors in the recycling process of carbon fiber-poly (Benzoxazine) composite waste by pyrolysis. Catalysts 2018, 8, 523. [Google Scholar] [CrossRef]
- Landa, L.; Remiro, A.; Valecillos, J.; Valle, B.; Sun, S.; Wu, C.; Bilbao, J.; Gayubo, A.G. Sorption enhanced steam reforming (SESR) of raw bio-oil with Ni based catalysts: Effect of sorbent type, catalyst support and sorbent/catalyst mass ratio. Fuel Process. Technol. 2023, 247, 107799. [Google Scholar] [CrossRef]
- Grams, J.; Jankowska, A.; Goscianska, J. Advances in design of heterogeneous catalysts for pyrolysis of lignocellulosic biomass and bio-oil upgrading. Microporous Mesoporous Mater. 2023, 362, 112761. [Google Scholar] [CrossRef]
- Gollakota, A.R.; Shu, C.-M.; Sarangi, P.K.; Shadangi, K.P.; Rakshit, S.; Kennedy, J.F.; Gupta, V.K.; Sharma, M. Catalytic hydrodeoxygenation of bio-oil and model compounds—Choice of catalysts, and mechanisms. Renew. Sustain. Energy Rev. 2023, 187, 113700. [Google Scholar] [CrossRef]
- Nabgan, W.; Tuan Abdullah, T.A.; Mat, R.; Nabgan, B.; Gambo, Y.; Moghadamian, K. Acetic acid-phenol steam reforming for hydrogen production: Effect of different composition of La2O3-Al2O3 support for bimetallic Ni-Co catalyst. J. Environ. Chem. Eng. 2016, 4, 2765–2773. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, N.; Yang, L.; Li, M.; Huang, L. Ni-Co bimetallic MgO-based catalysts for hydrogen production via steam reforming of acetic acid from bio-oil. Int. J. Hydrogen Energy 2014, 39, 18688–18694. [Google Scholar] [CrossRef]
- Bimbela, F.; Oliva, M.; Ruiz, J.; Garcia, L.; Arauzo, J. Hydrogen production via catalytic steam reforming of the aqueous fraction of bio-oil using nickel-based coprecipitated catalysts. Int. J. Hydrogen Energy 2013, 38, 14476–14487. [Google Scholar] [CrossRef]
- Adnan, M.A.; Adamu, S.; Muraza, O.; Hossain, M.M. Fluidizable NiO-Fe2O3/SiO2-γAl2O3 for tar (toluene) conversion in biomass gasification. Process Saf. Environ. Prot. 2018, 116, 754–762. [Google Scholar] [CrossRef]
- Rong, H.; He, P.; Luo, Y.; Cai, H.; Laghari, M.; Guo, D.; Ren, Y.; Cui, B. Research progress of main synthetic catalysts used in biomass pyrolysis. Process Saf. Environ. Prot. 2023, 179, 27–37. [Google Scholar] [CrossRef]
- Mann, U. Principles of Chemical Reactor Analysis and Design: New Tools for Industrial Chemical Reactor Operations, 2nd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2009. [Google Scholar]
- Lopez-Urionabarrenechea, A.; Gastelu, N.; Jiménez-Suárez, A.; Prolongo, S.G.; Serras-Malillos, A.; Acha, E.; Caballero, B.M. Secondary raw materials from residual carbon fiber-reinforced composites by an upgraded pyrolysis process. Polymers 2021, 13, 3408. [Google Scholar] [CrossRef]
- Lopez-Urionabarrenechea, A.; Gastelu, N.; Acha, E.; Caballero, B.; Orue, A.; Jiménez-Suárez, A.; Prolongo, S.; de Marco, I. Reclamation of carbon fibers and added-value gases in a pyrolysis-based composites recycling process. J. Clean. Prod. 2020, 273, 123173. [Google Scholar] [CrossRef]
- Basu, S.; Pradhan, N.C. Selective production of hydrogen by acetone steam reforming over Ni-Co/olivine catalysts. React. Kinet. Mech. Catal. 2019, 127, 357–373. [Google Scholar] [CrossRef]
