Improving Crystallization Properties, Thermal Stability, and Mechanical Properties of Poly(L-lactide)-b-poly(ethylene glycol)-b-poly(L-lactide) Bioplastic by Incorporating Cerium Lactate
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Cerium Lactate (Ce-LA)
2.3. Preparation of PLLA-PEG-PLLA/Ce-LA Composites
2.4. Characterization of PLLA-PEG-PLLA/Ce-LA Composites
3. Results and Discussion
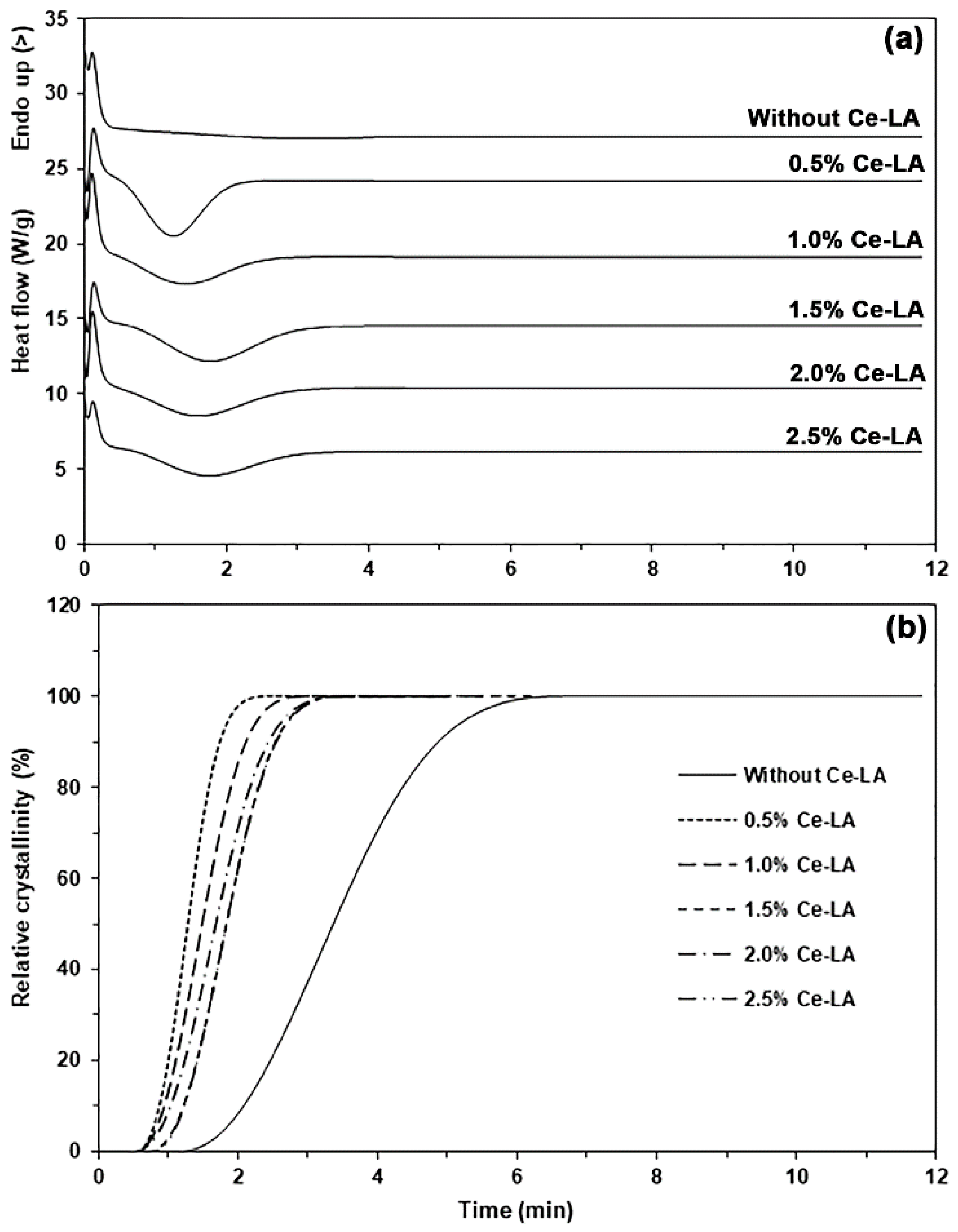
3.1. Thermal Transition Properties
3.2. Crystalline Structures
3.3. Phase Morphology
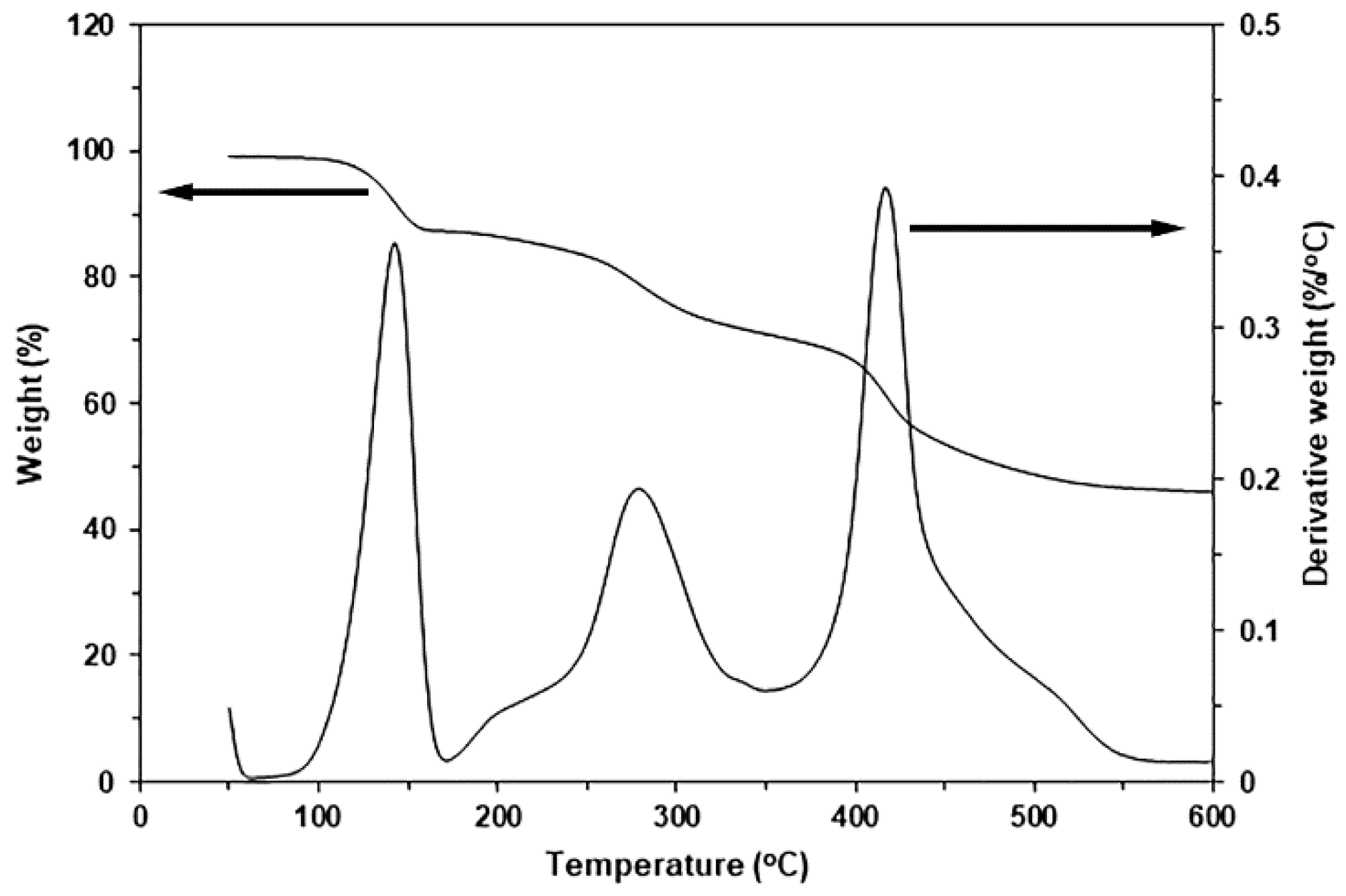
3.4. Thermal Decomposition Characteristics
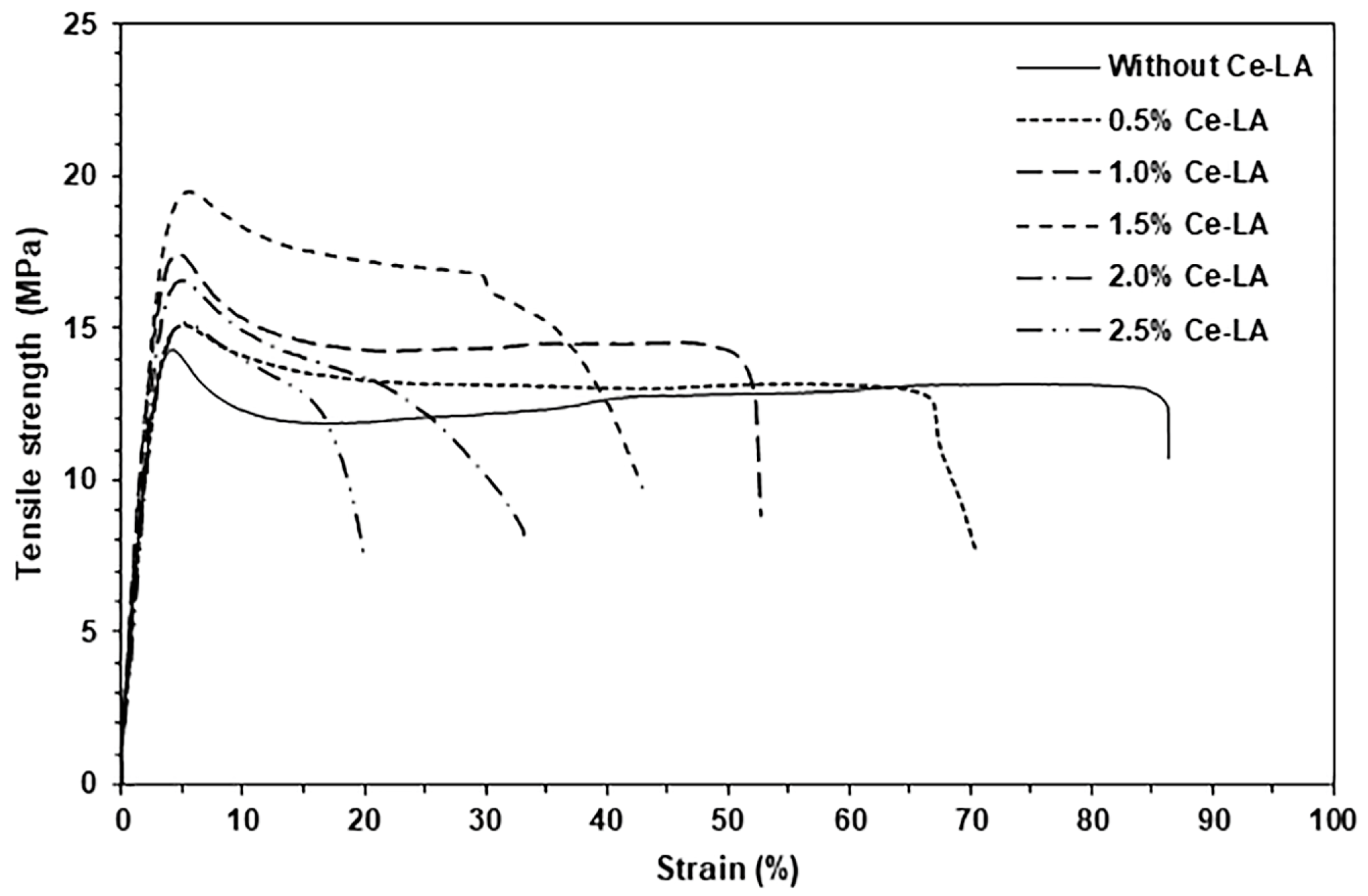
3.5. Tensile Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Tripathi, N.; Misra, M.; Mohanty, A.K. Durable polylactic acid (PLA)-based sustainable engineered blends and biocomposites: Recent developments, challenges, and opportunities. ACS Eng. Au 2021, 1, 7–38. [Google Scholar] [CrossRef]
- Yadav, D.; Srivastava, A.; Yadav, A.; Mehla, B.; Srivastava, M. Development and sustainability of bioplastics: A review. Asian J. Green Chem. 2022, 6, 112–128. [Google Scholar]
- Zhao, X.; Cornish, K.; Vodovotz, Y. Narrowing the gap for bioplastic use in food packaging: An update. Environ. Sci. Technol. 2020, 54, 4712–4732. [Google Scholar] [CrossRef] [PubMed]
- Jariyasakoolroj, P.; Leelaphiwat, P.; Harnkarnsujarit, N. Advances in research and development of bioplastic for food packaging. J. Sci. Food Agric. 2020, 100, 5032–5045. [Google Scholar] [CrossRef] [PubMed]
- Kakadellis, S.; Harris, Z.M. Don’t scrap the waste: The need for broader system boundaries in bioplastic food packaging life cycle assessment—A critical review. J. Clean. Prod. 2020, 274, 122831. [Google Scholar] [CrossRef]
