Thermal Condensation of Dehydrogenation Polymer (DHP) with Xylose
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
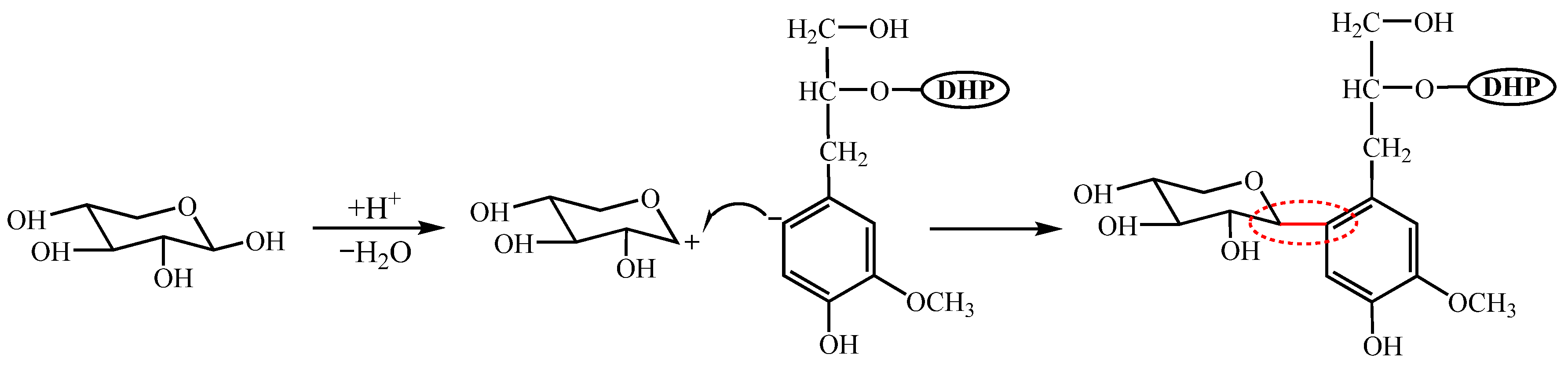
2.2. Preparation of DHP-Xylose Complex
2.3. Structural Characterization of DHP-Xylose Complex
3. Results and Discussions
3.1. FT-IR Analysis of DHP-Xylose Complex
3.2. 13C-NMR Analysis of DHP-Xylose Complex
3.3. Two-Dimensional-HSQC NMR Analysis of DHP-Xylose Complex
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Shen, R.; Hu, R.; Chen, Z. Discussions on the Developments of Aldehyde-Free Adhesive Technology and Aldehyde-Free Wood-based Panels in China. Mod. Chem. Res. 2022, 17, 6–9. [Google Scholar] [CrossRef]
- Shi, Y.; Cui, H. Patent Analysis of Formaldehyde-free Adhesive for Wood-based Panels. Stand. Sci. 2022, S1, 21–26. [Google Scholar] [CrossRef]
- Vázquez, G.; González, J.; Freire, S.; Antorrena, G. Effect of chemical modification of lignin on the gluebond performance of lignin-phenolic resins. Bioresour. Technol. 1997, 60, 191–198. [Google Scholar] [CrossRef]
- Khan, M.A.; Ashraf, S.M.; Malhotra, V.P. Development and characterization of a wood adhesive using bagasse lignin. Int. J. Adhes. Adhes. 2004, 24, 485–493. [Google Scholar] [CrossRef]
- Alonso, M.; Oliet, M.; Rodríguez, F.; Astarloa, G.; Echeverría, J. Use of a methylolated softwood ammonium lignosulfonate as partial substitute of phenol in resol resins manufacture. J. Appl. Polym. Sci. 2004, 94, 643–650. [Google Scholar] [CrossRef]