- Adrados, A.; Lopez-Urionabarrenechea, A.; Solar, J.; Requies, J.; De Marco, I.; Cambra, J.F. Upgrading of pyrolysis vapours from biomass carbonization. J. Anal. Appl. Pyrolysis 2013, 103, 293–299. [Google Scholar] [CrossRef]
- Miyazawa, T.; Kimura, T.; Nishikawa, J.; Kado, S.; Kunimori, K.; Tomishige, K. Catalytic performance of supported Ni catalysts in partial oxidation and steam reforming of tar derived from the pyrolysis of wood biomass. Catal. Today 2006, 115, 254–262. [Google Scholar] [CrossRef]
- Simell, P.A.; Bredenberg, J.-S. Catalytic purification of tarry fuel gas. Fuel 1990, 69, 1219–1225. [Google Scholar] [CrossRef]
- Garcia, L.; French, R.; Czernik, S.; Chornet, E. Catalytic steam reforming of bio-oils for the production of hydrogen: Effects of catalyst composition. Appl. Catal. A Gen. 2000, 201, 225–239. [Google Scholar] [CrossRef]
- Bizkarra, K.; Bermudez, J.M.; Arcelus-Arrillaga, P.; Barrio, V.L.; Cambra, J.F.; Millan, M. Nickel based monometallic and bimetallic catalysts for synthetic and real bio-oil steam reforming. Int. J. Hydrogen Energy 2018, 43, 11706–11718. [Google Scholar] [CrossRef]
- Anjalin, M.; Kanagathara, N.; Suganthi, A.B. A brief review on aniline and its derivatives. Mater. Today Proc. 2020, 33, 4751–4755. [Google Scholar] [CrossRef]
| Catalyst | BET (m2/g) | Pore Volume (cm3/g) | Average Pore Diameter (Å) | Metal (wt%) | ||
|---|---|---|---|---|---|---|
| Ni | Pd | Co | ||||
| Ni commercial | 99 | 0.404 | 163 | 39.3 | - | - |
| Ni–olivine | 4 | 0.016 | 35 | 29.9 | 0.8 | 8.6 |
| Catalyst | AMSA (m2/g) | MD (%) | Total Adsorbed CO (µmol/gsample) |
|---|---|---|---|
| Ni commercial | 0.85 | 0.32 | 21.7 |
| Ni–olivine | 0.02 | 0.01 | 0.5 |
| Catalyst | Total Acidity (mmolNH3/g) | Weak Acidity (mmolNH3/g) | Medium Acidity (mmolNH3/g) | Strong Acidity (mmolNH3/g) |
|---|---|---|---|---|
| Ni commercial | 0.850 | 0.071 | 0.239 | 0.540 |
| Tubular Reactor | No | Yes | |||||
|---|---|---|---|---|---|---|---|
| T tubular reactor (°C) | - | 700 | 800 | 900 | 700 | 700 | 800 |
| Catalyst | - | - | - | - | Ni commercial | Ni–olivine | Ni commercial |
| Solid (wt%) | 80.7 | 79.9 | 79.8 | 79.9 | 80.3 | 80.2 | 80.0 |
| Liquid (wt%) | 16.3 | 17.0 | 15.4 | 13.3 | 15.4 | 16.1 | 13.6 |
| Gas 1 (wt%) | 3.0 | 3.1 | 4.8 | 6.8 | 4.3 | 3.7 | 6.4 |
| Gases composition (vol%) and HHV (MJ/m3N) | |||||||
| H2 | 0.0 | 91.7 | 74.4 | 75.0 | 93.1 | 94.8 | 72.3 |
| CO | 97.0 | 8.0 | 20.6 | 20.5 | 5.9 | 5.2 | 20.9 |
| CO2 | 3.0 | <0.1 | 0.2 | 0.2 | <0.1 | <0.1 | 3.1 |
| CH4 | 0.0 | 0.3 | 4.8 | 4.3 | 1.0 | <0.1 | 3.7 |
| HHV (MJ/m3 in N.C.) | 11.2 | 11.8 | 12.9 | 12.7 | 11.9 | 11.7 | 12.2 |
| Collected liquids | |||||||
| Tubular Reactor | No | Yes | |||||
|---|---|---|---|---|---|---|---|
| T tubular reactor (°C) | - | 700 | 800 | 900 | 700 | 700 | 800 |
| Catalyst | - | - | - | - | Ni commercial | Ni–olivine | Ni commercial |
| Water (area%) | 34.5 | 45.2 | 46.8 | 92.1 | 74.7 | 58.4 | 97.5 |
| Identified organic compounds (area%) | 54.4 | 40.2 | 43.6 | 4.3 | 22.7 | 36.1 | 2.0 |
| Identified in total (area%) | 88.9 | 85.4 | 90.4 | 96.4 | 97.4 | 94.5 | 99.5 |
| Unidentified (area%) | 11.1 | 14.6 | 9.6 | 3.6 | 2.6 | 5.5 | 0.5 |
