Construction and Curing Behavior of Underwater In Situ Repairing Coatings for Offshore Structures
Abstract
1. Introduction
2. Experimental
2.1. Material
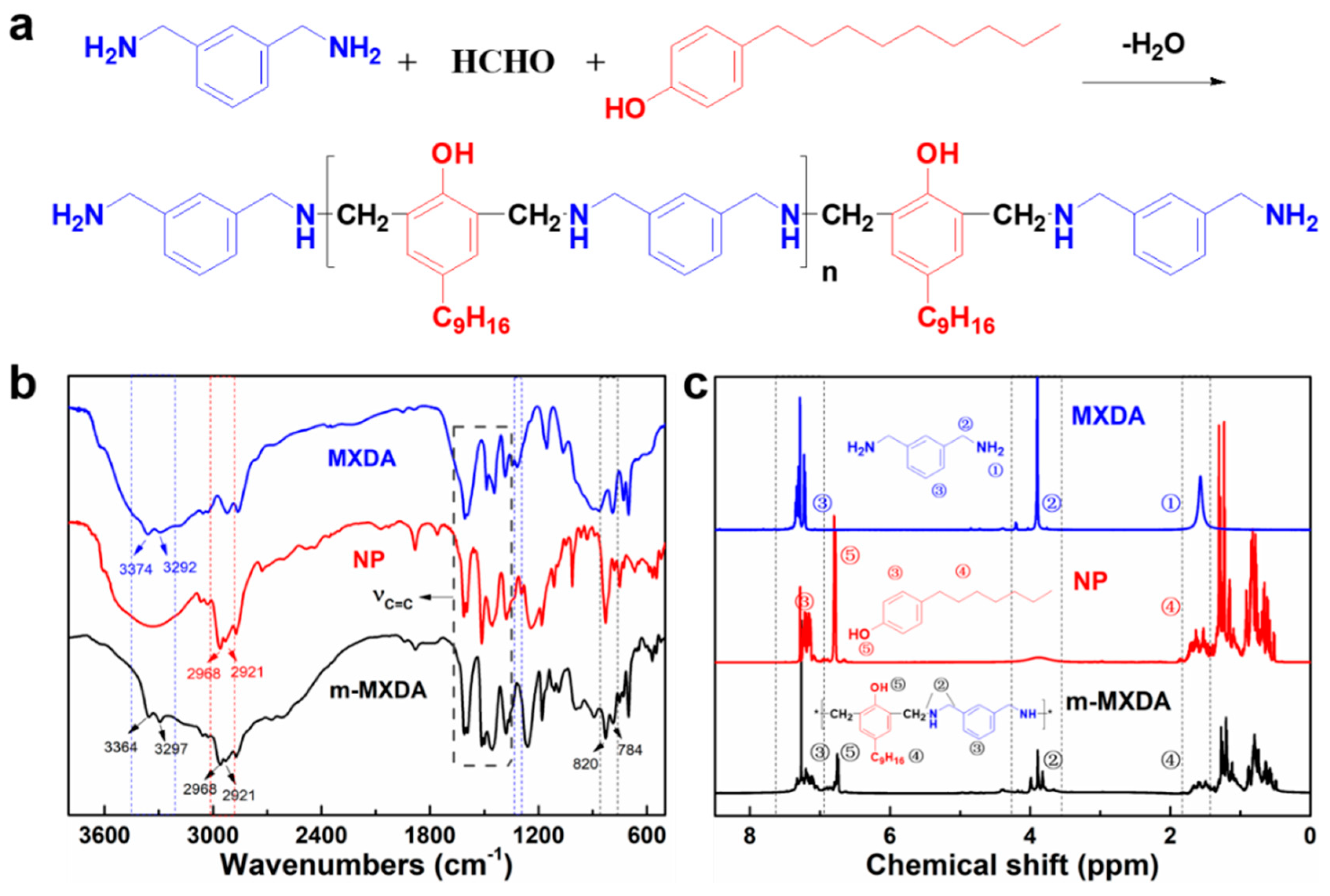
2.2. Synthesis of Mannich-Modified m-Xylylenediamine (m-MXDA)
2.3. Preparation of the In Situ Underwater Repairing Coating
2.4. Characterization
2.4.1. Fourier-Transform Infrared Spectroscopy (FTIR)
2.4.2. Nuclear Magnetic Resonance Spectroscopy
2.4.3. Pull-Off Tests