- Mu, Y.; Wang, C.; Zhao, L.; Chu, F. Study on Composite Adhesive of Hydroxymethylated Lignosulfonate/Phenol-formaldehyde Resin with Low Free Formaldehyde. Chem. Ind. For. Prod. 2009, 29, 38–42. [Google Scholar] [CrossRef]
- Mu, Y.; Wang, C.; Zhao, L.; Chun, F. Study on composite adhesive of alkali lignin/phenol formaldehyde resin for E0 grade plywood. In Proceedings of the Biorefining Technology Exchange and Industrialization Seminar and the 3rd National Chemical Application Technology Development Hot Spot Seminar, Xiamen, China, 12 November 2008; Volume 28, pp. 221–224. [Google Scholar]
- Alonso, M.V.; Oliet, M.; Rodrıguez, F.; Garcıa, J.; Gilarranz, M.; Rodrıguez, J. Modification of ammonium lignosulfonate by phenolation for use in phenolic resins. Bioresour. Technol. 2005, 96, 1013–1018. [Google Scholar] [CrossRef]
- Liu, G.; Qiu, X.; Xing, D. Phenolation Modification of Wheat Straw Soda Lignin and its Utilization in Preparation of Lignin-Based Phenolic Formaldehyde Resins Adhesive. J. Chem. Eng. Chin. Univ. 2007, 21, 678–684. [Google Scholar] [CrossRef]
- Khan, M.; Ashraf, S. Studies on thermal characterization of lignin: Substituted phenol formaldehyde resin as wood adhesives. J. Therm. Anal. Calorim. 2007, 89, 993–1000. [Google Scholar] [CrossRef]
- An, X. Application of Demethylated Lignosulfonate as a Substitute for Phenol in Wood Adhesives. Chem. Ind. For. Prod. 1995, 3, 36–42. [Google Scholar]