| Collected liquids |  |  |  |  |  |  |  |
| Identified organic compounds list (area%) | |||||||
| Toluene (C7H8) | 2.1 | - | - | - | - | 2.2 | - |
| Acetone (C3H6O) | 1.6 | - | - | - | - | - | - |
| N,N-dimethylbenzamide (C8H11N) | 0.4 | - | - | - | - | - | - |
| 2-methylbenzofuran (C9H8O) | 0.6 | 1.1 | - | - | - | 0.9 | - |
| N-methylaniline (C7H9N) | 1.5 | - | - | - | - | - | - |
| Aniline (C6H7N) | 11.6 | 30.2 | 26.2 | 1.3 | 19.1 | 14.9 | 0.9 |
| 1-phenyl-1H-pyrrole (C10H9N) | 0.4 | - | - | - | - | - | - |
| Quinoline (C6H9N3O2) | 1.0 | - | 0.5 | - | - | - | 0.6 |
| 2-methylphenol (C7H8O) | 2.7 | - | - | - | - | - | - |
| Phenol (C6H6O) | 10.9 | 6.2 | 2.8 | 1.3 | 2.4 | 6.1 | - |
| Diphenylether (C12H10O) | 3.8 | - | - | - | - | - | - |
| 6-methylquinoline (C10H9N) | 0.5 | - | - | - | - | - | 0.5 |
| 2-ethylphenol (C8H10O) | 0.8 | - | - | - | - | - | - |
| 4-methylphenol (C7H8O) | 2.2 | - | - | - | - | - | - |
| Caprolactam (C6H11NO) | 10.1 | - | - | - | - | - | - |
| 9H-Xanthene (C13H10O) | 0.5 | - | - | - | - | - | - |
| Diphenyl disulphide (C12H10S2) | 1.3 | - | - | - | - | - | - |
| Ethylbenzene (C8H10) | 0.4 | - | - | - | - | - | - |
| 1,3-dimethylbenzene (C8H10) | 0.2 | - | - | - | - | - | - |
| Methylthiobenzene (C7H8S) | 0.3 | - | - | - | - | - | - |
| p-aminotoluene (C7H9N) | 0.3 | - | - | - | - | - | - |
| 2,6-dimethylphenol (C8H10O) | 0.3 | - | - | - | - | - | - |
| 2-1-penthylfuran (E), (C9H12O) | 0.3 | - | - | - | - | - | - |
| 4-ethylphenol (C8H10O) | 0.3 | - | - | - | - | - | - |
| Diphenyl sulphide (C12H10S) | 0.3 | - | - | - | - | - | - |
| Styrene (C8H8) | - | 0.6 | - | - | - | 0.4 | - |
| Benzonitrile (C7H5N) | - | 2.1 | 2.8 | - | - | - | - |
| Benzene (C6H6) | - | - | 4.6 | - | - | - | - |
| Naphthalene (C10H8) | - | - | 6.0 | - | - | - | - |
| 2-ethylnaphthalene (C12H10) | - | - | 0.7 | - | - | - | - |
| Isoquinoline (C9H7N) | - | - | - | 1.7 | 1.2 | - | - |
| Benzofuran (C8H6O) | - | - | - | - | - | 1.6 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Acha, E.; Gastelu, N.; Lopez-Urionabarrenechea, A.; Caballero, B.M. Valorisation of Sub-Products from Pyrolysis of Carbon Fibre-Reinforced Plastic Waste: Catalytic Recovery of Chemicals from Liquid and Gas Phases. Polymers 2024, 16, 580. https://doi.org/10.3390/polym16050580
Acha E, Gastelu N, Lopez-Urionabarrenechea A, Caballero BM. Valorisation of Sub-Products from Pyrolysis of Carbon Fibre-Reinforced Plastic Waste: Catalytic Recovery of Chemicals from Liquid and Gas Phases. Polymers. 2024; 16(5):580. https://doi.org/10.3390/polym16050580
Chicago/Turabian StyleAcha, Esther, Naia Gastelu, Alexander Lopez-Urionabarrenechea, and Blanca María Caballero. 2024. "Valorisation of Sub-Products from Pyrolysis of Carbon Fibre-Reinforced Plastic Waste: Catalytic Recovery of Chemicals from Liquid and Gas Phases" Polymers 16, no. 5: 580. https://doi.org/10.3390/polym16050580
APA StyleAcha, E., Gastelu, N., Lopez-Urionabarrenechea, A., & Caballero, B. M. (2024). Valorisation of Sub-Products from Pyrolysis of Carbon Fibre-Reinforced Plastic Waste: Catalytic Recovery of Chemicals from Liquid and Gas Phases. Polymers, 16(5), 580. https://doi.org/10.3390/polym16050580