2.4.4. Dynamical Mechanical Analysis (DMA)
2.4.5. Electrochemical Impedance Spectroscopy (EIS) Measurements
3. Results and Discussion
3.1. Synthesis and Characterization of Mannich-Modified m-Xylylenediamine (m-MXDA)
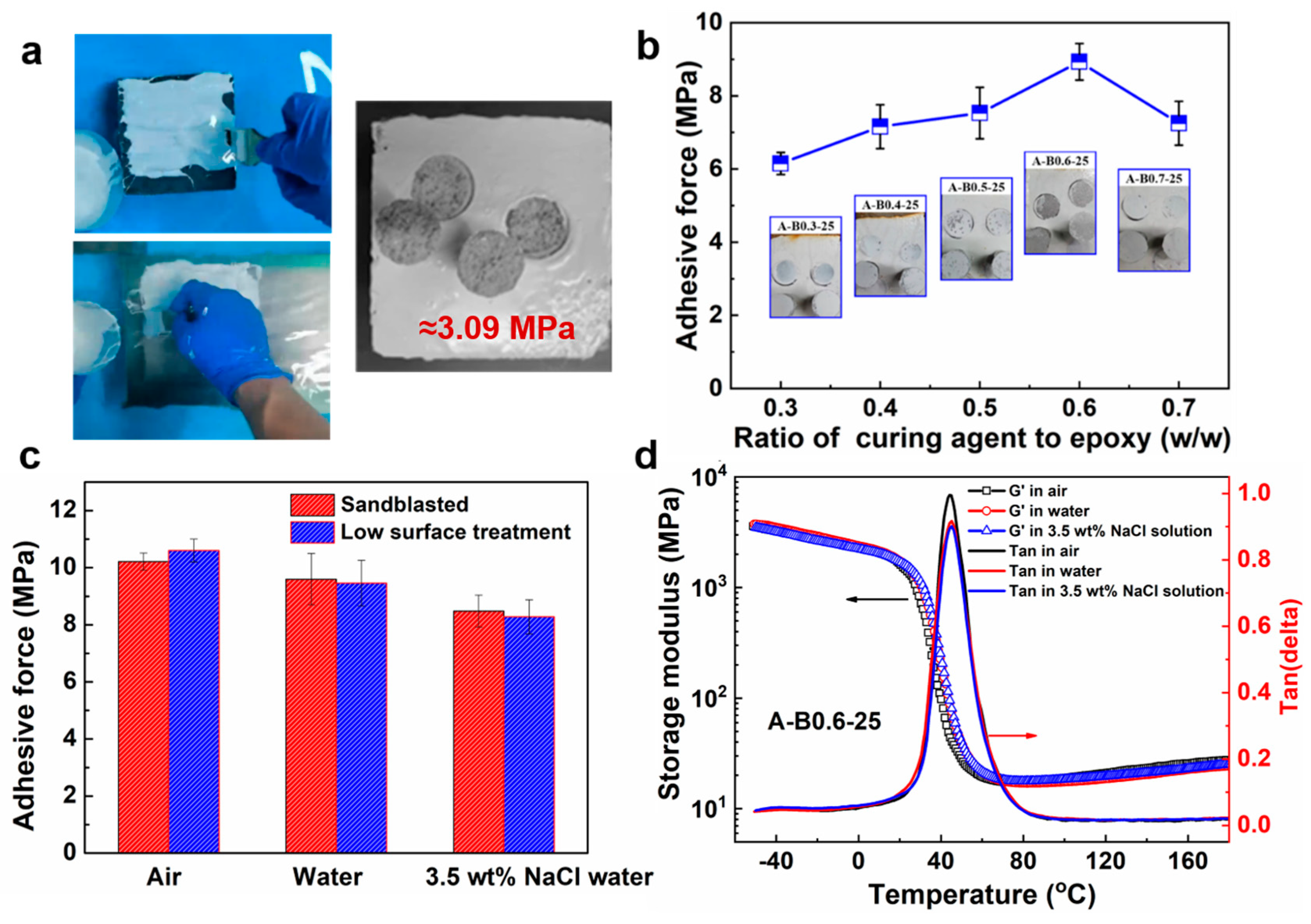
3.2. Adhesion and Properties of the Underwater In Situ Repairing Coatings
3.3. Anticorrosive Property of the Underwater In Situ Repairing Coatings
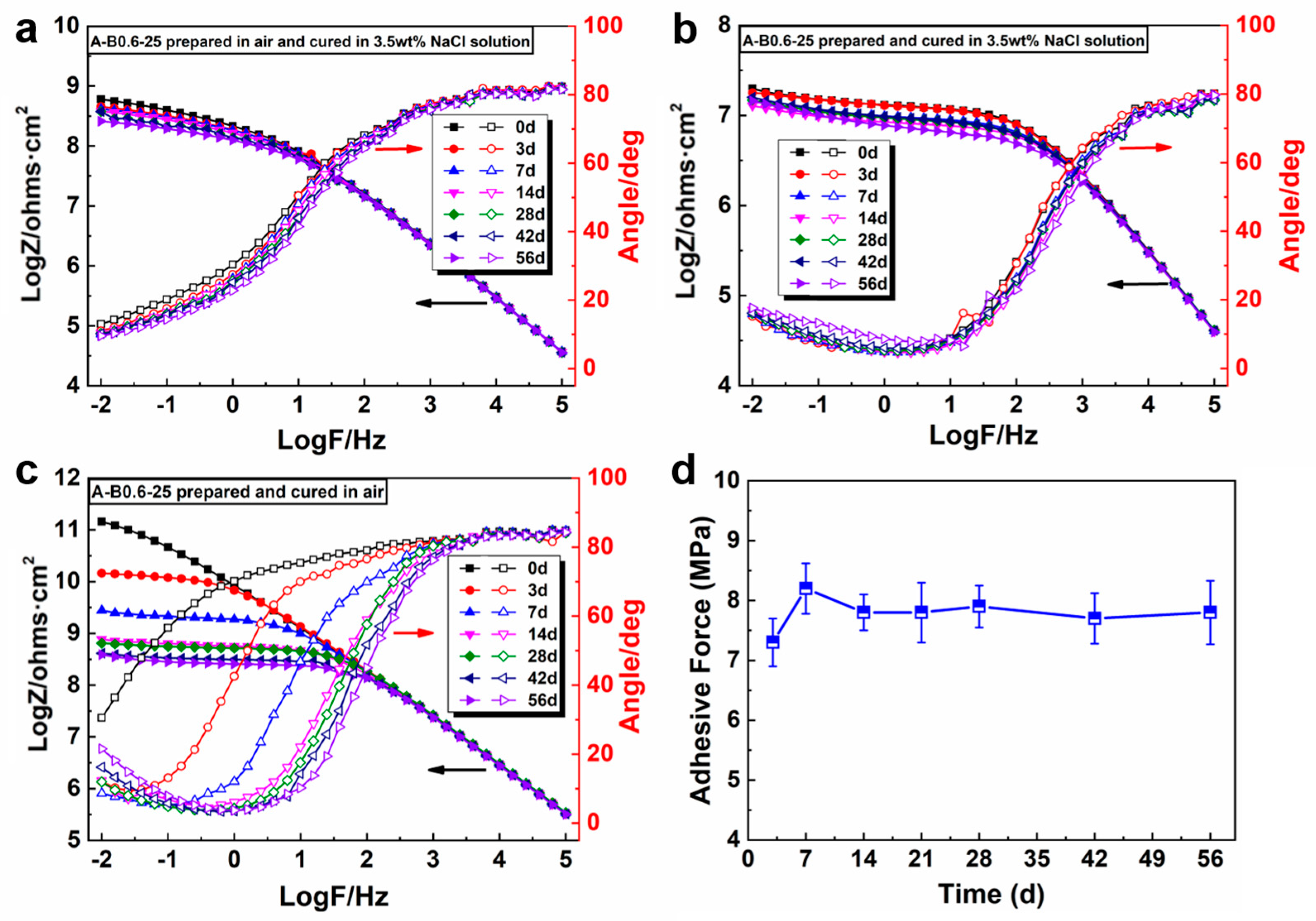