- Guo, T.; Chen, K.; Yang, S.; Li, S. Isolation of Lignin from Masson pine Kraft pulping black liqour and Preparation of Lignin-based Adhesives. China For. Prod. Ind. 1999, 26, 25–28. [Google Scholar] [CrossRef]
- Hüttermann, A.; Milstein, O.; Nicklas, B.; Trojanowski, J.; Haars, A.; Kharazipour, A. Enzymatic Modification of Lignin for Technical Use: Strategies and Results. ACS Symp. Ser. 1989, 27, 361–370. [Google Scholar]
- Haars, A.; Kharazipour, A.; Zanker, H.; Huttermann, A. Room-Temperature Curing Adhesives Based on Lignin and Phenoloxidases. ACS Symp. Ser. 1989, 385, 126–134. [Google Scholar]
- Cao, Y.; Duan, X.; Cao, Y.; Lu, J. Effect of Laccase Treatment Conditions of Lignosulphonate on Shearing Strength of Plywood. China For. Prod. Ind. 2007, 34, 13–17. [Google Scholar] [CrossRef]
- Yamaguchi, H.; Maeda, Y.; Sakata, I. Bonding among woody fibers by use of enzymatic phenol dehydrogenative polymerization. Mechanism of generation of bonding strength. Mokuzai Gakkaishi 1994, 40, 185–190. [Google Scholar]
- Yamaguchi, H.; Maeda, Y.; Sakata, I. Applications of phenol dehydrogenative polymerization by laccase to bonding among woody-fibers. Mokuzai Gakkaishi 1992, 38, 931–937. [Google Scholar]
- Yamaguchi, H.; Nagamori, N.; Sakata, I. Application of phenol dehydrogenative polymerization of vanillic acid to bonding of woody fibers. Mokuzai Gakkaishi 1993, 37, 220–226. [Google Scholar]
- Lund, M.; Felby, C. Wet strength improvement of unbleached kraft pulp through laccase catalyzed oxidation. Enzym. Microb. Technol. 2001, 28, 760–765. [Google Scholar] [CrossRef]