- Rodrigues, P.V.; Cunha, A.B.; Andrade, M.A.; Vilarinho, F.; Machado, A.V.; Castro, M.C.R. Blown film of PLA for packaging with green tea and fish industrial residues: An insight on their properties. Food Packag. Shelf Life 2024, 43, 101283. [Google Scholar] [CrossRef]
- Castro-Aguirre, E.; Iñiguez-Franco, F.; Samsudin, H.; Fang, X.; Auras, R. Poly(lactic acid)—Mass production, processing, industrial applications, and end of life. Adv. Drug Deliv. Rev. 2016, 107, 333–366. [Google Scholar] [CrossRef] [PubMed]
- Yanat, M.; Muthurajan, M.; Strubel, M.; Grolle, K.; Schroen, K. Polylactic acid films reinforced with chitin nanocrystals: Biodegradation and migration behavior. Food Packag. Shelf Life 2023, 40, 101217. [Google Scholar] [CrossRef]
- Petrovics, N.; Kirchkeszner, C.; Patkó, A.; Tábi, T.; Magyar, N.; Székely, I.K.; Szabó, B.S.; Nyiri, Z.; Eke, Z. Effect of crystallinity on the migration of plastic additives from polylactic acid-based food contact plastics. Food Packag. Shelf Life 2023, 36, 101054. [Google Scholar] [CrossRef]
- Vidal, C.P.; Luzi, F.; Puglia, D.; López-Carballo, G.; Rojas, A.; Galotto, M.J.; de Dicastillo, C.L. Development of a sustainable and antibacterial food packaging material based in a biopolymeric multilayer system composed by polylactic acid, chitosan, cellulose nanocrystals and ethyl lauroyl arginate. Food Packag. Shelf Life 2023, 36, 10150. [Google Scholar] [CrossRef]
- Wang, X.; Jang, J.; Su, Y.; Liu, J.; Zhang, H.; He, Z.; Ni, Y. Starting materials, processes and characteristics of bio-based foams: A review. J. Bioresour. Bioprod. 2024, 9, 160–173. [Google Scholar] [CrossRef]
- Tokiwa, Y.; Calabia, B.P. Biodegradability and biodegradation of poly(lactide). Appl. Microbiol. Biotechnol. 2006, 72, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Chotiprayon, P.; Chaisawad, B.; Yoksan, R. Thermoplastic cassava starch/poly(lactic acid) blend reinforced with coir fibres. Int. J. Biol. Macromol. 2020, 156, 960–968. [Google Scholar] [CrossRef] [PubMed]