3.4. Curing Behavior of the Underwater In Situ Repairing Coatings in 3.5 wt% NaCl Solution
3.4.1. Theoretical Basis of Measuring Curing Behavior of Thermosetting Resin Using Electrochemical Impedance Spectroscopy (EIS)
3.4.2. EIS Monitoring of the Coatings Cured in 3.5 wt% NaCl Solution
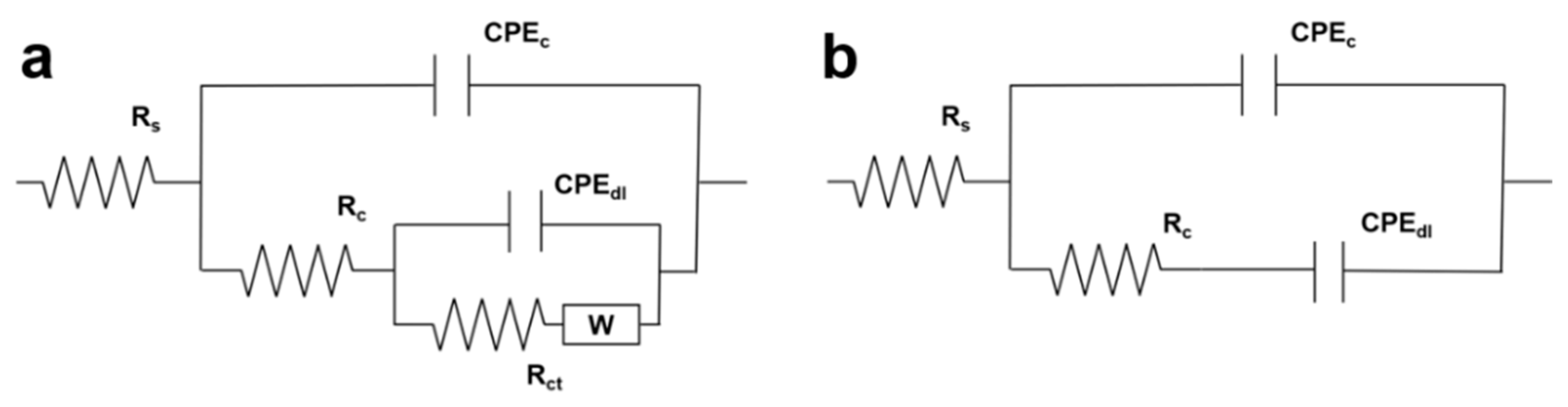
3.4.3. Equivalent Circuit Simulation of Coatings with Different Underwater Curing Times
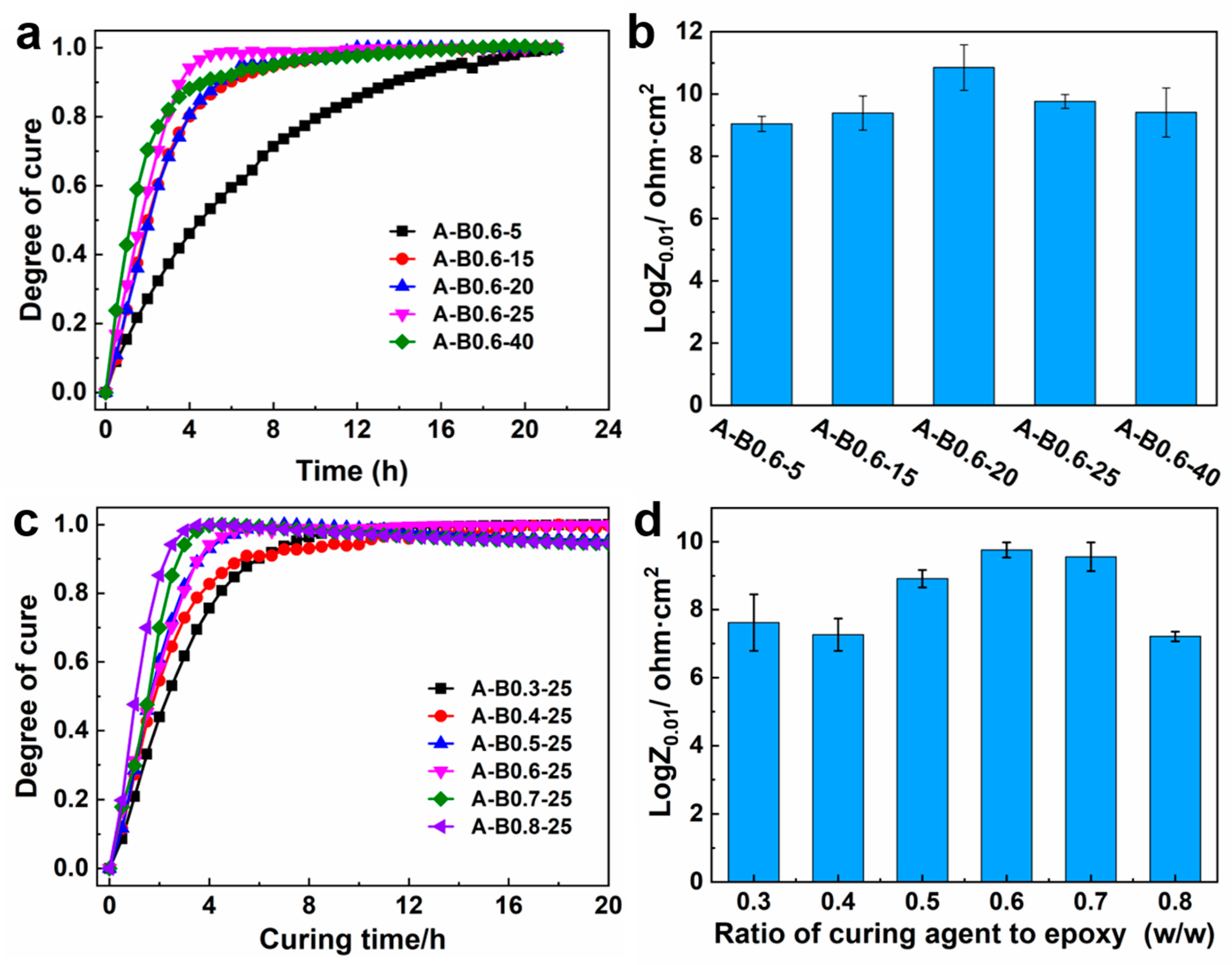
3.4.4. Curing Degree of Different Coating Systems
4. Conclusions
- (1)
- The prepared coatings had prominent adherence adhesion to the substrate whether in air, water, or 3.5 wt% NaCl solution. DMA analysis indicated that the mechanical properties of the coatings were not significantly impacted by the solidification environment. In addition, EIS results showed that the coatings exhibited excellent anticorrosion properties and could be applied to marine environments for long-term corrosion prevention.