- Chandra, R.P.; Ragauskas, A.J. Evaluating laccase-facilitated coupling of phenolic acids to high-yield kraft pulps. Enzym. Microb. Technol. 2002, 30, 855–861. [Google Scholar] [CrossRef]
- Chandra, R.P.; Lehtonen, L.K.; Ragauskas, A.J. Modification of high lignin content kraft pulps with laccase to improve paper strength properties. 1. Laccase treatment in the presence of gallic acid. Biotechnol. Prog. 2004, 20, 255–261. [Google Scholar] [CrossRef]
- Chandra, R.P.; Felby, C.; Ragauskas, A.J. Improving laccase-facilitated grafting of 4-hydroxybenzoic acid to high-kappa kraft pulps. J. Wood Chem. Technol. 2005, 24, 69–81. [Google Scholar] [CrossRef]
- Aracri, E.; Roncero, M.B.; Vidal, T. Studying the effects of laccase-catalysed grafting of ferulic acid on sisal pulp fibers. Bioresour. Technol. 2011, 102, 7555–7560. [Google Scholar] [CrossRef]
- Zhang, X.; Pei, J.; Zhang, F.; Yu, X.; Hu, H. Modification of OCC Pulp by Laccase with the Phenols Contained in APMP Waste Liquor. Trans. China Pulp Pap. 2011, 26, 16–20. [Google Scholar]
- Bolker, H.I. A lignin carbohydrate bond as revealed by infra-red spectroscopy. Nature 1963, 197, 489–490. [Google Scholar] [CrossRef]
- Wang, X. Study on Thermal Condensation Reaction Mechanism of Dehydrogenation Polymer Catalyzed with Acid. Master’s Thesis, Hubei University of Technology, Wuhan, China, 2022. [Google Scholar]