- Mastalygina, E.E.; Aleksanyan, K.V. Recent approaches to the plasticization of poly(lactic acid) (PLA) (A review). Polymers 2024, 16, 87. [Google Scholar] [CrossRef]
- Gholami, R.; Lawan, I.; Ebrahimi, S.; Pattulee, A.; Ahn, C.-H.; Rimdusit, S. Toughening polylactic acid with ultrafine fully vulcanized powdered natural rubber graft-copolymerized with poly(styrene-co-acrylonitrile): Tailoring the styrene–acrylonitrile ratio for enhanced interfacial interactions. Polymers 2024, 16, 2254. [Google Scholar] [CrossRef]
- Jha, S.; Akula, B.; Enyioma, H.; Novak, M.; Amin, V.; Liang, H. Biodegradable Biobased Polymers: A Review of the State of the Art, Challenges, and Future Directions. Polymers 2024, 16, 2262. [Google Scholar] [CrossRef]
- Yun, X.; Li, X.; Jin, Y.; Sun, W.; Dong, T. Fast crystallization and toughening of poly(L-lactic acid) by incorporating with poly(ethylene glycol) as a middle block chain. Polym. Sci. Ser. A 2018, 60, 141–155. [Google Scholar] [CrossRef]
- Baimark, Y.; Rungseesantivanon, W.; Prakymoramas, N. Synthesis of flexible poly(L-lactide)-b-polyethylene glycol-b-poly(L-lactide) bioplastics by ring-opening polymerization in the presence of chain extender. e-Polymers 2020, 20, 423–429. [Google Scholar] [CrossRef]
- Thongsomboon, W.; Srihanam, P.; Baimark, Y. Preparation of flexible poly(L-lactide)-b-poly(ethylene glycol)-b-poly(L-lactide)/talcum/thermoplastic starch ternary composites for use as heat-resistant and single-use bioplastics. Int. J. Biol. Macromol. 2023, 230, 123172. [Google Scholar] [CrossRef]
- Srihanam, P.; Thongsomboon, W.; Baimark, Y. Phase morphology, mechanical, and thermal properties of calcium carbonate-reinforced poly(L-lactide)-b-poly(ethylene glycol)-b-poly(L-lactide) bioplastics. Polymers 2023, 15, 301. [Google Scholar] [CrossRef]