- (2)
- Viscosity is a great important physical parameter during the curing period. Since impedance modules are attributed to ion mobility, which is related to the viscosity of the medium, electrochemical impedance spectroscopy (EIS) can be used to measure the curing behavior of a thermosetting resin. A formula, using the impedance modules as the primary variable, was put forward to evaluate the curing degree during the curing process.
- (3)
- The viscosity changes were well reflected by frequency response characteristics from Bode and Nyquist curves by EIS in different curing stages. Two EEC models were chosen to simulate the impedance date at the initial and final curing stage.
- (4)
- The curing degree increased obviously with increasing temperature. However, the dramatically increased viscosity would limit the further reaction of epoxy and amino residues in the system at a higher temperature. The ingredient ratio also had a significant influence on the reaction rate and the system. The optimal temperature and ratio were 20 °C and 0.6, respectively.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, H.; Feng, P.; Lv, Y.; Geng, Z.; Liu, Q.; Liu, X. A comparative study on UV degradation of organic coatings for concrete: Structure, adhesion, and protection performance. Prog. Org. Coat. 2020, 149, 105892. [Google Scholar] [CrossRef]
- Xu, J.; Gao, F.; Wang, H.; Dai, R.; Don, S.; Wang, H. Organic/inorganic hybrid waterborne polyurethane coatings with self-healing properties for anticorrosion application. Prog. Org. Coat. 2023, 174, 107244. [Google Scholar] [CrossRef]
- Bhandari, J.; Khan, F.; Abbassi, R.; Garaniya, V.; Ojeda, R. Modelling of pitting corrosion in marine and offshore steel structures-A technical review. J. Loss Prev. Process Ind. 2015, 37, 39–62. [Google Scholar] [CrossRef]
- Wang, Y.; Wharton, J.A.; Shenoi, R.A. Ultimate strength analysis of aged steel-plated structures exposed to marine corrosion damage: A review. Corros. Sci. 2014, 86, 42–60. [Google Scholar] [CrossRef]
- Narasi, S. Local corrosion chemistry—A review. Corrosion 2017, 73, 18–30. [Google Scholar]
- Yu, Z.; Lim, A.T.; Kollasch, S.L.; Jang, H.D.; Huang, J. Oil-Based Self-Healing Barrier Coatings: To Flow and Not to Flow. Adv. Funct. Mater. 2020, 30, 1906273. [Google Scholar] [CrossRef]
- Wang, L.; Li, S.; Fu, J. Self-healing anti-corrosion coatings based on micron-nano containers with different structural mor-phologies. Prog. Org. Coat. 2023, 175, 107381. [Google Scholar] [CrossRef]
- Pulikkalparambil, H.; Siengchin, S.; Parameswaranpillai, J. Corrosion protective self-healing epoxy resin coatings based on inhibitor and polymeric healing agents encapsulated in organic and inorganic micro and nanocontainers. Nano-Struct. Nano-Objects 2018, 16, 381–395. [Google Scholar] [CrossRef]
- Yin, X.; Zhang, Y.; Lin, P.; Li, Y.; Wang, Z.; Yu, B.; Zhou, F.; Xue, Q. Highly efficient thermogenesis from Fe3O4 nanoparticles for thermoplastic material repair both in air and underwater. J. Mater. Chem. A 2017, 5, 1221–1232. [Google Scholar] [CrossRef]
- Feng, Z.; Wan, R.; Chen, S.; Tang, X.; Ju, H.; Li, Y.; Song, G.L. In-situ repair of marine coatings by a Fe3O4 nanoparticle-modified epoxy resin under seawater. Chem. Eng. J. 2022, 430, 132827. [Google Scholar] [CrossRef]
- Fu, Y.; Guo, N.; Zhou, C.; Wang, G.; Feng, J. Investigation on in-situ laser cladding coating of the 304 stainless steel in water environment. J. Mater. Process. Technol. 2021, 289, 116949. [Google Scholar] [CrossRef]
- Shen, T.; Liang, Z.H.; Yang, H.C.; Li, W. Anti-corrosion coating within a polymer network: Enabling photothermal repairing underwater. Chem. Eng. J. 2021, 412, 128640. [Google Scholar] [CrossRef]
- Fan, H.; Gong, J.P. Bioinspired underwater adhesives. Adv. Mater. 2021, 33, e2102983. [Google Scholar] [CrossRef] [PubMed]
- Israelachvili, J.N. Intermolecular and Surface Forces; Academic Press: Cambridge, MA, USA, 2011. [Google Scholar]
- Croll, S.G. Stress and embrittlement in organic coatings during general weathering exposure: A review. Prog. Org. Coat. 2022, 172, 107085. [Google Scholar] [CrossRef]