- Terashima, N.; Ralph, S.A.; Landucci, L.L. New facile syntheses of monolignol glucosides; p-glucocoumaryl alcohol, coniferin and syringin. Holzforschung 1996, 50, 151–155. [Google Scholar]
- Yao, L.; Yang, H.; Yoo, C.G.; Chen, C.; Meng, X.; Dai, J.; Yang, C.; Yu, J.; Ragauskas, A.J.; Chen, X. A mechanistic study of cellulase adsorption onto lignin. Green Chem. 2021, 23, 333–339. [Google Scholar] [CrossRef]
- Zhang, M.; Yang, H.; Yao, L.; Xie, Y. Study on the Chemical Bond Connection between Lignin Dehydrogenation Polymer and Xylose. HuBei ZaoZhi 2012, 3, 41–45. [Google Scholar]
- Wen, J.L.; Sun, S.L.; Xue, B.L.; Sun, R.C. Recent Advances in Characterization of Lignin Polymer by Solution-State Nuclear Magnetic Resonance (NMR) Methodology. Materials 2013, 6, 359–391. [Google Scholar] [CrossRef]
- Holmgren, A.; Zhang, L.; Henriksson, G. Monolignol dehydrogenative polymerization in vitro in the presence of dioxane and a methylated β-β′ dimer model compound. Holzforschung 2008, 62, 508–513. [Google Scholar] [CrossRef]
- Richel, A.; Nicks, F.; Laurent, P.; Wathelet, B.; Wathelet, J.-P.; Paquot, M. Efficient microwave-promoted synthesis of glucuronic and galacturonic acid derivatives using sulfuric acid impregnated on silica. Green Chem. Lett. Rev. 2012, 5, 179–186. [Google Scholar] [CrossRef]
- Nicola, G.; Martin, L. Structural Insights on Recalcitrance during Hydrothermal Hemicellulose Extraction from Wood. ACS Sustain. Chem. Eng. 2017, 5, 5156–5165. [Google Scholar]