- Srisuwan, Y.; Srihanam, P.; Rattanasuk, S.; Baimark, Y. Preparation of poly(L-lactide)-b-poly(ethylene glycol)-b-poly(L-lactide)/zinc oxide nanocomposite bioplastics for potential use as flexible and antibacterial food packaging. Polymers 2024, 16, 1660. [Google Scholar] [CrossRef] [PubMed]
- Baimark, Y.; Srihanam, P.; Srisuwan, Y. Thermal, morphological, mechanical, and biodegradation properties of poly(L-lactide)-b-poly(ethylene glycol)-b-poly(L-lactide)/high-density polyethylene blends. Polymers 2024, 16, 2078. [Google Scholar] [CrossRef] [PubMed]
- Srihanam, P.; Pakkethati, K.; Srisuwan, Y.; Phromsopha, T.; Manphae, A.; Phinyocheep, P.; Masayuki Yamaguchi, M.; Baimark, Y. Utilization of bamboo biochar as a multi-functional filler of flexible poly(L-lactide)-b-poly(ethylene glycol)-b-poly(L-lactide) bioplastic. Sci. Rep. 2024, 14, 17601. [Google Scholar] [CrossRef]
- Wu, Y.; Hao, X.; Lin, F.; Wang, S.; Chen, L.; Lin, X.; Gan, D.; Fan, S.; Song, L.; Liu, Y. Developing a cerium lactate antibacterial nucleating agent for multifunctional polylactic acid packaging film. Int. J. Biol. Macromol. 2022, 220, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Cao, Z.Q.; Bao, R.Y.; Xie, B.H.; Yang, M.B.; Yang, W. Poly(L-lactic acid)-polyethylene glycol-poly(L-lactic acid) triblock copolymer: A novel macromolecular plasticizer to enhance the crystallization of poly(L-lactic acid). Eur. Polym. J. 2017, 97, 272–281. [Google Scholar] [CrossRef]
- Saeidlou, S.; Huneault, M.A.; Li, H.; Park, C.B. Poly(lactic acid) crystallization. Prog. Polym. Sci. 2012, 37, 1657–1677. [Google Scholar] [CrossRef]
- Shi, X.; Zhang, G.; Phuong, T.V.; Lazzeri, A. Synergistic effects of nucleating agents and plasticizers on the crystallization behavior of poly(lactic acid). Molecules 2015, 20, 1579–1593. [Google Scholar] [CrossRef]