- Nguyen, T.; Byrd, W.E.; Alshed, D.; Chin, J.; Clerici, C.; Martin, J. Relationship Between Interfacial Water Layer Adhesion Loss of Silicon/Glass Fiber-Epoxy Systems: A Quantitative Study. J. Adhes. 2007, 83, 587–610. [Google Scholar] [CrossRef]
- Westwood, G.; Horton, T.N.; Wilker, J.J. Simplified polymer mimics of cross-linking adhesive proteins. Macromolecules 2007, 40, 3960–3964. [Google Scholar] [CrossRef]
- Yang, N.; Yuan, R.; You, D.; Zhang, Q.; Wang, J.; Xuan, H.; Ge, L. Gallol-based constant underwater coating adhesives for severe aqueous conditions. Colloids Surf. A 2022, 634, 127948. [Google Scholar] [CrossRef]
- Ye, Y.; Zhang, D.; Liu, T.; Liu, Z.; Liu, W.; Pu, J.; Chen, H.; Zhao, H.; Li, X. Improvement of anticorrosion ability of epoxy matrix in simulate marine environment by filled with superhydrophobic POSS-GO nanosheets. J. Hazard. Mater. 2019, 364, 244–255. [Google Scholar] [CrossRef]
- Brusciotti, F.; Xue, H.; Montemor, M.F.; Lamaka, S.V.; Ferreira, M.G.S. Hybrid epoxy–silane coatings for improved corrosion protection of Mg alloy. Corros. Sci. 2013, 67, 82–90. [Google Scholar] [CrossRef]
- Yea, Y.; Yang, D.; Zhang, D.; Chen, H.; Zhao, H.; Li, X.; Wang, L. POSS-tetraaniline modified graphene for active corrosion protection of epoxy-based organic coating. Chem. Eng. J. 2020, 383, 123160. [Google Scholar] [CrossRef]
- Li, G.; Wu, Y.; Chen, Z.; Chen, M.; Xiao, P.; Li, X.; Zhang, H.; Zhang, P.; Cui, C.; Liu, W.; et al. Biomimetic epoxy adhesive capable of large-scale preparation: From structural underwater bonding to hydrothermal durability. Chem. Eng. J. 2022, 431, 134011. [Google Scholar] [CrossRef]
- Zhou, J.; Wan, Y.; Liu, N.; Yin, H.; Li, B.; Sun, D.; Ran, Q. Epoxy adhesive with high underwater adhesion and stability based on low viscosity modified Mannich bases. J. Appl. Polym. Sci. 2018, 135, 45688. [Google Scholar] [CrossRef]
- Cruz-Cruz, I.; Ramírez-Herrera, C.A.; Martínez-Romero, O.; Castillo-Márquez, S.A.; Jiménez-Cedeño, I.H.; Olvera-Trejo, D.; Elías-Zúñiga, A. Influence of epoxy resin curing kinetics on the mechanical properties of carbon fiber composites. Polymers 2022, 14, 1100. [Google Scholar] [CrossRef]
- Romberg, S.K.; Kotula, A.P. Simultaneous rheology and cure kinetics dictate thermal post-curing of thermoset composite resins for material extrusion. Addit. Manuf. 2023, 71, 103589. [Google Scholar] [CrossRef]
- Luo, S.; Hoang, P.T.; Liu, T. Direct laser writing for creating porous graphitic structures and their use for flexible and highly sensitive sensor and sensor arrays. Carbon 2016, 96, 522–531. [Google Scholar] [CrossRef]
- Rosu, D.; Mustatta, F.; Cascaval, C.N. Investigation of the curing reactions of some multifunctional epoxy resins using differential scanning calorimetry. Thermochim. Acta 2001, 370, 105–110. [Google Scholar] [CrossRef]
- Hardis, R.; Jessop, J.L.P.; Peters, F.E.; Kessler, M.R. Cure kinetics characterization and monitoring of an epoxy resin using DSC, Raman spectroscopy, and DEA. Compos. Part A Appl. Sci. Manuf. 2013, 49, 100–108. [Google Scholar] [CrossRef]
- Yang, T.; Pantawane, M.V.; Jin, Y.; Dahotre, N.B.; Neogi, A. Non-destructive evaluation of bulk material zones and interfaces in powder bed fusion additive manufactured Ti6Al4V. Mater. Sci. Eng. A 2024, 891, 145951. [Google Scholar] [CrossRef]
- Han, Y.; Wang, J.; Zhang, H.; Zhao, S.; Ma, Q.; Wang, Z. Electrochemical impedance spectroscopy (EIS): An efficiency method to monitor resin curing processes. Sens. Actuators A 2016, 250, 78–86. [Google Scholar] [CrossRef]
- Han, Y.; Wang, Z.; Zhao, S.; Wang, J. AC impedance function of electrochemical working station as novel curing degree monitor method: A model curing system of epoxy/anhydride/DMP-30. Measurement 2019, 145, 600–610. [Google Scholar] [CrossRef]
- ASTM D7234-21; Standard Test Method for Pull-Off Adhesion Strength of Coatings on Concrete Using Portable Pull-Off Adhesion Testers. ASTM: West Conshohocken, PA, USA, 2021.