- Matsushita, Y.; Kakehi, A.; Miyawaki, S.; Yasuda, S. Formation and chemical structures of acid-soluble lignin II: Reaction of aromatic nuclei model compounds with xylan in the presence of a counterpart for condensation, and behavior of lignin model compounds with guaiacyl and syringyl nuclei in 72% sulfuric acid. J. Wood Sci. 2004, 50, 136–141. [Google Scholar] [CrossRef]
- Yasuda, S.; Murase, N. Chemical Structures of Sulfuric Acid Lignin. Part XII. Reaction of Lignin Models with Carbohydrates in 72% H2SO4. Holzforschung 1995, 49, 418–422. [Google Scholar] [CrossRef]
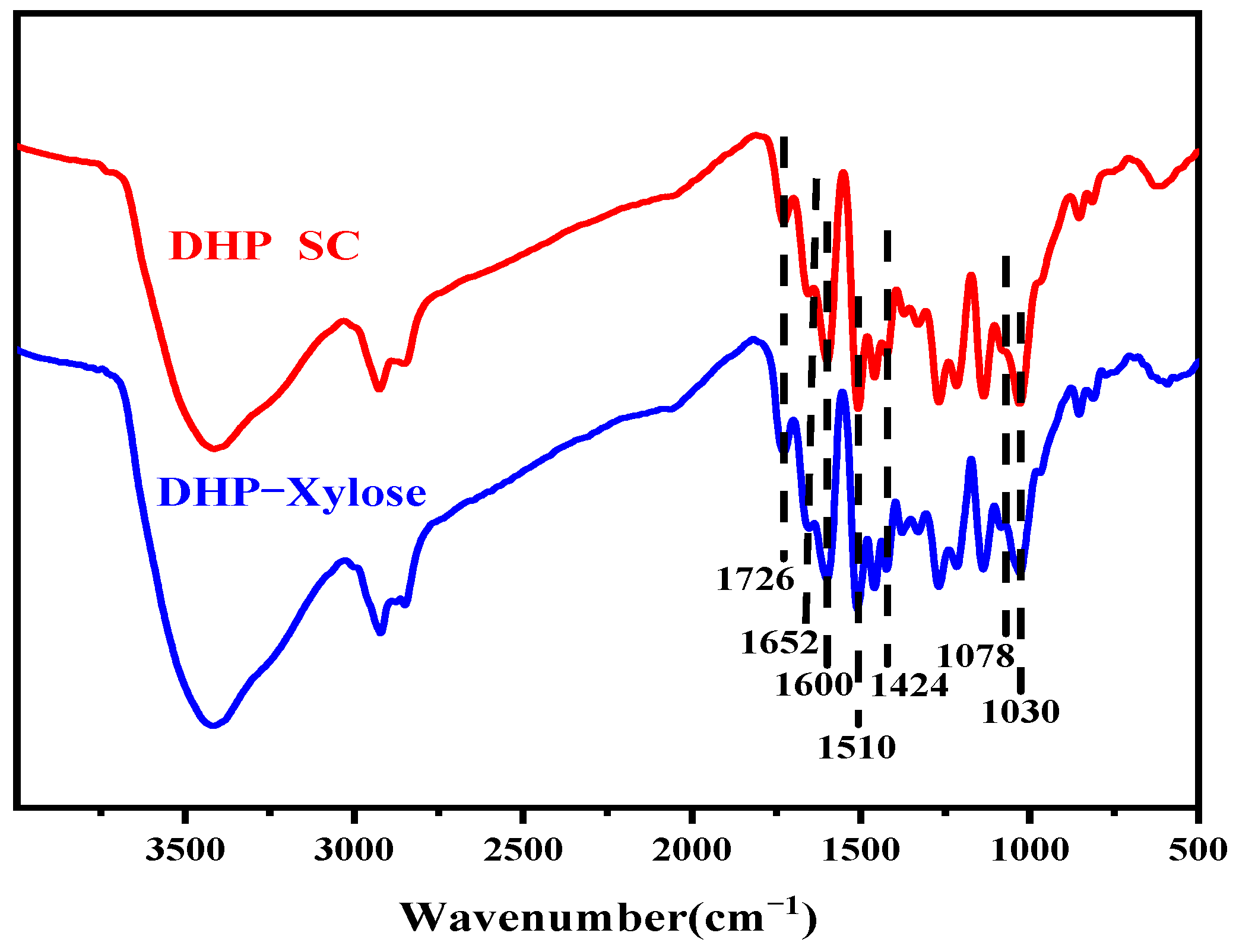
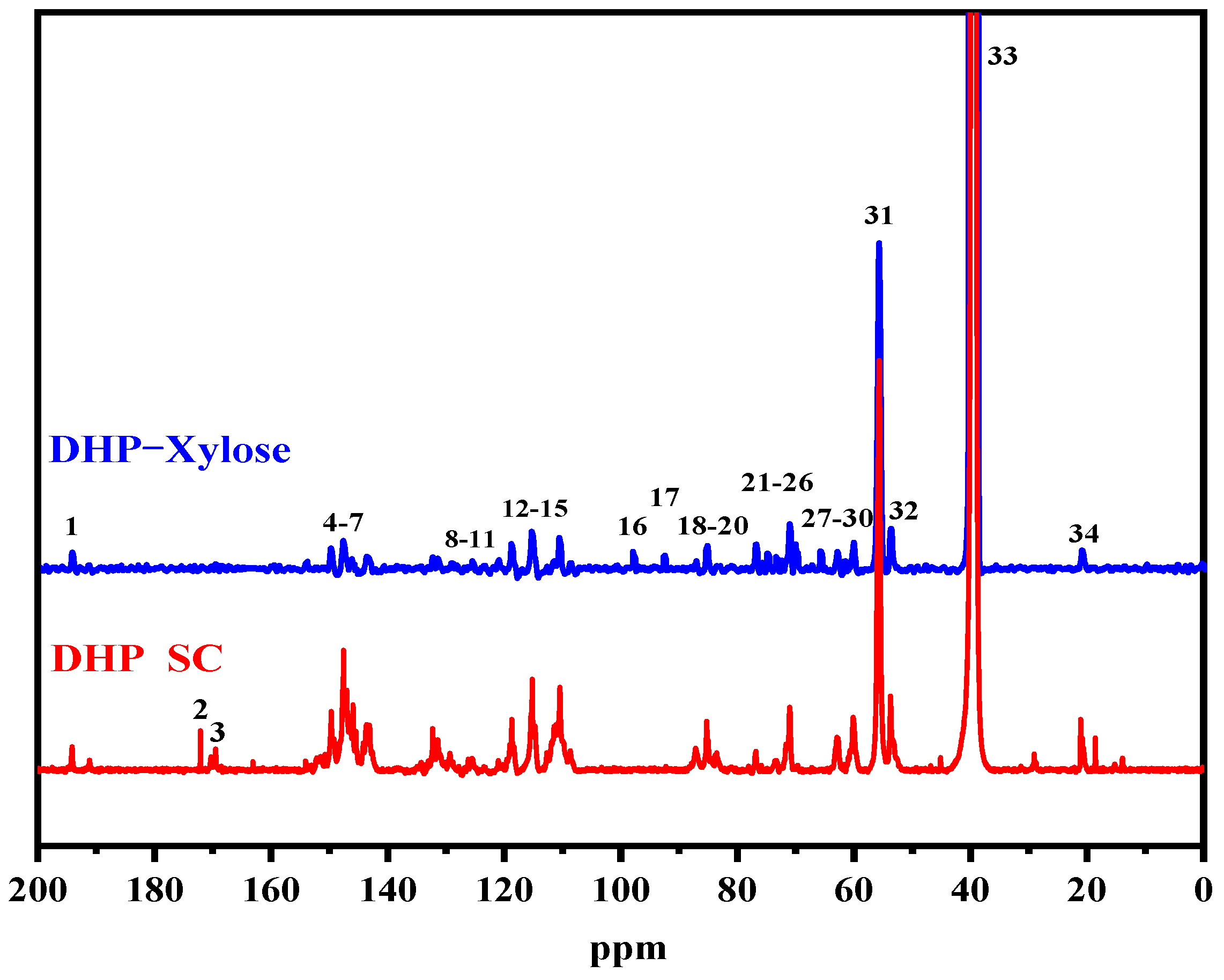
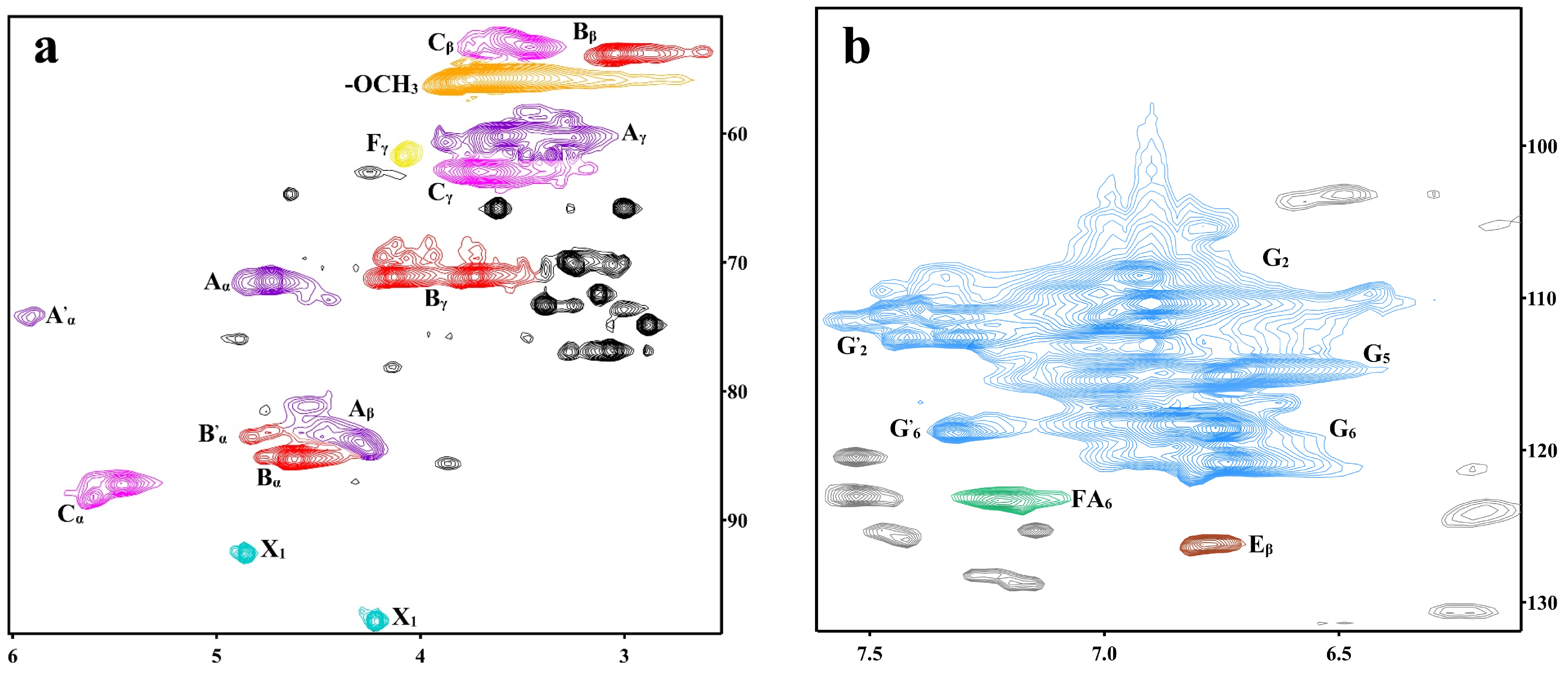
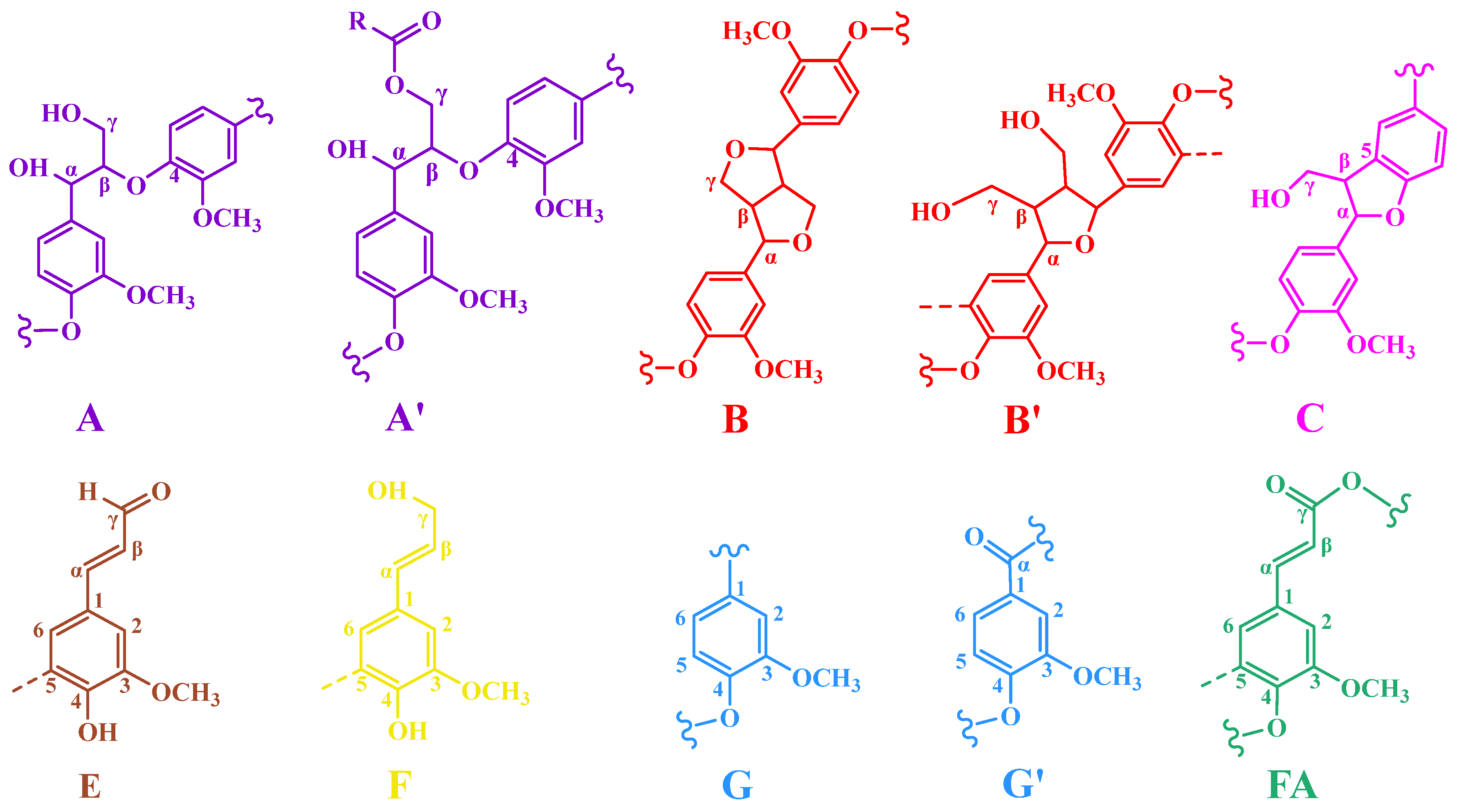
| Signal | Chemical Shifts (δ, ppm) | Assignments | |
|---|---|---|---|
| DHP SC | DHP-Xylose | ||
| 1 | 194.1 | 194.5 | γ-CHO in cinnamaldehyde |
| 2 | 172.1 | — | Cγ in Ferulic acid |
| 3 | 169.5 | — | -COO- in Ferulic acid ester |
| 4 | 150.6 | 150.1 | C3/C4 in guaiacyl |
| 5 | 147.5 | 148.1 | C3/C5 in guaiacyl, etherified |
| 6 | 145.9 | 146.6 | C4/C4′ in 5-5′, etherified |
| 7 | 143.6 | 144 | C4 in 5-5 |
| 8 | 132.2 | 132.7 | C1 in β-O-4 guaiacyl, non-etherified |
| 9 | 131.3 | 131.9 | C1/C1′ in β-5 |
| 10 | 129.3 | 129.4 | Cα in cinnamaldehyde |
| 11 | 128.7 | 128.6 | Cβ in cinnamaldehyde |
| 12 | 119.5 | 119.3 | C6 in guaiacyl |
| 13 | 118.7 | 118.6 | C5 in guaiacyl |
| 14 | 115.3 | 115.8 | C5 in guaiacyl |
| 15 | 110.4 | 110.9 | C2 in guaiacyl |