- Tang, Z.; Fan, F.; Chu, Z.; Fan, C.; Qin, Y. Barrier properties and characterizations of poly(lactic acid)/ZnO nanocomposites. Molecules 2020, 25, 1310. [Google Scholar] [CrossRef] [PubMed]
- Chu, Z.; Zhao, T.; Li, L.; Fan, J.; Qin, Y. Characterization of antimicrobial poly(lactic acid)/nano-composite films with silver and zinc oxide nanoparticles. Materials 2017, 10, 659. [Google Scholar] [CrossRef]
- Li, H.; Huneault, M.A. Effect of nucleation and plasticization on the crystallization of poly(lactic acid). Polymer 2007, 48, 6855–6866. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Y.N.; Huang, Z.G.; Weng, Y.X. Heat resistance, crystallization behavior, and mechanical properties of polylactide/nucleating agent composites. Mater. Des. 2015, 66, 7–15. [Google Scholar] [CrossRef]
- Chen, P.; Yu, K.; Wang, Y.; Wang, W.; Zhou, H.; Li, H.; Mi, J.; Wang, X. The effect of composite nucleating agent on the crystallization behavior of branched poly(lactic acid). J. Polym. Environ. 2018, 26, 3718–3730. [Google Scholar] [CrossRef]
- Li, Y.; Han, C. Isothermal and nonisothermal cold crystallization behaviors of asymmetric poly(L-lactide)/poly(D-lactide) blends. Ind. Eng. Chem. Res. 2012, 51, 15927–15935. [Google Scholar] [CrossRef]
- Jalali, A.; Huneault, M.A.; Elkoun, S. Effect of thermal history on nucleation and crystallization of poly(lactic acid). J. Mater. Sci. 2016, 51, 7768–7779. [Google Scholar] [CrossRef]
- Gao, P.; Alanazi, S.; Masato, D. Crystallization of polylactic acid with organic nucleating agents under quiescent conditions. Polymers 2024, 16, 320. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.D.; Kusmono, K.; Wildan, M.W.; Herianto, H. Preparation and properties of cellulose nanocrystals-reinforced Poly (lactic acid) composite filaments for 3D printing applications. Results Eng. 2023, 17, 100842. [Google Scholar] [CrossRef]