- Liu, M.; Han, S.; Pan, J.; Ren, W. Study on cohesion performance of waterborne epoxy resin emulsified asphalt as interlayer materials. Constr. Build. Mater. 2018, 177, 72–82. [Google Scholar] [CrossRef]
- May, M.; Wang, H.M.; Akid, R. Effects of the addition of inorganic nanoparticles on the adhesive strength of a hybrid sol–gel epoxy system. Int. J. Adhes. Adhes. 2010, 30, 505–512. [Google Scholar] [CrossRef]
- Dai, F.; Chen, F.; Wang, T.; Feng, S.; Hu, C.; Wang, X.; Zheng, Z. Effects of dopamine-containing curing agents on the water resistance of epoxy adhesives. J. Mater. Sci. 2016, 51, 4320–4327. [Google Scholar] [CrossRef]
- Rubino, F.; Nisticò, A.; Tucci, F.; Carlone, P. Marine application of fiber reinforced composites: A review. J. Mar. Sci. Eng. 2020, 8, 26. [Google Scholar] [CrossRef]
- Sarkar, N.; Sahoo, G.; Das, R.; Prusty, G.; Sahu, D.; Swain, S.K. Anticorrosion performance of three-dimensional hierarchical PANI@BN nanohybrids. Ind. Eng. Chem. Res. 2016, 55, 2921–2931. [Google Scholar] [CrossRef]
- Li, J.; Ecco, L.; Fedel, M.; Ermini, V.; Delmas, G.; Pan, J. In-situ AFM and EIS study of a solventborne alkyd coating with nanoclay for corrosion protection of carbon steel. Prog. Org. Coat. 2015, 87, 179–188. [Google Scholar] [CrossRef]
- Feng, Q.K.; Zhong, S.L.; Pei, J.Y.; Zhao, Y.; Zhang, D.L.; Dang, Z.M. Recent progress and future prospects on all-organic polymer dielectrics for energy storage capacitors. Chem. Rev. 2021, 122, 3820–3878. [Google Scholar] [CrossRef]
- Steinhaus, J.; Hausnerova, B.; Haenel, T.; Großgarten, M.; Möginger, B. Curing kinetics of visible light curing dental resin composites investigated by dielectric analysis (DEA). Dent. Mater. 2014, 30, 372–380. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, L.; Wu, H. Hydro/organo/ionogels: “Controllable” electromagnetic wave absorbers. Adv. Mater. 2022, 34, 2205376. [Google Scholar] [CrossRef]