| 16 | — | 98.3 | C1 in β-D-Xylose |
| 17 | — | 93 | C1 in α-D-Xylose |
| 18 | 87.1 | 87.5 | Cα in β-5 |
| 19 | 85 | 85.5 | Cα(β-β), Cβ(β-O-4) |
| 20 | 83.8 | 83.5 | Cβ in β-O-4 |
| 21 | 76.9 | 77.3 | Cα in β-O-4 |
| 22 | — | 75.3 | C3 in Xylose |
| 23 | — | 73.7 | C2 in β-D-Xylose |
| 24 | — | 72.9 | C2 in α-D-Xylose |
| 25 | 71 | 71.5 | Cγ in β-β |
| 26 | — | 70.1 | C4 in Xylose |
| 27 | — | 66.1 | C5 in Xylose |
| 28 | 62.9 | 63.2 | Cγ in β-5 |
| 29 | — | 62.1 | Cγ in cinnamylalcohol |
| 30 | 60.1 | 60.4 | Cγ in β-O-4 |
| 31 | 55.6 | 56.2 | -OCH3 |
| 32 | 53.7 | 54 | Cβ in β-5 |
| 33 | 39.4 | 40 | DMSO |
| 34 | 21 | 21.3 | -CH3 in acetyl group |
| Label | Chemical Shift | Assignments |
|---|---|---|
| δC/δH (ppm) | ||
| Cβ | 53.29/3.46 | Cβ-Hβ in phenylcoumaran (C) |
| Bβ | 53.82/3.04 | Cβ-Hβ in β-β (resinol) (B) |
| OCH3 | 55.82/3.76 | C-H in methoxyls |
| Aγ | 60.19/3.59 | Cγ-Hγ in β-O-4 substructures (A) |
| Fγ | 61.73/4.08 | Cγ-Hγ in cinnamyl alcohol end-groups (F) |
| Cγ | 62.96/3.71 | Cγ-Hγ in phenylcoumaran (C) |
| Bγ | 71.10/3.73 | Cγ-Hγ in β-β resinol (B) |
| 71.13/4.13 | ||
| Aα | 71.41/4.73 | Cα-Hα in β-O-4 unit (A) |
| A′α | 74.26/5.91 | β-O-4, Cα-Hα in guaiacyl units (G) |
| Aβ | 83.99/4.29 | Cβ-Hβ in β-O-4 substructures (A) |
| 81.18/4.56 | ||
| 83.50/4.48 | ||
| Bα | 85.28/4.62 | Cα-Hα in β-β resinol (B) |
| B′α | 83.40/4.83 | Cα-Hα in β-β (B′, tetrahydrofuran) |
| Cα | 87.22/5.46 | Cα-Hα in phenylcoumaran (C) |