- Younus, M.M.; Naguib, H.M.; Fekry, M.; Elsawy, M.A. Pushing the limits of PLA by exploring the power of MWCNTs in enhancing thermal, mechanical properties, and weathering resistance. Sci. Rep. 2023, 13, 16588. [Google Scholar] [CrossRef]
- Echeverría, C.; Limón, I.; Muñoz-Bonilla, A.; Fernández-García, M.; López, D. Development of highly crystalline polylactic acid with β-crystalline phase from the induced alignment of electrospun fibers. Polymers 2021, 13, 2860. [Google Scholar] [CrossRef]
- Zarei-Hanzaki, A.; Abedi, H.R.; Tayebi, L.; Mostafavi, E. Polylactic acid piezo-biopolymers: Chemistry, structural evolution, fabrication methods, and tissue engineering applications. J. Funct. Biomater. 2021, 12, 71. [Google Scholar] [CrossRef]
- Zhang, H.; Hortal, M.; Jordá-Beneyto, M.; Rosa, E.; Lara-Lledo, M.; Lorente, I. ZnO-PLA nanocomposite coated paper for anti-microbial packaging application. LWT Food Sci. Technol. 2017, 78, 250–257. [Google Scholar] [CrossRef]
- Akshaykranth, A.; Jayarambabu, N.; Venkatappa Rao, T.; Rakesh Kumar, R. Influence of MgO and ZnO nanofillers on morphology, structural, thermal and mechanical properties of polylactic acid films. Bull. Mater. Sci. 2022, 45, 247. [Google Scholar] [CrossRef]
- Bindhua, B.; Renishaa, R.; Robertsb, L.; Varghese, T.O. Boron Nitride reinforced polylactic acid composites film for packaging: Preparation and properties. Polym. Test. 2018, 66, 172–177. [Google Scholar] [CrossRef]
- Salimi, A.; Ahmadi, S.; Faramarzi, M.; Faghihi, J. Reactive blending of polylactic acid/polyethylene glycol toward biodegradable film. Macromol. Res. 2023, 31, 873–881. [Google Scholar] [CrossRef]
- Pakkethati, K.; Srihanam, P.; Manphae, A.; Rungseesantivanon, W.; Prakymoramas, N.; Lan, P.N.; Baimark, Y. Improvement in crystallization, thermal, and mechanical properties of flexible poly(L-lactide)-b-poly(ethylene glycol)-b-poly(L-lactide) bioplastic with zinc phenylphosphate. Polymers 2024, 16, 975. [Google Scholar] [CrossRef]