- Jorcin, J.B.; Orazem, M.E.; Pébère, N.; Tribollet, B. CPE analysis by local electrochemical impedance spectroscopy. Electrochim. Acta 2006, 51, 1473–1479. [Google Scholar] [CrossRef]
- Prieto, F.; Alvarez-Malmagro, J.; Rueda, M. Electrochemical Impedance Spectroscopy study of the adsorption of adenine on Au(111) electrodes as a function of the pH. J. Electroanal. Chem. 2017, 793, 209–217. [Google Scholar] [CrossRef]

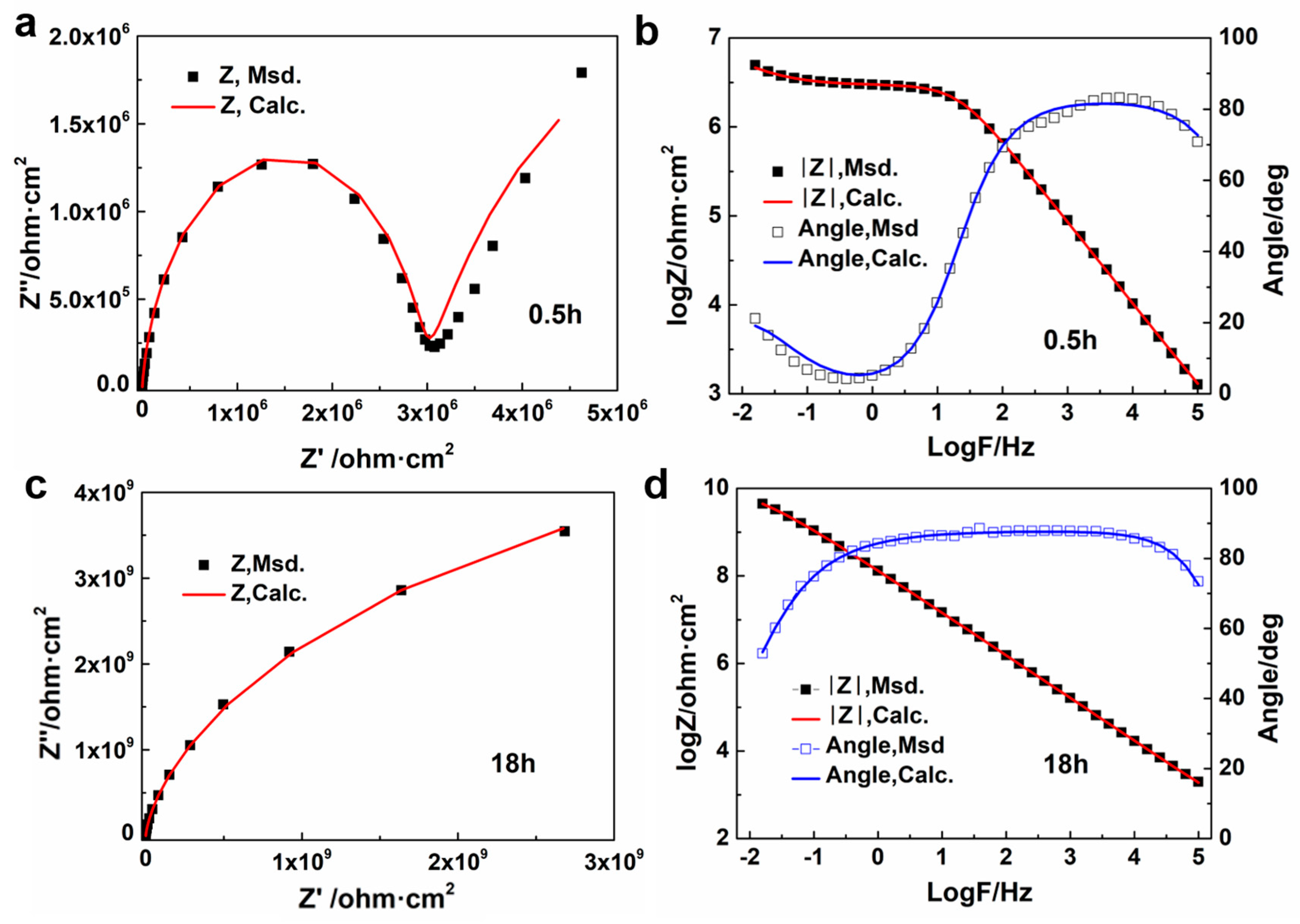
| Parameter | 0.5 h | 2 h | 8 h | 18 h |
|---|---|---|---|---|
| Rs (Ω·cm−2) | 224.5 | 351 | 412.2 | 508.4 |
| CPEc (S·cm−2·sn) * | 3.83 × 10−9 | 1.79 × 10−9 | 6.14 × 10−10 | 2.35 × 10−10 |
| Rc (Ω·cm−2) | 22.98 × 106 | 3.02 × 107 | 1.44 × 109 | 1.43 × 1010 |
| CPEdl (S·cm−2·sn) * | 2.07 × 10−6 | 8.88 × 10−9 | 1.44 × 10−9 | 1.20 × 10−9 |
| Rct (Ω·cm−2) | 6.09 × 106 | 1.16 × 108 | -- | -- |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Y.; Li, J.; Liu, Y.; Wu, W. Construction and Curing Behavior of Underwater In Situ Repairing Coatings for Offshore Structures. Polymers 2024, 16, 306. https://doi.org/10.3390/polym16030306
Xu Y, Li J, Liu Y, Wu W. Construction and Curing Behavior of Underwater In Situ Repairing Coatings for Offshore Structures. Polymers. 2024; 16(3):306. https://doi.org/10.3390/polym16030306
Chicago/Turabian StyleXu, Yao, Jiangbo Li, Yanxia Liu, and Wei Wu. 2024. "Construction and Curing Behavior of Underwater In Situ Repairing Coatings for Offshore Structures" Polymers 16, no. 3: 306. https://doi.org/10.3390/polym16030306
APA StyleXu, Y., Li, J., Liu, Y., & Wu, W. (2024). Construction and Curing Behavior of Underwater In Situ Repairing Coatings for Offshore Structures. Polymers, 16(3), 306. https://doi.org/10.3390/polym16030306