| X1 | 92.62/4.86 | C1-H1 in α-D-Xylose |
| 97.83/4.22 | C1-H1 in β-D-Xylose | |
| G2 | 108.55/6.92 | C2-H2 in guaiacyl units (G) |
| 110.38/6.90 | ||
| G′2 | 110.71/7.39 | α C2-H2 in G-type structural units with oxidized sites |
| 112.58/7.31 | ||
| G5 | 115.13/6.74 | C5-H5 in guaiacyl units (G) |
| 115.37/6.98 | ||
| G6 | 118.61/6.77 | C6-H6 in guaiacyl units (G) |
| 120.84/6.74 | ||
| G′6 | 118.81/7.32 | α C6-H6 in G-type structural units with oxidized sites |
| FA6 | 123.30/7.21 | C6-H6 in ferulate (p-FA) |
| Eβ | 126.17/6.78 | Cβ-Hβ in cinnamyl aldehyde end-groups (E) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, P.; Xie, J.; Peng, W.; Xie, J.; An, J.; Zhang, G.; Chen, J. Thermal Condensation of Dehydrogenation Polymer (DHP) with Xylose. Polymers 2024, 16, 3139. https://doi.org/10.3390/polym16223139
Wang P, Xie J, Peng W, Xie J, An J, Zhang G, Chen J. Thermal Condensation of Dehydrogenation Polymer (DHP) with Xylose. Polymers. 2024; 16(22):3139. https://doi.org/10.3390/polym16223139
Chicago/Turabian StyleWang, Peng, Jiaju Xie, Wenyao Peng, Junxian Xie, Junjian An, Guangyan Zhang, and Junjun Chen. 2024. "Thermal Condensation of Dehydrogenation Polymer (DHP) with Xylose" Polymers 16, no. 22: 3139. https://doi.org/10.3390/polym16223139
APA StyleWang, P., Xie, J., Peng, W., Xie, J., An, J., Zhang, G., & Chen, J. (2024). Thermal Condensation of Dehydrogenation Polymer (DHP) with Xylose. Polymers, 16(22), 3139. https://doi.org/10.3390/polym16223139