- Abbas, M.; Buntinx, M.; Deferme, W.; Peeters, R. (Bio)polymer/ZnO nanocomposites for packaging applications: A Review of gas barrier and mechanical properties. Nanomaterials 2019, 9, 1494. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, A.K.; Mohanty, S.; Nayak, S.K. Dynamic mechanical and thermal properties of polylactide-layered silicate nano-composites. J. Thermoplast. Compos. Mater. 2014, 27, 699–716. [Google Scholar] [CrossRef]
- Panicker, A.M.; Rajesh, K.A.; Varghese, T.O. Mixed morphology nanocrystalline cellulose from sugarcane bagasse fibers/poly(lactic acid) nanocomposite films: Synthesis, fabrication and characterization. Iran. Polym. J. 2017, 26, 125–136. [Google Scholar] [CrossRef]
- Díez-Pascual, A.M.; Díez-Vicente, A.L. Poly(3-hydroxybutyrate)/ZnO bionanocomposites with improved mechanical, barrier and antibacterial properties. Int. J. Mol. Sci. 2014, 15, 10950–10973. [Google Scholar] [CrossRef]

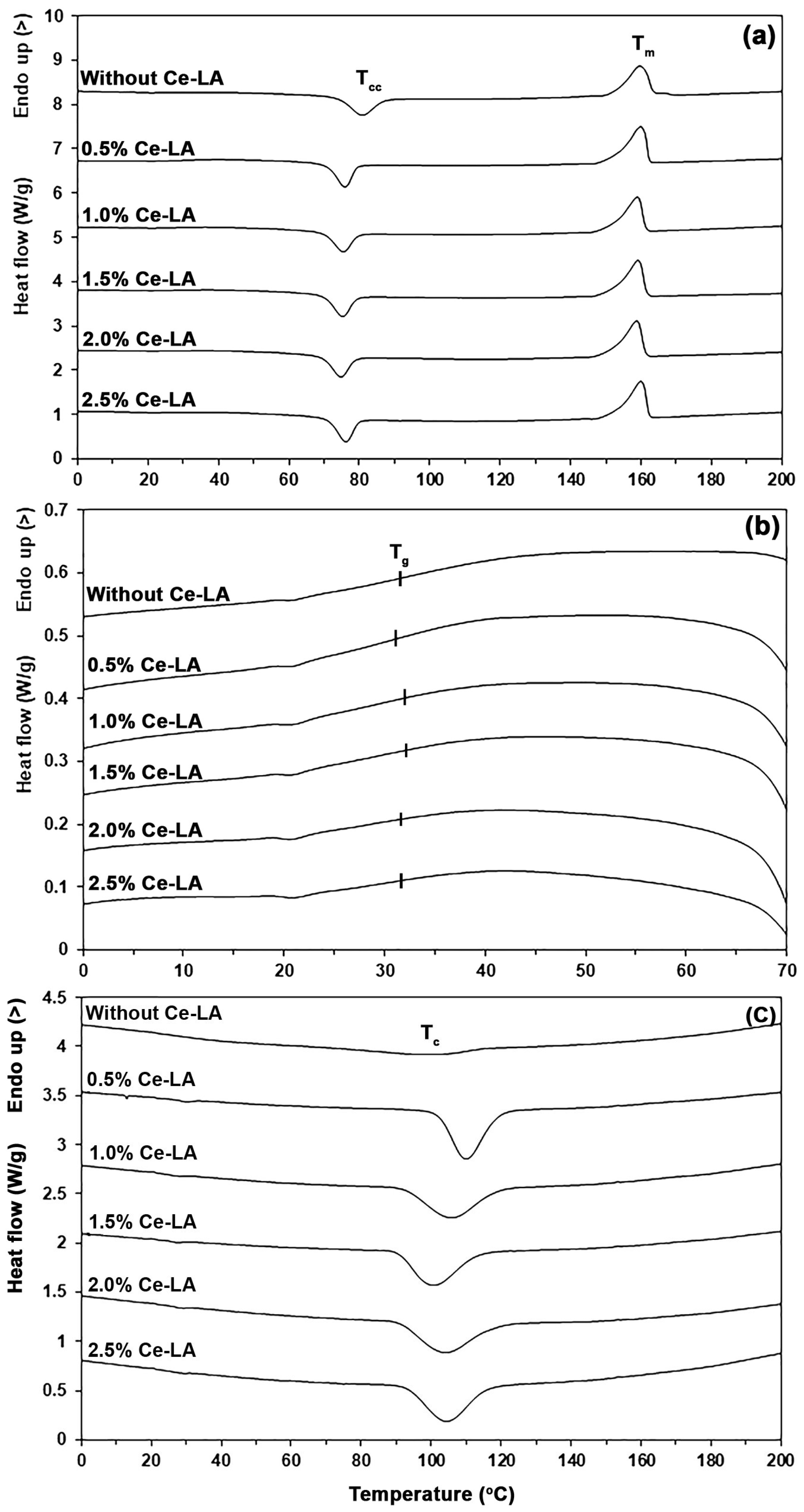



| Ce-LA Content (wt%) | Tg (°C) a | Tcc (°C) a | ΔHcc (J/g) a | Tm (°C) a | ΔHm (J/g) a | Xc (%) a | Tc (°C) b |
|---|---|---|---|---|---|---|---|
| - | 32 | 81 | 20.3 | 159 | 31.3 | 11.8 | 99 |
| 0.5 | 31 | 75 | 17.6 | 160 | 32.4 | 15.9 | 110 |
| 1.0 | 32 | 75 | 16.0 | 159 | 29.7 | 14.8 | 106 |
| 1.5 | 32 | 75 | 17.4 | 159 | 30.6 | 14.3 | 102 |
| 2.0 | 31 | 75 | 17.8 | 159 | 30.6 | 14.1 | 103 |
| 2.5 | 31 | 76 | 17.6 | 160 | 30.1 | 13.7 | 103 |
| Ce-LA Content (wt%) | t1/2 (min) | n | k (min−1) | R2 |
|---|---|---|---|---|
| - | 3.4 | 3.5916 | 0.0086 | 0.9962 |
| 0.5 | 1.3 | 3.3191 | 0.3590 | 0.9999 |
| 1.0 | 1.5 | 3.4340 | 0.1786 | 0.9997 |
| 1.5 | 1.8 | 3.5364 | 0.0862 | 0.9999 |
| 2.0 | 1.7 | 3.4335 | 0.1152 | 0.9988 |
| 2.5 | 1.8 | 3.4388 | 0.0919 | 0.9999 |
| Ce-LA Content (wt%) | Residue Weight at 600 °C (%) a | PLLA-Td,max (°C) b | PEG-Td,max (°C) b |
|---|---|---|---|
| - | 0.39 | 310 | 418 |
| 0.5 | 0.75 | 316 | 417 |
| 1 | 1.06 | 319 | 419 |
| 1.5 | 1.47 | 327 | 417 |
| 2 | 1.88 | 313 | 418 |
| 2.5 | 2.12 | 311 | 417 |
| Ce-LA Content (wt%) | Ultimate Tensile Strength (MPa) | Strain at Break (%) | Young’s Modulus (MPa) |
|---|---|---|---|
| - | 14.3 ± 0.6 | 86.3 ± 2.4 | 204 ± 18 |
| 0.5 | 15.1 ± 0.4 | 70.5 ± 5.1 | 230 ± 20 |
| 1 | 17.4 ± 0.6 | 52.6 ± 6.5 | 258 ± 15 |
| 1.5 | 19.5 ± 0.5 | 42.9 ± 5.2 | 312 ± 21 |
| 2 | 16.6 ± 0.3 | 33.2 ± 4.7 | 262 ± 24 |
| 2.5 | 15.2 ± 0.4 | 19.9 ± 6.8 | 254 ± 14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chuangchai, A.; Baimark, Y. Improving Crystallization Properties, Thermal Stability, and Mechanical Properties of Poly(L-lactide)-b-poly(ethylene glycol)-b-poly(L-lactide) Bioplastic by Incorporating Cerium Lactate. Polymers 2024, 16, 3367. https://doi.org/10.3390/polym16233367
Chuangchai A, Baimark Y. Improving Crystallization Properties, Thermal Stability, and Mechanical Properties of Poly(L-lactide)-b-poly(ethylene glycol)-b-poly(L-lactide) Bioplastic by Incorporating Cerium Lactate. Polymers. 2024; 16(23):3367. https://doi.org/10.3390/polym16233367
Chicago/Turabian StyleChuangchai, Arriya, and Yodthong Baimark. 2024. "Improving Crystallization Properties, Thermal Stability, and Mechanical Properties of Poly(L-lactide)-b-poly(ethylene glycol)-b-poly(L-lactide) Bioplastic by Incorporating Cerium Lactate" Polymers 16, no. 23: 3367. https://doi.org/10.3390/polym16233367
APA StyleChuangchai, A., & Baimark, Y. (2024). Improving Crystallization Properties, Thermal Stability, and Mechanical Properties of Poly(L-lactide)-b-poly(ethylene glycol)-b-poly(L-lactide) Bioplastic by Incorporating Cerium Lactate. Polymers, 16(23), 3367. https://doi.org/10.3390/polym16233367






